Abstract
Purpose: Medullary thyroid carcinoma (MTC) is a rare neuroendocrine malignancy with relatively early lymphatic metastatic spread. The clinical features of MTC remain controversial owing to the low incidence rate. This study aimed to analyze the clinical characteristics, prognostic factors, and long-term follow-up of MTC. Methods: Medical records of MTC patients treated at our hospital between December 2000 and November 2020 were reviewed retrospectively. Clinicopathologic features of MTC were analyzed using univariate and multivariate analyses. Cumulative survival rates were estimated using the Kaplan-Meier method. Results: In total, 152 patients with MTC were included. The rates of central and lateral lymph node metastases (LNM) were 52.0% and 42.8%, respectively. All patients were followed up with a median follow-up time of 43.5 (17.0−76.3) months. Univariate and multivariate analyses identified two independent factors associated with progressive disease. They were lateral LNM (p < 0.001) and lymph node ratio (LNR) >1/3 (p = 0.009). The 5-, 10-, and 15-year cumulative overall survival (OS) rates of MTC were 88.2%, 83.1%, and 76.2%, respectively. The 5-, 10-, and 15-year cumulative disease-free survival (DFS) rates were 61.8%, 48.6%, and 38.2%, respectively. Patients with stage I, II, and III disease had significantly longer OS and DFS than those with stage IV disease (p < 0.001). Conclusion: MTC is a rare endocrine malignancy and LNM is common. Patients with lateral LNM and LNR >1/3 are more likely to develop progressive disease. The long-term OS rates of MTC are good, but long-term DFS rates are poor.
Keywords: medullary thyroid carcinoma, lymph node metastases, lymph node ratio, prognostic factor, cumulative survival rates
Introduction
Medullary thyroid carcinoma (MTC) is a neuroendocrine thyroid carcinoma that originates from parafollicular C cells. 1 It is the third most common subtype of thyroid cancer, although it accounts for only 1–2% of all thyroid carcinomas.2,3 The incidence of MTC has increased by approximately 50% between 1983 and 2012 in the United States. 4 MTC carries the second worst prognosis of all thyroid carcinoma (after anaplastic thyroid carcinoma), and is responsible for 15% of thyroid carcinoma-related deaths.2,5 The 10-year mortality rate of MTC ranges from 13% to 38%. 6 MTC can be classified as hereditary or sporadic, with the latter accounting for 75% of the total. Hereditary MTC is caused by germline activating mutations in the RET proto-oncogene, and is a feature of multiple endocrine neoplasia type 2, an autosomal dominant disorder. However, the molecular mechanisms of sporadic MTC are less clear. Both somatic RET and RAS mutations may be responsible for sporadic MTC.
MTC is characterized by relatively early lymphatic metastatic spread and slow tumor growth.3,7 Its prognosis is related to various parameters, including distant metastases, lymph node metastases (LNM), local tumor invasion, and initial treatment. Currently, surgery is uniformly recommended as the preferred treatment for MTC.3,8,9 The minimum operative treatment is total thyroidectomy and central lymph node dissection (LND). However, due to the low incidence rate, the clinical characteristics and ideal surgical management of MTC remain largely unexplored, and prognostic factors have not been well characterized. The purpose of this study was to analyze the clinical features of MTC and to identify potential prognostic factors, with the aim of increasing the understanding of this rare disease among the medical community.
Materials and Methods
Patients
The medical records of MTC patients treated at our hospital between December 2000 and November 2020 were reviewed retrospectively. Patients were selected based on the following inclusion criteria: (1) radical surgery conducted at our hospital, (2) MTC confirmed via postoperative pathology, (3) patients were followed up at our hospital, and (4) complete perioperative records. Because our institution is a tertiary hospital, patients are transferred to our center for radical repeat surgery if they have undergone unsatisfactory primary surgery at a local hospital. Patients who did not have detailed pathological reports from their primary surgery were excluded. Clinical data were compiled from inpatient and outpatient medical records by two independent doctors. A retrospective database containing demographic characteristics, results of imaging and blood tests, operation information, pathology results, and follow-up information was constructed for analysis. The reporting of this study conforms to STROBE guidelines. 10
Treatment and Follow-up
All patients underwent preoperative thyroid function tests and ultrasound examinations. Fine-needle aspiration cytology is recommended for patients with high-risk nodules ≥1 cm, but is not required. An intra-operative frozen section analysis was routinely performed in patients who had not had a preoperative biopsy. If MTC was diagnosed on preoperative biopsy or intraoperative analysis, patients underwent a total thyroidectomy with central LND. For patients who had undergone primary surgery at a local hospital before being transferred to our center, residual lobectomy with central LND was performed. Lateral LND was performed in patients with clinically positive lateral neck lymph nodes on ultrasonography. Postoperative pathological diagnoses were confirmed by two pathologists. All patients were administered oral Euthyrox for thyroid hormone replacement, but no suppressive therapy was administered. Outpatient interviews, telephone calls, and WeChat were used for follow-up. Patients were first followed up 1 month after surgery, then every 3 months for 1 year, and then every 6 months thereafter. Ultrasonography and blood tests were performed routinely, and computed tomography was performed annually, or if other tests revealed abnormalities. All patients who did not undergo lateral LND had negative lateral neck ultrasonography result before surgery, and were reviewed 3 and 6 months after surgery by ultrasonography to exclude occult metastasis. Only lateral LNM found more than 6 months after surgery were considered to be recurrence.
Classifications
Postoperative complications were defined as any deviation from the normal postoperative course and classified according to the Clavien-Dindo classification of surgical complications. 11 The American Society of Anesthesiologists (ASA) classification was used to assess the risk of anesthesia, and an ASA grade ≥III was defined as high risk. 12 A tumor was considered multifocal if at least two foci were discovered in the unilateral or bilateral lobes. The major and total tumor size were defined as the diameter of the largest lesion and the sum of the diameters of all lesions, respectively, and 2 cm was used as a size threshold in this study. In patients with preoperatively suspected MTC and postoperative diagnosis of MTC, the baseline calcitonin level was tested preoperatively and at 1 month after surgery, respectively. The reference levels of calcitonin and carcinoembryonic antigen (CEA) were <10 pg/mL and ≤5 ng/mL, respectively. Lymph node ratio (LNR) was defined as the number of positive lymph nodes divided by the number of lymph nodes removed. For patients who did not have lateral LNM, LNR was calculated by dividing the number of positive central lymph nodes by the total number of removed lymph nodes.
Based on follow-up information, patients’ outcomes were classified into five categories: (1) disease-free, with normal calcitonin and no new lesions on imaging; (2) stable disease, with moderately elevated calcitonin (10−150 pg/mL) and no new lesions on imaging; (3) unstable disease, with markedly elevated calcitonin (>150 pg/mL) but no new lesions on imaging; (4) recurrence, with recurrent or metastatic lesions on imaging; and (5) death.
Statistical Analysis
The Statistical Package for Social Sciences software (version 25.0, IBM Corp., Armonk, NY, USA) was used for all analyses. Continuous variables with normal distribution were shown as the mean ± standard deviation, and continuous variables with skewed distribution were represented as median (25th−75th percentiles). Categorical variables were presented as absolute numbers or frequencies. Differences between study groups were analyzed using the χ2 test, Fisher’s exact test, or t-test, as appropriate. Logistic multivariate regression analyses were performed to identify independent prognostic factors. The Kaplan-Meier method with log rank test was used to estimate the survival probability. Statistical significance was set at p <0.05.
Results
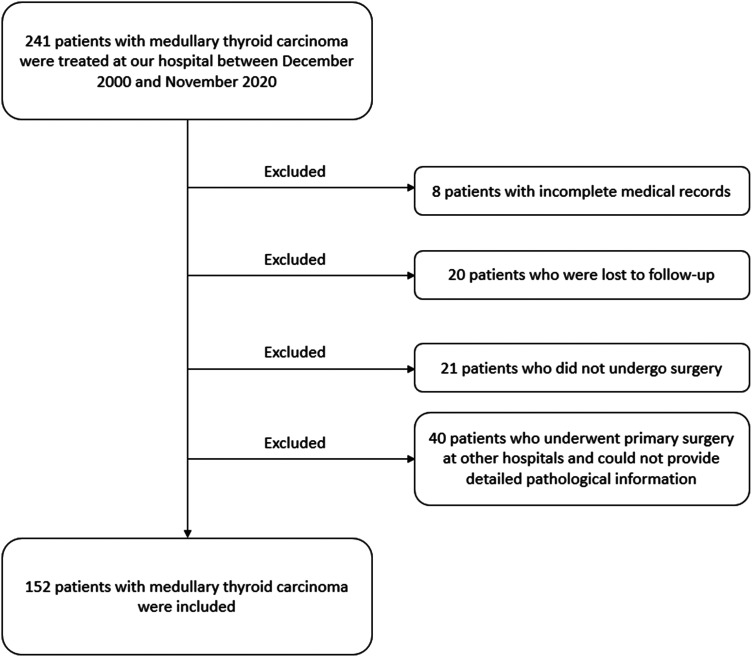
In total, 241 patients with MTC were treated at our hospital between December 2000 and November 2020. Based on the inclusion and exclusion criteria, 152 patients were included, while the remaining 89 patients were excluded (Figure 1). The clinicopathological features of the 152 included patients are listed in Table 1. There were 59 men (38.8%) and 93 women (61.2%), with a male to female ratio of 1:1.58. Only 36 patients (23.7%) had symptoms such as neck discomfort (n = 15), dysphagia (n = 4), headache (n = 4), palpitation (n = 3), diarrhea (n = 3), hoarseness (n = 3), dyspnea (n = 2), and general weakness (n = 2). Sporadic MTC accounted for 82.2% (125/152) of the total cases. Approximately 45.4% (69/152) of patients were classified as tumor stage IV according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (eighth edition). 13
Figure 1.
Flow diagram of the process of selection of the patients included in this study.
Table 1.
Clinicopathological features of the 152 patients with MTC.
| Characteristic | Value |
|---|---|
| Gender male/female (n) | 59/93 (38.8%/61.2%) |
| Age (years) | 46.5 ± 12.3 (range, 19 to 77) |
| BMI (kg/m2) | 23.5 (21.0−25.5) |
| Symptomatic (n) | 36 (23.7%) |
| Subtype (n) | |
| Sporadic MTC | 125 (82.2%) |
| Hereditary MTC | 27 (17.8%) |
| Surgery pattern (n) | |
| TT with central LND | 67 (44.1%) |
| TT with central and lateral LND | 85 (55.9%) |
| Multifocal tumor (n) | 43 (28.3%) |
| Bilateral lobes tumor (n) | 39 (25.7%) |
| Central LNM (n) | 79 (52.0%) |
| Lateral LNM (n) | 65 (42.8%) |
| Tumor staging (n) | |
| I | 41 (27.0%) |
| II | 19 (12.5%) |
| III | 23 (15.1%) |
| IV | 69 (45.4%) |
MTC: medullary thyroid carcinoma; BMI: body mass index; TT: total thyroidectomy; LND: lymph node dissection; LNM: lymph node metastases.
All patients underwent operation and the median operative time was 125 (90−185) min. The diagnosis of MTC was confirmed by postoperative pathology in all patients. The median major and total tumor size were 1.6 (1.0−2.5) cm and 1.8 (1.2−3.0) cm, respectively. Postoperative complications included hypocalcemia (n = 5), recurrent laryngeal nerve paralysis (n = 3), lymphatic leakage (n = 3), fever (n = 2), bleeding (n = 2), pulmonary infection (n = 2), Horner syndrome (n = 2), wound infection (n = 1), and jugular vein thrombus (n = 1). The two patients with postoperative bleeding underwent reoperation, and the other patients were treated conservatively. According to the Clavien-Dindo classification of surgical complications, 6, 13, and 2 patients were classified as grade I, II, and IIIb, respectively.
As of December 2020, 152 patients were followed up with a median follow-up time of 43.5 (17.0−76.3) months, and the range of follow up was 1–193 months. Ninety-three (61.2%) patients survived without disease, 10 (6.6%) survived with stable disease, 5 (3.3%) survived with unstable disease, 29 (19.1%) had recurrence, and 15 (9.9%) died. Local recurrence (primary tumor site), regional recurrence (central or lateral LNM) and distant metastasis were found in 5, 28 and 11 patients, respectively. Of the 28 patients with regional recurrence, 20 patients underwent initial lateral LND (19 and 1 with positive and negative lateral LNM, respectively), and the rest 8 patients did not (5 and 3 with central and lateral compartment recurrence, respectively). All patients were divided into two groups: survival (n = 137) and death (n = 15). The clinicopathological characteristics of the two groups are presented and compared in Table 2. Significantly fewer patients had central LNM (p = 0.022), lateral LNM (p = 0.002), contralateral central LNM (p = 0.031), contralateral lateral LNM (p = 0.022), and LNR ˃1/3 (p = 0.028) in the survival group. Multivariate logistic regression analyses were performed to examine the associations between the selected variables and prognosis (Table 3). No variables were independently associated with death. Meanwhile, all patients were also divided into two groups: disease-free group (n = 93) and others (n = 59). The differences between the two groups are presented in Table 4. The disease-free group had significantly fewer patients with male sex (p = 0.037), postoperative complications (p = 0.001), capsular invasion (p = 0.002), extrathyroidal invasion (p = 0.001), central LNM (p < 0.001), lateral LNM (p < 0.001), contralateral central LNM (p = 0.019), contralateral lateral LNM (p = 0.027), and LNR ˃1/3 (p < 0.001). The associations between the selected variables and prognosis were analyzed using multivariate logistic regression (Table 5). Two variables were independently associated with progressive disease: lateral LNM (odds ratio [OR] = 5.73, 95% confidence interval [CI]: 2.21−14.84; p < 0.001) and LNR ˃1/3 (OR = 4.52, 95% CI: 1.46−13.98; p = 0.009).
Table 2.
Comparison between MTC patients who survived and died.
| Survival (n = 137) | Death (n = 15) | P-value | |
|---|---|---|---|
| Male (n) | 51 (37.2%) | 8 (53.3) | 0.224 |
| Age (years) | 46.2 ± 12.4 | 49.3 ± 12.2 | 0.363 |
| BMI ≥28 (kg/m2) | 16 (11.7%) | 0 (0%) | 0.339 |
| Hereditary (n) | 26 (19.0%) | 1 (6.7%) | 0.407 |
| Hashimoto's disease (n) | 20 (14.6%) | 1 (6.7%) | 0.652 |
| Calcitonin ˃500 pg/mL (n) | 26 (19.0%) | 4 (26.7%) | 0.712 |
| CEA ˃50 ng/mL (n) | 28 (20.4%) | 2 (13.3%) | 0.753 |
| ASA ≥III (n) | 3 (2.2%) | 2 (13.3%) | 0.077 |
| Postoperative complications (n) | 18 (13.1%) | 3 (20.0%) | 0.736 |
| Multifocal tumor (n) | 39 (28.5%) | 4 (26.7%) | 1.000 |
| Bilateral tumor (n) | 35 (25.5%) | 4 (26.7%) | 1.000 |
| Major tumor size ˃2 cm (n) | 48 (35.0%) | 6 (40.0%) | 0.703 |
| Total tumor size ˃2 cm (n) | 57 (41.6%) | 8 (53.3%) | 0.383 |
| Capsular invasion (n) | 42 (30.7%) | 5 (33.3%) | 1.000 |
| Extrathyroidal invasion (n) | 13 (9.5%) | 4 (26.7%) | 0.116 |
| Central LNM (n) | 67 (48.9%) | 12 (80.0%) | 0.022 |
| Lateral LNM (n) | 53 (38.7%) | 12 (80.0%) | 0.002 |
| Contralateral central LNM (n) | 9 (6.6%) | 4 (26.7%) | 0.031 |
| Contralateral lateral LNM (n) | 4 (2.9%) | 3 (20.0%) | 0.022 |
| LNR ˃1/3 (n) | 32 (23.4%) | 8 (53.3%) | 0.028 |
MTC: medullary thyroid carcinoma; BMI: body mass index; CEA: carcinoembryonic antigen; ASA: American Society of Anesthesiologists; LNM: lymph nodes metastases; LNR: lymph node ratio.
Table 3.
Multivariate analyses for risk factors for death for patients with MTC.
| P-value | OR | 95% CI | |
|---|---|---|---|
| Central LNM | 0.683 | 1.409 | 0.272−7.291 |
| Lateral LNM | 0.095 | 3.708 | 0.798−17.234 |
| Contralateral central LNM | 0.371 | 2.069 | 0.420−10.196 |
| Contralateral lateral LNM | 0.389 | 2.364 | 0.334−16.755 |
| LNR ˃1/3 | 0.601 | 1.440 | 0.368−5.644 |
MTC: medullary thyroid carcinoma; OR: odds ratio; CI: confidence interval; LNM: lymph nodes metastases; LNR: lymph node ratio.
Table 4.
Comparison between MTC patients who survived without disease and others.
| Disease free (n = 93) | Others (n = 59) | P-value | |
|---|---|---|---|
| Male (n) | 30 (32.3%) | 29 (49.2%) | 0.037 |
| Age (years) | 46.4 ± 12.5 | 46.7 ± 12.1 | 0.850 |
| BMI ≥28 (kg/m2) | 11 (11.8%) | 5 (8.5%) | 0.511 |
| Hereditary (n) | 20 (21.5%) | 7 (11.9%) | 0.130 |
| Hashimoto's disease (n) | 15 (16.1%) | 6 (10.2%) | 0.299 |
| Calcitonin ˃500 pg/mL (n) | 14 (15.1%) | 16 (27.1%) | 0.069 |
| CEA ˃50 ng/mL (n) | 18 (19.4%) | 12 (20.3%) | 0.882 |
| ASA ≥III (n) | 1 (1.1%) | 4 (6.8%) | 0.146 |
| Postoperative complications (n) | 6 (6.5%) | 15 (25.4%) | 0.001 |
| Multifocal tumor (n) | 27 (29.0%) | 16 (27.1%) | 0.799 |
| Bilateral tumor (n) | 24 (25.8%) | 15 (25.4%) | 0.958 |
| Major tumor size ˃2 cm (n) | 32 (34.4%) | 22 (37.3%) | 0.718 |
| Total tumor size ˃2 cm (n) | 38 (40.9%) | 27 (45.8%) | 0.552 |
| Capsular invasion (n) | 20 (21.5%) | 27 (45.8%) | 0.002 |
| Extrathyroidal invasion (n) | 4 (4.3%) | 13 (22.0%) | 0.001 |
| Central LNM (n) | 35 (37.6%) | 44 (74.6%) | <0.001 |
| Lateral LNM (n) | 22 (23.7%) | 43 (72.9%) | <0.001 |
| Contralateral central LNM (n) | 4 (4.3%) | 9 (15.3%) | 0.019 |
| Contralateral lateral LNM (n) | 1 (1.1%) | 6 (10.2%) | 0.027 |
| LNR ˃1/3 (n) | 11 (11.8%) | 29 (49.2%) | <0.001 |
MTC: medullary thyroid carcinoma; BMI: body mass index; CEA: carcinoembryonic antigen; ASA: American Society of Anesthesiologists; LNM: lymph nodes metastases; LNR: lymph node ratio.
Table 5.
Multivariate analyses for risk factors for progressive disease for patients with MTC.
| P-value | OR | 95% CI | |
|---|---|---|---|
| Lateral LNM | <0.001 | 5.726 | 2.210−14.839 |
| LNR ˃1/3 | 0.009 | 4.521 | 1.462−13.977 |
| male gender | 0.059 | 2.354 | 0.968−5.721 |
| postoperative complications | 0.272 | 2.045 | 0.570−7.339 |
| capsular invasion | 0.738 | 1.202 | 0.410−3.525 |
| extrathyroidal invasion | 0.191 | 2.883 | 0.590−14.094 |
| central LNM | 0.722 | 0.818 | 0.269−2.482 |
| contralateral central LNM | 0.642 | 1.491 | 0.277−8.011 |
| contralateral lateral LNM | 0.580 | 0.484 | 0.037−6.323 |
MTC: medullary thyroid carcinoma; OR: odds ratio; CI: confidence interval; LNM: lymph nodes metastases; LNR: lymph node ratio.
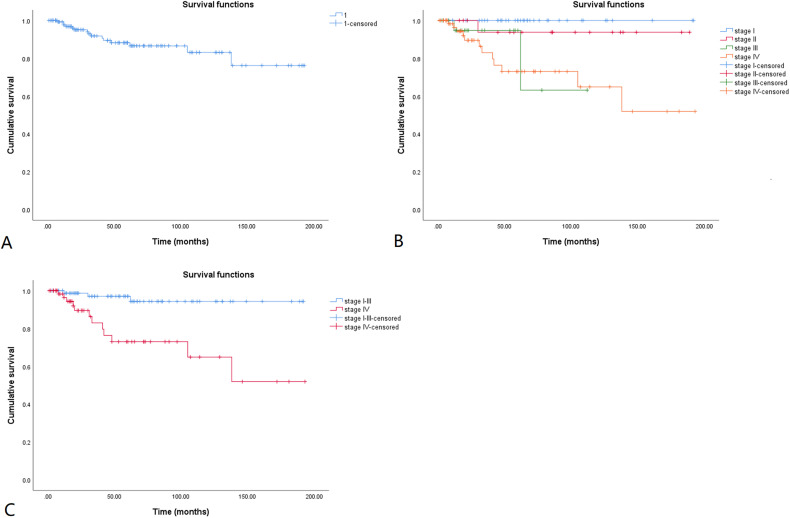
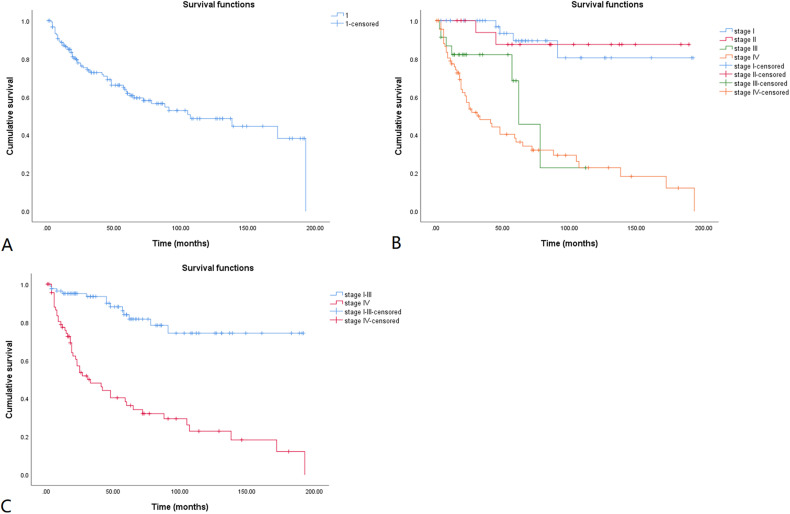
The cumulative overall survival (OS) rates for the 152 patients with MTC are shown in a Kaplan-Meier curve (Figure 2A). The 5-, 10-, and 15-year cumulative OS rates were 88.2%, 83.1%, and 76.2%, respectively. The cumulative OS rates calculated according to tumor stage are shown in Figure 2B. Because most of the deaths were in patients with stage IV disease, and the curves in Figure 2B overlap, patients with stage I, II, and III disease were combined into one group and compared with those with stage IV disease (Figure 2C). Patients with stage I, II, and III MTC had significantly longer OS than those with stage IV MTC (log rank, p < 0.001). The Kaplan-Meier method was also used to estimate the cumulative disease-free survival (DFS) rates for the 152 patients with follow-up data (Figure 3A). The 5-, 10-, and 15-year cumulative DFS rates were 61.8%, 48.6%, and 38.2%, respectively. The cumulative DFS rates calculated according to tumor stage are shown in Figure 3B, and the comparison of cumulative DFS rates between the stage IV and stage I to III groups is shown in Figure 3C. Patients with stage I to III MTC had significantly longer DFS than those with stage IV disease (log rank, p < 0.001).
Figure 2.
Kaplan-Meier overall survival curve for MTC patients. The cumulative overall survival rate is shown in Figure 2A. The cumulative overall survival rates calculated according to tumor stages are shown in Figure 2B. Tumor stage I-III is compared with stage IV in Figure 2C. The 5-, 10-, and 15-year cumulative overall survival rates were 88.2%, 83.1%, and 76.2%, respectively. MTC patients with stage I-III had significantly longer overall survival than those with stage IV (log rank, p < 0.001).
Figure 3.
Kaplan-Meier disease-free survival curve for MTC patients. The cumulative disease-free survival rate is shown in Figure 3A. The cumulative disease-free survival rates calculated according to tumor stages are shown in Figure 3B. Tumor stage I-III is compared with stage IV in Figure 3C. The 5-, 10-, and 15-year cumulative disease-free survival rates were 61.8%, 48.6%, and 38.2%, respectively. MTC patients with stage I-III had significantly longer disease-free survival than those with stage IV (log rank, p < 0.001).
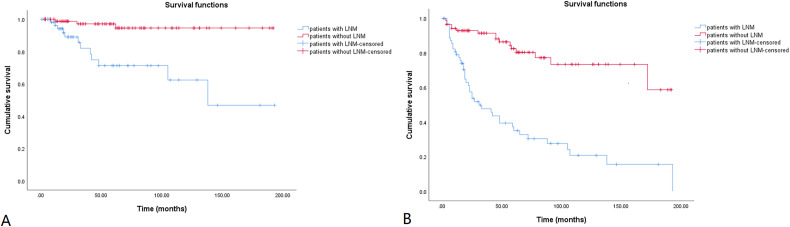
In order to verify the importance of lateral LNM, patients with (n = 65) and without (n = 87) lateral LNM were compared in Table 6. There were more patients with higher calcitonin and CEA levels, postoperative complications, multifocality, bilaterality, capsular invasion, extrathyroidal invasion, and LNR ˃1/3 in the lateral LNM group. The Kaplan-Meier curve was used to show the cumulative OS and DFS rates for patients with and without lateral LNM in Figure 4. The 15-year cumulative OS and DFS rates of patients with lateral LNM were 46.8% and 15.5%, respectively. Meanwhile, the 15-year cumulative OS and DFS rates of patients without lateral LNM were 94.6% and 58.7%, respectively. Patients with lateral LNM had significantly worse OS and DFS than those without (log rank, p < 0.001).
Table 6.
Comparison between MTC patients with and without lateral LNM.
| lateral LNM | P-value | ||
|---|---|---|---|
| yes (n = 65) | no (n = 87) | ||
| Male (n) | 29 (44.6%) | 30 (34.5%) | 0.205 |
| Age (years) | 47.0 ± 11.8 | 46.1 ± 12.8 | 0.643 |
| BMI ≥28 (kg/m2) | 7 (10.8%) | 9 (10.3%) | 0.933 |
| Hereditary (n) | 13(20.0%) | 14 (16.1%) | 0.533 |
| Hashimoto's disease (n) | 6 (9.2%) | 15 (17.2%) | 0.157 |
| Calcitonin ˃500 pg/mL (n) | 19 (29.2%) | 11 (12.6%) | 0.011 |
| CEA ˃50 ng/mL (n) | 21 (32.3%) | 9 (10.3%) | 0.001 |
| ASA ≥III (n) | 3 (4.6%) | 2 (2.3%) | 0.428 |
| Postoperative complications (n) | 18 (27.7%) | 3 (3.4%) | <0.001 |
| Multifocal tumor (n) | 24 (36.9%) | 19 (21.8%) | 0.041 |
| Bilateral tumor (n) | 22 (33.8%) | 17 (19.5%) | 0.046 |
| Major tumor size ˃2 cm (n) | 26 (40.0%) | 28 (32.2%) | 0.319 |
| Total tumor size ˃2 cm (n) | 30 (46.2%) | 35 (40.2%) | 0.465 |
| Capsular invasion (n) | 31 (47.7%) | 16 (18.4%) | <0.001 |
| Extrathyroidal invasion (n) | 12 (18.5%) | 5 (5.7%) | 0.014 |
| LNR ˃1/3 (n) | 29 (44.6%) | 11 (12.6%) | <0.001 |
MTC: medullary thyroid carcinoma; LNM, lymph node metastases; BMI: body mass index; CEA: carcinoembryonic antigen; ASA: American Society of Anesthesiologists; LNR: lymph node ratio.
Figure 4.
Kaplan-Meier survival curve for patients with and without lateral lymph node metastasis. The cumulative overall and disease-free survival rates are shown in Figure 4A and B, respectively. The 15-year cumulative OS rates were 46.8% and 94.6% for patients with and without lateral lymph node metastasis, respectively. The 15-year cumulative DFS rates were 15.5% and 58.7% for patients with and without lateral lymph node metastasis, respectively. Patients with lateral lymph node metastasis had worse overall (log rank, p < 0.001) and disease-free survival (log rank, p < 0.001).
Discussion
The present study found that the rates of central and lateral LNM in MTC were 52.0% (79/152) and 42.8% (65/152), respectively. Lateral LNM and LNR ˃1/3 were found to be independently associated with progressive disease. The 5-, 10-, and 15-year cumulative OS rates of MTC were 88.2%, 83.1%, and 76.2%, respectively. The 5-, 10-, and 15-year cumulative DFS rates were 61.8%, 48.6%, and 38.2%, respectively. Patients with stage I to III MTC had significantly longer OS and DFS than those with stage IV disease.
The clinical features, treatment, and prognosis of MTC are quite different from those of differentiated thyroid carcinomas due to their different origins.3,14 Due to its low incidence, the clinical characteristics of MTC remain understudied. Previous studies are mainly retrospective. The majority of MTC patients are diagnosed in the fourth and fifth decades of life. Ahn et al. 14 reported 1790 MTC patients and the mean age at diagnosis was 55.4 ± 12.5 years. Manjunath et al. 15 retrospectively analyzed 82 patients and found that the mean age was 42.07 ± 14.70 years. In the present study, the mean age of onset was similar, at 46.5 ± 12.3 years. A female preponderance was reported by most previous studies,2,14 although few studies have reported a similar ratio. 15 This study identified a male to female ratio of 1:1.58, supporting a female preponderance. LNM is common in MTC. Fan et al. 16 reported that LNM was found in 54.3% of patients with MTC. Meng et al. 17 used the Surveillance, Epidemiology, and End Results Program (SEER) database and found that the LNM rate was 35.54% in 1466 MTC patients. The results of our study are consistent with those reported in the literature.
In the present study, lateral LNM and LNR ˃1/3 were proven to be independently associated with progressive disease. Nodal status has been reported to be the single most important prognostic factor in MTC. 18 Kuo et al. 19 analyzed 609 MTC patients and reported that patients without LNM had similar survival to the general population. Regional LNM can result in poorer survival and lower biochemical cure rates.20-22 Momin et al. 23 retrospectively studied 67 patients with MTC and found that the presence of cervical metastases was a poor prognostic factor. In a previous study, the 10-year survival rates of MTC were 95.6% and 75.5% in patients with tumors limited to the gland and cervical LNM, respectively. 24 Lateral LNM results in worse TNM staging than central LNM. 13 The LNR reflects not only the extent of the tumor, but also the extent of surgery. Previous studies have shown that LNR can serve as a prognostic factor in several different tumors.25-27 LNR has also been recognized as an important prognostic factor for MTC. Kotwal et al. 28 reported 163 MTC patients and found that lateral LNM and LNR were significant predictors of loco-regional recurrence or persistent disease. Rozenblat et al. 7 performed a retrospective multicenter study and revealed that LNR was an independent factor for DFS. Chen et al. 29 used the SEER database to study 1237 patients. They found that LNR was an independent predictor of survival in MTC patients. The importance of lateral LNM and LNR was verified in the present study. For patients with these two characteristics, more attention should be paid to the possibility of recurrence and metastases during the follow-up process. Meanwhile, by comparing the clinicopathologic features and survival of patients with and without lateral LNM, we found that in the group of patients with lateral LNM, the OS and DFS were worse, and there were more patients with higher calcitonin and CEA levels, postoperative complications, multifocality, bilaterality, capsular invasion, extrathyroidal invasion, and LNR ˃1/3. This also revealed that lateral LNM could indicate tumor stage and prognosis. Based on these results, lateral LND should be considered for all the patients with MTC.
Several other factors have been associated with the prognosis of patients with MTC. Age at the time of diagnosis has been reported to be an independent prognostic factor. 18 Based on the SEER database, Gogna et al. 30 analyzed 2533 MTC patients aged ≥45 years and found that increasing age was detrimental to OS. In another study, age >55 years was recommended as the cutoff point for prognosis. 28 Male sex was also reported to be a risk factor associated with poor survival in several different studies.15,28,31 Meanwhile, it has been reported that increasing tumor size could increase the risk of tumor recurrence. 23 A larger tumor size was associated with lateral LNM and poor clinical outcomes.23,32,33 In a retrospective study, patients with T1 tumors had no difference in 5-year survival, regardless of whether they underwent total thyroidectomy. 34 The same result was also reported in patients with T2 tumors, although the follow-up time was shorter. 34 Hamdy et al. 2 reported that both the presence of LNM and the involved side were predictors of prognosis. Contralateral LNM might indicate a worse prognosis, although the staging according to the AJCC Cancer Staging Manual eighth was not different. All the above factors were analyzed in this study, but no significant result was obtained. This may be related to the limited number of patients in this single-center study.
Although MTC can be aggressive and standard chemotherapy, radiotherapy, and radioactive iodine are ineffective, it can run a relatively indolent course. Patients may survive for long periods of time. Momin et al. 23 reported a group of patients with an OS rate of 89.1% at 5 years. The 10-year survival rate of MTC has been reported to range from 69% to 89%. 20 Clark et al. 35 performed a retrospective cohort study and found that the 5-, 10-, and 20-year OS rates of MTC were 97%, 88%, and 84%, respectively, and the 5-, 10-, and 20-year DFS rates were 97%, 74%, and 29%, respectively. In the present study, the long-term OS rates of MTCs were similar. However, there have been some reports with different conclusions. A previous study reported that the 10-year survival rate was only 35% in South India. 15 Lennon et al. 36 reported a 10-year survival rate of 48.63% in MTC patients older than 45 years. Different survival rates may be affected by race, treatment method, and follow-up rate. In the present study, the mortality rate was relatively low, which might have been caused by the relatively short follow up. Because short follow up time could lead to the inability to see the final prognosis (death or survival) in some patients with progressive disease, it would preclude identifying factors associated with OS rather than DFS. Longer follow up was needed to get more accurate conclusions. AJCC stage had a negative association with prognosis, and stage IV had significantly lower survival than stage III and below. 15 This phenomenon was also observed and verified in this study.
There were some limitations to this study. First, the registration information, investigated variables, and sample size could not be planned beforehand because of the retrospective nature of the study. Second, the patient volume was limited owing to the low incidence of MTC and the study’s single-center nature. Prospective and multicenter clinical trials should be performed to overcome these limitations and to derive more reliable and robust data.
Conclusion
MTC is a rare endocrine malignancy with a female preponderance. The rates of central and lateral LNM were 52.0% and 42.8%, respectively. Lateral LNM and LNR ˃1/3 are independent risk factors for progressive disease. The 5-, 10-, and 15-year cumulative OS rates of MTC were 88.2%, 83.1%, and 76.2%, respectively. The 5-, 10-, and 15-year cumulative DFS rates of MTC were 61.8%, 48.6%, and 38.2%, respectively. Patients with stage I, II, and III MTC had significantly longer OS and DFS than those with stage IV disease.
Acknowledgments
We wish to thank our colleagues in the Department of Medical Records for their cooperation.
Abbreviations
- MTC
medullary thyroid carcinoma
- LNM
lymph node metastases
- LND
lymph node dissection
- ASA
American Society of Anesthesiologists
- CEA
carcinoembryonic antigen
- LNR
lymph node ratio
- AJCC
American Joint Committee on Cancer
- OR
odds ratio
- CI
confidence interval
- OS
overall survival
- DFS
disease-free survival
- SEER
Surveillance, Epidemiology, and End Results Program.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The study was supported by the Fundamental Research Funds for the Central Universities of China (3332019022).
Institutional Review Board Statement: The study was reviewed and approved by the Peking Union Medical College Hospital Institutional Review Board (Approval No. S-K1712).
Informed Consent Statement: The need for informed consent was waived due to the retrospective nature of the study.
ORCID iDs: Xin Wu https://orcid.org/0000-0002-3839-4768
Binglu Li https://orcid.org/0000-0002-9142-0793
References
- 1.Konstantinidis A, Stang M, Roman SA, Sosa JA. Surgical management of medullary thyroid carcinoma. Updates Surg. 2017;69(2):151-160. [DOI] [PubMed] [Google Scholar]
- 2.Hamdy O, Awny S, Metwally IH. Medullary thyroid cancer: epidemiological pattern and factors contributing to recurrence and metastasis. Ann R Coll Surg Engl. 2020;102(7):499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells SA, Jr, Asa SL, Dralle H, Elisei R, et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randle RW, Balentine CJ, Leverson GE, et al. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery. 2017;161(1):137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed SR, Ball DW. Clinical review: incidentally discovered medullary thyroid cancer: diagnostic strategies and treatment. J Clin Endocrinol Metab. 2011;96(5):1237-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maia AL, Wajner SM, Vargas CV. Advances and controversies in the management of medullary thyroid carcinoma. Curr Opin Oncol. 2017;29(1):25-32. [DOI] [PubMed] [Google Scholar]
- 7.Rozenblat T, Hirsch D, Robenshtok E, et al. The prognostic value of lymph node ratio in medullary thyroid carcinoma: a multi-center study. Eur J Surg Oncol. 2020;46(11):2023-2028. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell AL, Gandhi A, Scott-Coombes D, Perros P. Management of thyroid cancer: united Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130(S2):S150-S160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maia AL, Siqueira DR, Kulcsar MA, Tincani AJ, Mazeto GM, Maciel LM. Diagnosis, treatment, and follow-up of medullary thyroid carcinoma: recommendations by the thyroid department of the Brazilian society of endocrinology and metabolism. Arq Bras Endocrinol Metabol. 2014;58(7):667-700. [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. [DOI] [PubMed] [Google Scholar]
- 11.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurwitz EE, Simon M, Vinta SR, et al. Adding examples to the ASA-physical Status classification improves correct assignment to patients. Anesthesiology. 2017;126(4):614-622. [DOI] [PubMed] [Google Scholar]
- 13.Rosen JE, Lloyd RV, Brierley JD, et al. Thyroid - medullary. In: AJCC Cancer Staging Manual. 8th ed. Springer, 2017: 891-901. [Google Scholar]
- 14.Ahn HY, Chae JE, Moon H, Noh J, Park YJ, Kim SG. Trends in the diagnosis and treatment of patients with medullary thyroid carcinoma in korea. Endocrinol Metab (Seoul). 2020;35(4):811-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manjunath PR, Vadayath UM, Nair V, et al. Clinical profile of medullary thyroid carcinoma: audit from a tertiary care center in south India. Indian J Endocrinol Metab. 2020;24(4):355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan W, Xiao C, Wu F. Analysis of risk factors for cervical lymph node metastases in patients with sporadic medullary thyroid carcinoma. J Int Med Res. 2018;46(5):1982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng K, Luo H, Chen H, Guo H, Xia W. Prognostic value of numbers of metastatic lymph node in medullary thyroid carcinoma: a population-based study using the SEER 18 database. Medicine (Baltimore). 2019;98(1):e13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamatakos M, Paraskeva P, Katsaronis P, Tasiopoulou G, Kontzoglou K. Surgical approach to the management of medullary thyroid cancer: when is lymph node dissection needed? Oncology. 2013;84(6):350-355. [DOI] [PubMed] [Google Scholar]
- 19.Kuo EJ, Sho S, Li N, Zanocco KA, Yeh MW, Livhits MJ. Risk factors associated With reoperation and disease-specific mortality in patients With medullary thyroid carcinoma. JAMA Surg. 2018;153(1):52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin LX, Moley JF. Surgery for lymph node metastases of medullary thyroid carcinoma: a review. Cancer. 2016;122(3):358-366. [DOI] [PubMed] [Google Scholar]
- 21.Torresan F, Cavedon E, Mian C, Iacobone M. Long-Term outcome after surgery for medullary thyroid carcinoma: a single-center experience. World J Surg. 2018;42(2):367-375. [DOI] [PubMed] [Google Scholar]
- 22.Mathiesen JS, Kroustrup JP, Vestergaard P, et al. Survival and long-term biochemical cure in medullary thyroid carcinoma in Denmark 1997–2014: a nationwide study. Thyroid. 2019;29(3):368-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momin S, Chute D, Burkey B, Scharpf J. Prognostic variables affecting primary treatment outcome for medullary thyroid cancer. Endocr Pract. 2017;23(9):1053-1058. [DOI] [PubMed] [Google Scholar]
- 24.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107(9):2134-2142. [DOI] [PubMed] [Google Scholar]
- 25.Mansour J, Sagiv D, Alon E, Talmi Y. Prognostic value of lymph node ratio in metastatic papillary thyroid carcinoma. J Laryngol Otol. 2018;132(1):8-13. [DOI] [PubMed] [Google Scholar]
- 26.Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol. 2010;17(11):2847-2855. [DOI] [PubMed] [Google Scholar]
- 27.Mizrachi A, Hadar T, Rabinovics N, et al. Prognostic significance of nodal ratio in cutaneous squamous cell carcinoma of the head and neck. Eur Arch Otorhinolaryngol. 2013;270(2):647-653. [DOI] [PubMed] [Google Scholar]
- 28.Kotwal A, Erickson D, Geske JR, Hay ID, Castro MR. Predicting outcomes in sporadic and hereditary medullary thyroid carcinoma over Two decades. Thyroid. 2021;31(4):616-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Wang Y, Zhao K, Wang Y, He X. Postoperative nomogram for predicting cancer-specific and overall survival among patients with medullary thyroid cancer. Int J Endocrinol. 2020;2020:8888677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gogna S, Goldberg M, Samson D, et al. Medullary thyroid cancer in patients older than 45-epidemiologic trends and predictors of survival. Cancers (Basel). 2020;12(11):3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torresan F, Mian C, Cavedon E, Iacobone M. Cure and survival of sporadic medullary thyroid carcinoma following systematic preoperative calcitonin screening. Langenbecks Arch Surg. 2019;404(4):411-419. [DOI] [PubMed] [Google Scholar]
- 32.Oh HS, Kwon H, Song E, et al. Preoperative clinical and sonographic predictors for lateral cervical lymph node metastases in sporadic medullary thyroid carcinoma. Thyroid. 2018;28(3):362-368. [DOI] [PubMed] [Google Scholar]
- 33.Kim C, Baek JH, Ha E, et al. Ultrasonography features of medullary thyroid cancer as predictors of its biological behavior. Acta Radiol. 2017;58(4):414-422. [DOI] [PubMed] [Google Scholar]
- 34.Randle RW, Bates MF, Schneider DF, Sippel RS, Pitt SC. Survival in patients with medullary thyroid cancer after less than the recommended initial operation. J Surg Oncol. 2018;117(6):1211-1216. [DOI] [PubMed] [Google Scholar]
- 35.Clark JR, Fridman TR, Odell MJ, Brierley J, Walfish PG, Freeman JL. Prognostic variables and calcitonin in medullary thyroid cancer. Laryngoscope. 2005;115(8):1445-1450. [DOI] [PubMed] [Google Scholar]
- 36.Lennon P, Deady S, White N, et al. Aggressive medullary thyroid cancer, an analysis of the Irish national cancer registry. Ir J Med Sci. 2017;186(1):89-95. [DOI] [PubMed] [Google Scholar]