Abstract
Purpose: Medulloblastomas, comprising 20%–25% of all primary brain tumors in children are much rarer in adulthood. Disease biology varies substantially across different age groups; however, owing to rarity, adults with medulloblastoma are traditionally treated using pediatric protocols. This is a retrospective audit of adolescent and adult medulloblastoma from a comprehensive cancer center.
Methods: Data regarding demography, clinical presentation, imaging characteristics, histopathological features, molecular profiling, risk stratification, treatment details, and outcomes were retrieved from medical records. All time-to-event outcomes were analyzed using Kaplan–Meier method and compared with the log-rank test. Univariate and multivariate analysis of relevant prognostic factors was done with p value <0.05 being considered statistically significant.
Results: A total of 162 patients ≥15 years of age with medulloblastoma were included. The median age was 25 years (range: 15–59 years) with leptomeningeal metastases seen in 31 (19%) patients at initial diagnosis. Following surgery, patients were treated with appropriate risk-stratified adjuvant therapy comprising of craniospinal irradiation plus boost with or without systemic chemotherapy. At a median follow-up of 50 months, 5-year Kaplan–Meier estimates of progression-free survival and overall survival were 53.5% and 59.5%, respectively. The addition of adjuvant systemic chemotherapy did not impact upon survival in standard-risk medulloblastoma. High-risk (HR) disease and anaplastic histology emerged as significant and independent predictors of poor survival on multivariate analysis.
Conclusion: Medulloblastoma is a rare tumor in adolescents and adults with key differences in disease biology and resultant outcomes compared with the pediatric population. Contemporary management comprising maximal safe resection followed by appropriate risk-stratified adjuvant therapy provides acceptable survival outcomes.
Keywords: biology, medulloblastoma, outcomes, risk-stratification
Introduction
Medulloblastoma is a unique and aggressive malignant embryonal tumor arising from the cerebellum that comprises 20%–25% of all neoplasm of the central nervous system (CNS) in children.1,2 Medulloblastoma is much rarer in postpubertal children and adulthood constituting <1%–2% of all primary CNS tumors in adolescents and young adults (AYA) defined as 15–39 years of age with an estimated annual incidence of 0.6–1 per million population.2–4 The traditional clinicoradiological risk stratification schema5 remains widely prevalent in neuro-oncologic practice. Children over the age of 3 years with no or small residual tumor (<1.5 cm2) and absence of neuraxial metastases (M0 status) on magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) cytology are classified as having standard-risk disease with >80% long-term survival with standard therapy. The presence of any one or more adverse features such as age less than 3 years, residual tumor ≥1.5 cm2, or presence of metastases (M1–M4 status) makes it a high-risk (HR) disease with long-term survival ranging from 30% to 60% despite intensified adjuvant therapy. It is also well accepted that large-cell/anaplastic (LCA) histology is a significant adverse prognostic factor6,7 with consistently worse outcomes compared with nonanaplastic medulloblastoma.
Novel biological insights have led to consensus classification8 of medulloblastoma into four distinct molecular subgroups—wingless (WNT), sonic hedgehog (SHH), Group 3, and Group 4, respectively, each with different developmental origins, distinct phenotypes, unique transcription profile, and markedly variable prognosis, which has now been incorporated in the updated WHO classification9 and risk stratification of medulloblastoma.10 It is also important to note that clinical presentation, pathological characteristics, and molecular biology varies substantially across different age groups (infantile, pediatric, and adult) in medulloblastoma with resultant differences in therapy, prognosis, and outcomes.11–13 Owing to the rarity of disease, adolescent and adult medulloblastoma have traditionally been managed by extrapolation of data from pediatric protocols3,11,14–16 with no separate recommendations for them. Recently, the European Association of Neuro-Oncology (EANO) and EUropean RAre CANcer (EURACAN) have published guidelines17 for postpubertal and adult patients with medulloblastoma with an aim to provide direction for diagnostic and management decisions. This report is a retrospective audit of clinicoradiological characteristics, histopathological features, molecular profiling, and survival outcomes of adolescent and adult medulloblastoma treated at a comprehensive cancer center.
Materials and Methods
Consecutive adolescent and adult patients (≥15 years of age), with histologically confirmed diagnosis of medulloblastoma registered between 2002 and 2019 at an academic neuro-oncology unit of a tertiary-care cancer center, were identified from a prospectively maintained database. Following maximal safe resection of primary tumor, patients were treated with risk-stratified postoperative radiotherapy (RT) with or without adjuvant systemic chemotherapy. After completion of planned treatment, patients were followed up periodically (3–4 monthly in the first 2 years, 6-monthly until 5 years, and annually thereafter) with annual surveillance MRI scans as per institutional policy. Data regarding demography, clinical presentation, imaging characteristics, histopathological features, molecular profiling, risk stratification, treatment details, and outcomes were retrieved from hospital case files and electronic medical records. Molecular subgroup assignment was done on formalin-fixed paraffin-embedded tumor-tissue blocks based on the differential expression of 12 protein-coding genes and 9 microRNAs using real-time reverse transcriptase polymerase chain reaction (RT-PCR), which has been tested and validated previously.18 Recurrence or progression was defined as radiographic evidence of new tumor growth or progression of residual tumor on posttreatment follow-up imaging. Progression-free survival (PFS) was defined as the interval from diagnosis (date of surgery) until documented clinicoradiological progression or death. Overall survival (OS) was calculated from the date of diagnosis until death from any cause. All time-to event outcomes were analyzed using the Kaplan–Meier method and compared with the log-rank test using April 30, 2020 as the cutoff date for analysis. Univariate analysis of relevant patient, disease, and treatment-related characteristics was done to identify potential prognostic factors. All significant factors and factors with borderline statistical significance (p < 0.1) on univariate analysis were entered into a multivariate Cox proportional hazards model and expressed as hazard ratio with 95% confidence interval (CI). Any p value <0.05 was considered statistically significant. All statistical analysis was done on Statistical Package for Social Sciences. The study was duly reviewed and approved by the local ethics committee that granted waiver of consent owing to retrospective nature of the study.
Results
Electronic search of the neuro-oncology database identified 162 patients 15 years of age or more with newly diagnosed medulloblastoma between 2002 and 2019 that constitutes the present study cohort.
Baseline characteristics
Relevant baseline patient, disease, and treatment-related characteristics of the study cohort are described in Table 1. Briefly, the median age of the study cohort was 25 years (range: 15–59 years) with 106 (65%) patients in the 20–40 years age group and 16 (10%) patients over the age of 40 years at initial diagnosis. Large majority (n = 118, 73%) of patients were males resulting in skewed gender distribution. The most common presenting complaint was headache and vomiting due to raised intracranial pressure followed by gait disturbance, incoordination, ataxia, and visual impairment. Epicenter of tumor was located laterally (cerebellar hemispheric) in two-thirds (n = 106) of patients with remaining one third (n = 56) having midline vermian location. Complete neuraxial staging with spinal MRI and CSF cytology through lumbar puncture detected 31 (19%) patients with leptomeningeal metastases at initial diagnosis. Classic histology (n = 57, 35%) and desmoplastic medulloblastoma (n = 53, 33%) were the common morphological subtypes followed by LCA histology (n = 26, 16%) and medulloblastoma—not otherwise specified (n = 26, 16%). Molecular subgroup was available in 106 (65%) patients. Seventy-one (67%) of 106 patients with known molecular subgroup affiliation belonged to SHH subgroup, followed by 14 (13%), 15 (14.5%), and 6 (5.5%) patients each in WNT, Group 4, and Group 3, respectively.
Table 1.
Baseline Patient, Disease, and Treatment Characteristics of the Study Cohort (N = 162)
| Characteristics | N (%) |
|---|---|
| Age at initial diagnosis, years | |
| Median (range) | 25 (15–59) |
| Gender | |
| Male | 118 (73) |
| Female | 44 (27) |
| Epicenter of tumor | |
| Cerebellar hemispheric (lateralized) | 106 (65) |
| Midline vermian | 56 (35) |
| Metastasis at presentation | |
| No | 131 (81) |
| Yes | 31 (19) |
| Extent of surgery | |
| GTR/NTR | 101 (62) |
| STR | 61 (38) |
| Risk stratification | |
| SR disease | 80 (49) |
| HR disease | 82 (51) |
| Histological subtype | |
| Classic | 57 (35) |
| LCA | 26 (16) |
| Desmoplastic | 53 (33) |
| Not otherwise specified | 26 (16) |
| Molecular subgrouping | |
| WNT | 14 (9) |
| SHH | 71 (44) |
| Group 3 | 6 (4) |
| Group 4 | 15 (9) |
| Not known | 56 (34) |
| Time interval between surgery and RT | |
| ≤6 Weeks | 59 (36) |
| >6 Weeks | 103 (64) |
| Performance status at RT starting | |
| KPS ≥80 | 92 (57) |
| KPS <80 | 51 (31) |
| KPS: not known | 19 (12) |
| RT dose, Gy | |
| Median CSI dose (range) | 35 (23.4–40) |
| Median boost dose (range) | 19.8 (14.4–30.6) |
| Median primary-site dose (range) | 54.0 (54.4–54.8) |
| Adjuvant systemic chemotherapy | |
| Yes | 77 (47) |
| No | 71 (44) |
| Not known | 14 (9) |
CSI, craniospinal irradiation; GTR, gross total resection; HR, high-risk; KPS, Karnofsky performance status; LCA, large-cell/anaplastic; NTR, near-total resection; RT, radiotherapy; SHH, sonic hedgehog; SR, standard risk; STR, subtotal resection; WNT, wingless.
Treatment details
One hundred one (62%) patients underwent gross total resection/near-total resection with residual tumor <1.5 cm2 on postoperative imaging. Postoperative adjuvant therapy was based on traditional clinicoradiological risk stratification schema,5 with presence of residual tumor (≥1.5 cm2) and/or leptomeningeal metastases on complete neuraxial staging being defined as HR medulloblastoma. Since 2010, LCA histological subtype was also considered HR disease. Adult patients with standard-risk medulloblastoma typically received full-dose craniospinal irradiation (CSI) to a dose of 35 Gy/21 fractions plus posterior fossa/tumor-bed boost (19.8 Gy/11 fractions) for a total primary-site dose of 54.8 Gy/32 fractions over 6.5 weeks without adjuvant chemotherapy. Selected patients with standard-risk disease such as adolescents (15–18 years of age) were treated with reduced-dose CSI (23.4 Gy/14 fractions) plus posterior fossa/tumor-bed boost (30.6 Gy/17 fractions) for similar primary-site dose (54–55 Gy) followed by six cycles of adjuvant systemic chemotherapy starting at about 4 weeks from end of RT after complete myelo-recovery. It is widely believed that both these regimens that is, RT alone comprising full-dose CSI and boost irradiation for primary-site dose of 54–55 Gy and combined RT (reduced-dose CSI and boost for similar primary-site dose of 54–55 Gy) plus chemotherapy have similar efficacy in standard-risk medulloblastoma, with addition of chemotherapy compensating for reduction in CSI doses. Adjuvant systemic chemotherapy comprised cisplatin (75 mg/m2 intravenously only on D1 in alternate cycles 2, 4, 6), cyclophosphamide (1000 mg/m2 intravenously on D1 and D2 in cycles 1, 3, 5, and D2 and D3 in cycles 2, 4, 6) and vincristine (1.5 mg/m2 intravenously on D1 and D8 in all six cycles) administered at 3-weekly intervals with adequate hydration, forced saline diuresis, mesna prophylaxis and monitoring of toxicity with requisite dose modifications as appropriate. Patients with HR disease (n = 82, 51%) were offered full dose (35 Gy/21 fractions) or sometimes even extended-dose CSI (40 Gy/24 fractions) plus boost irradiation (14.4–19.8 Gy) for total primary-site dose of 54–55 Gy followed by six cycles of similar adjuvant systemic chemotherapy as above. Boost irradiation (5.4–9 Gy in 3–5 fractions) of metastatic sites was also considered at the discretion of the treating oncologist.
Clinical outcomes
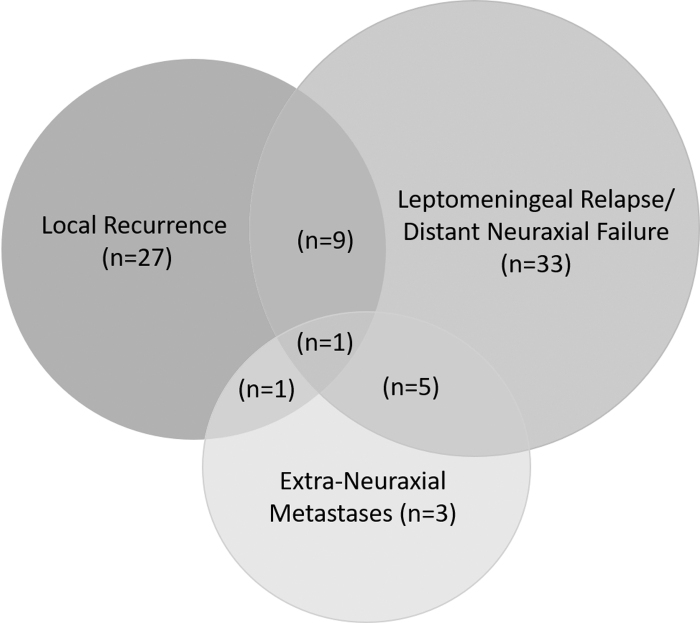
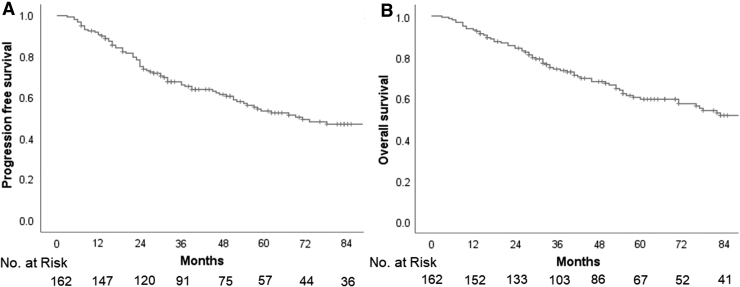
Seventy-nine patients in the study cohort developed recurrent/progressive disease with varying pattern of relapse such as local recurrence within the index tumor-bed/posterior fossa; leptomeningeal relapse/distant neuraxial failure (either focally in supratentorial brain/spine or diffuse leptomeningeal dissemination); or extraneuraxial metastases (ENM) alone or in combination (Fig. 1). Just less than half the patients with relapse (n = 38, 48%) had some component of local failure within the index tumor-bed/posterior fossa either as isolated local recurrence (n = 27, 34%) or in combination with other sites of disease (n = 11, 14%). Leptomeningeal relapse/distant neuraxial failure was seen in 48 (61%) patients commonly as diffuse neuraxial dissemination (n = 25, 32%), but also as focal leptomeningeal deposits either isolated or combined with local recurrence and/or ENM. Systemic metastases to bones, bone marrow, or lymph nodes were seen in a total 10 (13%) patients. The median time to first relapse was 25 months with an interquartile range (IQR) of 15–51 months. The median time to relapse was significantly longer in patients with isolated local recurrence in the tumor bed compared with patients with multifocal, spinal, or disseminated disease (49 months vs. 20 months; p = 0.04). Salvage therapy at relapse was discussed in a multidisciplinary neuro-oncology clinic and generally based on the patterns of failure, time-interval from index diagnosis, performance status of the patient, anticipated morbidity, and likely benefit. Patients with isolated tumor-bed relapse were generally treated more aggressively with re-excision (if feasible) followed by focal reirradiation and salvage systemic chemotherapy. By the time of this analysis, 74 patients had succumbed (72 to disease progression and 2 other causes), 84 patients were documented alive (81 without evidence of disease, including 4 patients who were salvaged after first relapse and 3 alive with disease), whereas 4 patients were lost to follow-up. At a median follow-up of 50 months (IQR: 29–84 months), the 5-year Kaplan–Meier estimates of PFS and OS for the entire study cohort were 53.5% (95% CI: 44.9%–62.1%) and 59.5% (95% CI: 51.1%–67.9%), respectively (Fig. 2).
FIG. 1.
Schematic representation of patterns of failure following risk-stratified therapy in adolescent and adult medulloblastoma.
FIG. 2.
Kaplan–Meier curves of progression-free survival (A) and overall survival (B) for the entire study cohort of adolescent and adult medulloblastoma.
Prognostic factors
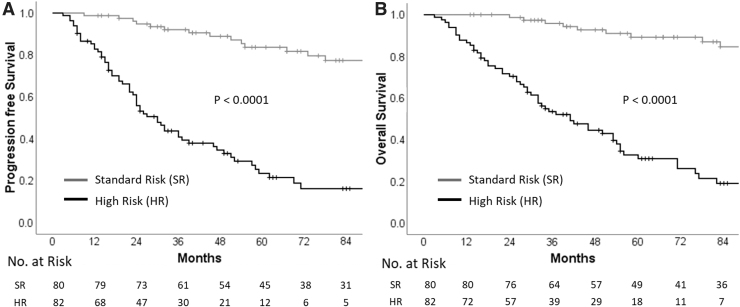
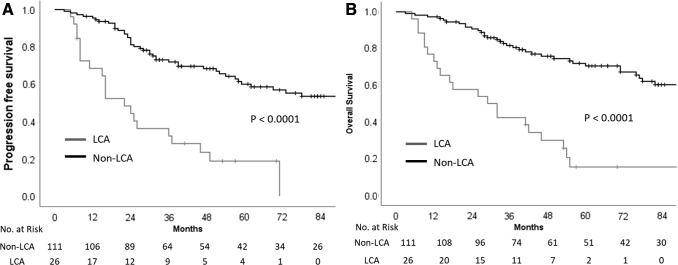
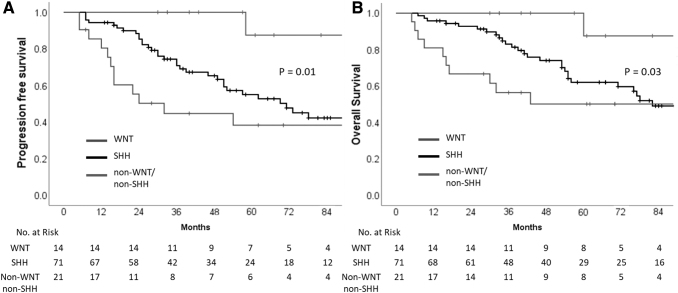
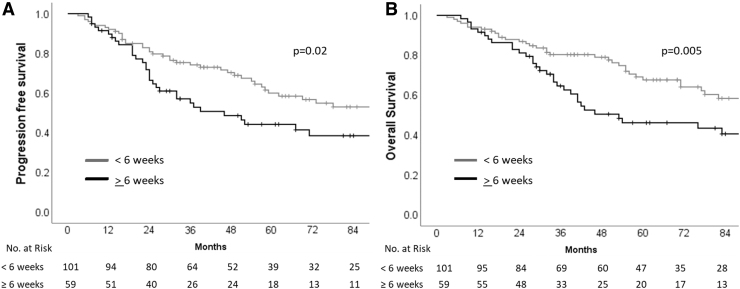
Univariate analysis of various patient, disease, and treatment-related characteristics (Table 2) identified risk stratification (including leptomeningeal metastasis and extent of resection), disease biology (incorporating histological subtype and molecular subgrouping), and time interval between surgery and RT as important determinants of survival. Patients with standard-risk medulloblastoma had significantly higher PFS and OS than patients with HR disease (Fig. 3). The presence of LCA histology was associated with significantly worse survival compared with nonanaplastic subtypes (Fig. 4). In patients with known molecular subgroup affiliation, WNT pathway medulloblastoma had the best outcomes, SHH subgroup tumors had intermediate outcomes, whereas non-WNT/non-SHH subgroup had the worst outcomes (Fig. 5). Finally, any delay of >6 weeks from surgery to initiation of adjuvant RT was also associated with significantly worse outcomes (Fig. 6). Age at initial diagnosis, gender, and anatomic location of tumor did not impact upon survival. In the overall cohort, there was no significant benefit of adjuvant systemic chemotherapy. To further investigate the role of adjuvant chemotherapy on outcome, subgroup analysis stratified by risk category was done. The addition of adjuvant chemotherapy did not impact upon PFS (p = 0.64) or OS (p = 0.16) in patients with standard-risk medulloblastoma. However, 5-year Kaplan–Meier estimates of PFS (31.5%, vs. 5.6%; p < 0.0001) and OS (42.3% vs. 5.8%; p < 0.0001) were significantly higher in patients with HR disease receiving adjuvant chemotherapy (n = 59) compared with no adjuvant chemotherapy (n = 18). This underlines the importance of adding chemotherapy to RT in patients with high-risk medulloblastoma, but an inherent negative selection bias cannot be completely ruled out in HR patients who did not receive adjuvant systemic chemotherapy either to poor performance status, organ dysfunction, comorbidity, disease progression early after RT, or personal/physician choice. On multivariate analysis, risk stratification, and histological subtype emerged as independent predictors of survival (Table 3). Late toxicity was difficult to ascertain due to lack of extractable data in a large majority of long-term surviving patients; however, one case of radiation-induced meningioma and two cases of cerebrovascular accidents were documented on follow-up in the survivor clinic. In contrast to pediatric medulloblastoma,19 documentation of therapy-related late effect risks and screening recommendations has been rather limited in AYA cohort highlighting the need for better coordination between oncologists and primary care providers.20
Table 2.
Univariate Analysis of Prognostic Factors Affecting Survival Outcomes
| Prognostic factors | No. of patients (N) | 5-Year PFS (%) | Log-rank p value | 5-Year OS (%) | Log-rank p value |
|---|---|---|---|---|---|
| Age at diagnosis | |||||
| ≤25 Years | 86 | 48.8 | 0.42 | 57.9 | 0.69 |
| >25 Years | 76 | 58.8 | 61.8 | ||
| Gender | |||||
| Male | 118 | 51.1 | 0.64 | 59.6 | 0.81 |
| Female | 44 | 60.9 | 59.8 | ||
| Epicenter of tumor | |||||
| Midline | 56 | 45.9 | 0.28 | 53.7 | 0.31 |
| Hemispheric | 106 | 57.5 | 62.7 | ||
| Metastasis at presentation | |||||
| No | 131 | 63.2 | <0.0001 | 68.1 | <0.0001 |
| Yes | 31 | 15.7 | 25.6 | ||
| Extent of resection | |||||
| GTR/NTR | 101 | 68.7 | <0.0001 | 74.7 | <0.0001 |
| STR | 61 | 27.2 | 33.8 | ||
| Risk stratification | |||||
| SR | 80 | 83.7 | <0.0001 | 89.2 | <0.0001 |
| HR | 82 | 23.4 | 30.8 | ||
| Histological subtype | |||||
| Non-LCA | 111 | 60.1 | <0.0001 | 70.5 | <0.0001 |
| LCA | 26 | 18.8 | 15.4 | ||
| Molecular subgrouping | |||||
| WNT | 14 | 87.5 | 0.01 | 87.5 | 0.03 |
| SHH | 71 | 55.2 | 62.2 | ||
| Othersa | 21 | 38.3 | 50.1 | ||
| Interval between surgery and RT | |||||
| >6 Weeks | 59 | 44.2 | 0.02 | 46.2 | 0.005 |
| ≤6 Weeks | 99 | 60.3 | 67.6 | ||
| Adjuvant systemic chemotherapy | |||||
| Yes | 77 | 44.3 | 0.12 | 54.2 | 0.13 |
| No | 71 | 60.9 | 64.7 | ||
All p values ≤0.05 are considered statistically significant and highlighted in bold.
Others pertains to non-WNT/non-SHH molecular subgroup.
OS, overall survival; PFS, progression-free survival.
FIG. 3.
Kaplan–Meier curves of progression-free survival (A) and overall survival (B) stratified by risk category (high-risk vs. standard-risk).
FIG. 4.
Kaplan–Meier curves of progression-free survival (A) and overall survival (B) stratified by histological subtype (large-cell/anaplastic vs. nonanaplastic).
FIG. 5.
Kaplan–Meier curves of progression-free survival (A) and overall survival (B) stratified by molecular subgrouping (WNT vs. SHH vs. non-WNT/non-SHH). SHH, sonic hedgehog; WNT, wingless.
FIG. 6.
Kaplan–Meier curves of progression-free survival (A) and overall survival (B) stratified by time interval from surgery to radiotherapy (<6 vs. ≥6 weeks).
Table 3.
Multivariate Analysis of Prognostic Factors in Adult Medulloblastoma
| Prognostic factors | PFS |
OS |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p | |
| Metastasis at presentation | ||||||
| No Yes (Ref.) |
0.76 | 0.35–1.60 | 0.47 | 0.97 | 0.44–2.17 | 0.95 |
| Extent of resection | ||||||
| GTR/NTR STR (Ref.) |
0.97 | 0.44–2.14 | 0.93 | 0.89 | 0.39–2.04 | 0.79 |
| Risk stratification | ||||||
| SR HR (Ref.) |
0.19 | 0.06–0.55 | 0.002 | 0.17 | 0.05–0.56 | 0.004 |
| Histological subtype | ||||||
| Non-LCA LCA (Ref.) |
0.47 | 0.23–0.93 | 0.03 | 0.38 | 0.18–0.78 | 0.009 |
| Molecular subgrouping | ||||||
| WNT SHH Othersa (Ref.) |
0.15 0.63 |
0.01–1.23 0.30–1.33 |
0.07 0.22 |
0.29 0.73 |
0.04–2.38 0.32–1.67 |
0.25 0.46 |
| Interval between surgery and RT | ||||||
| >6 Weeks ≤6 Weeks (Ref.) |
1.34 | 0.70–2.56 | 0.38 | 1.2 | 0.61–2.39 | 0.59 |
All p values ≤0.05 are considered statistically significant and highlighted in bold.
Others pertains to non-WNT/non-SHH molecular subgroup and was used as reference.
CI, confidence interval; HR, high-risk; Ref., reference.
Discussion
This large retrospective clinical audit reports acceptable 5-year PFS (53.5%) and OS (59.5%) and confirms HR disease and anaplastic histology as independent prognostic factors for survival in adolescent and adult medulloblastoma.
Data from large population-based registry, literature-based meta-analyses, multi-institutional collaborative network studies and prospective trials provide best guidance in the contemporary evidence-based management of adolescents and adults with medulloblastoma. In the first population-based analysis21 of Surveillance, Epidemiology, and End Results program (SEER) registry of the United States, 5- and 10-year survival of adult medulloblastoma diagnosed between 1973 and 2004 (N = 454) was 64.9% and 52.1%, respectively. In multivariable regression modeling, diagnoses in a later era (after 1980s), younger age at diagnosis (<20 years), gross total resection, and receipt of adjuvant RT were favorable prognostic factors, whereas LCA histology was associated with poor survival. An updated analysis22 of the SEER database (N = 857 patients diagnosed between 1973 and 2014) reported that adult medulloblastoma most commonly presented in the 20–29-year age group, with slight male preponderance (58.5%) with vast majority (91.6%) was located in the cerebellar region. Following surgical resection, 79% and 44.4% patients received RT and systemic chemotherapy, respectively. The median survival for adult medulloblastoma was 60 months, which was strongly correlated with anatomic location and adjuvant treatment on multivariate Cox proportional hazard models. Location of tumor outside the cerebellum predicted for worse survival compared with cerebellar location (HR = 1.69, 95% CI: 1.32–2.16; p = 0.001). Patients assigned to chemotherapy had shorter survival than those who were not (HR = 1.45, 95% CI: 1.26–1.67; p < 0.001), but receiving RT was associated with better survival compared with no RT (HR = 0.581, 95% CI: 0.48–0.70; p < 0.001). The benefit of upfront chemotherapy in adults with standard-risk medulloblastoma has been debatable and controversial. Kann et al.23 analyzed 751 adults (≥18 years) with medulloblastoma registered in the National Cancer Data Base (NCDB) from 2004 to 2012. The 5-year OS in propensity-matched patients who received RT plus upfront chemotherapy was significantly better than RT alone (84% vs. 74%; p = 0.01). Within the propensity-matched cohort, 5-year outcomes were improved on subgroup analysis for patients with nonmetastatic medulloblastoma (M0), patients receiving full-dose CSI, as well as for M0 patients receiving full-dose CSI, suggesting that patients with standard-risk disease also derive benefit from upfront systemic chemotherapy. Similar benefit from chemotherapy at initial diagnosis had also been reported previously in a literature-based meta-analysis24 involving AYA medulloblastoma (≥15 years) in 277 publications from 1969 to 2013. Of the 907 patients included, 94% and 71% had received RT and systemic chemotherapy, respectively. Patients who received chemotherapy at initial diagnosis did significantly better than those receiving RT alone and those receiving chemotherapy only as salvage for recurrent/progressive medulloblastoma. An updated NCBD analysis25 of 1144 patients of adult medulloblastoma from 2004 to 2016 reported the association of poorer survival with advancing age (p = 0.012), greater comorbidities (p = 0.039), and nonuninsured/private insurance (p < 0.05) on multivariate analysis. Data of 206 patients with adult medulloblastoma treated between 1976 and 2014 were pooled in a Rare Cancer Network (RCN) analysis involving 13 institutions globally.26 The median age of the RCN cohort was 31 years, with 98% and 48% patients receiving RT and chemotherapy, respectively. At a median follow-up of 31 months, 10-year estimates of local control, PFS, and OS were 46%, 38%, and 51%, respectively. On multivariate analyses, performance status and RT were significant prognostic factors for survival. The use of adjuvant systemic chemotherapy predicted for better local control but did not impact upon survival.
The conduct of prospective studies has been difficult and scarce in adolescents and adults with medulloblastoma. Patient characteristics, treatment details, and relevant outcomes of prospective clinical studies in adolescent and adult medulloblastoma are summarized in Table 4.27–31 Patients were treated with full-dose CSI regardless of risk stratification, whereas the use of chemotherapy was variable across the studies. Risk stratification and metastatic disease were independent prognostic factors in this cohort similar to childhood medulloblastoma. The impact of adjuvant therapy on neurocognitive functioning and quality-of-life (QOL) outcomes in adult medulloblastoma were recently reported32 by the German Neuro-Oncology Group (NOA). Three preselected QOL scales (role, social, and cognitive functioning) showed clinically relevant improvement in scores (≥10 points) compared with posttreatment levels until 30 months but decreased afterward. Verbal working memory and attention remained impaired until 18 months posttreatment. Coordination, processing speed, and verbal fluency improved compared with posttreatment scores and remained within normal range thereafter, pointing to modest impact of combined modality treatment on neurocognitive function and QOL in adults with medulloblastoma. The relatively poorer tolerance to standard adjuvant chemotherapy in adult medulloblastoma compared with children has prompted attenuated maintenance chemotherapy regimens with reduced toxicity.33
Table 4.
Summary of Prospective Clinical Studies in Adolescent and Adult Medulloblastoma
| First authorRef. | No. of patients | Age group (years) | RT details | Chemotherapy setting | Outcomes |
|---|---|---|---|---|---|
| Brandes27 | 95 | ≥18 | SR-MB: CSI (36 Gy) + Boost (18.8 Gy); HR-MB: CSI (36 Gy) + Boost (18.8 Gy) | No CTh; 2 cycles NACTh +4 cycles of adjuvant CTh | 10-Year PFS = 46%, 10-year OS = 65%; 10-year PFS = 36%, 10-year OS = 45%; risk strata and M+ disease—prognostic |
| Silvani28 | 28 | ≥21 | Full-dose CSI (36 Gy) + Boost (18 Gy) | 2 Cycles of NACTh | 5-year PFS = 57.6%, 5-year OS = 80% |
| Friedrich29 | 70 | ≥21 | Localized MB: CSI (35.2 Gy) + Boost (20 Gy) | Adjuvant CTh in 70% patients | 4-Year PFS = 68%, 4-year OS = 89% |
| von Bueren30 | 23 | ≥21 | Metastatic MB: HFRT CSI (40, 1 Gy twice daily) + Boost (28, 1 Gy twice daily) | Sandwich (CTh-RT-CTh) or sequential (RT-CTh) Rx | 4-year PFS = 51%, 4-year OS = 91%; no difference in sandwich versus sequential Rx |
| Beier31 | 30 | >21 | Chang stage: T1–T4, M0–M1 disease; full-dose CSI (35.2 Gy) + Boost (20 Gy) | 8 Cycles of adjuvant CTh | 70% Patients received >4 courses of CTh; 67% patients stopped CTh due to toxicity |
CTh, chemotherapy; HFRT, hyperfractionated radiation therapy; HR, high-risk; MB, medulloblastoma; NACTh, neoadjuvant chemotherapy; Rx, treatment.
Recently, the National Cancer Institute (NCI) through its Comprehensive Oncology Network Evaluating Rare CNS Tumors (CONNECT) program convened an adult medulloblastoma workshop34 to review advances, share scientific insights, and address challenges in this otherwise orphan disease. A collaborative working group with representation from leading clinicians, scientists, and patient advocacy organizations identified unmet needs in clinical trial design, tissue acquisition and testing, tumor modeling, and measurement of clinical outcomes and developed specific action items to expedite progress in adult medulloblastoma. Proposed recommendations34,35 included facilitating referral of adult medulloblastoma patients to centers of excellence; promoting participation in clinical trials; encouraging DNA methylation for confirmation of diagnosis and molecular subgrouping; offering counseling on contraception and fertility preservation; evaluating patients for symptoms and medical management of endocrine, vision, hearing, and neurocognitive deficits; providing psychosocial support and referral to neurorehabilitation; minimizing delays in therapy; and incorporating imaging standards and criteria for progression.
Strengths and limitations
This study represents the largest series of adolescent and adult medulloblastoma from South Asia treated appropriately using risk-stratified adjuvant radio(chemo)therapy. Availability of molecular profiling in two-thirds of the study cohort provides added value to the study. However, despite the aforesaid strengths, several caveats and limitations remain. Retrospective design of the study makes it susceptible to intrinsic biases that could potentially confound interpretation of results. Lack of data on neurocognitive functioning, endocrine status, and QOL precludes comments on the impact of adjuvant therapy on late toxicity and functional status in adolescents and adults with medulloblastoma.
Conclusions
Medulloblastoma is a rare tumor in adolescents and adults with key differences in clinicopathological spectrum, underlying molecular biology, prevalent treatment regimens, and outcomes compared with the pediatric population. Contemporary management comprising maximal safe resection followed by appropriate risk-stratified therapy results in acceptable survival outcomes. High-risk disease and anaplastic histology are significant and independent predictors of poor prognosis.
Acknowledgment
The authors thank the Brain Tumor Foundation (BTF) of India.
Author Disclosure Statement
None of the authors has any conflict of interest to declare.
Funding Information
No financial support was involved in the study.
References
- 1. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18:v1–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel AP, Fisher JL, Nichols E, et al. Global, regional, and national burden of brain and other CNS cancer, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:376–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Majd N, Penas-Prado M. Updates on management of adult medulloblastoma. Curr Treat Options Oncol. 2019;20:64. [DOI] [PubMed] [Google Scholar]
- 4. Vadgaonkar R, Epari S, Chinnaswamy G, et al. Distinct demographic profile and molecular markers of primary CNS tumor in 1873 adolescent and young adult patient population. Childs Nerv Syst. 2018;34:1489–95. [DOI] [PubMed] [Google Scholar]
- 5. Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–45. [DOI] [PubMed] [Google Scholar]
- 6. Massimino M, Antonelli M, Gandola L, et al. Histological variants of medulloblastoma are the most powerful clinical prognostic indicators. Pediatr Blood Cancer. 2013;60:210–6. [DOI] [PubMed] [Google Scholar]
- 7. Huang PI, Lin SC, Lee YY, et al. Large cell/anaplastic medulloblastoma is associated with poor prognosis—a retrospective analysis at a single institute. Childs Nerv Syst. 2017;33:1285–94. [DOI] [PubMed] [Google Scholar]
- 8. Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–20. [DOI] [PubMed] [Google Scholar]
- 10. Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131:821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korshunov A, Remke M, Werft W, et al. Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. J Clin Oncol. 2010;28:3054–60. [DOI] [PubMed] [Google Scholar]
- 12. Shonka N, Brandes A, De Groot JF. Adult medulloblastoma, from spongioblastoma cerebelli to the present day: a review of treatment and the integration of molecular markers. Oncology (Williston Park). 2012;26:1083–91. [PubMed] [Google Scholar]
- 13. Lassaletta A, Ramaswamy V. Medulloblastoma in adults: they're not just big kids. Neuro Oncol. 2016;18:895–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brandes AA, Bartolotti M, Marucci G, et al. New perspectives in the treatment of adult medulloblastoma in the era of molecular oncology. Crit Rev Oncol Hematol. 2015;94:348–59. [DOI] [PubMed] [Google Scholar]
- 15. Mascarin M, Coassin E, Franceschi E, et al. Medulloblastoma and central nervous system germ cell tumors in adults: is pediatric experience applicable? Childs Nerv Syst. 2019;35:2279–87. [DOI] [PubMed] [Google Scholar]
- 16. Spreafico F, Massimino M, Gandola L, et al. Survival of adults treated for medulloblastoma using paediatric protocols. Eur J Cancer. 2005;41:1304–10. [DOI] [PubMed] [Google Scholar]
- 17. Franceschi E, Hofer S, Brandes AA, et al. EANO–EURACAN clinical practice guideline for diagnosis, treatment, and follow-up of post-pubertal and adult patients with medulloblastoma. Lancet Oncol. 2019;20:e715–28. [DOI] [PubMed] [Google Scholar]
- 18. Kunder R, Jalali R, Sridhar E, et al. Real-time PCR assay based on the differential expression of microRNAs and protein-coding genes for molecular classification of formalin-fixed paraffin embedded medulloblastomas. Neuro Oncol. 2013;15:1644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salloum R, Chen Y, Yasui Y, et al. Late morbidity and mortality among medulloblastoma survivors diagnosed across three decades: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2019 20;37(9):731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howard AF, Tran J, Aparicio A, et al. Documentation of late-effects risks and screening recommendations for adolescent and young adult central nervous system, soft tissue, or bone tumor survivors treated with radiotherapy in British Columbia, Canada. J Adolesc Young Adult Oncol. 2019;8(2):142–8. [DOI] [PubMed] [Google Scholar]
- 21. Lai R. Survival of patients with adult medulloblastoma. Cancer. 2008;112:1568–74. [DOI] [PubMed] [Google Scholar]
- 22. Ma AK, Freedman I, Lee JH, et al. Tumor location and treatment modality are associated with overall survival in adult medulloblastoma. Cureus. 2020;12:e7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kann BH, Lester-Coll NH, Park HS, et al. Adjuvant chemotherapy and overall survival in adult medulloblastoma. Neuro Oncol. 2016;19:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kocakaya S, Beier CP, Beier D. Chemotherapy increases long-term survival in patients with adult medulloblastoma—a literature-based meta-analysis. Neuro Oncol. 2016;18:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haque W, Verma V, Brian Butler E, Teh BS. Prognostic role of chemotherapy, radiotherapy dose, and extent of surgical resection in adult medulloblastoma. J Clin Neurosci. 2020;76: 154–60. [DOI] [PubMed] [Google Scholar]
- 26. Atalar B, Ozsahin M, Call J, et al. Treatment outcome and prognostic factors for adult patients with medulloblastoma: the Rare Cancer Network (RCN) experience. Radiother Oncol. 2018;127:96–102. [DOI] [PubMed] [Google Scholar]
- 27. Brandes AA, Franceschi E, Tosoni A, et al. Efficacy of tailored treatment for high- and low-risk medulloblastoma in adults: a large prospective phase II trial. J Clin Oncol. 2010;28:2003. [Google Scholar]
- 28. Silvani A, Gaviani P, Lamperti E, et al. Adult medulloblastoma: multiagent chemotherapy with cisplatinum and etoposide: a single institutional experience. J Neurooncol. 2012;106:595–600. [DOI] [PubMed] [Google Scholar]
- 29. Friedrich C, von Bueren AO, von Hoff K, et al. Treatment of adult nonmetastatic medulloblastoma patients according to the paediatric HIT 2000 protocol: a prospective observational multicentre study. Eur J Cancer. 2013;49:893–903. [DOI] [PubMed] [Google Scholar]
- 30. von Bueren AO, Friedrich C, Von Hoff K, et al. Metastatic medulloblastoma in adults: outcome of patients treated according to the HIT2000 protocol. Eur J Cancer. 2015;51:2434–43. [DOI] [PubMed] [Google Scholar]
- 31. Beier D, Proescholdt M, Reinert C, et al. Multicenter pilot study of radiochemotherapy as first-line treatment for adults with medulloblastoma (NOA-07). Neuro Oncol. 2018;20:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dirven L, Luerding R, Beier D, et al. Neurocognitive functioning and health-related quality of life in adult medulloblastoma patients: long-term outcomes of the NOA-07 study. J Neurooncol. 2020;148:117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dagri JN, Evans A, Torkildson JC, et al. Feasibility of an attenuated maintenance chemotherapy regimen directed at adolescents and young adults with newly diagnosed localized medulloblastoma and other central rervous system embryonal tumors. J Adolesc Young Adult Oncol. 2014;3(3):106–11. [Google Scholar]
- 34. Penas-Prado M, Theeler BJ, Cordeiro B, et al. Proceedings of the Comprehensive Oncology Network Evaluating Rare CNS Tumors (NCI-CONNECT) Adult Medulloblastoma Workshop. Neuro-Oncology Adv. 2020;2. DOI: 10.1093/noajnl/vdaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Penas-Prado M, Armstrong TS, Gilbert MR. Proposed addition to the NCCN guidelines for adult medulloblastoma. J Natl Compr Canc Netw. 2020;18(11):1579–84. [DOI] [PubMed] [Google Scholar]