Abstract
Background:
Cervical cancer is the fourth most common cancer among women worldwide, with an estimate of 570,000 new cases and about 311,000 deaths annually. Low-resource countries, including those in sub-Saharan Africa, have the highest-burden with an estimate of 84% of all cervical cancers. This study examines the prevalence and socio-demographic-economic factors associated with cervical cancer screening in sub-Saharan Africa.
Methods:
A weighted population-based cross-sectional study using Demographic and Health Surveys data. We used available data on cervical cancer screening between 2011 and 2018 from the Demographic and Health Surveys for five sub-Saharan African countries (Benin, Ivory Coast, Kenya, Namibia, and Zimbabwe). The study population included women of childbearing age, 21–49 years (n=28,976). We fit a multivariable Poisson regression model to identify independent factors associated with cervical cancer screening.
Results:
The overall weighted prevalence of cervical cancer screening was 19.0% (95% CI: 18.5%−19.5%) ranging from 0.7% in Benin to 45.9% in Namibia. Independent determinants of cervical cancer screening were: older age (40–49 years) adjusted prevalence ratio (aPR)=1.77 (95% CI: 1.64, 1.90) compared with younger age (21–29 years), secondary/higher education (aPR= 1.51, 95 CI: 1.28–1.79) compared with no education, health insurance (aPR= 1.53, 95% CI: 1.44–1.61) compared with no insurance, and highest socioeconomic status (aPR= 1.39, 95% CI: 1.26–1.52) compared with lowest.
Conclusion:
The prevalence of cervical cancer screening is substantially low in sub-Saharan Africa countries and shows a high degree of between-country variation. Interventions aimed at increasing the uptake of cervical cancer screening in sub-Saharan Africa are critically needed.
Keywords: Cervical cancer screening, sub-Saharan Africa, Demographic and Health Surveys, women
1. Introduction
Globally, cancer is considered the second leading cause of death after cardiovascular diseases with an estimate of 9.6 million deaths in 2018 alone.1 The disability-adjusted life-years (DALYs) caused by cancer in 2015 for both men and women was 208.3 million worldwide.2 High-risk human papillomavirus (HPV) is a known causal agent of cervical cancer, a very common virus transmitted through sexual contact.3 Sexually active women are at high risk of getting infected with HPV during their lifetime, with an estimated lifetime highest prevalence reaching nearly 50% among those aged 20 to 24 years.4 To date, cervical cancer screening is the most effective cancer control method. Cervical cancer screening works by seeking abnormal changes in the cervical epithelial cells, which reduces the risk of cancer when discovered and treated early.5 Cervical cancer is a major cause of disease burden of public health significance affecting middle-aged women specifically, those living in low-resource countries such as sub-Saharan Africa (SSA).6 A recent study using data from 185 countries from the 2018 Global Cancer Observatory (GLOBOCAN ) database indicated that cervical cancer was the fourth most frequently diagnosed cancer and the fourth leading cause of cancer death among women globally with an estimated 570,000 incident cases and about 311,000 deaths annually.1,6 However, in most SSA countries, it is considered to be the most commonly diagnosed cancer and the leading cause of cancer-related death among women.1,7 Consequently, low-resource countries, including those in SSA, have a disproportionately high burden of all cervical cancers with an estimate of 84% and 88% of all deaths caused by the disease.6 This is in contrast to high-income countries (HICs), where the cumulative incidence rates of cervical cancer and mortality are two to four times lower than those in lower-resource countries.6
The use of advanced screening methods at a population-based level such as the use of cervical cytology (also called the Papanicolaou (Pap) test or Pap smear) and the HPV DNA test are some of the major attributable factors of low incidence and mortality in HICs.8,9 Most countries in SSA mainly focus on fighting infectious diseases such as tuberculosis, malaria, and HIV/AIDS and as such, non-communicable diseases such as cancers have received low priority and has led to a lack of effective population-based level cervical cancer screening programs, very limited resources and expertise, and poor awareness about disease prevention.5,10,11 Cervical cancer screening plays a significant role in the early detection of cervical cancer, increases treatment options for affected women, and improves cancer survival rates.6 The major attributable factors to a low survival rate of cervical cancer in SSA include late-stage diagnosis and lack of or delayed access to quality health care. 21 The vast majority of women in SSA do take the initiative to seek help by visiting healthcare facilities once they start to experience gynecological symptoms such as abnormal vaginal bleeding, offensive vaginal discharge, lower abdominal pain, and hematuria.12–15 However, at the time more serious symptoms, appear, it is probably too late and the advanced stage of the disease makes survival dismal.
To accommodate strategies to increase the uptake of cervical cancer screening among women in SSA, it is crucial to fully understand regional and country-specific variations in the prevalence of cervical cancer screening and associated factors. Such knowledge will guide the prioritization of intervention strategies to the most at-risk countries in SSA and assist stakeholders to adequately identify potential reasons for the low prevalence of cervical cancer screening. However, these estimates are lacking because most previous studies that have examined cervical cancer screening in SSA have either focused mainly on an individual country such as Kenya16 or was limited to an abstract presentation17 using Demographic and Health Surveys data (DHS). Thus, we aim to fill this critical knowledge gap by conducting a multi-country population-based study of the prevalence of cervical cancer screening in five combined SSA countries and examining the associated socio-demographic-economic factors using all available most recent DHS data from 2011–2018.
2. Methods
2.1. Data Source and Participants
The current study included all SSA countries which we had access to that participated in the most recent DHS surveys conducted from 2011–2018 and collected data on cervical cancer screening among women of childbearing age. These countries were: Benin, Ivory Coast, Kenya, Namibia, and Zimbabwe. Each country contributed one-year of survey data. Data were collected by each host country in coordination with ICF international located in Rockville, Maryland.18 The DHS surveys are nationally representative household surveys and are funded by the U.S. Agency for International Development (USAID). The survey data from each country was conducted using face-to-face questionnaire interviews and used multistage cluster sampling, stratified sampling design to collect detailed information about demographics and population health status, health behaviors, neonatal mortality, nutritional status, and family planning in each country.19,20 The initial stage involves the division of each country into geographic regions. Then within these regions, populations are stratified by urban or rural areas. The primary sampling units (PSUs) were selected with a probability proportional to size within each stratum. In the second stage of sampling, all households within the cluster were listed, and ~25 households were randomly selected for an interview using equal probability systematic sampling. The year of the administration of the relevant DHS survey for each country can be seen in Table 1. In 2012, there was consensus among professional organizations that issue cervical cancer screening guidelines to adopt the consistent recommendation of getting a Pap smear every three years for women aged 21 or older and no Pap smear for women younger than 21 years.21 In addition, the DHS data for cervical cancer screening questions for most countries included in this study (4 out 5) were limited to only women of childbearing age (15–49 years old). Therefore, the present weighted analysis was limited to women of childbearing age from 21–49 years (N=28,976) with cervical cancer screening data.
Table 1.
Background characteristics of the weighted survey participants, the prevalence of cervical cancer screening, and the multivariable-adjusted prevalence ratio by country and survey year (N=28,976)
| All Participants | Cervical Cancer Screening | Multivariable adjusted analysis | |||
|---|---|---|---|---|---|
|
| |||||
| Countries | Survey Year | Na (%b) | Nc (%) | (aPR) (95% CI) | P-Value |
| Overall | 28,976 | 5,512 (19.0) | |||
| Benin | 2017–18 | 5,708 (19.7) | 38 (0.7) | ref. | |
| Ivory Coast | 2011–12 | 2,968 (10.2) | 94 (3.2) | 3.14 (2.05, 4.82) | <.001 |
| Kenya | 2014 | 9,129 (31.5) | 1,943 (21.3) | 18.07 (12.45, 26.22) | <.001 |
| Namibia | 2013 | 4,872 (16.8) | 2,237 (45.9) | 37.13 (25.50, 54.05) | <.001 |
| Zimbabwe | 2015 | 6,299 (21.7) | 1,200 (19.1) | 18.12 (12.41, 26.46) | <.001 |
Na = Weighted sample size of the combined dataset that is represented by that survey for each country
%b = The % of the combined dataset that is represented by that survey.
Nc= Prevalence of cervical cancer screening.
ref=reference
STI: Sexually transmissible infection
Model fully adjusted for country, health insurance coverage (yes/no), pregnancy status (yes/no), breastfeeding status (yes/no), age (categorical), education status (categorical), marital status (categorical), wealth index status (categorical), place of residence (urban/rural), employment status (yes/no), number of living children (categorical), STI (yes/no), contraceptive use (yes/no), sexually active in the last 4 weeks (categorical), household having a radio (yes/no), household having a television (yes/no), visited health care facility in the last 12 months (yes/no)
2.2. Ethical Considerations
We first sent a written request to the DHS program for permission, which was granted to download and use the data from http://www.dhsprogram.com. Each country’s procedures and questionnaires for standard DHS surveys were reviewed and approved by the ICF Institutional Review Board (IRB) and the IRBs of each host country. Written or oral informed consent was obtained from each participant before the survey. Survey respondents were not coerced into participation22 and all data are completely de-identified with no names or household addresses in the data files. All the ethical matters were handled by the ICF IRB and the IRBs of the five host countries (Benin, Ivory Coast, Kenya, Namibia, and Zimbabwe) who conducted the primary surveys and not by the authors of this article. Thus, no further IRB approval was needed by the institutions of the authors of the present manuscript. Details on the ethical matters are described elsewhere.23
2.3. Assessment of Cervical Cancer Screening (outcome)
The outcome of interest for the present study was defined as self-reporting to have ever undergone a screening test or exam for cervical cancer. This was the most reliable variable available in all countries with only 0.3% missing information. Eligible respondent women were asked whether they underwent any cervical cancer screening prior to the survey and was measured using questions such as: ‘Have you ever been screened for cervical cancer?’, and ‘Have you ever had a cervical examination?’ Screening options include Pap smears and visual inspection with acetic acid. Detailed information about cervical cancer screening questionnaires is described elsewhere.24 The binary response of cervical cancer screening (yes/no) was used as our dependent variable as done by previous researchers using the DHS data.16 Women with a missing value for cervical cancer screening or who did not know about their screening status were excluded from this study (n=75).
2.4. Assessment of Potential Socio-demographic-economic Factors
Based on previous literature on cervical cancer screening,16 the following socio-demographic-economic factors were examined to determine whether they were associated with a higher likelihood of cervical cancer screening: health insurance coverage, contraceptive use, age, wealth index status, educational status, marital status, place of residence, employment status, number of living children, household owning a radio, household owning a television, sexual activity status, and visited healthcare facility in the last twelve months. The aforementioned potential factors were collected by self-report. Previous studies reported that these socio-demographic-economic factors may affect women’s cervical cancer screening behaviors.16,17 Wealth index was recategorized from five quintiles into three categories by combining poorest and poorer into one category (called “lowest”); middle wealth level into the second category (called “middle”); and richer and richest into the third category (called “highest”), as done by previous researchers.25–27 We also recategorized the age of participants from a continuous scale into three groups for this study (21–29, 30–39, and 40–49 years old). Given the high prevalence of HPV among sexually active people28, we selected the ‘sexually active in the past 4 weeks’ variable as one of the determinants of cervical cancer screening because it could be a proxy for a causal factor. Also, sexually active women may experience some early symptoms that can trigger them to seek preventive care services including cervical cancer screening earlier than non-sexually active women.
2.5. Statistical Analysis
All statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) to generate figure 1. Statistical tests were reported as significant at p values less than 0.05. significance. Each country’s data for cervical cancer screening questionnaires were extracted and then combined to create a single analytical dataset. To ensure that the estimates were nationally representative, we followed the DHS’s recommendation to analyze the DHS survey data by using the appropriate weight for our analysis based on the selected variables.29 Univariate analyses were conducted using frequency distributions for categorical variables to describe the characteristics of the study participants. To better understand between-country differences, we also analyzed each demographic/social factor of cervical cancer screening stratified by country. The prevalence of cervical cancer screening was calculated as the number of women who had cervical cancer screening divided by the total number of women interviewed in that category and multiplied by 100. The multivariable analysis was conducted using generalized estimating equations (GEE; SAS Institute) with an independent correlation structure to explore the association between socio-demographic-economic factors and cervical cancer screening adjusting for the country of residence, health insurance coverage, pregnancy status, breastfeeding status, age, educational status, marital status, wealth index status, place of residence (urban/rural), employment status, number of living children, sexually transmissible infection (STI), contraceptive use, sexually active, household having a radio, household having a television, visited health care facility in the last twelve months. To specify the use of the robust variance estimator for Poisson regression, the REPEATED statement (in SAS) was used to account for possible clustering by countries.30 The REPEATED statement is usually used for repeated measures or longitudinal data. However, the method can also be used for cross-sectional data with no repeated measurements to generate a robust estimate of variance.30 Descriptive statistics are presented as the weighted prevalence of cervical cancer screening and the multivariable Poisson regression results are presented as adjusted prevalence ratios (aPR) with 95% confidence intervals (CIs). Benin was selected as the reference country because it was the country with the lowest rate of cervical cancer screening
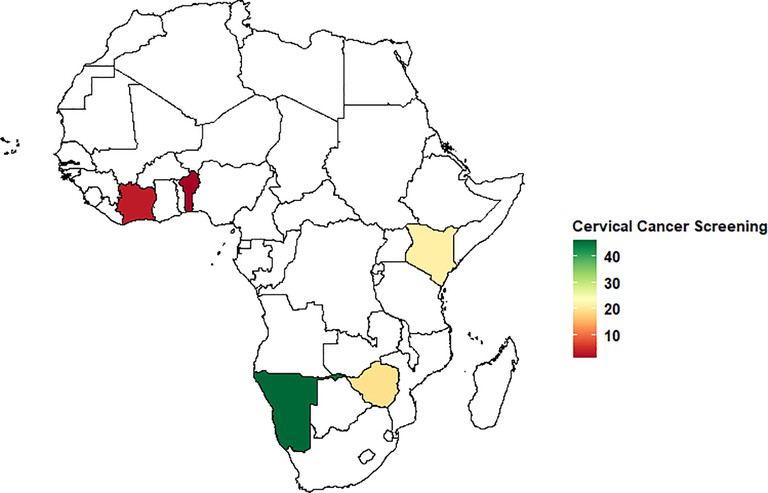
Figure 1.
Weighted Prevalence of Cervical Cancer Screening in sub-Saharan Africa (%) in women of childbearing age 21–49 years shaded by Sub-Saharan countries in Africa. Countries shaded white were not included in the analysis.
3. Results
3.1. Sociodemographic Characteristics of the Participants
A total of 28,976 weighted participants were included in the current analysis (Table 1). The mean (SD) age was 32.6±7.8 years old which was close to the mean age of the women excluded from the study (31.8 ±8.3). More than two-thirds of the participants reported not having health insurance coverage (84.1%). The majority of the participants were from a wealthy household (53.5%) and over 50.0% of women had secondary/higher education. More than one-third of the participants were middle-aged women between the ages of 30–39 years old (36.1%). More than half of participants were married/living with a partner (69.4%), currently employed (68.9%), resided in urban areas (50.3%), sexually active in the last 4 weeks (59.6%), or visited a healthcare facility in the last 12 months (61.1%). The majority of women had access to a radio in the household (63.5%) but most did not have a television (52.9%). Women with one or more living children less than 5 years old at the time of the DHS interview constituted 70.0% of the survey respondents (Table 2).
Table 2.
Weighted Prevalence of cervical cancer screening by country stratify (N=28,976)
| Benin (N=5,708) | Ivory Coast (N=2,968) | Kenya (N=9,129) | Namibia (N=4,872) | Zimbabwe (N=6,299) | |
|---|---|---|---|---|---|
|
| |||||
| Characteristic | N (%) | N (%) | N (%) | N (%) | N (%) |
| Age group | |||||
| 21–29 | 10 (0.17) | 25 (0.84) | 691 (7.57) | 637 (13.07) | 295 (4.69) |
| 30–39 | 19 (0.33) | 39 (1.30) | 754 (8.25) | 884 (18.15) | 546 (8.67) |
| 40–49 | 9 (0.16) | 30 (1.02) | 498 (5.46) | 716 (14.70) | 359 (5.70) |
| Health insurance | |||||
| No | 34 (0.60) | 71 (2.38) | 1,172 (12.84) | 1,447 (29.7) | 833 (13.24) |
| Yes | 4 (0.06) | 23 (0.78) | 771 (8.44) | 790 (16.21) | 367 (5.82) |
| Wealth index status | |||||
| Lowest | 3 (0.05) | 7 (0.25) | 311 (3.41) | 473 (9.71) | 168 (2.68) |
| Middle | 4 (0.07) | 5 (0.17) | 298 (3.27) | 418 (8.58) | 113 (1.79) |
| Highest | 31 (0.54) | 82 (2.74) | 1,334 (14.61) | 1,346 (27.62) | 919 (14.59) |
| Place of residence | |||||
| Urban | 27 (0.47) | 78 (2.62) | 1,059 (11.60) | 1,516 (31.11) | 789 (12.54) |
| Rural | 11 (0.19) | 16 (0.54) | 884 (9.69) | 721 (14.81) | 411 (6.52) |
| Education | |||||
| No education | 9 (0.15) | 20 (0.67) | 29 (0.32) | 63 (1.31) | 11 (0.18) |
| Primary | 5 (0.08) | 35 (1.16) | 868 (9.51) | 310 (6.36) | 182 (2.90) |
| Secondary/Higher | 24 (0.43) | 39 (1.33) | 1,046 (11.46) | 1,864 (38.25) | 1,007 (15.98) |
| Marital status | |||||
| Never married | 6 (0.10) | 17 (0.57) | 198 (2.17) | 992 (20.35) | 47 (0.74) |
| Married/Living with partner | 26 (0.46) | 69 (2.33) | 1,458 (15.97) | 1,061 (21.79) | 934 (14.83) |
| Widowed/Divorced/Separated | 6 (0.10) | 8 (0.26) | 287 (3.15) | 184 (3.78) | 219 (3.49) |
| Employment status | |||||
| No | 6 (0.10) | 18 (0.60) | 262 (3.97) | 722 (14.91) | 429 (6.82) |
| Yes | 32 (0.56) | 76 (2.57) | 1,581 (17.34) | 1,504 (31.06) | 771 (12.24) |
| Pregnancy status | |||||
| No | 34 (0.60) | 89 (3.00) | 1,851 (20.28) | 2,087 (42.84) | 1,138 (18.06) |
| Yes | 4 (0.06) | 5 (0.16) | 92 (1.00) | 150 (3.08) | 62 (1.00) |
| Breastfeeding status | |||||
| No | 30 (0.53) | 78 (2.62) | 1,579 (17.30) | 1,942 (39.87) | 1,033 (16.41) |
| Yes | 8 (0.13) | 16 (0.54) | 364 (3.98) | 295 (6.05) | 167 (2.65) |
| Number of living children | |||||
| None | 4 (0.06) | 16 (0.55) | 200 (2.19) | 267 (5.48) | 60 (0.96) |
| 1 to 4 | 30 (0.53) | 60 (2.01) | 1,480 (16.21) | 1,738 (35.69) | 1,021 (16.21) |
| More than 4 | 4 (0.07) | 18 (0.59) | 263 (2.88) | 232 (4.75) | 119 (1.89) |
| Contraceptive use | |||||
| No | 27 (0.47) | 67 (2.25) | 756 (8.29) | 838 (17.19) | 387 (6.14) |
| Yes | 11 (0.19) | 27 (0.90) | 1,187 (13.00) | 1,399 (28.73) | 813 (12.92) |
| STI | |||||
| No | 37 (0.64) | 87 (2.95) | 1,863 (20.52) | 2,139 (44.14) | 1,162 (18.50) |
| Yes | 1 (0.02) | 5 (0.16) | 66 (0.73) | 84 (1.74) | 33 (0.53) |
| Sexually active in the last 4 weeks | |||||
| Never had sex | 1 (0.02) | 0 (0.00) | 9 (0.10) | 19 (0.40) | 1 (0.01) |
| Active last 4 weeks | 21 (0.36) | 58 (1.96) | 1,348 (14.7 8) | 1,153 (23.94) | 848 (13.56) |
| Not active last 4 weeks | 16 (0.28) | 33 (1.12) | 576 (6.32) | 1,038 (21.56) | 344 (5.50) |
| Household has radio | |||||
| No | 7 (0.13) | 32 (1.09) | 395 (4.43) | 505 (10.68) | 642 (10.65) |
| Yes | 27 (0.49) | 60 (2.08) | 1,507 (16.90) | 1,656 (35.00) | 519 (8.61) |
| Household has television | |||||
| No | 10 (0.18) | 16 (0.57) | 798 (8.94) | 839 (17.74) | 415 (6.89) |
| Yes | 25 (0.44) | 76 (2.61) | 1,105 (12.38) | 1,322 (27.95) | 745 (12.37) |
| Visited healthcare facility last 12mths | |||||
| No | 16 (0.29) | 32 (1.09) | 501 (5.49) | 820 (16.84) | 312 (4.95) |
| Yes | 21 (0.37) | 61 (2.07) | 1,442 (15.81) | 1,415 (29.05) | 888 (14.11) |
In table 2, we only reported the number (%) of cervical cancer screening for each variable by country stratify. The numbers are not meant to sum up. We used the total number for each country as the denominator when calculating the prevalence of cervical cancer screening.
3.2. The Prevalence of Cervical Cancer Screening in SSA
The overall weighted prevalence of cervical cancer screening was 19.0%, 95% CI: (18.5–19.5%) during the study period, ranging from 0.7% in Benin to 45.9% in Namibia (Table 1 and Figure 1). The prevalence of cervical cancer screening was higher among women who were older 40–49 years old (25.6%), those who had access to health insurance coverage (42.4%), from higher socioeconomic status (23.9%), and those with a secondary/higher education (26.4%). Furthermore, cervical cancer screening was also higher among women using contraceptives (25.2%) and sexually active in the last 4 weeks (19.9%). The prevalence of cervical screening was lower among women with STI. Lastly, women living in urban areas had a higher prevalence of cervical cancer screening (23.8%) compared to those living in rural areas (14.2%) (Table 1). Country-stratified analysis (Table 2) indicated that the prevalence of cervical cancer screening also varied widely between countries in relation to different factors such as age, health insurance, wealth status, education level, employment status, and visited healthcare facility in the last 12 months. Namibia, Zimbabwe, and Kenya had the highest prevalence of cervical cancer screening among women aged 30–39 compared with the other countries. Additionally, Namibia and Kenya had the highest prevalence of cervical cancer screening among women in the highest socioeconomic status. Regarding the educational level, Namibia and Zimbabwe had the highest prevalence of cervical cancer screening among women with higher education.
3.3. Multivariable Analysis of Determinants of Cervical Cancer Screening
Results from the multivariable analysis indicated that the country of residence was the factor most strongly associated with cervical cancer screening in the present study (Table 1). The aPR of cervical cancer screening was highest in Namibia (aPR= 37.13, 95%CI: 25.50–54.05) followed by Zimbabwe (aPR= 18.12, 95%CI: 12.41–26.46), Kenya (aPR= 18.07, 95%CI: 12.45–26.22), and Ivory Coast (aPR= 3.14, 95%CI: 2.05–4.82). Sexually active status in the last 4 weeks was the strongest factor associated with cervical cancer screening (aPR=3.27, 95% CI: 2.29–4.97) (Table 3). We observed a dose-response relationship between age and cervical cancer screening, where increasing age was positively associated with a higher cervical cancer screening rate (Ptrend <.0001). Older women 40–49 years old were more likely to get screened for cervical cancer (aPR=1.77, 95% CI: 1.64–1.90) compared with younger women (21–29 years old). Women with secondary/higher education were more likely to undergo cervical cancer screening (aPR= 1.51, 95% CI: 1.28–1.79) compared with no education. Having health insurance coverage was also positively associated with cervical cancer screening (aPR= 1.53, 95% CI: 1.44–1.61) compared to those with no health insurance. Women in the highest socioeconomic status were more likely to undergo cervical cancer screening (aPR= 1.39, 95% CI: 1.26–1.52) compared to those in the lowest socioeconomic status. Participants living in rural areas were less likely to undergo cervical cancer screening (aPR=0.86, 95% CI: 0.81–0.92). Possession of a radio or television was not significantly associated with cervical cancer screening (Table 3).
Table 3.
Background characteristics of the weighted survey participants, the prevalence of cervical cancer screening, and the multivariable-adjusted prevalence patio (N=28,976)
| All Participants | Cervical Cancel Screening | Multivariable-Adjusted analysis | ||
|---|---|---|---|---|
|
| ||||
| Characteristic | Na (%b) | Nc (%) | (aPR) (95% CI) | P Value |
| Age group | ||||
| 21–29 | 12,200 (42.1) | 1,658 (13.6) | ref. | |
| 30–39 | 10,470 (36.1) | 2,241 (21.4) | 1.46 (1.37, 1.55) | <.001 |
| 40–49 | 6,306 (21.8) | 1,613 (25.6) | 1.77 (1.64, 1.90) | <.001 |
| Health insurance | ||||
| No | 24,367 (84.1) | 3,558 (14.6) | ref. | |
| Yes | 4,605 (15.9) | 1,953 (42.4) | 1.53 (1.44, 1.61) | <.001 |
| Wealth index status | ||||
| Lowest | 8,283 (28.6) | 963 (11.6) | ref. | |
| Middle | 5,190 (17.9) | 838 (16.2) | 1.17 (1.07, 1.28) | 0.001 |
| Highest | 15,503 (53.5) | 3,711 (23.9) | 1.39 (1.26, 1.52) | <.001 |
| Place of residence | ||||
| Urban | 14,569 (50.3) | 3,469 (23.8) | ref. | |
| Rural | 14,407 (49.7) | 2,043 (14.2) | 0.86 (0.81, 0.92) | <.001 |
| Education | ||||
| No education | 5,232 (18.1) | 133 (2.5) | ref. | |
| Primary | 8,663 (29.9) | 1,399 (16.2) | 1.38 (1.17, 1.63) | 0.002 |
| Secondary/Higher | 15,081 (52.1) | 3,980 (26.4) | 1.51 (1.28, 1.79) | <.001 |
| Marital status | ||||
| Never married | 5,445 (18.8) | 1,258 (23.1) | ref. | |
| Married/Living with partner | 20,102 (69.4) | 3,549 (17.7) | 1.05 (0.98, 1.12) | 0.21 |
| Widowed/Divorced/Separated | 3,429 (11.8) | 705 (20.5) | 0.99 (0.91, 1.08) | 0.78 |
| Employment status | ||||
| No | 8,983 (31.1) | 1,537 (17.1) | ref. | |
| Yes | 19,940 (68.9) | 3,964 (19.9) | 1.13 (1.07, 1.20) | <.001 |
| Pregnancy status | ||||
| No | 26,777 (92.4) | 5,199 (19.4) | ref. | |
| Yes | 2,199 (7.6) | 313 (14.2) | 1.14 (1.02, 1.27) | 0.02 |
| Breastfeeding status | ||||
| No | 22,617 (78.1) | 4,663 (20.6) | ref. | |
| Yes | 6,359 (22.0) | 849 (13.4) | 0.99 (0.93, 1.08) | 0.99 |
| Number of living children | ||||
| None | 3,661 (12.6) | 547 (14.9) | ref. | |
| 1 to 4 | 20,271 (70.0) | 4,329 (21.4) | 1.20 (1.09, 1.32) | 0.003 |
| More than 4 | 5,044 (17.4) | 636 (12.6) | 0.98 (0.86, 1.11) | 0.76 |
| Contraceptive use | ||||
| No | 15,358 (53.0) | 2,075 (13.5) | ref. | |
| Yes | 13,618 (47.0) | 3,437 (25.2) | 1.12 (1.06, 1.18) | <.001 |
| STI | ||||
| No | 27,655 (95.9) | 5,288 (19.1) | ref. | |
| Yes | 1,174 (4.1) | 190 (16.2) | 1.22 (1.09, 1.38) | 0.001 |
| Sexually active in the last 4 weeks | ||||
| Never had sex | 625 (2.2) | 30 (4.8) | ref. | |
| Active last 4 weeks | 17,212 (59.6) | 3,428 (19.9) | 3.37 (2.29, 4.97) | <.001 |
| Not active last 4 weeks | 11,026 (38.2) | 2,007 (18.2) | 3.24 (2.21, 4.78) | <.001 |
| Household has radio | ||||
| No | 10,294 (36.5) | 1,581 (15.4) | ref. | |
| Yes | 17,888 (63.5) | 3,771 (21.1) | 1.01 (0.95, 1.06) | 0.84 |
| Household has television | ||||
| No | 14,895 (52.9) | 2,079 (14.0) | ref. | |
| Yes | 13,286 (47.2) | 3,273 (24.6) | 1.02 (0.95, 1.10) | 0.57 |
| Visited healthcare facility last 12mths | ||||
| No | 11,279 (38.9) | 1,682 (14.9) | ref. | |
| Yes | 17,687 (61.1) | 3,828 (21.6) | 1.26 (1.18, 1.32) | <.001 |
Na = Weighted sample size of the combined dataset that is represented by that survey for each country
%b = The % of the combined dataset that is represented by that survey.
Nc= Prevalence of cervical cancer screening.
ref=reference
Model fully adjusted for country, health insurance coverage (yes/no), pregnancy status (yes/no), breastfeeding status (yes/no), age (categorical), education status (categorical), marital status (categorical), wealth index status (categorical), place of residence (urban/rural), employment status (yes/no), number of living children (categorical), STI (yes/no), contraceptive use (yes/no), sexually active in the last 4 weeks (categorical), household having a radio (yes/no), household having a television (yes/no), visited health care facility in the last 12 months (yes/no)
4. Discussion
In this large population-based cross-sectional study of more than 28,000 women of childbearing age from five countries in SSA, we found for the first-time a high degree of heterogeneity and disparities in the prevalence of cervical cancer screening across SSA countries. The overall weighted prevalence of cervical cancer screening in SSA was only 19.0% compared to 81.1% in HICs (e.g. the United States)31 with Benin having the lowest prevalence at 0.7%. The low rate of cervical cancer screening among women of childbearing age in these low-resource countries is concerning and can increase the risk of cervical cancer in the future. West African countries had the lowest prevalence of cervical cancer screening than countries located in the East and South. The low prevalence of cervical cancer screening in our study is consistent with previous studies that also found low cervical screening rates in other SSA countries – 4.8% in Uganda32 and 6% both in Kenya33 and Tanzania.34
While cervical cytology (Pap test or Pap smear) and HPV DNA test are well-established screening methods for the early detection of cervical cancer in HICs, implementing such screening services can be cost-prohibitive and may not be feasible in many SSA countries.6,8,9,35,36 In contrast, most SSA countries lack national screening programs and have insufficient funds, inadequate infrastructure, and resources to screen all eligible women.36 In the absence of Pap smears or HPV DNA tests in most low and middle-income countries (LMICs), including those in SSA, a visual inspection of the cervix with acetic acid is the most effective and common screening technique implemented in these settings. However, this screening method has a lower sensitivity of 82.4% and a specificity of 87.4% when compared to Pap smears.37–40 Despite financial constraints, the Cancer Association of South Africa’s guidelines advocate for Pap smears at least every three years among women 25 years and older to detect abnormal cells as early as possible leading to better treatment and improved survivorship.41 Furthermore, the low prevalence of cervical cancer screening could also be explained by the fact that most countries in this region have competing health needs with a high burden of infectious diseases, maternal and child health problems, coupled with limited health resources thereby limiting prioritization on cancer prevention methods such as HPV vaccinations and national cervical cancer screening programs.5,42,43 In addition, lack of awareness about cervical cancer screening could also play a significant role because most women in SSA delay preventive care services and only seek medical assistance after noticing gynecological signs and symptoms such as abnormal vaginal bleeding, foul-smelling vaginal discharge, and hematuria.12–15 These clinical symptoms often herald advanced stage malignancy with consequent poor prognosis and increased risk of mortality.5,12,44
Findings from this study suggest that there are substantial variations in the prevalence of cervical cancer screening across these five countries. Such between-country variation is of public health and clinical significance because screening for early detection is a significant aspect in the control of cervical cancer and greatly improves survivorship.45,46 Our findings underscore the need to deploy more efforts in the field to encourage and promote early screening, which could have significant public health impacts in these low-resource countries.
The multivariable analysis of five combined countries indicated that factors independently associated with cervical cancer screening were country of residence, age, health insurance coverage, wealth index status, place of residence, education level, employment status, pregnancy status, contraceptive use, STI, being sexually active in the last 4 weeks, visiting a health care facility in the last twelve months, and the number of living children a woman has. The positive association between increased age and cervical cancer screening could be explained by older women being more informed about the potential health benefits of screening for preventing cervical cancer. This finding is consistent with previous studies that also found older women were more likely to be screened for cervical cancer than younger women.16,47,48 There was evidence of a “dose-response relationship” between age and cervical cancer screening. However, it should be noted that between country age differences regarding cervical cancer screening are mostly driven by three countries (Namibia, Zimbabwe, and Kenya). As anticipated, having health insurance coverage was positively associated with cervical cancer screening a finding that is consistent with other previous studies.16,49 Health insurance coverage provides an opportunity for women to undergo preventive care services at no additional out-of-pocket cost. Higher education was positively associated with higher cervical cancer screening in the present study because educated women are more likely to know about the adverse outcomes of cervical cancer.48 This also highlights the need for more educational-based programs about cervical cancer to promote and increase awareness about cervical cancer screening in low resource settings. Our finding agrees with previous studies that indicated that higher educated women were more likely to undergo cervical cancer screening than lower-level education.47,48 Furthermore, we found that family wealth status was positively associated with cervical cancer screening during our study period. A plausible explanation is that stronger financial power increases health insurance coverage, indirectly translating to an increased routine in preventive care services.50 Moreover, in most countries included in this study, access to cervical cancer screening is not freely available to all women. For example, one study noted that at Kenyatta National Referral Hospital, the cost of a pap smear test is approximately $7. However, the client is required to pay roughly $6.5 for a file or identification card.15 In Zimbabwe, visual inspection with acetic acid is the screening method offered in all public health care clinics for free.51 In addition, a previous study conducted in SSA indicated that only 18% of women screened for free would participate if there was a fee associated with the screening.52 This finding is also consistent with previous studies conducted in this region.16,48 Interestingly, women who were sexually active in the last 4 weeks and those who had STI, were more likely to undergo cervical cancer screening. A possible explanation could be that those who may have experienced some early signs or symptoms may trigger the need to seek medical care or that being sexually active leads to more hospital visits such as for contraceptives need or treatment of STIs. Consistent with previous studies, the negatively observed association between cervical cancer screening and living in rural areas was not surprising and could be due to disparities in access to health care services and lack of healthcare facilities.32,48 Lastly, in agreement with a previous study, we found that women who visited health care facilities within the last twelve months were likely to undergo cervical cancer screening.16
4.1. Study Strengths and Limitations
Our study has several strengths. To the best of our knowledge, this is one of the few comprehensive studies, and perhaps the largest to date, to examine the prevalence and determinants of cervical cancer screening across multiple SSA countries. The findings from this study are useful in enhancing the uptake of cervical cancer screening, counseling practices, and interventions in these countries and the entire SSA regions. Nevertheless, our study has a few limitations that should be addressed. First, the cross-sectional nature of the survey does not allow for the determination of temporal relationships and outcome measures. Secondly, this study was limited to only five of the 48 countries in SSA and thus, our finding may lack external validity especially in countries we did not include in the analysis. Thirdly, our study population was limited to only women of childbearing age (15–49 years), and yet cancer incidence increases as a function of age. Therefore, the true cancer screening uptake beyond 49 years of age could be slightly different from our findings, which could be subject to exclusion bias. However, because only 1 country out of the 5 collected data among women over age 49, it is unlikely that exclusion bias affects our findings. In addition, information on why women were screened such as signs or symptoms, part of prenatal care, or routine interaction with health care providers were not included in the survey. Lastly, the method of cervical cancer screening such as Pap smear was available only for 2 out of 5 countries. Nevertheless, this study provides important information regarding the prevalence of cervical cancer screening and associated factors among women of childbearing age for the first time in five SSA countries.
5. Conclusions
The overall prevalence of cervical cancer screening in SSA is low, which suggesting the need for targeted interventions to lower the burden of cervical cancer, improve the quality of life, and increase survivorship among women. Findings from this study indicated great heterogeneity in cervical cancer screening behaviors across SSA countries and are related to women’s demographic and personal characteristics. Being sexually active in the last 4 weeks, being older, having health insurance coverage, higher socioeconomic status, a higher level of education, and contraceptive use were some of the leading factors independently and positively associated with cervical cancer screening. The findings highlight an urgent need for interventions through health education to encourage screening behaviors for detecting cervical cancer in this region.
Novelty and Impact.
To the best of our knowledge, this is one of the few comprehensive studies, and perhaps the largest to date, to examine the prevalence and determinants of cervical cancer screening across multiple SSA countries. The prevalence of cervical cancer screening is substantially low in sub-Saharan Africa and shows for the first time a high degree of between-country variation.
Highlights.
Prevalence and determinants of cervical cancer screening in SSA was examined
The prevalence of cervical cancer screening is substantially low in SSA countries
There is a high degree of between-country variation in cervical cancer screening.
Older age, having health insurance coverage, and higher socioeconomic status are some of the independent leading factors associated with cervical cancer screening.
Acknowledgments
The authors thank the DHS program implemented by ICF for granting access to the original data. The authors also thank Kani Dembele for her assistance with the literature search.
Funding Source: JM received funding through an International Research Career Development Award from the NIH/FIC (grant #K43TW011416) that provided research-protected time for reviewing and writing of this manuscript. PS was aslo supported by the NIH Director’s Transformative Award 1R01AI145057. The funding agencies cited here did not play a role in the design, collection of data, analysis, interpretation, and writing of this manuscript or decision to publish the results. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIH/Fogarty International Center.
Abbreviations
- aPR
adjusted prevalence ratios
- DHS
Demographic and Health Surveys data
- STI
sexually transmissible infection
- IC
confidence intervals
- IRB
Institutional Review Board
- HICs
high-income countries
- HPV
Human papillomavirus
- Pap
Papanicolaou
- PSU
Primary sampling units
- LMICs
Low and middle-income countries (LMICs)
- SSA
sub-Saharan Africa
- STI
Sexually Transmissible Infection
Footnotes
Disclosure of conflicts of interest
All authors have no conflict of interest to disclose as it relates to this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer C, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstock H, Berman S, Cates W. Sexually transmitted infections in American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6–1014982671. [DOI] [PubMed] [Google Scholar]
- 4.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV Infection Among Females in the United States. JAMA. 2007;297(8):813–819. [DOI] [PubMed] [Google Scholar]
- 5.Stewart TS, Moodley J, Walter FM. Population risk factors for late-stage presentation of cervical cancer in sub-Saharan Africa. Cancer Epidemiology. 2018;53:81–92. [DOI] [PubMed] [Google Scholar]
- 6.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer (IARC). GLOBOCAN http://gco.iarc.fr/tomorrow/home (2018).
- 8.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. 2010;127(12):2893–2917. [DOI] [PubMed] [Google Scholar]
- 9.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337. [DOI] [PubMed] [Google Scholar]
- 10.Cumber SN, Nchanji KN, Tsoka-Gwegweni JM. Breast cancer among women in sub-Saharan Africa: prevalence and a situational analysis. Southern African Journal of Gynaecological Oncology. 2017;9(2):35–37. [Google Scholar]
- 11.Kangmennaang J, Mkandawire P, Luginaah I. Breast cancer screening among women in Namibia: explaining the effect of health insurance coverage and access to information on screening behaviours. Glob Health Promot. 2019;26(3):50–61. [DOI] [PubMed] [Google Scholar]
- 12.Mwaka AD, Okello ES, Wabinga H, Walter FM. Symptomatic presentation with cervical cancer in Uganda: a qualitative study assessing the pathways to diagnosis in a low-income country. BMC Women’s Health. 2015;15(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Momberg M, Botha MH, Van der Merwe FH, Moodley J. Women’s experiences with cervical cancer screening in a colposcopy referral clinic in Cape Town, South Africa: a qualitative analysis. BMJ Open. 2017;7(2):e013914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mwaka AD, Wabinga HR, Mayanja-Kizza H. Mind the gaps: a qualitative study of perceptions of healthcare professionals on challenges and proposed remedies for cervical cancer help-seeking in post conflict northern Uganda. BMC Family Practice. 2013;14(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kivuti-Bitok LW, Pokhariyal GP, Abdul R, McDonnell G. An exploration of opportunities and challenges facing cervical cancer managers in Kenya. BMC Research Notes. 2013;6(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiruneh FN, Chuang K-Y, Ntenda PAM, Chuang Y-C. Individual-level and community-level determinants of cervical cancer screening among Kenyan women: a multilevel analysis of a Nationwide survey. BMC women’s health. 2017;17(1):109–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon M, Krantz E, Casper C. Correlates of cervical cancer screening from four Sub-Saharan African (SSA) countries: Results from the Demographic Health Survey (DHS)—2013–2015. Journal of Clinical Oncology. 2017;35(15_suppl):e18006–e18006. [Google Scholar]
- 18.Kent EE, Forsythe LP, Yabroff KR, et al. Are Survivors Who Report Cancer-Related Financial Problems More Likely to Forgo or Delay Medical Care? Cancer-Am Cancer Soc. 2013;119(20):3710–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutstein S, Rojas G. Guide to DHS statistics. Demographic and Health Surveys. Calverton, Maryland: ORCMacro, 2003. [Google Scholar]
- 20.Macro International Inc. Measure DHS: demographic and healthsurveys. Available from: http://www.measuredhs.com/countries/browse_country.cfm?selected=2.
- 21.American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology, 2016. American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Gynecology : ACOG Practice bulletin no. 168: cervical cancer screening and prevention. Obstet. Gynecol. 2016; 128: pp. e111–30. [DOI] [PubMed] [Google Scholar]
- 22.Mishra V, Vaessen M, Boerma JT, et al. HIV testing in national population-based surveys: experience from the Demographic and Health Surveys. Bulletin of the World Health Organization. 2006;84(7):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macro International Inc. Measure DHS: demographic and healthsurveys. Available from: https://dhsprogram.com/What-We-Do/Protecting-the-Privacy-of-DHS-Survey-Respondents.cfm. Accessed 13th June 2020.
- 24.Viens L, Perin D, Senkomago V, Neri A, Saraiya M. Questions About Cervical and Breast Cancer Screening Knowledge, Practice, and Outcomes: A Review of Demographic and Health Surveys. J Womens Health (Larchmt). 2017;26(5):403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lunani LL, Abaasa A, Omosa-Manyonyi G. Prevalence and Factors Associated with Contraceptive Use Among Kenyan Women Aged 15–49 Years. AIDS and behavior. 2018;22(Suppl 1):125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titilayo A, Palamuleni ME, Olaoye-Oyesola JO, Owoeye OM. Religious Perceptions and Attitudes of Men towards Discontinuation of Female Genital Cutting in Nigeria: Evidence from the 2013 Nigeria Demographic and Health Survey. African journal of reproductive health. 2018;22(1):20–28. [DOI] [PubMed] [Google Scholar]
- 27.Ba DM, Ssentongo P, Kjerulff KH, et al. Adherence to Iron Supplementation in 22 Sub-Saharan African Countries and Associated Factors among Pregnant Women: A Large Population-Based Study. Curr Dev Nutr. 2019;3(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd JP, Frampton GK, Harris P. Interventions for encouraging sexual behaviours intended to prevent cervical cancer. Cochrane Database Syst Rev. 2011;2011(4):Cd001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macro International Inc. Measure DHS: demographic and healthsurveys. Using Datasets for Analysis. Available from: https://www.dhsprogram.com/data/Using-Datasets-for-Analysis.cfm. Accessed 13th June 2020.
- 30.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Institute: cancer Trends Progress Report. Available from https://progressreport.cancer.gov/detection/cervical_cancer. Accessed 10th October 2020.
- 32.Ndejjo R, Mukama T, Musabyimana A, Musoke D. Uptake of Cervical Cancer Screening and Associated Factors among Women in Rural Uganda: A Cross Sectional Study. PloS one. 2016;11(2):e0149696–e0149696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudenga SL, Rositch AF, Otieno WA, Smith JS. Knowledge, attitudes, practices, and perceived risk of cervical cancer among Kenyan women: brief report. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2013;23(5):895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham MS, Skrastins E, Fitzpatrick R, et al. Cervical cancer screening and HPV vaccine acceptability among rural and urban women in Kilimanjaro Region, Tanzania. BMJ Open. 2015;5(3):e005828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Restivo V, Costantino C, Marras A, et al. Pap Testing in a High-Income Country with Suboptimal Compliance Levels: A Survey on Acceptance Factors among Sicilian Women. Int J Environ Res Public Health. 2018;15(9):1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Vuyst H, Alemany L, Lacey C, et al. The burden of human papillomavirus infections and related diseases in sub-saharan Africa. Vaccine. 2013;31 Suppl 5(0 5):F32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sankaranarayanan R, Nessa A, Esmy PO, Dangou JM. Visual inspection methods for cervical cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2012;26(2):221–232. [DOI] [PubMed] [Google Scholar]
- 38.Fokom-Domgue J, Combescure C, Fokom-Defo V, et al. Performance of alternative strategies for primary cervical cancer screening in sub-Saharan Africa: systematic review and meta-analysis of diagnostic test accuracy studies. BMJ : British Medical Journal. 2015;351:h3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakisige C, Schwartz M, Ndira AO. Cervical cancer screening and treatment in Uganda. Gynecol Oncol Rep. 2017;20:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sankaranarayanan R, Anorlu R, Sangwa-Lugoma G, Denny LA. Infrastructure requirements for human papillomavirus vaccination and cervical cancer screening in sub-Saharan Africa. Vaccine. 2013;31 Suppl 5:F47–52. [DOI] [PubMed] [Google Scholar]
- 41.Cancer Association of South Africa (CANSA). CANSA Educates on Rights of Women to Cancer Screening Available from : https://cansa.org.za/cansa-educates-on-rights-of-women-to-cancer-screening/. Accessed 20th September 2020.
- 42.Gouda HN, Charlson F, Sorsdahl K, et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7(10):e1375–e1387. [DOI] [PubMed] [Google Scholar]
- 43.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018;392(10159):1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishra K, Desai A, Patel S, Mankad M, Dave K. Role of percutaneous nephrostomy in advanced cervical carcinoma with obstructive uropathy: a case series. Indian J Palliat Care. 2009;15(1):37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrae B, Andersson TML, Lambert PC, et al. Screening and cervical cancer cure: population based cohort study. BMJ. 2012;344:e900–e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jayant K, Sankaranarayanan R, Thorat RV, et al. Improved Survival of Cervical Cancer Patients in a Screened Population in Rural India. Asian Pacific journal of cancer prevention : APJCP. 2016;17(11):4837–4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phaswana-Mafuya N, Peltzer K. Breast and Cervical Cancer Screening Prevalence and Associated Factors among Women in the South African General Population. Asian Pacific journal of cancer prevention : APJCP. 2018;19(6):1465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Compaore S, Ouedraogo CMR, Koanda S, Haynatzki G, Chamberlain RM, Soliman AS. Barriers to Cervical Cancer Screening in Burkina Faso: Needs for Patient and Professional Education. J Cancer Educ. 2016;31(4):760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cowburn S, Carlson MJ, Lapidus JA, DeVoe JE. The association between insurance status and cervical cancer screening in community health centers: exploring the potential of electronic health records for population-level surveillance, 2008–2010. Prev Chronic Dis. 2013;10:E173–E173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ba DM, Ssentongo P, Agbese E, et alPrevalence and determinants of breast cancer screening in four sub-Saharan African countries: a population-based study BMJ Open 2020;10:e039464. doi: 10.1136/bmjopen-2020-039464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuguyo O, Matimba A, Tsikai N, et al. Cervical cancer in Zimbabwe: a situation analysis. Pan Afr Med J. 2017;27:215–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chigbu CO, Onyebuchi AK, Ajah LO, Onwudiwe EN. Motivations and preferences of rural Nigerian women undergoing cervical cancer screening via visual inspection with acetic acid. Int J Gynaecol Obstet. 2013;120(3):262–265. [DOI] [PubMed] [Google Scholar]