Abstract
Numerous biological mechanisms contribute to outcome after stroke, including brain injury, inflammation, and repair mechanisms. Clinical genetic studies have the potential to discover biological mechanisms affecting stroke recovery in humans and identify intervention targets. Large sample sizes are needed to detect commonly occurring genetic variations related to stroke brain injury and recovery. However, this usually requires combining data from multiple studies where consistent terminology, methodology, and data collection timelines are essential. Our group of expert stroke and rehabilitation clinicians and researchers with knowledge in genetics of stroke recovery here present recommendations for harmonizing phenotype data with focus on measures suitable for multicenter genetic studies of ischemic stroke brain injury and recovery. Our recommendations have been endorsed by the International Stroke Genetics Consortium.
Keywords: Data collection, genetics, ischemic stroke, outcome, phenotype, recovery, standardization
Introduction
Genetic studies can potentially discover biological mechanisms affecting stroke recovery with treatment implications. However, they need large sample sizes only achievable by combining data from multiple studies, where harmonized terminology, methodology, and data collection timelines are essential.
The terms stroke outcome and stroke recovery differ in meaning. Stroke outcome describes the degree of function at specific time points; stroke recovery encompasses the degree of improvement (or deterioration) over time and better captures dynamic biological processes. Stroke recovery evaluation requires initial stroke severity data, without which only stroke outcome is measurable. It is also important to distinguish restitution (“true”) recovery from behavioral compensation. For example, “true” motor recovery suggests restoration of pre-stroke movement patterns 1 whereas “compensation,” implies new (possibly dysfunctional) movement patterns for accomplishing functional tasks. 2
The dynamics of stroke recovery depend on intrinsic and extrinsic factors. 3 Each patient’s recovery pattern uniquely reflects the combined influences of lesion size and location, biological mechanisms of brain repair, comorbidities, pre-morbid health status, and post-stroke factors including acute recanalization, rehabilitation, psychosocial factors, and environmental influences. Consequently, the degree of stroke recovery varies considerably between individuals, and even skilled clinicians have difficulty making accurate recovery predictions. 4
The need for improved predictive models of stroke recovery has become a major research focus5,6 and recent studies suggest that genetic variations influence recovery after stroke.7–9 Despite multiple studies, findings remain heterogeneous, due to differences in populations, recovery metrics, assessment time points, and study designs. Most studies using global assessments incorporate the modified Rankin Scale (mRS) 10 while some use more detailed modality-specific functions, for example, upper extremity (UE) motor function, language or cognitive function,3,11 or patient-reported outcome measures (PROMs). Few studies use repeated measures, leading to knowledge gaps on stroke recovery time course. To standardize timing and metric choices across studies, the Stroke Recovery and Rehabilitation Roundtable taskforce in 2017 recommended core outcomes for trials and standardized measurement time points to reduce heterogeneity. 11
Here, we focus specifically on design of prospective genetic studies of ischemic stroke (IS) recovery, aiming to ascertain the underlying genetic influences on stroke recovery biology. Our recommendations complement existing advise for standardizing phenotype data 12 and biological sample collection 13 for stroke risk and recovery studies11,14 by providing recommendations for pre-specified harmonized data sets suitable for large, high-quality, multi-center collaborations in prospective stroke genetic recovery studies. We propose measures comprehensive enough to provide both stroke- and domain-specific data, but simple enough to allow collection of large sample sizes across numerous and diverse enrollment sites. This will allow opportunities to discover genetic factors influencing hitherto unknown biological pathways affecting the dynamics of IS recovery. We do not here consider intracerebral hemorrhage (ICH) given ICH recovery mechanisms differ from IS.
Methods
Methods for reaching a consensus on these recommendations are described in the Supplement.
Results
Overview of phenotypic variables
We prioritized phenotypic variables into categories: (1) minimum variables—mandatory for all studies, (2) preferred variables— recommended but may be precluded by practical limitations, and (3) optional variables—interesting for some multi-center projects. Table 1 shows the minimum (mandatory) variable set. Supplemental Table 1 lists a detailed comprehensive set. Supplemental Table 2 suggests variable formats to facilitate compilation of joint data. Regularly updated versions will be kept at the Global Alliance for International Stroke Genetics Consortium (ISGC) Acute and Long-term Outcome studies (https://genestroke.wixsite.com/alliesinstroke).
Table 1.
Recommended minimum variable sets for genetic studies of ischemic stroke recovery.
| Evaluation time | Clinical | Stroke clinical | Stroke imaging | Treatment | Functional |
|---|---|---|---|---|---|
| Stroke onset- two days | Pre-stroke/demographics a • Pre-stroke mRS • Charlson Comorbidity Index EP • Age at time of stroke onset • Sex • Race Risk factors at stroke onset a • Hypertension • Atrial fibrillation • Coronary heart disease • Diabetes mellitus • Smoking • Hypercholesterolemia • Previous stroke | • Main stroke type b • TOAST/CCS subtype • Survival LP • Time from stroke to death LP | • Initial CT/MRI examination performed • Time to initial CT/MRI scan LO • CT at 24 h LO • Time to CT 24 h scan LO • Hemorrhagic transformation on 24 h CT scan LO | • Thrombolysis • Thrombectomy | • Initial stroke severity: NIHSS c within 6 h d after hospital presentation (when possible) or just before recanalization therapy • Time from stroke onset to initial NIHSS LP • NIHSS c 24 h after recanalization therapy/24 h after baseline NIHSS, if no recanalization therapy LO • Time from stroke onset to 24 h NIHSS LO |
| Seven days/ discharge | As above, if not already done | • Survival • Time from stroke to death LP | • NIHSS c at seven days or at discharge, if earlier EP • Time from stroke onset to NIHSS at seven days/discharge EP | ||
| Three months | • Time of evaluation EO • Survival EO • Recurrent stroke EO | • NIHSSc,EO • mRS EO | |||
| 12 months, yearly thereafter | • Time of evaluation EO • Survival EO • Recurrent stroke EO | • NIHSSc,EO • mRS EO |
Listed variables are recommended as minimum for Early Phase Studies (with focus on 0–48 h and seven days/hospital discharge) and Later Phase Studies (with focus on three months and beyond), unless otherwise specified. A comprehensive outline of all suggested minimum, preferred, and optional variables are shown in Supplemental Table 1. Time of evaluation after stroke onset (hours for up to 72 h; days thereafter) should be registered.
mRS: modified Rankin Scale; IS: ischemic stroke; ICH: intracerebral haemorrhage; h: hours; NIHSS: National Institutes of Health Stroke Scale; CT: computed tomography; MRI: magnetic resonance imaging; TOAST: Trial of ORG 10172 in Acute Stroke Treatment; CCS: Causative Classification of Stroke.
Can often be collected somewhat later.
Only IS in this manuscript.
Including individual subitems.
For Later Phase studies: NIHSS within 6 h = preferred, NIHSS within 24 h = minimum.
Early Phase studies, preferred.
Later Phase studies, preferred.
Early Phase studies, optional.
Later Phase studies, optional.
Timing of recovery assessment
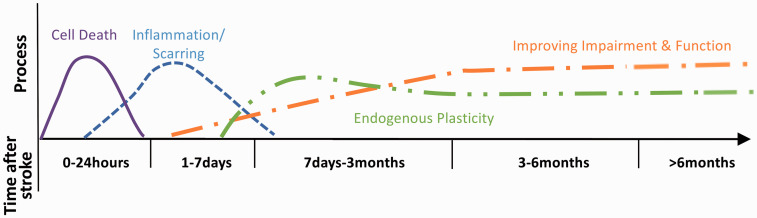
Stroke recovery starts immediately at symptom onset and continues for years thereafter (Figure 1). Blood biomarkers, and other biomarker evaluations, for example, magnetic resonance imaging (MRI), often vary across time points. To provide simplification, we recommend the time course for assessment of evolution and recovery into three phases post-stroke (Day 0 is day of stroke onset): 0 to 24–48 h, seven days, and approximately Day 90 after stroke onset and when possible one year and later. When appropriate, studies may choose additional precisely defined time periods.
Figure 1.
Framework showing time points post stroke related to current known biology of stroke recovery. Time post stroke should always be included in data acquisition. Adapted to represent ischaemic stroke only, from: Bernhardt J et al, Int J Stroke 2017, Vol. 12(5) 444–450, copyright © 2017 by World Stroke Organization. Reprinted by permission of SAGE Publications, Ltd.
Studies of hyperacute recovery and therapy should perform evaluations within 6 h (when possible) or at least within 24 h after stroke onset and before revascularization therapy, followed by a new evaluation at 24 h post stroke 15 or 24 h after recanalization therapy (see below).
Seven days post-stroke is often recommended for evaluation. 1 However, because many patients leave hospital earlier, we recommend evaluation either at seven days or discharge, whichever occurs earlier.
IS studies often conclude evaluations at three months.16,17 However, improvement may occur at 6–12 months and possibly beyond. 18 Recovery is not linear, and time frames may vary by different domains, for example, cognitive versus motor function. 19 To evaluate three-month recovery independently of early acute phases, sometimes influenced by treatments, for example, revascularization, we recommend measuring recovery as functional change between Day 7 (or discharge), and three months. If possible, additional evaluations at one and three years are strongly recommended to evaluate longer term recovery.
Recommended phenotypic variables
Pre-stroke variables, demographic data
Pre-stroke functional status affects stroke outcome and should be measured as mRS, ideally specifying whether due to a stroke preceding the index stroke versus other conditions. We also recommend the Charlson Comorbidity Index, 20 with information about pre-existing key medical conditions. For further details, see Table 1 and Supplemental Table 1.
All studies should provide demographics: age at stroke onset, sex, race/ethnicity, residential area type (urban/rural), educational status, living situation (housing type), and available social support (living alone/with someone). 21
Baseline clinical and imaging information
Baseline characteristics of current IS should include initial National Institutes of Health Stroke Scale (NIHSS) total and individual component scores, and Trial of ORG 10172 in Acute Stroke Treatment 22 and/or Causative Classification of Stroke subtype. 23 Specific “other determined” stroke etiologies (e.g., cervical artery dissection) could be detailed. Laboratory parameters and Glasgow Coma Scale may be recorded.
We recommend baseline registration of head computed tomography/magnetic resonance (CT/MRI), and CT/MRI angiography and perfusion, because, for example, dynamic blood flow changes may be related to genetic influences. 24
Stroke treatment and neuroimaging at 0–48 h and seven days/hospital discharge
Treatment with thrombolysis and thrombectomy should be noted. Final expanded thrombolysis in cerebral infarction (TICI) score 25 indicating degree of revascularization achieved should be mandated in large vessel occlusion stroke studies. Additional treatments possibly affecting recovery include carotid endarterectomy/stenting and pharmacologic interventions for blood pressure, dyslipidemia, or atrial fibrillation.
Follow-up imaging at 24 h after recanalization therapy with CT/MRI is valuable to evaluate location and extent of the acute ischemic lesion(s). When possible, MRI with FLAIR, DWI, MRI angiography, and GRE/T2*/SWI is recommended within 24 h (or within seven days at the latest) after stroke onset. MRI performed later might also have value. Imaging measures of leukoaraiosis, microhemorrhages, prior infarcts, and arterial stenoses could be considered. Injury extent to specific neural structures, such as corticospinal tract, may be useful for some hypotheses.
Neuroimaging biomarkers can serve as endophenotypes. For examples, please see Supplement.
Clinical measures at 0–48 h and seven days/hospital discharge
In the first days after stroke, neurological deficits can be highly unstable, with patients rapidly improving, or deteriorating. Serial NIHSS scores, 26 often standard of care in acute stroke, capture these changes. A change in NIHSS between baseline (<6 h from onset) and 24 h (ΔNIHSS6–24 h) is related to 90-day outcome independent of baseline NIHSS 27 with a genome wide association study (GWAS) of ΔNIHSS6–24 h having revealed genes potentially involved in ischemic brain injury (data not shown). We therefore recommend NIHSS (including subitems) at baseline <6 h or at least within 24 h after stroke onset, and short-term follow-up at 24 h after stroke onset/after recanalization therapy, noting number of hours since stroke onset.
Recovery during the initial days after stroke onset is difficult to measure, and we recommend evaluations including NIHSS (with subitems) either at seven days or discharge from hospital, whichever occurs earlier.
The Shoulder Abduction Finger Extension score during the first three days after stroke predicts upper limb motor outcome. 28 This complements the NIHSS and is useful as an early marker, easier to assess than more complex motor assessments such as the Fugl-Meyer (FM) or Action Research Arm Test (ARAT).
Gait performance measured as walking speed predicts walking recovery and falls risk. Gait is also linked with quality of life and participation level, and testing does not require much time. On Day 7, we recommend recording the ability to walk 10 m independently (yes/no), and for those able, a 10 -m walk test. This may be repeated at later time points (see below).
Early complications such as infections and recurrent stroke may also influence recovery and should be considered.
Considerations and treatment information up to three months and beyond
Stroke recurrence, with a 30%–40% cumulative risk among first stroke survivors, is a common cause of worsening disability and requires tracking.29,30 Secondary prevention measures, complications (e.g., depression, infections, seizures, fractures after falls), level of physical activity, and socioeconomic factors may substantially affect outcome and recovery. At the designing stage, studies should define which of these variables to collect as confounding factors, exclusion criteria, or endpoint/dependent variables.
Rehabilitation treatment is heterogeneous across centers and difficult to uniformly register. We suggest registering how often the treatment is administered per week or month and duration of rehabilitation in days. The starting day after stroke onset and treatment dose (minutes per day) may be recorded.
Treatment with antidepressants and other psychotropic medication 31 should be noted as should other rehabilitation adjuncts, whether pharmacologic or device-based (e.g., transcranial magnetic stimulation).
Evaluation at three months and beyond
Factors influencing long-term recovery (improvement/deterioration) may differ from those important in earlier time periods. As mentioned above, we recommend evaluation at Day 7, or discharge (if earlier) as a new baseline for long-term recovery at three months.
Stroke variably affects different functional domains. 32 We recommend that specific domains are considered separately and only in more detail where appropriate. For example, if a motor deficit is detected on the NIHSS, more in-depth motor testing can be performed (Figure 2 and Supplemental Table 1). In this way, the NIHSS subitems provide screening for deficits requiring more detailed evaluation, saving time, and resources.
Figure 2.
Suggested domain-specific screening by using NIHSS. Detected deficits are subsequently assessed with more detailed evaluations. NIHSS: National Institutes of Health Stroke Scale.
Evaluation of specific recovery domains
Motor function
Motor deficits are seen in >80% of IS 33 and can be screened by NIHSS items 5 and 6. A more detailed assessment of motor deficit changes over time is of great importance to evaluate recovery. The FM-UE motor scale 34 is well known and recommended but requires trained personnel. 35 The FM lower extremity motor scale may be considered, 34 but limited reproducibility, a high concordance with UE weakness, and overlapping recovery mechanisms may limit its value. UE motor function is best captured with ARAT, but this requires equipment. 11
Gait velocity (see above) is also useful for long-term motor function evaluation.
Sensory function
The FM Sensory Examination or the Nottingham Sensory Scale could be considered.
Cognitive function
Combining the four NIHSS items, Orientation (1b), Executive function (1c), Language (9), and Inattention (11), has similar value as the Mini-Mental State Examination in detecting severe cognitive impairment. 36 A more elaborate cognitive evaluation with the Montreal Cognitive Assessment Scale 37 is recommended when possible. When even more detailed or longitudinal understanding of specific cognitive domains is needed, an in-depth neuropsychological assessment may encompass multiple cognitive domains, especially verbal episodic memory, executive function, and processing speed. Pre-stroke cognitive assessment with tools such as the informant questionnaire on cognitive decline in the elderly (IQCODE) 38 is important, as pre-stroke cognitive impairment is frequent and associated with post-stroke dementia. 39 The genetics of post-stroke cognitive impairment are not covered here but addressed in separate working groups of the ISGC (www.strokegenetics.org) and the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium.
Speech function
NIHSS item 9 provides a screening tool for aphasia. Aphasia evaluations are hampered by language differences between populations. We favor the Western Aphasia Battery-Revised version bedside screening test, which takes 10–15 min and is well-accepted by researchers. 40 Language evaluation items in cognitive tests are also a possibility.
Neglect
NIHSS item 11 provides a screening tool for neglect and hemi-inattention. Among the many available bedside assessments, the Star cancellation test is recommended.
Mood
The Hospital Anxiety and Depression Scale 41 had most consensus in our group for utility across different time points. Alternatives have been recommended by others. 42
Other specific domains
Post-stroke visual field loss, eye movement abnormalities, dysphagia, balance disorders, fatigue, frailty, and urinary incontinence are all important aspects of post-stroke recovery. We agreed that no specific recommendations can be made for these domains at this time but provide some suggestions in the Supplement.
Global assessment
The three-month mRS is used in most stroke trials and should be performed in studies of stroke recovery genetics to facilitate comparison across cohorts. Evaluation of mRS at other time points (e.g., 6 months, 12 months, and yearly thereafter) may be useful. The mRS offers advantages of ease of administration, good inter-observer reproducibility, certification, and available phone-based evaluation.10,43. The mRS score has been analyzed both as a continuous and an ordinal variable,44,45 but dichotomization may lose information and statistical power.
Other functional scales, such as Barthel Index and the Nottingham extended activities of daily living scale (ADL), have limitations such as ceiling effects or rarer usage.
Patient-reported outcome measures
Outcome and recovery evaluations important to clinicians are not always congruent with those of patients. When possible, PROMs should be included in recovery studies to support the validity of other measures and reflect meaningful stroke outcomes and recovery. PROMs can assess disability, mood, cognitive function, pain, mobility, and fatigue. The Patient-Reported Outcome Measurement Information System, 36-Item Short Form Survey, EuroQuality of life 5 dimension questionnaire (EQ-5D), and Stroke Impact Scale are frequently used PROMs. 46
Combining dynamic changes from different domains
Genetic correlates of recovery mechanisms may influence several functional domains. Combined measurements across domains can be obtained by quantification of the domain with greatest impairment in individual subjects (defined as the system with the worst baseline subscore from the baseline NIHSS), and computing the percentage of the maximum possible score for this domain followed by comparing these measures on Day 7 and Day 90. Recovery is calculated as the remaining deficit (% recovery = 100 × (1−(ScoreMax − Scored90)/(ScoreMax − Scored7))) for each subject. 47
Neuroimaging
Neuroimaging after stroke can detect new infarcts, hemorrhages, and small vessel disease including white matter changes and brain atrophy. For these purposes, MRI including FLAIR and GRE/T2*/SWI sequences could be considered at three months, one year, and later.
Several other forms of neuroimaging and associated methods have been examined in relation to genetic variation, for examples—please see Supplement.
Discussion
We here recommend a specific set of phenotype outcome variables, time frames, and covariates for prospective genetic studies of recovery after IS. To detect changes in the patient-specific evolution of symptoms the same variables should, when possible, be measured at the different time points.
Our suggested time points for evaluations and the assessments categorized as minimum, preferred, or optional can be useful tools for individual studies, comparative studies, and multi-center studies on stroke recovery genetics. Of the large number of potential evaluation tools available for assessment of IS recovery, we suggested tools that should be simple and accessible while detailed enough to capture dynamic changes in the designated domains.
Physical follow-up examinations after the acute phase of stroke are labour intensive. Patient telephone interviews may be an alternative. Live examinations permit detailed determination of many neurological features but come at a higher price such as cost and travel. Phone and video-based examinations are less expensive, but more limited in the data that can be reliably measured. Given the focus of the current recommendations, we advise live examinations for studies focusing on recovery at 90 days and beyond to be performed whenever resources permit.
We stress the use of NIHSS, including its subscores, for screening because it is already widely utilized in clinical routine, clinical trials, and recovery studies. More elaborate evaluations focusing on specific domains can be complementary, as can combined measures such as the predict recovery potential 2 algorithm evaluating clinical function, MRI, and surrogate parameters to predict three-month UE motor function. 28 Other clinical evaluations to predict recovery such as sitting balance for independent walking and ability to comprehend and repeat spoken language are uncommonly standardized and systematically investigated and may currently have less value for genetic studies of stroke recovery. Increasing importance is being placed on PROMs to ensure that recovery measured using tools based on neurological impairment is meaningful from the patient’s perspective, although the role of PROMs in stroke genetics research has not been established.
Training, certification, and recertification are essential to reduce error and inter-rater variance. A plan for training, certification, and recertification for each behavioral scale should be a part of every stroke recovery study or trial.
Statistical considerations are important. Many scales are ordinal and non-linear. An improvement in the NIHSS of 10 points, for instance, may signify different degrees of improvement when a patient improves from 20 to 10 versus from 10 to 0. Additional concerns regarding repeated measurements include regression to the mean and management of missing data. Analyses must consider collinearity when employing the same variable to calculate both the independent and the dependent variables to avoid misinterpretation of paired observations when comparing baseline scores with follow-up results. 48 Analyses combining different domains may be considered for detecting genetic influence on general stroke recovery.
Conclusions
The rapid progress of genetic research methodologies provides excellent opportunities to discover new factors influencing stroke recovery. However, to obtain optimal efficiency, harmonized and well-accepted phenotyping instruments across studies are required. We suggest selected evaluations of stroke recovery to measure important recovery domains. Harmonization of these evaluations between studies will allow performance of large prospective studies of genetic influence on recovery dynamics in the early and later phases after stroke.
Supplemental Material
Supplemental material, sj-pdf-1-wso-10.1177_17474930211007288 for International stroke genetics consortium recommendations for studies of genetics of stroke outcome and recovery by Arne G Lindgren, Robynne G Braun, Jennifer Juhl Majersik, Philip Clatworthy, Shraddha Mainali, Colin P Derdeyn, Jane Maguire, Christina Jern, Jonathan Rosand, John W Cole, Jin-Moo Lee, Pooja Khatri, Paul Nyquist, Stéphanie Debette, Loo Keat Wei, Tatjana Rundek, Dana Leifer, Vincent Thijs, Robin Lemmens, Laura Heitsch, Kameshwar Prasad, Jordi Jimenez Conde, Martin Dichgans, Natalia S Rost, Steven C Cramer, Julie Bernhardt, Bradford B Worrall, Israel Fernandez-Cadenas and International Stroke Genetics Consortium in International Journal of Stroke
Supplemental material, sj-pdf-2-wso-10.1177_17474930211007288 for International stroke genetics consortium recommendations for studies of genetics of stroke outcome and recovery by Arne G Lindgren, Robynne G Braun, Jennifer Juhl Majersik, Philip Clatworthy, Shraddha Mainali, Colin P Derdeyn, Jane Maguire, Christina Jern, Jonathan Rosand, John W Cole, Jin-Moo Lee, Pooja Khatri, Paul Nyquist, Stéphanie Debette, Loo Keat Wei, Tatjana Rundek, Dana Leifer, Vincent Thijs, Robin Lemmens, Laura Heitsch, Kameshwar Prasad, Jordi Jimenez Conde, Martin Dichgans, Natalia S Rost, Steven C Cramer, Julie Bernhardt, Bradford B Worrall, Israel Fernandez-Cadenas and International Stroke Genetics Consortium in International Journal of Stroke
Supplemental material, sj-pdf-3-wso-10.1177_17474930211007288 for International stroke genetics consortium recommendations for studies of genetics of stroke outcome and recovery by Arne G Lindgren, Robynne G Braun, Jennifer Juhl Majersik, Philip Clatworthy, Shraddha Mainali, Colin P Derdeyn, Jane Maguire, Christina Jern, Jonathan Rosand, John W Cole, Jin-Moo Lee, Pooja Khatri, Paul Nyquist, Stéphanie Debette, Loo Keat Wei, Tatjana Rundek, Dana Leifer, Vincent Thijs, Robin Lemmens, Laura Heitsch, Kameshwar Prasad, Jordi Jimenez Conde, Martin Dichgans, Natalia S Rost, Steven C Cramer, Julie Bernhardt, Bradford B Worrall, Israel Fernandez-Cadenas and International Stroke Genetics Consortium in International Journal of Stroke
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Apart from what is mentioned under Disclosures in the Supplement, there is no other potential conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institute of Child Health and Human Development (K12HD093427); Lund University; National Institute of Neurological Disorders and Stroke (K23NS099487-01, R01-NS082285, R01-NS086905, U19-NS115388 and 5U10NS086606); National Institutes of Health (KL2TR003016, R01-NS085419, R01-NS100178, R01-NS105150, R01-NS114045, R21-NS106480, U24-NS107230, U24-NS107237 and U24-NS107222); Maestro Project and Ibiostroke project funded by Eranet-Neuron, ISCIII and FEDER; CaNVAS project funded by National Institutes of Health; Department of Biotechnology, Ministry of Science and Technology, India; Skåne University Hospital; Region Skåne; The Swedish Heart and Lung Foundation; and The Swedish Research Council (2018–02543 and 2019–01757); The Swedish Government (under the “Avtal om Läkarutbildning och Medicinsk Forskning, ALF”); Sparbanksstiftelsen Färs och Frosta; Freemason's Lodge of Instruction Eos, Lund; FWO Flanders (1841918N); US Department of Veteran Affairs, the American Heart Association (15GPSG23770000, 17IBDG3300328).
Supplementary material: Supplementary material is available for this article online.
ORCID iDs
Arne G Lindgren https://orcid.org/0000-0003-1942-7330
Paul Nyquist https://orcid.org/0000-0001-6078-3543
Vincent Thijs https://orcid.org/0000-0002-6614-8417
Julie Bernhardt https://orcid.org/0000-0002-2787-8484
References
- 1.Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke 2017; 12: 444–450. [DOI] [PubMed] [Google Scholar]
- 2.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair 2009; 23: 313–319. [DOI] [PubMed] [Google Scholar]
- 3.Lindgren A, Maguire J. Stroke recovery genetics. Stroke 2016; 47: 2427–2434. [DOI] [PubMed] [Google Scholar]
- 4.Nijland RH, van Wegen EE, Harmeling-van der Wel BC, Kwakkel G. Accuracy of physical therapists’ early predictions of upper-limb function in hospital stroke units: the EPOS study. Phys Ther 2013; 93: 460–469. [DOI] [PubMed] [Google Scholar]
- 5.Stinear CM. Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol 2017; 16: 826–836. [DOI] [PubMed] [Google Scholar]
- 6.Carrera C, Cullell N, Torres-Aguila N, et al. Validation of a clinical-genetics score to predict hemorrhagic transformations after rtPA. Neurology 2019; 93: e851–e863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeiffer D, Chen B, Schlicht K, et al. Genetic imbalance is associated with functional outcome after ischemic stroke. Stroke 2019; 50: 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Söderholm M, Pedersen A, Lorentzen E, et al. Genome-wide association meta-analysis of functional outcome after ischemic stroke. Neurology 2019; 92: e1271–e1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mola-Caminal M, Carrera C, Soriano-Tarraga C, et al. PATJ low frequency variants are associated with worse ischemic stroke functional outcome. Circ Res 2019; 124: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinohara Y, Minematsu K, Amano T, Ohashi Y. Modified Rankin Scale with expanded guidance scheme and interview questionnaire: Interrater agreement and reproducibility of assessment. Cerebrovasc Dis 2006; 21: 271–278. [DOI] [PubMed] [Google Scholar]
- 11.Kwakkel G, Lannin NA, Borschmann K, et al. Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Int J Stroke 2017; 12: 451–461. [DOI] [PubMed] [Google Scholar]
- 12.Majersik JJ, Cole JW, Golledge J, et al. Recommendations from the international stroke genetics consortium, Part 1: Standardized phenotypic data collection. Stroke 2015; 46: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battey TW, Valant V, Kassis SB, et al. Recommendations from the international stroke genetics consortium, Part 2: Biological sample collection and storage. Stroke 2015; 46: 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd LA, Hayward KS, Ward NS, et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017; 12: 480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres-Aguila NP, Carrera C, Muino E, et al. Clinical variables and genetic risk factors associated with the acute outcome of ischemic stroke: A systematic review. J Stroke 2019; 21: 276–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen stroke study. Arch Phys Med Rehabil 1995; 76: 406–412. [DOI] [PubMed] [Google Scholar]
- 17.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke 1992; 23: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 18.Cortes JC, Goldsmith J, Harran MD, et al. A short and distinct time window for recovery of arm motor control early after stroke revealed with a global measure of trajectory kinematics. Neurorehabil Neural Repair 2017; 31: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballester BR, Maier M, Duff A, et al. A critical time window for recovery extends beyond one-year post-stroke. J Neurophysiol 2019; 122: 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 21.Counsell C, Dennis M, McDowall M. Predicting functional outcome in acute stroke: comparison of a simple six variable model with other predictive systems and informal clinical prediction. J Neurol Neurosurg Psychiatry 2004; 75: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of ORG 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 23.Ay H, Benner T, Arsava EM, et al. A computerized algorithm for etiologic classification of ischemic stroke: The Causative Classification of Stroke system. Stroke 2007; 38: 2979–2984. [DOI] [PubMed] [Google Scholar]
- 24.Lucitti JL, Sealock R, Buckley BK, et al. Variants of Rab GTPase-Effector Binding Protein-2 cause variation in the collateral circulation and severity of stroke. Stroke 2016; 47: 3022–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg 2019; 11: 433–438. [DOI] [PubMed] [Google Scholar]
- 26.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989; 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 27.Heitsch L, Ibanez L, Carrera C, et al. Early neurological change after ischemic stroke is associated with 90-day outcome. Stroke 2021; 52: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stinear CM, Byblow WD, Ackerley SJ, Smith M–C, Borges VM, Barber PA. REP2: A biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol 2017; 4: 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire community stroke project. Stroke 1994; 25: 333–337. [DOI] [PubMed] [Google Scholar]
- 30.Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth community stroke study. Stroke 2004; 35: 731–735. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein LB. Common drugs may influence motor recovery after stroke. The Sygen in acute stroke study investigators. Neurology 1995; 45: 865–871. [DOI] [PubMed] [Google Scholar]
- 32.Cramer SC, Koroshetz WJ, Finklestein SP. The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke 2007; 38: 1393–1395. [DOI] [PubMed] [Google Scholar]
- 33.Rathore SS, Hinn AR, Cooper LS, Tyroler HA, Rosamond WD. Characterization of incident stroke signs and symptoms: Findings from the atherosclerosis risk in communities study. Stroke 2002; 33: 2718–2721. [DOI] [PubMed] [Google Scholar]
- 34.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31. [PubMed] [Google Scholar]
- 35.See J, Dodakian L, Chou C, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair 2013; 27: 732–741. [DOI] [PubMed] [Google Scholar]
- 36.Cumming TB, Blomstrand C, Bernhardt J, Linden T. The NIH stroke scale can establish cognitive function after stroke. Cerebrovasc Dis 2010; 30: 7–14. [DOI] [PubMed] [Google Scholar]
- 37.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 38.Jorm AF. The informant questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr 2004; 16: 275–293. [DOI] [PubMed] [Google Scholar]
- 39.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 40.Wallace SJ, Worrall L, Rose T, et al. A core outcome set for aphasia treatment research: The ROMA consensus statement. Int J Stroke 2019; 14: 180–185. [DOI] [PubMed] [Google Scholar]
- 41.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 42.Towfighi A, Ovbiagele B, El Husseini N, et al. Poststroke depression: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e30–e43. [DOI] [PubMed] [Google Scholar]
- 43.Weimar C, Kurth T, Kraywinkel K, et al. Assessment of functioning and disability after ischemic stroke. Stroke 2002; 33: 2053–2059. [DOI] [PubMed] [Google Scholar]
- 44.Nunn A, Bath PM, Gray LJ. Analysis of the modified rankin scale in randomised controlled trials of acute ischaemic stroke: A systematic review. Stroke Res Treat 2016; 2016: 9482876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhemtulla M, Brosseau-Liard PE, Savalei V. When can categorical variables be treated as continuous? A comparison of robust continuous and categorical SEM estimation methods under suboptimal conditions. Psychol Methods 2012; 17: 354–373. [DOI] [PubMed] [Google Scholar]
- 46.Salinas J, Sprinkhuizen SM, Ackerson T, et al. An international standard set of patient-centered outcome measures after stroke. Stroke 2016; 47: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liew SL, Zavaliangos-Petropulu A, Jahanshad N, et al. The ENIGMA stroke recovery working group: Big data neuroimaging to study brain-behavior relationships after stroke. Hum Brain Mapp 2020. DOI:10.1002/hbm.25015. [DOI] [PMC free article] [PubMed]
- 48.Hope TMH, Friston K, Price CJ, Leff AP, Rotshtein P, Bowman H. Recovery after stroke: not so proportional after all? Brain 2019; 142: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-wso-10.1177_17474930211007288 for International stroke genetics consortium recommendations for studies of genetics of stroke outcome and recovery by Arne G Lindgren, Robynne G Braun, Jennifer Juhl Majersik, Philip Clatworthy, Shraddha Mainali, Colin P Derdeyn, Jane Maguire, Christina Jern, Jonathan Rosand, John W Cole, Jin-Moo Lee, Pooja Khatri, Paul Nyquist, Stéphanie Debette, Loo Keat Wei, Tatjana Rundek, Dana Leifer, Vincent Thijs, Robin Lemmens, Laura Heitsch, Kameshwar Prasad, Jordi Jimenez Conde, Martin Dichgans, Natalia S Rost, Steven C Cramer, Julie Bernhardt, Bradford B Worrall, Israel Fernandez-Cadenas and International Stroke Genetics Consortium in International Journal of Stroke
Supplemental material, sj-pdf-2-wso-10.1177_17474930211007288 for International stroke genetics consortium recommendations for studies of genetics of stroke outcome and recovery by Arne G Lindgren, Robynne G Braun, Jennifer Juhl Majersik, Philip Clatworthy, Shraddha Mainali, Colin P Derdeyn, Jane Maguire, Christina Jern, Jonathan Rosand, John W Cole, Jin-Moo Lee, Pooja Khatri, Paul Nyquist, Stéphanie Debette, Loo Keat Wei, Tatjana Rundek, Dana Leifer, Vincent Thijs, Robin Lemmens, Laura Heitsch, Kameshwar Prasad, Jordi Jimenez Conde, Martin Dichgans, Natalia S Rost, Steven C Cramer, Julie Bernhardt, Bradford B Worrall, Israel Fernandez-Cadenas and International Stroke Genetics Consortium in International Journal of Stroke
Supplemental material, sj-pdf-3-wso-10.1177_17474930211007288 for International stroke genetics consortium recommendations for studies of genetics of stroke outcome and recovery by Arne G Lindgren, Robynne G Braun, Jennifer Juhl Majersik, Philip Clatworthy, Shraddha Mainali, Colin P Derdeyn, Jane Maguire, Christina Jern, Jonathan Rosand, John W Cole, Jin-Moo Lee, Pooja Khatri, Paul Nyquist, Stéphanie Debette, Loo Keat Wei, Tatjana Rundek, Dana Leifer, Vincent Thijs, Robin Lemmens, Laura Heitsch, Kameshwar Prasad, Jordi Jimenez Conde, Martin Dichgans, Natalia S Rost, Steven C Cramer, Julie Bernhardt, Bradford B Worrall, Israel Fernandez-Cadenas and International Stroke Genetics Consortium in International Journal of Stroke