Abstract
Introduction: The cannabinoid Δ9-tetrahydrocannabinolic acid (Δ9-THCA) has long been suggested in review articles and anecdotal reports to be anticonvulsant; yet, there is scant evidence supporting this notion. The objective of this study was to interrogate the anticonvulsant potential of Δ9-THCA in various seizure models—the Scn1a+/− mouse model of Dravet syndrome, the 6-Hz model of psychomotor seizures and the maximal electroshock (MES) model of generalized tonic-clonic seizures.
Materials and Methods: We examined the effect of acute Δ9-THCA treatment against hyperthermia-induced seizures, and subchronic treatment on spontaneous seizures and survival in the Scn1a+/− mice. We also studied the effect of acute Δ9-THCA treatment on the critical current thresholds in the 6-Hz and MES tests using outbred Swiss mice. Highly purified Δ9-THCA was used in the studies or a mixture of Δ9-THCA and Δ9-THC.
Results: We observed mixed anticonvulsant and proconvulsant effects of Δ9-THCA across the seizure models. Highly pure Δ9-THCA did not affect hyperthermia-induced seizures in Scn1a+/− mice. A Δ9-THCA/Δ9-THC mixture was anticonvulsant in the 6-Hz threshold test, but purified Δ9-THCA and Δ9-THC had no effect. Conversely, both Δ9-THCA and Δ9-THC administered individually were proconvulsant in the MES threshold test but had no effect when administered as a Δ9-THCA/Δ9-THC mixture. The Δ9-THCA/Δ9-THC mixture, however, increased spontaneous seizure severity and increased mortality of Scn1a+/− mice.
Discussion: The anticonvulsant profile of Δ9-THCA was variable depending on the seizure model used and presence of Δ9-THC. Because of the unstable nature of Δ9-THCA, further exploration of Δ9-THCA through formal anticonvulsant drug development is problematic without stabilization. Future studies may better focus on determining the mechanisms by which combined Δ9-THCA and Δ9-THC alters seizure thresholds, as this may uncover novel targets for the control of refractory partial seizures.
Keywords: medicinal cannabis, epilepsy, THCA, Dravet syndrome, seizure
Introduction
Epilepsy is a common neurological disease with a lifetime prevalence of 7.6 per 1000 persons.1 Approximately 30% of epilepsy patients are refractory to currently available treatments, motivating the quest for novel treatment options.2 In recent years, there has been increasing interest in cannabis-based medicines as a source of novel anticonvulsant agents. This follows numerous media stories illuminating remarkable improvements in intractable childhood epilepsy patients using cannabis-based products,3,4 as well as the cannabidiol (CBD) formulation Epidiolex™ being approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of Dravet syndrome and Lennox–Gastaut syndrome.5–7
Despite CBD gaining regulatory approval, many patients continue to use unregulated, artisanal cannabis-based products that contain a multitude of cannabinoids. Frequently, these artisanal extracts contain very low amounts of CBD, leading to speculation that constituents beyond CBD have anticonvulsant activity.8 Indeed, many believe that Δ9-tetrahydrocannabinolic acid (Δ9-THCA), the biosynthetic precursor of Δ9-tetrahydrocannabinol (Δ9-THC), mediates the anticonvulsant efficacy of these products.8–10 Community use of Δ9-THCA for epilepsy occurs despite scant evidence to support its anticonvulsant properties.
Clinical evaluation of Δ9-THCA as an anticonvulsant is limited to two published studies, a case series and an open-label retrospective chart review, both in pediatric populations.9,10 The case series reported conflicting reductions and exacerbations of seizure frequency in four patients after the addition of relatively low doses (0.02–2.2 mg/kg/day oral) of Δ9-THCA to existing anticonvulsant regimens.9 The chart review reported that Δ9-THCA was ineffective in five patients using Δ9-THCA-only extracts.10
Over 40 years ago, a preclinical study showed that 200 mg/kg Δ9-THCA was anticonvulsant in the mouse maximal electroshock (MES) test.11 Since this study, Δ9-THCA has been attributed anticonvulsant activity in several reviews and lay media; yet, the evidence to support these assertions has not advanced beyond this original preclinical report.12–14
In this study, we evaluated the anticonvulsant potential of Δ9-THCA in the Scn1a+/− mouse model of Dravet syndrome. In addition, we examined its effects in two conventional seizure models: the 6-Hz threshold (6-HzT) model of psychomotor seizures and the MES threshold (MEST) model of generalized tonic-clonic seizures (GTCS).
Materials and Methods
Drugs
Δ9-THCA was isolated from hemp extracts. In brief, crude cannabis extract was dissolved in methanol (LiChrosolv®; Merck, Darmstadt, Germany) and treated overnight with activated charcoal (Ajax Finechem, Wollongong, Australia) at 4°C. The solution was filtered through a Büchner funnel and the filtrate was collected. The solvent was removed under pressure, and then reverse phase column chromatography (Büchi Reveleris PREP; Büchi AG, Flawil, Switzerland) with a C18 column (Büchi AG) was used to purify the residue and elute Δ9-THCA. Purity of Δ9-THCA isolated was 97% with 3% Δ9-THC. In addition, we purchased Δ9-THCA-A with a purity of 99.5% (<0.5% Δ9-THC content) and Δ9-THC (dronabinol, 100% purity) from THC Pharm GmbH (Frankfurt, Germany). Cannabinoids were stored protected from light at −30°C. Sodium valproate was purchased from Sigma-Aldrich, Inc. (St. Louis, MO). Analytical standards were purchased from Novachem Pty Ltd (Heidelberg West, Australia).
Drug administration
Drug solutions were prepared fresh and were administered acutely as an intraperitoneal (i.p.) injection in a volume of 10 ml/kg. For conventional seizure model experiments (6-Hz and MEST), Δ9-THCA and Δ9-THC were prepared in 0.5% ethanol in vegetable oil. For hyperthermia-induced seizure experiments, Δ9-THCA was prepared in vegetable oil. Sodium valproate was prepared in saline.
Purity analysis
Purity of Δ9-THCA was assessed by UV chromatography using Zorbax XDB-C18 column (Agilent Technologies, Inc., Santa Clara, CA) with a Shimadzu Nexera ultrahigh-performance liquid chromatograph coupled to a Shimadzu SPD-20AV photodiode array detector (Shimadzu Corp., Kyoto, Japan). Purity was calculated as a percent of the Δ9-THCA peak area to total peak area in the chromatogram at 272 nm (measured UV maxima of Δ9-THCA). Peak identity was confirmed by comparing retention time and UV spectra to a certified Δ9-THCA reference standard.
Animals
All animal care and experimental procedures were approved by the University of Sydney Animal Ethics Committee in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (2016/1035 and 2018/1395). Swiss outbred mice were purchased from Animal Resources Centre (stock ARC(S); Canning Vale, Australia) and singly housed after arrival for 7 days before experimentation. Scn1a+/− mice were purchased from The Jackson Laboratory (stock 37107-JAX; Bar Harbor, ME) and generated for experiments as previously described.15,16 Scn1a+/− mice were group housed. All mice were housed under a 12-h light/12-h dark cycle (07:00–19:00 light) with ad libitum access to food and water.
Hyperthermia-induced seizures in Scn1a+/− mice
Hyperthermia-induced seizure experiments were conducted on male and female Scn1a+/− mice at postnatal days 14–16 (P14–16) as previously described.15 This model has been validated with first-line treatments, clobazam and valproic acid, and the phytocannabinoid CBD is anticonvulsant against hyperthermia-induced seizures in Scn1a+/− mice.17,18 In brief, mice received a single i.p. injection of vehicle or Δ9-THCA by a researcher blinded to treatment and the hyperthermia protocol commenced immediately. Instantly following the hyperthermia-induced seizure protocol (duration ∼15 min), plasma and brains samples were collected and stored at −80°C until assayed.
Spontaneous seizures and survival in Scn1a+/− mice
Male and female Scn1a+/− mice were exposed to a single hyperthermia-induced seizure event at P18 as described previously.16 Mice were randomly assigned to treatment groups after the thermally induced seizure. The mice were administered the cannabinoids orally through supplementation in chow. Δ9-THCA was dissolved in cold-pressed hemp seed oil (HempFoods Australia; Bangalow, Australia) and then formulated in R&M Standard Diet powder (Specialty Feeds; Glen Forrest, Australia). The final hemp seed oil concentration was 25% (v/w).
The groups tested were as follows: (1) control (hemp seed oil), (2) 250 mg Δ9-THCA/kg chow, and (3) 2000 mg Δ9-THCA/kg chow. An observer blinded to treatment quantified the number of spontaneous GTCS in a 60 h window.15 Mice continued drug treatment to P30 to monitor survival. Plasma and brain samples were collected on P31 within 30 min of lights on.
MEST and 6-HzT tests
MEST and 6-HzT tests were conducted in Swiss male mice (9–12 weeks old) using a rodent electroconvulsive therapy (ECT) unit (Model 57800; Ugo Basile, Gemonio, Italy) as described previously.19 Mice were pretreated with vehicle, Δ9-THCA, Δ9-THC, or sodium valproate by i.p. injection 15 min before seizure induction. A 0.5% tetracaine in saline solution was applied to both corneas to induce local anesthesia. Pretreatment time (15 min) was based on previously determined time-to-peak plasma concentrations.16,17 Immediately before the electrical stimulation, saline was applied to each cornea to ensure electrical conductivity.
Corneal electroshocks (6 Hz, 3 s shock duration, 0.2 ms rectangular pulse width) starting at 20 mA and moving in 2 mA increments to a maximum of 50 mA were used for 6-HzT seizure experiments. Shocks were delivered and seizures were scored by an observer blinded to treatment for the presence of a psychomotor seizure occurring within 30 s of the shock delivery. Seizure response was characterized by the presence of rhythmic jaw, forelimb clonus, immobility, and/or Straub tail.20
For MEST-induced seizures a modified paradigm was used to adapt to the ECT unit.21 Corneal electroshocks (60 Hz, 0.4 s shock duration, 0.5 ms rectangular pulse width) were administered starting at 50 mA and moving in 2 mA increments to a maximum of 60 mA. Mice were shocked and scored by an observer blinded to treatment for the presence of GTCS with full hindlimb extension (hindlimbs at a 180° angle to the torso).
For both MEST and 6-HzT tests, the critical current (mA) at which 50% of mice seized (CC50) was determined using the “up-and-down” method described by Kimball et al.22
Separate cohorts of mice were used to collect plasma and brain samples to mimic the concentrations of Δ9-THCA and Δ9-THC at the time of 6-HzT seizure testing. Mice (n=6 per group) received an i.p. injection of Δ9-THCA (200 mg/kg, 97% purity) or Δ9-THC (6 mg/kg) and tetracaine was applied to corneas. Fifteen minutes later, saline was applied and mice received a standardized electroshock of 28 mA or 16 mA, the previously determined CC50 values for Δ9-THCA and Δ9-THC, respectively. Immediately after the electroshock, plasma and brain samples were collected through cardiac puncture.
Analytical chemistry
Cannabinoid concentrations in biological samples were assayed by liquid chromatography–mass spectrometry (LC-MS)/MS as previously described.17 In brief, plasma samples were prepared using supported-liquid extraction (SLE) with methyl tert-butyl ether. Brain samples were prepared by filtering homogenates through Amicon Ultracel-3K (Merck-Millipore, Burlington, VT) filtration devices before SLE. Plasma and brain samples were reconstituted in acetonitrile and 0.1% formic acid in water (1:1, v/v) for analysis.
Samples were assayed by LC-MS/MS as previously described.17,23 The mass spectrometer operated in negative (Δ9-THCA) and positive (Δ9-THC) electrospray ionization modes with multiple reaction monitoring and the following mass transition pairs: m/z 357.20→245.35, 357.20→191.30 (Δ9-THCA) and m/z 315.15→193.15, 315.15→259.20 (Δ9-THC). Quantification was achieved by comparing experimental samples to 8-point standard curves prepared with analytical standards. Limits of quantification (LOQ) were 0.04 ng/mg brain and <50 ng/ml plasma (Δ9-THCA), 0.1 ng/ml plasma and 0.005 ng/mg brain (Δ9-THC).
Statistical analysis
Hyperthermia-induced seizure threshold temperatures and survival data were analyzed using the Mantel–Cox log-rank test. Statistical comparisons of spontaneous seizure data were made using Fisher's exact test (proportion of mice seizure free) or one-way analysis of variance (ANOVA) followed by Dunnett's post hoc (seizure frequency and seizure severity). MEST and 6-HzT data were analyzed using one-way ANOVA followed by Dunnett's post hoc comparisons. Plasma Δ9-THC concentrations were analyzed using a Student's t-test. p<0.05 was considered statistically significant for all analyses.
Results
Purified Δ9-THCA is ineffective against hyperthermia-induced seizures in Scn1a+/− mice
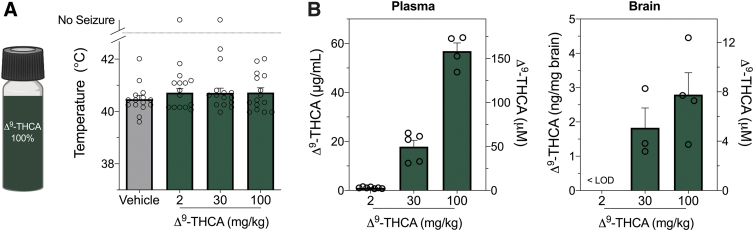
We evaluated pure Δ9-THCA against hyperthermia-induced seizures in the Scn1a+/− mouse model of Dravet syndrome (Fig. 1A). Based on allometric scaling, a low Δ9-THCA dose (2 mg/kg) was administered to approximate low doses administered to childhood epilepsy patients.8–10 The highest dose tested (100 mg/kg) matched the dose of CBD that has been shown to be anticonvulsant against hyperthermia-induced seizures in Scn1a+/− mice.17,24 No effect was observed on the temperature threshold for thermally induced seizures at any dose (Fig. 1A). Despite Δ9-THCA having a low brain-to-plasma ratio (<10%), micromolar concentrations were found in the brain at doses ≥30 mg/kg (Fig. 1B).
FIG. 1.
The effects of Δ9-THCA on hyperthermia-induced seizures in Scn1a+/− mice. (A) Pure Δ9-THCA that contained <0.5% THC impurity was used for hyperthermia-induced seizure experiments in Scn1a+/− mice. Threshold temperature of individual mice for GTCS induced by hyperthermia after acute i.p. treatment with vehicle (VEH, gray bar) or varying doses of pure Δ9-THCA (dark green bars). Δ9-THCA had no effect on the temperature threshold for hyperthermia-induced seizures. The average temperatures of seizure induction are depicted by the bars and error bars represent SEM, with n=15 per group (log-rank Mantel–Cox). (B) Concentrations of Δ9-THCA in plasma (left panel) and brain (right panel) from individual experimental animals. Concentrations are depicted as both mass concentrations (left y-axis) and molar concentrations (right y-axis). Error bars represent SEM, with n=4–7 per treatment. Δ9-THCA, Δ9-tetrahydrocannabinolic acid; GTCS, generalized tonic-clonic seizures; i.p., intraperitoneal; LOD, limit of detection; SEM, standard error of the mean.
Combined Δ9-THCA and Δ9-THC is anticonvulsant in the 6-HzT seizure model
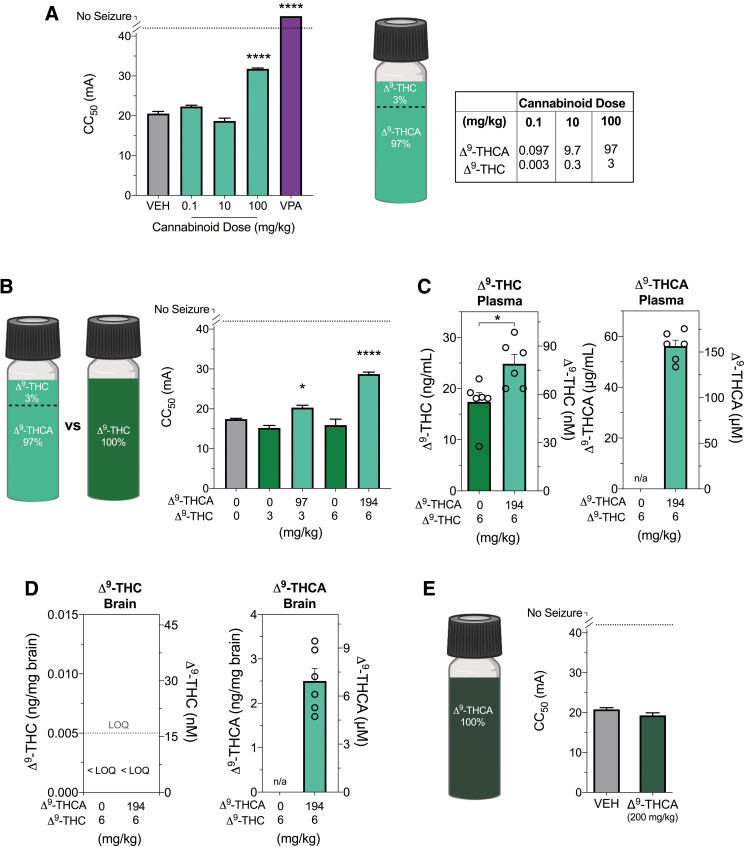
We then sought to examine the effects of Δ9-THCA (97% Δ9-THCA and 3% Δ9-THC) on psychomotor seizures using the 6-HzT test. Although initially the presence of Δ9-THC was undesirable, the effects of this mixture remains highly relevant to community usage of Δ9-THCA-dominant oils that contain both Δ9-THCA and Δ9-THC.7 This Δ9-THCA/Δ9-THC mixture was anticonvulsant in the 6-HzT seizure model (one-way ANOVA; F4,4=332.5, p<0.0001); the 100 mg/kg dose significantly increased the CC50 compared with vehicle-treated mice (p=0.0002) (Fig. 2A); however, the effect size was small compared with sodium valproate (300 mg/kg), which yielded 100% protection (Fig. 2A).
FIG. 2.
The effects of Δ9-THCA in the 6-HzT test. (A) The CC50 exhibit a psychomotor seizure in the 6-HzT seizure model after acute i.p. treatment with vehicle (VEH, gray bar), varying doses of a Δ9-THCA/Δ9-THC mixture (light green bars) or sodium valproate (VPA, purple bar). Δ9-THCA/Δ9-THC mixture (100 mg/kg) significantly increased the CC50 threshold. Sodium valproate (300 mg/kg) treatment protected mice from psychomotor seizures. Error bars represent SEM, with n=11–12 per treatment (****p<0.0001; one-way ANOVA followed by Dunnett's post hoc compared with vehicle-treated mice). Cannabinoid content of the Δ9-THCA/Δ9-THC mixture was 97% Δ9-THCA and 3% Δ9-THC, with the corresponding doses. (B) The 6-HzT test was repeated to compare CC50 values after treatment with Δ9-THCA/Δ9-THC mixture (light green bar) with those following treatment with matched doses of pure Δ9-THC (green bars). Δ9-THCA/Δ9-THC mixture (100 and 200 mg/kg) significantly increased the CC50, with n=12 per treatment (*p<0.05, ****p<0.0001; one-way ANOVA followed by Dunnett's post hoc compared with vehicle-treated mice). (C) Plasma and (D) brain concentrations of Δ9-THC (left panel) and Δ9-THCA (right panel) in individual animals after treatment with 6 mg/kg Δ9-THC (green bar) or 200 mg/kg Δ9-THCA/Δ9-THC formulation (light green bars). Significantly higher plasma Δ9-THC concentrations were observed after treatment with the Δ9-THCA/Δ9-THC mixture (*p<0.05, Student's t-test). Concentrations of Δ9-THC in brain samples were below the LOQ, depicted by the dashed line. Concentrations are depicted as both mass concentrations (left y-axis) and molar concentrations (right y-axis). Error bars represent SEM, with n=6 per treatment. (E) The CC50 value in the 6-HzT seizure model after acute i.p. treatment with vehicle (VEH, grey bar) or 200 mg/kg pure Δ9-THCA (dark green bar) that contained <0.5% Δ9-THC impurity. Error bars represent SEM, with n=12 per treatment (Student's t-test). ANOVA, analysis of variance; CC50, critical current at which 50% of mice seized; 6-HzT, 6-Hz threshold; LOQ, limit of quantification.
We then determined whether the anticonvulsant effect observed at 100 mg/kg was simply attributed to Δ9-THC and whether a higher dose of the Δ9-THCA/Δ9-THC mixture had a more robust anticonvulsant effect. We repeated the experiment with 100 and 200 mg/kg doses of the Δ9-THCA/Δ9-THC mixture and Δ9-THC alone (3 and 6 mg/kg) matching the Δ9-THC doses found in the mixture (Fig. 2B). Again the Δ9-THCA/Δ9-THC mixture was anticonvulsant (F4,55=45.64, p<0.0001). The CC50 values of the Δ9-THCA/Δ9-THC mixture were significantly greater than vehicle (100 mg/kg, p=0.0497 and 200 mg/kg, p<0.0001).
Neither dose of Δ9-THC had any effect, suggesting that Δ9-THC within the Δ9-THCA/Δ9-THC mixture was not responsible for the anticonvulsant effect. We compared plasma and brain concentrations of Δ9-THC and Δ9-THCA from mice treated with 200 mg/kg of the Δ9-THCA/Δ9-THC mixture with those treated with a matched Δ9-THC (6 mg/kg) dose (Fig. 2C, D). Of interest, the addition of Δ9-THCA increased plasma Δ9-THC concentrations, with higher Δ9-THC concentrations observed in Δ9-THCA/Δ9-THC mixture group than the matched Δ9-THC-alone group (p=0.0170). Brain Δ9-THC concentrations were detectable but below the LOQ (Fig. 2D). The brain Δ9-THC concentrations would have been low and rising 15 min postdose, as the brain tmax of Δ9-THC is 60–120 min.25,26 A mean Δ9-THCA concentration of 6.98 (±1.93) μM was measured in brain tissue (Fig. 2D).
Subsequently, we sourced pure Δ9-THCA (<0.5% THC impurity) to examine its effects in the 6-HzT test (Fig. 2E). Pure Δ9-THCA (200 mg/kg) had no effect on the threshold of seizures induced by 6-Hz electroshock.
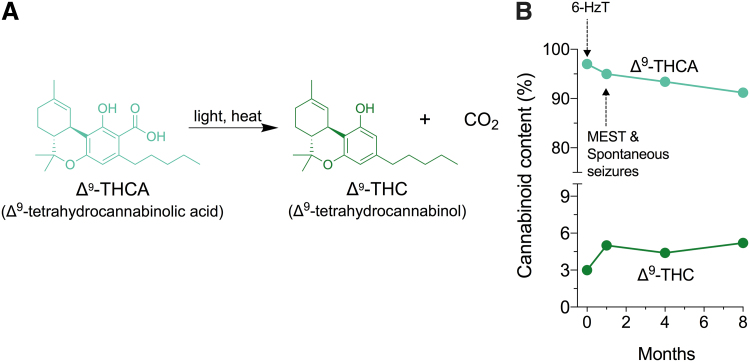
Δ9-THCA is chemically unstable under controlled storage conditions
When exposed to light and/or heat, Δ9-THCA readily decarboxylates to Δ9-THC (Fig. 3A). The Δ9-THCA-dominant mixture was stored protected from light at −30°C and cannabinoid content was assessed over time (Fig. 3B). Despite these storage conditions, Δ9-THCA was not stable and degraded to 91% over 8 months explaining the different Δ9-THCA to Δ9-THC ratios across our experiments.
FIG. 3.
Δ9-THCA is chemically unstable. (A) Chemical structure of Δ9-THCA and schematic of its decarboxylation to Δ9-THC. Decarboxylation of Δ9-THCA is catalyzed by light and heat. (B) Cannabinoid content of the Δ9-THCA-dominant cannabinoid extract over time. Δ9-THCA was stored protected from light at −30°C. Arrows represent when experiments were conducted.
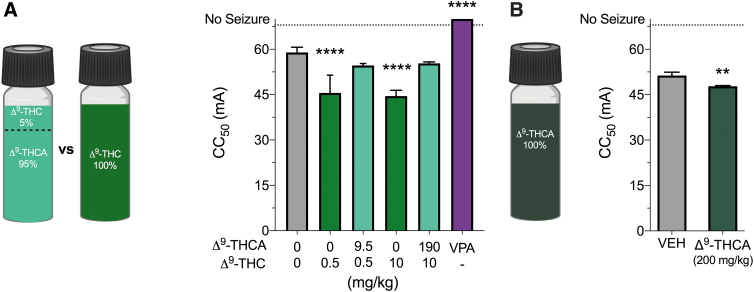
Purified Δ9-THCA and Δ9-THC administered alone are proconvulsant in the MEST seizure model
We examined the effect of the Δ9-THCA/Δ9-THC mixture (95% Δ9-THCA, 5% Δ9-THC) in the MEST model of GTCS (Fig. 4A). We conducted a MEST test with a 200 mg/kg dose of the Δ9-THCA/Δ9-THC mixture and purified Δ9-THC at 10 mg/kg to match the dose in the mixture (Fig. 4A). To assess potential low-dose effects of Δ9-THCA and Δ9-THC, we also examined the effect of a lower dose of the Δ9-THCA/Δ9-THC mixture (10 mg/kg) and purified Δ9-THC (0.5 mg/kg).
FIG. 4.
The effects of Δ9-THCA in the MEST test. (A) A Δ9-THCA/Δ9-THC mixture and pure Δ9-THC were used in the MEST acute seizure model. Cannabinoid content of the Δ9-THCA/Δ9-THC mixture was 95% Δ9-THCA and 5% Δ9-THC. The CC50 exhibits a seizure with maximal hindlimb extension after acute i.p. treatment with vehicle (VEH, gray bar), pure Δ9-THC (green bar), a Δ9-THCA/Δ9-THC mixture (light green bar), or sodium valproate (VPA, purple bar). Dose of pure Δ9-THC matches that in the Δ9-THCA/Δ9-THC mixture. Δ9-THC (0.5 and 10 mg/kg) significantly reduced the CC50 threshold for MES seizures. Sodium valproate (300 mg/kg) treatment protected mice from MES-induced tonic extension. Error bars represent SEM, with n=12 per treatment (****p<0.0001; one-way ANOVA followed by Dunnett's post hoc compared with vehicle-treated mice). (B) The MEST test was repeated to compare CC50 values after treatment with vehicle (VEH, grey bar) or 200 mg/kg pure Δ9-THCA (dark green bar) that contained <0.5% Δ9-THC impurity. Δ9-THCA treatment significantly reduced the CC50 compared with vehicle treatment. Error bars represent SEM, with n=12 per treatment (**p<0.01; Student's t-test). MEST, maximal electroshock threshold.
Δ9-THC was proconvulsant in the MEST test (F5,65=52.55, p<0.0001). The CC50 values of both 0.5 and 10 mg/kg doses of Δ9-THC alone were significantly decreased compared with vehicle (p<0.0001 and p<0.0001, respectively). Neither dose of the Δ9-THCA/Δ9-THC mixture affected the CC50 in the MEST test. In contrast, sodium valproate (300 mg/kg) achieved 100% seizure protection (p<0.0001).
Subsequently, we procured a pure Δ9-THCA formulation and examined its effects in the MEST test (Fig. 4B). Of interest, Δ9-THCA (200 mg/kg) was proconvulsant with a CC50 significantly lower than in vehicle-treated mice (p=0.0054).
Combined Δ9-THCA and Δ9-THC increased the severity of spontaneous seizures and reduced the lifespan of Scn1a+/− mice
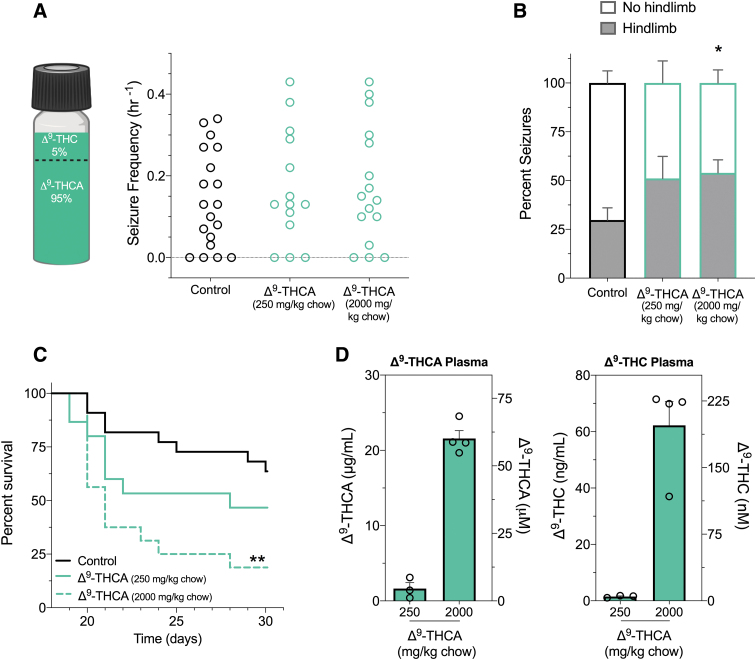
We then evaluated the effect of the Δ9-THCA/Δ9-THC mixture (95% Δ9-THCA, 5% Δ9-THC) against spontaneous seizures in Scn1a+/− mice (Fig. 5A). Because these experiments require subchronic drug administration, it was not possible to procure sufficient quantities of purified Δ9-THCA. Treatment delivered through supplementation in chow (250 or 2000 mg/kg chow) had no effect on the proportion of mice that experienced spontaneous seizures or spontaneous seizure frequency (Fig. 5A). Treatment with Δ9-THCA/Δ9-THC mixture (2000 mg/kg chow) increased the severity of spontaneous seizures, as the percentage of seizures that advanced to hindlimb extension was significantly higher than control-treated mice (p=0.0139) (Fig. 5B).
FIG. 5.
The effects of Δ9-THCA on spontaneous seizures and survival in Scn1a+/− mice. (A) A Δ9-THCA/Δ9-THC mixture was used for spontaneous seizure and survival experiments in Scn1a+/− mice. Cannabinoid content of the Δ9-THCA/Δ9-THC mixture used was 95% Δ9-THCA and 5% Δ9-THC. GTCS frequency of individual untreated and Δ9-THCA-treated mice is given. Treatments were administered orally through supplementation in chow, which was initiated after the induction of a single thermally induced seizure. Unprovoked, spontaneous GTCS were quantified over a 60-h recording period. Treatment with the Δ9-THCA/Δ9-THC mixture had no effect on incidence or frequency of seizures, with n=14–19 per group (Fisher's exact text and one-way ANOVA followed by Bonferroni's post hoc, respectively). (B) Proportion of spontaneous GTCS with (gray bars) or without (white bars) full tonic hindlimb extension is given. Subchronic treatment with high-dose Δ9-THCA/Δ9-THC mixture (2000 mg/kg chow) significantly increased the severity of GTCS in Scn1a+/− mice. The proportion of GTCS with tonic hindlimb extension was significantly greater compared with control-treated mice (*p<0.05; Bonferroni's planned comparisons). Error bars represent SEM with n=11–15. (C) Survival curves comparing control and Δ9-THCA/Δ9-THC mixture-treated mice are given. Treatment began at postnatal day 18 (P18) and survival was monitored until P30. Survival of Scn1a+/− mice was significantly worse with high-dose Δ9-THCA/Δ9-THC mixture (2000 mg/kg chow), with n=15–22 per group (**p<0.005; log-rank Mantel–Cox). (D) Plasma concentrations of Δ9-THCA (left panel) and Δ9-THC (right panel) from individual experimental animals treated with a Δ9-THCA/Δ9-THC mixture. Concentrations are depicted as both mass concentrations (left y-axis) and molar concentrations (right y-axis). Error bars represent SEM, with n=3–4 per treatment.
Increased seizure severity was associated with poor survival, with only 19% survival to P30 compared with 64% survival of controls (p=0.0012) (Fig. 5C). Treatment with a lower dose of the Δ9-THCA/Δ9-THC mixture (250 mg/kg chow) had no effect on survival compared with controls. Steady-state plasma concentrations of Δ9-THCA and Δ9-THC measured in Scn1a+/− experimental mice after subchronic treatment are given in Figure 5D. Concentrations of Δ9-THCA in the brain were below the LOQ for mice treated with the 250 mg/kg chow and 0.3±0.1 ng/mg brain (873±278 nM) with the 2000 mg/kg chow doses. Concentrations of Δ9-THC in brain samples were below the limit of detection and LOQ, respectively.
Discussion
Δ9-THCA-dominant cannabis extracts are being used in the community to treat epilepsy despite insufficient evidence. We aimed to fill the knowledge gap by assessing the anticonvulsant properties of Δ9-THCA across several mouse seizure models. Our results highlight great complexity in the action of Δ9-THCA, with both anticonvulsant and proconvulsant effects being observed depending on the seizure model and presence of Δ9-THC. Against 6-Hz-induced seizures, Δ9-THCA was anticonvulsant only when Δ9-THC was present. However, in the MEST model, the Δ9-THCA/Δ9-THC mixture was ineffective and even proconvulsant when purified Δ9-THCA or Δ9-THC was administered alone. Finally, purified Δ9-THCA had no effect on hyperthermia-induced seizures in the Scn1a+/− mouse model, whereas a Δ9-THCA/Δ9-THC mixture worsened spontaneous seizure severity and reduced survival.
This study further highlights the difficulties posed by the instability of Δ9-THCA for pharmacological research. Stability studies show that Δ9-THCA decarboxylates even when stored at 4 and 18°C, so Δ9-THC contamination in Δ9-THCA is “nearly unavoidable.”27,28 We observed significant decarboxylation of Δ9-THCA under conditions where it was stored protected from light at −30°C, with short exposures to air and ambient temperatures for drug preparation. Investigators characterizing the pharmacology of Δ9-THCA should be cognizant of its handling and storage conditions and routinely perform analytical tests to confirm purity.
In addition, those considering use of Δ9-THCA as a single molecule for pharmaceutical development might consider strategies to improve stability such as bioisosteric replacement of the carboxylic acid group.29 Although the instability of Δ9-THCA would need to be resolved before a formal drug development pathway, its degradation to Δ9-THC was not necessarily a disadvantage here. Understanding the effects of coadministered Δ9-THCA and Δ9-THC is highly relevant for epilepsy patients using Δ9-THCA-dominant cannabis extracts that invariably contain both cannabinoids, often with greater relative doses of Δ9-THCA to Δ9-THC.8
This study provides novel evidence that a Δ9-THCA/Δ9-THC mixture dose dependently reduced seizures in the 6-HzT test, although with mild effect sizes at very high doses (>100 mg/kg i.p.). Of importance, purified Δ9-THCA or Δ9-THC was ineffective when administered alone, which suggests a potential synergistic interaction between the two cannabinoids when combined. However, it is important to note that an isobolographic study would need to be conducted to draw a firm conclusion on the presence of cannabinoid synergy.
The current data are insufficient to draw such a conclusion. The interaction between Δ9-THCA and Δ9-THC might have pharmacodynamic and/or pharmacokinetic explanations. Because both cannabinoids were present in the brain, there could be a pharmacodynamic interaction at a common anticonvulsant target such as cannabinoid CB1 receptors. Recently, Δ9-THCA was reported to be a positive allosteric modulator of CB1 receptors.30 Alternatively, our observation that Δ9-THCA increased plasma concentrations of Δ9-THC points to a pharmacokinetic interaction that could be explored in future studies.
Although the Δ9-THCA/Δ9-THC mixture was effective in the 6-HzT model, it had no effect in the MEST test, and highly purified Δ9-THCA (200 mg/kg) was proconvulsant. This conflicts with a previous report showing that 200 mg/kg Δ9-THCA was anticonvulsant in the MES model.11 Unfortunately, this early study did not describe the purity of the Δ9-THCA that was used. Considerable Δ9-THC contamination might account for the effect because Δ9-THC is anticonvulsant in this model (Effective dose for 50% of cohort=35–43.8 mg/kg dose range).31,32 We found Δ9-THC to be proconvulsant at lower doses (0.5 and 10 mg/kg). This is consistent with a study reporting biphasic effects of Δ9-THC on the severity of MES seizures, with low doses having proconvulsant effects and high doses being anticonvulsant.33
Within the community, Δ9-THCA-dominant cannabis extracts are being used to treat Dravet syndrome patients even in the absence of evidence supporting its efficacy.8,9 In this study, highly purified Δ9-THCA had no effect on hyperthermia-induced seizures in the Scn1a+/− mouse model of Dravet syndrome despite Δ9-THCA attaining >1 μM brain concentrations.
This is the first report of appreciable Δ9-THCA concentrations in brain tissue after systemic administration. However, it is important to clarify that Δ9-THCA does not readily accumulate in brain tissue as it has a low brain-to-plasma ratio (Fig. 1B, <10%). Our study's lowest dose corresponds to the highest dose reported by Sulak et al.9 in a case series of pediatric patients. It is possible that lower doses of Δ9-THCA might be effective given we have observed low-dose effects of Δ9-THC (0.1–0.3 mg/kg) against hyperthermia-induced seizures in Scn1a+/− mice. Furthermore, very low doses of Δ9-THCA have been reported to reduce nausea and vomiting in rodents.22,34 The effects of lower doses of Δ9-THCA could be explored in a future study.
We also examined the effect of the Δ9-THCA/Δ9-THC mixture on spontaneous seizures and lifespan of Scn1a+/− mice. This yielded catastrophic effects with the mixture worsening the severity of spontaneous seizures and reducing survival. A similar exacerbation of premature mortality was observed after cotreatment of CBD with Δ9-THC.17 A commonality between these studies is a pharmacokinetic interaction with the perpetrator drugs (CBD or Δ9-THCA) increasing the plasma concentrations of the victim drug (Δ9-THC). A recent study showed cannabinoid-induced convulsions may be a species-specific phenomenon that is restricted to rodents. However, the use of a high-dose Δ9-THCA-dominant extract was noted to exacerbate seizures in a Dravet syndrome patient, potentially refuting this possibility.9,33
The anticonvulsant efficacy of the Δ9-THCA/Δ9-THC mixture in the 6-HzT model warrants further exploration. Following the pathway of the NIH Epilepsy Therapy Screening Program, the Δ9-THCA/Δ9-THC mixture could be examined in the lamotrigine-resistant amygdala-kindled seizure model, which is used when an investigational drug is anticonvulsant in the 6-Hz but not the MES test. Levetiracetam, used to treat refractory partial seizures, is anticonvulsant in the 6-Hz but not the MES model.20,35 Therefore, it is conceivable that a Δ9-THCA/Δ9-THC mixture may have potential in treating therapy-resistant partial seizures, although the proconvulsant effects of purified Δ9-THCA in the MEST test and the THCA/Δ9-THC combination in Scn1a+/− mice complicates its further development. In any case, Δ9-THCA is inferior to CBD as an anticonvulsant, with CBD displaying efficacy in the 6-Hz and MES seizure models, as well as the Scn1a+/− mouse model of Dravet syndrome.17,24,36–38
Conclusion
Our results suggest that Δ9-THCA-dominant medicinal cannabis formulations might be, at best, highly circumscribed in the treatment of epilepsy. Future studies may be better focused in determining the potential mechanisms by which Δ9-THCA alters seizure thresholds, as this may uncover novel targets for refractory seizure control.
Acknowledgments
The authors gratefully acknowledge Barry and Joy Lambert for their continued support of the Lambert Initiative for Cannabinoid Therapeutics. In addition, we thank Katelyn Lambert for inspiring our work on novel cannabinoid therapies for childhood epilepsy. The authors also thank Rebecca Vogel for technical assistance and Anastasia Suraev for her important insights around the use of artisanal cannabis extracts in childhood epilepsy patients.
Abbreviations Used
- Δ9-THCA
Δ9-tetrahydrocannabinolic acid
- ANOVA
analysis of variance
- ARC
Australian Research Council
- CBD
cannabidiol
- CC50
critical current at which 50% of mice seized
- EMA
European Medicines Agency
- FDA
US Food and Drug Administration
- GTCS
generalized tonic-clonic seizures
- 6-HzT
6-Hz threshold
- i.p.
intraperitoneal
- LOD
limit of detection
- LOQ
limit of quantification
- MEST
maximal electroshock threshold
- NHMRC
National Health and Medical Research Council
- SEM
standard error of the mean
- VEH
vehicle
- VPA
valproate
- WHO
World Health Organization
Author Disclosure Statement
J.C.A. is Deputy Academic Director of the Lambert Initiative for Cannabinoid Therapeutics. He has served as an expert witness in various medicolegal cases involving cannabis and served as a temporary advisor to the World Health Organization (WHO) on their review of cannabis and the cannabinoids. He receives funding from the Australian National Health and Medical Research Council (NHMRC) (APP1161571) and the Lambert Initiative for Cannabinoid Therapeutics. I.S.M. is Academic Director of the Lambert Initiative for Cannabinoid Therapeutics. He has served as an expert witness in various medicolegal cases involving cannabis use, has received honoraria from Janssen, is currently a consultant to Kinoxis Therapeutics and has received research funding and fellowships from the NHMRC and Australian Research Council (ARC). J.C.A, I.S.M., and L.L.A are inventors on several patents involving cannabinoid therapeutics. M.J.B receives personal fees from Applied Cannabis Research, an industry contract research organization.
Funding Information
This work was supported by the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded center for medicinal cannabis research at the University of Sydney and a NHMRC Project Grant (APP1161571).
Cite this article as: Benson MJ, Anderson LL, Low IK, Luo JL, Kevin RC, Zhou C, McGregor IS, Arnold JC (2022) Evaluation of the possible anticonvulsant effect of Δ9-tetrahydrocannabinolic acid in murine seizure models, Cannabis and Cannabinoid Research 7:1, 46–57, DOI: 10.1089/can.2020.0073.
References
- 1. Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia. 2011;52:657–678. [DOI] [PubMed] [Google Scholar]
- 3. Friedman D, Devinsky O. Cannabinoids in the treatment of epilepsy. N Engl J Med. 2015;373:1048–1058. [DOI] [PubMed] [Google Scholar]
- 4. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–2020. [DOI] [PubMed] [Google Scholar]
- 6. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med. 2018;378:1888–1897. [DOI] [PubMed] [Google Scholar]
- 7. Sekar K, Pack A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: a comprehensive review with a focus on adverse effects. F1000Research. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suraev A, Lintzeris N, Stuart J, et al. Composition and use of cannabis extracts for childhood epilepsy in the Australian community. Sci Rep. 2018;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sulak D, Saneto R, Goldstein B. The current status of artisanal cannabis for the treatment of epilepsy in the United States. Epilepsy Behav. 2017;70:328–333. [DOI] [PubMed] [Google Scholar]
- 10. Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav. 2015;45:49–52. [DOI] [PubMed] [Google Scholar]
- 11. Karler R, Turkanis SA. Cannabis and epilepsy: Oxford, United Kingdom: Elsevier, 1979. [Google Scholar]
- 12. Moreno-Sanz G. Can you pass the acid test? Critical review and novel therapeutic perspectives of Δ9-tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res. 2016;1:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perucca E. Cannabinoids in the treatment of epilepsy: hard evidence at last? J Epilepsy Res. 2017;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russo EB. Cannabis therapeutics and the future of neurology. Front Integr Neurosci. 2018;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller AR, Hawkins NA, McCollom CE, et al. Mapping genetic modifiers of survival in a mouse model of Dravet syndrome. Genes Brain Behav. 2014;13:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hawkins NA, Anderson LL, Gertler TS, et al. Screening of conventional anticonvulsants in a genetic mouse model of epilepsy. Ann Clin Transl Neur. 2017;4:326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson LL, Low IK, McGregor IS, et al. Interactions between cannabidiol and Δ9-tetrahydrocannabinol in modulating seizure susceptibility and survival in a mouse model of Dravet syndrome. Br J Pharmacol. 2020; DOI: 10.1111/bph.15181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hawkins NA, Zachwieja NJ, Miller AR, et al. Fine mapping of a Dravet syndrome modifier locus on mouse chromosome 5 and candidate gene analysis by RNA-seq. PLoS Genet. 2016;12:e1006398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benson MJ, Thomas NK, Talwar S, et al. A novel anticonvulsant mechanism via inhibition of complement receptor C5ar1 in murine epilepsy models. Neurobiol Dis. 2015;76:87–97. [DOI] [PubMed] [Google Scholar]
- 20. Barton ME, Klein BD, Wolf HH, et al. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. [DOI] [PubMed] [Google Scholar]
- 21. Frankel WN, Taylor L, Beyer B, et al. Electroconvulsive thresholds of inbred mouse strains. Genomics. 2001;74:306–312. [DOI] [PubMed] [Google Scholar]
- 22. Kimball A, Burnett W, Doherty DG. Chemical protection against ionizing radiation: i. sampling methods for screening compounds in radiation protection studies with mice. Radiat Res. 1957;7:1–12. [PubMed] [Google Scholar]
- 23. Anderson LL, Low IK, Banister SD, et al. Pharmacokinetics of phytocannabinoid acids and anticonvulsant effect of cannabidiolic acid in a mouse model of Dravet syndrome. J Nat Prod. 2019;82:3047–3055. [DOI] [PubMed] [Google Scholar]
- 24. Anderson LL, Absalom NL, Abelev SV, et al. Coadministered cannabidiol and clobazam: preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia. 2019;60:2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torrens A, Vozella V, Huff H, et al. Comparative pharmacokinetics of Δ9-tetrahydrocannabinol in adolescent and adult male mice. J Pharmacol Exp Ther. 2020;374:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spiro AS, Wong A, Boucher AA, et al. Enhanced brain disposition and effects of Δ9-tetrahydrocannabinol in P-glycoprotein and breast cancer resistance protein knockout mice. PLoS One. 2012;7:e35937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taschwer M, Schmid MG. Determination of the relative percentage distribution of THCA and Δ9-THC in herbal cannabis seized in Austria—impact of different storage temperatures on stability. Forensic Sci Int. 2015;254:167–171. [DOI] [PubMed] [Google Scholar]
- 28. McPartland JM, MacDonald C, Young M, et al. Affinity and efficacy studies of tetrahydrocannabinolic acid A at cannabinoid receptor types one and two. Cannabis Cannabinoid Res. 2017;2:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pertwee RG, Rock EM, Guenther K, et al. Cannabidiolic acid methyl ester, a stable synthetic analogue of cannabidiolic acid, can produce 5-HT1A receptor-mediated suppression of nausea and anxiety in rats. Br J Pharmacol. 2018;175:100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palomares B, Garrido-Rodriguez M, Gonzalo-Consuegra C, et al. Δ9-Tetrahydrocannabinolic acid alleviates collagen-induced arthritis: role of PPARγ and CB1 receptors. Br J Pharmacol. 2020;177:4034–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sofia RD, Solomon TA, Barry III H. Anticonvulsant activity of Δ9-tetrahydrocannabinol compared with three other drugs. Eur J Pharmacol. 1976;35:7–16. [DOI] [PubMed] [Google Scholar]
- 32. Consroe P, Wolkin A. Cannabidiol—antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther. 1977;201:26–32. [PubMed] [Google Scholar]
- 33. Chesher G, Jackson DM. Anticonvulsant effects of cannabinoids in mice: drug interactions within cannabinoids and cannabinoid interactions with phenytoin. Psychopharmacologia. 1974;37:255–264. [DOI] [PubMed] [Google Scholar]
- 34. Rock E, Kopstick R, Limebeer C, et al. Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus. Br J Pharmacol. 2013;170:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toman J. The search for new drugs against epilepsy. Texas Rep Biol Med. 1952;10:96–104. [PubMed] [Google Scholar]
- 36. Kaplan JS, Stella N, Catterall WA, et al. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. PNAS. 2017;114:11229–11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patra PH, Barker-Haliski M, White HS, et al. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia. 2019;60:303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klein BD, Jacobson CA, Metcalf CS, et al. Evaluation of cannabidiol in animal seizure models by the Epilepsy Therapy Screening Program (ETSP). Neurochem Res. 2017;42:1939–1948. [DOI] [PubMed] [Google Scholar]