Abstract
Introduction
People with type 2 diabetes have increased risk of dementia. Glucagon‐like peptide‐1 (GLP‐1) receptor agonists (RAs) are among the promising therapies for repurposing as a treatment for Alzheimer's disease; a key unanswered question is whether they reduce dementia incidence in people with type 2 diabetes.
Methods
We assessed exposure to GLP‐1 RAs in patients with type 2 diabetes and subsequent diagnosis of dementia in two large data sources with long‐term follow‐up: pooled data from three randomized double‐blind placebo‐controlled cardiovascular outcome trials (15,820 patients) and a nationwide Danish registry‐based cohort (120,054 patients).
Results
Dementia rate was lower both in patients randomized to GLP‐1 RAs versus placebo (hazard ratio [HR]: 0.47 (95% confidence interval [CI]: 0.25–0.86) and in the nationwide cohort (HR: 0.89; 95% CI: 0.86–0.93 with yearly increased exposure to GLP‐1 RAs).
Discussion
Treatment with GLP‐1 RAs may provide a new opportunity to reduce the incidence of dementia in patients with type 2 diabetes.
Keywords: dementia, glucagon‐like peptide‐1 receptor agonists, randomized controlled trial, real‐world evidence, type 2 diabetes
1. BACKGROUND
Both diabetes and dementia are highly prevalent age‐related diseases constituting major health and socioeconomic challenges worldwide. 1 , 2 Patients with diabetes have an accelerated rate of cognitive decline 3 and a 1.6‐fold increased risk of developing dementia, 4 including Alzheimer's disease (AD) and vascular dementia, compared to people without diabetes. 5 Yearly screening for mild cognitive impairment is recommended in patients with diabetes from the age of 65 years. 6 However, current treatment options are limited with no globally approved new treatments over the last 20 years, 1 and just one recently approved new therapy with modest benefits in the United States. 7 There are approximately 50 million people worldwide with dementia; of these, it is estimated that more than 7 million have diabetes. 4 , 8 The number of people developing dementia is projected to reach 152 million by 2050, with a current estimated worldwide cost of US$818 billion, 9 making better pharmacological therapies for patients with dementia an urgent priority. Given the large number of people worldwide with type 2 diabetes, and the substantially increased risk of dementia incidence among these individuals, a pharmacological treatment that reduces the incidence of dementia in people with type 2 diabetes would have a large impact.
Glucagon‐like peptide‐1 (GLP‐1) receptor agonists (RAs) are used in the treatment of type 2 diabetes and obesity, where they effectively lower glucose levels, body weight, and risk of cardiovascular disease. 10 , 11 , 12 However, GLP‐1 may also be important for cognition, 13 suggested by findings from GLP‐1 receptor knockout mice that have a phenotype characterized by a learning deficit that can be restored after hippocampal GLP‐1 receptor gene transfer. 14 GLP‐1 is an established neurotransmitter and receptors for GLP‐1 are expressed in numerous brain regions including the striatum, nucleus accumbens, and hippocampus. 15 Studies in animals have shown that administration of the GLP‐1 RA liraglutide can improve memory and learning, increase CA1 neurons in the hippocampus, reduce amyloid beta (Aβ), and prevent tau tangle depositions. 16 , 17 , 18 GLP‐1 RAs are considered among the most promising therapies for repurposing as a treatment for AD. 19 Preliminary evidence shows that liraglutide improves cerebral glucose metabolism in patients with mild or moderate AD dementia. 20
While further randomized controlled trials (RCTs) are needed to fully explore the potential role of GLP‐1 RAs in treating and preventing AD and vascular dementia, a more immediate and more focused question is whether GLP‐1 RAs can reduce dementia incidence in people with type 2 diabetes. Recently, a potentially pivotal study reported that an exploratory analysis of the Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial suggested that the GLP‐1 RA dulaglutide could reduce cognitive impairment in patients aged 50 years or above with type 2 diabetes and additional cardiovascular risk factors. 21 During a median follow‐up of 5.4 (interquartile range [IQR] 5.1–5.9) years, 8828 participants provided a baseline and one or more follow‐up Montreal Cognitive Assessment (MoCA) or Digit Symbol Substitution Test (DSST) scores. The cognitive outcome, which was the first occurrence of a follow‐up score on MoCA or DSST that was 1.5 standard deviations (SDs) or more below the baseline mean score in the participant's country, occurred in 4.05 per 100 patient‐years in participants assigned dulaglutide and 4.35 per 100 patient‐years in people assigned placebo. After post hoc adjustment for individual standardized baseline scores, the hazard of substantive cognitive impairment was reduced by 14% in those assigned dulaglutide (hazard ratio [HR]: 0.86; 95% confidence interval [CI]: 0.79–0.95; P = .0018). This suggests an opportunity to reduce cognitive impairment in people with type 2 diabetes, but it is unclear whether this impact on cognitive decline impacts on the incidence of dementia, a much more crucial clinical outcome.
To address this key clinical question of whether GLP‐1 RAs reduce the incidence of dementia in people with type 2 diabetes, we assessed exposure to GLP‐1 RAs and subsequent diagnosis of dementia in data sources with long‐term treatment exposure, including pooled data from three double‐blind RCTs and a nationwide cohort of patients with diabetes.
2. METHODS
2.1. Trials and registries
For RCT data, all subjects provided informed consent. For the nationwide registry data, ethical permission is not required for register‐based retrospective research in Denmark. This report used a triangulation approach integrating study designs and data sources with different key sources of potential bias to obtain a more reliable answer. 22 First, we pooled data from three large multicenter, double‐blind, placebo‐controlled cardiovascular outcome trials with GLP‐1 RAs: liraglutide (LEADER; 10 9340 patients), subcutaneous semaglutide (SUSTAIN‐6; 11 3297 patients), and oral semaglutide (PIONEER 6; 12 3183 patients). Patients with type 2 diabetes at high risk for or with established cardiovascular disease were randomly assigned in a 1:1 ratio to receive placebo or liraglutide (LEADER), or semaglutide (SUSTAIN‐6; PIONEER 6), in addition to standard of care. Investigators were encouraged to treat all patients according to local guidelines to achieve the most effective glycemic and cardiovascular risk management; accordingly, additional non‐investigational anti‐hyperglycemic (except incretin‐based), anti‐hypertensive, anti‐thrombotic, and lipid‐lowering medication could be added or adjusted. 10 , 11 , 12
Next, we used the Danish National Prescription Register, which holds information on all redeemed prescriptions in Denmark since January 1, 1995 (Table S1 in supporting information), to identify a nationwide cohort of patients treated for diabetes. We identified everyone with a first prescription of a second‐line diabetes treatment (Table S2 in supporting information) between January 1, 1995, and December 31, 2017.
Follow‐up for onset of dementia started on January 1, 2009 (Figure S1 in supporting information), as this was when GLP‐1 RAs could be considered a well‐known and available treatment for diabetes in Denmark. To correctly identify patients with a first‐ever prescription for a second‐line diabetes treatment, we excluded those who had a prescription between January 1, 1995 through June 30, 1995. Furthermore, patients with dementia before start of follow‐up or who developed dementia before age 50 were excluded. For the main analysis, we included everyone with at least 5 years since initial exposure to second‐line diabetes treatment.
2.2. GLP‐1 RAs
The predefined treatment durations for the three RCTs are listed in Table S3 in supporting information. In the nationwide cohort, all dispensed prescriptions for GLP‐1 RAs were identified and years of cumulative GLP‐1 RA exposure were updated throughout the follow‐up period. At each date throughout follow‐up, the current treatment exposure was defined by looking 5 years back in time. To summarize the exposure duration, we counted the number of 6‐month subintervals in which the patient redeemed at least one prescription.
1. Research in Context
Systematic review: The authors reviewed the literature using PubMed. Patients with type 2 diabetes have a 1.6‐fold increased risk of dementia, and there are an estimated 7 million people with both diabetes and dementia worldwide. Studies show promising effects with glucagon‐like peptide‐1 (GLP‐1) receptor agonists (RAs) on cognitive decline and Alzheimer's disease hallmarks, but it is unclear whether this translates to an impact on the incidence of dementia, a more crucial clinical outcome.
Interpretation: We determined the impact of GLP‐1 RAs in patients with type 2 diabetes on subsequent development of dementia in three large clinical trials and a Danish nationwide study. Our results suggest that GLP‐1 RAs may provide a new opportunity to reduce dementia incidence in patients with type 2 diabetes.
Future directions: Results support the need for a well‐powered randomized controlled trial to determine whether GLP‐1 RAs may have a broader role in preventing dementia.
HIGHLIGHTS.
GLP‐1 RA exposure and dementia incidence were assessed in RCTs and nationwide registries
Treatment with GLP‐1 RAs associated with a lower rate of dementia in type 2 diabetes may provide new opportunity to reduce dementia incidence in type 2 diabetes
2.3. Other diabetes treatments
Other second‐line diabetes treatments that were available as alternative treatment options to GLP‐1 RAs during the entire follow‐up period in the nationwide cohort (insulin, sulfonylureas, dipeptidyl peptidase‐4 inhibitors, meglitinides) were assessed to test whether a potential influence of GLP‐1 RAs on dementia prevention was specific for treatment with GLP‐1 RAs. Monotherapy with metformin was defined as a first‐line treatment for type 2 diabetes and not considered a comparable treatment option to GLP‐1 RAs.
2.4. Dementia
In the RCTs, the standardized Medical Dictionary for Regulatory Activities (MedDRA, version 21.1) was used to identify dementia‐related adverse events (AEs) using the narrow scope search terms for dementia (Table S4 in supporting information). AE data collection across the trials differed in line with the regulatory requirements at the time of trial conduct. In LEADER and PIONEER 6, only serious AEs were systematically collected, while all AEs were collected in SUSTAIN‐6.
In the nationwide cohort, dementia was defined as a diagnosis of dementia in the National Patient Register or first‐ever prescription for approved dementia specific treatment in the National Prescription Register (cholinesterase inhibitors and memantine; Tables S1 and S2 in supporting information).
2.5. Statistical analysis
For the pooled RCTs, an intention‐to‐treat analysis was performed using Cox regression with treatment assignment as the only explanatory variable to determine the HR for developing dementia with GLP‐1 RAs versus placebo. We reported the HR for dementia for patients randomized to a GLP‐1 RA versus placebo. The absolute risk of dementia (with death without dementia as a competing risk) was calculated using the Aalen‐Johansen estimator.
In the nationwide cohort, we used a nested case‐control study design in which each patient at case date (date of dementia diagnosis) was matched on age, sex, and calendar date to 10 controls without dementia. The effects of differences in cumulative exposure to GLP‐1 RAs for developing dementia were modelled with Cox regression in a 5‐year exposure window prior to case date (Figure S1 in supporting information) and reported as HRs for each 1‐year increase in GLP‐1 RA exposure for cases versus controls. Reported were HRs for each 1‐year increase in exposure duration. The model was adjusted for age, sex, and calendar date via matching, and information on diabetes duration (years since first‐ever prescription of any second‐line diabetes treatment), stroke, myocardial infarction, hypertension, chronic renal disease, and educational attainment (Table S1 and S2 in supporting information) at the start of the exposure window. A similar Cox regression model was used for each of the other second‐line diabetes treatments. Furthermore, the HR for dementia with exposure to GLP‐1 RAs was investigated across subgroups, including sex, age, calendar period, insulin exposure, and cardiovascular status, with cardiovascular disease defined as prior stroke or myocardial infarction, and in various prespecified sensitivity analyses (Table S5 in supporting information). The level of statistical significance was set at 5%.
2.6. Role of the funding source
The funder of the study participated in the study design, data collection (RCT), data analysis (RCT), data interpretation, writing this paper, and in the decision to submit this paper for publication. The corresponding author had full access to all the data in the study.
3. RESULTS
3.1. Study populations
In total, 15,820 patients at high risk for or with established cardiovascular disease were randomized to a GLP‐1 RA or placebo in the pooled RCTs. Baseline characteristics are presented in Table 1.
TABLE 1.
Baseline characteristics in the pooled RCTs
| GLP‐1 RA (N = 7907) | Placebo (N = 7913) | |
|---|---|---|
| Male sex, n (%) | 5108 (64.6) | 5073 (64.1) |
| Age in years, mean (SD) | 64.6 (7.2) | 64.8 (7.3) |
| Age, n (%) | ||
| < 70 years | 5942 (75.2) | 5850 (73.9) |
| 70–80 years | 1764 (22.3) | 1864 (23.6) |
| 80–90 years | 198 (2.5) | 198 (2.5) |
| > 90 years | 3 (< 0.1) | 1 (< 0.1) |
| Diabetes duration, mean (±SD) | 13.5 (8.2) | 13.5 (8.2) |
| Stroke, n (%) | 1229 (15.5) | 1299 (16.4) |
| Myocardial infarction, n (%) | 2554 (32.3) | 2531 (32.0) |
| Hypertension, n (%) a | 5804 (91.9) | 5766 (91.2) |
| Chronic renal disease, n (%) b | 189 (2.4) | 173 (2.2) |
Not including the PIONEER 6 trial.
Chronic renal disease is defined as eGFR < 30 mL/minute/1.73 m2.
Abbreviations: eGFR, estimated glomerular filtration rate; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; RCTs, randomized controlled trials; SD, standard deviation.
In the nationwide cohort of 120,054 patients with at least 5 years since initiation of a second‐line diabetes treatments, 4849 patients developed dementia during the follow‐up period from 2009 through 2017. Characteristics of case and control patients are presented in Table 2. Liraglutide comprised 95% of all prescriptions for GLP‐1 RAs.
TABLE 2.
Characteristics of the case and control patients in the nationwide cohort
| Dementia cases (N = 4849) | Controls (N = 48,506) | |
|---|---|---|
| Male sex, n (%) | 2299 (47.4) | 22,998 (47.4) |
| Age, n (%) | ||
| < 70 years | 1278 (26.4) | 12,784 (26.4) |
| 70–80 years | 2268 (46.8) | 22,688 (46.8) |
| 80–90 years | 1260 (26.0) | 12,612 (26.0) |
| > 90 years | 43 (0.9) | 422 (0.9) |
| Diabetes duration, median (IQR) a , b | 6.0 (3.0–10.0) | 6.0 (3.0–9.0) |
| GLP‐1 RAs, n (%) | ||
| 0 years | 4575 (94.3) | 44,594 (91.9) |
| 1–2 years | 59 (1.2) | 623 (1.3) |
| 2–3 years | 35 (0.7) | 586 (1.2) |
| 3–4 years | 35 (0.7) | 483 (1.0) |
| 4–5 years | 74 (1.5) | 1076 (2.2) |
| Stroke, n (%) b | 760 (15.7) | 5628 (11.6) |
| Myocardial infarction, n (%) b | 527 (10.9) | 5241 (10.8) |
| Hypertension, n (%) b | 3252 (67.1) | 31,961 (65.9) |
| Chronic renal disease, n (%) b | 233 (4.8) | 2287 (4.7) |
| Educational attainment, n (%) b , c | ||
| Basic | 2490 (51.4) | 23,920 (49.3) |
| Medium | 1508 (31.1) | 14,971 (30.9) |
| Advanced | 427 (8.8) | 4681 (9.7) |
Years since initiation of second‐line diabetes treatment.
At beginning of 5‐year exposure window.
Educational status unknown in 424 (8.7%) cases and in 4934 (10.2%) controls.
Abbreviations: GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; IQR, interquartile range.
3.2. Dementia in pooled RCTs
In the pooled RCT analysis, 15 patients randomized to a GLP‐1 RA and 32 patients randomized to placebo developed dementia during a median follow‐up of 3.61 years (Table S3 in supporting information).
Patients randomized to GLP‐1 RAs had a lower rate of developing dementia compared to those randomized to placebo (HR: 0.47; 95% CI: 0.25–0.86; Figure 1 and Table S6 in supporting information).
FIGURE 1.
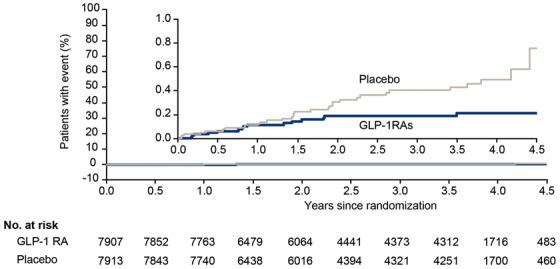
Time to dementia with GLP‐1 RAs versus placebo in pooled RCTs. In the pooled RCTs, 15 patients randomized to a GLP‐1 RA and 32 patients randomized to placebo developed dementia. GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; RCT, randomized controlled trial
3.3. Dementia in the nationwide cohort
The median follow‐up time in the nationwide cohort was 7.4 years. The analysis nested in the nationwide cohort was specifically designed to examine long‐term effects by ensuring at least 5 years of treatment with second‐line diabetes treatment. The result was a reduced rate of dementia with increasing exposure to GLP‐1 RAs compared to other second‐line diabetes treatments (Figure 2). The reduced rate of dementia with GLP‐1 RA exposure was similar across subgroups stratified by sex, age, calendar period, co‐exposure to insulin, and cardiovascular status (Figure 3).
FIGURE 2.
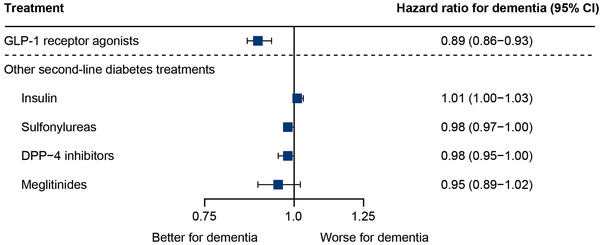
HRs for dementia with yearly increase in exposure duration to GLP‐1 RAs and other second‐line diabetes treatments in the nationwide cohort. Cox proportional hazards regression models conducted for exposure to each treatment. Estimates denote the HR for yearly increase in duration of exposure. The models were adjusted for history of stroke, myocardial infarction, hypertension, educational attainment, and diabetes duration. Sex, age, and calendar date were included via matching. SGLT‐2 inhibitors (HR: 0.81; 95% CI: 0.68–0·97) were not available throughout the entire study period. CI, confidence interval; DPP‐4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; HR, hazard ratio; SGLT‐2, sodium‐glucose co‐transporter‐2
FIGURE 3.
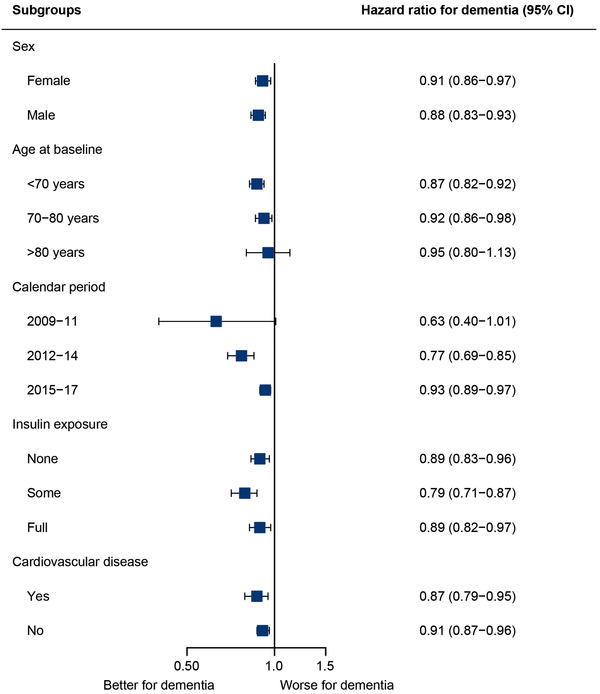
HRs for dementia with yearly increase in exposure duration to GLP‐1 RAs according to subgroup in the nationwide cohort. Cox proportional hazards regression models conducted for exposure to GLP‐1 in various subgroups. Estimates denote the HR for yearly increase in duration of exposure. The models were adjusted for history of stroke, myocardial infarction, hypertension, educational attainment, and diabetes duration. Sex, age, and calendar date were included via matching. CI, confidence interval; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; HR, hazard ratio
Exposure to other second‐line diabetes treatments available in the study period was not associated with a decrease in HR, except for sulfonylureas (P = .04, Figure 2). Ignoring prescriptions for second‐line diabetes treatments in the 2 years prior to the diagnosis of dementia, GLP‐1 RAs were the only treatment significantly associated with a reduced rate of dementia (Figure S2 in supporting information). The result for GLP‐1 RA exposure did not change when diabetes duration was assessed as “time since first treatment with metformin or second‐line diabetes treatment” (Figure S3 in supporting information); when exposure to GLP‐1 RAs was assessed within 3 and 10 years before diagnosis of dementia (Figure S4 in supporting information); and when adjusting the analysis for only age, sex, and calendar date via matching (Figure S5 in supporting information). Lastly, the competing risk analysis of “death without dementia” as an endpoint also resulted in a lower mortality rate with GLP‐1 RA exposure compared to other second‐line treatments (Figure S6 in supporting information).
4. DISCUSSION
We found that treatment with GLP‐1 RAs was associated with a reduced incidence of dementia in patients with type 2 diabetes in three pooled RCTs and in a nationwide cohort.
Intensive pharmacotherapeutic efforts have so far failed to identify treatments that prevent, delay, or arrest the disease course of dementia, including recent unsuccessful trials focusing on reducing production or enhancing clearance of Aβ, 23 , 24 and the urgent need for new therapeutic approaches is increasingly evident. The presented results indicate a beneficial opportunity of delaying progression to dementia with GLP‐1 RAs in people with type 2 diabetes. Given the significantly increased 1.6‐fold risk of dementia, 4 and the 7 million people with type 2 diabetes estimated already to have dementia, the potential opportunities for clinical practice and improved outcomes for people with type 2 diabetes are apparent.
Our findings build upon the recently published exploratory analysis from the cardiovascular outcome trial REWIND showing that dulaglutide might reduce cognitive impairment. The adjusted hazard of that composite cognitive outcome (decline of 1.5 SDs or more on either the MoCA or DSST) was reduced by 14% with dulaglutide compared to placebo (HR: 0.86; 95% CI; 0.79–0.95; P = .0018). 21 The current article, examining more than 15,000 participants from three RCTs and a nationwide cohort from Denmark, demonstrates that the impact on cognitive impairment from REWIND translates into the key clinical outcome of a reduced incidence of dementia, and emphasizes the opportunity to potentially reduce dementia incidence in people with type 2 diabetes.
An RCT to determine whether GLP‐1 RAs have therapeutic benefits for the prevention or treatment of dementia beyond people with type 2 diabetes is now an important priority.
There are several potential mechanisms of action. For example, it is plausible that the effect of GLP‐1 RAs on dementia could be related to amelioration of dementia‐related risk factors, such as glycated hemoglobin, body weight, systolic blood pressure, and cardiovascular disease observed in the included RCTs. 10 , 11 , 12 However, animal studies point to more general neuroprotective mechanisms of GLP‐1 RAs, 13 which could have implications for a broader patient population. The role of GLP‐1 RAs in reducing neuroinflammation 25 , 26 may be important, given the recent focus on microglia in neurodegenerative diseases. 27 It has been shown that semaglutide specifically decreases markers of systemic inflammation in patients, 28 , 29 and that both liraglutide and semaglutide attenuate development of atherosclerotic plaques in APOE–/– and LDLr–/– mice through an anti‐inflammatory mechanism. 30 A recent study showed that exenatide, another GLP‐1 RA, improved brain vascular health in an aged mouse model in which transcriptome analysis showed enrichment for human AD genome‐wide association study genes. 31 In humans, it was recently demonstrated that liraglutide slowed down the decline in memory function independently of weight loss in a group of patients with obesity and prediabetes or early type 2 diabetes. 32 Thus, in addition to its glucose‐lowering effect, GLP‐1 RAs may have specific properties for preventing dementia. This is supported by our finding from the nationwide cohort that other second‐line diabetes treatments available were not associated with a lower incidence of dementia. Exposure to sulfonylureas (borderline significant) and sodium‐glucose co‐transporter‐2 inhibitors (marketed since December 2012) was found to be associated with a decreased rate of dementia in the main analysis but was not associated with a significant reduction in dementia when accounting for reverse causation, that is, ignoring prescriptions in the 2 years leading up to a diagnosis of dementia. To further explore confounding by indication and whether income level could have biased the results, the analyses were repeated with recently available register data that extended the study period until 2018, which showed consistent results for the main analysis and when accounting for reverse causation (Figure S7 in supporting information).
A Danish case control study recently showed that exposure to GLP‐1 RAs was associated with a decreased rate of dementia in patients with diabetes. This study addressed “ever use” of treatments and included patients independent of diabetes duration, making the study vulnerable to bias, including not accounting for lag time between exposure and dementia, which may explain why multiple drugs were found to be protective for dementia. 33
The current pooled analysis of double‐blind RCTs is important for interpretation, as randomization limits sources of confounding. The similarity in study populations in LEADER, SUSTAIN‐6, and PIONEER 6 supports the scientific validity of a combined analysis. 10 , 11 , 12 Further, the high completion rate (> 96%) and available information about vital status for all (> 99%) of the patients by the end of the trials indicate high validity for the conduct of the trials. 10 , 11 , 12 It is, however, a limitation that dementia was not a prespecified endpoint in any of the three RCTs, and in accordance with the regulatory requirements at the time of trial conduct, only serious AEs were reported in LEADER and PIONEER 6 whereas both serious and non‐serious AEs were reported in SUSTAIN‐6. Despite similar dementia rates reported with placebo in the two trials of longest duration (1.41 in LEADER and 1.47 in SUSTAIN‐6, Table S3 in supporting information), underreporting cannot be excluded, particularly in the trials requiring only serious AE reporting. This would, however, not bias the treatment effect but could reduce the power. Despite a small number of events, we found a protective effect of GLP‐1 RAs versus placebo. The influence of GLP‐1 RAs was not apparent at randomization and though the number of events is limited, a differentiation between the curves became apparent after 1 year of treatment, which could indicate that this was due to treatment exposure and not inherent group differences. Furthermore, as there was a similar 11 or lower 10 , 12 risk of death reported in patients randomized to GLP‐1 RAs versus placebo, death as a competing risk should not have influenced the results.
Due to the large size of the nationwide cohort with more than 4000 patients developing dementia over 9 years of follow‐up, we were able to test the results in different subgroups of clinical importance and sensitivity analyses. The decreased rate of dementia with increasing exposure to GLP‐1 RAs was statistically significant throughout different calendar intervals, although the HR became less pronounced in recent years. This trend may be influenced by changes in guidelines to initiate treatment with GLP‐1 RAs earlier and increased use of newer second‐line diabetes treatments. The results were consistent in all investigated subgroups that stratified patients by sex, age group, insulin exposure, calendar period, and cardiovascular disease status, implying that the effect of GLP‐1 RA may be present in patients both with and without cardiovascular disease. However, it was not possible within the available measurements to assess how much of the effect of GLP‐1 RAs was potentially mediated through reduced cardiovascular disease. Further investigations of mode of action and clinically relevant subgroups are warranted, but are beyond the scope of this study. The results remained unchanged after ignoring exposure 2 years before a diagnosis of dementia, indicating that our results were not influenced by a pattern of fewer GLP‐1 RA prescriptions with an impending diagnosis of dementia. Because our hypothesis was that any beneficial effect of treatment on development of dementia would take place over a prolonged period of time, we required patients to have at least 5 years of any second‐line diabetes treatment for inclusion. Yet, results remained consistent with both 3 and 10 years of required second‐line diabetes treatment.
The outcome was “all‐cause dementia”, as the dementia diagnosis of any type has previously been evaluated to have a high validity with a correct diagnosis in 85.8% of cases in the Danish National Patient Register, in contrast to the validity of registered dementia subtypes, which is less reliable. 34 Because of the completeness of the nationwide register information on diagnoses and redeemed prescriptions, 35 the cohort is representative of the population treated for diabetes in Denmark.
The register‐based information permitted us to incorporate time‐varying exposure information about cumulative exposure to different diabetes treatments. We were able to adjust for several potential confounders, including cardiovascular disease, hypertension, chronic renal disease, and educational attainment. Diabetes duration was measured as “time since first second‐line diabetes treatment” as we expected disease progression to be comparable across patients at this time, given that addition of a second‐line drug often reflects a state of disease progression in which blood glucose can no longer be controlled with metformin alone. The result for GLP‐1 RAs remained consistent with the main analysis when the measure for diabetes duration also included monotherapy with metformin.
We were not able to adjust for lifestyle factors such as body mass index, smoking, and physical activity. Thus, unknown and residual confounding could still exist in this observational data source. However, it is noteworthy that the results remained unchanged when we adjusted for only age, sex, and calendar date. Also, in the analysis of death without prior dementia, GLP‐1 RAs were associated with a lower rate of death, further supporting the robustness of the results. Supported by the result from the pooled RCTs in which unknown confounders per design were balanced between treatment and placebo groups, our results suggest a potential for GLP‐1 RAs to reduce the risk of dementia in patients with type 2 diabetes.
In conclusion, the potential for applying a licensed therapeutic option with a well‐established safety profile calls for further investigations to elucidate the effects of GLP‐1 RAs on preventing cognitive decline and dementia.
CONFLICTS OF INTEREST
CHN, TG, EH, and CTP report no conflicts of interests. SF reports grants from Novo Nordisk related to the manufacture of GLP‐1 RAs during the conduct of the study and support for attending meetings and/or travel from the German Research Foundation (payments made to institution); she has also been secretary of the German Consortium in Statistics (DAGStat). CTMH, DVM, KK are Novo Nordisk employees and report personal fees from Novo Nordisk A/S related to the manufacture of GLP‐1 RAs (salary and shareholder), during the conduct of the study. KK also reports Novo Nordisk stock in pension funds. CTMH is also inventor on a patent application related to GLP‐1 compounds and indications (patent is owned by Novo Nordisk and she receives no financial or other benefits from it) and is a minor stockholder of Novo Nordisk A/S. CB reports grants and personal fees from Acadia, Addex, Exciva, Janssen, Suven, and Lundbeck; personal fees from Roche, Otsuka, Biogen, Eli Lilly, Sunovion, Novo Nordisk, and AARP; grants and personal fees from Synexus, outside the submitted work; he also reports the following grants to his institution (UoE) from: 2021 ADDF, 2020 UKRI, 2019 IMI2, NIH, Charles Wolfson Foundation, Novo Nordisk, 2018 MRC, Synexus, Capital, award from Dennis and Mireille Gillings Foundation, Novartis, and Oryzon; honoraria from Harvard University (to institution, UoE), GE Healthcare, Acadia, AARP, and Addex. LBK is a Novo Nordisk employee and an inventor on numerous patents and applications related to GLP‐1 compounds and indications; all patents are owned by Novo Nordisk, which markets liraglutide and semaglutide, and she receives no financial or other benefits from them. BZ reports grants and personal fees from Novo Nordisk during the conduct of the study, and personal fees from Eli Lilly, Merck, Boehringer Ingelheim, and Janssen, outside the submitted work. LSM is a Novo Nordisk employee and reports personal fees from Novo Nordisk A/S related to the manufacture of GLP‐1 RA (salary) during the conduct of the study; she is also vice chair of the Danish Society for Pharmacoepidemiology and is on the executive committee for the Nordic PharmacoEpidemiological Network.
Supporting information
Supporting Information
ACKNOWLEDGMENT
Editing assistance (limited to formatting) was supported financially by Novo Nordisk and provided by Clare Lowe, of Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc, during preparation of this article. This work was supported by Novo Nordisk.
Nørgaard CH, Friedrich S, Hansen CT, et al. Treatment with glucagon‐like peptide‐1 receptor agonists and incidence of dementia: Data from pooled double‐blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer's Dement. 2022;8:e12268. 10.1002/trc2.12268
REFERENCES
- 1. Prince M, Karagiannidou M, Comas‐Herrera A, Knapp M. World Alzheimer Report 2016. Improving healthcare for people living with dementia. Alzheimers Dis Int. 2016:1‐127. https://www.alzint.org/u/WorldAlzheimerReport2016.pdf. [Google Scholar]
- 2. International Diabetes Federation. IDF Diabetes Atlas Ninth Edition 2019. 2019; [PubMed]
- 3. Rawlings AM, Sharrett AR, Schneider AL, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161(11):785‐793. 10.7326/m14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England). 2020;396(10248):413‐446. 10.1016/s0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta‐analysis of prospective observational studies. J Diabetes Invest. 2013;4(6):640‐650. 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association . 12. Older Adults: standards of Medical Care in Diabetes ‐ 2019. Diabetes Care. 2019;42(1):S139‐47. [DOI] [PubMed] [Google Scholar]
- 7. Food and Drug Administration . FDA's Decision to Approve New Treatment for Alzheimer's Disease. https://wwwfdagov/drugs/news‐events‐human‐drugs/fdas‐decision‐approve‐new‐treatment‐alzheimers‐disease 2021.
- 8. World Health Organization . Diabetes. https://www.who.int/health‐topics/diabetes#tab=tab_1
- 9. World Health Organization . Dementia fact sheet. https://www.who.int/news‐room/fact‐sheets/detail/dementia
- 10. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. The N Engl J Med. 2016;375(4):311‐322. 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375(19):1834‐1844. 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 12. Husain M, Birkenfeld AL, Donsmark M, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381(9):841‐851. 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 13. Grieco M, Giorgi A, Gentile MC, et al. Glucagon‐Like Peptide‐1: a Focus on Neurodegenerative Diseases. Front Neurosci. 2019;13:1112. 10.3389/fnins.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. During MJ, Cao L, Zuzga DS, et al. Glucagon‐like peptide‐1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9(9):1173‐1179. 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 15. Heppner KM, Kirigiti M, Secher A, et al. Expression and distribution of glucagon‐like peptide‐1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology. 2015;156(1):255‐267. 10.1210/en.2014-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansen HH, Barkholt P, Fabricius K, et al. The GLP‐1 receptor agonist liraglutide reduces pathology‐specific tau phosphorylation and improves motor function in a transgenic hTauP301L mouse model of tauopathy. Brain Res. 2016;1634:158‐170. 10.1016/j.brainres.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 17. Hansen HH, Fabricius K, Barkholt P, et al. The GLP‐1 Receptor Agonist Liraglutide Improves Memory Function and Increases Hippocampal CA1 Neuronal Numbers in a Senescence‐Accelerated Mouse Model of Alzheimer's Disease. J Alzheimers Dis. 2015;46(4):877‐888. 10.3233/jad-143090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Duffy KB, Ottinger MA, et al. GLP‐1 receptor stimulation reduces amyloid‐beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19(4):1205‐1219. 10.3233/jad-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corbett A, Pickett J, Burns A, et al. Drug repositioning for Alzheimer's disease. Nat Rev Drug Discov. 2012;11(11):833‐846. 10.1038/nrd3869. [DOI] [PubMed] [Google Scholar]
- 20. Gejl M, Gjedde A, Egefjord L, et al. Alzheimer's Disease, 6‐Month Treatment with GLP‐1 Analog Prevents Decline of Brain Glucose Metabolism: randomized, Placebo‐Controlled, Double‐Blind Clinical Trial. Front Aging Neurosci. 2016;8:108. 10.3389/fnagi.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cukierman‐Yaffe T, Gerstein HC, Colhoun HM, et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 2020;19(7):582‐590. 10.1016/s1474-4422(20)30173-3. [DOI] [PubMed] [Google Scholar]
- 22. Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45(6):1866‐1886. 10.1093/ije/dyw314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egan MF, Kost J, Voss T, et al. Randomized Trial of Verubecestat for Prodromal Alzheimer's Disease. N Engl J Med. 2019;380(15):1408‐1420. 10.1056/NEJMoa1812840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henley D, Raghavan N, Sperling R, Aisen P, Raman R, Romano G. Preliminary Results of a Trial of Atabecestat in Preclinical Alzheimer's Disease. N Engl J Med. 2019;380(15):1483‐1485. 10.1056/NEJMc1813435. [DOI] [PubMed] [Google Scholar]
- 25. Yun SP, Kam TI, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med. 2018;24(7):931‐938. 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Secher A, Normann Hansen S, Thim Hansen C, et al. The GLP‐1 receptor agonist semaglutide reduces neuroinflammation in a lipopolysaccharide mouse model. The 15th International Conference on Alzheimer's and Parkinson's Diseases (AD/PD); 9‐14 March. Abstract ID 1791; 2021. https://cslide.ctimeetingtech.com/adpd21/attendee/confcal/session/calendar/2021‐03‐14. [Google Scholar]
- 27. Mathys H, Davila‐Velderrain J, Peng Z, et al. Single‐cell transcriptomic analysis of Alzheimer's disease. Nature. 2019;570(7761):332‐337. 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes Care. 2019;42(9):1724‐1732. 10.2337/dc19-0749. [DOI] [PubMed] [Google Scholar]
- 29. Rodbard HW, Rosenstock J, Canani LH, et al. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: the PIONEER 2 Trial. Diabetes Care. 2019;42(12):2272‐2281. 10.2337/dc19-0883. [DOI] [PubMed] [Google Scholar]
- 30. Rakipovski G, Rolin B, Nøhr J, et al. The GLP‐1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE(‐/‐) and LDLr(‐/‐) Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl Sci. 2018;3(6):844‐857. 10.1016/j.jacbts.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao L, Li Z, Vong JSL, et al. Pharmacologically reversible zonation‐dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat Commun. 2020;11(1):4413. 10.1038/s41467-020-18249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vadini F, Simeone PG, Boccatonda A, et al. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. Int J Obes (2005). 2020;44(6):1254‐1263. 10.1038/s41366-020-0535-5. [DOI] [PubMed] [Google Scholar]
- 33. Wium‐Andersen IK, Osler M, Jørgensen MB, Rungby J, Wium‐Andersen MK. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case‐control study. Eur J Endocrinol. 2019;181(5):499‐507. 10.1530/eje-19-0259. [DOI] [PubMed] [Google Scholar]
- 34. Phung TK, Andersen BB, Høgh P, Kessing LV, Mortensen PB, Waldemar G. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2007;24(3):220‐228. 10.1159/000107084. [DOI] [PubMed] [Google Scholar]
- 35. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. 10.2147/clep.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


