Abstract
Serology remains the method of choice for laboratory diagnosis of Mycoplasma pneumoniae infection. Currently available serological tests employ complex cellular fractions of M. pneumoniae as antigen. To improve the specificity of M. pneumoniae diagnosis, a recombinant protein was assessed as a serodiagnostic reagent. A panel of recombinant proteins were expressed from a cloned M. pneumoniae gene that encodes a 116-kDa surface protein antigen. The recombinant proteins were assessed for reactivity with patient sera and the most antigenic was further assessed for its serodiagnostic potential by indirect enzyme-linked immunosorbent assay (ELISA). The ELISA based on the recombinant protein was equivalent in sensitivity to the commercial test (Serodia Myco II; Fujirebio Inc.) to which it was compared. Southern and Western blotting data suggested that the recombinant protein derived from the 116-kDa protein of M. pneumoniae could provide a species-specific diagnostic tool, although further assessment is required.
Laboratory tests are important in the diagnosis of Mycoplasma pneumoniae infection, as it is difficult to differentiate, by clinical signs, between the etiological agents responsible for primary atypical pneumonia. Serological diagnosis of M. pneumoniae infection is commonly performed by the complement fixation test (15, 30, 33), but it is a time-consuming assay with low specificity (27, 30) and lower sensitivity than immunofluorescence or enzyme-linked immunosorbent assay (ELISA) (24, 30). Diagnosis using PCR is sensitive but expensive, requires specialized equipment, and is prone to false-positive results from cross-contamination of samples and prolonged carriage of M. pneumoniae (23, 28, 34). Other methods of direct detection are less sensitive than serology (19, 23).
An ELISA employing a purified recombinant protein encoded by an M. pneumoniae gene as antigen would have the inherent advantage of improved specificity over existing commercial serological tests, which employ complex cellular fractions of M. pneumoniae (7, 18). The P1 protein is the only purified M. pneumoniae protein to have been used in serological diagnosis (3, 18). The cross-reactivity of the P1 protein with both the Mycoplasma genitalium MgPa protein and eukaryotic proteins has been circumvented by the use of species-specific, P1-derived, synthetic octapeptides as antigen (15). However, in comparison to a large recombinant protein antigen, the use of short synthetic peptides as antigen reduces the number of epitopes available for binding and may thus reduce the sensitivity of the test. In addition, the expense of producing synthetic peptides for use as antigen may prohibit commercial development.
A 116-kDa protein of M. pneumoniae was recently characterized as an antigenic surface protein, and its gene was identified (accession no. Z71425) and compared to its homologue (MG075) in M. genitalium (9). The aims of this work were to identify the immunodominant region of this protein and to assess a purified recombinant protein including this region as an ELISA antigen for the detection of immunoglobulin G (IgG) to determine whether the single recombinant protein has potential as a serodiagnostic antigen.
MATERIALS AND METHODS
Bacterial strains.
M. pneumoniae FH (ATCC 15531) was donated by Vicki Peters, Department of Virology, Royal Children’s Hospital, Melbourne, Victoria, Australia. M. pneumoniae M129-B7 (ATCC 29342), M. pneumoniae PI1428 (ATCC 29085), M. genitalium G37 (ATCC 33530), M. pirum HRC/70-159 (ATCC 25960), and M. penetrans GTU-54-6AI (ATCC 55252) were obtained from the American Type Culture Collection.
Other mycoplasma strains used were M. gallisepticum S6 (39), M. imitans 4229T (2), and M. iowae DJA (supplied by S. D. Levisohn, Kimron, Israel). Escherichia coli DH5α was obtained from Gibco BRL.
M. pneumoniae, M. genitalium, and M. penetrans were grown in modified SP4 medium (37). M. pirum was grown in ATCC medium 243 (American Type Culture Collection, Manassas, Va.). M. gallisepticum, M. imitans, and M. iowae were grown in mycoplasma broth (38). Mycoplasma cells were harvested from broth by centrifugation and resuspended in phosphate-buffered saline (PBS) (9). E. coli cells were grown in SOC medium and on Luria-Bertani medium with agar (29).
DNA extraction and Southern blotting.
M. pneumoniae, M. penetrans, and M. pirum DNAs were extracted by the method of Su et al. (31). DNA was extracted from M. genitalium by the method of Peterson et al. (26). M. gallisepticum, M. iowae, and M. imitans DNA were prepared by the method of Kleven et al. (20). Southern blots of BglII- and EcoRI-digested DNAs from the three M. pneumoniae strains, M. genitalium, M. pirum, M. penetrans, M. gallisepticum, M. imitans, and M. iowae were probed with [α-32P]dCTP-labelled fragments generated from plasmid pGEX-1N-MP3, a 730-bp region which is relatively conserved between the 116-kDa gene of M. pneumoniae and MG075 of M. genitalium by randomly primed labelling (Boehringer Mannheim). The region of the gene encoding the 116-kDa protein of M. pneumoniae with the greatest identity (96%) to its M. genitalium homologue, MG075, was used to design oligonucleotide 116kcon (ATAAACTCCAAGGTGAGTTTGATAA). This oligonucleotide spans bases 284 to 307 of the 116-kDa protein gene and bases 287 to 310 of MG075. This oligonucleotide was also used to probe Southern blots following labelling by 3′ phosphorylation using polynucleotide kinase (Promega) and [γ-32P]ATP. The blots were hybridized with the probes at 50°C overnight. The blot hybridized with the pGEX-1N-MP3-derived probe was washed three times for 60 min each time at 60°C in 1× SSC (0.15 M NaCl plus 0.015 sodium citrate)–0.1% sodium dodecyl sulfate (SDS). The blot hybridized with oligonucleotide 116kcon was rinsed twice at room temperature and then washed twice at 50°C for 10 min each time with 6× SSC–0.5% SDS. Following initial autoradiographic exposure, this blot was washed twice again, but at 60°C for 10 min each time, with 6× SSC–0.5% SDS.
SDS-PAGE and Western blotting.
Proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene difluoride membrane (Immobilon P; Millipore), and probed by a method adapted for M. pneumoniae (17). The immunoaffinity-purified polyclonal rabbit antibodies raised to both the 116-kDa protein of M. pneumoniae and glutathione S-transferase (GST) have been described previously (9). Bound human IgG was detected with rabbit anti-human γ chain conjugated to horseradish peroxidase (HRP; Dako) at a dilution of 1/800. The variable region of glycoprotein G of equine herpesvirus type 1, expressed from the pGEX expression vector as a fusion with GST and purified by glutathione affinity chromatography (4), was used as a negative control antigen.
Human sera.
Twenty-four single serum samples and 30 paired serum samples were obtained from individuals who had a suspected M. pneumoniae infection. All serum samples were tested for antibodies to M. pneumoniae by using a gelatin particle agglutination assay with undefined membrane components of M. pneumoniae Mac as antigen (Serodia Myco II; Fujirebio Inc.).
Serum samples from 29 of these individuals (including five sets of paired serum sample) were assessed for IgG reactivity with recombinant proteins by Western blotting. Of these, 27 had been found to contain antibodies against M. pneumoniae by agglutination in accordance with the manufacturer’s guidelines.
The 30 paired serum samples were assessed by indirect ELISA for IgG reactive with a recombinant protein that was encoded by a region of the gene encoding the 116-kDa protein of M. pneumoniae. Cryoproteins and insoluble lipid were removed from all sera by centrifugation at 10000 × g for 60 min at 4°C prior to use in the ELISA (12).
Expression of regions of the 116-kDa protein.
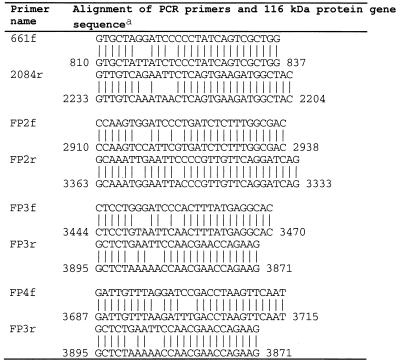
The generation of recombinant plasmids pGEX-1N-MP3 and pGEX-1N-MP10 has been described previously (9). Regions of the gene for the 116-kDa protein not contained in these two plasmids were amplified by PCR using the primer pairs shown in Table 1. PCR products were purified by electrophoresis in agarose gels, extraction from gel slices with the Wizard DNA Clean-Up System (Promega), and then ligated to the pGEM-T vector (Promega). The ligated plasmids were used to transform competent E. coli DH5α cells. Each cloned PCR product was excised from pGEM-T by using BamHI and EcoRI, purified by agarose gel electrophoresis as described above, and ligated to pGEX-3X (Pharmacia) digested with BamHI and EcoRI. The ligated plasmids were used to transform E. coli DH5α. The identity of the cloned DNA was confirmed by DNA sequencing using Deaza G/A T7Sequencing Mixes (Pharmacia).
TABLE 1.
Oligonucleotides used to amplify expressed PCR products
Nucleotide numbering corresponds to that of the operon encoding the 16- and 116-kDa proteins (accession no. Z71425).
E. coli transformed with recombinant plasmids pGEX-3X-MP661, pGEX-3X-MPFP2, pGEX-3X-MPFP3, and pGEX-3X-MPFP4, which contained cloned PCR products, and E. coli transformed with recombinant plasmids pGEX-1N-MP3 and pGEX-1N-MP10 were incubated for 2 h in the presence of 2 mM isopropyl-β-d-thiogalactopyranoside. Expression of fusion proteins from the recombinant plasmids was assessed by SDS-PAGE and Western blotting of whole-cell lysates.
Fusion proteins 661, 10c, and FP3 were purified by affinity binding to glutathione Sepharose 4B (Pharmacia) and competitive elution with free glutathione (4). Fusion proteins 3c, FP2, and FP4 were purified as inclusion bodies (25). Purified inclusion bodies were resuspended in 0.4 ml of 8 M urea, to which 0.4 ml of PBS was then added.
Fusion protein 661 was further purified by anion-exchange chromatography or by cleavage of 661 from GST bound to glutathione Sepharose 4B using the Restriction Protease Factor Xa Cleavage and Removal Kit (Boehringer Mannheim). For anion-exchange chromatography, a Mono Q HR5/5 column (Pharmacia) was equilibrated with buffer A (50 mM Tris-HCl [pH 8], 5 mM 2 mercaptoethanol, 0.1 mM EDTA, 10% [vol/vol] glycerol). A 0.1-mg sample of 661 was loaded, the column was washed with 10 ml of buffer A, and the proteins were then eluted with a concentration gradient of buffer B (buffer A containing 0.4 M NaCl) increasing to 20% over 15 ml and then to 100% over 10 ml, followed by a further 5 ml of buffer B. Fractions with peak optical density at 214 nm were collected.
Purification by cleavage from the fusion partner was done as suggested by the manufacturer but with a final incubation of the cleaved polypeptide 661 with 50 μl of washed 50% glutathione Sepharose 4B at 4°C for 30 min to remove residual contaminants. The glutathione Sepharose 4B was then precipitated by centrifugation, and phenylmethylsulfonyl fluoride was added to the supernatant to a final concentration of 50 μg/ml.
ELISA.
The ELISA method used was adapted from a previously published method (13). Washing at each stage was done three times with PBS–0.05% Tween 20 (PBS-T), and sera and antibodies were diluted in 5% skim milk powder in PBS-T. Each of the inner 60 wells of MaxiSorp Immunoplates (Nunc) was coated at 4°C overnight with 100 μl of protein 661 diluted in carbonate-bicarbonate buffer (32 mM Na2CO3, 64 mM NaHCO3). The plate was washed, 100 μl of 5% (wt/vol) skim milk powder in PBS-T was added to each well, and the plate was incubated for 1 h at room temperature. After washing, 100 μl of diluted primary antibody was added to each well, and the plate was incubated for 1 h at room temperature. The plate was washed, and 100 μl of diluted rabbit antibody to human γ chain conjugated to HRP (Dako) was added to each well. The plate was incubated for 1 h at room temperature. The plate was washed, 100 μl of substrate solution (0.32 mM 3,3′,5,5′-tetramethylbenzidine dihydrochloride [Sigma], 85 mM sodium acetate, 12 mM acetic acid) was added, and the plate was incubated at room temperature for 5 min. The reactions were stopped by addition of 25 μl of 1 M HCl per well, and the A450 of the contents of each well was determined.
Determination of serum reactivity.
The same positive serum (95508680) was assayed in duplicate at 1/100 and serial fourfold dilutions to 1/25,600 on each plate to obtain a standard curve. Each serum sample was assayed in duplicate. If the difference in optical density between the unblanked duplicate wells was greater than 10% of their mean value, they were assayed again in triplicate. The mean optical density of duplicate wells containing antigen incubated with conjugated antibody, but not primary antibody, was subtracted as a blank value from the optical density of assayed sera. The four-parameter curve fit method was applied to the standard curve data points by using the program Delta Soft 3 (E. Bechtold and BioMetallics, Inc.). Averages of the optical densities of duplicate serum dilutions of 1/100 were interpolated on the standard curve, and the reactivity of the sera was expressed in units relative to units of reactivity per microliter of 95508680. The reactivity of serum 95508680 with 661 was defined as 2,560 relative units (RU) of IgG/μl. The use of a single reference serum, tested in a serial dilution series on each plate, has been shown to eliminate systematic errors in such assays, allowing greater analytical consistency. Furthermore, it has been shown that interpolation of relative antibody units from such a standard curve yields more reliable antibody measurement than titration to an endpoint or assessment solely on the basis of absorbance measurements (22).
RESULTS
Hybridization of probes derived from the 116-kDa protein gene to Southern blots of mycoplasma genomic DNA.
The 7.9- and 3.3-kb EcoRI and 10.3-kb BglII fragments of all three strains of M. pneumoniae hybridized to the probe derived from plasmid pGEX-1N-MP3, but no fragments of the other species hybridized to this probe. The 730-nucleotide fragment of the 116-kDa protein gene contained in pGEX-1N-MP3 had 66% identity with MG075, the M. genitalium homologue of the 116-kDa protein gene of M. pneumoniae (11). To increase the probability of detecting homologues of the 116-kDa protein in other species, the 116kcon oligonucleotide was used as a probe.
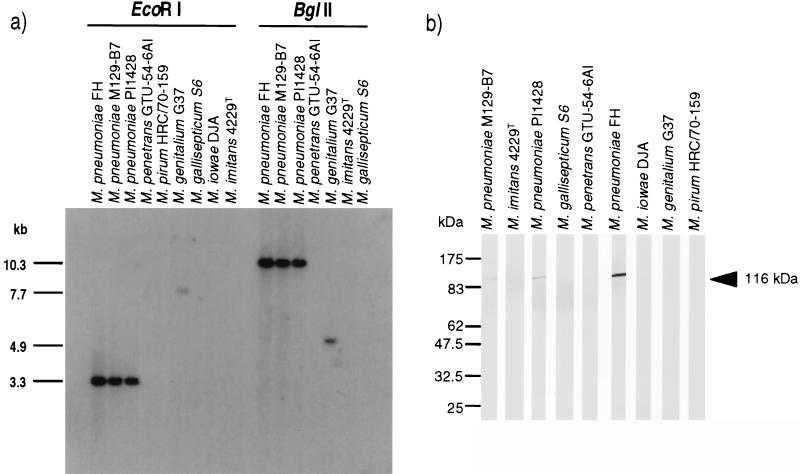
The melting temperatures of the duplexes formed between M. pneumoniae DNA and 116kcon and between M. genitalium DNA and 116kcon were predicted to be 71 and 65°C, respectively (29). Exposure after the initial washing at 50°C revealed hybridization of 116kcon to the 3.3-kb EcoRI and 10.3-kb BglII fragments of the M. pneumoniae strains and the 7.7-kb EcoRI and 4.9-kb BglII fragments of M. genitalium, as predicted from the M. genitalium genomic sequence (11). Washing of both membranes at 60°C reduced the background considerably but did not reveal hybridization to any other fragments (Fig. 1a).
FIG. 1.
Southern and Western blot analyses to identify homologues of the M. pneumoniae 116-kDa protein in other Mycoplasma species. (a) Southern blot of genomic DNAs of species in the M. pneumoniae group, M. penetrans, and M. iowae (2, 25) digested with the enzymes indicated above the lanes and probed with oligonucleotide 116kcon. The sizes of the M. pneumoniae and M. genitalium genomic DNA fragments that hybridized with 116kcon are indicated to the left of the autoradiograph. Note that the M. pirum and M. iowae DNA were not digested by BglII (or BamHI), suggesting GATm5T methylation. (b) Whole-cell proteins from the Mycoplasma species indicated at the top were Western blotted and probed with immunoaffinity-purified rabbit antibodies to the 116-kDa protein. The position of the 116-kDa protein band is indicated to the right. The positions of New England BioLabs broad-range prestained protein markers are indicated to the left. These images were captured by using a Nikon Touchscan and Adobe Photoshop.
Western blotting of mycoplasma whole-cell proteins probed with affinity-purified rabbit antibodies to the 116-kDa protein.
Immunoaffinity-purified rabbit antibodies to the 116-kDa protein gave strong reactions with the 116-kDa protein of M. pneumoniae FH and weaker reactions with the 116-kDa protein of strains PI1428 and M129 (Fig. 1b). No other Mycoplasma species contained proteins reactive with the antibodies to the 116-kDa protein.
Expression of different regions of the 116-kDa protein gene.
Six different, nonoverlapping regions of the 116-kDa protein gene were cloned and expressed in pGEX vectors as fusion proteins with GST. The only regions of the gene not included in one of the expression clones were those encoding amino acids 1 to 8 and 1030 of the 116-kDa protein (Table 2). In addition, amino acids 963 and 968 of the 116-kDa protein were tryptophan residues encoded by TGA codons, and thus, amino acids 963 to 968 were not expressed.
TABLE 2.
Fusion proteins spanning the 116-kDa protein
| Plasmid | Fusion protein | Amino acid range(s)a | Predicted molecular mass (kDa) |
|---|---|---|---|
| pGEX-3X-MP661 | 661 | 9–474 | 53 (79.6)c |
| pGEX-1N-MP3 | 3c | 467–709 | 27.4 (54) |
| pGEX-3X-MPFP2 | FP2 | 709–850 | 17 (43.7) |
| pGEX-1N-MP10 | 10c | 846–896 | 5.7 (32.3) |
| pGEX-3X-MPFP3 | FP3 | 887–962, 887–967, 887–1029b | 9.2 (35.8) |
| pGEX-3X-MPFP4 | FP4 | 969–1029 | 7.4 (34) |
The positions of the first and last amino acids of the 116-kDa protein contained in each fusion protein are shown.
The PCR product generated by FP3F and FP3R spanned two TGA stop codons (TGA codes for Trp in mycoplasma). Consequently, three fusion proteins were expressed from plasmid pGEX-3X-MPFP3, but most of the fusion proteins terminated at the first TGA (encoding Trp amino acid 963).
Each value in parentheses is the molecular mass of the protein fused to GST.
GST was affinity purified from E. coli transformed with pGEX-3X alone and used as a control in subsequent Western blotting experiments. Western blot analysis demonstrated successful expression of the predicted fusion proteins 3c, 10c, 661, FP2, FP3, and FP4 from plasmids pGEX-1N-MP3, pGEX-1N-MP10, pGEX-3X-MP661, pGEX-3X-MPFP2, pGEX-3X-MPFP3, and pGEX-3X-MPFP4, respectively. FP3 did not react, and FP2 and FP4 reacted only weakly, with the immunoaffinity-purified rabbit antibodies to the 116-kDa protein. However, all fusion proteins were reactive with the immunoaffinity-purified rabbit antibodies to GST.
Contaminants ranging from 45 to 84 kDa and reactive with human sera persisted through glutathione Sepharose 4B affinity purification of the fusion proteins. They were easily discriminated from all of the purified fusion proteins except 661 in Western blots. As some contaminants comigrated with fusion protein 661 on SDS-PAGE, it was difficult to determine the reactivity of human sera with it by Western blotting. The immunoreactive contaminants were removed by either anion-exchange chromatography or Factor Xa cleavage of recombinant protein 661 from its GST fusion partner while attached to glutathione Sepharose 4B.
Assessment of fusion protein reactivity with sera from humans infected with M. pneumoniae.
The proportions (percentages) of the 29 serum samples tested that were immunoreactive with each of the different regions of the 116-kDa protein in Western blots were as follows: GST, 1 (3); 661, 26 (90); 3c, 15 (52); FP2, 6 (21); 10c, 11 (38); FP3, 15 (52); FP4, 5 (26). (FP4 was tested with serum samples from 19 individuals.) Only 1 of the 29 individuals had serum IgG reactive with GST. The most antigenic recombinant protein was 661. It failed to react with sera from four individuals. Sera from these individuals were further assessed by Western blotting using whole-cell proteins of M. pneumoniae as antigen. One of the single sera not reactive with 661 was positive by particle agglutination, weakly reactive with FP3, and also weakly reactive with the P1 protein and a 40-kDa protein of M. pneumoniae. The other single serum not reactive with 661 was negative by particle agglutination and did not react with any other fusion protein but did react with a 40-kDa protein of M. pneumoniae. The acute-phase serum not reactive with 661 did not react with any other fusion protein but was positive by particle agglutination and weakly reactive with the P1, 90-kDa, 70-kDa, and 40-kDa proteins of M. pneumoniae. However, the convalescent-phase serum from this individual did react with 661. The paired sera not reactive with 661 were also both negative by particle agglutination but weakly reactive with the P1 protein and a 40-kDa protein of M. pneumoniae.
Optimization of ELISA based on protein 661.
While protein 661 could be purified by either anion-exchange chromatography or cleavage from the fusion partner, the occasional presence of anti-GST antibodies in human sera and the higher yields achievable by cleavage led to use of protein purified by cleavage for all ELISA evaluations. Recombinant protein 661 was used at a concentration of 20 μg/ml, and at 11 threefold dilutions to 110 pg/ml, to coat duplicate wells of microtiter plates. The bound antigen was tested at a dilution of 1/500 with a human serum which had IgG strongly reactive with 661 by Western blotting. IgG bound to 661 was detected with HRP-conjugated rabbit anti-human γ-chain antibody diluted 1/1,600. Antigen incubated with the conjugate only (not the primary antibody) provided a control for nonspecific binding of the conjugate. Nonspecific binding was eliminated at an antigen concentration of 2 μg/ml or less. Reactivity with 661 was maximal in wells coated with 2-μg/ml 661 or more, so subsequent assays were performed by using 2-μg/ml 661.
A serum strongly reactive with 661 in a Western blot assay was assayed at 1/100 and serial twofold dilutions to 1/25,600 with the conjugate at 1/800 and serial twofold dilutions to 1/25,600. A serum that was not reactive with 661 in the Western blot assay was tested in an identical manner. Six serial twofold dilutions of the conjugate were incubated with 661 in the absence of the primary antibody as a control for nonspecific binding. The binding of the unreactive serum was reduced to background levels when the HRP-conjugated rabbit anti-human γ-chain antibody was used at a dilution of 1/12,800. This conjugate concentration was used in all subsequent assays. Maximal discrimination between the negative and positive sera occurred at a serum dilution of 1/100, so subsequent assays were performed with sera diluted 1/100.
Assessment of paired sera by 661 ELISA.
Individuals were defined as seropositive if they had a fourfold or greater rise in agglutination titer (10, 21) or a fourfold or greater rise in RU of reactivity, as determined by ELISA. The agglutination assay and ELISA results for all paired sera are presented in Table 3. Of the 30 individuals, 15 were positive by the ELISA and 14 were positive by the agglutination assay. Thirteen individuals were positive and 14 were negative by both tests. Of the three individuals with discrepant results, one had a fourfold rise in agglutination titer and a twofold rise in IgG RU (patient 2), one had a high but stationary titer in the agglutination assay and a fourfold increase in IgG RU (patient 3), and the last was negative by agglutination assay for both sera but had a fourfold rise in IgG RU (patient 24).
TABLE 3.
IgG ELISA and agglutination assay results for paired sera from 30 individuals
| Patient no. | Agglutination titer
|
Anti-M. pneumoniae IgG (RU)
|
||
|---|---|---|---|---|
| Acute phase | Convalescent phase | Acute phase | Convalescent phase | |
| 1 | 160 | >640a | 5 | 115a |
| 2 | 40 | 160a | 144 | 388 |
| 3 | 2,560 | 2,560 | 32 | 578a |
| 4 | 80 | 40 | 17 | 15 |
| 5 | 160 | 160 | 692 | 197 |
| 6 | 320 | 640 | 274 | 926 |
| 7 | 160 | 1,280a | 10 | 1,106a |
| 8 | <40 | 320a | 172 | >2,560a |
| 9 | <40 | >640a | 10 | 287a |
| 10 | 80 | >640a | 291 | >2,560a |
| 11 | 40 | 160a | 121 | >2,560a |
| 12 | 40 | 640a | 70 | >2,560 |
| 13 | 80 | >640a | 9 | 57a |
| 14 | <40 | 80 | 113 | 126 |
| 15 | <40 | >640a | 14 | 413a |
| 16 | <40 | 160a | 54 | 7,679a |
| 17 | 160 | >640a | 13 | 1,125a |
| 18 | <40 | 80 | >2,560 | >2,560 |
| 19 | <40 | >640a | 12 | 9,903a |
| 20 | 80 | >640a | 150 | >2,560a |
| 21 | 80 | 160 | 155 | 286 |
| 22 | <40 | <40 | 172 | 298 |
| 23 | <40 | <40 | 395 | 306 |
| 24 | <40 | <40 | 274 | 1,166a |
| 25 | <40 | <40 | 174 | 308 |
| 26 | <40 | <40 | 93 | 136 |
| 27 | <40 | <40 | 142 | 185 |
| 28 | <40 | <40 | 30 | 31 |
| 29 | <40 | <40 | 293 | 219 |
| 30 | <40 | <40 | 66 | 73 |
Fourfold or greater change in RU or agglutination titer.
DISCUSSION
Assessment of the species specificity of antibody to the 116-kDa protein.
Species of the M. pneumoniae phylogenetic group and the phylogenetically related species M. penetrans and M. iowae (2, 25) were examined for genes homologous to that encoding the 116-kDa protein by using probes from two different regions of the gene which were relatively conserved between the two characterized homologues in M. pneumoniae and M. genitalium and for proteins recognized by antisera to the 116-kDa protein. In the species examined, genes homologous to that encoding the 116-kDa protein were only detected in M. pneumoniae and M. genitalium and cross-reactive proteins were only present in M. pneumoniae. As the amount of total cell protein loaded in the Western blots was similar for each strain of M. pneumoniae and there are only three predicted amino acid differences between the 116-kDa proteins of M. pneumoniae FH and M129 (11), the differences in 116-kDa protein reactivity with the immunoaffinity-purified rabbit antibodies to the 116-kDa protein is unlikely to be attributable to differences between strains in the antigenicity of the 116-kDa protein.
It is most likely that the differences observed reflect differences between strains in the level of expression of the 116-kDa protein in vitro. In a previous study, a 110-kDa protein of M. pneumoniae FH had greater reactivity with sera from 12 humans infected with M. pneumoniae than did the 110-kDa protein that was expressed by M. pneumoniae isolated from the same 12 infected humans (36). Assuming that this 110-kDa protein is identical to the 116-kDa protein, the high levels of antibody against the 116-kDa protein detected in our study suggest that its expression in some strains may be greater in vivo than in vitro. However, variation in the level of expression of the 116-kDa protein cannot be explained by current sequence data. The 116-kDa protein is encoded by the 3′ gene of a dicistronic operon (9). There are no nucleotide differences between M. pneumoniae FH and M129 in the intergenic sequence between the open reading frames (ORFs) of this operon and only a single variant nucleotide in the 287 nucleotides 5 prime to the 5′ ORF, but this is 113 nucleotides 3 prime to the site of transcriptional initiation. Thus, differences in expression were not due to differences in translational or transcriptional regulatory sequences. It may be that variation in the expression of products of this operon is effected by either differing levels of a transcriptional factor acting on the promoter or variations in amounts of posttranslational processing.
The predicted amino acid sequence of the protein encoded by ORF MG075 of M. genitalium had only 52% amino acid identity with the predicted amino acid sequence of the 116-kDa protein. While this suggests that there is unlikely to be cross-reactivity between the 116-kDa protein of M. pneumoniae and the MG075 product of M. genitalium, our failure to detect a cross-reactive protein by Western blotting could also be due to lack of expression in vitro. Ultimately, determination of the species specificity of antibodies to 116-kDa protein homologues will depend on the expression of regions of the gene from M. genitalium.
The immunodominant region of the 116-kDa protein of M. pneumoniae as a serodiagnostic antigen.
Recombinant protein 661 was the most antigenic of the recombinant proteins derived from the 116-kDa protein. Of the 29 individuals with suspected M. pneumoniae infection, 3 did not develop serum IgG reactive with 661 in a Western blot assay. Two of these three were also negative by particle agglutination and had low levels of IgG reactive with whole-cell proteins of M. pneumoniae and thus were probably not infected with M. pneumoniae. The third had a low level of reactivity with M. pneumoniae proteins by Western blot but was strongly positive by the particle agglutination assay, which detects primarily IgM (1). This single serum was described as an acute-phase sample and thus may have had detectable M. pneumoniae-specific IgM but not IgG (17, 35).
Performance of the 661 ELISA.
Protein 661 was used as an antigen in an indirect ELISA. Cutoff values for the IgG ELISA were not calculated, as anti-M. pneumoniae IgG remains elevated for at least a year following infection and is therefore only of value in demonstrating a rise in titer (35). The sensitivity of the indirect ELISA (94%) was not significantly greater than the sensitivity of the Serodia Myco II particle agglutination assay (88%) (P = 0.5, Fisher’s exact test). Only 3 of the 30 individuals tested had different results by the two tests. These discrepant results could be due to the different isotypes detected by the agglutination assay (primarily IgM) (1) and the indirect ELISA (solely IgG).
The data from this limited trial indicated that recombinant protein 661 could be used as a serodiagnostic reagent. The inherent advantage of serodiagnosis using a purified single polypeptide rather than complex cellular fractions is improved specificity (7, 18). The cross-reactivity with human tissues observed in the complement fixation test for detection of M. pneumoniae-specific antibodies is due to the glycolipid antigen (27), but proteins of M. pneumoniae also cause cross-reactivity. The P1 and the P30 proteins both have homology with eukaryotic structural proteins, sera raised to both proteins cross-react with human proteins (5, 6, 16), and antibody against P1 is cross-reactive with MgPa, the homologue in M. genitalium, necessitating the use of species-specific, P1-derived, synthetic peptides as antigens in a diagnostic ELISA (15).
The sera tested in our study were all collected in Victoria from 1995 to 1997. It is possible that they are representative of infection with only a limited number of M. pneumoniae strains. However, the nucleotide sequence of the gene encoding the 116-kDa protein, and consequently also the epitopes of the protein, is highly conserved between M. pneumoniae M129 (14) and FH (9), which are representatives of the two M. pneumoniae groups expressing variant P1 proteins (32). It should be noted that the two major antigenic groups described for M. pneumoniae have been classified as such solely on the basis of their P1 proteins and there is no evidence that this variability is reflected in other proteins. Indeed, the variation in P1 has been suggested to result from recombination between the functional gene and a reservoir of pseudogenes found throughout the genome (32) and is thus unlikely to reflect evolutionary divergence between the genomes of strains assigned to either group.
While our trial demonstrated that the indirect ELISA using recombinant protein 661 as antigen had sensitivity comparable to that of existing tests, it was performed on a selective set of sera from patients thought to be infected with M. pneumoniae on clinical grounds. Thus, a more extensive prospective trial is required to compare the sensitivity of the assay using recombinant protein 661 as an antigen with the sensitivity of existing serological tests. Similarly, further investigation is required to determine to what extent specificity is improved by using purified recombinant protein 661 as antigen rather than the M. pneumoniae cellular fractions currently employed in serology. Such a trial should include a parallel assay developed by expressing the homologous region of the M. genitalium gene so that the extent of cross-reactivity of current tests can be assessed, as well as the specificity of the ELISA described in this paper. The availability of these parallel assays would then allow comprehensive assessment of the epidemiology and relative significance of these two organisms in respiratory disease.
ACKNOWLEDGMENTS
This work was supported by grants from the Australian Research Council, an Australian Postgraduate Award (Industry), and Bioproperties (Australia) Pty. Ltd.
We thank I. D. Walker for advice on the conduct of this work.
REFERENCES
- 1.Barker C E, Sillis M, Wreghitt T G. Evaluation of Serodia Myco II particle agglutination test for detecting Mycoplasma pneumoniae antibody: comparison with mu-capture ELISA and indirect immunofluorescence. J Clin Pathol. 1990;43:163–165. doi: 10.1136/jcp.43.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradbury J M, Abdul-Wahab O M S, Yavari C A, Dupiellet J-P, Bové J M. Mycoplasma imitans sp. nov. is related to Mycoplasma gallisepticum and found in birds. Int J Syst Bacteriol. 1993;43:721–728. doi: 10.1099/00207713-43-4-721. [DOI] [PubMed] [Google Scholar]
- 3.Cimolai N, Mah D, Thomas E, Middleton P J. Rapid immunoblot method for diagnosis of acute Mycoplasma pneumoniae infection. Eur J Clin Microbiol Infect Dis. 1990;9:223–226. doi: 10.1007/BF01963844. [DOI] [PubMed] [Google Scholar]
- 4.Crabb B S, MacPherson C M, Reubel G H, Browning G F, Studdert M J, Drummer H E. A type-specific serological test to distinguish antibodies to equine herpesviruses 4 and 1. Arch Virol. 1995;140:245–258. doi: 10.1007/BF01309860. [DOI] [PubMed] [Google Scholar]
- 5.Dallo S F, Chavoya A, Baseman J B. Characterization of the gene for a 30-kilodalton adhesion-related protein of Mycoplasma pneumoniae. Infect Immun. 1990;58:4163–4165. doi: 10.1128/iai.58.12.4163-4165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallo S F, Lazzell A L, Chavoya A, Reddy S P, Baseman J B. Biofunctional domains of the Mycoplasma pneumoniae P30 adhesin. Infect Immun. 1996;64:2595–2601. doi: 10.1128/iai.64.7.2595-2601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies C. Principles. In: Wild D, editor. The immunoassay handbook—1994. New York, N.Y: Stockton Press; 1994. p. 38. [Google Scholar]
- 8.Del Sal G, Manfioletti G, Schneider C. A one tube plasmid DNA mini-preparation suitable for sequencing. Nucleic Acids Res. 1988;16:9878. doi: 10.1093/nar/16.20.9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy M F, Walker I D, Browning G F. The immunoreactive 116 kDa surface protein of Mycoplasma pneumoniae is encoded in an operon. Microbiology. 1997;143:3391–3402. doi: 10.1099/00221287-143-10-3391. [DOI] [PubMed] [Google Scholar]
- 10.Echevarria J M, Leon P, Balfagon P, Lopez J A, Fernandez M V. Diagnosis of Mycoplasma pneumoniae infection by microparticle agglutination and antibody-capture enzyme-immunoassay. Eur J Clin Microbiol Infect Dis. 1990;9:217–220. doi: 10.1007/BF01963842. [DOI] [PubMed] [Google Scholar]
- 11.Fraser C M, Gocayne J D, White O, Adams M D, Clayton R A, Fleischmann R D, Bult C J, Kerlavage A R, Sutton G, Kelley J M, Fritchman J L, Weidman J F, Small K V, Sandusky M, Fuhrmann J, Nguyen D, Utterback T R, Saudek D M, Phillips C A, Merrick J M, Tomb J F, Dougherty B A, Bott K F, Hu P C, Lucier T S, Peterson S N, Smith H O, Hutchinson III C A, Venter J C. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 12.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 13.Higgins P A, Whithear K G. Detection and differentiation of Mycoplasma gallisepticum and Mycoplasma synoviae antibodies in chicken serum using enzyme linked immunosorbent assay. Avian Dis. 1986;30:160–168. [PubMed] [Google Scholar]
- 14.Himmelreich R, Hilbert H, Plagens H, Pirkl E, Li B C, Herrmann R. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 1996;24:4420–4449. doi: 10.1093/nar/24.22.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs E. Serological diagnosis of Mycoplasma pneumoniae infections: a critical review of current procedures. Clin Infect Dis. 1993;17:S79–S82. doi: 10.1093/clinids/17.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs E, Bartl A, Oberle K, Schiltz E. Molecular mimicry by Mycoplasma pneumoniae to evade the induction of adherence inhibiting antibodies. J Med Microbiol. 1995;43:422–429. doi: 10.1099/00222615-43-6-422. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs E, Bennewitz A, Bredt W. Reaction pattern of human anti-Mycoplasma pneumoniae antibodies in enzyme-linked immunosorbent assays and immunoblotting. J Clin Microbiol. 1986;23:517–522. doi: 10.1128/jcm.23.3.517-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs E, Fuchte K, Bredt W. A 168 kilodalton protein of Mycoplasma pneumoniae used as antigen in a dot-linked enzyme-linked immunosorbent assay. Eur J Clin Microbiol. 1986;5:435–440. doi: 10.1007/BF02075700. [DOI] [PubMed] [Google Scholar]
- 19.Kleemola M, Raty R, Karjalainen J, Schuy W, Gerstenecker B, Jacobs E. Evaluation of an antigen-capture enzyme immunoassay for rapid diagnosis of Mycoplasma pneumoniae infection. Eur J Clin Microbiol Infect Dis. 1993;12:872–875. doi: 10.1007/BF02000413. [DOI] [PubMed] [Google Scholar]
- 20.Kleven S H, Browning G F, Bulach D M, Ghiocas E, Morrow C J, Whithear K G. Examination of Mycoplasma gallisepticum strains using restriction endonuclease DNA analysis and DNA-DNA hybridisation. Avian Pathol. 1988;17:559–570. doi: 10.1080/03079458808436477. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman D, Lieberman D, Horowitz S, Horovitz O, Schlaeffer F, Porath A. Microparticle agglutination versus antibody-capture enzyme immunoassay for diagnosis of community-acquired Mycoplasma pneumoniae pneumonia. Eur J Clin Microbiol Infect Dis. 1995;14:577–584. doi: 10.1007/BF01690728. [DOI] [PubMed] [Google Scholar]
- 22.Malvano R, Boniolo A, Dovis M, Zannino M. ELISA for antibody measurement: aspects related to data expression. J Immunol Methods. 1982;48:51–60. doi: 10.1016/0022-1759(82)90209-5. [DOI] [PubMed] [Google Scholar]
- 23.Marmion B P, Williamson J, Worswick D A, Kok T W, Harris R J. Experience with newer techniques for the laboratory detection of Mycoplasma pneumoniae infection: Adelaide, 1978–1992. Clin Infect Dis. 1993;17(Suppl. 1):S90–S99. doi: 10.1093/clinids/17.supplement_1.s90. [DOI] [PubMed] [Google Scholar]
- 24.Moule J H, Caul E O, Wreghitt T H. The specific IgM response to Mycoplasma pneumoniae infection: interpretation and application to early diagnosis. Epidemiol Infect. 1987;99:685–692. doi: 10.1017/s0950268800066541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai K, Thøgersen H C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- 26.Peterson S N, Hu P C, Bott K F, Hutchison C A I. A survey of the Mycoplasma genitalium genome by using random sequencing. J Bacteriol. 1993;175:7918–7930. doi: 10.1128/jb.175.24.7918-7930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponka A, Ponka T, Sarna S, Pentinnen K. Questionable specificity of lipid antigen in the Mycoplasma pneumoniae complement-fixation test in patients with extrapulmonary manifestations. J Infect. 1981;3:332–338. doi: 10.1016/s0163-4453(81)91901-0. [DOI] [PubMed] [Google Scholar]
- 28.Razin S. DNA probes and PCR in diagnosis of Mycoplasma infections. Mol Cell Probes. 1994;8:497–511. doi: 10.1006/mcpr.1994.1071. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Sillis M. The limitations of IgM assays in the serological diagnosis of Mycoplasma pneumoniae infections. J Med Microbiol. 1990;33:253–258. doi: 10.1099/00222615-33-4-253. [DOI] [PubMed] [Google Scholar]
- 31.Su C J, Chavoya A, Baseman J B. Regions of Mycoplasma pneumoniae cytadhesin P1 structural gene exist as multiple copies. Infect Immun. 1988;56:3157–3161. doi: 10.1128/iai.56.12.3157-3161.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su C J, Dallo S F, Chavoya A, Baseman J B. Possible origin of sequence divergence in the P1 cytadhesin gene of Mycoplasma pneumoniae. Infect Immun. 1993;61:816–822. doi: 10.1128/iai.61.3.816-822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thacker W L, Talkington D F. Comparison of two rapid commercial tests with complement fixation for serologic diagnosis of Mycoplasma pneumoniae infections. J Clin Microbiol. 1995;33:1212–1214. doi: 10.1128/jcm.33.5.1212-1214.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Kuppeveld F J, Johansson K E, Galama J M, Kissing J, Bolske G, Hjelm E, van der Logt J T, Melchers W J. 16S rRNA based polymerase chain reaction compared with culture and serological methods for diagnosis of Mycoplasma pneumoniae infection. Eur J Clin Microbiol Infect Dis. 1994;13:401–405. doi: 10.1007/BF01971997. [DOI] [PubMed] [Google Scholar]
- 35.Vikerfors T, Brodin G, Grandien M, Hirschberg L, Krook A, Pettersson C A. Detection of specific IgM antibodies for the diagnosis of Mycoplasma pneumoniae infections: a clinical evaluation. Scand J Infect Dis. 1988;20:601–610. doi: 10.3109/00365548809035660. [DOI] [PubMed] [Google Scholar]
- 36.Vu A C, Foy H M, Cartwright F D, Kenny G E. The principal protein antigens of isolates of Mycoplasma pneumoniae as measured by levels of immunoglobulin G in human serum are stable in strains collected over a 10-year period. Infect Immun. 1987;55:1830–1836. doi: 10.1128/iai.55.8.1830-1836.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitcomb R F. Mycoplasma characterisation. In: Razin S, Tully J G, editors. Methods in mycoplasmology—1983. New York, N.Y: Academic Press Inc.; 1983. pp. 147–158. [Google Scholar]
- 38.Whithear K G. Avian mycoplasmosis. In: Corner L A, editor. Australian standard diagnostic techniques for animal diseases—1992. East Melbourne, Victoria, Australia: Australian Agricultural Council (Standing Committee on Agriculture); 1992. pp. 1–12. [Google Scholar]
- 39.Zander D V. Origin of S6 strain Mycoplasma. Avian Dis. 1961;5:154–156. [Google Scholar]