Abstract
Few studies have explored interventions to improve adolescent and young adult (AYA) cancer care delivery. While many AYAs receive cancer care at NCI Community Oncology Research Program (NCORP) sites, few enroll on clinical trials. Barriers and facilitators to pediatric oncologist activation of and enrollment on an AYA cross-network National Clinical Trials Network (NCTN) supportive care trial were assessed using a survey that was administered to 162 stakeholders representing all 47 children's oncology group (COG) institutions affiliated to an NCORP. Fifty-eight stakeholders participated representing 62% of all sites surveyed. Approximately half of participants (45%) were unaware of the trial. Seven sites had the study open and one enrolled a patient. Reasons for not opening and enrolling on the trial included limited research staff and resources, low anticipated accrual, and lower prioritization of the trial. Enrollment facilitators included having a local “AYA champion,” improving communication between pediatric and medical oncology, and having site education on available AYA trials. Interventions focused on increasing site and provider awareness of AYA trials and decreasing local barriers to AYA enrollment are needed.
Keywords: adolescent and young adult, NCORP, cancer clinical trial, enrollment, disparity, oncofertility
Introduction
Enrollment of adolescents and young adults (AYAs, 15–39 years) onto cancer clinical trials is significantly lower than that of younger pediatric patients (≤15 years) and similar to the extremely low enrollment reported in older adults.1–3 Poor participation of AYAs on clinical trials limits improvement in survival and hinders determination of optimal therapeutic and supportive care approaches.4–6
NCI Community Oncology Research Program (NCORP) consists of three major components: seven Research bases that develop and conduct studies; Community Sites, and Minority/Underserved Community Sites that implement the studies. There are 32 Community Sites and 14 Minority/Underserved Community Sites, each, composed of a consortium of hospitals, oncology practices, and/or integrated health care systems. While there are 46 NCORPs, not every NCORP has a pediatric component. At the time of this survey, there were 47 children's oncology group (COG) NCORP institutions. The NCORP Community Sites accrue individuals to NCI-approved cancer clinical trials and research studies. The sites are consortia of researchers, public hospitals, physician practices, academic medical centers, and other groups that provide health care services in communities across the U.S. NCORP Minority Underserved Community Sites have a patient population that comprised at least 30% racial/ethnic minorities or rural residents.7
The Community Clinical Oncology Program (CCOP), predecessor to the NCORP, was launched in 1983 to increase underrepresented patient enrollment on NCI-sponsored clinical trials.8 The NCORP offers unique opportunities to conduct care delivery research in community settings where the majority of cancer patients receive their treatment. The NCORP network is the primary source of accrual to NCI cancer control symptom management trials and to health-related quality of life trials that are embedded into National Clinical Trials Network (NCTN) treatment trials. NCORP is committed to integrating health disparities research questions across all studies in the network.7
While most CCOP institutions are located in the community setting, few AYAs enroll onto cancer clinical trials at these sites despite the NCI-supported clinical research infrastructure and a commitment to enrolling underrepresented populations. Barriers limiting the enrollment of AYAs onto cancer trials need further exploration.9,10
AYA cancer patients have unique needs and face unique challenges. In addition to providing access to relevant medical care, programs that support AYA patients with cancer must be aware of ancillary and support service needs that vary from younger and older patients with cancer. In particular, AYAs often do not have the same access to key clinical trials or comprehensive supportive care services, including psychosocial support and fertility preservation. Disparate access to care and services might influence the overall outcomes and quality of life of AYA patients with cancer.11–13 Accrual of AYAs to supportive care studies can be particularly challenging since they are often prioritized less than therapeutic studies by clinicians and institutions. Further, there have been very few AYA-focused supportive care clinical trials within the NCTN infrastructure. To date, no studies have assessed the participation of COG NCORP sites in cross-network supportive care trials led by the adult research bases. The purpose of the study was to identify barriers to COG NCORP site enrollment of AYAs onto an AYA-focused cross-network NCTN supportive care trial, as well as opportunities to enhance enrollment.
Methods
Study design and survey instrument
The COG NCORP Committee developed a brief survey to assess barriers and facilitators to activation and enrollment on a cross-network adult research base led interventional supportive care trial at COG NCORP sites in February 2020 (Table 1). This particular trial was selected as the focus of the survey because few cross-network supportive care trials have been conducted in the NCORP. The supportive care trial, referenced in the article as “Study X” is not being identified by name because the trial is currently open to enrollment and we do not want the findings in this study to influence enrollment. The trial was activated in 2015 and was open to patients aged ≥15. The web-based self-administered survey consisted of 10 questions, including multiple choice and free text responses, and was administered using the Qualtrics survey platform (www.qualtrics.com). The final question asked participants how the COG NCORP Committee can help sites increase their enrollment of AYAs onto clinical trials.
Table 1.
Barriers to Opening Cross-Network Supportive Care Trial Survey Tool
| Q1. Name of the Institution |
| Q2. Role in your institution |
| Q3. Are you aware of study X? |
| Q4. Is study X open at your institution? |
| Q5. Did your site enroll any patients on study X? |
| Q6. What are the reasons that your site did not enroll patients on this study? |
| Q7. Are you planning to open study X at your institution? |
| Q8. What are the reasons why your site is not planning on opening this study? |
| Q9. What has delayed the opening of this study at your site? |
| Q10. Does your site have a “Champion” to promote opening AYA studies? |
| Q11. Does your site have a “Champion” to promote enrolling AYAs on studies? |
| Q12. How can the COG NCORP Committee help your site maximize enrollments onto AYA studies? |
AYA, adolescent and young adult; COG, children's oncology group; NCORP, NCI Community Oncology Research Program.
The survey was sent electronically to 162 stakeholders involved in the clinical trial enrollment process at the 47 COG NCORP sites that existed at the time of this survey and was open to responses from February 10th to 27th, 2020. Stakeholder roles included NCORP COG site Principal investigators, clinical research associates, AYA responsible investigators, and cancer control and nursing responsible investigators. Ten sites had two respondents each in differing roles to capture the perspective of various team members involved in the clinical trial enrollment process. Over the past decade, the COG cancer control, nursing, and AYA committees have developed networks of site champions identified as “responsible investigators” whose charge is to advocate for committee initiatives at their institutions. Two reminder emails were sent to all participants within 2 weeks of the initial invitation. Institutional Review Board approval was not sought as these activities were deemed as quality improvement projects because they were designed to implement processes to improve AYAs access to supportive care trials at COG NCORP sites.
Results
Demographics
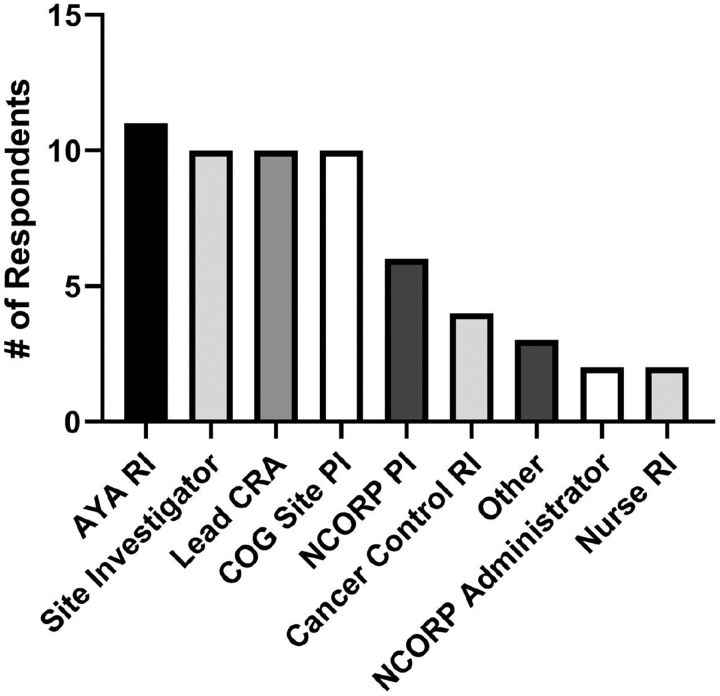
Of the 162 individuals surveyed, 58 responded, representing 29 of the 47 COG NCORP sites for a total site response rate of 63%. Respondents represented a variety of COG NCORP site roles (Fig. 1).
FIG. 1.
Roles of the survey respondents at their respective sites. Fifty-eight stakeholders involved in the enrollment of AYAs onto cancer clinical trials representing 29 COG NCORP components responded to the survey. Some participants serve more than one role at their site. AYA, adolescent and young adult; COG, children's oncology group; NCORP, NCI Community Oncology Research Program.
Barriers to AYA enrollment
Eighty-three percent of participants stated that the trial was not open for enrollment at their site and only one participant had enrolled an AYA on the trial. Approximately half (45%) of all respondents were not aware of the trial before this survey. When respondents who were aware of the study but did not currently have it open was asked if their site planned on opening the study, 77% stated they planned to do so. Limited responses were provided reporting the reasons for deciding to not activate the study, however, they included that medical oncologists were not interested in opening the study and expected accrual would be too low. Sites that planned to open the study were asked what, if anything, delayed study opening. Responses included limited overall staff to open trials and high staff turnover, high burden of study opening processes, lack of knowledge about the study, regulatory delays, and the study was deemed a lower priority for the site.
Opportunities to increase AYA enrollment
Sixty-four percent of respondents reported having an “AYA champion” at their site that was responsible for promoting opening AYA trials and 55% reported having an “AYA champion” to promote enrolling AYAs on trials. Sites that have an AYA champion for opening AYA trials were more likely to be aware of the trial compared with sites without this role (77% vs. 18%, p < 0.001) and were more likely to have the study open for accrual at the time of the survey (30% vs. 0%, p = 0.07), although this difference was not statistically significant. Sites that have an AYA champion for enrolling AYAs on trials were more likely to be aware of the trial compared with sites without this role (65% vs. 43%, p = 0.15) and more likely to have the study open at the time of the survey (25% vs. 15%, p = 0.07), although these differences were not statistically significant.
Participants provided suggestions on how the COG NCORP Committee can further support AYA enrollment at NCORP sites (Table 2). More than half of respondents reported the need to receive communication regarding AYA relevant studies from NCTN groups and education on strategies to communicate with medical oncologists (55%). Respondents suggested that additional ways, in which the committee can foster accrual on trials, include providing resources to assist opening AYA relevant studies for enrollment (13%) and designating AYA champions at NCORP sites (10%). It was also suggested that additional clinical research associate and regulatory staff support from the committee could facilitate accrual (10%).
Table 2.
Potential Interventions to Improve Adolescent and Young Adult Accrual at Children's Oncology Group Sites
| Increase communication about AYA trials to sites |
| Improve communication with medical oncology and increase their awareness of AYA trials |
| Designate AYA champions at NCORP sites |
| Assist with opening and prioritization of AYA studies |
| Provide logistical support and CRA support to sites |
CRA, clinical research associate.
Discussion
To our knowledge, this is the first study that explores barriers to activation of and enrollment on an AYA cross-network NTCN trial at COG NCORP sites. Study X was selected to examine the barriers to activation and opportunities to improve enrollment as it is an AYA focused cross-network supportive care trial that was open across the NCTN groups to AYA enrollment. The survey found that few sites had opened the trial and most stakeholders were unaware of the trial and the opportunity to enroll their patients.
Supportive care and health-related quality of life issues, including mental health, physical functioning, onco-fertility, body image, and sexuality, are often underaddressed in the clinical care of AYAs.14–18 Thus, it is essential that when supportive care trials are developed, they be opened in a timely manner, and have diverse representation of AYAs across all cancer treatment settings, including community sites. AYA enrollment on trials that are focused on an age range and are not disease specific might be expected to be more rapid because the pool of eligible patients is larger. However, many more local stakeholders need to be engaged in the enrollment process when eligibility spans across cancer subtypes and cancer care teams. It is concerning that, at the time of this survey, few COG NCORP sites had opened study X and half of the stakeholders were not even aware of the study's existence. Prior studies and publications have suggested that lack of available AYA relevant clinical trials is the major barrier to enrolling more AYAs with cancer on trials, however, the current survey highlights that developing AYA trials is not sufficient to drive enrollment.19,20 Lack of institution and provider awareness of available AYA trials and how to open trials led by an adult versus pediatric research base appear to be a significant barriers limiting AYA access to trials that are available at the national level as these trials are not being activated at the site level. Freyer and Seibel identified the steps needed to successfully enroll AYAs on trial, which requires that a trial exists, the trial is open, and available at the local site, is presented to the patient, and is accepted.21 In theory, supportive care trials might be easier to recruit due to potentially fewer regulatory challenges, less cost, lower likelihood of adverse events, and larger number of eligible patients. However, at most sites, therapeutic trials are higher priority studies than supportive care trials.
This survey has identified several barriers similar to those reported for enrollment on therapeutic studies. Participants who were aware of the trial provided some suggestions as to why their sites have not yet activated the trial. Barriers identified were limited resources to focus on opening and activating AYA trials, regulatory and financial burden of conducting NCTN studies at community sites, and limited prioritization of these trials. Dickens et al. recently reported similar findings across COG NCORP sites, highlighting the need for additional clinical and regulatory research support.22 Given the large number of AYAs treated in the community setting and its focus on enrolling underserved populations, the NCORP is well-positioned to enroll a diverse population of AYAs, however, increased enrollment cannot be reasonably expected until identified barriers are addressed.
There are other challenges unique to recruiting AYAs to NCTN cross-network clinical trials as opposed to COG–wide clinical trials. A cross-network clinical trial open through the NCTN is a clinical trial led by one or jointly led by multiple NCTN groups, but open to accrual across all NCTN groups. An AYA relevant trial may be sponsored by either COG or adult NCTN groups, and if it is a “cross-network NCTN trial,” it means that it is an adult-sponsored trial that is accessible to the patients of pediatric oncologists in COG, and if it is a COG led trial, it is available to medical oncologists at their institution through an adult NCTN group. For example, the S1826 is a Hodgkin lymphoma SWOG led cross-group clinical trial that is open to accrual across all NCTN groups. As most adult NCTN trials have age restrictions younger than 18 years and some COG trials have restrictions older than age 21 years, a cross-group trial directly addresses the restriction of age limits on single group trials. Significant confusion exists regarding the opening of enrollment on and auditing of cross-network clinical trials and this can be a deterrent to sites opening and enrolling on them. In addition, awareness and interest in these clinical trials may be limited and lack of dissemination of this knowledge even within an institution may limit enrollment by other NCTN research bases that are participating. Local AYA champions from pediatric and medical oncology can be helpful in advocating for activation of and enrollment on clinical trials.23 For example, COG institutions have contributed >30% of the enrollments on the S1826 trial. This may be accomplished through formal shared tumor boards, newsletters, and standard operating procedures or less formal communications such as email and phone consultations.24,25
In response to concerns of low AYA enrollment onto cancer clinical trials at NCORP sites, the COG NCORP committee has developed a series of initiatives to directly address barriers related to lack of awareness of trials, lack of knowledge on enrolling AYAs onto intergroup NCTN trials, and limited communication between pediatric and medical oncologists. In early 2020, the COG NCORP Committee launched a quarterly newsletter, which provides information about available AYA trials and a series of webinars that highlight specific trials such as study X. In addition, along with the COG AYA Oncology Discipline Committee, they helped develop a Frequently Asked Questions document that addresses the opening of, enrollment on, and auditing of cross-network AYA NCTN trials. The COG AYA Committee has also led efforts to identify AYA “champions” across sites from various disciplines, including pediatric and medical oncology, whose purpose is to ensure that the unique needs of AYAs are addressed, including fostering their participation on clinical trials.
The current study has a number of limitations. There was a lower individual participation rate than was ideal. However, the individuals who responded represent a large proportion of NCORP sites and a diverse group of stakeholders involved in clinical trial enrollment. Those who responded may have more of an interest in AYA enrollment, potentially biasing the results and perhaps suggesting that limited awareness of AYA trials may be even more significant than identified in the current survey. The authors selected key stakeholders across COG sites to participate, and responses may not be fully representative of the providers' and research offices' perspectives on barriers to enrollment. In addition, the survey is subject to a response bias with stakeholders potentially more likely to answer that they are planning to open the study when asked based on the perception that this is the answer the survey developers hoped to receive. In addition, the authors acknowledge that the results from a single cross-network NCTN trial may not be fully generalizable to the entire NCORP/NCTN network; however, this is a first effort to understand barriers and facilitators to enrollment on a supportive care AYA trial that is available to enrollment across the NCTN.
Awareness and education are important first steps in the process, but there is a continuum of events as that need to happen for successful accrual of the AYA patient on a clinical trial. This study highlights that the time and resources required to conduct clinical research, specifically in rare cancers, is underresourced. Until this is addressed, progress in increasing AYA enrollment in the community setting will likely remain slow, however, we hope that acknowledging these barriers will lead to the increased site awareness and prioritization of opening and activating AYA trials at NCORP sites.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by grants U10-CA180886 (Michael Roth), P30 CA016672 (Michael Roth), and UG1-CA189955 (Brad H. Pollock) from the National Cancer Institute at the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Bleyer A, Montello M, Budd T, Saxman S. National survival trends of young adults with sarcoma: lack of progress is associated with lack of clinical trial participation. Cancer. 2005;103(9):1891–7. [DOI] [PubMed] [Google Scholar]
- 2. Trama A, Bernasconi A, McCabe MG, et al. Is the cancer survival improvement in European and American adolescent and young adults still lagging behind that in children? Pediatr Blood Cancer. 2019;66(1):e27407. [DOI] [PubMed] [Google Scholar]
- 3. Liu L, Moke DJ, Tsai K-Y, et al. A reappraisal of sex-specific cancer survival trends among adolescents and young adults in the United States. J Natl Cancer Inst. 2019;111(5):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chow CJ, Habermann EB, Abraham A, et al. Does enrollment in cancer trials improve survival? J Am Coll Surg 216:774–80, 2013; discussion 780–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albritton K, Caligiuri M, Anderson B, et al. Closing the gap: research and care imperatives for adolescents and young adults with cancer. Bethesda, MD: Department of Health and Human Services, National Institute of Health, National Cancer Institute, and the LiveStrong Young Adult Alliance; 2006.
- 6. Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin. 2007;57(4):242–55. [DOI] [PubMed] [Google Scholar]
- 7. NCORP.Cancer.gov Last accessed April 8, 2021.
- 8. McCaskill-Stevens W, Lyss AP, Good M, et al. The NCI Community Oncology Research Program: what every clinician needs toknow. Am Soc ClinOncolEduc Book. 2013;33:e84. [DOI] [PubMed] [Google Scholar]
- 9. Minasian LM, Carpenter WR, Weiner BJ, et al. Translating research into evidence-based practice: the National Cancer Institute Community Clinical Oncology Program. Cancer. 2010;116(19):4440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roth ME, Unger JM, O'Mara AM, et al. Enrollment of adolescents and young adults onto SWOG cancer research network clinical trials: a comparative analysis by treatment site and era. Cancer Med. 2020;9(6):2146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernbach A, Lockart B, Armus CL, et al. Evidence-based recommendations for fertility preservation options for inclusion in treatment protocols for pediatric and adolescent patients diagnosed with cancer. J Pediatr Oncol Nurs. 2014;31(4):211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campbell JE, Assanasen C, Robinson RD, Knudtson JF. Fertility preservation counseling for pediatric and adolescent cancer patients. J Adolesc Young Adult Oncol. 2016;5(1):58–63. [DOI] [PubMed] [Google Scholar]
- 13. Wong AWK, Chang TT, Christopher K, et al. Patterns of unmet needs in adolescent and young adult (AYA) cancer survivors: in their own words. J Cancer Surviv. 2017;11:751–64. [DOI] [PubMed] [Google Scholar]
- 14. Keegan TH, Lichtensztajn DY, Kato I, et al. AYA HOPE Study Collaborative Group. Unmet adolescent and young adult cancer survivor's information and service needs: a population-based cancer registry study. J Cancer Surviv. 2012;6(3):239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith AW, Keegan T, Hamilton A, et al. Understanding care and outcomes in adolescents and young adult with Cancer: a review of the AYA HOPE study. Pediatr Blood Cancer. 2019;66(1):e27486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheung CK, Zebrack B. What do adolescents and young adults want from cancer resources? Insights from a Delphi panel of AYA patients. Support Care Cancer. 2017;25(1):119–26. [DOI] [PubMed] [Google Scholar]
- 17. Dauti A, Gerstl B, Chong S, Chisholm O, Anazodo A. Improvements in clinical trials information will improve the reproductive health and fertility of cancer patients. J Adolesc Young Adult Oncol. 2017;6(2):235–69. [DOI] [PubMed] [Google Scholar]
- 18. Thomas SM, Malvar J, Tran HH, et al. A prospective comparison of cancer clinical trial availability and enrollment among adolescents/young adults treated at an adult cancer hospital or affiliated children's hospital. Cancer. 2018;124(20):4064–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith AW, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: an update on progress and recommendations for the future. Cancer. 2016;122(7):988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaw PH, Ritchey AK. Different rates of clinical trial enrollment between adolescents and young adults aged 15 to 22 years old and children under 15 years old with cancer at a children's hospital. J Pediatr Hematol Oncol. 2007;29(12):811–4. [DOI] [PubMed] [Google Scholar]
- 21. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. 2015;3(2):137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dickens DS, Roth ME, Pollock BH, Langevin AM. Understanding the barriers to pediatric oncologist engagement and accrual to clinical trials in National Cancer Institute-Designated Community Oncology Research Programs. JCO Oncol Pract. 2020;16(10):e1060–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reed DR, Oshrine B, Pratt C, et al. Sink or collaborate: how the immersive model has helped address typical adolescent and young adult barriers at a single institution and kept the adolescent and young adult program afloat. J Adolesc Young Adult Oncol. 2017;6(4):503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mobley EM, Swami U, Mott S, et al. A retrospective analysis of clinical trial accrual of patients presented in a multidisciplinary tumor board at a tertiary health care center and associated barriers. Oncol Res Treat. 2020;43(5):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roth M, Mittal N, Saha A, et al. The Children's Oncology Group Adolescent and Young Adult Responsible Investigator Network: a new model for addressing site-level factors impacting clinical trial enrollment. J Adolesc Young Adult Oncol. 2020;9(4):522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]