Abstract
Background: Alterations of astrocyte function play a crucial role in neuroinflammatory diseases due to either the loss of their neuroprotective role or the gain of their toxic inflammatory properties. Accumulating evidence highlights that cannabinoids and cannabinoid receptor agonists, such as WIN55,212-2 (WIN), reduce inflammation in cellular and animal models. Thus, the endocannabinoid system has become an attractive target to attenuate chronic inflammation in neurodegenerative diseases. However, the mechanism of action of WIN in astrocytes remains poorly understood.
Objective: We studied the immunosuppressive property of WIN by examining gene expression patterns that were modulated by WIN in reactive astrocytes.
Materials and Methods: Transcriptomic analysis by RNA-seq was carried out using primary human astrocyte cultures stimulated by the proinflammatory cytokine interleukin 1 beta (IL1β) in the presence or absence of WIN. Real-time quantitative polymerase chain reaction analysis was conducted on selected transcripts to characterize the dose-response effects of WIN, and to test the effect of selective antagonists of cannabinoid receptor 1 (CB1) and peroxisome proliferator-activated receptors (PPAR).
Results: Transcriptomic analysis showed that the IL1β-induced inflammatory response is robustly inhibited by WIN pretreatment. WIN treatment alone also induced substantial gene expression changes. Pathway analysis revealed that the anti-inflammatory properties of WIN were linked to the regulation of kinase pathways and gene targets of neuroprotective transcription factors, including PPAR and SMAD (mothers against decapentaplegic homolog). The inhibitory effect of WIN was dose-dependent, but it was not affected by selective antagonists of CB1 or PPAR.
Conclusions: This study suggests that targeting the endocannabinoid system may be a promising strategy to disrupt inflammatory pathways in reactive astrocytes. The anti-inflammatory activity of WIN is independent of CB1, suggesting that alternative receptors mediate the effects of WIN. These results provide mechanistic insights into the anti-inflammatory activity of WIN and highlight that astrocytes are a potential therapeutic target to ameliorate neuroinflammation in the brain.
Keywords: immunosuppression, inflammation, neurobiology, synthetic cannabinoids
Introduction
Neuroinflammation is a key component of neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease, and HIV-associated neurocognitive disorders (HAND).1,2 Neuroinflammation is facilitated by astrogliosis, a process wherein astrocytes react to injuries in the central nervous system with an increase in proliferation and pronounced morphological changes.3 Reactive astrocytes also exhibit altered functional properties that affect blood–brain barrier permeability, extracellular levels of neurotransmitters such as glutamate, and availability of energy substrates to neurons.4–12 A variety of factors can elicit the activation of astrocytes, including those released by reactive microglia (e.g., cytokines), injured neurons (e.g., glutamate and reactive oxygen species), and pathogenic proteins from exogenous sources (e.g., viruses and bacteria).13,14 Astrocyte reactivity triggers several intracellular signaling cascades that activate transcription factors (TFs) such as NF-κB, CCAAT-enhancer-binding proteins (CEBP), activator protein (AP) 1, and others.15–17 Upon activation, these TFs regulate the expression of genes involved in inflammation, mitochondrial function, and cell adhesion, all of which are altered in neurodegenerative diseases.2,18 Reactive astrocytes express inflammatory genes, including cytokines such as interleukin 6 (IL6) and interferon, and complement proteins such as complement component 3 (C3), which are all implicated in the pathogenesis of neurodegenerative diseases.19–23 C3 expression is implicated in inflammatory responses in AD and HAND.24–28 Astrocytes also express TFs that suppress inflammatory transcriptional responses such as peroxisome proliferator-activated receptors (PPAR)α and γ, which are implicated in neuroprotective mechanisms,29–32 or members of the SMAD family of TFs, which are known mediators of the transforming growth factor-β anti-inflammatory function.33 Due to these dynamic functions of astrocytes, they are increasingly recognized as potential therapeutic targets to mitigate chronic neuroinflammation in neurodegenerative diseases.34
Multiple lines of evidence indicate that cannabinoids and cannabinoid receptor agonists exert anti-inflammatory properties,35–37 in part, by activating PPARα or γ.37–41 Specifically, WIN-55212-2 (WIN) is an aminoalkylindole that acts as potent agonist of cannabinoid receptors 1 and 2 (CB1 and CB2) at concentrations in the nanomolar range and with 20-fold higher affinity for CB2.42 WIN has been shown to attenuate inflammatory responses in cellular or animal models of neuroinflammation.36,37,43–47 However, the effects of WIN on the regulation of global gene expression changes in reactive astrocytes have not been studied. The identification of genes and pathways modulated by WIN in reactive astrocytes may reveal a promising therapeutic strategy for the prevention and treatment of neurodegenerative diseases.
In this study, we profiled the transcriptome of human primary astrocyte cultures after stimulation with recombinant IL1β in the presence or absence of WIN. Bioinformatic analysis showed that the IL1β-induced inflammatory response is robustly inhibited by WIN pretreatment. Pathway analysis suggested that the anti-inflammatory properties of WIN were linked to the regulation of kinase pathways and gene targets of neuroprotective TFs, including PPAR and SMAD. Using astrocytes from a different donor, we showed that the inhibitory effects of WIN were independent of CB1 or PPARα/γ receptors. Overall, these data support the therapeutic potential of targeting astrocytes to prevent or reverse inflammatory gene expression and reveal a potential mechanism, by which cannabinoids ameliorate inflammatory responses in astrocytes.
Materials and Methods
Primary human astrocyte cultures
The cell model for astrocytes was approved by the University of California San Diego Human Research Protections Program and the National Institutes of Health as part of a grant actively funded by the National Institute for Mental Health. Astrocytes were isolated from fetal human brain tissue from elective terminated pregnancy between 12 and 16 weeks of gestation, acquired from Advanced Bioscience Resources. Donors gave written informed consent for research-use of the cells and tissue. Tissue was fragmented and mechanically dissociated using a scalpel and washed three times with HBSS media (cat. no. 14175-095; Gibco) with 1 mM Glutamax (cat. no. 35050-061; Gibco), 20 μg/mL Gentamicin (cat. no. 15710-064; Gibco), and 5 mM HEPES (cat. no. 15630-080; Gibco). The tissue was homogenized with the addition of 15 mL of 0.25% trypsin EDTA (cat. no. 25200-056; Gibco) for 5 min in a 37°C incubator. After 5 min, 1 mL of a trypsin inhibitor (cat. no. 10109; Roche) and 24 mL of DMEM (cat. no. 11960-044; Gibco) with human serum (cat. no. 35-060-cl; Corning) was added. The mixture was then centrifuged for 5 min at 4°C to pellet the cells. The cells were resuspended in 5 mL of DMEM and strained with a 70 μM strainer (cat. no. 352350; Falcon). The cell suspension was underlaid with 7 mL of a solution of filtered 8% BSA in PBS and cells were centrifuged at 1×104 rpm at 4°C for 10 min. The cells were resuspended in DMEM with human serum, 1 mM GlutaMAX, and 20 μg/mL gentamicin. Astrocytes were plated at a density of 1×107/T75 flask and cultured as adherent monolayers. After 1 week, the DMEM with human serum was replaced with DMEM with 10% fetal bovine serum (cat. no. 16000044; Gibco) and 1% penicillin/streptomycin (cat. no. 30-001-CI-1; Corning). Every 3 days, a half media exchange was performed. One donor line was used for the transcriptomic analysis and different passages of an additional donor line were used for the real-time quantitative polymerase chain reaction (RT-qPCR) experiments.
Treatments for RNA-seq experiment
Primary astrocytes were treated with WIN (cat. no. 1038; R&D Systems) and IL1β (cat. no. rcyec-hil1b; InvivoGen) at concentrations of 10 μM and 10 ng/mL, respectively. DMSO was used as vehicle treatment. After treatment for 24 h with vehicle and WIN, IL1β was added for 6 h before RNA extraction. IL1β was used because of its widespread role in neuroinflammatory diseases.48–50 While many inflammatory cytokines are implicated in neurodegenerative diseases, IL1β is one of first and most robustly expressed cytokines in models for inflammation, including in vitro astrocyte cultures.51–54
RNA isolation and RT-qPCR
RNA was extracted with RNeasy plus mini kit (cat. no. 74136; Qiagen) according to manufacturer's instructions. After cDNA synthesis, gene expression was determined using TaqMan gene expression assays using primers specific to IL6 (cat. no. hs00174131; TaqMan), C3 (cat. no. hs00163811; TaqMan), and ActB (cat. no. 1612290; Applied Biosystems). The PCRs were carried out using 2×Fast advanced master mix (cat. no. 4444557; Thermo Fisher Scientific) at 48°C for 30 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Fold-change of mRNA transcripts compared to vehicle-treated cells was calculated using the comparative CT method, as previously described.9,55
WIN dose-response on IL1β-stimulated human astrocytes
Astrocyte cultures were treated with WIN at 100 nM, 1 mM, 10 μM, and 20 μM for 24 h and then treated with IL1β (10 ng/mL) for 6 h before RNA isolation for analysis by RT-qPCR. The concentration of IL1β used (10 ng/mL) is consistent with the relevant literature.56,57 Moreover, mouse models utilizing adenovirus-driven IL1β overexpression in the brain achieved expression levels of ∼10 ng/mg total protein 7 days postinjections58 or a mean of 41 ng in the whole striatum 8 days postinjections.59
Time-dependent effects of WIN on IL1β-stimulated human astrocytes
To determine how time of exposure to WIN affects IL1β-induced changes in astrocyte gene expression, astrocytes were treated with WIN (20 μM) in one of three ways: (1) 24 h before IL1β, (2) same time as IL1β, or (3) 1 h after IL1β. IL1β was added at 10 ng/mL for 6 h.
Treatment of astrocytes with WIN and IL1β, and with PPARα, PPARγ, CB1, and CB2 selective inhibitors
To inhibit PPARα, PPARγ, and CB1, the following antagonists we used, respectively: GW9662 (cat. no. M6191; 10 μM; Sigma-Aldrich), GW6471 (10 μM), and SR141617 (16 nM). Inhibitors were added to the cells for 30 min followed by WIN (10 μM) for 24 h and then IL1β at 10 ng/mL for 6 h before RNA isolation.
RNA-seq library preparation
RNA was isolated as described above. RNA integrity was measured using an Agilent 2100 Bioanalyzer (Santa Clara, CA) with an RNA Integrity Number ≥8.5. cDNA libraries were prepared with 1 μg of starting total RNA using the Illumina TruSeq RNA Library Kit (Illumina, Inc., San Diego, CA). The libraries were amplified via 15 cycles of PCR and the amplified library was sequenced using an Illumina HiSeq 4000.
RNA-seq bioinformatics analysis
Raw sequencing data were processed using Rosalind developed by OnRamp BioInformatics, Inc., (San Diego, CA). Reads were trimmed using cutadapt.60 Quality scores were assessed using FastQC. Reads were aligned to the Homo sapiens genome build hg19 using STAR.61 Individual sample reads were quantified using HTseq62 and normalized via Relative Log Expression using DESeq2 R library. Sample-to-sample variation was assessed using a correlation matrix of Pearson's correlation coefficients (Supplementary Fig. S1A). Downstream analysis was performed using raw counts and R packages supported by integrated Differential Expression and Pathway analysis.63 Exploratory data analysis was performed after data were transformed using the regularized-logarithm (rlog) transformation function of the DESeq2 package.64 Hierarchical clustering was computed by ranking all genes by standard deviation across all samples and the top 2000 genes were selected based on the distribution of variance (Supplementary Fig. S1B). Principal component analysis (PCA) was performed using the matrix of rlog normalized read counts. The first and second principal components are shown to describe the largest variability in the dataset. Differential gene expression analysis was conducted using DESeq2 with false discovery rate (FDR) smaller than 0.01 and fold change (FC) larger than 2 as cutoffs. All comparisons were generated using the vehicle-treated samples as baseline. Volcano plots for each comparison show statistical significance (negative log of FDR) versus FC. Functional annotation of differentially expressed genes (DEGs) was done with enrichment analysis of biological pathways supplied by KEGG (Kyoto Encyclopedia of Genes and Genomes). For k-means clustering, we used the rlog transformed data and ranked the first 2000 genes by standard deviation. Based on the within-group sum of squares plot (Supplementary Fig. 1C) as a reference, we chose k=4. Enrichment analysis is conducted for each cluster using Gene Ontology (GO) biological process terms. For pathway analysis, we used the FC values returned by DESeq2 and applied Parametric Analysis of Gene Set Enrichment using FDR <0.05 as cutoff.65 Gene sets associated to TFs were identified using the Transcriptional Regulatory Element Database.66
Statistical analysis
RT-qPCR experiments using astrocyte cell cultures were performed in biological duplicates in astrocytes derived from an independent donor. Different passages were used to run different experiments in Figures 5–7. The bars represent the mean of biological replicates. The error bars represent standard error of the mean. Data were analyzed by one-way analysis of variance (ANOVA) and post hoc analyses using Tukey's Test using GraphPad Prism. Differences in means were considered significant if p<0.05.
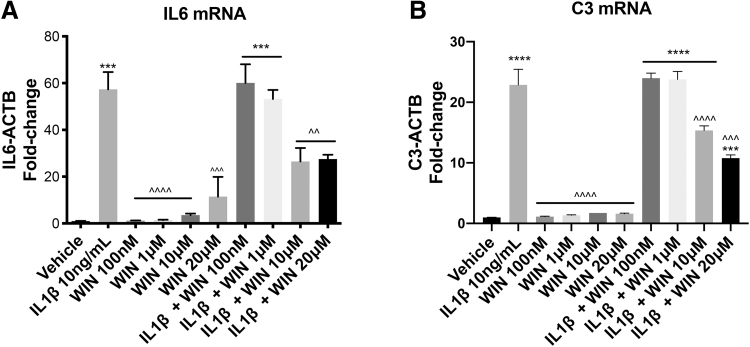
FIG. 5.
WIN reduced IL1β-induced inflammatory gene expression in astrocytes in a dose-dependent manner. Fold-change of IL6 (A) or C3 (B) mRNA levels normalized to ACTB mRNA levels in total RNA isolated from human astrocytes. One-way ANOVA was conducted for the effect of treatment on IL6 [F (9, 10)=24.83, p<0.0001] and C3 [F (9, 10)=107.3, p<0.0001]. A post hoc Tukey's test was conducted; corrected p-values are shown (***p<0.001; ****p<0.0001 vs. vehicle; ^^p<0.01, ^^^p<0.001, ^^^^p<0.0001 vs. IL1β-treated cells). ANOVA, analysis of variance.
FIG. 6.
WIN treatment before, at the same time, and after IL1β treatment robustly blocked IL1β-induced inflammatory gene expression. (A–C) Fold-change of IL6 mRNA transcript levels normalized to ACTB mRNA levels in total RNA isolated from human astrocytes. One-way ANOVA was conducted for WIN 24 h before IL1β [F (3, 4)=377.6, p<0.0001], WIN at the same time as IL1β [F (3, 4)=91.26, p=0.0004], WIN after IL1β [F (3, 4)=90.3, p=0.0004]. (D–F) Fold-change of C3 mRNA transcript levels normalized to ACTB mRNA levels in total RNA isolated from human astrocytes. One-way ANOVA was conducted for WIN 24 h before IL1β [F (3, 4)=233.8, p<0.0001], WIN at the same time as IL1β [F (3, 4)=148.4, p=0.0001], WIN after IL1β [F (3, 4)=270.0, p<0.0001]. A post hoc Tukey's test was conducted; corrected p-values are shown (**p<0.01; ***p<0.001; ****p<0.0001 vs. vehicle, ^^p<0.01, ^^^p<0.001, ^^^^p<0.0001 vs. IL1β-treated cells).
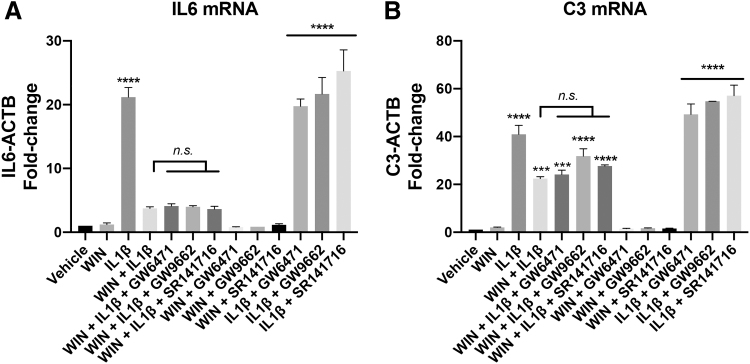
FIG. 7.
PPARα, PPARγ, and CB1 antagonists did not block the inhibitory effect of WIN on the IL1β-induced inflammatory gene transcription. (A) Fold-change of IL6 mRNA transcript levels normalized to ACTB mRNA levels in total RNA isolated from human astrocytes. One-way ANOVA was conducted [F (12, 13)=55.96, p<0.0001]. (B) Fold-change of C3 mRNA transcript levels normalized to ACTB mRNA levels in total RNA isolated from human astrocytes. One-way ANOVA was conducted [F (12, 13)=90.84, p<0.0001]. A post hoc Tukey's test was conducted; corrected p-values are shown (***p<0.001, ****p<0.0001 vs. vehicle; n.s. vs. WIN+IL1β). CB1, cannabinoid receptor 1; PPAR, peroxisome proliferator-activated receptors.
Data availability
Raw sequencing files and processed transcript counts generated in this study are deposited on the GEO at accession GSE160092.
Results
RNA-seq analysis showed that substantial transcriptional changes were induced by IL1β and WIN in primary astrocytes
To evaluate the effect of WIN on the global transcriptional responses induced by IL1β in astrocytes, we performed transcriptomic analysis by RNA-seq. Astrocytes were treated with or without WIN (24 h) before stimulation with IL1β (6 h). Control RNA was extracted from vehicle-treated cultures. To assess variability among replicate samples, we used a Pearson's correlation matrix of normalized read counts, which showed that the variation was minimal with correlation coefficients (r) >0.9 (Supplementary Fig. S1A). This result was confirmed by different clustering methods, including hierarchical clustering (Fig. 1A), PCA (Fig. 1B) and k-means clustering (Fig. 3). To explore the overall variation in gene expression, we used hierarchical clustering and showed that both WIN and IL1β treatments induced substantial gene expression changes in astrocytes (Fig. 1A). The PCA showed expected grouping among replicates and sample groups spread across the two PCs (Fig. 1B). The PCA plot highlighted that there was a clear difference between WIN- and IL1β-treated samples. Overall, these results demonstrated that astrocytes showed a strong transcriptional response following IL1β and WIN treatments.
FIG. 1.
Exploratory data analysis of transcriptomic data. (A) Hierarchical clustering of top 2000 genes showing that the variation between replicates was minimal and that substantial changes were induced by IL1β and WIN treatments. (B) PCA plot showing that the majority of variance is explained by PC1 (68%) and PC2 (21%). IL, interleukin; PCA, principal component analysis. Color images are available online.
FIG. 3.
WIN diminished the IL1β-induced inflammatory response. (A) k-Means clustering visualizing 4 groups using the top 2000 differentially expressed genes. (B) The top GO biological process terms from each cluster is shown with associated p values. (C) For each cluster, the normalized expression values for three representative genes are plotted and error bars represent standard deviation values. GO, Gene Ontology. Color images are available online.
The IL1β-induced inflammatory transcriptional response in astrocytes was ameliorated by the cannabinoid receptor agonist WIN
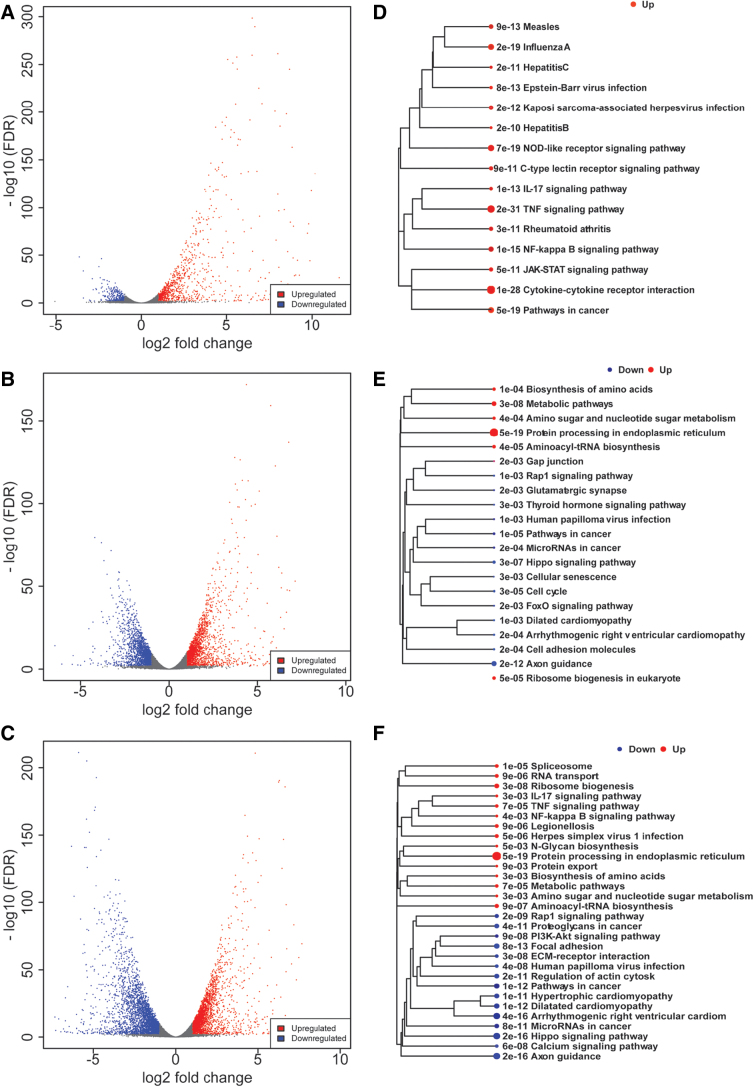
Comparing IL1β, WIN, and IL1β+WIN-stimulated cells with vehicle-treated cells, we identified 1204, 3827, and 6046 genes differentially regulated genes, respectively (Fig. 2A–C). To functionally annotate the up- and downregulated genes, we performed pathway enrichment analysis using KEGG pathways (Fig. 2D–F). As expected, the IL1β-induced genes (n=914) were significantly enriched with pathways related to inflammatory response, such as TNF signaling, cytokine–cytokine receptor interaction, and NF-κB signaling (Fig. 2D). We did not identify pathways significantly enriched for the IL1β repressed genes, consistent with the small fraction of genes downregulated in this group (n=290). For WIN-regulated genes, the enriched pathways were distinct for up- and downregulated genes (Fig. 2E). The genes repressed by WIN (n=1872) were involved in axon guidance and hippo signaling pathways. The genes induced by WIN (n=1955) were related in protein processing in endoplasmic reticulum. For genes regulated in the presence of both WIN and IL1β (Fig. 2F), the upregulated genes (n=2797) were related to inflammation and protein processing in the endoplasmic reticulum, consistent with the pathways modulated by each individual treatment. The enrichment analysis of downregulated genes (n=3249) in presence of WIN and IL1β identified several kinase pathways, including PI3K-Akt signaling and Hippo signaling, suggesting that WIN activates specific signaling cascades and that the modulation of these pathways may be involved in the immunosuppressive action of WIN.
FIG. 2.
IL1β and WIN treatments induced substantial changes in gene expression. Volcano plots visualizing the up- and downregulated genes by plotting the statistical significance (−log 10 FDR) versus the log2-fold change for pairwise comparisons between IL1β versus vehicle (A), WIN versus vehicle (B) and WIN+IL1β versus vehicle (C). (D–F) Enrichment KEGG pathways analysis of DEGs is shown for each pairwise comparison. The relationship among enriched KEGG pathways is visualized as a tree. DEGs, differentially expressed genes; FDR, false discovery rate; KEGG, Kyoto Encyclopedia of Genes and Genomes. Color images are available online.
To gain insight into the molecular pathways underlying different patterns of gene expression in activated astrocytes that are exposed to WIN, we used k-means clustering, an unsupervised method for clustering genes into groups based on their expression pattern across all samples. Figure 3 shows the gene clusters (Fig. 3A), associated functional annotations (Fig. 3B and Table 1) based on the most enriched GO terms, and representative genes from each cluster (Fig. 3C). Clusters C and D were strongly enriched in genes related to inflammatory responses. These clusters of genes were upregulated in astrocytes stimulated with IL1β and downregulated in astrocytes pretreated with WIN. On the contrary, genes in clusters A and B were independent of IL1β and showed to be regulated by WIN. Cluster A included genes induced by WIN that were enriched in response to unfolded proteins. Cluster B contained genes repressed by WIN that were related to cell migration. These GO enrichment results confirmed the pathway enrichment analysis of DEGs in Figure 2.
Table 1.
Gene Ontology Terms Enriched for Different Clusters
| Cluster | Adj.p | Pathways |
|---|---|---|
| A | 3.00E-17 | Endoplasmic reticulum unfolded protein response |
| A | 1.93E-16 | Response to endoplasmic reticulum stress |
| A | 1.20E-15 | Response to unfolded protein |
| A | 1.20E-15 | Cellular response to unfolded protein |
| A | 2.43E-15 | Response to topologically incorrect protein |
| A | 2.48E-14 | Cellular response to topologically incorrect protein |
| A | 5.93E-09 | IRE1-mediated unfolded protein response |
| A | 8.19E-06 | Carboxylic acid metabolic process |
| A | 9.96E-06 | Oxoacid metabolic process |
| A | 1.29E-04 | ER-nucleus signaling pathway |
| A | 1.35E-04 | Organic substance transport |
| A | 1.62E-04 | PERK-mediated unfolded protein response |
| A | 4.27E-04 | Protein exit from endoplasmic reticulum |
| A | 4.27E-04 | Golgi vesicle transport |
| B | 7.97E-31 | Anatomical structure morphogenesis |
| B | 4.46E-28 | Cell migration |
| B | 2.07E-27 | Movement of cell or subcellular component |
| B | 2.04E-26 | Regulation of developmental process |
| B | 2.65E-26 | Cell motility |
| B | 4.25E-25 | Locomotion |
| B | 2.67E-24 | Tissue development |
| B | 8.43E-23 | Cellular developmental process |
| B | 2.62E-22 | Regulation of cell migration |
| B | 6.94E-22 | Regulation of multicellular organismal process |
| B | 7.77E-22 | Regulation of cell motility |
| B | 2.64E-21 | Animal organ development |
| B | 3.38E-21 | Cell differentiation |
| B | 3.73E-21 | Regulation of signaling |
| C | 1.44E-24 | Response to cytokine |
| C | 8.86E-24 | Immune system process |
| C | 1.93E-23 | Cellular response to chemical stimulus |
| C | 2.21E-23 | Defense response |
| C | 5.56E-23 | Response to organic substance |
| C | 5.58E-22 | Response to virus |
| C | 5.58E-22 | Cellular response to organic substance |
| C | 6.63E-22 | Cellular response to cytokine stimulus |
| C | 5.98E-21 | Response to stress |
| C | 1.89E-20 | Regulation of multicellular organismal process |
| C | 5.45E-20 | Response to external stimulus |
| C | 5.45E-20 | Defense response to virus |
| C | 3.05E-19 | Positive regulation of response to stimulus |
| C | 4.27E-19 | Positive regulation of multicellular organismal process |
| C | 7.77E-19 | Cell proliferation |
| D | 5.83E-51 | Response to cytokine |
| D | 4.71E-49 | Response to external biotic stimulus |
| D | 9.40E-48 | Cytokine-mediated signaling pathway |
| D | 4.51E-46 | Defense response |
| D | 1.20E-45 | Cellular response to cytokine stimulus |
| D | 2.07E-41 | Immune response |
| D | 1.27E-39 | Immune system process |
| D | 2.37E-37 | Response to external stimulus |
| D | 2.47E-32 | Cellular response to organic substance |
| D | 4.94E-32 | Response to organic substance |
| D | 2.22E-30 | Multiorganism process |
| D | 1.06E-29 | Response to stress |
| D | 1.59E-29 | Inflammatory response |
Overall, this unsupervised analysis showed that the inflammatory response activated by IL1β was strongly ameliorated in the astrocytes pretreated with WIN (Fig. 3B) and suggested that modulation of specific signaling pathways (e.g., PI3K-Akt) could mediate the repressive action of WIN. Moreover, these results showed that WIN induced substantial gene expression changes in resting astrocytes independently of IL1β stimulation.
TF enrichment analysis revealed TFs associated with inflammatory responses in astrocytes
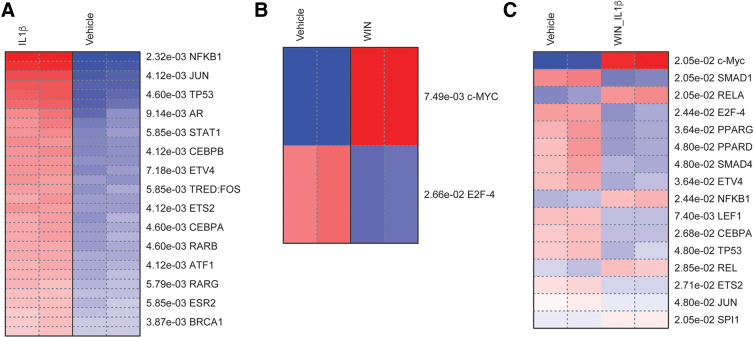
To explore gene regulatory mechanisms underlying different patterns of gene expression, we performed pathway analysis to identify coherently altered upstream TFs associated to DEGs. This analysis confirmed that IL1β induced the activity of TFs known to regulate inflammatory genes induced by cytokines in immune cells, such as NF-κB, CEBP, and STAT (Fig. 4A).15–17,67 The analysis of genes regulated by WIN suggested that MYC was associated with upregulated genes and that E2F4 was associated with downregulated genes. Finally, we analyzed the genes regulated in presence of WIN and IL1β (Fig. 4C). Among TFs associated with the action of WIN in presence of IL1β, PPAR and SMAD were of particular interest, as they have been linked to neuroprotective and anti-inflammatory pathways (Fig. 4C).8,38,68 Overall, this analysis revealed potential gene regulatory mechanisms underlying the anti-inflammatory properties of cannabinoid receptor agonists.
FIG. 4.
TFs associated with inflammatory responses in astrocytes. Pathway analysis of DEGS was performed using the TRED database. Enriched TF are ranked by p-values (FDR <0.05 cutoff). Heatmaps are shown for pairwise comparisons between IL1β versus vehicle (A), WIN versus vehicle (B) and WIN+IL1β versus vehicle (C). TF, transcription factor; TRED, Transcriptional Regulatory Element Database. Color images are available online.
WIN inhibits the transcriptional activation of IL1β-induced inflammatory genes
To determine the dose-response curve of WIN-mediated inhibition of IL1β-induced genes, we used primary human astrocyte cultures that were exposed to increasing doses of WIN and then treated with vehicle or IL1β for 6 h. The expression of two inflammatory genes, IL6 and C3, was analyzed by RT-qPCR. IL1β robustly increased (∼55-fold) levels of IL6 mRNA, while increasing doses of WIN had no effect on levels of IL6 mRNA (Fig. 5A). WIN blocked IL1β-induced IL6 mRNA starting at 10 μM and no further reduction was seen with 20 μM WIN (Fig. 5A). IL1β induced a ∼23-fold increase in C3 mRNA levels compared to vehicle and this induction was also blocked by 10 and 20 μM WIN by ∼35% and 55%, respectively (Fig. 5B). These results showed that WIN exhibited a repressive effect on the transcriptional activation of inflammatory genes starting at concentration of 10 μM in vitro.
WIN treatment before, at the same time, and after IL1β treatment robustly blocked IL1β-induced inflammatory gene expression
To better understand how WIN affects inflammatory gene expression in astrocytes, cells were exposed to WIN 24 h before, at the same time, and 1 h after exposure to IL1β. After 6 h of IL1β treatment, total RNA was isolated and analyzed for IL6 and C3 mRNA levels. IL1β induced a robust increase in IL6 mRNA levels (Fig. 6A–C). WIN treatment 24 h before IL1β reduced IL1β mRNA by over 90% (Fig. 6A), while WIN treatment at the same time or 1 h after IL1β treatment resulted in a decrease of ∼75% compared to IL1β treated cells (Fig. 6B, C). WIN treatment for 30 h induced a significant increase in C3 mRNA compared to vehicle-treated cells (Fig. 6D). WIN-mediated effects on C3 mRNA levels were not as robust as the effects on IL6 levels, with WIN mediating ∼30% reductions in IL1β-induced C3 mRNA levels when WIN was used 24 h before, at the same time, or after IL1β exposure (Fig. 6D–F). Collectively, these data suggest that the anti-inflammatory effects of WIN affect the expression of genes differently, likely depending upon the gene-specific regulatory mechanisms of transcription. The anti-inflammatory effects of WIN are, however, robust when used before, concomitant with, or after the inflammatory cytokine IL1β.
PPARα, PPARγ, and CB1 antagonists did not block the inhibitory effect of WIN on the IL1β-induced inflammatory gene transcription
To determine the involvement of PPAR and cannabinoid receptors, we sought to use selective antagonists against PPARα, PPARγ, and CB1 receptors to modulate the inhibitory effects of WIN on the activation of IL6 and C3 mRNAs. We did not include CB2 due to undetectable or very low levels of expression of this gene in the primary astrocytic cultures (Supplementary Fig. S1D).
Astrocyte cultures were treated with or without WIN in the presence of GW6471 (10 μM, PPARα), GW9662 (10 μM, PPARγ), and SR141617 (16 nM, CB1). Treatment with IL1β induced a 20-fold increase in IL6 mRNA levels and this was reduced by ∼85% by WIN pretreatment. None of the selective inhibitors had a significant effect of reversing WIN-mediated inhibition (Fig. 7A). The PPARγ inhibitor with IL1β showed modest increases in IL6 levels compared to IL1β alone, but the differences were not significant (Fig. 7A). The lack of a modulatory effect of the PPARγ inhibitor is in contrast to results that we previously reported in astrocytes derived from a different donor, suggesting that the pathways mediating IL1β and WIN activity may vary by donor.8
IL1β induced a ∼40-fold increase in C3 mRNA levels compared to vehicle-treated cells. WIN reduced the IL1β-induced changes by ∼50% and treatment with a PPARα and γ, and CB1 antagonists did not reverse the effects of WIN (Fig. 7B). The selective antagonists had no effect on C3 mRNA levels in the presence of WIN, but they all increased the levels of IL1β-induced C3 mRNA compared to IL1β alone, although the differences did not reach significance (Fig. 7B). Overall, these data suggest that the repressive effects of WIN did not depend on CB1 or PPAR. However, the involvement of these pathways may vary by donor.
Discussion
This work provides, for the first time, detailed transcriptomic analyses of IL1β-activated human astrocytes in the presence or absence of the cannabinoid receptor agonist WIN. The analysis of differentially expressed genes revealed the anti-inflammatory effect of WIN at a genome-wide level and identified specific signaling cascades regulated by IL1β and/or WIN. By using selective inhibitors and independent lines of human astrocytes, we showed that the anti-inflammatory effects of WIN are independent of canonical CB1 receptor.
The robust induction of gene expression by IL1β is consistent with published findings showing that astrocytes are highly reactive to inflammatory cytokines and conditioned media from immunoactivated monocyte-derived macrophages and microglia.15,69 Upon stimulation, astrocytes secrete inflammatory cytokines that act in autocrine and paracrine manners.70,71 Reactive astrocytes also express adhesion molecules and chemokines that influence blood–brain barrier permeability and the passage of immune cells from the periphery into the brain.72 Unchecked, the inflammatory cycle may lead to neurodegeneration. Indeed, the transcriptomic changes presented here are consistent with these astrocytic properties and with molecular signatures of neuroinflammatory diseases such as AD and HAND.25,73,74 In particular, activation of transcriptional networks involving responses to viral infection, autoimmunity, and cancer shown in Figure 2D are consistent with in vitro and in vivo studies on astrocytes in neurodegenerative diseases.2,25,75
WIN has been studied extensively for its anti-inflammatory effects, but no studies have detailed the genome-wide effects of WIN on human astrocytes. WIN-mediated activation of the unfolded protein response corroborates previous studies showing that the cannabinoid receptor agonist stresses the function of the endoplasmic reticulum.76 The activation of PI3 kinase signaling in activated astrocytes treated with WIN is consistent with previous reports suggesting that PI3 kinase mediates a neuroprotective role of WIN.77
Clustering analysis highlighted the suppressive effects of WIN on transcriptional responses induced by IL1β. These data are consistent with studies in animal models that show that cannabinoids can slow progression of neurodegenerative disease and behavioral deficits through reductions in inflammatory responses.78–80 Follow-up experiments are needed to determine if the immunosuppressive effect is a property of other cannabinoids. In future studies, it will be important to assess toxicity using in vitro and in vivo models to optimize the therapeutic use of WIN and other cannabinoids. However, the fact that WIN robustly reversed many of the IL1β-induced transcriptomic changes bodes well for therapeutic targeting of astrocytes to disrupt chronic inflammation in the brain as is prevalent in the neurodegenerative diseases AD and HAND.
Bioinformatic analysis of the promoter of DEGs revealed that the consensus motif of PPAR and SMAD TFs are enriched in IL1β-induced genes that are inhibited by WIN. Previous studies have suggested that the anti-inflammatory effects of WIN and other cannabinoids may be independent of CB1 and CB2 receptors.81–83 Other studies, including our own, support a potential role for the PPAR family in mediating the anti-inflammatory effects of WIN and possibly other cannabinoids.8,38,40 However, using a new donor line, the immunosuppressive effect of WIN on gene transcription was not reversed by selective antagonists of PPARα and PPARγ, suggesting that the pathways mediating the effects of WIN on astrocytes may vary by donor. Follow-up experiments comparing different donor lines are needed to confirm whether genetic background is an important factor in determining the effects of WIN in astrocytes.
This comprehensive dataset generated from primary human astrocytes should be viewed in the context of the following limitations. Any study of only a single brain cell in a culture is limited in its implications as astrocytes function in concert with other brain cell types.69,84,85 It will be important in future studies to compare the transcriptome of astrocytes from in vitro cultures with those isolated from diseased brain tissues with the goal of determining the degree to which in vitro reactive astrocytes reflect the condition in neurodegenerative diseases. Moreover, donor-specific effects should be addressed when using human tissues. Using two donor lines, we showed that the immunosuppressive effect of WIN was independent of the specific donor line; however, we did not reproduce the modulatory effect of PPARγ inhibitors in contrast to results previously published using a different astrocyte donor line. Further studies using astrocytes generated from multiple independent genetic backgrounds are needed to capture interindividual variabilities in the response to inflammatory stimuli and cannabinoid receptor agonists. These interindividual differences in anti-inflammatory effects highlight the potential of using personalized medicine to identify therapeutic strategies for neurodegenerative diseases. An additional limitation is the use of a single inflammatory cytokine, which is of limited relevance to the multifactorial aspect of neuroinflammatory conditions. Moreover, these data represent only a single snapshot of the transcriptome of astrocytes, which likely changes over time.
In conclusion, our results suggest that reactive astrocytes may contribute to some of the molecular signatures associated with inflammation in neurodegenerative diseases, and that these signatures and therapeutic strategies to reverse them can be modeled in vitro by stimulating primary human astrocytes with the inflammatory cytokine IL1β. The global anti-inflammatory activity of WIN may explain recent studies that suggest cannabis is neuroprotective in AD and HAND.78,86 Future studies examining the effects of different stimuli and combinations of varying proportions on astrocytes and other brain cells may lead to better modeling of and therapeutic testing for neurodegenerative diseases.
Supplementary Material
Acknowledgments
The authors thank Drs. Dario Meluzzi and Hairi Li for assistance with server management for bioinformatic analysis and data submission to GEO database.
Abbreviations Used
- AD
Alzheimer's disease
- ANOVA
analysis of variance
- AP
activator protein
- CB1
cannabinoid receptor 1
- CEBP
CCAAT-enhancer-binding proteins
- DEGs
differentially expressed genes
- FC
fold change
- FDR
false discovery rate
- GO
Gene Ontology
- HAND
HIV-associated neurocognitive disorders
- IL1β
interleukin 1 beta
- PCA
principal component analysis
- PPAR
peroxisome proliferator-activated receptors
- rlog
regularized-logarithm
- RT-qPCR
real-time quantitative polymerase chain reaction
- TFs
transcription factors
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by National Institutes of Mental Health (grants MH115819, NS105177 to J.A.F.); the National Institute on Aging (grant AG066215 to J.A.F.); and the National Institute of Drug Abuse (DP1DA042232 to F.T.).
Supplementary Material
Cite this article as: Fields JA, Swinton MK, Montilla-Perez P, Ricciardelli E, Telese F (2022) The cannabinoid receptor agonist, WIN-55212-2, suppresses the activation of proinflammatory genes induced by interleukin 1 beta in human astrocytes, Cannabis and Cannabinoid Research 7:1, 78–92, DOI: 10.1089/can.2020.0128.
References
- 1. Colombo E, Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunol. 2016;37:608–620. [DOI] [PubMed] [Google Scholar]
- 2. Canchi S, Swinton MK, Rissman RA, et al. . Transcriptomic analysis of brain tissues identifies a role for CCAAT enhancer binding protein beta in HIV-associated neurocognitive disorder. J Neuroinflammation. 2020;17:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2014;7:a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Norden DM, Trojanowski PJ, Villanueva E, et al. . Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liddelow SA, Guttenplan KA, Clarke LE, et al. . Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang T, Cadenas E. Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell. 2014;13:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yin F, Sancheti H, Patil I, et al. . Energy metabolism and inflammation in brain aging and Alzheimer's disease. Free Radic Biol Med. 2016;100:108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swinton MK, Carson A, Telese F, et al. . Mitochondrial biogenesis is altered in HIV+ brains exposed to ART: implications for therapeutic targeting of astroglia. Neurobiol Dis. 2019;130:104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fields JA, Swinton MK, Carson A, et al. . Tenofovir disoproxil fumarate induces peripheral neuropathy and alters inflammation and mitochondrial biogenesis in the brains of mice. Sci Rep. 2019;9:17158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Natarajaseenivasan K, Cotto B, Shanmughapriya S, et al. . Astrocytic metabolic switch is a novel etiology for Cocaine and HIV-1 Tat-mediated neurotoxicity. Cell Death Dis. 2018;9:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr HIV Res. 2012;10:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cisneros IE, Ghorpade A. Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes. Neuropharmacology. 2014;85:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. [DOI] [PubMed] [Google Scholar]
- 14. Efremova L, Chovancova P, Adam M, et al. . Switching from astrocytic neuroprotection to neurodegeneration by cytokine stimulation. Arch Toxicol. 2017;91:231–246. [DOI] [PubMed] [Google Scholar]
- 15. Fields J, Ghorpade A. C/EBPbeta regulates multiple IL-1beta-induced human astrocyte inflammatory genes. J Neuroinflammation. 2012;9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li YX, Sibon OCM, Dijkers PF. Inhibition of NF-kappaB in astrocytes is sufficient to delay neurodegeneration induced by proteotoxicity in neurons. J Neuroinflammation. 2018;15:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao K, Wang CR, Jiang F, et al. . Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia. 2013;61:2063–2077. [DOI] [PubMed] [Google Scholar]
- 18. Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harb Persp Med. 2012;2:a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cisneros IE, Erdenizmenli M, Cunningham KA, et al. . Cocaine evokes a profile of oxidative stress and impacts innate antiviral response pathways in astrocytes. Neuropharmacology. 2018;135:431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rogers J, Cooper N, Webster S, et al. . Complement activation by beta-amyloid in Alzheimer's disease. Proc Natl Acad Sci USA. 1992;89:10016–10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goetzl EJ, Schwartz JB, Abner EL, et al. . High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol. 2018;83:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fiala M, Rhodes RH, Shapshak P, et al. . Regulation of HIV-1 infection in astrocytes: expression of Nef, TNF-alpha and IL-6 is enhanced in coculture of astrocytes with macrophages. J Neurovirol. 1996;2:158–166. [DOI] [PubMed] [Google Scholar]
- 23. Heyser C, Masliah E, Samimi A, et al. . Progressive cognitive decline paralleled by inflammatory neurodegeneration in Interleukin-6 transgenic mice. Proc Natl Acad Sci USA. 1996;94:1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nitkiewicz J, Borjabad A, Morgello S, et al. . HIV induces expression of complement component C3 in astrocytes by NF-kappaB-dependent activation of interleukin-6 synthesis. J Neuroinflammation. 2017;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sekar S, McDonald J, Cuyugan L, et al. . Alzheimer's disease is associated with altered expression of genes involved in immune response and mitochondrial processes in astrocytes. Neurobiol Aging. 2015;36:583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGuire JL, Gill AJ, Douglas SD, et al. ; Group CHATER. The complement system, neuronal injury, and cognitive function in horizontally-acquired HIV-infected youth. J Neurovirol. 2016;22:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bryant AK, Fazeli PL, Letendre SL, et al. . Complement component 3 is associated with metabolic comorbidities in older HIV-positive adults. AIDS Res Hum Retroviruses. 2016;32:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hong S, Beja-Glasser VF, Nfonoyim BM, et al. . Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pascual G, Fong AL, Ogawa S, et al. . A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mannelli LD, D'Agostino G, Pacini A, et al. . Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: pain relief and neuroprotection share a PPAR-alpha-mediated mechanism. Mediators Inflamm. 2013;2013:328797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schintu N, Frau L, Ibba M, et al. . PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur J Neurosci. 2009;29:954–963. [DOI] [PubMed] [Google Scholar]
- 32. Paterniti I, Impellizzeri D, Crupi R, et al. . Molecular evidence for the involvement of PPAR-delta and PPAR-gamma in anti-inflammatory and neuroprotective activities of palmitoylethanolamide after spinal cord trauma. J Neuroinflammation. 2013;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. von Bernhardi R, Cornejo F, Parada GE, et al. . Role of TGFbeta signaling in the pathogenesis of Alzheimer's disease. Front Cell Neurosci. 2015;9:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lowin T, Pongratz G, Straub RH. The synthetic cannabinoid WIN55,212-2 mesylate decreases the production of inflammatory mediators in rheumatoid arthritis synovial fibroblasts by activating CB2, TRPV1, TRPA1 and yet unidentified receptor targets. J Inflamm (Lond). 2016;13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Su SH, Wu YF, Lin Q, et al. . Cannabinoid receptor agonist WIN55,212-2 and fatty acid amide hydrolase inhibitor URB597 ameliorate neuroinflammatory responses in chronic cerebral hypoperfusion model by blocking NF-kappa B pathways. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:1189–1200. [DOI] [PubMed] [Google Scholar]
- 37. Fakhfouri G, Ahmadiani A, Rahimian R, et al. . WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-gamma pathway. Neuropharmacology. 2012;63:653–666. [DOI] [PubMed] [Google Scholar]
- 38. O'Sullivan SE. An update on PPAR activation by cannabinoids. Br J Pharmacol. 2016;173:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O'Sullivan SE, Kendall DA, Randall MD. Time-dependent vascular effects of Endocannabinoids mediated by peroxisome proliferator-activated receptor gamma (PPARgamma). PPAR Res. 2009;2009:425289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Payandemehr B, Ebrahimi A, Gholizadeh R, et al. . Involvement of PPAR receptors in the anticonvulsant effects of a cannabinoid agonist, WIN 55,212-2. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:140–145. [DOI] [PubMed] [Google Scholar]
- 41. Enayatfard L, Rostami F, Nasoohi S, et al. . Dual role of PPAR-gamma in induction and expression of behavioral sensitization to cannabinoid receptor agonist WIN55,212-2. Neuromolecular Med. 2013;15:523–535. [DOI] [PubMed] [Google Scholar]
- 42. Felder CC, Joyce KE, Briley EM, et al. . Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- 43. Hao MX, Jiang LS, Fang NY, et al. . The cannabinoid WIN55,212-2 protects against oxidized LDL-induced inflammatory response in murine macrophages. J Lipid Res. 2010;51:2181–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lisboa SF, Niraula A, Resstel LB, et al. . Repeated social defeat-induced neuroinflammation, anxiety-like behavior and resistance to fear extinction were attenuated by the cannabinoid receptor agonist WIN55,212-2. Neuropsychopharmacology. 2018;43:1924–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marchalant Y, Rosi S, Wenk GL. Anti-inflammatory property of the cannabinoid agonist WIN-55212-2 in a rodent model of chronic brain inflammation. Neuroscience. 2007;144:1516–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang DP, Yin H, Kang K, et al. . The potential protective effects of cannabinoid receptor agonist WIN55,212-2 on cognitive dysfunction is associated with the suppression of autophagy and inflammation in an experimental model of vascular dementia. Psychiatry Res. 2018;267:281–288. [DOI] [PubMed] [Google Scholar]
- 47. Zhao Y, Liu Y, Zhang WP, et al. . WIN55212-2 ameliorates atherosclerosis associated with suppression of pro-inflammatory responses in ApoE-knockout mice. Eur J Pharmacol. 2010;649:285–292. [DOI] [PubMed] [Google Scholar]
- 48. Shaftel SS, Griffin WS, O'Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hewett SJ, Jackman NA, Claycomb RJ. Interleukin-1beta in central nervous system injury and repair. Eur J Neurodegener Dis. 2012;1:195–211. [PMC free article] [PubMed] [Google Scholar]
- 50. Claycomb RJ, Hewett SJ, Hewett JA. Neuromodulatory role of endogenous interleukin-1beta in acute seizures: possible contribution of cyclooxygenase-2. Neurobiol Dis. 2012;45:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choi SS, Lee HJ, Lim I, et al. . Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One. 2014;9:e92325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jang E, Kim JH, Lee S, et al. . Phenotypic polarization of activated astrocytes: the critical role of lipocalin-2 in the classical inflammatory activation of astrocytes. J Immunol. 2013;191:5204–5219. [DOI] [PubMed] [Google Scholar]
- 53. Knoll JG, Krasnow SM, Marks DL. Interleukin-1beta signaling in fenestrated capillaries is sufficient to trigger sickness responses in mice. J Neuroinflammation. 2017;14:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krasnow SM, Knoll JG, Verghese SC, et al. . Amplification and propagation of interleukin-1beta signaling by murine brain endothelial and glial cells. J Neuroinflammation. 2017;14:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 56. Dhar A, Gardner J, Borgmann K, et al. . Novel role of TGF-beta in differential astrocyte-TIMP-1 regulation: implications for HIV-1-dementia and neuroinflammation. J Neurosci Res. 2006;83:1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gardner J, Borgmann K, Deshpande MS, et al. . Potential mechanisms for astrocyte-TIMP-1 downregulation in chronic inflammatory diseases. J Neurosci Res. 2006;83:1281–1292. [DOI] [PubMed] [Google Scholar]
- 58. Ferrari CC, Pott Godoy MC, Tarelli R, et al. . Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol Dis. 2006;24:183–193. [DOI] [PubMed] [Google Scholar]
- 59. Ferrari CC, Depino AM, Prada F, et al. . Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol. 2004;165:1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marcel M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. 2011:10–12. [Google Scholar]
- 61. Dobin A, Davis CA, Schlesinger F, et al. . STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ge SX, Son EW, Yao RN. iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinformatics. 2018;19:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim SY, Volsky DJ. PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics. 2005;6:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jiang C, Xuan Z, Zhao F, et al. . TRED: a transcriptional regulatory element database, new entries and other development. Nucleic Acids Res. 2007;35:D137–D140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ben Haim L, Ceyzeriat K, Carrillo-de Sauvage MA, et al. . The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer's and Huntington's diseases. J Neurosci. 2015;35:2817–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee MY, Lim HW, Lee SH, et al. . Smad, PI3K/Akt, and Wnt-dependent signaling pathways are involved in BMP-4-induced ESC self-renewal. Stem Cells. 2009;27:1858–1868. [DOI] [PubMed] [Google Scholar]
- 69. Rizzo MD, Crawford RB, Bach A, et al. . Delta(9)-tetrahydrocannabinol suppresses monocyte-mediated astrocyte production of monocyte chemoattractant protein 1 and interleukin-6 in a toll-like receptor 7-stimulated human coculture. J Pharmacol Exp Ther. 2019;371:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wahl S, Allen J, McCartney-Francis N, et al. . Macrophage- and astrocyte-derived transforming growth factor b as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991;173:981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Morganti-Kossmann MC, Kossmann T, Brandes ME, et al. . Autocrine and paracrine regulation of astrocyte function by transforming growth factor-beta. J Neuroimmunol. 1992;39:163–173. [DOI] [PubMed] [Google Scholar]
- 72. Haseloff RF, Blasig IE, Bauer HC, et al. . In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Levine AJ, Quach A, Moore DJ, et al. . Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neurovirol. 2016;22:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Levine AJ, Soontornniyomkij V, Achim CL, et al. . Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J Neurovirol. 2016;22:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cuevas-Diaz Duran R, Wang CY, Zheng H, et al. . Brain region-specific gene signatures revealed by distinct astrocyte subpopulations unveil links to glioma and neurodegenerative diseases. eNeuro. 2019;6:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang G, Bi H, Gao J, et al. . Inhibition of autophagy and enhancement of endoplasmic reticulum stress increase sensitivity of osteosarcoma Saos-2 cells to cannabinoid receptor agonist WIN55,212-2. Cell Biochem Funct. 2016;34:351–358. [DOI] [PubMed] [Google Scholar]
- 77. Su SH, Wang YQ, Wu YF, et al. . Cannabinoid receptor agonist WIN55,212-2 and fatty acid amide hydrolase inhibitor URB597 may protect against cognitive impairment in rats of chronic cerebral hypoperfusion via PI3K/AKT signaling. Behav Brain Res. 2016;313:334–344. [DOI] [PubMed] [Google Scholar]
- 78. Bilkei-Gorzo A, Albayram O, Draffehn A, et al. . A chronic low dose of Delta(9)-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat Med. 2017;23:782–787. [DOI] [PubMed] [Google Scholar]
- 79. Cheng D, Spiro AS, Jenner AM, et al. . Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer's disease transgenic mice. J Alzheimers Dis. 2014;42:1383–1396. [DOI] [PubMed] [Google Scholar]
- 80. Cheng D, Low JK, Logge W, et al. . Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1E9 mice. Psychopharmacology (Berl). 2014;231:3009–3017. [DOI] [PubMed] [Google Scholar]
- 81. Sheng WS, Hu S, Min X, et al. . Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia. 2005;49:211–219. [DOI] [PubMed] [Google Scholar]
- 82. Shim JY, Welsh WJ, Cartier E, et al. . Molecular interaction of the antagonist N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide with the CB1 cannabinoid receptor. J Med Chem. 2002;45:1447–1459. [DOI] [PubMed] [Google Scholar]
- 83. Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. [DOI] [PubMed] [Google Scholar]
- 84. Montes de Oca Balderas P, Montes de Oca Balderas H. Synaptic neuron-astrocyte communication is supported by an order of magnitude analysis of inositol tris-phosphate diffusion at the nanoscale in a model of peri-synaptic astrocyte projection. BMC Biophys. 2018;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J Immunol. 2001;166:6383–6391. [DOI] [PubMed] [Google Scholar]
- 86. Watson CW, Paolillo EW, Morgan EE, et al. . Cannabis exposure is associated with a lower likelihood of neurocognitive impairment in people living with HIV. J Acquir Immune Defic Syndr. 2020;83:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing files and processed transcript counts generated in this study are deposited on the GEO at accession GSE160092.