Abstract
Background:
The development of an integral and global treatment to improve the quality of life in those with fibromyalgia syndrome (FMS) is challenging. The aim of this study is to investigate the impact of whole-body photobiomodulation (PBM) on pain perception, functionality, quality of soft tissue, central sensitisation and psychological factors in patients suffering with FMS.
Methods:
This study is a randomised, placebo-controlled clinical trial. A total of 44 participants will be recruited in a private care practice and randomised to receive either a whole-body PBM therapy programme or placebo in the same care centre. The parameters of the PBM programme are as follows: wavelengths of red and near-infrared LEDs 50:50 ratio with 660–850 nanometers; fluence of 25.2 J/cm2; treatment time of 1200 s and a total power emitted of 967 W. Treatment sessions will be 3 times weekly for a period of 4 weeks, totalling 12 treatment sessions. Primary outcome will be pain (Numeric Pain Rating Scale; Widespread Pain Index; Symptom Severity Score). Secondary outcomes will be functionality (Fibromyalgia Impact Questionnaire; the Leisure Time Physical Activity Instrument), quality of soft tissue (elastography), central sensitisation (pain pressure threshold and the Autonomic Symptom Profile) and psychological factors (Pain Catastrophising scale, Tampa Scale, Self-Efficacy questionnaire). Assessments will be at baseline (T1), after session 6 (T2), after treatment (T3) and 2 weeks (T4), 3 (T5) and 6 (T6) month follow-up.
Discussion:
PBM therapy has been shown to reduce pain and inflammation and to increase the rate of tissue repair for a wide range of conditions, but its potential use as a whole-body treatment in FM is yet to be explored. This trial will investigate whether whole-body PBM therapy is effective at reducing pain intensity, improving functionality, quality of soft tissue, central sensitisation symptoms and psychological measurements. Furthermore, 3- and 6-month follow-up will investigate long-term efficacy of this treatment.
Trial registration:
NCT04248972. Registered on January 29, 2020, https://clinicaltrials.gov/ct2/show/NCT04248972?term=navarro-ledesma+santiago&draw=2&rank=2.
Keywords: chronic pain, elasticity imaging techniques, fibromyalgia, pain perception, photobiomodulation therapy, pressure algometry, pressure pain threshold, psychology, ultrasonography
Introduction
Fibromyalgia syndrome (FMS) is a chronic and multicomponent illness with unknown etiology and is considered the most frequent cause of diffuse chronic musculoskeletal pain. 1 Following the American College of Rheumatology (ACR), different criteria have been included in FMS diagnosis, such as digital pain pressure sensitivity at a pressure of 4 kg and widespread pain, criteria which cannot be explained by the presence of degenerative or inflammatory disorders, cognitive behaviour disorders, restless sleep, fatigue and somatic symptoms.2,3 This syndrome can occur in all ages, but it is more common in middle-aged adults. 4 In the general population, the range is from 0.5% to 5%, and up to 15.7% in a clinical setting. In Spain, the estimated prevalence is 4.2% in women and 0.2% in men. 1
Despite some physical therapy interventions, such as exercise and cognitive behaviour therapy showing some therapeutic benefit,2,4,5 FMS is a complex syndrome and there is little evidence to confirm if the condition is fully improved in all aspects using these treatment programmes. Thus, a multifactorial and definitive treatment is currently lacking.2,5,6
Photobiomodulation (PBM) therapy, formerly known as low-level laser therapy (LLLT), is an emerging, noninvasive and promising therapy for those suffering from FMS because it has shown positive impact on relieving musculoskeletal and neuropathic pain, with consequent improvement on quality of life. 6 Current research has established effective wavelengths of light used for PBM to range from 600 to 1070 nm, with a fluence (energy density) range of between 1 and 20 J/cm2. Effective tissue penetration is maximised in this range, as the principal tissue chromophores (haemoglobin and melanin) have high absorption bands at wavelengths shorter than 600 nm. Wavelengths in the range 600–700 nm are used to treat superficial tissue (skin, subcutaneous tissue, superficial fascia and muscles), and longer wavelengths in the range 780–950 nm, which penetrate further, are used to treat deeper-seated tissues (deep fascia and muscles, bone, brain).7–9
Previous studies have shown positive effects of PBM in patients suffering from FMS, such as a decrease in pain, sleep disorders, tiredness, muscle spasm, morning stiffness and tender point numbers.6,10–12 Recently, the possibility of a whole-body PBM has been shown, offering not only a local but also a systemic response. In this regard, improvements in neuronal bioenergetic functions, cerebral blood flow, oxidative stress, neuroinflammation, neural apoptosis, neurotrophic factors, neurogenesis and effects on intrinsic brain networks have been proposed, thus including a brain PBM treatment. This is currently used in a wide range of neurological and psychological conditions. 7 Thus, a whole-body PBM treatment is presented as a new possibility of treatment with potential benefits for those with FMS and its results are still to be investigated.
Current literature only shows a whole-body PBM trial carried out in a sports population, and showed short-term effects without conclusive changes. 13
People suffering from FMS usually present with tender points in a number of anatomical areas; in fact, 95% of people with chronic pain disorders have been shown to present with myofascial pain. 14 Tender or trigger points are the result of a nonspecific response of the central nervous system in its interaction with the autonomic nervous system. 15
Ultrasound elastography (USE) imaging can provide an objective and reproducible measure of a change in the status of myofascial trigger points as determined by physical examination because palpably stiff nodules vibrate with lower amplitude than healthy tissue when using ultrasound. 14 The USE was first described in the 1990s, and recently developed to assess quantitatively tissue stiffness; thus, there has been an increase in the use of USE to measure changes in elasticity of soft tissues in the study of physiological processes and pathology.16,17
In addition to pain, presence of tender points and central sensitisation, other postural, balance and functional symptoms have also been presented in those with FMS5,18–20 affecting psychological health, ability to enjoy leisure activities and consequently quality of life.18,21,22 It is therefore important to also include outcome measures of these symptoms in investigative research.
We hypothesise that a whole-body application of PBM will improve pain primary. Secondary, functionality, quality of soft tissue, central sensitisation and psychological symptoms are expected to improve in patients suffering from FMS.
To our knowledge, this is the first study to use whole-body PBM in FMS patients.
Methods
Design
This is a triple-blinded, randomised, placebo-controlled clinical trial with blinding of participants, therapists, evaluators and statisticians to active or placebo whole-body PBM.
Setting
Participants will be recruited in a private care practice in Malaga, Spain. Potential referrals will be informed of the trial through formal meetings and trial information sheets. This study is reported in line with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Statement (Supplemental information). 23 This study protocol has received ethical approval by Ethics Committee of Human Research of the University of Granada, Spain (1044/CEIH/2020). All the participants will accept and sign an informed consent before beginning the study.
Patient involvement
Patients will be involved in the design and conduct of this research. During the feasibility stage, priority of the research question, choice of outcome measures and methods of recruitment will be informed by discussions with patients through a focus group session. Once the trial has been published, participants will be informed of the results through a new session and they could ask for the details of the results.
Participants
Participants will be screened by a physiotherapist to determine whether they meet the following inclusion and exclusion criteria:
Inclusion criteria
Aged between 34 and 64 years
FMS diagnosis from a rheumatologist according to the ACR classification criteria (modified 2010/2011). 24 To diagnose fibromyalgia in adults, it is necessary for all the following criteria to be met: (1) Present generalised pain, that is, in at least four of the five regions, (2) present symptoms for at least 3 months at similar levels, (3) symptom severity scale (SSS) score ⩾5 and Widespread Pain Index (WPI) ⩾7, or SSS score ⩾9 and WPI between 4 and 6, and (4) a diagnosis of fibromyalgia does not exclude the presence of other illnesses and is valid irrespective of other diagnoses.
Exclusion criteria
Presenting any inflammatory, neurological or orthopaedic disease which can alter balance, hearing and vision, or cognitive impairment, which might impact the ability to answer questions. Furthermore, fascial muscle disorders such as trigger points, myofascial syndrome pain and neck pain.
Participants will be randomised to receive either a whole-body PBM therapy or placebo.
Patients will be required to not receive or participate in any other FMS study or treatment during the study period. Any change in medication type or dosage during the study period will be recorded, and prescribed medication from medical doctors will be kept. Therefore, the placebo will be related only to the use of PBM. Patients who have already undergone previous treatments will be accepted because FMS patients need continuous care. In addition, the accepted treatments previously received before the start of the trial would be those related to manual therapy and physical activity.
The interventions are described following Template for Intervention Description and Replication (TIDieR) Checklist recommendations. 25
PBM therapy programme
Participants randomised to this treatment will receive a whole-body PBM treatment using a NovoTHOR® whole body light bed (Figure 1). For each treatment session, participants will lie supine in the treatment bed for 20 min, with no or minimal attire (underwear). Treatment sessions will be 3 times weekly for a period of 4 weeks, totalling 12 treatment sessions. The parameters of the equipment are shown in Table 1.
Figure 1.

NovoTHOR bed.
Table 1.
NovoTHOR parameters.
| NovoTHOR XL parameters | Unit | |
|---|---|---|
| Wavelengths of red and near-infrared (NIR) LEDs 50:50 ratio | 660 850 |
nm nm |
| Number of LEDs | 2880 | |
| Power emitted per LED | 0.336 | W |
| Beam area per LED (at the lens/skin contact surface) | 12.0 | cm2 |
| Total power emitted | 967 | W |
| Total area of NovoTHOR emitting surfaces | 34,544 | cm2 |
| Treatment time | 1200 | s |
| Continuous wave (CW) (not pulsed) | CW | |
| Irradiance | 0.028 | W/cm2 |
| Fluence | 25.2 | J/cm2 |
Placebo feature
The placebo feature of the whole-body PBM bed provides controls that select active or placebo (sham) treatments in a way undetectable by participant, operator or observers, such that no one is aware whether the participant is receiving an active or placebo treatment. There is a switch box (see Figure 2) that randomises participants to active or placebo; no other randomisation is necessary. With this system, if the operator becomes unblinded, they will only discover which treatment that particular participant is getting, and hence, only that particular participant would be excluded from the trial and not the operator. A blocked randomisation system (randomly varying the block size) to ensure that comparison groups will be generated in a ratio of 1:1 of approximately the same size will be used. For every block of 10 participants, five would be allocated to each arm of the trial. In the worst scenario, the allocation could be unbalanced by as much as two.
Figure 2.

NovoTHOR randomising switch box.
Furthermore, special goggles that block the PBM light are worn by the participant, operator and observers. These emit LED light inside (behind the lenses, so that the wearer sees some red light) to make it harder for participants, operator or observers to detect if the PBM bed is active or placebo. The goggles are designed to accommodate spectacles.
Heating elements also come on in the NovoTHOR bed when the PBM bed is in placebo mode, so that participants feel like they are in the real treatment.
PBM is safe, and easy to administer, is noninvasive and has no known side effects, with few reported contraindications. 26
For each treatment session, participants will lie supine in the treatment bed for 20 min, with no or minimal attire (underwear). Treatment sessions will be 3 times weekly for a period of 4 weeks, totalling 12 treatment sessions.
Data collection
Assessment of primary and secondary outcome measures will be at baseline, after treatment 6, immediately following the last treatment (4 weeks) and then 2 weeks and at 3 monthly follow-up intervals to 6 months after completion of treatment. A flow diagram illustrates these assessment times (Figure 3).
Figure 3.
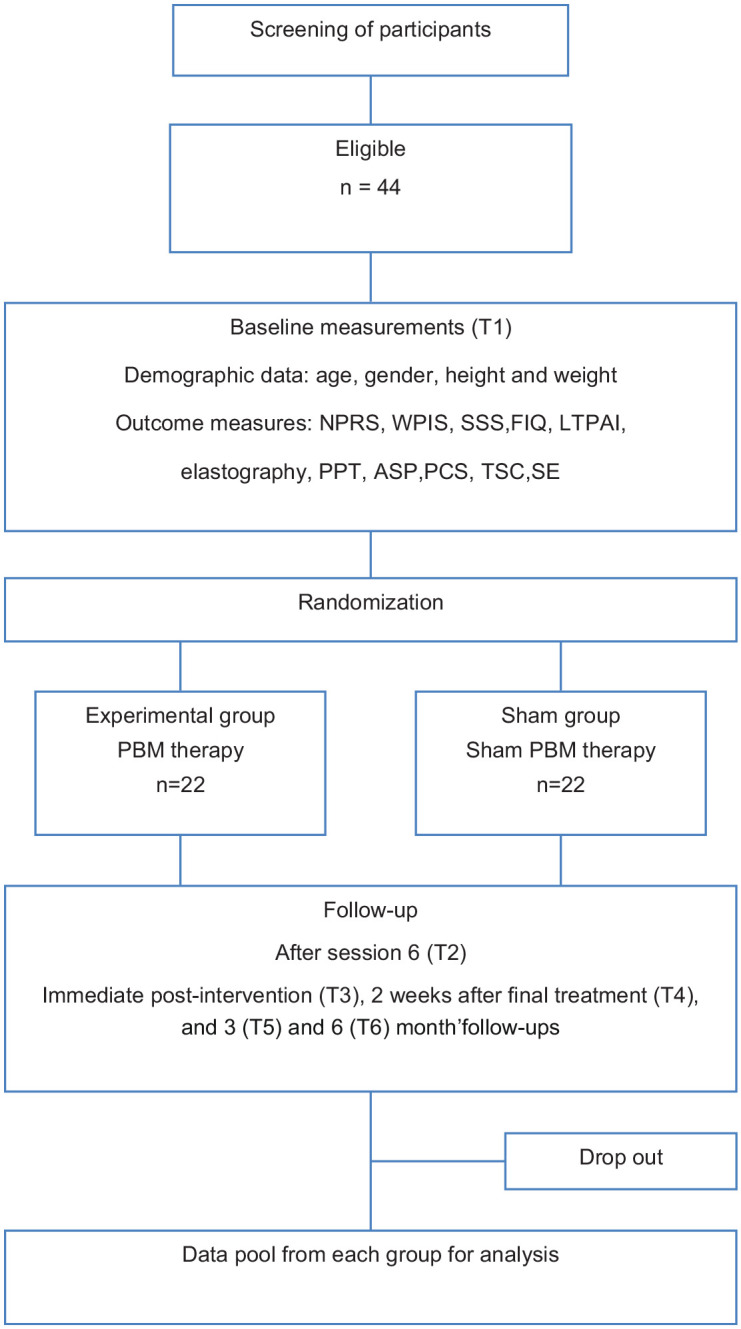
Flow diagram illustrating the assessment times.
Outcome measures
Primary outcome measures
The Numeric Pain Rating Scale (NPRS), where 0 indicates ‘no pain’, and 10 indicates ‘worst possible pain’. At each measurement point of the study, patients of both groups will be asked to rate the average intensity of their pain over the past 7 days. This procedure has demonstrated a high degree of validity and reliability. 27
The revised Fibromyalgia Impact Questionnaire (FIQR), a self-administered questionnaire comprising 21 individual questions, with a rating scale of 0–10. The questions compose three different domains: function, overall impact and symptoms score (range: 0–30, 0–20 and 0–50, respectively).3,28 The FIQR total score ranges from 0 to 100, with a higher score indicating a greater impact of the condition on the person’s life.
WPI, a questionnaire which shows appropriate distribution and a sufficient number of body quadrants and axial skeleton pain representation. It is part of the FMS diagnosis. 29
Symptom Severity Score (SSS), a questionnaire which is part of the FMS diagnosis. 29
Secondary outcome measures
The Leisure Time Physical Activity Instrument (LTPAI), used to measure the physical activity. This has four components, each with three levels of activity: light, medium and vigorous. Scores indicate the number of hours which these activity levels had been carried out each week in the last 4 weeks summing as the total number of hours of physical activity. 30 This tool has shown satisfactory test–retest reliability for the total score, that is, intraclass correlation coefficient (ICC) = 0.86 [confidence interval (CI): 0.79–0.93], and for the PAHWI (ICC = 0.91, CI: 0.82–9.96). 30
Pain pressure threshold (PPT): 12 tender points will be assessed according to the ACR criteria using a standard pressure algometer of 1 cm2 (FPK 20; Wagner Instruments, Greenwich, CT, USA), exerting a pressure of up to 4 kg. The algometer will be positioned perpendicular to the tender point, and the pressure continuously increased until the patient expressed a sensation of pain. The points assessed will be occiput at the suboccipital muscle insertions, low cervical at the anterior aspects of the intertransverse spaces at C5–C7, trapezius at the midpoint of the upper border, supraspinatus at origins, above the scapula spine near the medial border, paraspinous 3 cm lateral to the midline at the level of the mid-scapula, second rib at the second costochondral junctions, just lateral to the junctions on the upper surfaces, lateral pectoral at the level of the fourth rib at the anterior axillary line, lateral epicondyle 2 cm distal to the epicondyles, medial epicondyle at the epicondyles, gluteal at the upper outer quadrants of buttocks in the anterior fold of muscle, greater trochanter just posterior to the trochanteric prominence and knees at the medial fat pad proximal to the joint line, forearm at the distal dorsal third of the forearm, thumbnail and midfoot at the midpoint of the dorsal third metatarsal. 3 The mean of two measurements at each tender point will be used for the analysis. The total count of positive tender points will be recorded for each participant (Figure 4). 3
The Autonomic Symptom Profile (ASP) is a validated self-report questionnaire that comprehensively assesses autonomic symptoms across 11 subscales and yields a composite autonomic symptom score. 31
Quantified USE in tender points. Changes in the status of myofascial trigger points can be demonstrated with an objective and reproducible USE measure. 8 Methodology used in previous studies will be followed. 32 All measurements will be performed with the Logiq S7 using a 15-MHz linear probe (GE Healthcare, Milwaukee, WI) by a physiotherapist expert in musculoskeletal ultrasound imaging with 10 years of experience in ultrasound imaging. The strain elastography (SEL) will be obtained in the same position that the PPT assessment was carried out. A transverse glide will then be performed at the exact position that the PPT assessment was developed and SEL measurements will be taken. At this point, the tissue will be compressed approximately 2–5 mm, and a software-incorporated quality control (expressed as one to five green bars being displayed, with five bars being the most acceptable) will be used to evaluate the recommended compression size. The exact raw strain value (0–6; with 0 being softest and 6 being the hardest tissue) will be calculated using a 5-mm circular region in a soft part of the area of interest, as indicated by the manufacturer’s instructions and shown in previous studies. 33 A mean of three measurements at each point will be calculated to minimise intraobserver variation. Only sequences with the highest image quality (with green bars on the quality assessment) were used as recommended by the manufacturer.
The Pain Catastrophising Scale, a validated questionnaire to assess the mechanism by which catastrophising impacts pain experience. 34
The Spanish version of the Tampa Scale of Kinesophobia, a valid and reliable measure of fear of movement. 35
The self-efficacy questionnaire, which assesses personal confidence to carry out an activity with the aim of successfully achieving a desired outcome. 36
Figure 4.
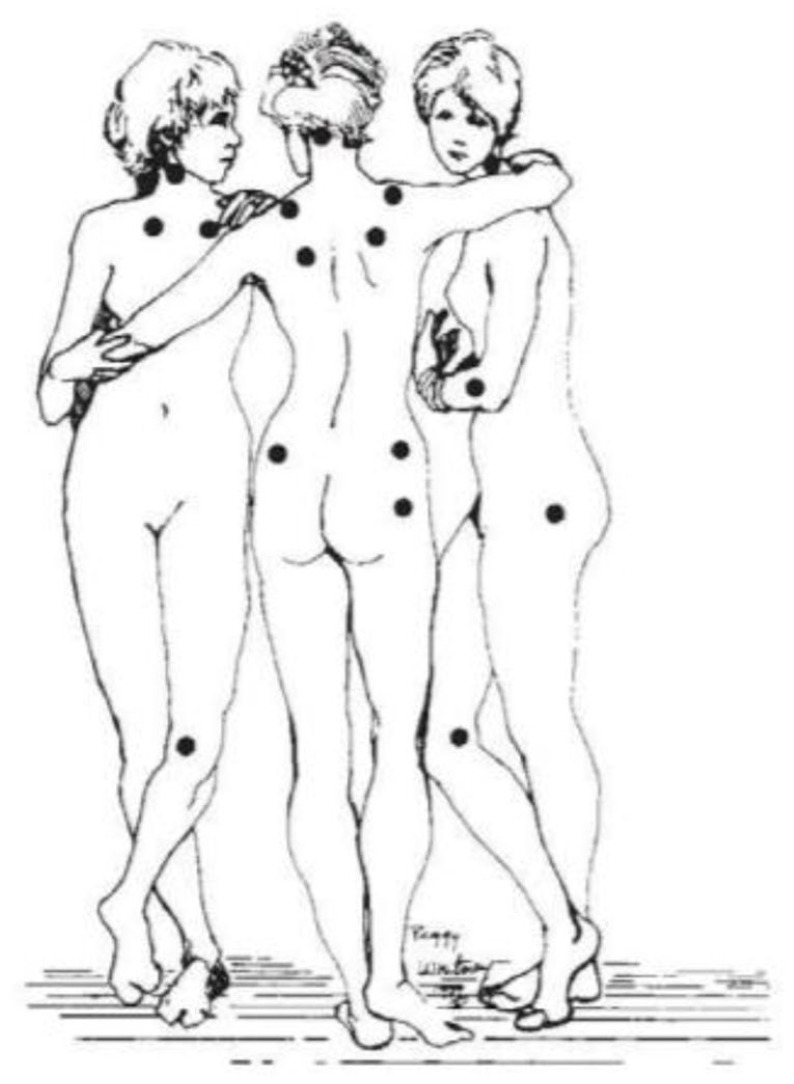
Location of tender points established as criteria for fibromyalgia syndrome (FMS) diagnosis by the American College of Rheumatology (ACR). 3 Image based on the original ‘The Three Graces’ by the French 154 painter Jean-Baptiste Regnault (1793).
Recruitment procedures
Participants will be recruited from a private clinic and rehabilitation service of Malaga (Spain). In addition, advertisements on social media will be placed to increase the potential number of participants in the study. The physiotherapist in contact with the participants for recruitment will provide information about the study, including details of eligibility criteria. Following informed consent, participants will be randomised to an active or placebo whole-body treatment.
To improve the adherence to the treatment, the physiotherapist administering the treatment will be in regular contact with the participants in reminding of the time schedule and follow-up sessions to them.
Statistical analysis
SPSS® Statistics version 21.0 (IBM, Chicago, IL, USA) will be used for all analyses. The Shapiro–Wilk test will be used to verify data distribution normality. To study intragroup mean differences for all the outcomes between the six assessment times [baseline (T1), after session 6 (T2), immediate postintervention (T3), 2 weeks after the final treatment (T4) and 3 (T5) and 6 (T6) month follow-up], repeated-measures analysis of variance (ANOVA) will be used. To compare the two groups (PBM intervention and placebo groups) at baseline and follow-ups regarding clinical characteristics, a six-way repeated-measures ANOVA will be conducted, with six levels corresponding to every time of assessment (T1, T2, T3, T4, T5 and T6), and the two intervention groups as independent factors. A value of p < 0.05 will be considered to be statistically significant.
Between- and within-group effect sizes for all quantitative variables will be measured with the Cohen d coefficient. An effect size greater than 0.8 will be considered large, around 0.5 moderate, and less than 0.2 small. 37
Sample size calculation
Sample size for this trial is based on an expected mean difference between groups of 2 points of the NPRS, which is the minimum clinically important difference. 38 Based on results of other randomised clinical trials32,39 and previous reviews, 40 assuming the standard deviation of the NPRS of 2.0 units to detect this difference between the intervention and placebo groups, with a value of α = 0.05 and a statistical power of 90%, a minimum of 22 patients per group is needed.
Data management
Data from the study will be only accessible to the research team and will be stored on password-protected computers at the University of Granada. Paper-form data will be stored in locked cabinets located at the Department of Physiotherapy of that same university. To preserve data confidentiality, study participants will be assigned an identification number which will be kept for the duration of the study. A list of participant identification numbers will be created and separated from the deidentified data.
Statistical analyses will be performed keeping participant anonymity by using patient identification numbers and the statistician will be blinded to group allocation. Confidentiality will also be preserved when disseminating results by using group data.
Trial status
This trial is recruiting participants from 30 January 2021, and will be completed on 30 December 2021. The protocol version number is PBMFM-3 with date 22 January 2020.
Discussion
The aim of this study is to investigate the impact of whole-body PBM on pain perception, functionality, quality of soft tissue, central sensitisation and psychological factors in patients suffering from FMS.
We hypothesise that the use of a whole-body PBM application will improve the FMS condition, in terms of pain, functionality, quality of soft tissue, central sensitisation and consequently psychological factors and quality of life.
Moreover, we will investigate the long-term impact of the PBM programme and will further investigate with the follow-up of outcome measures after treatment 6, immediately following the last treatment (4 weeks) and then 2 weeks and at 3 monthly follow-up intervals to 6 months after completion of treatment.
To our knowledge, this is the first study developed in participants with FMS using a whole-body PBM approach. PBM acts on the mitochondria, specifically photoreceptors within the mitochondrial respiratory chain. Those suffering from FMS may present compromised mitochondrial respiration and decreased ATP synthesis. Therefore, they may show a lack of energy in response to any physical activity.14,41,42 This allows an increase in sensitisation of the whole body over time, leading to chronicity of pain and tenderness of soft tissue in those with FMS.6,14,15 Similarly, chronic pain is associated with fatigue as well as having psychological impact. 42
Against this background, a PBM treatment is proposed to stimulate an upregulation of mitochondrial activity through acting on the mitochondrial respiratory chain, which consequently increases ATP production into muscle cells and decreases oxidative stress and reactive oxygen species production. Furthermore, a single irradiation with PBM has been demonstrated to increase cytochrome c-oxidase activity in intact skeletal muscle tissue 24 h after irradiation. Importantly, immune cells (mast cells in particular) appear to be strongly affected by PBM, and there is considered to be a crucial role of the movement of leukocytes in inflammation. PBM causes an increase not only in ATP but also in NADH, protein and RNA, as well as a reciprocal augmentation in oxygen consumption. 43
Mechanistic actions of PBM at the cellular level, with resultant changes in downstream signalling pathways, may lead to improvements in pain, function, quality of soft tissue, central/peripheral sensitisation and consequently psychological impact in FMS patients. Furthermore, the proposed whole-body PBM treatment will offer the possibility of both central and peripheral effects, resulting in a systemic response.
The strength of the presented study will be to show changes in pain intensity, functionality, quality of soft tissue, central sensitisation symptoms and psychological aspects after a whole-body PBM treatment, being the first in this line. Furthermore, 3- and 6-month follow-up will investigate the long-term efficacy of this treatment. On the other hand, some limitations may be recognised. Given the expected systematic response after the treatment, outcome measures related to neuroendocrine and immunological responses may be added. Sample size calculation was based on NPRS changes; nevertheless, FMS presents with not only localised pain but widespread pain as well; thus, adding another primary outcome for sample size calculation may be indicated. In this regard, the FIQR may be used. Based on previous studies, the minimally clinically important difference in patients with fibromyalgia is reported to be 27 points 44 ; therefore, to detect an effect size of 0.67 between groups, with a two-sided 0.05 level test and to achieve a statistical power of 80%, a group of 36 participants in each group would be necessary for this study. 45
The results of this study will elucidate the therapeutic benefits of a whole-body PBM approach as a novel treatment for patients with FMS, for which there is little evidence of positive impact of other treatments.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221078095 for Short- and long-term effects of whole-body photobiomodulation on pain, functionality, tissue quality, central sensitisation and psychological factors in a population suffering from fibromyalgia: protocol for a triple-blinded randomised clinical trial by Santiago Navarro-Ledesma, Ana Gonzalez-Muñoz, James Carroll and Patricia Burton in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors want to thank all participants who voluntarily want to collaborate in this research project.
Footnotes
Authors’ note: The trial has been registered at Clinicaltrials.gov with the following identifier: NCT04248972. The results of the study will be disseminated at several research conferences and as published articles in peer-reviewed journals. The full protocol and participant-level dataset will be available when this study will be finished. There is a preprint available on https://www.researchsquare.com/article/rs-654227/v1
Author contributions: Santiago Navarro-Ledesma: Conceptualisation; Investigation; Methodology; Project administration; Supervision; Validation; Visualisation; Writing – original draft; Writing – review & editing.
Ana Gonzalez-Muñoz: Conceptualisation; Investigation; Project administration; Resources.
James Carroll: Funding acquisition; Investigation; Project administration; Resources; Supervision; Writing – review & editing.
Patricia Burton: Conceptualisation; Investigation; Methodology; Project administration; Supervision; Validation; Visualisation; Writing – review & editing.
Availability of data and materials: Protocol modifications will be notified to relevant parties. All members of the research team will have access to the final trial dataset. They will not have any responsibility for the coordinating centre, steering committee, endpoint adjudication committee, data management team and other individuals or groups overseeing the trial.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research has been funded by THOR Photomedicine by providing the equipment needed.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: James Carroll is the owner of THOR Photomedicine, a company which sells LLLT devices. Patricia Burton affiliation is THOR Photomedicine.
Ethics approval and consent to participate: This study protocol has received ethical approval by Ethics Committee of Human Research of the University of Granada, Spain (1044/CEIH/2020). All the participants will accept and sign an informed consent before beginning the study.
ORCID iD: Santiago Navarro-Ledesma  https://orcid.org/0000-0002-4302-1106
https://orcid.org/0000-0002-4302-1106
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Santiago Navarro-Ledesma, Department of Physiotherapy, Faculty of Health Sciences, Campus of Melilla, University of Granada, Querol Street, 5, 52004 Melilla, Spain.
Ana Gonzalez-Muñoz, Clinica Ana Gonzalez, Malaga, Spain.
James Carroll, THOR Photomedicine Ltd, Chesham, Buckinghamshire, UK.
Patricia Burton, THOR Photomedicine Ltd, Chesham, Buckinghamshire, UK.
References
- 1. Mas AJ, Carmona L, Valverde M, et al. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: results from a nationwide study in Spain. Clin Exp Rheumatol 2008; 26: 519–526. [PubMed] [Google Scholar]
- 2. Bellato E, Marini E, Castoldi F, et al. Fibromyalgia syndrome: etiology, pathogenesis, diagnosis, and treatment. Pain Res Treat 2012; 2012: 426130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolfe F, Smythe HA, Yunus MB, et al. Criteria for the classification of fibromyalgia. Arthritis Rheumat 1990; 33: 71–73. [DOI] [PubMed] [Google Scholar]
- 4. Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311: 1547–1555. [DOI] [PubMed] [Google Scholar]
- 5. García-Ríos MC, Navarro-Ledesma S, Tapia-Haro RM, et al. Effectiveness of health education in patients with fibromyalgia: a systematic review. Eur J Phys Rehabil Med 2019; 55: 301–313. [DOI] [PubMed] [Google Scholar]
- 6. Yeh SW, Hong CH, Shih MC, et al. Low-level laser therapy for fibromyalgia: a systematic review and meta-analysis. Pain Physician 2019; 22: 241–254. [PubMed] [Google Scholar]
- 7. Salehpour F, Mahmoudi J, Kamari F, et al. Brain photobiomodulation therapy: a narrative review. Mol Neurobiol 2019; 55: 6601–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karu TI, Pyatibrat LV, Kolyakov SF, et al. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B Biol 2005; 81: 98–106. [DOI] [PubMed] [Google Scholar]
- 9. Karu TI, Pyatibrat LV, Kolyakov SF, et al. Absorption measurements of cell monolayers relevant to mechanisms of laser phototherapy: reduction or oxidation of cytochrome c oxidase under laser radiation at 632.8 nm. Photomed Laser Surg 2008; 26: 593–599. [DOI] [PubMed] [Google Scholar]
- 10. Gur A, Karakoc M, Cevik R, et al. Efficacy of low power laser therapy and exercise on pain and functions in chronic low back pain. Lasers Surg Med 2003; 32: 233–238. [DOI] [PubMed] [Google Scholar]
- 11. Fernández García R, Suárez Holgado JD, Formieles Ortiz I, et al. Utilización de un programa con láser en pacientes diagnosticados de fibromialgia. Reumatol Clin 2011; 7: 94–97. [DOI] [PubMed] [Google Scholar]
- 12. Honda Y, Sakamoto J, Hamaue Y, et al. Effects of physical-agent pain relief modalities for fibromyalgia patients: a systematic review and meta-analysis of randomized controlled trials. Pain Res Manag 2018; 2018: 2930632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghigiarelli JJ, Fulop AM, Burke AA, et al. The effects of whole-body photobiomodulation light-bed therapy on creatine kinase and salivary interleukin-6 in a sample of trained males: a randomized, crossover study. Front Sports Act Living 2020; 2: 48–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chung H, Dai TK, Sharma S, et al. The nuts and Bolts of low-level laser (light) therapy. Ann Biomed Eng 2012; 40: 516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kisselev SB, Moskvin SV. The use of laser therapy for patients with fibromyalgia: a critical literary review. J Lasers Med Sci 2019; 10: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sigrist RMS, Liau J, Kaffas A, et al. Ultrasound elastography: review of techniques and clinical applications. Theranostics 2017; 7: 1303–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: basic principles and technology. Ultraschall Med 2013; 34: 169–184. [DOI] [PubMed] [Google Scholar]
- 18. Alvarez-Gallardo I, Soriano-Maldonado A, Segura Jiménez V, et al. High levels of physical fitness are associated with better health-related quality of life in women with fibromyalgia: the al-Ándalus project. Phys Ther 2019; 25: 1481–1494. [DOI] [PubMed] [Google Scholar]
- 19. Góes SM, Leite N, Shay BL, et al. Functional capacity, muscle strength and falls in women with fibromyalgia. Clin Biomech (Bristol, Avon) 2012; 27: 578–583. [DOI] [PubMed] [Google Scholar]
- 20. Lorente LC, Ríos MCG, Ledesma SN, et al. Functional status and body mass index in postmenopausal women with fibromyalgia: a case–control study. Int J Environ Res Public Health 2019; 16: 4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Felipe García-Bardón V, Castel-Bernal B, Vidal-Fuentes J. Evidencia científica de los aspectos psicológicos en la fibromialgia. Posibilidades de intervención. Reumatol Clin 2006; 2: 38–43. [DOI] [PubMed] [Google Scholar]
- 22. Villarraga AR, Ligia A, Castellanos Z, et al. Predictores de calidad de vida en pacientes. Revista Colombiana de Reumatología 2005; 12: 295–300, http://www.sld.cu/galerias/pdf/sitios/rehabilitacion-doc/predictorescalidaddevida.pdf [Google Scholar]
- 23. Chan A, Tetzlaff J, Gotzsche P, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013; 8: e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han C, Lee SJ, Lee SY, et al. Available therapies and current management of fibromyalgia: focusing on pharmacological agents. Drugs Today 2011; 47: 539–557. [DOI] [PubMed] [Google Scholar]
- 25. Yamato T, Maher C, Saragiotto B, et al. The TIDieR checklist will benefit the physiotherapy profession. Physiother Theory Pract 2017; 33: 267–268. [DOI] [PubMed] [Google Scholar]
- 26. Huang YY, Sharma SK, Carroll J, et al. Biphasic dose response in low level light therapy – an update. Dose Response 2011; 9: 602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jensen MP, Turner JA, Romano JM, et al. Comparative reliability and validity of chronic pain intensity measures. Pain 1999; 83: 157–162. [DOI] [PubMed] [Google Scholar]
- 28. Bennett RM, Friend R, Jones KD, et al. The revised fibromyalgia impact questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther 2009; 11: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Segura-Jiménez V, Aparicio VA, Álvarez-Gallardo IC, et al. Validation of the modified 2010 American College of Rheumatology diagnostic criteria for fibromyalgia in a Spanish population. Rheumatology 2014; 53: 1803–1811. [DOI] [PubMed] [Google Scholar]
- 30. Mannerkorpi K, Hernelid C. Leisure time physical activity instrument and physical activity at home and work instrument. Development, face validity, construct validity and test-retest reliability for subjects with fibromyalgia. Disabil Rehabil 2005; 27: 695–701. [DOI] [PubMed] [Google Scholar]
- 31. Sletten DM, Suarez GA, Low PA, et al. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc 2012; 87: 1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Navarro-Ledesma S, Gonzalez-Muñoz A. Short-term effects of 448 kilohertz radiofrequency stimulation on supraspinatus tendon elasticity measured by quantitative ultrasound elastography in professional badminton players: a double- blinded randomized clinical trial. Int J Hyperthermia 2021; 38: 421–427. [DOI] [PubMed] [Google Scholar]
- 33. Brage K, Hjarbaek J, Kjaer P, et al. Ultrasonic strain elastography for detecting abnormalities in the supraspinatus tendon: an intra-and inter-rater reliability study. BMJ Open 2019; 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Darnall BD, Sturgeon JA, Cook KF, et al. Development and validation of a daily pain catastrophizing scale. J Pain 2017; 18: 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gómez-Pérez L, López-Martínez AE, Ruiz-Párraga GT. Psychometric properties of the Spanish version of the Tampa Scale for Kinesiophobia (TSK). J Pain 2011; 12: 425–435. [DOI] [PubMed] [Google Scholar]
- 36. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1997; 84: 191–215. [DOI] [PubMed] [Google Scholar]
- 37. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, MI: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 38. Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94: 149–158. [DOI] [PubMed] [Google Scholar]
- 39. Bourgault P, Lacasse A, Marchand S, et al. Multicomponent interdisciplinary group intervention for self-management of fibromyalgia: a mixed-methods randomized controlled trial. PLoS ONE 2015; 10: e0126324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008; 9: 105–121. [DOI] [PubMed] [Google Scholar]
- 41. Antonialli FC, De Marchi T, Tomazoni SS, et al. Phototherapy in skeletal muscle performance and recovery after exercise: effect of combination of super-pulsed laser and light-emitting diodes. Lasers Med Sci 2014; 29: 1967–1976. [DOI] [PubMed] [Google Scholar]
- 42. Jonsjö MA, Åström J, Jones MP, et al. Patients with ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome) and chronic pain report similar level of sickness behavior as individuals injected with bacterial endotoxin at peak inflammation. Brain Behav Immun: Heal 2019; 2: 100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Passarella S, Casamassima E, Molinari S, et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett 1984; 175: 95–99. [DOI] [PubMed] [Google Scholar]
- 44. Loftus N, Dobbin N, Crampton JS. The effects of a group exercise and education programme on symptoms and physical fitness in patients with fibromyalgia: a prospective observational cohort study. Disabil Rehabil. Epub ahead 1 March 2021. DOI: 10.1080/09638288.2021.1891463. [DOI] [PubMed] [Google Scholar]
- 45. Wang C, Schmid CH, Fielding RA, et al. Effect of Tai Chi versus aerobic exercise for fibromyalgia: comparative effectiveness randomized controlled trial. BMJ 2018; 360: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221078095 for Short- and long-term effects of whole-body photobiomodulation on pain, functionality, tissue quality, central sensitisation and psychological factors in a population suffering from fibromyalgia: protocol for a triple-blinded randomised clinical trial by Santiago Navarro-Ledesma, Ana Gonzalez-Muñoz, James Carroll and Patricia Burton in Therapeutic Advances in Chronic Disease


