Abstract
Colorectal cancer (CRC) is a major contributor to cancer-associated morbidity worldwide and over one-third of CRC is located in the rectum. Neoadjuvant chemoradiotherapy (nCRT) followed by surgical resection is commonly applied to treat locally advanced rectal cancer (LARC). In this review, we summarize current and novel concepts of neoadjuvant therapy for LARC such as total neoadjuvant therapy and describe how these developments impact treatment response. Moreover, as response to nCRT is highly divergent in rectal cancers, we discuss the role of potential predictive biomarkers. We review recent advances in biomarker discovery, from a clinical as well as a histopathological and molecular perspective. Furthermore, the role of emerging predictive biomarkers derived from the tumor environment such as immune cell composition and gut microbiome is presented. Finally, we describe how different tumor models such as patient-derived cancer organoids are used to identify novel predictive biomarkers for chemoradiotherapy (CRT) in rectal cancer.
Keywords: biomarkers, neoadjuvant chemoradiotherapy, organoids, pathological complete response, predictive markers, rectal cancer
Introduction
Colorectal cancer (CRC) is the third most frequently diagnosed cancer entity worldwide and a leading cause of cancer-related mortality. 1 Rectal cancers account for over one-third of CRC and are frequently diagnosed at a locally advanced stage, which is commonly defined as Union for International Cancer Control (UICC) T3/T4 stage and node negative or positive disease.2,3 Neoadjuvant chemoradiotherapy (nCRT) followed by surgical resection is a standard treatment for locally advanced rectal cancer (LARC) that is located in the middle or lower rectum. 3 The benefits of nCRT comprise a better local disease control and a higher sphincter preservation rate in tumors of the lower rectum compared to postoperative chemoradiotherapy (CRT) in patients with LARC. 4 Pathologic complete response (pCR) is defined as the absence of viable tumor cells in the rectal wall and lymph nodes upon histological examination of the resected specimen.5,6 It is a frequently used surrogate endpoint to evaluate response to neoadjuvant treatment in LARC, as pCR correlates with a significant reduction of local recurrences and an improved overall survival (OS). 7 In contrast, clinical complete response (cCR) refers to the absence of residual tumors, ulcerations, or rectal wall irregularities on both clinical and radiological assessment. It is used as a surrogate marker for pCR in clinical trials that assess treatment response of LARC to nCRT.8,9 Observations from many clinical studies demonstrate that the response to nCRT is highly variable in LARC.6,10 While approximately 20–30% of patients with rectal cancers achieve either pCR or cCR with conventional nCRT, there is a significant proportion of tumors that do not respond to nCRT.11–13 Since nCRT can cause specific, treatment-associated toxicities,14,15 it is important to select the intensity of the neoadjuvant therapy based on the potential benefit for the patient. Therefore, identifying biomarkers that predict response to nCRT is an important clinical challenge in the management of LARC. In this review, we present current and novel approaches of neoadjuvant therapy for LARC and their impact on tumor response. Recent advances in the discovery of predictive biomarkers for nCRT of rectal cancer are outlined and critically discussed, including clinical, histopathological, and molecular markers. Furthermore, we describe how different rectal cancer models including cancer organoids can be used to identify novel predictive biomarkers for CRT response.
Current concepts of nCRT of rectal cancer
Based on the results of the landmark CAO/ARO/AIO-94 trial, the current standard neoadjuvant treatment of LARC is a conventionally fractionated radiation (usually a total dose of 50.4 Gy in 28 fractions of 1.8 Gy) with concurrent fluoropyrimidine-based chemotherapy.4,16,17 Six to eight weeks after nCRT, the tumor is surgically removed by total mesorectal excision (TME). Results of the randomized CAO/ARO/AIO-94 trial showed a superiority of nCRT over adjuvant CRT with respect to patient compliance, rate of local recurrence, toxicity, and sphincter preservation for cancers located in the lower rectum.4,18 However, OS rate and the occurrence of distant metastasis were not improved by nCRT. As concomitant chemotherapy, either infusional 5-fluorouracil or oral capecitabine is used, and both agents showed similar clinical outcomes.17,19,20
Another standard regimen of neoadjuvant therapy for LARC is a short-course preoperative radiotherapy (SCPRT) with a total of 25 Gy in five fractions of 5 Gy, followed by surgery within 10 days from the first radiation. 3 The Dutch TME trial showed a reduced rate of local recurrences by preoperative SCPRT compared with surgery alone. 21 Either CRT or SCPRT can be performed as neoadjuvant therapy for rectal cancer according to the European Society for Medical Oncology (ESMO) guidelines. 3 Two randomized trials showed no significant difference in local disease control or survival between these two approaches.22,23 However, in case of borderline resectable tumors, CRT rather than SCPRT is recommended due to superior oncological outcomes.3,24
An ongoing effort is to improve the response to nCRT by adding chemotherapeutic and targeted agents to the above-mentioned standard nCRT. Whether the addition of oxaliplatin is beneficial in LARC remains an open question. Both the CAO/ARO/AIO-04 trial as well as the ADORE trial demonstrated an improvement of disease-free survival (DFS) by adding oxaliplatin to fluorouracil-based regimen in patients with LARC.25,26 On the contrary, the FOWARC, PETACC-6, STAR-01, NSABP, and ACCORD-12 trials failed to demonstrate an improved local tumor response (pCR) or long-term survival (DFS or OS) when oxaliplatin was combined with 5-fluorouracil-based CRT.27–30 In a recent meta-analysis of seven randomized clinical trials including 5782 patients comparing oxaliplatin-based versus standard nCRT, an improved DFS and pCR rate, but also a higher rate of grade 3–4 diarrhea, was observed in the oxaliplatin group. 31 The overall benefit with an hazard ratio for DFS of 0.9, however, was not clinically relevant. Interestingly, a post hoc analysis of the aforementioned CAO/ARO/AIO-04 trial revealed a significant survival benefit by adding oxaliplatin to nCRT in younger patients aged <60 years, whereas those ⩾70 years had no survival benefit. 32 These results are supported by a meta-analysis of three randomized trials. 33 Thus, although oxaliplatin is recommended as a standard in the context of nCRT for LARC, 3 it cannot be ruled out that younger patients might derive a benefit. Another attempt to improve oncological outcomes is the incorporation of irinotecan in nCRT. The randomized phase III CinClare trial compared tumor response of patients with LARC who received capecitabine-based CRT with or without concurrent irinotecan. 34 The dose of irinotecan was adapted based on the uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotype. The pCR rate, which was the primary endpoint, was higher in the irinotecan group (30% versus 15%), but grade 3–4 toxicities also occurred more frequently. There was a tendency toward improved DFS and OS in the irinotecan group in the long-term follow-up.35,36 In contrast, results of the phase III ARISTOTLE trial showed that the addition of irinotecan did not improve the pCR rate but increased the rate of adverse events and substantially reduced treatment compliance and dose intensities of both chemoradiotherapy and radiotherapy. 37 Hence, the value of adding irinotecan to nCRT for rectal cancer is unclear. Other agents that have been extensively studied as an addition to conventional nCRT include epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) targeting antibodies/biologicals. The results of these clinical trials have been summarized in a recent review 38 and do not support the use of these agents beyond clinical trials.
Novel approaches in the neoadjuvant treatment of rectal cancer
Total neoadjuvant therapy
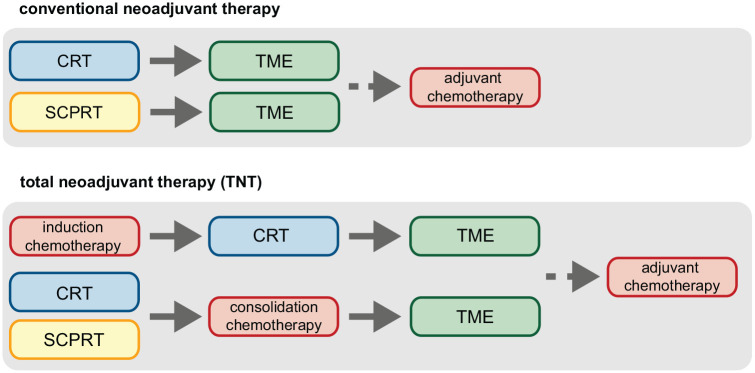
Many ongoing clinical studies are assessing different therapeutic strategies to improve the outcome of nCRT in LARC. One major field of research is total neoadjuvant therapy (TNT), which is defined as neoadjuvant radiotherapy or chemoradiotherapy with additional chemotherapy administered either before (as induction therapy) or after CRT (as consolidation therapy), 7 as illustrated in Figure 1. The original goal of TNT was to improve DFS in patient with LARC and such a benefit was in fact observed in most studies that compared TNT with standard nCRT (see Table 1). Interesting, results of these trials also demonstrate a considerably higher pCR rate in patient who receive TNT, despite differences in study designs.39–43 Therapy-associated toxicities were also more frequently observed in the TNT group but did not result in differences in therapy adherence, surgical management, or rate of postoperative complications.39,44 Furthermore, the CAO/ARO/AIO-12 and organ preservation of rectal adenocarcinoma (OPRA) study investigated different sequences of TNT, namely, induction chemotherapy followed by CRT or consolidation chemotherapy after CRT. In both trials, consolidation treatment was superior in terms of cCR or pCR rate allowing for organ preservation strategies which amounted to about 55–60% in the OPRA trail in the consolidation arm.45,46 In all, the mentioned TNT studies demonstrate that TNT (especially with consolidation chemotherapy) improves DFS rates and substantially increases cCR and pCR rates, allowing for organ preservation and watch-and-wait strategies, which is outlined in the following section, in patients with LARC.39,42
Figure 1.
Schematic overview of regimen for standard neoadjuvant therapy and total neoadjuvant therapy for locally advanced rectal cancer (LARC). In conventional neoadjuvant chemoradiotherapy (CRT), patients with LARC are treated with radiation (usually 50.4 Gy in 28 fractions of 1.8 Gy) and concurrent infusional 5-fluorouracil or oral capecitabine, followed by total mesorectal excision (TME). In total neoadjuvant therapy, CRT is either preceded by induction chemotherapy (with, for instance, fluoropyrimidine- and oxaliplatin-based regimens) or short-course preoperative radiotherapy (SCPRT, 5×5 Gy), or followed by consolidation chemotherapy, prior to TME. Adjuvant chemotherapy is recommended by many national guidelines.
Table 1.
Clinical trials that investigate total neoadjuvant therapy in rectal cancer.
| Trial | N | Preoperative treatment | Postoperative treatment | Results |
|---|---|---|---|---|
| RAPIDO40,41,44 | 920 | Arm A: SCPRT + 9 cycles FOLFOX4 or 6 cycles
CAPOX Arm B: 5-FU-based CRT |
Optional (12 cycles FOLFOX4 or 8 cycles CAPOX) | ● pCR: 28% versus 14%
(p < 0.001) ● 3-year DrTF (primary endpoint): 23.7% versus 30.4% (p = 0.019) |
| Polish-II47,48 | 515 | Arm A: 5× 5 Gy + 3 cycles of FOLFOX4 Arm B: 5-FU-based CRT |
At discretion of treating physicians | ● pCR: 16% versus 12%
(n.s.) ● R0-resection (primary endpoint): 77% versus 71% (p = 0.07, n.s.) ● 3-year DFS: 53% versus 52% (p = 0.85, n.s.) ● 7-year OS: n.s. |
| PRODIGE 23 39 | 461 | Arm A: 6 cycles FOLFIRINOX + CRT Arm B: 5-FU-based CRT |
FOLFOX or capecitabine | ● pCR: 27.5% versus 11.7%
(p < 0.001) ● 3-year DFS (primary endpoint): 75.7% versus 68.5% (p = 0.034) |
| CAO/ARO/AIO-12 45 | 306 | Arm A (induction CT): 3 cycles FOLFOX + CRT Arm B (consolidation CT): CRT + 3 cycles FOLFOX |
No | ● pCR (primary endpoint): Arm A, 17% (n.s.); Arm B, 25%
(p < 0.001) Survival data pending |
| OPRA 46 | 306 | I-Arm (induction): 8 cycles FOLFOX or 5 cycles
CAPOX + CRT C-Arm (consolidation): CRT + 8 cycles FOLFOX or 5 cycles CAPOX |
No | ● 3-year DFS (primary endpoint): 78% in I-Arm versus 77% in C-Arm (n.s.) |
| NCT0033581642,43 | 292 | Arm 1: 5-FU-based CRT Arm 2: CRT + 2 cycles FOLFOX6 Arm 3: CRT + 4 cycles FOLFOX6 Arm 4: CRT + 6 cycles FOLFOX6 |
Optional, investigator’s choice | ● pCR (primary endpoint): 18%, 25%, 30%, and 38% in Arms
1, 2, 3, and 4
(p = 0.0036) ● 5-year DFS: 50%, 81%, 86%, and 76% in Arms 1, 2, 3, and 4 (p = 0.004) ● 5-year OS: 79%, 92%, 88%, and 84% in Arms 1, 2, 3, and 4 (n.s.) |
| STELLAR 49 | 599 | Arm 1: SCPRT + 4× CAPOX Arm 2: CRT |
CAPOX | ● pCR + cCR rate (primary endpoint): 22.5%
versus 12.6%
(p = 0.001) 3-year DFS: 64.5% versus 62.3% (noninferiority: p < 0.001) 3-year OS: 86.5% versus 75.1% (p = 0.036) |
CAPOX, capecitabine/oxaliplatin; cCR, clinical complete response; CRT, chemoradiotherapy; CT, chemotherapy; DFS, disease-free survival; DrTF, disease-related treatment failure; FOLFIRINOX, leucovorin/fluorouracil/irinotecan/oxaliplatin; FOLFOX, leucovorin/fluorouracil/oxaliplatin; N, number of evaluable patients; n.s., not significant; OS, overall survival; pCR, pathological complete response; SCPRT, short-course preoperative radiotherapy.
Watch-and-wait strategy
The watch-and-wait strategy was initially introduced by Habr-Gama in 2004 and is increasingly gaining attention. In the original study by Habr-Gama, 265 patients with distal, resectable, rectal adenocarcinoma were enrolled and received nCRT. 50 Of those patients, 71 achieved cCR after nCRT and did not undergo subsequent surgery. Instead, they were closely surveilled. All other patients underwent surgery and 22 of those had a pCR upon pathological examination. Comparison of patients who achieved pCR with those who received close surveillance after cCR showed no significant differences in systemic recurrence, DFS and OS during a mean follow-up of 54.9 months. These results have challenged the standard concept of LARC therapy which required surgical resection of the primary tumor after nCRT and stimulated further studies. To date, accumulating data show that the watch-and-wait approach can lead to an excellent rectal preservation rate after neoadjuvant therapy for LARC.51–56 A large proportion of patients could avoid surgery and of those who experienced local tumor regrowth during follow-up examinations could undergo surgical or endoscopic salvage therapy in most cases. However, evidence in favor of watch-and-wait is primarily based on retrospective or registry data, while evidence from randomized trials is lacking. Furthermore, whether the watch-and-wait approach is noninferior regarding long-term survival compared with surgery is currently unclear. Renehan et al. 55 demonstrated no difference in 3-year OS between patients in the watch-and-wait and surgery group. In contrast, Smith et al. 53 showed that survival in the watch-and-wait group was inferior compared with patients who underwent surgical resection and achieved pCR. Therefore, the implementation of the watch-and-wait strategy into clinical practice is still facing challenges. Nevertheless, as response to neoadjuvant therapy will be improved by approaches such as TNT, more patients might achieve cCR and can be offered the option of watch-and-wait in the future, indicating a growing importance to define predictive biomarkers.
Predictive biomarkers for response to nCRT in rectal cancer
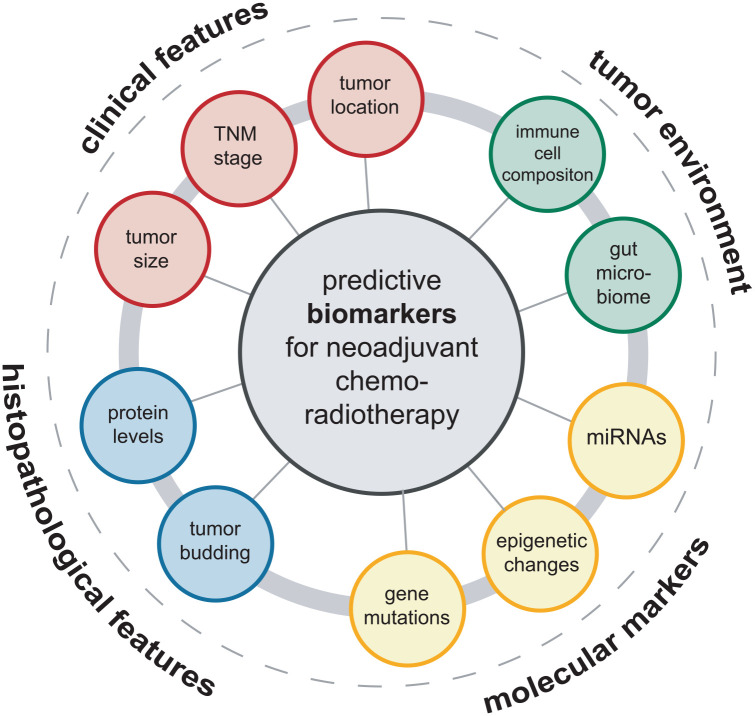
Over the past decade, a large spectrum of markers was reported to predict response to nCRT, specifically long-course radiotherapy with concomitant fluoropyrimidines in LARC. These markers include clinical features such as the tumor stage according to the TNM classification, tumor size, and location within the rectum. In addition, a number of histopathological markers were identified, ranging from tumor-intrinsic features such as tumor budding, grade of differentiation to the altered levels of marker proteins. At the molecular level, markers including gene mutations, microRNAs (miRNAs), and epigenetic changes were found to be predictive. More recently, factors derived from the tumor environment such as the immune cell composition or the gut microbiome were reported to have predictive value. A summary of markers is presented in Figure 2. Selected biomarkers are described and critically discussed in the following section.
Figure 2.
Overview of biomarkers that predict response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. A spectrum of clinical, histopathological, molecular, and tumor environment-derived factors can influence and predict the local response to neoadjuvant radiochemotherapy in locally advanced rectal cancer.
Clinical and histological predictors of response to neoadjuvant therapy
Clinical features
A number of clinical features were shown to predict treatment response to nCRT in LARC, including tumor size, clinical stage, and distance of the tumor from the anal verge (DTAV). Pretreatment tumor size was found to be associated with treatment response in LARC in several retrospective studies. In a cohort of 138 patients with LARC and locally unresectable T1–T2 tumors, Bitterman et al. 57 demonstrated that pretreatment tumor size <3 cm was an independent predictor of pCR and cCR following nCRT. Similar results have been demonstrated recently in a larger population-based study, showing that patients with tumors <3 cm are more likely to achieve pCR after nCRT, SCPRT, or chemotherapy, regardless of their pretreatment clinical stage. 8 These findings are supported by several additional studies that did not use a specific cutoff value for tumor size.58–60 However, it should be noted that tumor size was measured with different methods in these studies, including endorectal ultrasound, digital rectal examination, and flexible endoscopy. Furthermore, the difference in mean tumor size between patients with and without pCR was only minor (0.5–1 cm) in some studies,58–61 limiting its predictive value in a clinical scenario.
Tumor stage, as determined by the TNM system, is a more comprehensive predictive marker. 62 For the T stage, several studies comprising large patient cohorts found a lower pCR rate in patients with cT4 LARC.63,64 Accordingly, patients with cT1-2 tumors were more likely to exhibit cCR/pCR after nCRT in another large patient series. 8 These observations are confirmed by data from the American College of Surgeons’ National Cancer database which comprised a total of 23,747 patients with LARC treated with nCRT. 65 Nodal status was also found to be predictive for tumor response as patients with cN2 stage LARC were found to have a significantly lower pCR rate after nCRT. 64 Accordingly, a series of studies demonstrated that clinical node-positivity at diagnosis (cN+) was significantly associated with a lower pCR or cCR rate.57,58
The association between DTAV and treatment response to nCRT remains controversial. In a retrospective study comprising 173 LARC patients, a DTAV <5 cm was significantly associated with favorable pathological response (defined as ypT0-1N0). 66 Similarly, a positive correlation of DTAV <3 cm with complete response was reported by other investigators. 57 However, these findings could not be confirmed by other studies,59,67 and even opposite results were described. For instance, Restivo et al. 68 demonstrated that a DTAV of >5 cm was a predictor for pCR in their cohort of 260 LARC patients who underwent nCRT. Interestingly, Patel et al. 69 presented a bimodal association between DTAV and pCR in a cohort of 827 patients by showing that both tumors located in the lower (<4 cm) and upper (>8 cm) rectum were less likely to achieve pCR. So far, no studies were able to identify the underlying reason for the association between DTAV and nCRT response. Thus, the value of DTAV as a predictive marker remains unclear.
Histopathological markers
Histopathological features of rectal cancer have been widely investigated for their potential to predict response to nCRT. Mucinous rectal adenocarcinomas (defined by the presence of more than 50% mucin content in the tumor specimen) were associated with a poorer response to nCRT.64,70 Mucinous adenocarcinomas had more advanced tumor stages and a lower proliferative activity compared with nonmucinous tumors, possibly explaining the different response to nCRT.71,72 Tumor differentiation was also reported to be a predictive factor. Patients with well-differentiated tumors, as determined in pretreatment tumor biopsies, were found to more likely achieve complete response to nCRT.59,73,74 Another predictive marker is tumor budding, which is characterized by the presence of isolated single tumor cells or clusters of up to four tumor cells at the tumor invasive front. 75 Tumor budding was found in approximately 20% of pretreatment rectal cancer tissues and shown to be a significant predictor of poor response to nCRT in LARC as well as a negative prognostic factor. 76
In addition, the prognostic value of tumor invasion-related histopathological factors has been widely demonstrated in rectal cancer, including lymphovascular invasion (LVI) and perineural invasion (PNI).77–79 The presence of LVI and/or PNI after CRT was associated with worse survival in RC patients.80,81 Furthermore, the rate of distant recurrence was higher in RC patients after nCRT and TME if LVI and PNI were observed.82,83 However, there is considerably less data supporting a predictive role of LVI and PNI for treatment response. In a retrospective analysis, Agarwal et al. 84 found that the LVI rate in RC patients after nCRT was higher in the poor response group (⩾50% residual cancer cells), while no difference was found for the PNI rate. In contrast, other studies reported that the absence of both LVI and PNI was associated with a better response to nCRT in retrospective cohorts.85,86 Since the comprehensive assessment of LVI and PNI requires the histological examination of large volumes of tumor tissue, confirming their predictive value by prospective studies that rely on endoscopically obtained pretreatment tumor samples will be challenging.
Protein-based markers
The correlation between protein levels of specific genes and response to nCRT in rectal cancer has been extensively investigated by immunohistochemistry analysis of single candidate proteins and by explorative, proteomic approaches. The identified protein biomarkers are involved in diverse biological processes, such as DNA repair, 87 oncogenic signaling, 88 or apoptosis. 89 A selection of predictive, tissue-based protein markers is summarized in Table 2. For instance, Yu et al. 90 determined the protein levels of multidrug resistance–associated protein 3 in rectal cancer biopsies using immunohistochemistry and found that higher expression was predictive of poor response to nCRT and associated with a lower 5-year survival rate. Other studies used a combination of different protein markers to improve the response prediction. For instance, Dalle Fratte et al. 91 determined protein levels of a panel of 11 cancer-related proteins by immunohistochemistry and found that the combination of low Ki67 and high CXCR4 levels had the highest predictive potential. To obtain a more global view of altered protein levels, proteomic methods were applied to identify predictive protein signatures in pretreatment tumor tissue. Croner et al. 92 compared protein levels of responders versus poor responders by isotope-coded protein label analysis and identified a panel of 140 differentially regulated proteins that can potentially predict response to nCRT. Similarly, Chauvin et al. 93 identified 384 proteins with differential abundance between responders and nonresponders. In a technically different approach, another group used peptide microarrays with tyrosine kinase substrate and found that basal phosphorylation levels of 21 substrates were also feasible in predicting poor response to preoperative CRT in LARC patients. 94 However, to date, none of these identified protein markers or signatures have been independently validated in additional patient cohorts. Therefore, their robustness and clinical utility are yet unclear.
Table 2.
Selection of protein-based predictive markers for response to nCRT in rectal cancer.
| Protein marker | Treatment response assessment | Tumor tissue | Cohort | Predictive value | Reference |
|---|---|---|---|---|---|
| RAD18 | Sensitivity and nonresponders | Pre-nCRT | 51, LARC | Low expression is associated with favorable response | Yan et al. 87 |
| TCF-4 | Dworak’s TRG | Pre-nCRT | 96, LARC | Low expression is associated with favorable response | Dou et al. 88 |
| Beclin 1 | pCR, residual microscopic disease | Pre-nCRT | 96, LARC | High expression is associated with poor response | Zaanan et al. 89 |
| MRP3 | TRG | Pre-nCRT | 144, LARC | High expression is associated with poor response | Yu et al. 90 |
| Fibrinogen β chain | TRG | Pre-nCRT | 20, RC | High expression is associated with poor response | Repetto et al. 95 |
| DUOX2 | Dworak’s TRG | Pre-nCRT | 172, LARC | High expression is associated with poor response | Lin et al. 96 |
| FAK | Ryan’s TRG | Pre-nCRT | 73, LARC | Low expression is associated with poor response | Gómez del Pulgar et al. 97 |
| VRK1 and VRK2 | Ryan’s TRG | Pre-nCRT | 67, LARC | High expression is associated with favorable response | del Puerto-Nevado et al. 98 |
| SDF-1α and PLGF | pCR | Pre-nCRT and postsurgery | 55, LARC | High expression of SDF-1α and positive PLGF staining after nCRT is associated with resistance to nCRT | Kim et al. 99 |
| Survivin | Dworak’s TRG | Pre-nCRT | 54, LARC | High expression is associated with poor response | Kim et al. 100 |
| FOXK1 and FOXK2 | pCR | Pre-nCRT and postsurgery | 256, LARC | High expression is associated with poor response | Zhang et al. 101 |
| ALDOB | Dworak’s TRG | Pre-nCRT | 172, LARC | High expression is associated with poor response | Tian et al. 102 |
| CCR6 | Mandard’s TRG | Pre-nCRT | 95, LARC | High expression is associated with poor response | Chang et al. 103 |
| PLK1 | TRG | Pre-nCRT | 75, LARC | Low expression is associated with poor response | Cebrián et al. 104 |
| COX2 | Mandard’s TRG | Pre-nCRT | 49, LARC | High expression is associated with poor response | Smith et al. 105 |
| CA9 | TRG | Pre-nCRT | 61, LARC | High expression is associated with poor response | Guedj et al. 106 |
ALDOB, aldolase B; CA9, carbonic anhydrase 9; CCR6, C-C motif chemokine receptor 6; COX2, cyclooxygenase 2; DUOX2, dual oxidase 2; FAK, focal adhesion kinase; FOX, forkhead box; LARC, locally advanced rectal cancer; MRP3, multidrug resistance–associated protein 3; nCRT, neoadjuvant chemoradiotherapy; pCR, pathological complete response; PLGF, placental growth factor; PLK1, polo-like kinase 1; RAD18, RAD18 E3 Ubiquitin Protein Ligase; SDF-1α, stromal cell-derived factor 1α; TCF-4, T-cell factor 4; TRG, tumor regression grade; VRK1: vaccinia related kinase 1; VRK2: vaccinia related kinase 2.
Tumor environment
Immune microenvironment
The tumor microenvironment in solid tumors is highly complex and consists of tumor, immune, and stroma cells that interact with the extracellular matrix. 107 The composition of the tumor microenvironment and the subtle interactions between its components determine cancer development and progression. 107 The immune cell component of the microenvironment can elicit both tumor-inhibiting and tumor-promoting effects: while cytotoxic T cells and natural killer cells can cause cytolysis of tumor cells, regulatory T cells and M2 macrophages are immunosuppressive and can support tumor survival. 108 The composition of immune cells in the tumor microenvironment of CRC was shown to have prognostic and predictive values. 109 To characterize the immune microenvironment in CRC, an immunoscore was developed based on cellular densities of CD3(+) and CD8(+) lymphocytes in the tumor center and at the invasive margin.110,111 Accumulating evidence suggests that alterations in immune cell composition in the tumor microenvironment might influence the response to nCRT. In LARC, high immunoscores in pretreatment tumor tissues are associated with a higher tumor downstaging rate (partial and complete response) after nCRT. 112 To further characterize the subtypes of tumor-infiltrating lymphocytes (TILs), several studies measured the densities of CD8+ and CD4+ TILs in rectal cancer samples before nCRT.113–117 These studies consistently found that high CD8+ TIL levels were associated with a favorable response to nCRT. Furthermore, many of those studies also observed high CD4+ TIL density to be a favorable predictive marker.113,115 Interestingly, the pretreatment CD8+ TIL count also showed a prognostic value, as it was associated with a superior 5-year-DFS and OS rate.117,118 Regulatory T cells mediate peripheral immune tolerance in the tumor microenvironment, thus playing an important role in suppressing antitumor immunity. 119 To investigate if regulatory T cells can impact response to nCRT, McCoy et al. analyzed post-CRT tissue samples of 135 patients with LARC. The study showed that a low density of stromal FOXP3+ regulatory T cells in these samples correlated significantly with occurrence of pCR. 120 Similarly, Zhang et al. 121 reported that high levels of FOXP3+ TILs were associated with poor response to neoadjuvant therapy in LARC. Tumor-associated macrophages (TAMs) are another important cellular component of the cancer immune microenvironment and known for their functional plasticity. TAMs can be classified into pro-inflammatory (M1) and immunosuppressive (M2) subsets depending on their polarization status, and these subtypes can profoundly affect tumor biology. 122 Intratumoral CD163 levels, a marker of M2 polarized macrophages, were found to be increased in rectal cancer tissues upon short-course irradiation. 123 This observation is supported by transcriptome profiling of pre- and post-CRT rectal cancer tissues, which demonstrated an enrichment of signatures specific for M2 macrophages. Interestingly, this increase was particularly pronounced in nonresponders to nCRT. 113
Programmed cell death-ligand 1 (PD-L1) pathway mediates immune escape and is a potent target for anticancer immunotherapy. 124 Interestingly, multiple studies reported that PD-L1 expression and T-cell infiltration were increased after nCRT in LARC.117,125,126 Hecht et al. 126 studied 103 pre- and 159 post-CRT samples of LARC, and found that low PD-L1 expression in cancer and inflammatory immune cells, either in pre-CRT samples or in the invasive front of post-CRT samples, was an independent negative prognostic marker for OS. However, Saigusa et al. 127 reported that high PD-L1 expression was associated with an inferior recurrence-free survival and OS rate of rectal cancer patients after nCRT. Whether PD-L1 expression has also a predictive value for nCRT in LARC is yet unclear.
Cytokines and chemokines
Systemic cytokine and chemokine levels indicate inflammatory processes which play an important role in CRC progression. 128 Thus, serum levels of specific cytokines have been studied in the context of rectal cancer treatment. Interleukin-6 (IL-6) is a pro-inflammatory cytokine, and serum levels of IL-6 were determined in patients at different time points during nCRT. IL-6 levels tended to be lower in patients who achieved complete response. 129 In another study, levels of soluble CD40L, CCL-5, and a set of cytokines were analyzed during the course of nCRT. A decrease in soluble CD40L levels was associated with a favorable response, while higher post-CRT levels of IL-6 were associated with nonresponse. 130 However, in another large study that analyzed levels of a set of blood-based markers including IL-6 and IL-8 (interleukin-8), only IL-8 could predict response to nCRT. 131
Extracellular matrix
The composition of the extracellular matrix can vary in rectal cancers, and altered levels of specific components are associated with specific responses to nCRT in LARC. Jayne et al. 132 determined protein abundances of fibronectin, collagen IV, laminin, and the fibronectin receptor in pretreatment rectal cancer tissues and found that levels of the fibronectin receptor, α5β1 integrin, were significantly higher in nonresponders. Similarly, by using a proteomic approach, a set of proteins was found to be more highly expressed in poor responders. Among these proteins, fibronectin beta chain could be validated in an independent cohort. 95 Furthermore, high expression of matrix metallopeptidase 9 (MMP 9) was also associated with poor response to nCRT. 133 In a technically sophisticated approach, Goncalves-Ribeiro et al. performed laser-capture microdissection of stroma and tumor glands from pretreatment rectal cancer samples, followed by comparative transcriptomic profiling. Interestingly, differential expression of genes between responders and nonresponders was mostly found in the stromal compartment. Based on these data, a two-protein classifier was built consisting of FN1 and COL3A1, and immunohistochemistry staining of these two proteins showed a high positive predictive value in a validation cohort. 134 These findings underline an important but less extensively explored role of the extracellular matrix as a predictive marker for nCRT in rectal cancer.
Gut microbiome
Over the last decade, accumulating data from microbial metagenomics studies suggest a link between the composition of the gut microbiome and specific diseases, including cancer.135,136 The gut microbiome is not only linked to intestinal tumorigenesis 137 but also evolves during cancer therapy such as chemoradiation.138–140 Conversely, the gut microbiota may modulate response to cancer treatment by a diverse set of potential mechanisms, including direct enzymatic degradation and metabolism of drugs by specific bacterial species, alteration of bacterial diversity resulting in local tissue inflammation, and modulation of tumor immune response.141,142 The effect of the microbiome on response to radiotherapy is less extensively investigated, but studies in preclinical models support an underlying immunomodulatory effect of gut bacteria.140,143 Recently, clinical studies investigating the predictive value of the gut microbiome for response to nCRT of LARC have been reported. The largest prospective, longitudinal study to date compared fecal samples of 45 responders and 38 nonresponders with LARC before and after nCRT. 144 Dorea and Anaerostipes species were reported to be enriched in feces of responders, whereas Coriobacteriaceae and Fusobacterium were overrepresented in feces of nonresponders before nCRT. Moreover, the authors established a predictive random forest classifier for response to nCRT based on the identified microbial biomarkers. Another study analyzed fecal samples of 45 patients with rectal cancer prior to CRT. 145 Bacteroidales species were enriched in the noncomplete response group, which is in line with findings from another study with 22 patients. 146 The latter study also reported that Shuttleworthia species were enriched in responders, while several bacteria taxa of Clostridiales were enriched in nonresponders. In general, higher microbial diversity and richness were observed in good responders compared with poor responders as reported from a longitudinal study of 39 patients. 138 Taken together, using pretreatment gut microbial features to predict response to nCRT is promising, but studies with larger cohorts are warranted to shed more light on this emerging field of research.
Molecular predictors of response to neoadjuvant therapy
Gene mutations
Activating mutations of KRAS are one of the most common genetic alterations in CRC 147 and have been extensively studied as prognostic markers for LARC. However, their predictive value remains controversial. Several studies reported that KRAS mutations detected in pretreatment cancer tissue of LARC patients were significantly associated with a lower pCR rate.148,149 Moreover, this correlation was independent of other confounding factors such as clinical stage or number of cycles of FOLFOX (leucovorin/fluorouracil/oxaliplatin) treatment. 148 However, a large retrospective study including 1886 patients with UICC stage II–III and a systematic review both failed to confirm the relationship between KRAS mutation and decreased rates of pCR.150,151 Noteworthy, the systematic review did not discriminate between different genotypes of KRAS mutations, 151 while Duldulao et al. 149 found that KRAS mutations in different codons resulted in a differential resistance profile to nCRT. In particular, rectal cancers with KRAS codon 13 and G12V mutations were reported to less likely exhibit a pCR.148,149 These genotype-specific differences might partly explain the inconsistent results when analyzing the predictive potential of KRAS mutations and underline the necessity for precise genomic analysis.
Mutations in TP53 are detected in most CRC. 147 It was shown that rectal cancer patients harboring combined KRAS and TP53 mutations were more resistant to nCRT, 152 and presence of both mutations was also independently associated with lymph node metastasis in LARC. 148 However, the question whether TP53 mutations alone could predict response to nCRT remains open. While early studies found no correlation between TP53 status (mutations, allelic loss, and nuclear TP53 overexpression) and response to nCRT in LARC, 153 a meta-analysis including 1830 cases indicated that both a low expression of TP53 protein and/or presence of wild-type TP53 were correlated with favorable response to nCRT. 154 Moreover, in a recent large prospective study of LARC patients, TP53 mutations were shown to be associated with poorer pathological tumor regression and a worse 5-year progression-free survival (PFS) after nCRT. 155 Other mutations in genes such as BRAF and SMAD4 were found to be associated with treatment resistance to nCRT in LARC patients.155,156 But since mutations in these genes occur less frequently than KRAS or TP53 mutations, validation of the findings in larger cohorts is yet lacking.
DNA methylation
DNA methylation is an epigenetic process that affects cytosines in CpG-rich promoters and thereby modulates transcriptional activity of genes. 157 Increased methylation of CpG island, also described as CIMP, is a prevalent biological feature of CRC. 158 CpG island methylator phenotype (CIMP) status has been assessed as a predictive biomarker for treatment response to nCRT in LARC, but the results vary depending on the method applied. Jo et al. 159 assessed the CIMP status of 150 LARC patients treated within the CAO/ARO/AIO-94 and CAO/ARO/AIO-04 trials, using a marker panel covering the gene promoters of RUNX3, SOCS1, NEUROG1, IGF2, and CACNA1G. The authors found that a positive CIMP status (defined by at least three methylated promoters) was associated with a worse 3- and 5-year DFS after nCRT. Another study profiled the methylation level of 24 tumor suppressor genes in pretreatment LARC samples, and revealed that only high TIMP3 methylation was significantly associated with pCR to nCRT. 160 In a genome-wide methylation analysis of 45 tissue samples of LARC patients, Ha et al. 161 discovered that the methylation status of the KLHL34 cg14232291 locus is a predictor for sensitivity to nCRT in LARC. In a similar genome-wide profiling approach in 32 pretreatment LARC biopsies, a classifier based on differentially methylated-CpG loci in OBSL1, GPR1, and INSIG1 was developed. 162 This classifier was shown to discriminate between complete and incomplete responders with 100% sensitivity and 90% specificity, and this predictive value was further verified in an independent cohort of 77 LARC patients. 162
MicroRNA
miRNAs are short noncoding RNAs, sized from 19 to 25 nucleotides, that posttranscriptionally regulate the expression of target genes and are functionally involved in many biological processes in rectal cancer. 163 Several differentially expressed miRNAs in rectal cancer were reported as biomarkers that can predict response to nCRT.164,165 For instance, expression level of miR-21, a well-characterized miRNA, was found to discriminate between complete responders and noncomplete responders in rectal cancer.166,167 However, while one study demonstrated that overexpression of miR-21-5p was associated with complete regression after nCRT, 166 another study found the opposite. 167 Similarly, Eriksen et al. 168 described that miR-21 downregulation was related to enhanced response to nCRT in a test cohort with 55 LARC patients but observed an opposite association in a larger validation cohort. In this context, Campayo et al. 169 found that the combined expression level of miR-21, miR-99b, and miR-375 could improve the prediction of excellent responders to nCRT. A number of other studies used high-throughput methods such as expression microarray and small RNA sequencing to comprehensively characterize the expression of miRNAs in rectal cancer tissues, and yielded a large number of miRNAs that are differentially expressed between responders and nonresponders to nCRT.170–172 In a recent systematic review, Izzotti et al. identified 77 miRNAs that have a potential value for predicting response to nCRT. However, only six of them were differentially expressed in two or more independent studies. 164 Hence, the role of miRNA as predictive biomarkers for rectal cancer remains yet elusive and single candidate miRNA will require validation in independent cohorts.
Liquid biopsies
Liquid biopsy is a powerful diagnostic tool to monitor changes in tumor genetics. In comparison with tissue biopsies, liquid biopsy is noninvasive and can be collected serially, thereby facilitating a real-time assessment of the mutational landscape. 173 Circulating tumor DNA (ctDNA) has been extensively studied as a diagnostic tool in patients with LARC, but the data regarding its predictive value remain controversial. 174 Several studies investigated ctDNA levels at three time points: pretreatment/baseline, post-CRT, and postsurgery.175,176 Murahashi et al. found that changes in ctDNA levels at baseline were predictive for therapy response, 175 but this association was not confirmed in a larger study with 159 patients. 176 Depending on the size of the nucleic acid, long fragments of ctDNA were proposed as more tumor-specific than short fragments of ctDNA. 177 Agostini et al. 178 reported that a decrease of long fragments of ctDNA after nCRT was associated with superior therapy response in 67 patients with LARC. Furthermore, epigenetic alterations including methylation of DNA can be analyzed in liquid biopsy. As such, Sun et al. observed an association between a higher methylation status of O6-methylguanine-DNA methyltransferase (MGMT) promoter at baseline and a better tumor response in a cohort of 34 patients with LARC, 179 which is confirmed by results of another study. 180 Also, genetic mutations detected in liquid biopsy were proposed as potential biomarkers. Yang et al. 181 reported an association between mutations of TP53 and APC gene detected in pretreatment liquid biopsies and worse therapy response to nCRT in 119 patients with LARC. Due to the heterogeneous study designs and applied analytic methods (digital droplet polymerase chain reaction, amplicon-based sequencing, and whole genome sequencing), different time points of sample collection, and relatively small cohort in most cases, larger and well-designed studies are needed in the future to verify the predictive value of liquid biopsy for LARC.
Tumor models to investigate response to radiochemotherapy in rectal cancer
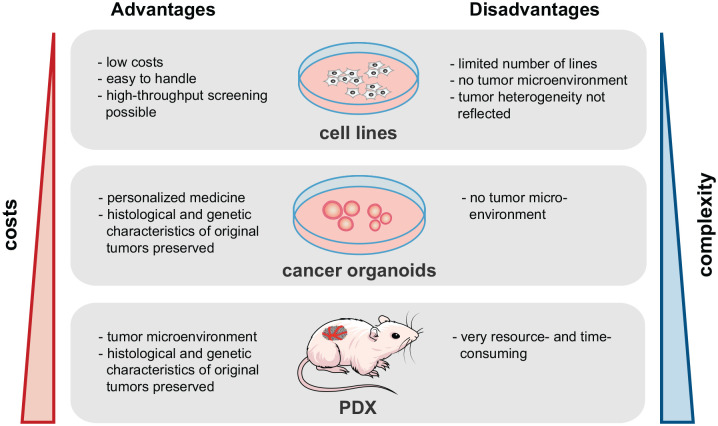
The identification and subsequent functional characterization of predictive biomarkers in rectal cancer requires modeling of the disease and the therapy response in vitro and in vivo. Several models of rectal cancer have been developed for this purpose, with specific advantages and disadvantages. Here we provide an overview of existing rectal cancer models that have been used to study the effect of CRT (see Figure 3).
Figure 3.
Tumor model systems to study treatment response in rectal cancer. Cancer cell lines, tumor organoids, and patient-derived mouse xenograft models can be used to study treatment response. Advantages and disadvantages of each model system are described.
Cancer cell lines are the most commonly used model of CRC, as they are easy to cultivate and amenable to most genetic manipulations. Hence, they have been extensively used to study the impact of candidate genes and cellular processes on radiosensitivity. CRC cell lines were used to decipher the role of oncogenic signaling in response to CRT, including Wnt, RAS, and PI3K signaling.182–184 Furthermore, the role of many novel genes that modulate radiosensitivity were identified and characterized in CRC cell lines, including COASY, 184 XPO1, 185 CRBP1, 186 or inducible nitric oxide synthase, 187 among others. While providing interesting insights into the mechanistic function of these genes for radiosensitivity, a major drawback is that these potential biomarkers have not been confirmed in independent clinical cohorts. The impact of different miRNAs on radiosensitivity in rectal cancer was also elucidated in CRC cell line models, thereby identifying transcriptional targets that mediate the phenotypic effects of the miRNAs.188,189 Finally, CRC cell lines were used for drug screens to identify novel radiosensitizing compounds. 190 One limitation of most cell line models is that they originate from cancer tissues of the colon, and only a very limited number of rectal cancer cell lines are used in experimental studies so far. Furthermore, primarily rectal cancer is genetically heterogeneous and different single cell clones from the same tumor can respond differently to radiotherapy due to their genetic diversity and differential activation of oncogenic pathways. 191 Thus, while cell lines are a suitable model to characterize the function of single genes, recapitulating the in vivo response to CRT requires the use of more complex models of rectal cancer.
One strategy to overcome this challenge is the development of patient-derived tumor xenografts (PDX). In this approach, cells from tumor tissues are implanted subcutaneously or orthotopically into immunodeficient rodent lines such as athymic nude mice. A multitude of evidence demonstrates that PDX preserves the histological architecture and genetic characteristics of the original tumors. 192 Hence, several studies have been performed with colon cancer PDX models to assess drug response and drug resistance, as well as to discover new therapeutic targets and predictive biomarkers.193,194 However, only one study used PDX models of primary rectal cancer tissue to investigate the response to 5-FU-based CRT. This study demonstrated that PDX models could reproduce the heterogeneous response of primary tumors to nCRT. 195 Despite this finding, PDX models have several limitations. For instance, the impact of the immune system on therapy response is not adequately modeled in immunocompromised host animals. In addition, experiments with PDX are time-consuming and resource-intensive, limiting their use for large-scale screening experiments.
A novel approach to modeling CRC is the use of patient-derived organoids (PDOs). To establish PDO lines, cancer stem cells are isolated from tumor biopsies, seeded in base matrix, and cultured with defined culture medium containing specific growth factors.196–198 Under these conditions, an outgrowth of a self-organizing, three-dimensional mini-organ, termed organoid, can be achieved in vitro. Most PDO studies showed that these model systems recapitulate the histologic and genetic characters of original tumors of various entities and their specific drug responses.198–200 Recently, several studies investigated rectal cancer organoids as a model to predict the patient’s response to radiotherapy.201–203 Yao et al. examined the response of organoids derived from LARC to CRT in vitro. It was observed that the effect in organoids corresponds significantly to the clinical response. 203 Similarly, Ganesh et al. 202 also showed a high correlation between the response in PDOs of LARC and of patient’s tumors to chemotherapy or radiation. Furthermore, the group performed endoluminal transplantation of PDOs into the murine rectum. The transplanted organoids developed similar clinical courses as in the corresponding patients, including metastasis formation. Thus, both studies demonstrate that PDOs can be used as tumor models that share disease characteristics as the primary tumor and are predictive for therapy response. Nonetheless, there are several experimental challenges in the current use of organoid models, particularly the lack of an intact tumor microenvironment. Organoid co-cultures with other cell types such as immune cells and fibroblasts have been described but are yet in nascent stage. 198 Further advances in the field of organoid culture are expected and will improve the development of personalized in vitro models to predict and model the response to CRT.
Conclusion
Over the last decade, significant progress has been made in the treatment of LARC by the introduction and refinement of nCRT. 38 Accumulating evidence from prospective clinical trials demonstrates that a fraction of patients can achieve pCR and therefore might not require tumor resection. 5 Therefore, current efforts aim to improve the treatment response by adding an intensified chemotherapy regimen to nCRT (‘total neoadjuvant therapy’). Results from advanced phase clinical trials indicate that TNT can further increase the fraction of patients with clinical or pathological complete response and improve DFS. However, intensifying neoadjuvant therapy is inevitably associated with increased risk for and severity of adverse effects. Due to inter- and intratumoral heterogeneity, some tumors will not respond completely to the intensified neoadjuvant treatment. 204 Therefore, predictive biomarkers are needed to optimize patient selection for intensified neoadjuvant therapy regimens. In this review, we provide a broad overview of biomarkers that predict response to nCRT in LARC. While the predictive value of some markers, such as the tumor stage, has been well characterized, others including the tumor immune microenvironment and the gut microbiome are yet emerging. However, only few of these predictive markers are used in the clinical routine, as their potential utility is limited for several reasons. First, biomarker studies in the field of rectal cancer are conceptually very heterogeneous, with large differences in the selected therapy regimen, radiation dose, inclusion of clinical confounders, and grading system of tumor response. This heterogeneity limits the comparability and robustness of the identified biomarkers. Second, for the majority of predictive markers, validation by independent cohorts is lacking or produced conflicting results. Currently, the predictive value of only a few novel biomarkers could be consistently confirmed, including TILs or specific combination of oncogenic mutations. Third, it is questionable if a single biomarker will be sufficient for response prediction or a combination of several markers is needed to achieve substantial predictive power. To address these shortcomings, future prospective clinical trials that evaluate novel nCRT regimens should be complemented by biomarker discovery programs. These should include the collection of tumor tissues, blood, and stool samples, enabling a comprehensive multi-omics analysis. In this context, an important question is how to rationally select candidate markers for clinical validation. For instance, it is unclear whether biomarkers that were identified in patients who received fluoropyrimidine-based nCRT retain their predictive power when TNT is used instead. Therefore, it is necessary to understand the molecular mechanisms underlying the radiosensitizing effects of individual compounds and to define biomarkers based on this knowledge. This translational approach will be facilitated by the recent development of PDO models which can recapitulate the clinical response of individual patients to nCRT.201–203 Future studies with large panels of organoids that combine response assessment to nCRT and comprehensive molecular characterization will reveal novel molecular modulators of response. In summary, predictive biomarkers will become an important part in the management of LARC. The most important challenge ahead is to validate biomarkers discovered in preclinical or translational studies in well-designed, prospective clinical trials, and to integrate these multidimensional markers in clinically useful scores.
Acknowledgments
We would like to thank Leonhard Bamberg for helpful comments on the manuscript.
Footnotes
Author contributions: Moying Li: Writing – original draft.
Qiyun Xiao: Writing – original draft.
Nachiyappan Venkatachalam: Visualization.
Ralf-Dieter Hofheinz: Writing – review & editing.
Marlon R. Veldwijk: Writing – review & editing.
Carsten Herskind: Writing – review & editing.
Matthias P. Ebert: Writing – review & editing.
Tianzuo Zhan: Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: T.Z. and M.L. were supported by the Clinician Scientist program ‘Interfaces and Interventions in Chronic Complex Conditions’ funded by the DFG (EB 187/8-1). Q.X. was supported by a fellowship of the Chinese Scholarship Council (CSC). M.P.E. was supported by the DFG (GRK2727) and a grant provided by the MERCK Heidelberg Innovation Call (Darmstadt, Germany). N.V. was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), SFB 1324, project number 331351713.
Contributor Information
Moying Li, Medical Faculty Mannheim, Heidelberg University, Mannheim.
Qiyun Xiao, Department of Medicine II, Mannheim University Hospital, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany.
Nachiyappan Venkatachalam, Department of Medicine II, Mannheim University Hospital, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany.
Ralf-Dieter Hofheinz, Department of Medicine III, Mannheim University Hospital, Medical Faculty Mannheim, Heidelberg University, Mannheim, GermanyMannheim Cancer Center, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany.
Marlon R. Veldwijk, Department of Radiation Oncology, Mannheim University Hospital, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
Carsten Herskind, Department of Radiation Oncology, Mannheim University Hospital, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany.
Matthias P. Ebert, Department of Medicine II, Mannheim University Hospital, Medical Faculty Mannheim, Heidelberg University, Mannheim, GermanyMannheim Cancer Center, Medical Faculty Mannheim, Heidelberg University, Mannheim, GermanyDKFZ-Hector Cancer Institute, University Medical Center Mannheim, Mannheim, Germany
Tianzuo Zhan, Department of Internal Medicine II, Mannheim University Hospital, Medical Faculty Mannheim, Heidelberg University, Theodor-Kutzer-Ufer 1-3, D-68167 Mannheim, GermanyMannheim Cancer Center, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Aklilu M, Eng C. The current landscape of locally advanced rectal cancer. Nat Rev Clin Oncol 2011; 8: 649–659. [DOI] [PubMed] [Google Scholar]
- 3. Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28: iv22–iv40. [DOI] [PubMed] [Google Scholar]
- 4. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740. [DOI] [PubMed] [Google Scholar]
- 5. Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010; 11: 835–844. [DOI] [PubMed] [Google Scholar]
- 6. Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg 2020; 271: 440–448. [DOI] [PubMed] [Google Scholar]
- 7. Rödel C, Hofheinz R, Fokas E. Rectal cancer: neoadjuvant chemoradiotherapy. Best Pract Res Clin Gastroenterol 2016; 30: 629–639. [DOI] [PubMed] [Google Scholar]
- 8. Hammarström K, Imam I, Mezheyeuski A, et al. A comprehensive evaluation of associations between routinely collected staging information and the response to (chemo)radiotherapy in rectal cancer. Cancers 2020; 13: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hiotis SP, Weber SM, Cohen AM, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg 2002; 194: 131–135; discussion 135. [DOI] [PubMed] [Google Scholar]
- 10. Loos M, Quentmeier P, Schuster T, et al. Effect of preoperative radio(chemo)therapy on long-term functional outcome in rectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013; 20: 1816–1828. [DOI] [PubMed] [Google Scholar]
- 11. Buckley AM, Lynam-Lennon N, O’Neill H, et al. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol 2020; 17: 298–313. [DOI] [PubMed] [Google Scholar]
- 12. Cercek A, Roxburgh CS, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol 2018; 4: e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Sluis FJ, Couwenberg AM, de Bock GH, et al. Population-based study of morbidity risk associated with pathological complete response after chemoradiotherapy for rectal cancer. Br J Surg 2020; 107: 131–139. [DOI] [PubMed] [Google Scholar]
- 14. Pucciarelli S, Del Bianco P, Efficace F, et al. Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg 2011; 253: 71–77. [DOI] [PubMed] [Google Scholar]
- 15. West MA, Loughney L, Barben CP, et al. The effects of neoadjuvant chemoradiotherapy on physical fitness and morbidity in rectal cancer surgery patients. Eur J Surg Oncol 2014; 40: 1421–1428. [DOI] [PubMed] [Google Scholar]
- 16. Cunningham D, Atkin W, Lenz H-J, et al. Colorectal cancer. Lancet 2010; 375: 1030–1047. [DOI] [PubMed] [Google Scholar]
- 17. Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst 2015; 107: djv248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012; 30: 1926–1933. [DOI] [PubMed] [Google Scholar]
- 19. Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012; 13: 579–588. [DOI] [PubMed] [Google Scholar]
- 20. O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from national surgical adjuvant breast and bowel project trial R-04. J Clin Oncol 2014; 32: 1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011; 12: 575–582. [DOI] [PubMed] [Google Scholar]
- 22. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006; 93: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 23. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J Clin Oncol 2012; 30: 3827–3833. [DOI] [PubMed] [Google Scholar]
- 24. Brændengen M, Tveit KM, Berglund A, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008; 26: 3687–3694. [DOI] [PubMed] [Google Scholar]
- 25. Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015; 16: 979–989. [DOI] [PubMed] [Google Scholar]
- 26. Hong YS, Kim SY, Lee JS, et al. Oxaliplatin-based adjuvant chemotherapy for rectal cancer after preoperative chemoradiotherapy (ADORE): long-term results of a randomized controlled trial. J Clin Oncol 2019; 37: 3111–3123. [DOI] [PubMed] [Google Scholar]
- 27. Deng Y, Chi P, Lan P, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial. J Clin Oncol 2019; 37: 3223–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmoll H-J, Haustermans K, Price TJ, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine +/– oxaliplatin in locally advanced rectal cancer: final results of PETACC-6. J Clin Oncol 2018; 36: 3500. [Google Scholar]
- 29. Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010; 28: 1638–1644. [DOI] [PubMed] [Google Scholar]
- 30. Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 2012; 30: 4558–4565. [DOI] [PubMed] [Google Scholar]
- 31. Des Guetz G, Landre T, Larrouy A, et al. Is there a benefit of oxaliplatin in neoadjuvant treatment of locally advanced rectal cancer? An updated meta-analysis. J Clin Oncol 2020; 38: 4098. [Google Scholar]
- 32. Hofheinz RD, Arnold D, Fokas E, et al. Impact of age on the efficacy of oxaliplatin in the preoperative chemoradiotherapy and adjuvant chemotherapy of rectal cancer: a post hoc analysis of the CAO/ARO/AIO-04 phase III trial. Ann Oncol 2018; 29: 1793–1799. [DOI] [PubMed] [Google Scholar]
- 33. Fontana E, Zichi C, Smyth EC, et al. Neoadjuvant chemoradiation (CRT) for locally advanced rectal cancer (LARC) with or without oxaliplatin (OX): individual patient data (IPD) meta-analysis of three randomized controlled trials (RCTs) with subgroup analyses of age cohorts. J Clin Oncol 2020; 38: 4074. [Google Scholar]
- 34. Zhu J, Liu A, Sun X, et al. Multicenter, randomized, phase III trial of neoadjuvant chemoradiation with capecitabine and irinotecan guided by UGT1A1 status in patients with locally advanced rectal cancer. J Clin Oncol 2020; 38: 4231–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu J, Sun X, Liu A, et al. Long-term outcome of a phase III trial on neoadjuvant chemoradiation with capecitabine and irinotecan in patients with locally advanced rectal cancer: updated results of the CinClare trial. J Clin Oncol 2021; 39: 3603. [Google Scholar]
- 36. Wang J, Fan J, Li C, et al. The impact of chemotherapy completion on the efficacy of irinotecan in the preoperative chemoradiotherapy of locally advanced rectal cancer: an expanded analysis of the CinClare phase III trial. Clin Colorectal Cancer 2020; 19: e58–e69. [DOI] [PubMed] [Google Scholar]
- 37. Sebag-Montefiore D, Adams R, Gollins S, et al. ARISTOTLE: a phase III trial comparing concurrent capecitabine with capecitabine and irinotecan (Ir) chemoradiation as preoperative treatment for MRI-defined locally advanced rectal cancer (LARC). J Clin Oncol 2020; 38: 4101. [Google Scholar]
- 38. Roeder F, Meldolesi E, Gerum S, et al. Recent advances in (chemo-)radiation therapy for rectal cancer: a comprehensive review. Radiat Oncol 2020; 15: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Conroy T, Lamfichekh N, Etienne PL, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol 2020; 38: 4007. [Google Scholar]
- 40. Hospers G, Bahadoer RR, Dijkstra EA, et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: the randomized RAPIDO trial. J Clin Oncol 2020; 38: 4006. [Google Scholar]
- 41. Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021; 22: 29–42. [DOI] [PubMed] [Google Scholar]
- 42. Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015; 16: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marco MR, Zhou L, Patil S, et al. Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: final results of a multicenter phase II trial. Dis Colon Rectum 2018; 61: 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Valk MJM, Marijnen CA, van Etten B, et al. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer – results of the international randomized RAPIDO-trial. Radiother Oncol 2020; 147: 75–83. [DOI] [PubMed] [Google Scholar]
- 45. Fokas E, Allgäuer M, Polat B, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ArO/AIO-12. J Clin Oncol 2019; 37: 3212–3222. [DOI] [PubMed] [Google Scholar]
- 46. Garcia-Aguilar J, Patil S, Kim JK, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol 2020; 38: 4008. [Google Scholar]
- 47. Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol 2016; 27: 834–842. [DOI] [PubMed] [Google Scholar]
- 48. Ciseł B, Pietrzak L, Michalski W, et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol 2019; 30: 1298–1303. [DOI] [PubMed] [Google Scholar]
- 49. Jin J, Tang Y, Hu C, et al. A multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR): the final reports. J Clin Oncol 2021; 39: 3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004; 240: 711–717; discussion 717–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maas M, Beets-Tan RGH, Lambregts DMJ, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011; 29: 4633–4640. [DOI] [PubMed] [Google Scholar]
- 52. Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 2015; 16: 919–927. [DOI] [PubMed] [Google Scholar]
- 53. Smith JJ, Strombom P, Chow OS, et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol 2019; 5: e185896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dossa F, Chesney TR, Acuna SA, et al. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017; 2: 501–513. [DOI] [PubMed] [Google Scholar]
- 55. Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 2016; 17: 174–183. [DOI] [PubMed] [Google Scholar]
- 56. van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 2018; 391: 2537–2545. [DOI] [PubMed] [Google Scholar]
- 57. Bitterman DS, Resende Salgado L, Moore HG, et al. Predictors of complete response and disease recurrence following chemoradiation for rectal cancer. Front Oncol 2015; 5: 286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garland ML, Vather R, Bunkley N, et al. Clinical tumour size and nodal status predict pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Int J Colorectal Dis 2014; 29: 301–307. [DOI] [PubMed] [Google Scholar]
- 59. Huh JW, Kim HR, Kim YJ. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum 2013; 56: 698–703. [DOI] [PubMed] [Google Scholar]
- 60. Wallin U, Rothenberger D, Lowry A, et al. CEA – a predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis Colon Rectum 2013; 56: 859–868. [DOI] [PubMed] [Google Scholar]
- 61. Moureau-Zabotto L, Farnault B, de Chaisemartin C, et al. Predictive factors of tumor response after neoadjuvant chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2011; 80: 483–491. [DOI] [PubMed] [Google Scholar]
- 62. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J Clin 2017; 67: 93–99. [DOI] [PubMed] [Google Scholar]
- 63. Peng H, Wang C, Xiao W, et al. Analysis of clinical characteristics to predict pathologic complete response for patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. J Cancer 2018; 9: 2687–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tan Y, Fu D, Li D, et al. Predictors and risk factors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer: a population-based analysis. Front Oncol 2019; 9: 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Al-Sukhni E, Attwood K, Mattson DM, et al. Predictors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Ann Surg Oncol 2016; 23: 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shao K, Zheng R, Li A, et al. Clinical predictors of pathological good response in locally advanced rectal cancer. Radiat Oncol 2021; 16: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kleiman A, Al-Khamis A, Farsi A, et al. Normalization of CEA levels post-neoadjuvant therapy is a strong predictor of pathologic complete response in rectal cancer. J Gastrointest Surg 2015; 19: 1106–1112. [DOI] [PubMed] [Google Scholar]
- 68. Restivo A, Zorcolo L, Cocco IM, et al. Elevated CEA levels and low distance of the tumor from the anal verge are predictors of incomplete response to chemoradiation in patients with rectal cancer. Ann Surg Oncol 2013; 20: 864–871. [DOI] [PubMed] [Google Scholar]
- 69. Patel SV, Roxburgh CS, Vakiani E, et al. Distance to the anal verge is associated with pathologic complete response to neoadjuvant therapy in locally advanced rectal cancer. J Surg Oncol 2016; 114: 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Simha V, Kapoor R, Gupta R, et al. Mucinous adenocarcinoma of the rectum: a poor candidate for neo-adjuvant chemoradiation? J Gastrointest Oncol 2014; 5: 276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vernmark K, Sun XF, Holmqvist A. Mucinous and non-mucinous rectal adenocarcinoma – differences in treatment response to preoperative radiotherapy. J Pers Med 2020; 10: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hugen N, Verhoeven RH, Radema SA, et al. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann Oncol 2013; 24: 2819–2824. [DOI] [PubMed] [Google Scholar]
- 73. Ryan JE, Warrier SK, Lynch AC, et al. Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review. Colorectal Dis 2016; 18: 234–246. [DOI] [PubMed] [Google Scholar]
- 74. García-Flórez LJ, Gómez-Álvarez G, Frunza AM, et al. Predictive markers of response to neoadjuvant therapy in rectal cancer. J Surg Res 2015; 194: 120–126. [DOI] [PubMed] [Google Scholar]
- 75. Lugli A, Zlobec I, Berger MD, et al. Tumour budding in solid cancers. Nat Rev Clin Oncol 2021; 18: 101–115. [DOI] [PubMed] [Google Scholar]
- 76. Rogers AC, Gibbons D, Hanly AM, et al. Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod Pathol 2014; 27: 156–162. [DOI] [PubMed] [Google Scholar]
- 77. Betge J, Pollheimer MJ, Lindtner RA, et al. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer 2012; 118: 628–638. [DOI] [PubMed] [Google Scholar]
- 78. Faiz Z, Huijgen LJW, Alqethami HJ, et al. Prevalence and prognostic significance of extramural venous invasion in patients with locally advanced esophageal cancer. Ann Surg Oncol 2018; 25: 1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Knijn N, Mogk SC, Teerenstra S, et al. Perineural invasion is a strong prognostic factor in colorectal cancer. Am J Surg Pathol 2016; 40: 103–112. [DOI] [PubMed] [Google Scholar]
- 80. Cienfuegos JA, Rotellar F, Baixauli J, et al. Impact of perineural and lymphovascular invasion on oncological outcomes in rectal cancer treated with neoadjuvant chemoradiotherapy and surgery. Ann Surg Oncol 2015; 22: 916–923. [DOI] [PubMed] [Google Scholar]
- 81. Song JH, Yu M, Kang KM, et al. Significance of perineural and lymphovascular invasion in locally advanced rectal cancer treated by preoperative chemoradiotherapy and radical surgery: can perineural invasion be an indication of adjuvant chemotherapy? Radiother Oncol 2019; 133: 125–131. [DOI] [PubMed] [Google Scholar]
- 82. Lee JH, Jang HS, Kim JG, et al. Lymphovascular invasion is a significant prognosticator in rectal cancer patients who receive preoperative chemoradiotherapy followed by total mesorectal excision. Ann Surg Oncol 2012; 19: 1213–1221. [DOI] [PubMed] [Google Scholar]
- 83. Chablani P, Nguyen P, Pan X, et al. Perineural invasion predicts for distant metastasis in locally advanced rectal cancer treated with neoadjuvant chemoradiation and surgery. Am J Clin Oncol 2017; 40: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Agarwal A, Chang GJ, Hu CY, et al. Quantified pathologic response assessed as residual tumor burden is a predictor of recurrence-free survival in patients with rectal cancer who undergo resection after neoadjuvant chemoradiotherapy. Cancer 2013; 119: 4231–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yildirim E, Bektas S, Pelen Z, et al. Histopathological, radiological, and demographic factors predicting the response to neoadjuvant therapy for rectal cancer. J Gastrointest Cancer. Epub ahead of print 1 September 2021. DOI: 10.1007/s12029-021-00697-9. [DOI] [PubMed] [Google Scholar]
- 86. Malekzadeh Moghani M, Alahyari S, Moradi A, et al. Pathological predictors of response to neoadjuvant treatment in rectal carcinoma. J Gastrointest Cancer 2021; 52: 690–695. [DOI] [PubMed] [Google Scholar]
- 87. Yan X, Chen J, Meng Y, et al. RAD18 may function as a predictor of response to preoperative concurrent chemoradiotherapy in patients with locally advanced rectal cancer through caspase-9-caspase-3-dependent apoptotic pathway. Cancer Med 2019; 8: 3094–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dou X, Wang R, Meng X, et al. The prognostic role of TCF4 expression in locally advanced rectal cancer patients treated with neoadjuvant chemoradiotherapy. Cancer Biomark 2015; 15: 181–188. [DOI] [PubMed] [Google Scholar]
- 89. Zaanan A, Park JM, Tougeron D, et al. Association of beclin 1 expression with response to neoadjuvant chemoradiation therapy in patients with locally advanced rectal carcinoma. Int J Cancer 2015; 137: 1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yu Z, Zhang C, Wang H, et al. Multidrug resistance-associated protein 3 confers resistance to chemoradiotherapy for rectal cancer by regulating reactive oxygen species and caspase-3-dependent apoptotic pathway. Cancer Lett 2014; 353: 182–193. [DOI] [PubMed] [Google Scholar]
- 91. Dalle Fratte C, Mezzalira S, Polesel J, et al. A panel of tumor biomarkers to predict complete pathological response to neoadjuvant treatment in locally advanced rectal cancer. Oncol Res 2021; 28: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Croner RS, Sevim M, Metodiev MV, et al. Identification of predictive markers for response to neoadjuvant chemoradiation in rectal carcinomas by proteomic isotope coded protein label (ICPL) analysis. Int J Mol Sci 2016; 17: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chauvin A, Wang CS, Geha S, et al. The response to neoadjuvant chemoradiotherapy with 5-fluorouracil in locally advanced rectal cancer patients: a predictive proteomic signature. Clin Proteomics 2018; 15: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Folkvord S, Flatmark K, Dueland S, et al. Prediction of response to preoperative chemoradiotherapy in rectal cancer by multiplex kinase activity profiling. Int J Radiat Oncol Biol Phys 2010; 78: 555–562. [DOI] [PubMed] [Google Scholar]
- 95. Repetto O, De Re V, De Paoli A, et al. Identification of protein clusters predictive of tumor response in rectal cancer patients receiving neoadjuvant chemo-radiotherapy. Oncotarget 2017; 8: 28328–28341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lin SC, Chang IW, Hsieh PL, et al. High immunoreactivity of DUOX2 is associated with poor response to preoperative chemoradiation therapy and worse prognosis in rectal cancers. J Cancer 2017; 8: 2756–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gomez del Pulgar T, Cebrián A, Fernández-Aceñero MJ, et al. Focal adhesion kinase: predictor of tumour response and risk factor for recurrence after neoadjuvant chemoradiation in rectal cancer. J Cell Mol Med 2016; 20: 1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. del Puerto-Nevado L, Marin-Arango JP, Fernandez-Aceñero MJ, et al. Predictive value of vrk 1 and 2 for rectal adenocarcinoma response to neoadjuvant chemoradiation therapy: a retrospective observational cohort study. BMC Cancer 2016; 16: 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kim HJ, Bae SB, Jeong D, et al. Upregulation of stromal cell-derived factor 1α expression is associated with the resistance to neoadjuvant chemoradiotherapy of locally advanced rectal cancer: angiogenic markers of neoadjuvant chemoradiation. Oncol Rep 2014; 32: 2493–2500. [DOI] [PubMed] [Google Scholar]
- 100. Kim K, Chie EK, Wu HG, et al. High survivin expression as a predictor of poor response to preoperative chemoradiotherapy in locally advanced rectal cancer. Int J Colorectal Dis 2011; 26: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 101. Zhang Y, Xu M, Chen J, et al. Prognostic value of the FOXK family expression in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Onco Targets Ther 2020; 13: 9185–9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tian YF, Hsieh PL, Lin CY, et al. High expression of aldolase B confers a poor prognosis for rectal cancer patients receiving neoadjuvant chemoradiotherapy. J Cancer 2017; 8: 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chang H, Wei JW, Tao YL, et al. CCR6 is a predicting biomarker of radiosensitivity and potential target of radiosensitization in rectal cancer. Cancer Res Treat 2018; 50: 1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cebrián A, Del Pulgar TG, Fernández-Aceñero MJ, et al. Decreased PLK1 expression denotes therapy resistance and unfavourable disease-free survival in rectal cancer patients receiving neoadjuvant chemoradiotherapy. Pathol Res Pract 2016; 212: 1133–1137. [DOI] [PubMed] [Google Scholar]
- 105. Smith FM, Reynolds JV, Kay EW, et al. COX-2 overexpression in pretreatment biopsies predicts response of rectal cancers to neoadjuvant radiochemotherapy. Int J Radiat Oncol Biol Phys 2006; 64: 466–472. [DOI] [PubMed] [Google Scholar]
- 106. Guedj N, Bretagnol F, Rautou PE, et al. Predictors of tumor response after preoperative chemoradiotherapy for rectal adenocarcinomas. Hum Pathol 2011; 42: 1702–1709. [DOI] [PubMed] [Google Scholar]
- 107. Giraldo NA, Sanchez-Salas R, Peske JD, et al. The clinical role of the TME in solid cancer. Br J Cancer 2019; 120: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Guo L, Wang C, Qiu X, et al. Colorectal cancer immune infiltrates: significance in patient prognosis and immunotherapeutic efficacy. Front Immunol 2020; 11: 1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Corrò C, Dutoit V, Koessler T. Emerging trends for radio-immunotherapy in rectal cancer. Cancers 2021; 13: 1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018; 391: 2128–2139. [DOI] [PubMed] [Google Scholar]
- 111. Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313: 1960–1964. [DOI] [PubMed] [Google Scholar]
- 112. Anitei MG, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res 2014; 20: 1891–1899. [DOI] [PubMed] [Google Scholar]
- 113. Kamran SC, Lennerz JK, Margolis CA, et al. Integrative molecular characterization of resistance to neoadjuvant chemoradiation in rectal cancer. Clin Cancer Res 2019; 25: 5561–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Matsutani S, Shibutani M, Maeda K, et al. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci 2018; 109: 966–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yasuda K, Nirei T, Sunami E, et al. Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol 2011; 6: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Akiyoshi T, Gotoh O, Tanaka N, et al. T-cell complexity and density are associated with sensitivity to neoadjuvant chemoradiotherapy in patients with rectal cancer. Cancer Immunol Immunother 2021; 70: 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Teng F, Meng X, Kong L, et al. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res 2015; 166: 721–732.e1. [DOI] [PubMed] [Google Scholar]
- 118. Shinto E, Hase K, Hashiguchi Y, et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol 2014; 21(Suppl. 3): S414–S421. [DOI] [PubMed] [Google Scholar]
- 119. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006; 6: 295–307. [DOI] [PubMed] [Google Scholar]
- 120. McCoy MJ, Hemmings C, Miller TJ, et al. Low stromal Foxp3+ regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. Br J Cancer 2015; 113: 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang S, Bai W, Tong X, et al. Correlation between tumor microenvironment-associated factors and the efficacy and prognosis of neoadjuvant therapy for rectal cancer. Oncol Lett 2019; 17: 1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017; 14: 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shabo I, Olsson H, Sun XF, et al. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. Int J Cancer 2009; 125: 1826–1831. [DOI] [PubMed] [Google Scholar]
- 124. Gutting T, Burgermeister E, Härtel N, et al. Checkpoints and beyond – immunotherapy in colorectal cancer. Semin Cancer Biol 2019; 55: 78–89. [DOI] [PubMed] [Google Scholar]
- 125. Ogura A, Akiyoshi T, Yamamoto N, et al. Pattern of programmed cell death-ligand 1 expression and CD8-positive T-cell infiltration before and after chemoradiotherapy in rectal cancer. Eur J Cancer 2018; 91: 11–20. [DOI] [PubMed] [Google Scholar]
- 126. Hecht M, Büttner-Herold M, Erlenbach-Wünsch K, et al. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur J Cancer 2016; 65: 52–60. [DOI] [PubMed] [Google Scholar]
- 127. Saigusa S, Toiyama Y, Tanaka K, et al. Implication of programmed cell death ligand 1 expression in tumor recurrence and prognosis in rectal cancer with neoadjuvant chemoradiotherapy. Int J Clin Oncol 2016; 21: 946–952. [DOI] [PubMed] [Google Scholar]
- 128. West NR, McCuaig S, Franchini F, et al. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol 2015; 15: 615–629. [DOI] [PubMed] [Google Scholar]
- 129. Debucquoy A, Goethals L, Geboes K, et al. Molecular responses of rectal cancer to preoperative chemoradiation. Radiother Oncol 2006; 80: 172–177. [DOI] [PubMed] [Google Scholar]
- 130. Tada N, Tsuno NH, Kawai K, et al. Changes in the plasma levels of cytokines/chemokines for predicting the response to chemoradiation therapy in rectal cancer patients. Oncol Rep 2014; 31: 463–471. [DOI] [PubMed] [Google Scholar]
- 131. Buijsen J, van Stiphout RG, Menheere PP, et al. Blood biomarkers are helpful in the prediction of response to chemoradiation in rectal cancer: a prospective, hypothesis driven study on patients with locally advanced rectal cancer. Radiother Oncol 2014; 111: 237–242. [DOI] [PubMed] [Google Scholar]
- 132. Jayne DG, Heath RM, Dewhurst O, et al. Extracellular matrix proteins and chemoradiotherapy: α5β1 integrin as a predictive marker in rectal cancer. Eur J Surg Oncol 2002; 28: 30–36. [DOI] [PubMed] [Google Scholar]
- 133. Unsal D, Uner A, Akyurek N, et al. Matrix metalloproteinase-9 expression correlated with tumor response in patients with locally advanced rectal cancer undergoing preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007; 67: 196–203. [DOI] [PubMed] [Google Scholar]
- 134. Gonçalves-Ribeiro S, Sanz-Pamplona R, Vidal A, et al. Prediction of pathological response to neoadjuvant treatment in rectal cancer with a two-protein immunohistochemical score derived from stromal gene-profiling. Ann Oncol 2017; 28: 2160–2168. [DOI] [PubMed] [Google Scholar]
- 135. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016; 375: 2369–2379. [DOI] [PubMed] [Google Scholar]
- 136. Elinav E, Garrett WS, Trinchieri G, et al. The cancer microbiome. Nat Rev Cancer 2019; 19: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol 2017; 32: 43–53. [DOI] [PubMed] [Google Scholar]
- 138. Sun Y, Dou X, Li W, et al. Longitudinal analysis of fecal microbiome diversity during the neoadjuvant concurrent chemoradiotherapy of patients with locally advanced rectal cancer. Int J Radiat Oncol 2020; 108: e579–e580. [Google Scholar]
- 139. Li W, Jin J, Tang Y, et al. Hypofractionated radiation changes the gut flora into a inflammatory activation pattern which is related with tumor complete regression in local advanced rectum cancer. Int J Radiat Oncol 2020; 108: e533. [Google Scholar]
- 140. Tonneau M, Elkrief A, Pasquier D, et al. The role of the gut microbiome on radiation therapy efficacy and gastrointestinal complications: a systematic review. Radiother Oncol 2021; 156: 1–9. [DOI] [PubMed] [Google Scholar]
- 141. McQuade JL, Daniel CR, Helmink BA, et al. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol 2019; 20: e77–e91. [DOI] [PubMed] [Google Scholar]
- 142. Alexander JL, Wilson ID, Teare J, et al. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 2017; 14: 356–365. [DOI] [PubMed] [Google Scholar]
- 143. Liu J, Liu C, Yue J. Radiotherapy and the gut microbiome: facts and fiction. Radiat Oncol 2021; 16: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yi Y, Shen L, Shi W, et al. Gut microbiome components predict response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a prospective, longitudinal study. Clin Cancer Res 2021; 27: 1329–1340. [DOI] [PubMed] [Google Scholar]
- 145. Jang BS, Chang JH, Chie EK, et al. Gut microbiome composition is associated with a pathologic response after preoperative chemoradiation in patients with rectal cancer. Int J Radiat Oncol Biol Phys 2020; 107: 736–746. [DOI] [PubMed] [Google Scholar]
- 146. Shi W, Shen L, Zou W, et al. The gut microbiome is associated with therapeutic responses and toxicities of neoadjuvant chemoradiotherapy in rectal cancer patients – a pilot study. Front Cell Infect Microbiol 2020; 10: 562463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chow OS, Kuk D, Keskin M, et al. KRAS and combined KRAS/TP53 mutations in locally advanced rectal cancer are independently associated with decreased response to neoadjuvant therapy. Ann Surg Oncol 2016; 23: 2548–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Duldulao MP, Lee W, Nelson RA, et al. Mutations in specific codons of the KRAS oncogene are associated with variable resistance to neoadjuvant chemoradiation therapy in patients with rectal adenocarcinoma. Ann Surg Oncol 2013; 20: 2166–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhou P, Goffredo P, Ginader T, et al. Impact of KRAS status on tumor response and survival after neoadjuvant treatment of locally advanced rectal cancer. J Surg Oncol 2021; 123: 278–285. [DOI] [PubMed] [Google Scholar]
- 151. Clancy C, Burke JP, Coffey JC. KRAS mutation does not predict the efficacy of neo-adjuvant chemoradiotherapy in rectal cancer: a systematic review and meta-analysis. Surg Oncol 2013; 22: 105–111. [DOI] [PubMed] [Google Scholar]
- 152. Garcia-Aguilar J, Chen Z, Smith DD, et al. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann Surg 2011; 254: 486–492; discussion 492–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lopez-Crapez E, Bibeau F, Thezenas S, et al. p53 status and response to radiotherapy in rectal cancer: a prospective multilevel analysis. Br J Cancer 2005; 92: 2114–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chen MB, Wu XY, Yu R, et al. P53 status as a predictive biomarker for patients receiving neoadjuvant radiation-based treatment: a meta-analysis in rectal cancer. PLoS ONE 2012; 7: e45388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sclafani F, Wilson SH, Cunningham D, et al. Analysis of KRAS, NRAS, BRAF, PIK3CA and TP53 mutations in a large prospective series of locally advanced rectal cancer patients. Int J Cancer 2020; 146: 94–102. [DOI] [PubMed] [Google Scholar]
- 156. Jiang D, Wang X, Wang Y, et al. Mutation in BRAF and SMAD4 associated with resistance to neoadjuvant chemoradiation therapy in locally advanced rectal cancer. Virchows Arch 2019; 475: 39–47. [DOI] [PubMed] [Google Scholar]
- 157. Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer 2004; 4: 988–993. [DOI] [PubMed] [Google Scholar]
- 158. Zhang B, Wang J, Wang X, et al. Proteogenomic characterization of human colon and rectal cancer. Nature 2014; 513: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Jo P, Jung K, Grade M, et al. CpG island methylator phenotype infers a poor disease-free survival in locally advanced rectal cancer. Surgery 2012; 151: 564–570. [DOI] [PubMed] [Google Scholar]
- 160. Molinari C, Casadio V, Foca F, et al. Gene methylation in rectal cancer: predictive marker of response to chemoradiotherapy? J Cell Physiol 2013; 228: 2343–2349. [DOI] [PubMed] [Google Scholar]
- 161. Ha YJ, Kim CW, Roh SA, et al. Epigenetic regulation of KLHL34 predictive of pathologic response to preoperative chemoradiation therapy in rectal cancer patients. Int J Radiat Oncol Biol Phys 2015; 91: 650–658. [DOI] [PubMed] [Google Scholar]
- 162. Do Canto LM, Barros-Filho MC, Rainho CA, et al. Comprehensive analysis of DNA methylation and prediction of response to neoadjuvant therapy in locally advanced rectal cancer. Cancers 2020; 12: 3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Imedio L, Cristóbal I, Rubio J, et al. MicroRNAs in rectal cancer: functional significance and promising therapeutic value. Cancers 2020; 12: 2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Izzotti A, Ceccaroli C, Geretto M, et al. Predicting response to neoadjuvant therapy in colorectal cancer patients the role of messenger- and micro-RNA profiling. Cancers 2020; 12: 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Pettit C, Walston S, Wald P, et al. Molecular profiling of locally-advanced rectal adenocarcinoma using microRNA expression (review). Int J Oncol 2017; 51: 393–404. [DOI] [PubMed] [Google Scholar]
- 166. Lopes-Ramos CM, Habr-Gama A, de Souza Quevedo B, et al. Overexpression of miR-21-5p as a predictive marker for complete tumor regression to neoadjuvant chemoradiotherapy in rectal cancer patients. BMC Med Genomics 2014; 7: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Caramés C, Cristóbal I, Moreno V, et al. MicroRNA-21 predicts response to preoperative chemoradiotherapy in locally advanced rectal cancer. Int J Colorectal Dis 2015; 30: 899–906. [DOI] [PubMed] [Google Scholar]
- 168. Eriksen AHM, Sørensen FB, Andersen RF, et al. Association between the expression of microRNAs and the response of patients with locally advanced rectal cancer to preoperative chemoradiotherapy. Oncol Lett 2017; 14: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Campayo M, Navarro A, Benítez JC, et al. MiR-21, miR-99b and miR-375 combination as predictive response signature for preoperative chemoradiotherapy in rectal cancer. PLoS ONE 2018; 13: e0206542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Nakao T, Iwata T, Hotchi M, et al. Prediction of response to preoperative chemoradiotherapy and establishment of individualized therapy in advanced rectal cancer. Oncol Rep 2015; 34: 1961–1967. [DOI] [PubMed] [Google Scholar]
- 171. Machackova T, Trachtova K, Prochazka V, et al. Tumor microRNAs identified by small RNA sequencing as potential response predictors in locally advanced rectal cancer patients treated with neoadjuvant chemoradiotherapy. Cancer Genomics Proteomics 2020; 17: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lopes-Ramos C, Koyama FC, Habr-Gama A, et al. Comprehensive evaluation of the effectiveness of gene expression signatures to predict complete response to neoadjuvant chemoradiotherapy and guide surgical intervention in rectal cancer. Cancer Genet 2015; 208: 319–326. [DOI] [PubMed] [Google Scholar]
- 173. Cescon DW, Bratman SV, Chan SM, et al. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer 2020; 1: 276–290. [DOI] [PubMed] [Google Scholar]
- 174. Morais M, Pinto DM, Machado JC, et al. ctDNA on liquid biopsy for predicting response and prognosis in locally advanced rectal cancer: a systematic review. Eur J Surg Oncol. Epub ahead of print 3 September 2021. DOI: 10.1016/j.ejso.2021.08.034. [DOI] [PubMed] [Google Scholar]
- 175. Murahashi S, Akiyoshi T, Sano T, et al. Serial circulating tumour DNA analysis for locally advanced rectal cancer treated with preoperative therapy: prediction of pathological response and postoperative recurrence. Br J Cancer 2020; 123: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Tie J, Cohen JD, Wang Y, et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut 2019; 68: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Wang BG, Huang HY, Chen YC, et al. Increased plasma DNA integrity in cancer patients. Cancer Res 2003; 63: 3966–3968. [PubMed] [Google Scholar]
- 178. Agostini M, Pucciarelli S, Enzo MV, et al. Circulating cell-free DNA: a promising marker of pathologic tumor response in rectal cancer patients receiving preoperative chemoradiotherapy. Ann Surg Oncol 2011; 18: 2461–2468. [DOI] [PubMed] [Google Scholar]
- 179. Sun W, Sun Y, Zhu M, et al. The role of plasma cell-free DNA detection in predicting preoperative chemoradiotherapy response in rectal cancer patients. Oncol Rep 2014; 31: 1466–1472. [DOI] [PubMed] [Google Scholar]
- 180. Shalaby SM, El-Shal AS, Abdelaziz LA, et al. Promoter methylation and expression of DNA repair genes MGMT and ERCC1 in tissue and blood of rectal cancer patients. Gene 2018; 644: 66–73. [DOI] [PubMed] [Google Scholar]
- 181. Yang L, Wang Y, Bao H, et al. ctDNA as a potential prognostic marker for locally advanced rectal cancer patients with ‘watch and wait’ approach. J Clin Oncol 2019; 37: 3544. [Google Scholar]
- 182. Emons G, Spitzner M, Reineke S, et al. Chemoradiotherapy resistance in colorectal cancer cells is mediated by Wnt/β-catenin signaling. Mol Cancer Res 2017; 15: 1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Carón RW, Yacoub A, Mitchell C, et al. Radiation-stimulated ERK1/2 and JNK1/2 signaling can promote cell cycle progression in human colon cancer cells. Cell Cycle 2005; 4: 456–464. [DOI] [PubMed] [Google Scholar]
- 184. Ferrandon S, DeVecchio J, Duraes L, et al. CoA synthase (COASY) mediates radiation resistance via PI3K signaling in rectal cancer. Cancer Res 2020; 80: 334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ferreiro-Neira I, Torres NE, Liesenfeld LF, et al. XPO1 inhibition enhances radiation response in preclinical models of rectal cancer. Clin Cancer Res 2016; 22: 1663–1673. [DOI] [PubMed] [Google Scholar]
- 186. Yokoi K, Yamashita K, Ishii S, et al. Comprehensive molecular exploration identified promoter DNA methylation of the CRBP1 gene as a determinant of radiation sensitivity in rectal cancer. Br J Cancer 2017; 116: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Chung P, Cook T, Liu K, et al. Overexpression of the human inducible nitric oxide synthase gene enhances radiation-induced apoptosis in colorectal cancer cells via a caspase-dependent mechanism. Nitric Oxide 2003; 8: 119–126. [DOI] [PubMed] [Google Scholar]
- 188. Zhu Y, Wang C, Becker SA, et al. miR-145 antagonizes SNAI1-mediated stemness and radiation resistance in colorectal cancer. Mol Ther 2018; 26: 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ji D, Zhan T, Li M, et al. Enhancement of sensitivity to chemo/radiation therapy by using miR-15b against DCLK1 in colorectal cancer. Stem Cell Reports 2018; 11: 1506–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kleiman LB, Krebs AM, Kim SY, et al. Comparative analysis of radiosensitizers for K-RAS mutant rectal cancers. PLoS ONE 2013; 8: e82982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Braun R, Anthuber L, Hirsch D, et al. Single-cell-derived primary rectal carcinoma cell lines reflect intratumor heterogeneity associated with treatment response. Clin Cancer Res 2020; 26: 3468–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol 2012; 9: 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Fichtner I, Slisow W, Gill J, et al. Anticancer drug response and expression of molecular markers in early-passage xenotransplanted colon carcinomas. Eur J Cancer 2004; 40: 298–307. [DOI] [PubMed] [Google Scholar]
- 194. Krumbach R, Schüler J, Hofmann M, et al. Primary resistance to cetuximab in a panel of patient-derived tumour xenograft models: activation of MET as one mechanism for drug resistance. Eur J Cancer 2011; 47: 1231–1243. [DOI] [PubMed] [Google Scholar]
- 195. Janakiraman H, Zhu Y, Becker SA, et al. Modeling rectal cancer to advance neoadjuvant precision therapy. Int J Cancer 2020; 147: 1405–1418. [DOI] [PubMed] [Google Scholar]
- 196. Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009; 459: 262–265. [DOI] [PubMed] [Google Scholar]
- 197. Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011; 141: 1762–1772. [DOI] [PubMed] [Google Scholar]
- 198. Tuveson D, Clevers H. Cancer modeling meets human organoid technology. Science 2019; 364: 952–955. [DOI] [PubMed] [Google Scholar]
- 199. Kolahi KS, Nakano M, Kuo CJ. Organoids as oracles for precision medicine in rectal cancer. Cell Stem Cell 2020; 26: 4–6. [DOI] [PubMed] [Google Scholar]
- 200. van de Wetering M, Francies HE, Francis JM, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015; 161: 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Park M, Kwon J, Kong J, et al. A patient-derived organoid-based radiosensitivity model for the prediction of radiation responses in patients with rectal cancer. Cancers 2021; 13: 3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Ganesh K, Wu C, O’Rourke KP, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med 2019; 25: 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Yao Y, Xu X, Yang L, et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell 2020; 26: 17–26.e6. [DOI] [PubMed] [Google Scholar]
- 204. Frydrych LM, Ulintz P, Bankhead A, et al. Rectal cancer sub-clones respond differentially to neoadjuvant therapy. Neoplasia 2019; 21: 1051–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]