Abstract
Purpose: Adolescents and young adults (AYAs) have experienced inferior improvements in cancer survival outcomes. One potential explanation is the low rate of enrollment in cancer clinical trials. While the reasons behind this are multifactual, sociodemographic factors are probably contributory. We examined the impact of factors such as insurance type and race/ethnicity on clinical trial enrollment among AYAs treated for cancer at an academic medical center.
Methods: We identified AYAs (ages 15–39 years) treated for cancer at the University of North Carolina between April 2014 and April 2019. Cancer registry data were linked to electronic health record data to associate treatment and sociodemographic factors with clinical trial enrollment. A multivariable log-binomial model was used to estimate adjusted risk ratios.
Results: In a 5-year period, 1574 AYA patients were identified, 59% female, 21% non-Hispanic Black and 9% Hispanic. Overall, 37% of AYAs participated in any clinical trial and 14% enrolled on a therapeutic trial. When compared to publicly insured AYAs, those with private insurance [adjusted RR: 1.52, 95% CI: 1.05–2.22] or with no insurance [adjusted RR: 2.12, 95% CI: 1.34–3.33] were more likely to enroll in a therapeutic clinical trial. Hispanic AYAs were less likely to enroll [adjusted RR: 0.50, 95% CI: 0.27–0.93] when compared to non-Hispanic White patients.
Conclusions: Rates of clinical trial enrollment among AYAs vary based on health insurance type and race/ethnicity, suggesting possible disparities in access. Attention to resource, cultural, and language barriers may improve trial enrollment and cancer outcomes among vulnerable AYA subpopulations.
Keywords: clinical trials, insurance coverage, uninsured, ethnicity
Purpose
Each year about 90,000 adolescents and young adults (AYAs), individuals between the ages of 15 and 39, are diagnosed with cancer in the United States.1 Compared to other age groups, AYAs have experienced inferior improvements in cancer survival rates.2–4 While this discrepancy is likely a result of multiple interacting elements, such as tumor biology, access to and nature of treatment, and socioeconomic factors, it is probable that the underrepresentation of AYAs in cancer clinical trials is a significant contributing factor.3,5,6
Several studies have observed low therapeutic trial enrollment rates among AYAs of 6%–14%,7 in stark contrast to 60%–70% estimated enrollment of children under the age of 15.6,8,9 Enrollment in clinical trials is of particular concern because participation can directly benefit patients allowing for early access to novel therapies and providing scientists with data to optimize treatment protocols for this age group. Further, decreased participation in clinical trials has been shown to be strongly correlated with inferior survival outcomes among AYAs.6
Despite substantial evidence of lower enrollment rates of AYAs in national cancer treatment trials, the causes for this phenomenon are not fully understood. Researchers have hypothesized that disease and treatment-related factors (cancer type, cancer stage, treatment facility type [pediatric vs. adult or academic vs. community], trial availability) likely contribute to varying rates of enrollment.6,9 Multiple studies have shown that as AYAs age, they are less likely to receive care in pediatric hospitals from pediatric oncologists and are therefore less likely to enroll in clinical trials.9,10 Additionally, teenagers and young adults who are treated outside of pediatric hospitals may not have access to appropriate trials for their age and type of tumor.9 Potential delays in cancer diagnosis and inconsistent access to care for the age group may also be contributing factors.11 Creation of AYA-focused oncology programs with clinical trial “champions,” broadening eligibility for clinical trials across cooperative groups (such as by expanding pediatric trials to young adults or vice-versa), and supporting development of trials targeting common AYA cancers have all been approaches to these barriers. While these strategies are necessary underpinnings to improved AYA trial enrollment, alone they may not be sufficient.
In contrast to treatment-related factors, limited data are available regarding the role of sociodemographic factors (insurance status, race/ethnicity) on AYA cancer trial enrollment and the extant literature reports discordant findings. Race and ethnicity have previously been reported as important factors associated with trial enrollment12 while others have not observed such associations.3,10,13 Decreased enrollment among Black when compared to White AYAs12 and higher enrollment among Hispanic AYAs compared to AYAs of other ethnicities12 have both been previously reported. However, these findings have not been confirmed in other studies.3,10,13
Similarly, prior studies have reported conflicting results regarding the impact of insurance type on trial enrollment,10,12–14 an important factor for AYAs who experience the highest rates of noninsurance.15 Previous reports have observed higher enrollment among privately insured AYAs when compared to those with public insurance12 or who are uninsured,10,13 but most of these studies considered only a limited spectrum of cancer types and were conducted before the initiation of the Affordable Care Act, which significantly altered access to health insurance coverage for AYAs.
Using a retrospective cohort study design and consideration of the broad range of AYA cancer types, we sought to describe patterns of clinical trial enrollment at an academic comprehensive cancer center with both pediatric and adult programs and to examine the potential associations of sociodemographic factors (health insurance, marital status, and race/ethnicity) with clinical trial participation. We hypothesized that AYAs who are uninsured, who identify as a racial or ethnic minority, and who are unmarried would have lower rates of clinical trial enrollment. By identifying potential sociodemographic factors associated with lower trial enrollment, researchers and providers may be better equipped to support trial enrollment among vulnerable AYA subpopulations.
Methods
Study design and data source
We conducted a retrospective cohort study to assess the impact of sociodemographic factors on cancer clinical trial enrollment among AYAs treated within an academic health care system. Data were obtained from linkage of the University of North Carolina (UNC) Cancer Registry with electronic health record (EHR) data including a discrete field for clinical trial enrollment including both therapeutic and supportive care studies.
Participants and sampling methods
Cohort eligibility included patients in the UNC Cancer Registry with a newly diagnosed, invasive cancer treated between April 2014 and April 2019. Participants were limited to AYAs defined by the National Cancer Institute (NCI) as being 15 and 39 years old at the time of cancer diagnosis.16 From the cancer registry we recorded patient date of diagnosis, age at diagnosis, stage of cancer, and cancer site. The UNC Cancer Registry was linked to the EHR using patient name, medical record number, sex, and date of birth. From the UNC EHR we obtained patient date of birth, biological sex, race and ethnicity, marital status, primary health insurer at diagnosis, and clinical trial enrollment status (any, therapeutic, and nontherapeutic). At UNC, clinical trial enrollment status is recorded as a discrete field within the EHR, which is linked to the central cancer clinical trial database “OnCore.” Studies are classified within OnCore as therapeutic (including drug, diagnostic, or behavioral interventions), correlative, or observational (biology and registries).
Independent variables and outcomes
The primary outcome of interest was patient enrollment in a therapeutic cancer clinical trial. Enrollment in a nontherapeutic or any type of cancer clinical trial were secondary outcomes. Patients were required to complete trial enrollment within 90 days after cancer diagnosis. Independent variables included patient age at diagnosis, race/ethnicity, sex, marital status, and health insurance status. Insurance status was recorded based on the primary payer for patient care at the time of diagnosis as private, public (any Medicaid or Medicare), military, or uninsured/self-pay. Cancer type and stage at diagnosis were also considered. Related cancers were grouped into 10 categories and analyzed separately. The remaining types that could not be categorized within these 10 groups (4%) were combined into a separate “other” category.
Statistical analysis
Study population characteristics were reported using standard measures of frequencies. Bivariable associations were assessed with chi-squared tests. Using log-binomial models, we estimated unadjusted risk ratios describing associations of therapeutic clinical trial enrollment with sociodemographic and disease variables. A multivariable log-binomial model was used to estimate risk ratios for therapeutic trial enrollment by insurance type while adjusting for age at diagnosis, race/ethnicity, marital status, cancer diagnosis, and cancer stage. A separate model considering Medicare as an independent insurance type was performed as a sensitivity analysis.
The study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Results
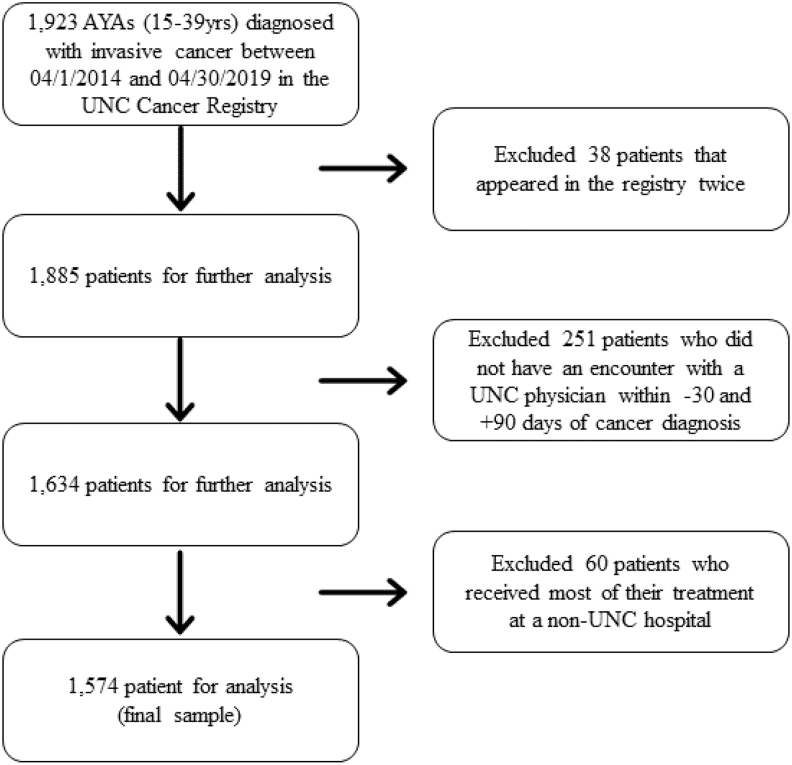
Using the UNC Cancer Registry, we identified 1923 AYAs diagnosed with invasive cancer within the UNC health system between April 2014 and April 2019. After excluding patients with duplicate entries in the registry, with no care encounter at UNC within −30/+90 days of diagnosis, and who received most of their care at a non-UNC facility, 1574 remained in the study population (Fig. 1). A description of the full study population by health insurance type is presented in Table 1. Fifty-nine percent of the study population was female, 64% was non-Hispanic White, 21% was non-Hispanic Black, 9% was Hispanic, and 6% was non-Hispanic other. Slightly more than half of patients were not married. Of the 303 subjects with public insurance, 250 (83%) had Medicaid, 43 (14%) had Medicare, and 10 (3%) had both Medicaid and Medicare. Health insurance types at diagnosis were not evenly distributed among subgroups (Table 1). For example, proportionately more Hispanic patients were uninsured when compared to patients of other ethnicities.
FIG. 1.
Identification of study population of AYAs with cancer.
Table 1.
Patient Characteristics by Primary Health Insurance Payer Type
| |
Total n = 1574 |
Private n = 968 |
Public n = 303 |
Military n = 109 |
Uninsured n = 194 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | N | % | n | % | |
| Age at diagnosis | ||||||||||
| 15–19 | 131 | 8 | 71 | 7 | 48 | 16 | 9 | 8 | 3 | 2 |
| 20–24 | 178 | 11 | 100 | 10 | 28 | 9 | 23 | 21 | 27 | 14 |
| 25–29 | 270 | 17 | 163 | 17 | 55 | 18 | 18 | 17 | 34 | 18 |
| 30–34 | 417 | 27 | 265 | 27 | 77 | 25 | 28 | 26 | 47 | 24 |
| 35–39 | 578 | 37 | 369 | 38 | 95 | 31 | 31 | 28 | 83 | 43 |
| Sex | ||||||||||
| Female | 923 | 59 | 581 | 60 | 178 | 59 | 50 | 46 | 114 | 59 |
| Male | 651 | 41 | 387 | 40 | 125 | 41 | 59 | 54 | 80 | 41 |
| Race/Ethnicity | ||||||||||
| Non-Hispanic White | 974 | 64 | 685 | 74 | 142 | 48 | 66 | 64 | 81 | 43 |
| Non-Hispanic Black | 313 | 21 | 150 | 16 | 106 | 36 | 16 | 16 | 41 | 22 |
| Hispanic | 140 | 9 | 44 | 5 | 30 | 10 | 11 | 11 | 55 | 29 |
| Non-Hispanic Other | 93 | 6 | 53 | 6 | 17 | 6 | 10 | 10 | 13 | 7 |
| Marital status | ||||||||||
| Single | 732 | 50 | 404 | 45 | 194 | 66 | 29 | 29 | 105 | 57 |
| Married | 663 | 45 | 456 | 51 | 73 | 25 | 69 | 69 | 65 | 35 |
| Separated/Divorced | 79 | 5 | 36 | 4 | 26 | 9 | 2 | 2 | 15 | 8 |
| Cancer stage | ||||||||||
| In situ | 56 | 4 | 45 | 5 | 5 | 2 | 1 | 1 | 5 | 3 |
| Local | 705 | 46 | 447 | 47 | 116 | 40 | 50 | 47 | 92 | 49 |
| Regional | 427 | 28 | 261 | 28 | 79 | 27 | 32 | 30 | 55 | 29 |
| Distant | 339 | 22 | 190 | 20 | 91 | 31 | 23 | 22 | 35 | 19 |
| Cancer site | ||||||||||
| Breast | 169 | 11 | 109 | 11 | 25 | 8 | 13 | 12 | 22 | 11 |
| Gynecologic | 220 | 14 | 130 | 13 | 42 | 14 | 6 | 6 | 42 | 22 |
| Testicular | 86 | 6 | 55 | 6 | 10 | 3 | 7 | 6 | 14 | 7 |
| CNS | 84 | 5 | 44 | 5 | 25 | 8 | 5 | 5 | 10 | 5 |
| GI | 131 | 8 | 79 | 8 | 28 | 9 | 4 | 4 | 20 | 10 |
| Head/Neck/Lung | 82 | 5 | 45 | 5 | 17 | 6 | 11 | 10 | 9 | 5 |
| Hematologic | 271 | 17 | 159 | 16 | 69 | 23 | 21 | 19 | 22 | 11 |
| Sarcoma | 85 | 5 | 42 | 4 | 22 | 7 | 10 | 9 | 11 | 6 |
| Skin | 253 | 16 | 199 | 21 | 24 | 8 | 13 | 12 | 17 | 9 |
| Thyroid | 124 | 8 | 73 | 8 | 22 | 7 | 12 | 11 | 17 | 9 |
| Other | 69 | 4 | 33 | 3 | 19 | 6 | 7 | 6 | 10 | 5 |
Of the 1574 subjects, 37% were enrolled on any cancer clinical trial within 90 days of their cancer diagnosis. The proportion of enrolled AYAs varied among therapeutic trials (14%) and nontherapeutic trials (33%). Unadjusted associations between clinical trial enrollment and pertinent covariates are presented in Table 2. Notably, neither race/ethnicity nor insurance type had a statistically significant association with therapeutic trial enrollment rate in the bivariable analysis. Higher rates of therapeutic trial enrollment were associated with younger age, cancer type, and advanced cancer stage.
Table 2.
Clinical Trial Enrollment by Demographic, Disease, and Treatment Factors
| |
Any trial n = 580 (37%) |
Therapeutic trials n = 213 (14%) |
Nontherapeutic trials n = 520 (33%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | p | n | % | p | N | % | p | |
| Primary insurance | 1.00 | 0.17 | 0.98 | ||||||
| Private | 356 | 37 | 133 | 14 | 321 | 33 | |||
| Public | 111 | 37 | 31 | 10 | 101 | 33 | |||
| Military | 41 | 38 | 16 | 15 | 34 | 31 | |||
| Uninsured/Self Pay | 72 | 37 | 33 | 17 | 64 | 33 | |||
| Age at diagnosis | 0.13 | 0.04 | 0.21 | ||||||
| 15–19 | 56 | 43 | 29 | 22 | 45 | 34 | |||
| 20–24 | 58 | 33 | 19 | 11 | 52 | 29 | |||
| 25–29 | 100 | 37 | 33 | 12 | 93 | 34 | |||
| 30–34 | 139 | 33 | 53 | 13 | 123 | 30 | |||
| 35–39 | 227 | 39 | 79 | 14 | 207 | 36 | |||
| Sex | 0.14 | 0.23 | 0.10 | ||||||
| Female | 354 | 38 | 133 | 14 | 320 | 35 | |||
| Male | 226 | 35 | 80 | 12 | 200 | 31 | |||
| Race/Ethnicity | 0.03 | 0.11 | 0.04 | ||||||
| Non-Hispanic White | 364 | 37 | 132 | 14 | 330 | 34 | |||
| Non-Hispanic Black | 133 | 43 | 51 | 16 | 116 | 37 | |||
| Hispanic | 39 | 28 | 11 | 8 | 33 | 24 | |||
| Non-Hispanic Other | 32 | 34 | 14 | 15 | 30 | 32 | |||
| Marital status | 0.84 | 0.49 | 0.74 | ||||||
| Single | 283 | 39 | 100 | 14 | 251 | 34 | |||
| Married | 249 | 38 | 102 | 15 | 223 | 34 | |||
| Separated/Divorced | 32 | 41 | 9 | 11 | 30 | 38 | |||
| Cancer stage | <0.001 | <0.001 | <0.001 | ||||||
| In situ | 5 | 9 | 0 | 0 | 5 | 9 | |||
| Local | 219 | 31 | 68 | 10 | 205 | 29 | |||
| Regional | 187 | 44 | 76 | 18 | 165 | 39 | |||
| Distant | 157 | 46 | 66 | 20 | 133 | 39 | |||
| Cancer site | <0.001 | <0.001 | <0.001 | ||||||
| Breast | 91 | 54 | 49 | 29 | 75 | 44 | |||
| Gynecologic | 106 | 48 | 38 | 17 | 103 | 47 | |||
| Testicular | 28 | 33 | 8 | 9 | 23 | 27 | |||
| CNS | 28 | 33 | 7 | 8 | 27 | 32 | |||
| GI | 56 | 43 | 9 | 7 | 53 | 41 | |||
| Head/Neck/Lung | 34 | 42 | 17 | 21 | 31 | 38 | |||
| Hematologic | 107 | 40 | 49 | 18 | 90 | 33 | |||
| Sarcoma | 26 | 31 | 10 | 12 | 19 | 22 | |||
| Skin | 41 | 16 | 8 | 3 | 37 | 15 | |||
| Thyroid | 34 | 27 | 8 | 7 | 34 | 27 | |||
| Other | 29 | 42 | 10 | 15 | 28 | 41 | |||
In multivariable analysis controlling for patient age at diagnosis, health insurance type, race/ethnicity, marital status, cancer site, and cancer stage, statistically significant differences in therapeutic trial enrollment by insurance type and race/ethnicity emerged. The uninsured [2.12, 95% CI: 1.34–3.33] and the privately insured [RR: 1.52, 95% CI: 1.05–2.22] AYAs were significantly more likely than publicly insured AYAs to enroll in a therapeutic clinical trial (Table 3). Repeating this model with privately insured AYAs as the referent, the likelihood of therapeutic trial enrollment among uninsured AYAs was higher than that of privately insured AYAs although it did not quite reach statistical significance [RR: 1.39, 95% CI: 0.99–1.95]. A sensitivity analysis was performed designating Medicare as a separate insurance category from public that found no significant differences from the reported final multivariable model. Additionally, adjusted multivariable analysis showed that Hispanic AYAs were significantly less likely than non-Hispanic White AYAs to participate in a therapeutic trial [RR: 0.50, 95% CI: 0.27–0.93]. The youngest AYAs and those with more advanced cancers were also more likely to be enrolled on a study. AYAs with skin and thyroid cancers were less likely, and AYAs with breast or gynecological cancers were more likely, than those with hematological malignancies to enroll on a therapeutic trial.
Table 3.
Multivariable Log-Binomial Model Estimating Risk Ratios for Therapeutic Trial Enrollment Controlling for Key Demographic and Disease Covariates
| n | % | RR unadjusted | 95% CI | RR adjusted | 95% CI | |
|---|---|---|---|---|---|---|
| Primary insurance | ||||||
| Public | 133 | 14 | Reference | Reference | ||
| Private | 31 | 10 | 1.34 | 0.93–1.94 | 1.52 | 1.05–2.22 |
| Military | 16 | 15 | 1.43 | 0.82–2.52 | 1.45 | 0.82–2.56 |
| Uninsured/Self Pay | 33 | 17 | 1.66 | 1.05–2.62 | 2.12 | 1.34–3.33 |
| Age at diagnosis | ||||||
| 15–19 | 29 | 22 | Reference | Reference | ||
| 20–39 | 184 | 13 | 0.58 | 0.41–0.82 | 0.48 | 0.32–0.71 |
| Race/Ethnicity | ||||||
| Non-Hispanic White | 132 | 14 | Reference | Reference | ||
| Non-Hispanic Black | 51 | 16 | 1.20 | 0.89–1.62 | 1.00 | 0.75–1.34 |
| Hispanic | 11 | 8 | 0.58 | 0.32–1.04 | 0.50 | 0.27–0.93 |
| Non-Hispanic Other | 14 | 15 | 1.11 | 0.67–1.85 | 0.93 | 0.57–1.50 |
| Marital status | ||||||
| Single | 100 | 14 | Reference | Reference | ||
| Married | 102 | 15 | 1.13 | 0.87–1.45 | 1.23 | 0.92–1.64 |
| Separated/Divorced | 9 | 11 | 0.83 | 0.44–1.58 | 0.79 | 0.40–1.54 |
| Cancer stage | ||||||
| In situ/Local | 68 | 9 | Reference | Reference | ||
| Regional | 76 | 18 | 1.99 | 1.47–2.70 | 1.64 | 1.20–2.25 |
| Distant | 66 | 20 | 2.18 | 1.59–2.98 | 2.14 | 1.47–3.11 |
| Cancer site | ||||||
| Hematologic | 49 | 18 | Reference | Reference | ||
| Breast | 49 | 29 | 1.60 | 1.13–2.27 | 2.33 | 1.52–3.58 |
| Gynecologic | 38 | 17 | 0.96 | 0.65–1.40 | 1.63 | 1.04–2.56 |
| Testicular | 8 | 9 | 0.51 | 0.25–1.04 | 0.77 | 0.37–1.60 |
| CNS | 7 | 8 | 0.46 | 0.22–0.98 | 1.07 | 0.46–2.50 |
| GI | 9 | 7 | 0.38 | 0.19–0.75 | 0.51 | 0.25–1.02 |
| Head/Neck/Lung | 17 | 21 | 1.15 | 0.70–1.88 | 1.48 | 0.89–2.47 |
| Sarcoma | 10 | 12 | 0.65 | 0.34–1.23 | 0.92 | 0.48–1.79 |
| Skin | 8 | 3 | 0.17 | 0.08–0.36 | 0.36 | 0.16–0.79 |
| Thyroid | 8 | 7 | 0.36 | 0.17–0.73 | 0.36 | 0.14–0.92 |
| Other | 10 | 15 | 0.80 | 0.43–1.50 | 1.44 | 0.73–2.82 |
RR, risk ratio; CI, confidence interval.
Discussion
In an analysis of nearly 1600 AYAs diagnosed and treated for cancer over 5 years at an academic cancer center, we found that 14% of AYAs were enrolled in a therapeutic clinical trial (37% were enrolled in any clinical trial), proportions of enrollment that are among the highest reported for this population.7 When compared to those with private insurance and those without health insurance, publicly insured AYAs were less likely to participate in therapeutic clinical trials. Surprisingly, uninsured AYAs in our study had the highest rates of therapeutic trial enrollment. These findings stand in contrast to two prior reports in which uninsured AYAs identified from SEER-based populations were less likely to enroll in trials compared to those with health insurance coverage.10,13 One of these studies compared enrollment rates in 2006 to 2012–2013 (following implementation of the ACA) and found that the lower likelihood for enrollment among uninsured AYAs in 2006 did not persist in 2012–2013. Enrollment rates among uninsured AYAs doubled between these time periods.13 The authors hypothesize that the lower overall proportion of uninsured AYAs made resources (foundational and institutional) more available for the remaining uninsured individuals, helping to support their enrollment on trials. Since our study drew exclusively from the post-ACA implementation period, the high proportion of enrollment observed among AYAs overall potentially reflects the impacts of increased access to health insurance for AYAs.
Additionally, an uninsured status may not serve as a proxy for limited access to financial and social resources as public health insurance may. Many AYAs are uninsured but this may be related to personal preference and priorities as opposed to lack of access to health insurance, at least for a subgroup of AYAs. A 2019 National Health Interview Survey found that 24% of uninsured 18–29 and 21% of 30–49 year-olds were uninsured because “coverage was not needed or wanted”.17 Interestingly, of the 194 uninsured AYAs in our study, 84 (43%) changed insurance status within 90 days of diagnosis. In total, 20 (24%) switched to private health plans, 53 (63%) switched to public, and 8 (10%) switched to military. This finding could also help explain why an unexpectedly high proportion of uninsured individuals at diagnosis were able to enroll on a trial. Providers may understand that many uninsured AYAs likely qualify for health insurance policies and proceed with presenting trial options and enrollment with the expectation of future coverage. Without data regarding provider motivation for trial presentation and enrollment, our study is unable to answer this question. Lastly, uninsured AYAs may have motivation to enroll in a clinical trial in which some of their medical care may be reimbursed by the trial sponsor.18
Despite these encouraging findings for uninsured AYAs, enrollment of publicly insured AYAs continues to lag behind as has been previously reported.12 Privately insured AYAs were 50% more likely and uninsured AYAs over twice as likely to participate in a therapeutic clinical trial when compared to publicly insured AYAs. We hypothesize that AYAs with public insurance may have less access to social support and financial resources (such as reliable transportation19) compared to those with private insurance, making participation in clinical trials more challenging despite having access to health insurance.
While lower enrollment among Black AYAs compared to Whites has been previously reported,12 we did not observe this difference in our study. However, as hypothesized, Hispanic AYAs in our study were less likely to enroll in a clinical trial when compared to other AYAs. This could be due to multiple factors including cultural barriers or preferences, systemic racism resulting in distrust in the medical establishment, study eligibility restrictions regarding primary language, availability of resources such as interpreters to help complete the informed consent process, provider/investigator time constraints, and health system bias. These results highlight the importance of measures to support enrollment of underrepresented populations such as through recruitment of ethnically diverse team members, support for more robust interpreter services, more time for providers and study staff to conduct interactions with non-English speaking patients, and the availability of translated study materials.
Other sociodemographic factors also impacted clinical trial enrollment among our study population. Younger AYAs were significantly more likely to enroll in trials than older age groups, which we anticipated based on findings from previous studies of increased enrollment at pediatric centers.9,10 Our findings also contribute to the mixed information from the literature about the significance of sex3,10,20 in trial enrollment among AYAs. We found that sex was not significantly associated with enrollment. While not statistically significant, married AYAs were more likely than single or separated AYAs to participate in a therapeutic trial even with controlling for insurance type. This finding suggests that perhaps married AYAs have access to higher levels of support enabling trial participation.
While not primary outcomes for our study, we observed strong associations of therapeutic trial enrollment with both cancer site and stage. Compared to hematological malignancies, an archetypal AYA cancer type, trial enrollment was higher among AYAs with breast or gynecological cancers. This is likely at least in part due to UNC's strong history of caring for patients with these cancers and a robust investigator-initiated study system leading to increased trial availability. We were not surprised to observe lower enrollment for patients with thyroid and skin cancers as these cancers are often cured surgically and have high cure rates limiting the impact of clinical trials for these cancers. Higher rates of enrollment were observed for cancers at more advanced stage (regional or distant) when compared to localized cases. With poorer outcomes among patients with more advanced cancers, clinical trials are desperately needed to advance care and improve outcomes. Interestingly, we observed a higher proportion of advanced cancers among publicly insured AYAs suggesting possible delays in diagnosis within this subgroup, a finding consistent with prior reports warranting further study.10,21
Our findings should be considered within the limitations of this study. The patients in our sample were all treated at an academic health care center. Thus, we are unable to comment on enrollment at community cancer centers where many AYAs receive cancer treatment and the impact of sociodemographic factors on enrollment in these settings. Additionally, we do not have information regarding trial availability and individual patient eligibility. Details regarding patient motivators to participate in trials or to decline were also not available based on our study methods. Finally, classification of the main independent variable of interest, health insurance payer type, was assigned at the time of diagnosis. As we presented earlier, insurance status is a fluid characteristic that changes over time and we are unable to determine whether expected insurance access impacted presentation of trial availability and enrollment. Finally, the classification of therapeutic trials at our institution includes diagnostic and behavioral interventions in addition to drug trials. Due to this classification, our estimate for the rate of therapeutic trial enrollment may be higher than that of studies that only considered therapeutic drug trials.
In summary, sociodemographic factors such as insurance payer type and race/ethnicity are associated with differential enrollment of AYAs on cancer clinical trials. Factors likely associated with fewer social, supportive, and financial resources (public health insurance and single status) may identify AYAs at higher risk for trial non-enrollment. Lower rates of enrollment among Hispanic patients compared to others point to opportunities for addressing cultural and logistical barriers to improve enrollment. By recognizing sociodemographic barriers to clinical trial enrollment among AYAs with cancer, health care systems can enact changes to enhance both trial access and enrollment to improve outcomes among vulnerable AYA subpopulations.
Acknowledgment
The authors would like to thank Emily Pfaff, MS for her help in data linkage and extraction.
Author Disclosure Statement
The authors confirm that no competing financial interests exist.
Funding Information
No funding sources for this study.
References
- 1. American Cancer Society. Cancer Facts & Figures 2020. 2020.
- 2. Barakat LP, Schwartz LA, Reilly A, et al. A Qualitative Study of Phase III Cancer Clinical Trial Enrollment Decision-Making: perspectives from Adolescents, Young Adults, Caregivers, and Providers. J Adolesc Young Adult Oncol. 2014;3(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins CL, Malvar J, Hamilton AS, et al. Case-linked analysis of clinical trial enrollment among adolescents and young adults at a National Cancer Institute-designated comprehensive cancer center. Cancer. 2015;121(24):4398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harlan LC, Lynch CF, Keegan TH, et al. Recruitment and follow-up of adolescent and young adult cancer survivors: the AYA HOPE Study. J Cancer Surviv. 2011;5(3):305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bleyer WA, Tejeda H, Murphy SB, et al. National cancer clinical trials: children have equal access; adolescents do not. J Adolesc Health. 1997;21(6):366–73. [DOI] [PubMed] [Google Scholar]
- 6. Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107(7 Suppl):1645–55. [DOI] [PubMed] [Google Scholar]
- 7. Keegan THM, Parsons HM. Adolescent angst: enrollment on clinical trials. Hematology Am Soc Hematol Educ Program. 2018;2018(1):154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiss AR, Hayes-Lattin B, Kutny MA, et al. Inclusion of Adolescents and Young Adults in Cancer Clinical Trials. Semin Oncol Nurs. 2015;31(3):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fern LA, Whelan JS. Recruitment of adolescents and young adults to cancer clinical trials—international comparisons, barriers, and implications. Semin Oncol. 2010;37(2):e1–8. [DOI] [PubMed] [Google Scholar]
- 10. Parsons HM, Harlan LC, Seibel NL, et al. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29(30):4045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin Z, Griffith MA, Rosenthal AC. Identifying and meeting the needs of adolescents and young adults with cancer. Curr Oncol Rep. 2021;23(2):17. [DOI] [PubMed] [Google Scholar]
- 12. Sanford SD, Beaumont JL, Snyder MA, et al. Clinical research participation among adolescent and young adults at an NCI-designated Comprehensive Cancer Center and affiliated pediatric hospital. Support Care Cancer. 2017;25(5):1579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parsons HM, Penn DC, Li Q, et al. Increased clinical trial enrollment among adolescent and young adult cancer patients between 2006 and 2012–2013 in the United States. Pediatr Blood Cancer. 2019;66(1):e27426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sreeraman Kumar R, Thapa R, Kim Y, et al. Higher than reported adolescent and young adult clinical trial enrollment during the “Golden Age” of melanoma clinical trials. Cancer Med. 2018;7(4):991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berchick, Edward R., Jessica C.. Barnett, and Rachel D. Upton Current Population Reports, P60-267(RV), Health Insurance Coverage in the United States:2018, U.S. Government Printing Office, Washington, DC, 2019. [Google Scholar]
- 16. National Cancer Institute. Adolescents and Young Adults with Cancer: National Cancer Institute; 2018. Accessed March 21, 2021 from: https://www.cancer.gov/types/aya
- 17. Cha A, Cohen R. Reasons for being uninsured among adults aged 18–64 in the United States, 2019. NCHS Data Brief 2020;382:1–8. [PubMed] [Google Scholar]
- 18. Cho HL, Danis M, Grady C. The ethics of uninsured participants accessing healthcare in biomedical research: a literature review. Clin Trials. 2018;15(5):509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38(5):976–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zullig LL, Fortune-Britt AG, Rao S, et al. Enrollment and racial disparities in cancer treatment clinical trials in north carolina. NC Med J. 2016;77(1):52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin S, Ulrich C, Munsell M, et al. Delays in cancer diagnosis in underinsured young adults and older adolescents. Oncologist. 2007;12(7):816–24. [DOI] [PubMed] [Google Scholar]