Abstract
Background: Pre-clinical studies have demonstrated the potential anticancer activity of cannabinoids, yet little clinical data exist to support this. Nearly 40% of patients with cancer using cannabis believe it will treat their cancer with numerous anecdotal reports shared online through social media platforms. Case reports have been published in peer-reviewed journals, but often lack key clinical information to validate anticancer claims.
Methods: We reviewed literature in PubMed and EBSCO databases that evaluated the relationship between cannabis or the endocannabinoid system and potential anticancer activity. We also reviewed online sources, books, and ClinicalTrials.gov for reports or studies on using cannabis as cancer treatment. All case reports published in peer-reviewed journals were compiled and appraised as weak, moderate, or strong based on the quality of evidence provided supporting an anticancer effect. Strong reports met three criteria; (a) active cancer at time of cannabis administration, (b) validated laboratory or radiographic responses were reported, and (c) cannabis used without concurrent anticancer treatments.
Results: Of the 207 pre-clinical articles reviewed, 107 (52%) were pre-clinical studies with original data. A total of 77 unique case reports described patients with various cancers (breast, central nervous system, gynecological, leukemia, lung, prostate, and pancreatic) using cannabis. Our appraisal showed 14% of the case reports were considered strong, 5% moderate, and the remaining 81% were weak. Ten percent of cases were in pediatric patients. Cannabidiol use was most often reported as the anticancer cannabinoid with daily doses ranging from 10 to 800 mg. Tetrahydrocannabinol use was reported in six studies, with doses ranging from 4.8 to 7.5 mg. Two small trials published data on survival in patients with recurrent glioblastoma multiforme.
Conclusion: This review of clinical data suggests most published, peer-reviewed case reports provide insufficient data to support the claim for cannabis as an anticancer agent, and should not be used in place of evidence-based, traditional treatments outside of a clinical trial. No strong clinical trial data exist to confirm the pre-clinical studies that suggest cannabinoids may have an anticancer benefit. Future studies exploring anticancer potential of cannabis in patients with metastatic cancers who have not responded to traditional therapy are needed.
Keywords: cannabis, marijuana, cancer, antineoplastic
Introduction
Increased use of social media and marijuana legalization has led to greater claims about cannabis as a cancer cure. A 2019 study using a Google Trends' tool analyzed the search activity on cannabis and cancer and found, “online search volume for cannabis and cancer increased at 10 times the rate of standard therapies. Cannabis as a cancer cure comprised the largest category of social media content on alternative cancer treatments at 23.5%.”1 A large survey conducted at a National Cancer Institute-designated cancer center found that 24% of the 926 respondents used cannabis in the last year and 74% wanted more information about cannabis from their providers.2 This survey also reported nearly one in four patients use cannabis in an attempt to help treat their cancer.
In 1975, the first study to demonstrate potential antineoplastic activity of cannabinoids reported that delta9-tetrahydrocannabinol (THC), delta8-tetrahydrocannabinol, and cannabinol reduced the tumor size and increased mean survival time in mice implanted with Lewis lung adenocarcinoma cells, but cannabidiol (CBD) did not have such effects.3 Other in vitro and in vivo studies using a variety of cannabinoids (both THC and CBD) have shown potential anticancer effects in several cell lines (e.g., brain, breast, cervical, colon, leukemia, lymphoma, and prostate).4–6 Proposed mechanisms of cannabinoid anticancer actions include (a) inhibiting tumor growth, (b) reducing cell viability, (c) inducing apoptosis, (d) inhibiting angiogenesis, and (e) inhibiting invasion/metastasis.7
Despite these promising pre-clinical studies, human trials with clinical end-points pertaining to tumor control are extremely limited. A phase I clinical trial in patients with recurrent glioblastomas assessed safety and efficacy of intrathecal THC use, but could not demonstrate improved tumor control or longer survival.8 A recent phase 1b trial using nabiximols (a 1:1 THC/CBD oromucosal spray) with dose-intense temozolomide (DIT) did report increased survival in the nabiximols group, but the objective of this feasibility study was not to investigate survival differences.9 As of March 2021, there are only seven active, interventional studies using medical cannabis in the cancer population that are recruiting patients, all of which are aimed at symptom control based on listings in ClinicalTrials.gov The case reports and case series that have been published claiming anticancer benefits of cannabinoids often lack important details (e.g., pertinent disease history, review of concomitant medications, supporting response assessment).
With over 600,000 projected deaths due to cancer in 2020, patients are increasingly seeking alternative treatments with hope of finding a “miracle cure.”10 A 2012 study in patients with cancer across 18 countries found that the current use of complementary and alternative medicine (CAM) across all studies was 40%, with the highest being in the United States.11 From 1970s to 2000s, the overall prevalence of CAM use doubled from 25% to 49%. While cannabis may help to improve the debilitating symptoms patients with cancer experience, any impact on cancer control is far from established. Our goal was to summarize current data and assess the clinical evidence for claims that cannabis works as an anticancer agent in humans. We conducted a thorough review and analysis of cannabis as an anticancer agent to inform patients, cancer providers, and researchers of what data exist and how to help move the field forward.
Materials and Methods
Search criteria
We created a search query in the PubMed and EBSCO databases to identify any articles including cannabis and cancer (Appendix A1). All abstracts were reviewed and only articles addressing the relationship between cannabis or the endocannabinoid system and potential anticancer activity were included.
We also searched for articles, books, presentations, and online materials pertaining to cancer as an anticancer agent to find additional potentially relevant studies or case reports. We reviewed all published abstracts and oral presentations from the annual scientific meetings from 2018 to 2020 at the American Society for Clinical Oncology and the International Cannabis Research Society. Two books were reviewed (Cannabis for the Treatment of Cancer and Nature's Answer for Cancer).12,13 A search of www.clinicaltrials.gov was performed to find current and past clinical trials.
Case report appraisal
Key findings for each patient discussed in published peer-reviewed case reports or case series was abstracted and summarized in Table 2. We abstracted the following data if available for each report: (a) traditional therapy used (surgery, radiation, chemotherapy, hormone therapy), (b) details on THC or CBD use (e.g., dose, timing in relation to other concurrent anticancer therapies), (c) cancer status at initiation of cannabis (e.g., in remission, active/metastatic disease), and (d) validated clinical responses (e.g., radiological or laboratory/physical exam response). A number of studies claimed a radiographic or laboratory response, but relied on experimental labs or subjective statements in place of detailed imaging reports, and were not considered to have sufficient evidence reported. Using information/evidence presented in each report, we appraised the potential anticancer impact of cannabis as strong, moderate, or weak. Strong cases met ALL of the following criteria: (a) active cancer at time of cannabis initiation, (b) a radiographic OR clinically validated laboratory response/physical exam improvement was documented, and (c) cannabis was utilized without other concurrent anticancer therapies (e.g., chemotherapy, hormonal therapy, or radiation). If case reports met the first two criteria, but other anticancer therapies were used concurrently, we graded these as moderate. All other reports were graded as weak.
Table 2.
Appraisal of peer-reviewed, published case reports claiming anticancer activity in patients using cannabis
| Cancer type | Reference | Age | Gender | Surgery | Radiation | Chemotherapy | Hormone therapy | Maximum THC dose, mg/day | Maximum CBD dose, mg/day | Active cancer at time of cannabis initiation? | Cannabis utilized without other concurrent anticancer therapies? | Radiographic response or improvement reported? | Clinically validated laboratory and/or improvement on physical exam reported? | Appraisal of evidence supporting cannabis anticancer impact |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast | 15 | 93 | F | 0 | 20 | Y | Y | N | Y | Strong | ||||
| Breast | 15 | 63 | F | 0 | 20 | Y | Y | N | Y | Strong | ||||
| Breast | 15 | 43 | F | 0 | 20 | Y | Y | Y | Y | Strong | ||||
| Breast | 15 | 70 | F | X | 0 | 40 | Y | N | N | Y | Moderate | |||
| Breast | 15 | 63 | F | 0 | 40 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 73 | F | 0 | 40 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 69 | F | 0 | 20 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 77 | F | X | X | 0 | 20 | a | N | N | N | Weak | ||
| Breast | 15 | 42 | F | X | X | 0 | 20 | Y | N | N | N | Weak | ||
| Breast | 15 | 49 | F | 0 | 40 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 63 | F | X | 0 | 30 | Y | Y | N | N | Weak | |||
| Breast | 15 | 64 | F | 0 | 20 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 65 | F | 0 | 20 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 61 | F | 0 | 20 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 73 | F | X | 0 | 20 | Y | Y | N | N | Weak | |||
| Breast | 15 | 48 | F | X | X | X | 0 | 20 | a | Y | N | N | Weak | |
| Breast | 15 | 47 | F | 0 | 20 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 79 | F | 0 | 10 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 75 | F | 0 | 20 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 49 | F | 0 | 20 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 59 | F | 0 | 20 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 56 | F | X | X | 0 | 20 | a | Y | N | N | Weak | ||
| Breast | 15 | 67 | F | X | X | 0 | 20 | a | Y | N | N | Weak | ||
| Breast | 15 | 61 | F | 0 | 20 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 64 | F | X | X | 0 | 20 | Y | Y | N | N | Weak | ||
| Breast | 15 | 62 | F | X | 0 | 20 | Y | Y | N | N | Weak | |||
| Breast | 15 | 42 | F | 0 | 10–60 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 44 | F | 0 | 40 | Y | Y | N | N | Weak | ||||
| Breast | 15 | 75 | F | 0 | 40 | Y | Y | N | N | Weak | ||||
| CNS | 17 | 5 | M | X | X | X | 0 | 10–60 | Y | Y | Y | N | Strong | |
| CNS | 17 | 59 | M | X | X | 0 | 10–60 | Y | Y | Y | N | Strong | ||
| CNS | 24 | 38 | M | X | X | X | 0 | 300–450 | Y | N | Y | N | Moderate | |
| CNS | 24 | 38 | M | X | X | X | 0 | 100–200 | Y | N | Y | N | Moderate | |
| CNS | 25 | 41 | M | X | X | X | 0 | 400 | Y | N | N | N | Weak | |
| CNS | 25 | 40 | M | X | X | X | 0 | 400 | Y | Y | N | N | Weak | |
| CNS | 25 | 44 | F | X | 0b | 600 | Y | Y | N | N | Weak | |||
| CNS | 25 | 60 | M | X | X | X | 0 | 200 | Y | N | N | N | Weak | |
| CNS | 25 | 61 | M | X | X | X | 7.5 | 400 | Y | N | N | N | Weak | |
| CNS | 25 | 49 | F | X | X | X | 0 | 400 | Y | N | N | N | Weak | |
| CNS | 25 | 38 | F | X | 0 | 400 | Y | Y | N | N | Weak | |||
| CNS | 25 | ∼37 | M | X | 0 | 400 | N | Y | N | N | Weak | |||
| CNS | 25 | 35 | F | X | X | X | 0 | 200 | Y | N | N | N | Weak | |
| CNS | 17 | 10 | F | 0 | 10–60 | Y | Y | N | N | Weak | ||||
| CNS | 17 | 7 | M | X | X | X | 0 | 10–60 | Y | Y | N | N | Weak | |
| CNS | 17 | 57 | M | X | X | 0 | 10–60 | Y | Y | N | N | Weak | ||
| CNS | 17 | 68 | F | X | X | X | 0 | 10–60 | Y | Y | N | N | Weak | |
| CNS | 17 | 10 | M | 0 | 10–60 | Y | Y | N | N | Weak | ||||
| CNS | 17 | 14 | F | X | X | X | 0 | 10–60 | Y | Y | N | N | Weak | |
| CNS | 17 | 69 | M | X | X | X | 0 | 10–60 | Y | Y | N | N | Weak | |
| CNS | 17 | 47 | M | 0 | 10–60 | Y | Y | N | N | Weak | ||||
| CNS | 17 | 42 | M | X | X | X | 0 | 10–60 | Y | Y | N | N | Weak | |
| CNS | 17 | 6 | F | X | X | X | 0 | 10–60 | Y | Y | N | N | Weak | |
| CNS | 17 | 56 | M | 0 | 10–60 | Y | Y | N | N | Weak | ||||
| CNS | 17 | 9 | F | X | X | X | 0 | 10–60 | Y | Y | N | N | Weak | |
| GYN | 26 | 81 | F | 0 | 1c | Y | Yd | Y | Y | Strong | ||||
| Leukemia | 20 | 14 | F | X | X | 0e | 0e | Y | Y | N | Y | Strong | ||
| Lung | 27 | 81 | M | 0 | ∼12 | Y | Y | Y | N | Strong | ||||
| Pancreatic | 28 | 70 | F | X | 0 | 100 | Y | N | N | N | Weak | |||
| Pancreatic | 28 | 47 | M | 7.5 | 400 | Y | Y | N | N | Weak | ||||
| Pancreatic | 28 | 47 | M | X | 0 | 400 | Y | N | N | N | Weak | |||
| Pancreatic | 28 | 67 | F | X | 7.5 | 400 | Y | N | N | N | Weak | |||
| Pancreatic | 28 | 70 | M | X | 0 | 200 | Y | N | N | N | Weak | |||
| Pancreatic | 28 | 68 | F | X | 4.8 | 400 | Y | N | N | N | Weak | |||
| Pancreatic | 28 | 53 | F | X | 0 | 400 | Y | N | N | N | Weak | |||
| Pancreatic | 28 | 45 | M | 7.5 | 800 | Y | Y | N | N | Weak | ||||
| Pancreatic | 28 | 50 | F | X | 4.8 | 400 | Y | N | N | N | Weak | |||
| Prostate | 16 | 70 | M | 0 | 10–60 | Y | Y | N | Y | Strong | ||||
| Prostate | 16 | 81 | M | 0 | 10–60 | Y | Y | N | Y | Strong | ||||
| Prostate | 16 | 75 | M | 0 | 10–60 | Y | Y | N | Y | Strong | ||||
| Prostate | 16 | 82 | M | X | 0 | 40 | Y | N | N | Y | Moderate | |||
| Prostate | 16 | 59 | M | X | 0 | 10–60 | N | Y | N | N | Weak | |||
| Prostate | 16 | 79 | M | X | 0 | 10–60 | Y | N | N | N | Weak | |||
| Prostate | 16 | 76 | M | X | X | 0 | 10–60 | Y | N | N | N | Weak | ||
| Prostate | 16 | 74 | M | 0 | 10–60 | Y | Y | N | N | Weak | ||||
| Prostate | 16 | 83 | M | 0 | 10–60 | Y | Y | N | N | Weak | ||||
| Prostate | 16 | 84 | M | X | 0 | 10–60 | Y | N | N | N | Weak | |||
| Prostate | 16 | 71 | M | X | 0 | 10–60 | Y | N | N | N | Weak |
All peer-reviewed, published case reports that claimed anticancer activity of cannabis were analyzed and appraised as either having weak, moderate, or strong evidence that cannabis may have worked as an antineoplastic.
Cannabis use started after standard curative treatment for unspecified “breast cancer.” Unclear if disease was still active at time of administration.
Intermittent dronabinol use reported but the dose was not provided.
One drop of CBD oil each evening.
Cannabis was used without standard therapies, but was used with Laetrile, a naturally occurring substance that is falsely promoted as an anticancer treatment.
Various cannabis strains were used to make homemade hemp oil per Rick Simpson's instruction. Doses ranged from 0.02 to 1.0 mL at a time, up to three times a day.
GYN, gynecological; N, no; Y, yes.
Results
The PubMed and EBSCO searches yielded 4202 and 4786 results, respectively, and consisted of a variety of reports including journal articles, systematic reviews, case studies, clinical trials, and opinion pieces. All but four articles (which were sent to us by the authors) were found in this search. Ultimately, 218 peer-reviewed were included in our review. A total of 207 were pre-clinical reports, 9 involved case reports/case series (totaling 77 cases), and 2 were other clinical studies.
Pre-clinical data summary
A total of 207 pre-clinical articles were reviewed and the original articles were summarized in Table 1. About 100 articles (48%) were reviews, commentaries, interviews, or other sources without original data. The remaining 107 articles (52%) included original data from animal models (11%), in vitro assays (55%), or a combination of both in vivo and in vitro studies (34%). Cannabinoid utilized was also reported; 11% used THC alone, 21% used CBD alone, 17% used a combination of THC, CBD, and other cannabinoids (non-CBD or –THC phytocannabinoid or synthetic) and the remaining 50% of articles used other cannabinoids. The “other” category primarily comprised of cannabinoid receptor agonists alone such as JWH-015, JWH-133, and WIN 55,212-2.
Table 1.
Pre-clinical studies of cannabinoids in cancer models that report original data from PubMed and EBSCO literature searches, categorized by study type and compound studied
| Cancer type | No. of original data | Study type |
Compound studied |
|||||
|---|---|---|---|---|---|---|---|---|
| In vivo | In vitro | Both in vivo and in vitro | THC alone | CBD alone | Multiple including THC or CBDa | Other cannabinoidb | ||
| Breast | 19 | 4 | 9 | 6 | 4 | 6 | 1 | 8 |
| CNS | 20 | 2 | 12 | 6 | 2 | 4 | 5 | 9 |
| Colorectal | 10 | 0 | 5 | 5 | 0 | 2 | 2 | 6 |
| Gynecological | 4 | 0 | 3 | 1 | 0 | 3 | 1 | 0 |
| Leukemia | 6 | 0 | 4 | 2 | 1 | 3 | 1 | 1 |
| Lung | 9 | 1 | 3 | 5 | 1 | 2 | 2 | 4 |
| Melanoma | 4 | 3 | 1 | 0 | 1 | 0 | 2 | 1 |
| Prostate | 6 | 1 | 3 | 2 | 0 | 0 | 1 | 5 |
| Multiple | 8 | 0 | 5 | 3 | 1 | 1 | 1 | 5 |
| Otherc | 21 | 1 | 14 | 6 | 2 | 1 | 3 | 15 |
| Total | 107 | 12 | 59 | 36 | 12 | 22 | 19 | 54 |
Articles that studied THC and CBD, in combination or separately, THC with other cannabinoid (non-CBD or –THC phytocannabinoid or synthetic), in combination or separately, or CBD with other cannabinoid (non-CBD or –THC phytocannabinoid or synthetic), in combination or separately.
Articles that studied non-CBD or –THC phytocannabinoid or synthetic cannabinoids.
Other cancers include Bile duct, Gastric, Head and Neck, Kidney, Liver, Lymphoma, Non-melanoma, Pancreas, Testicular, Thyroid, and Urological.
CBD, cannabidiol; CNS, central nervous system; THC, delta9-tetrahydrocannabinol.
Case reports in peer-reviewed literature
The 77 case reports reviewed included a variety of cancers such as breast (n=29), central nervous system (CNS; n=25), prostate (n=11), and pancreatic (n=9) (Table 2). One case report was available for ovarian cancer, non-small cell lung cancer, and pediatric acute lymphocytic leukemia. The majority of cases came from four references from a single author (an original study and three follow-up articles), and only the original article is indexed in PubMed.14–17 Only 11 reports (14%) met criteria to be appraised as showing strong evidence of cannabis having anticancer potential, while 4 (5%) were graded moderate, and the remaining 62 were weak (81%). Ten percent of all cases were reported in pediatric patients (nine in CNS tumors and one leukemia).
As there is no established protocol for cannabis dosing with an anticancer intent, all dosing data were listed for each patient when reported. Daily doses of CBD ranged widely from 10 to 800 mg while daily doses of THC were lower at 4.8 to 7.5 mg. Only six cases reported THC doses, all in combination with CBD and all with the primary goal of symptom management. While most cases used cannabis daily, a few reports on prostate, breast, and glioma patients administered cannabis 3 days on and 3 days off, with an average of 20 mg of synthetic CBD a day.
Case reports from non-peer-reviewed sources
Discussions of cannabis use to treat cancer are widespread online on social media platforms, blogs, and various cannabis-specific websites, making it impossible to compile and review all of them. A multi-part documentary by CNN reporter Dr. Sanjay Gupta investigated the rising use of cannabis and CBD products to treat cancer and a variety of other medical conditions.18 In 2018, a documentary called Weed the People followed families of children with cancer and interviewed patients, clinicians, researchers, and the larger cannabis community to portray how cannabis is being used to treat cancers.19
In Cannabis for the Treatment of Cancer, a review of in vitro and in vivo studies was conducted to look at cannabinoids as a treatment for cancer. The author included over 150 case reports (some of which were published in peer-reviewed journals and are included in Table 2). Rick Simpson Oil (RSO) is widely discussed online as a potential cure for cancer. In Nature's Answer for Cancer, the author provides dosing information as well as instructions on how to produce RSO suggesting that patients ingest 60 g over the span of 90 days in treatment naïve patients (or up to 180 g over a 6 month period to “undo the harm all the chemo and radiation has left behind”). The case reports given are vague and provide little clinical evidence to substantiate any anticancer claims. Despite this, the author advises patients to discontinue any standard treatments (e.g., chemotherapy) and claims that the medical community causes more harm than good. In Table 2, only one published case report listed use of RSO.20
The above examples from non-peer reviewed sources show there are numerous anecdotal stories found online claiming cannabis had potential anticancer effects, however, the vast majority of the cases lacked clinically important details and were cited from sources such as news articles, blogs, or brief patient interviews. As a result, these reports were not appraised or included in Table 2.
Clinical trials using cannabis as an anticancer agent
Data from published human trials are limited to two phase I clinical trials. The first used THC in nine patients with recurrent glioblastoma multiforme (GBM). After surgical resection, THC was administered directly to the tumor cavity via an intracranial subcutaneous reservoir. The primary goal was to study the safety of intracranial THC administration and the THC impact on tumor cells. All tumors were found to express varying amounts of CB1 and CB2 receptors, but there was no correlation between receptor-type expression and survival. When biopsies from the patient tumors were isolated, THC did decrease the number of viable cells in vitro, alluding to an anticancer effect.8 However, no clear clinical benefit was seen in terms of imaging response or prolonged survival. The second was a two-part randomized double-blind, placebo-controlled study in patients with recurrent GBM following standard therapy who used nabiximols in conjunction with DIT. Part 1 found no grade 3 or 4 toxicities. Part 2 was a placebo-controlled study in 21 patients who either received nabiximols or placebo plus DIT. Median overall survival in the placebo group was 12.1 months versus 21.8 months in the nabiximols group, however, MGMT-methylation status (an important prognostic indicator and indicator of responsiveness to DIT) was not reported.9 While not initially found in our search criteria, a phase I study of dexanabinol (a synthetic analog of THC that acts more on N-methyl-d-asparate receptors than cannabinoid receptors) found infusions to be safe in patients with treatment refractory gliomas, but none of the 26 patients treated had radiographic improvement based on Response Assessment in Neuro-Oncology (RANO) criteria.21
A search of the term “medical cannabis” and “cancer” as the main disease under study on ClinicalTrials.gov in March 2021 revealed a total of 16 studies investigating medical cannabis in the cancer population. All seven of the active interventional trials have primary outcome measures focused on either management of cancer-related symptoms, and/or safety of medical cannabis.
Discussion
While pre-clinical studies suggest cannabis may have anticancer properties, our thorough review and appraisal of case reports and clinical trials found little evidence to support any clinically meaningful benefit in patients. We analyzed data from 77 peer-reviewed, published case reports and concluded 81% of cases lacked sufficient evidence to support claims that cannabis had an anticancer effect. Clinical trials focus predominately on symptom control, with only two small trials in patients with recurrent GBM reporting clinical end-points of disease control (e.g., progression-free or overall survival). Ultimately, patients and cancer providers need more robust case reports to provide compelling pilot data for researchers to conduct properly designed interventional trials to move this field forward.
A number of well-conducted in vitro and in vivo experiments provide data on the potential mechanisms by which cannabis can lead to tumor cell death. While we focused on clinical reports, our review of the pre-clinical articles show only 52% of published articles contain original data (Table 1). CNS tumors (often GBM and high grade gliomas) are most commonly studied, followed by breast, colorectal, lung, and prostate cancer. The pre-clinical articles without original data are often review articles that tend to have grand conclusions about the promise of using cannabis to treat cancer. This may lead to inaccurate assumptions by patients and the general public that the results of pre-clinical studies can be immediately translated into clinical practice.
The most comprehensive review of the relationship between cannabis and cancer was reported in the National Academy of Science Engineering and Medicine (NASEM) 2017 publication, The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. While our review focused on potential anticancer properties of cannabis, there have been large epidemiologic studies examining the association between cannabis use and development of new cancers. The NASEM committee found moderate evidence suggesting no association between cannabis use and new cancer development. When responding to the question of “Are Cannabis or Cannabinoids an Effective Treatment for Cancer?” the committee's review of primary literature led to this conclusion, “There is insufficient evidence to support or refute the conclusion that cannabinoids are an effective treatment for cancers, including glioma.”22
As clinical trials assessing an anticancer impact of cannabis are lacking, we chose to critically analyze all peer-reviewed, published case reports to highlight potential cancer types or cannabis doses that may be worthwhile exploring in prospective studies. Only 11 case reports met a strong appraisal for anticancer impact and included adult patients with cancers of the breast (n=3), prostate (n=3), CNS (n=2), ovary (n=1), and lung (n=1), and one 14-year-old patient with pediatric leukemia. Nearly 29% (n=22) of cases reported cannabis use in conjunction with standard treatments such as chemotherapy, hormone therapy, surgery, or radiation, significantly confounding any claimed anticancer effect of cannabis. Finally, 81% (n=62) of reports did not provide validated laboratory or radiographic evidence (e.g., relied on research-based assays of circulating tumor cells [CTCs], or used unclear language when reporting imaging results).
All strong cases used some formulation of CBD oil. The majority (73%) used CBD on a schedule of 3 days on 3 days off, while the rest used CBD daily. The doses ranged from 1 to 60 mg of CBD per day, with no cases reporting any THC dose. All but two strong cases were in adults, otherwise there were no notable differences in age or gender or tumor type. However, many of the moderate and weak cases had similar cannabinoid usage and patient/tumor characteristics, making it difficult to determine which factors contribute to a strong anticancer response. In addition, many of the weak/moderate cases lacked sufficient clinical details. As a result, current case reports do not help researchers determine which tumor types, cannabinoid formulations, or cannabinoid doses to use when designing future interventional trials.
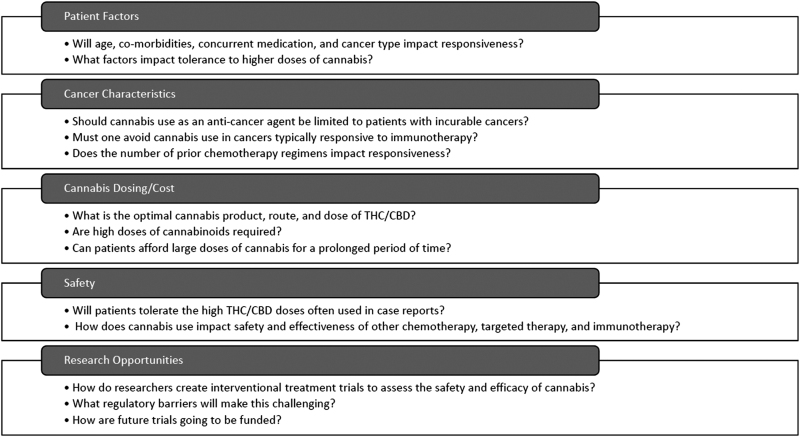
There are many variables to consider when patients and clinicians consider using cannabis as an anticancer agent (Fig. 1). Factors such as age, comorbidities, and concomitant medications can all influence a patient's ability to tolerate and metabolize cannabis, and these may impact its potential effectiveness as an anticancer agent. Important questions regarding patient/cancer characteristics, cannabis dosing, and safety cannot be answered without critical appraisal of case reports. We provided details on cannabis products and found in most reports patients utilized CBD alone, at times in doses as high as 800 mg/day. This dose appears somewhat discordant with the online/social media reports showing patients using extremely high doses of concentrated oils that include THC (e.g., RSO). Determining the best formulation, route, THC/CBD ratio, and total amounts of cannabinoids will certainly challenge researchers aiming to conduct prospective interventional trials.
FIG. 1.
Key considerations when using cannabis as an anticancer agent in patients.
Our review and appraisal of case reports have limitations. First, the three criteria we used to generate an appraisal of evidence supporting the anticancer impact of cannabis may not have been valid. We included criteria that we felt were clinically relevant and, if met, would be more convincing to both patients and cancer clinicians. As a result, patients using cannabis in adjuvant fashion to “prevent recurrence” or “maintain remissions” were considered weak. Reports using cannabis concurrently with other standard anticancer therapies (e.g., chemotherapy, radiation) would similarly be graded as moderate or weak, even though there could be a potential for a synergistic effect of cannabis. Regarding radiographic and laboratory/physical exam response, we used each author's interpretations and did not assess based on formal criteria for imaging response (e.g., RECIST measurements) or a certain percentage reduction in tumor markers. Only a few reports listed the tumor size and/or provided the radiographic images to independently interpret the claims made by the author. Ultimately, imaging or lab studies had to be clinically validated. Simply stating “scans” or “labs” (without specifying type/dates/etc.) were “normal,” “stable,” or “clear,” or those using experimental CTCs, were not considered sufficient evidence reported. We did consider detailed reports stating improvements on physical examination findings as strong evidence. While we searched for evidence outside of peer-reviewed medical literature, we limited our final analysis to only peer-reviewed published case reports. Online social media sites (with groups focusing on cannabis killing/curing cancers) were perused, but the anecdotal posts from patients were too numerous and too devoid of important details to be included.
We are currently conducting an online, anonymous survey in patients with cancer using cannabis to learn about patterns of use and characterize patient perceptions about the anticancer impact of cannabis (work in progress, www.catasurvey.com). Preliminary results show nearly 4 in 10 patients with cancer believe cannabis use improved control of their cancer.23 We plan to compile a detailed case series from a subset of these survey respondents using rigorous review of medical records. Advancing this field of research will require other novel studies as well. Creating prospective registries that track cancer treatments, cannabis use, and clinical outcomes is needed. Developing small, well-designed interventional pilot studies in specific cohorts may also allow researchers to formally assess any anticancer properties of cannabis. Ultimately, placebo-controlled randomized trials comparing standard of care cancer treatments with or without cannabis protocols will be needed.
Conclusion
In conclusion, pre-clinical studies describing the anticancer potential of cannabis have led to public perception that cannabis can help treat cancer. Our extensive review of clinical data suggests most published, peer-reviewed case reports are of insufficient quality to support these beliefs and show that clinical trials remain elusive. While cannabis may be used for symptom management in patients with cancer, it should not be used in place of evidence-based anticancer treatments outside of a clinical trial. Future studies using cannabis as an anticancer agent in patients with metastatic cancers refractory to traditional therapy will be needed to identify cancer types and cannabinoid dosing worth exploring in larger placebo-controlled randomized trials.
Acknowledgment
The authors thank Donald Abrams, MD, for review of the article.
Abbreviations Used
- CAM
complementary and alternative medicine
- CBD
cannabidiol
- CNS
central nervous system
- CTC
circulating tumor cell
- DIT
dose-intense temozolomide
- GBM
glioblastoma multiforme
- GYN
gynecological
- N
no
- NASEM
National Academy of Science Engineering and Medicine
- RANO
Response Assessment in Neuro-Oncology
- RSO
Rick Simpson Oil
- THC
delta9-tetrahydrocannabinol
- Y
yes
Appendix
Appendix A1. Search Queries Used in PubMed and EBSCO Databases
Pub Med search query:
(neoplasms[mh] OR neoplas*[tiab] OR cancer*[tw] OR tumor[tiab] OR tumors[tiab] OR tumour[tiab] OR tumours[tiab] OR carcinoma*[tiab] OR precancerous[tiab] OR malign*[tiab] OR oncolog*[tiab] OR metasts*[tiab] OR metastic[tiab] OR chemotherap*[tw] OR antineoplastic*[tw] OR anticancer[tw] OR adenocarcinoma*[tiab] OR sarcoma*[tiab] OR glioblastoma*[tiab] OR leukemia*[tiab] OR squamous[tiab] OR lymphoma*[tiab] OR melanoma*[tiab] OR paraneoplas*[tw] OR “antineoplastic agents”[pharmacological action] OR “antineoplastic agents”[Majr]) AND (“cannabis”[mh] OR “cannabinoids”[mh] OR “marijuana use”[mh] OR cannabis[tiab] OR cannabinoid*[tiab] OR cannabidiol*[tiab] OR CBD[tiab] OR THC[tiab] OR marijuana[tiab] OR tetrahydrocannabinol*[tiab] OR “11-hydroxy-delta(9)-tetrahydrocannabinol” [Supplementary Concept])
EBSCO search query:
((MH (neoplasms OR “antineoplastic agents”)) OR (TX (squamous OR lymphoma OR melanoma OR paraneoplas OR “antineoplastic agents” OR leukemia OR glioblastoma OR sarcoma OR adenocarcinoma OR anticancer OR antineoplastic OR chemotherapy OR metastic OR metastasis OR oncology OR malignancy OR precancerous OR carcinoma OR neoplasms OR cancer OR tumor OR tumors OR tumour OR tumours))) AND ((MH (“marijuana use” OR cannabinoids OR Cannabis OR cannabinoids)) OR (TX (cannabis OR cannabinoid OR cannabidiol OR “CBD” OR “THC” OR “marijuana” OR “tetrahydrocannabinol” OR “11 hydroxy delta 9 tetrahydrocannabinol”)))
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Cite this article as: Guggisberg J, Schumacher M, Gilmore G, Zylla DM (2022) Cannabis as an anti-cancer agent: a review of clinical data and assessment of case reports, Cannabis and Cannabinoid Research 7:1, 24–33, DOI: 10.1089/can.2021.0045.
References
- 1. Shi S, Brant AR, Sabolch A, et al. . False news of a cannabis cancer cure. Cureus. 2019;11:e3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pergam SA, Woodfield MC, Lee CM, et al. . Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer. 2017;123:4488–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munson AE, Harris LS, Friedman MA, et al. . Antineoplastic activity of cannabinoids. J Natl Cancer Inst. 1975;55:597–602. [DOI] [PubMed] [Google Scholar]
- 4. Fowler CJ. Delta(9)-tetrahydrocannabinol and cannabidiol as potential curative agents for cancer: a critical examination of the preclinical literature. Clin Pharmacol Ther. 2015;97:587–596. [DOI] [PubMed] [Google Scholar]
- 5. Ladin DA, Soliman E, Griffin L, et al. . Preclinical and clinical assessment of cannabinoids as anti-cancer agents. Front Pharmacol. 2016;7:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vecera L, Gabrhelik T, Prasil P, et al. . The role of cannabinoids in the treatment of cancer. Bratisl Lek Listy. 2020;121:79–95. [DOI] [PubMed] [Google Scholar]
- 7. Afrin F, Chi M, Eamens AL, et al. . Can Hemp Help? Low-THC cannabis and non-THC cannabinoids for the treatment of cancer. Cancers. 2020;12:1033–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guzmán M, Duarte MJ, Blázquez C, et al. . A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Twelves C, Sabel M, Checketts D, et al. . A phase 1b randomised, placebo-controlled trial of nabiximols cannabinoid oromucosal spray with temozolomide in patients with recurrent glioblastoma. Br J Cancer. 2021;124:1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 11. Horneber M, Bueschel G, Dennert G, et al. . How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11:187–203. [DOI] [PubMed] [Google Scholar]
- 12. Kander J. Cannabis for the treatment of cancer: the anticancer activity of phytocannabinoids and endocannabinoids. In: Hill D (ed.). 2020, pp. 75–217. [Google Scholar]
- 13. Simpson, R. Rick Simpson Oil — Nature's Answer for Cancer. SimpsonRamaDur d.o.o., Jurja Dobrile 20, 10000 Zagreb, Croatia, 2013, pp. 46–56;73–82;107–117. [Google Scholar]
- 14. Kenyon J, Liu W, Dalgleish A. Report of objective clinical responses of cancer patients to pharmaceutical-grade synthetic cannabidiol. Anticancer Res. 2018;38:5831–5835. [DOI] [PubMed] [Google Scholar]
- 15. Kenyon J, Davies A, Stott C. Report of Objective Responses of Breast Cancer Patients to Pharmaceutical Grade Synthetic Cannabidiol. Case Reports and Images in Oncology. 2020, pp. 1–6. Available at: https://www.ijcrioncology.com/about-us.php (last accessed February 22, 2021).
- 16. Kenyon J, Liu W, Dalgleish, et al. Report of Objective Responses of Prostate Cancer Patients to Pharmaceutical Grade Synthetic Cannabidiol. Case Reports and Images in Oncology. 2020, pp. 1–5. Available at: https://www.ijcrioncology.com/about-us.php (last accessed February 22, 2021).
- 17. Kenyon J, Davies A, Stott C. Report of Objective Responses of Glioma Patients to Pharmaceutical Grade Synthetic Cannabidiol. Case Reports and Images in Oncology. 2020, pp. 1–5. Available at: https://www.ijcrioncology.com/about-us.php (last accessed February 22, 2021).
- 18. Dr. Sanjay Gupta: Weed—CNN Special Documentary. 2015, p. 43:38. [Google Scholar]
- 19. Epstein A. Weed the People. 2018. Available at: https://www.netflix.com/title/81016247 (last accessed August 12, 2020).
- 20. Singh Y, Bali C. Cannabis extract treatment for terminal acute lymphoblastic leukemia with a Philadelphia chromosome mutation. Case Rep Oncol. 2013;6:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juarez TM, Piccioni D, Rose L, et al. . Phase I dose-escalation, safety, and CNS pharmacokinetic study of dexanabinol in patients with brain cancer. Neurooncol Adv. 2021;3:vdab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press: Washington, DC, 2017. [PubMed] [Google Scholar]
- 23. Zylla D, Gilmore, G, Eklund, J, et al. . A survey of patients with cancer who report anti-cancer benefits of cannabis use (abstract). International Cannabinoid Research Society Annual Meeting: Jerusalem, Israel, 2021. [Google Scholar]
- 24. Dall'Stella PB, Docema MFL, Maldaun MVC, et al. . Case report: Clinical outcome and image response of two patients with secondary high-grade glioma treated with chemoradiation, pcv, and cannabidiol. Front Oncol. 2018;8:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Likar R, Koestenberger M, Stultschnig M, et al. . Concomitant treatment of malignant brain tumours with cbd — a case series and review of the literature. Anticancer Res. 2019;39:5797–5801. [DOI] [PubMed] [Google Scholar]
- 26. Barrie AM, Gushue AC, Eskander RN. Dramatic response to laetrile and cannabidiol (cbd) oil in a patient with metastatic low grade serous ovarian carcinoma. Gynecol Oncol Rep. 2019;29:10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sulé-Suso J, Watson NA, van Pittius DG, et al. . Striking lung cancer response to self-administration of cannabidiol: A case report and literature review. SAGE Open Med Case Rep. 2019;7:2050313X19832160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nahler G, Likar R. Cannabidiol possibly improves survival of patients with pancreatic cancer: A case series. Clinical Oncology and Research. 2020:1–4. [Google Scholar]