Abstract
Background:
Preliminary data suggest that psilocybin-assisted treatment produces substantial and rapid antidepressant effects in patients with major depressive disorder (MDD), but little is known about long-term outcomes.
Aims:
This study sought to examine the efficacy and safety of psilocybin through 12 months in participants with moderate to severe MDD who received psilocybin.
Methods:
This randomized, waiting-list controlled study enrolled 27 patients aged 21–75 with moderate to severe unipolar depression (GRID-Hamilton Depression Rating Scale (GRID-HAMD) ⩾ 17). Participants were randomized to an immediate or delayed (8 weeks) treatment condition in which they received two doses of psilocybin with supportive psychotherapy. Twenty-four participants completed both psilocybin sessions and were followed through 12 months following their second dose.
Results:
All 24 participants attended all follow-up visits through the 12-month timepoint. Large decreases from baseline in GRID-HAMD scores were observed at 1-, 3-, 6-, and 12-month follow-up (Cohen d = 2.3, 2.0, 2.6, and 2.4, respectively). Treatment response (⩾50% reduction in GRID-HAMD score from baseline) and remission were 75% and 58%, respectively, at 12 months. There were no serious adverse events judged to be related to psilocybin in the long-term follow-up period, and no participants reported psilocybin use outside of the context of the study. Participant ratings of personal meaning, spiritual experience, and mystical experience after sessions predicted increased well-being at 12 months, but did not predict improvement in depression.
Conclusions:
These findings demonstrate that the substantial antidepressant effects of psilocybin-assisted therapy may be durable at least through 12 months following acute intervention in some patients.
Keywords: Insight, long-term effects, major depressive disorder, mystical experience, psilocybin
Major depressive disorder (MDD) affects over 260 million people worldwide and is a leading cause of disability and healthcare expenditures (James et al., 2018). First-line treatments, including pharmacotherapy and psychotherapy, may take weeks or months to produce clinically meaningful symptom reduction, and patients can have difficulty with treatment adherence (Cuijpers et al., 2008; Kolovos et al., 2017; Lam, 2012). At least 30% of patients ultimately meet criteria for treatment-resistant depressive illness after failing to respond to multiple attempts at treatment (Nemeroff, 2007). MDD also has a highly recurrent course, with 40–60% of those diagnosed with a single episode eventually relapsing, and rate of relapse increasing with each subsequent episode (Richards, 2011; Solomon et al., 2000). Novel interventions are needed that can act rapidly and produce sustained remission.
Several preliminary studies suggest that psilocybin-assisted treatment may have substantial antidepressant effects in patients with MDD, with treatment response occurring within a week of administration of just one or two doses in the context of psychotherapy (Carhart-Harris et al., 2018, 2021; Davis et al., 2021). In an initial report of primary outcomes following two doses of psilocybin using a randomized waitlist-control study design, we reported a large effect size (Cohen d = 2.3) and high rates of treatment response and remission (71% and 54%) at 1 month following intervention (Davis et al., 2021). Treatment-resistant patients also appear to have a favorable response rate (Carhart-Harris et al., 2018). A more recent study used a double-blind double dummy design to compare high-dose psilocybin plus 6 weeks of placebo with very low dose psilocybin plus 6 weeks of escitalopram (Carhart-Harris et al., 2021). The authors failed to show a significant difference between the two groups at 6 weeks in their designated primary outcome measure (Quick Inventory of Depressive Symptoms). The majority of results for secondary outcome measures including other depression severity scores favored the high-dose psilocybin group, though analyses were not corrected for multiple comparisons.
Although psilocybin treatment of MDD appears promising, little is known about long-term efficacy and safety. The three studies conducted to date demonstrated efficacy at their longest follow-up assessments of 4 weeks (Davis et al., 2021), 6 weeks (Carhart-Harris et al., 2021), and 6 months (Carhart-Harris et al., 2018), although depression severity scores were trending upward at 3- and 6-month follow-up timepoints. Carhart-Harris et al. (2018), with the longest period of follow-up, had an open-label design. Given the chronicity and relapsing disease course of MDD (Richards, 2011), the present study represents a significant extension of these previous findings by assessing efficacy and safety of a psilocybin intervention throughout a 12-month follow-up period.
Methods
Study design
Full details of the study design and inclusion criteria have been described previously (Davis et al., 2021). All procedures involving subjects were approved by The Johns Hopkins Medicine Institutional Review Board. Written informed consent was obtained from all participants. Participants were 21–75 years of age, medically stable, and met criteria for a moderate to severe episode of MDD as defined by a score of ⩾17 on the GRID-Hamilton Depression Rating Scale (GRID-HAMD) assessed by blinded clinician raters. Individuals with personal or first- or second-degree relative history of psychotic or bipolar I or II disorder were excluded. To avoid interactions with psychoactive drugs including those used to treat depression, participants were required to refrain from using such medications for at least five half-lives before screening and for at least 1 month following the second psilocybin session. Following medical and psychological screening and baseline assessments, participants were randomized to an immediate or delayed treatment condition. Participants in the immediate treatment group began the intervention after screening, while those in the delayed treatment group began the intervention after an 8-week delay interval.
After participants entered the intervention period, they were provided with 6–8 h of preparatory meetings with two facilitators. At least one facilitator in each dyad had a master’s or doctoral level of clinical training in mental health (e.g. master of social work, PhD in clinical psychology, MD specializing in psychiatry). Following preparation, participants received two doses of psilocybin at 20 mg/70 kg and 30 mg/70 kg spaced approximately 2 weeks apart. Psilocybin was administered in a comfortable room under the supervision of both facilitators following established safety guidelines (Johnson et al., 2008). A nondirective psychotherapeutic approach was taken on session days. Participants returned for follow-up at 1 day and 1 week following each drug administration session, and then at 1, 3, 6, and 12 months following the second session, during which depression severity was assessed with participant- and clinician-rated measures. Each follow-up visit included a 1–2 h meeting with at least one of the therapist facilitators. Functional magnetic resonance imaging was completed at baseline and 1 week after the second psilocybin session (Doss et al., 2021).
Outcome measures
Measures of depression severity
The primary outcome measure was the GRID-HAMD (Depression Rating Scale Standardization Team, 2003), which was assessed by blinded clinician raters via telephone as described previously (Davis et al., 2021). Inter-rater reliability at the 3-, 6-, and 12-month timepoints was 87.5% (see online supplement for additional information). Depression was also assessed with two self-report questionnaires: the Quick Inventory of Depressive Symptoms (QIDS) (Rush et al., 2003), and the Beck Depression Inventory II (BDI-II) (Beck et al., 1996). Depression severity was assessed at baseline and at each of the follow-up timepoints.
Participant-rated measures of acute psilocybin effects
Various measures of acute psilocybin effects assessed at the end of the session or the following day were reported previously (Davis et al., 2021). Of interest in this follow-up analysis was whether a subset of these acute measures would predict subsequent follow-up results. Based on previous studies showing associations between acute psilocybin measures and subsequent positive effects in healthy and patient samples (Bogenschutz et al., 2015; Davis et al., 2020; Garcia-Romeu et al., 2014; Griffiths et al., 2008, 2016, 2018), the following measures were examined: the Mystical Experience Questionnaire (MEQ30) (Barrett et al., 2015) and four single-item measures (Carbonaro et al., 2020) on which participants rated the degree to which the session experience was personally meaningful, spiritually significant, psychologically insightful, and psychologically challenging on a scale from 1 = no more than routine, everyday experiences to 8 = the single most (meaningful, spiritually significant, psychologically insightful, or psychologically challenging experience) of my life. The psychological challenge item was included as a comparison because it was not expected to predict subsequent positive outcomes. The MEQ30 was completed at the conclusion of each psilocybin session and the single-item measures were completed on the day following each session.
Overall well-being attributed to psilocybin
At the 1-, 3-, 6-, and 12-month follow-up timepoints, participants completed the Persisting Effects Questionnaire (Griffiths et al., 2018), which involved rating on a 6-point scale current persisting effects that they attributed to their psilocybin experiences (see online supplement for more information). For this study, an overall well-being score was calculated as the grand mean of the five subscales of positive change: attitudes about life, attitudes about self, mood, relationships, and behavior, with each expressed as a percentage of maximum possible score.
Safety measures
At each follow-up timepoint, adverse events were recorded, suicidal ideation was assessed using the Columbia Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2008), and symptoms indicative of hallucinogen persisting perceptual disorder (HPPD) were solicited (e.g. “Since your drug session have you experienced any uncontrolled or disturbing return of drug-like effects?”).
Statistical analyses
A repeated-measures analysis of variance was conducted with time (baseline, 1 week post-treatment, and 1, 3, 6, and 12 months post-treatment) and condition (immediate and delayed treatment) as factors on the primary depression outcome (GRID-HAMD score), with effect sizes calculated using partial eta squared ( ). This analysis showed a significant effect of time, but no significant effect of condition or a time-by-condition interaction. Therefore, data were collapsed across the conditions and a series of paired t tests compared baseline scores with scores at each of the follow-up timepoints with Bonferroni adjustment for multiple comparisons. Paired t test effect sizes were calculated using Cohen d. Descriptive statistics of follow-up measures were calculated, including treatment response (⩾50% reduction in depression scores from baseline) and remission (GRID-HAMD ⩽ 7, QIDS ⩽ 5, BDI-II ⩽ 9) for measures of depression (Beck et al., 1996; Rush et al., 2003; Zimmerman et al., 2013). The relationship between acute measures of session experiences and follow-up measures of acute psilocybin effects (MEQ30 and ratings of meaning, insight, spiritual significance and psychological challenge) were examined with Spearman’s correlations (rs). For these calculations, the highest ratings or scores from Session 1 and Session 2 for each participant were used. For the follow-up measures, the overall well-being score was expressed as a percentage of maximum possible score, and the depression measures were expressed as percentage change from the baseline score for each participant. Analyses of several group-based comparisons were conducted using χ2 for categorical variables and t test for continuous variables. A two-tailed significance level of p < .05 was used for between-group comparisons and correlations. Analyses were completed using SPSS 26 and 27.
Results
Participants
As described in more detail previously (Davis et al., 2021), 27 participants were randomized and 24 completed both psilocybin sessions, with 13 and 11 assigned to the immediate and delayed treatment groups, respectively. All 24 participants completed all long-term follow-up assessment visits (see online supplement, CONSORT diagram). The group was 67% female and 92% Caucasian. One participant identified as Black and another as Asian; none identified as Hispanic. Participants had a mean (SD) age of 39.8 (12.2) years. Mean duration of illness (years since diagnosis of MDD) was 21.5 (12.2) years, and mean time in current major depressive episode was 24.4 (22.0) months. Of the participants, 88% had previously attempted treatment with an antidepressant (e.g. a selective serotonin, norepinephrine, or dopamine reuptake inhibitor, etc.) and 58% reported previous use of such medication in the current depressive episode. Twenty-five percent had previously used a psychedelic drug, with an average of 3.3 previous uses and an average time of 9.2 years since last use.
Changes in depressive symptoms
As reported previously (Davis et al., 2021), GRID-HAMD scores were significantly lower in the immediate treatment group at 1 and 4 weeks post treatment when compared with corresponding timepoints after randomization in the delayed treatment group. After completing the delay period, participants in the delayed treatment group completed the psilocybin intervention and follow-up assessments. In this follow-up study, analysis of variance with GRID-HAMD scores showed a significant effect of time (baseline and 5 post-treatment timepoints) (F(4.4, 96.3) = 34.9, p < 0.001; = .61), but no significant effect of condition (immediate vs delayed treatment) or a time-by-condition interaction. Thus, the following results are from data collapsed across conditions.
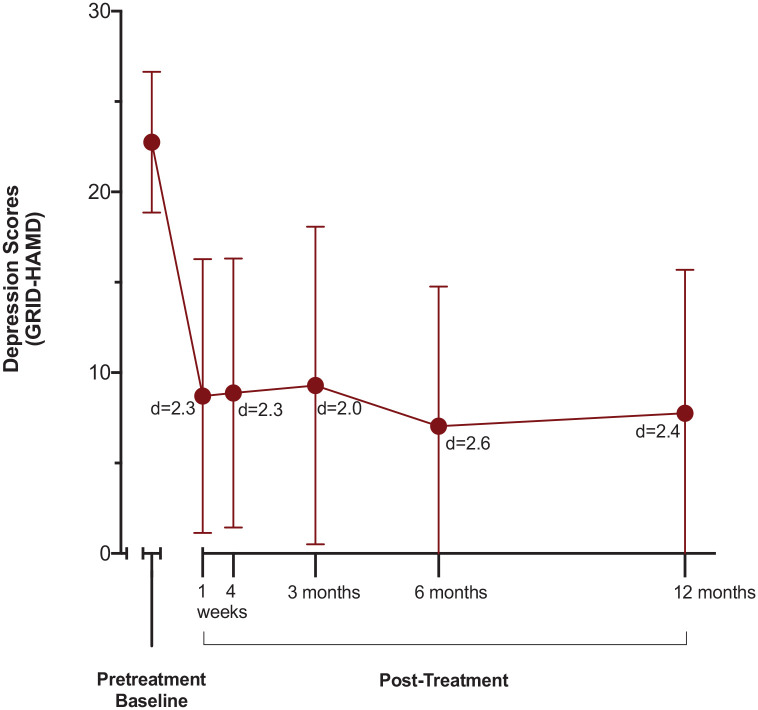
As shown in Figure 1, mean GRID-HAMD scores for the overall treatment sample decreased from a mean (SD) of 22.8 (3.9) at pretreatment baseline to 8.7 (7.6) at 1 week, 8.9 (7.4) at 4 weeks, 9.3 (8.8) at 3 months, 7.0 (7.7) at 6 months, and 7.7 (7.9) at 12 months post-treatment (p > .001 at all timepoints, paired t tests with Bonferroni correction). The effect sizes for these differences were large, with Cohen d (95% CI) being 2.3 (1.5, 3.1) at 1 week, 2.3 (1.5, 3.1) at 4 weeks, 2.0 (1.3, 2.7) at 3 months, 2.6 (1.7, 3.4) at 6 months, and 2.4 (1.6, 3.2) at 12 months. Similar significant, large magnitude, and sustained decreases in depression from pretreatment across the five follow-up assessments occurred with the two patient-rated depression assessment questionnaires (QIDS and BDI-II, online supplement Table S1, Figures S1 and S2).
Figure 1.
Decrease in GRID-HAMD depression scores over time from baseline through the 12-month follow-up (N = 24).
Data points are means and brackets are ±1 SD; lower brackets are truncated at GRID-HAMD scores of 0. Mean GRID-HAMD was 22.8 (3.9) at baseline, 8.7 (7.6) at 1 week, 8.9 (7.4) at 4 weeks, 9.3 (8.8) at 3 months, 7.0 (7.7) at 6 months, and 7.7 (7.9) at 12 months post-treatment. All timepoints were significantly different from baseline (p < 0.001). Cohen d effect size is shown for each timepoint. Cohen d (95% CI) was 2.3 (1.5–3.1) at 1 week, 2.3 (1.5–3.1) at 4 weeks, 2.0 (1.3–2.7) at 3 months, 2.6 (1.7–3.4) at 6 months, and 2.4 (1.6–3.2) at 12 months.
As previously reported (Davis et al., 2021), at 1 week after treatment, 17 of the 24 participants (71%) showed a clinical response rate on the GRID-HAMD (⩾50% reduction from pretreatment) and 14 (58%) were in remission (GRID-HAMD score ⩽ 7) (Zimmerman et al., 2013). As shown in Table 1, the response and remission rates were generally sustained through the 12-month follow-up assessment, with final response and remission rates of 75% and 58%, respectively. Table 1 shows similar or greater response and remission rates with the two patient-rated measures of depression (QIDS and BDI-II).
Table 1.
Percentage of total sample (N = 24) meeting criteria for treatment response (reduction in depression ⩾ 50% from pretreatment baseline) or remission.
| Post-Treatment Follow-Up
Time |
|||||
|---|---|---|---|---|---|
| 1 week | 4 weeks | 3 months | 6 months | 12 months | |
| GRID-HAMD | |||||
| Response rate | 71% | 71% | 67% | 79% | 75% |
| Remission rate a | 58% | 54% | 54% | 71% | 58% |
| QIDS | |||||
| Response rate | 79% | 71% | 79% | 79% | 79% |
| Remission rate b | 54% | 54% | 58% | 67% | 67% |
| BDI-II | |||||
| Response rate | 79% | 79% | 79% | 88% | 83% |
| Remission rate c | 67% | 63% | 58% | 75% | 75% |
GRID-HAMD: Hamilton Depression Rating Scale; QIDS: Quick Inventory of Depressive Symptoms; BDI-II: Beck Depression Inventory II.
Remission = GRID-HAMD score ⩽ 7 (Zimmerman et al., 2013).
Remission = QIDS score ⩽ 5 (Rush et al., 2003).
Remission = BDI-II score ⩽ 9 (Beck et al., 1996).
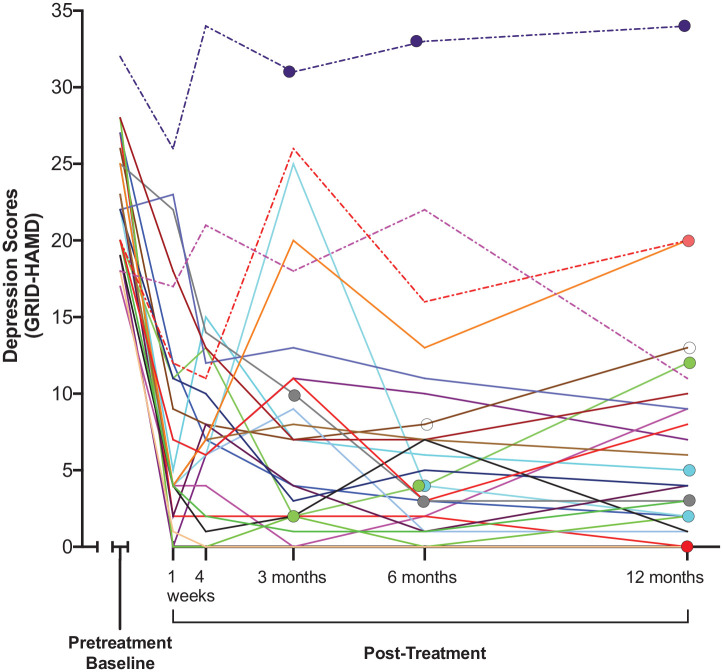
Figure 2 shows GRID-HAMD depression scores for each of the 24 study participants from pretreatment through the 12-month follow-up assessment. Most participants showed large decreases in their depression score at the first follow-up interval at 1-week post-treatment, consistent with the 71% response rate and 58% remission rate shown in Table 1. The figure shows that psilocybin did not exacerbate depression in any participant and that 3 of 24 participants (13%) did not meet criteria for a treatment response at any post-treatment timepoint.
Figure 2.
Depression scores (GRID-HAMD) for each of 24 study participants at baseline and each of 5 follow-up assessment timepoints.
Individual participants are represented with different colors. Dashed lines indicate three participants who did not fulfill criteria for a treatment response at any post-treatment timepoint. Enlarged data points indicate participants who reported treatment with antidepressant medication, with the left-most enlarged data points showing the first follow-up timepoint at which medication use was reported.
Figure 2 also provides detailed information about participants who started or resumed daily use of an antidepressant medication for depression (i.e. a selective serotonin reuptake inhibitor, a serotonin-norepinephrine reuptake inhibitor, or a norepinephrine-dopamine reuptake inhibitor) after psilocybin treatment. Of the 24 participants, 0 (0%), 3 (12.5%), 5 (20.8%), 8 (33.3%), and 8 (33.3%), respectively, reported daily antidepressant use at the 4-week and 3-, 6-, and 12-month follow-up assessments. The 8 participants who started antidepressant treatment by the 12-month timepoint had higher baseline GRID-HAMD scores (mean 25.3 vs 21.5, p = .02) compared with those who did not report antidepressant use; however, they were not statistically different in age, sex, years with depression, duration of current depressive episode, or history of medication use in the current depressive episode. Mean GRID-HAMD scores and overall well-being scores at 12 months did not significantly differ between those who did and did not begin antidepressant treatment.
Overall well-being attributed to psilocybin across the 1-, 3-, 6-, and 12-month follow-up timepoints was intermediate and stable. The grand mean (SD) overall well-being score, expressed as percentage of maximum possible score, was 63.9 (22.6), 60.0 (21.3), 59.0 (24.0), and 65.0 (20.0), respectively.
Participant-rated measures of session experiences as predictors of subsequent overall well-being and changes in depression severity
Correlations between participant-rated measures of psilocybin experiences at the time of the session and subsequent measures of well-being and depression were examined. At the first long-term follow-up assessment (Table 2, Week 4), ratings of personal meaning, psychological insight, spiritual significance, and the mystical experience (MEQ30) correlated significantly with well-being, and ratings of personal meaning and spiritual significance correlated significantly with improvement in depression (GRID-HAMD). However, at subsequent follow-up timepoints, none of these session experience measures were significantly correlated with improvements in depression (Table 2). The MEQ30 significantly correlated with well-being at all four follow-up timepoints, and ratings of personal meaning and spiritual significance were significantly correlated at three of four timepoints. Ratings of psychological challenge during the session were not significantly correlated with subsequent measures of well-being or depression at any follow-up timepoint.
Table 2.
Relationship between measures of psilocybin experiences assessed at the end of the session or the following day with the follow-up measures of well-being and improvement in depression assessed at 4 weeks and 3, 6, and 12 months. a
| Session measures b | 4 weeks |
Follow-up |
3 months |
Follow-up |
6 months |
Follow-up |
12 months |
Follow-up |
|---|---|---|---|---|---|---|---|---|
| Well-being c | GRID-HAMD d | Well-being c | GRID-HAMD d | Well-being c | GRID-HAMD d | Well-being c | GRID-HAMD d | |
| Personal Meaning e | 0.70* | 0.67* | 0.43 | 0.34 | 0.51 | 0.44 | 0.45 | 0.43 |
| Psychological Insight e | 0.49 | 0.36 | 0.35 | 0.04 | 0.27 | 0.34 | 0.39 | 0.25 |
| Spiritual Significance e | 0.67* | 0.56* | 0.54 | 0.16 | 0.44 | 0.28 | 0.60* | 0.40 |
| Psychological Challenge e | 0.12 | 0.32 | 0.09 | 0.18 | 0.13 | 0.31 | 0.06 | 0.13 |
| Mystical Experience MEQ30 | 0.71* | 0.38 | 0.43 | 0.17 | 0.50 | 0.05 | 0.50 | 0.19 |
HAMD: Hamilton Depression Rating Scale; MEQ30: Mystical Experience Questionnaire.
Data show Spearman’s correlations (rs); bold font indicates p < .05 and asterisks indicate p < .01.
Assessments shown in this column were the highest rating or score from Sessions 1 and 2 for each participant.
Well-being scores were expressed as percentage of maximum possible score.
GRID-HAMD depression scores were expressed as percentage change from baseline for each participant.
Only 20 of 24 participants completed these measures due to an error in the survey programming.
Safety outcomes
During the follow-up period, there were no serious adverse events, suicidal ideation remained low, there were no instances of self-injurious behavior, no reported use of psilocybin or other psychedelics, and no participant met criteria for HPPD. Further details on adverse events are available in the online supplement.
Discussion
The present study suggests that two doses of psilocybin provided in the context of supportive therapy for MDD produced large and stable antidepressant effects throughout a 12-month follow-up period. More specifically, depression, as measured by blinded clinician-rated assessments (GRID-HAMD), decreased substantially after treatment and remained low at 1, 3, 6, and 12 months post-treatment. The effect size at 12 months was very large (Cohen d = 2.4). Likewise, high and stable rates of response and remission occurred throughout the follow-up period (75% response and 58% remission at 12 months). Two patient-rated measures of depression (QIDS and BDI-II) showed similar large magnitude and stable antidepressant effects on mean scores and on response and remission rates. These findings suggesting enduring antidepressant effects of psilocybin 1 year after treatment significantly extend the previous results in this and two other trials that showed antidepressant effects through 4 weeks (Davis et al., 2021), 6 weeks (Carhart-Harris et al., 2021), and 6 months (Carhart-Harris et al., 2018). Notably, the remission rate and magnitude of the effect in the current study at both 6 and 12 months were substantially greater than those in a previous study at 6 months (QIDS, remission rate 67% and 67% vs 32%, Cohen d 2.2 and 2.3 vs 1.6, respectively) (Carhart-Harris et al., 2018). Whether this difference reflects population differences in severity of illness or procedural differences is unknown. Future research is needed to explore the possibility that efficacy of psilocybin treatment in MDD may be substantially longer than the 12 months observed in the present study, as has been suggested in a study that documented decreases in depressive symptoms up to 4.5 years following psilocybin treatment in patients with cancer-related distress (Agin-Liebes et al., 2020).
Of note, eight patients (33%) reported beginning a new course of treatment with a daily antidepressant drug at some point during the 12-month follow-up period, which is similar to the 32% of patients in a previous trial that did so by 6 months (Carhart-Harris et al., 2018). Although patients who used antidepressants during the follow-up period had higher GRID-HAMD scores at baseline, at 12 months they did not significantly differ from those who did not initiate medications. Determining the extent of the contribution of psilocybin vs other medications to clinical improvement in those who resumed antidepressant use is not possible. However, participant ratings of persisting well-being attributed to the psilocybin sessions were not significantly different from those who did not use antidepressant medications, suggesting that psilocybin treatment resulted in some independent benefit.
Although relapse and remission rates at 12 months were favorable, the ability to accurately compare the long-term efficacy of psilocybin-assisted treatment to that of standard antidepressant treatment is limited. The majority of recent studies of long-term antidepressant efficacy drop non-responders from follow-up and focus on rate of relapse among those who respond to a particular drug, which is often a minority of the intention-to-treat sample (McGrath et al., 2006; Trivedi et al., 2006). In our sample, of the 17 participants who met criteria for treatment response at 1-month follow-up, 12 (71%) continued to meet criteria for treatment response at all subsequent timepoints. An additional 3 participants who responded at 1 month also met criteria for treatment response at 12 months, but had one or more interim assessments during which their GRID-HAMD score was elevated out of the range for treatment response. The 71% continuous treatment response at 12 months is somewhat higher than the 54% rate reported in a study of fluoxetine responders who were maintained on fluoxetine, and much higher than those switched to placebo (28%) (McGrath et al., 2006).
The present study provides new information about qualitative features of the acute psilocybin experience that predict subsequent enduring effects. Patient ratings of personal meaning, spiritual significance, and MEQ30 scores after psilocybin sessions significantly correlated with a measure of overall well-being at most follow-up timepoints. However, except for ratings of personal meaning and spiritual significance at the first long-term follow-up assessment at 4 weeks, none of patient ratings of psilocybin experience at the time of the session were predictive of improvements in depression. Notably, two previous studies in individuals with cancer-related depression and anxiety (Griffiths et al., 2016; Ross et al., 2016) showed positive associations of MEQ30 session experiences with improvements in depression symptoms at 5 or 6 weeks. Considering that the direction of correlation at 4 weeks was in the predicted direction (rs = 0.38, p = .066), it is possible that the present study was underpowered to detect such an effect with MEQ30 or other measures of psilocybin experience. Alternatively, this difference may reflect a lack of such relationship in a sample of individuals with MDD as opposed to depressive symptoms secondary to a cancer diagnosis.
There were no serious adverse events, depression symptoms were not significantly exacerbated in any participant, and there was no reported use of psilocybin or other psychedelic drugs during the follow-up period. This latter observation contrasts with a previous study in which 5 of 19 participants reported use of psilocybin outside of the research setting by the end of the 6-month follow-up period (Carhart-Harris et al., 2018). Reasons for this difference are unknown, but the observation indicates the importance of assessing use of psychedelics outside of a clinical trial. Although the safety results presented herein are favorable, larger phase 3 and 4 studies will be needed to more fully assess safety.
Strengths and limitations
Strengths of this study of psilocybin-facilitated treatment of depression include a primary outcome measure that was assessed by blinded clinician raters, the longest post-treatment follow-up interval to date, and excellent participant retention. Although no participants reported extraneous psilocybin use, 33% reported using antidepressants during the follow-up period, which precludes determination of the effects of psilocybin alone in those patients. Although the randomized waiting list-control design of the study allowed for comparison of short-term treatment effects to the control group, as described previously (Davis et al., 2021), the design did not allow for a comparison group at long-term follow-up. A recent study suggests that expectancy effects and psychotherapy may account for some of the clinical benefit of psychedelic-assisted therapy (Carhart-Harris et al., 2021). In that study, which utilized a double-blind, double dummy design, both the high-dose and very low-dose psilocybin groups showed significant immediate decreases in depression, suggesting that the preparation and drug administration day procedures may reduce depressive symptoms even in the absence of high-dose psilocybin. Studies of other types of interventions for patients with MDD have demonstrated that placebo effects may last for weeks or months beyond intervention, and a lack of a comparator group makes it difficult to account for such effects in our study (Khan et al., 2008). Other limitations include the small sample size, the predominately Caucasian, non-Hispanic study sample, and exclusion of those judged to be at elevated risk of suicide.
Clinical implications
As novel antidepressants, classic psychedelics are commonly compared to ketamine and its analogues. Despite distinct mechanisms of pharmacologic action, both have rapid antidepressant effects and both have garnered concern about their potential for non-medical use (Schak et al., 2016; Shalit et al., 2019). Ketamine has nontrivial abuse potential and there may be overlap between mechanisms underlying its antidepressant effects and abuse potential, which may be exacerbated by the requirement for repeated administration to maintain therapeutic efficacy (Kokane et al., 2020; Liu et al., 2016). Although evidence to date suggests that psilocybin has relatively low abuse potential (Johnson et al., 2018), there remains concern for its potential to cause harm or encourage substance misuse in vulnerable populations (Reiff et al., 2020; Schatzberg, 2020). The present study highlights a key potential advantage of psilocybin treatment over ketamine in that antidepressant effects after just two administrations of psilocybin paired with psychological support appear to be sustained through 12 months, which is well beyond the duration of effects reported with ketamine (McIntyre et al., 2021; Salloum et al., 2020). It will be important for future research to determine the risks and benefits of additional psilocybin administration for those who failed to respond or experienced early relapse.
Conclusions
The results of this long-term follow-up of participants who were not blinded to the drug condition suggest that psilocybin-assisted treatment for MDD produces large and stable antidepressant effects throughout at least 12 months after treatment. These data document larger effects of longer duration than previous studies of psilocybin in depressed patients. Further studies are needed with active treatment or placebo comparison controls in larger and more diverse populations.
Supplemental Material
Supplemental material, sj-doc-1-jop-10.1177_02698811211073759 for Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: Prospective 12-month follow-up by Natalie Gukasyan, Alan K Davis, Frederick S Barrett, Mary P Cosimano, Nathan D Sepeda, Matthew W Johnson and Roland R Griffiths in Journal of Psychopharmacology
Acknowledgments
Darrick May, MD, Annie Umbricht, MD, and Eric Strain, MD, provided medical oversight during the study sessions. Patrick H. Finan, PhD, Jessiy Salwen, PhD, and Mary Bailes, LCPC, served as blinded clinician raters. Laura Doyle, BA, John Clifton, BS, Kasey Cox, MS, and Rhiannon Mayhugh, PhD, facilitated the intervention sessions and data collection. We thank Darrick G. May, MD, for help in the design and conduct of the study, and James B. Potash, MD, for his helpful comments on this article. These individuals, from Johns Hopkins University, received no additional compensation, outside of their usual salary, for their contributions.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AKD is a board member of Source Research Foundation. MWJ has received grant support from the Heffter Research Institute unrelated to this study and he is an advisor to the following companies: AJNA Labs LLC, AWAKN Life Sciences Inc., Beckley Psytech Ltd., Entheon Biomedical Corp., Field Trip Psychedelics Inc., Mind Medicine Inc., Otsuka Pharmaceutical Development & Commercialization Inc., and Silo Pharma, Inc. RRG is a board member of the Heffter Research Institute and has received grant support from the Heffter Research Institute unrelated to this study. RRG is site principal investigator, and MWJ and NG are co-investigators for a multi-site trial of psilocybin-assisted therapy for major depressive disorder sponsored by Usona Institute.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by a crowd-sourced funding campaign organized by Tim Ferriss and by grants from the Riverstyx Foundation and Dave Morin. Effort for AKD and NG was provided by NIH grant T32DA07209 from NIDA. Effort for authors was also provided by The Center for Psychedelic and Consciousness Research which is funded by the Steven and Alexandra Cohen Foundation, Tim Ferriss, Matt Mullenweg, Craig Nerenberg, and Blake Mycoskie. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Natalie Gukasyan  https://orcid.org/0000-0003-3567-1421
https://orcid.org/0000-0003-3567-1421
Alan K Davis  https://orcid.org/0000-0003-4770-8893
https://orcid.org/0000-0003-4770-8893
Roland R Griffiths  https://orcid.org/0000-0001-5185-7854
https://orcid.org/0000-0001-5185-7854
References
- Agin-Liebes GI, Malone T, Yalch MM, et al. (2020) Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. Journal of Psychopharmacology 34(2): 155–166. [DOI] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, Griffiths RR. (2015) Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. Journal of Psychopharmacology 29(11): 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, et al. (1996) Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. Journal of Personality Assessment 67(3): 588–597. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, et al. (2015) Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. Journal of Psychopharmacology 29(3): 289–299. [DOI] [PubMed] [Google Scholar]
- Carbonaro TM, Johnson MW, Griffiths RR. (2020) Subjective features of the psilocybin experience that may account for its self-administration by humans: A double-blind comparison of psilocybin and dextromethorphan. Psychopharmacology 237: 2293–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Day C, et al. (2018) Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 235: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Giribaldi B, Watts R, et al. (2021) Trial of psilocybin versus escitalopram for depression. New England Journal of Medicine 384(15): 1402–1141. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Van Straten A, Andersson G, et al. (2008) Psychotherapy for depression in adults: A meta-analysis of comparative outcome studies. Journal of Consulting and Clinical Psychology 76(6): 909–922. [DOI] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, Griffiths RR. (2020) Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. Journal of Contextual Behavioral Science 15: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, May DG, et al. (2021) Effects of psilocybin-assisted therapy on major depressive disorder: A randomized clinical trial. JAMA Psychiatry 78(5): 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depression Rating Scale Standardization Team (2003) GRID-HAMD-17, GRID-HAMD-21 structured interview guide. San Diego, CA: International Society for CNS Drug Development. [Google Scholar]
- Doss MK, Považan M, Rosenberg MD, et al. (2021) Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Translational Psychiatry 11(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu A, Griffiths RR, Johnson MW. (2014) Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Current Drug Abuse Reviews 7(3): 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. Journal of Psychopharmacology 30(12): 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2018) Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. Journal of Psychopharmacology 32(1): 49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, Johnson MW, et al. (2008) Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. Journal of Psychopharmacology 22(6): 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, et al. (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet 392(10159): 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR, Hendricks PS, et al. (2018) The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology 142: 143–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Richards WA, Griffiths RR. (2008) Human hallucinogen research: Guidelines for safety. Journal of Psychopharmacology 22(6): 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Redding N, Brown WA. (2008) The persistence of the placebo response in antidepressant clinical trials. Journal of Psychiatric Research 42(10): 791–796. [DOI] [PubMed] [Google Scholar]
- Kokane SS, Armant RJ, Bolaños-Guzmán CA, et al. (2020) Overlap in the neural circuitry and molecular mechanisms underlying ketamine abuse and its use as an antidepressant. Behavioural Brain Research 384: 112548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolovos S, van Tulder MW, Cuijpers P, et al. (2017) The effect of treatment as usual on major depressive disorder: A meta-analysis. Journal of Affective Disorders 210: 72–81. [DOI] [PubMed] [Google Scholar]
- Lam RW. (2012) Onset, time course and trajectories of improvement with antidepressants. European Neuropsychopharmacology 22: S492–S498. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lin D, Wu B, et al. (2016) Ketamine abuse potential and use disorder. Brain Research Bulletin 126: 68–73. [DOI] [PubMed] [Google Scholar]
- McGrath PJ, Stewart JW, Quitkin FM, et al. (2006) Predictors of relapse in a prospective study of fluoxetine treatment of major depression. American Journal of Psychiatry 163(9): 1542–1548. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Rosenblat JD, Nemeroff CB, et al. (2021) Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: An international expert opinion on the available evidence and implementation. American Journal of Psychiatry 178(5): 383–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. (2007) Prevalence and management of treatment-resistant depression. Journal of Clinical Psychiatry 68(8): 17–25. [PubMed] [Google Scholar]
- Posner K, Brent D, Lucas C, et al. (2008) Columbia-suicide severity rating scale (C-SSRS). New York: Columbia University Medical Center. [Google Scholar]
- Reiff CM, Richman EE, Nemeroff CB, et al. (2020) Psychedelics and psychedelic-assisted psychotherapy. American Journal of Psychiatry 177(5): 391–410. [DOI] [PubMed] [Google Scholar]
- Richards D. (2011) Prevalence and clinical course of depression: A review. Clinical Psychology Review 31(7): 1117–1125. [DOI] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, et al. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. Journal of Psychopharmacology 30(12): 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, et al. (2003) The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry 54(5): 573–583. [DOI] [PubMed] [Google Scholar]
- Salloum NC, Fava M, Hock RS, et al. (2020) Time to relapse after a single administration of intravenous ketamine augmentation in unipolar treatment-resistant depression. Journal of Affective Disorders 260: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schak KM, Vande Voort JL, Johnson EK, et al. (2016) Potential risks of poorly monitored ketamine use in depression treatment. American Journal of Psychiatry 173(3): 215–218. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF. (2020) Some comments on psychedelic research. American Journal of Psychiatry 177(5): 368–369. [DOI] [PubMed] [Google Scholar]
- Shalit N, Rehm J, Lev-Ran S. (2019) Epidemiology of hallucinogen use in the US results from the National epidemiologic survey on alcohol and related conditions III. Addictive Behaviors 89: 35–43. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, et al. (2000) Multiple recurrences of major depressive disorder. American Journal of Psychiatry 157(2): 229–233. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, et al. (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. American Journal of Psychiatry 163(1): 28–40. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Martinez JH, Young D, et al. (2013) Severity classification on the Hamilton depression rating scale. Journal of Affective Disorders 150(2): 384–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-jop-10.1177_02698811211073759 for Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: Prospective 12-month follow-up by Natalie Gukasyan, Alan K Davis, Frederick S Barrett, Mary P Cosimano, Nathan D Sepeda, Matthew W Johnson and Roland R Griffiths in Journal of Psychopharmacology