Abstract
The association between human papillomavirus (HPV) DNA copy number and cervical disease was investigated. Viral DNA copy number for the most common high-risk HPV types in cervical cancer (types 16, 18, 31, and 45) was determined in cervical cytobrush specimens from 149 women with high-grade cervical intraepithelial neoplasias (CIN II-CIN III), 176 with low-grade CIN (CIN I), and 270 with normal cytology. Quantitative, PCR-based fluorescent assays for each of the HPV genotypes and for the β-globin gene were used. The amount of cellular DNA increased significantly with increasing disease; thus, HPV was expressed as copies per microgram of cellular DNA. The assay had a dynamic range of >107, allowing documentation for the first time of the wide range of HPV copy numbers seen in clinical specimens. Median HPV DNA copy number varied by more than 104 among the viral types. HPV16 was present in the highest copy number; over 55% of HPV16-positive samples contained more than 108 copies/μg. Median copy number for HPV16 showed dramatic increases with increasing epithelial abnormality, an effect not seen with the other HPV types. HPV16 increased from a median of 2.2 × 107 in patients with normal cytology, to 4.1 × 107 in CIN I patients, to 1.3 × 109 copies/μg in CIN II-III patients. Even when stratified by cervical disease and viral type, the range of viral DNA copies per microgram of cellular DNA was quite large, precluding setting a clinically significant cutoff value for “high” copy numbers predictive of disease. This study suggests that the clinical usefulness of HPV quantitation requires reassessment and is assay dependent.
Human papillomaviruses (HPVs) induce a variety of proliferative lesions, but only the “high-risk” genotypes are associated with anogenital cancers (15, 16). The most common high-risk types of HPV in cervical cancer in the United States are HPV16, -18, -31, and -45 (2). Cervical cancer is thought to develop from cervical intraepithelial neoplasias (CIN), which are graded from I to III depending on the degree of epithelial abnormality (11). The prevalence of high-risk HPV types increases with the grade of CIN (4, 10).
Average HPV DNA copy number has been shown to increase significantly with the grade of CIN for HPV16 but not for the other high-risk types, suggesting a genotype-specific association between HPV DNA load and neoplastic progression. However, viral DNA load (5, 12) determinations have thus far been limited by the sensitivity and specificity of the tests used. Some determinations have been made with the Hybrid Capture system (Digene Diagnostics, Silver Spring, Md.), which is quantitative between approximately 5 × 104 and 5 × 107 viral DNA copies, but this does not cover the full range observed in clinical specimens (about 102 to 109 copies) (3). Hybrid Capture uses probe mixes, and viral load results are thus an average of all the HPVs present. Viral load determinations for HPV types 31 and 45 in CIN have not been reported.
The present study reports HPV DNA load for four high-risk HPV types (types 16, 18, 31, and 45) in women with various degrees of cervical abnormality, from cytologically normal through CIN II-III. A single type-specific, quantitative assay, with an effective range of 102 to 109 copies of HPV DNA, was used for each genotype. Viral DNA load was found to vary by orders of magnitude with HPV genotype and cervical disease grade.
MATERIALS AND METHODS
Study population.
Both patients and healthy subjects were nonpregnant white, African-American, or Hispanic women, aged 18 years and older, and residents of Harris County, Tex., at the time of the study. Other eligibility criteria for both groups included no previous history of cervical neoplasia, of treatment for cervical neoplasia or cancer, or of hysterectomy. Patients with a confirmed histological diagnosis of CIN were identified among women referred to the University of Texas M. D. Anderson Cancer Center Colposcopy Clinic (UTMDACC) between September 1991 and August 1994 for further evaluation of an abnormal Pap smear. Of 640 women, 399 were confirmed with CIN; 325 met the other eligibility requirements and agreed to participate. Healthy women were selected from women attending family planning and screening services at two Harris County Health Department clinics serving large, multiethnic populations. Women were eligible for the healthy group when the cytological smear at the time of recruitment was normal and when they had no history of abnormal Pap smear or cervical biopsy. Of 414 women who met all eligibility criteria, 270 agreed to participate.
Patient data collection.
Patients had a complete physical examination, a repeat Pap smear, a colposcopic examination, colposcopically directed biopsies of abnormal areas, and two cervical samples collected for HPV testing. Cytologically normal women had a complete physical examination, a Pap smear, and two cervical samples collected for HPV testing. Exams and specimen collection for cytologically normal women were performed by nurse practitioners trained at the UTMDACC. The first samples for HPV testing were collected with a cotton swab and preserved in the transport medium provided by the manufacturer (Digene). The samples for PCR analysis were then collected with cervical brushes which were placed in vials and frozen immediately.
Cytological and histological diagnoses.
Cytological and histological specimens for all patients were interpreted at the UTMDACC Department of Pathology. Two independent readers at the UTMDACC reviewed each Pap smear and biopsy. A committee of staff members including the Director of the Colposcopy Clinic (M.F.M.) reviewed discrepant cases monthly and reached a final diagnosis. Cytological specimens for the healthy women were read and interpreted at the San Antonio Chest Hospital, in San Antonio, Tex. A high level of agreement in Pap smear diagnoses between that hospital and the UTMDACC was observed (kappa coefficient, 0.85).
DNA extraction-identification of HPV DNA-positive specimens.
The cytobrush specimens were thawed and vortexed in 1 ml of 0.01 M phosphate-buffered saline–5 mM EDTA, pH 7.4. A contamination control, consisting of 1 ml of water, was inserted after every 10th patient sample and subjected to the entire extraction and DNA detection protocol. Specimens were centrifuged at 1,000 × g for 5 min at room temperature. DNA was isolated from each cell pellet by standard phenol-chloroform extraction. Each supernatant from the DNA extraction was centrifuged in a Centricon 100 microconcentrator (Amicon, Inc., Beverly, Mass.) at 1,000 × g for 30 min. Retentates were collected and diluted to 200 μl with water. Ten microliters of this DNA was used in each fluorogenic PCR.
Each DNA specimen was tested for overall HPV positivity by PCR with L1 consensus primers (13) followed by electrophoresis in ethidium bromide-containing gels. HPV-positive samples were then tested by the quantitative fluorescent probe assay for HPV types 16, 18, 31, and 45.
Probes and primers.
The fluorogenic probe assay is based on the increase in fluorescent signal which occurs when probes are degraded by the 5′→3′ exonuclease activity of Taq polymerase (9). After degradation, the reporter dyes, FAM (6-carboxyfluorescein) and HEX (hexachlorofluorescein), present at the 5′ ends of the probes can diffuse away from a quencher dye, TAMRA (6-carboxy-tretramethyl-rhodamine), present on or near the 3′ end of each probe, thereby increasing the fluorescent signal from the reporter dyes. The probe sequences for each of the high-risk HPVs (Table 1) were selected and synthesized as described previously (18). The primer sequences (Table 1) were selected by using the Oligo 5.0 primer analysis program (National Biosciences, Inc., Plymouth, Minn.). The primer pairs for each of the HPV types were selected based on having a Tm of approximately 65°C, predicted lack of cross-hybridization to other common HPV types, no predicted loop formation, and no predicted dimer formation with the other primer.
TABLE 1.
Primers and probes used in HPV fluorogenic assay
| Gene | Primer-probeab | Sequence |
|---|---|---|
| HPV16/L1 | U primer 6564 | CCT TAT TGG TTA CAA CGA GCA C |
| L primer 7012 | GCG TCC TAA AGG AAA CTG ATC TA | |
| U probe 6862 | CCC CAG GAG GCA CAC TAG AAG A(Tc) | |
| HPV18/L1 | U primer 6548 | GTT ACA TAA GGC ACA GGG TCAT |
| L primer 6993 | CGT CCA AGG GGA TAT TGA TC | |
| U probe 6902 | AAA GGA TGC TGC ACC GGC Tc | |
| HPV31/L1 | U primer 6490 | GAT GCA ACG TGC TCA GGG A |
| L primer 6930 | GCG ACC CAG TGG AAA CTG ATC TA | |
| U probe 6852 | CCC AAA AGC CCA AGG AAG ATcC | |
| HPV45/L1 | U primer 6536 | TTA ATA AGC CAT ATT GGT TAC ATA AG |
| L primer 6950 | TTA GGT CAA CAG TCC AAA ACT TTA | |
| L probe 6910 | CCT GCT TTT CTG GAG GTG TAG TATcC | |
| Globin | U primer 61992 | GAA GAG CCA AGG ACA GGT AC |
| L primer 62240 | CAA CTT CAT CCA CGT TCA CC | |
| U probe 62049 | CCC TAG GGT TGG CCA ATC TAC TcC |
The primer-probe name denotes, in order, the strand sense and the end nucleotide position (5′ terminus in U and 3′ terminus in L primers-probes) as numbered in the GenBank reference sequences (accession numbers: HPV16, K02718; HPV18, X05015; HPV31, J04353; HPV45, X74479; and globin, U01317).
HEX and FAM reporter dyes are incorporated at the 5′ end of probes. Probes are blocked at the 3′ end by a 3′ phosphate.
Denotes the T to which the TAMRA is coupled; when the T was not present in the target DNA sequence, it is shown in parentheses.
Assay controls.
Control templates for HPV types 16, 18, 31, 33, 35, 45, 51, 52, and 56 were prepared by PCR amplification of cloned DNA with L1 type-specific primers (sequences available on request). The DNA concentrations were determined by fluorometry (DyNA Quant 200; Amersham Pharmacia Biotech, Piscataway, N.J.). Assay controls, consisting of a dilution series of the homologous template (1 × 105 to 3 × 101 copies) and a set of heterologous templates (2 × 103 copies each of HPV types 6, 11, 16, 18, 31, 33, 35, 45, 51, 52, and 56 in separate tubes), were included in each run. Each control sample also contained 50 ng of human placental DNA. Significant cross-reactivity was not normally observed with any of the heterologous templates. Data was utilized only from assays in which the controls registered <50 copies of each heterologous template.
Fluorogenic PCR.
The 50-μl PCR mixtures contained 10 mM Tris (pH 8.3), 50 mM KCl, 4.5 mM MgCl2, 200 μM deoxynucleoside triphosphates, 0.3 μM (each) primer, 50 nM (each) fluorogenic probe (FAM-HPV probe and HEX-globin probe), 0.025 U of AmpliTaq Gold DNA polymerase (The Perkin-Elmer Corp., Norwalk, Conn.) per μl, and 10 μl of template DNA. Following Taq polymerase activation and template denaturation for 12 min at 95°C, amplification conditions were as follows: 40 cycles of 30 s at 94°C, 10 s at 60°C, and 2 min at 65°C. Amplification was carried out in a Perkin-Elmer 9600 thermal cycler (Perkin-Elmer), after which the samples were transferred to a MicroFLUOR W, 96-well, white microtiter plate (Dynatech Industries, Inc., McLean, Va.), and the fluorescence was measured in a Perkin-Elmer LS-50B luminescence spectrometer. Data acquisition and analysis were performed with the TaqMan Fluorescence Data Manager (Perkin-Elmer) and Excel 5.0 (Microsoft Corporation, Redmond, Wash.). None of the contamination controls tested positive.
Copy number determination.
The spillover fluorescence from the FAM (HPV) channel into the HEX (globin) channel and vice versa was calculated from two sets of control samples, one containing both probes but only HPV template and the second containing both probes but only globin template. Included with each set of patient specimens were the assay controls and a dilution series of non-HPV-containing human cellular DNA. Plots of the homologous template dilution series fluorescence versus log (template copies) were linear over the range of 50 to 109 copies, thus allowing HPV copy number to be determined from the fluorescence in patient samples. Globin copy numbers in each specimen were determined similarly. All patient samples were assayed at least twice; samples with copy numbers >109 were diluted and retested. Patient copy numbers were the average of at least two determinations.
Deletion of DNA from the HPV genome is known to occur on integration; however, in most cases integration occurs in the E1-E2 region and the L1 open reading frame is retained (17, 20). In addition, integration occurs only rarely in CIN lesions (6, 8). Thus, the L1 copy numbers reported here should reflect the complete genome copy numbers.
Data analysis.
The positive threshold for each assay was the average signal in all contamination controls plus two standard deviations. The thresholds were different for each assay: HPV16, 37 copies; HPV18, 60 copies; HPV31, 34 copies; and HPV45, 96 copies. The sensitivity of the consensus primer PCR test used to select HPV-positive specimens (see above) was also about 100 copies. Comparison of median copy numbers between types requires that the threshold be set at or above the highest value for any type. The threshold in the present study was set at 100 copies. From the globin and HPV copy numbers, HPV copies per microgram of human cellular DNA were calculated. Determinations of the percentage of infected cells in each sample were not made, and hence copies per infected cell could not be calculated.
RESULTS
We detected HPV in 50% (298 of 595) of the samples. The four high-risk types included in the fluorogenic assay accounted for 55% (164 of 298) of the infections. Of these, HPV16 was the most common HPV DNA detected (78 single infections and 105 in multiple infections). HPV showed the expected association with cervical disease status (Table 2), although only HPV16 showed a statistically significant increase in prevalence with increasing severity of cervical disease.
TABLE 2.
HPV type distribution in different disease classifications
| Type | No. of normalsa (%) | No. of CIN Ib (%) | No. of CIN II-IIIb (%) |
|---|---|---|---|
| HPV16 | 7 (2.6) | 15 (8.5) | 56 (37.6) |
| HPV18 | 4 (1.5) | 10 (5.7) | 6 (4.0) |
| HPV31 | 2 (0.7) | 5 (2.8) | 12 (8.1) |
| HPV45 | 3 (1.1) | 10 (5.7) | 2 (1.3) |
| Multiple types | 2 (0.7) | 15 (8.5) | 15 (10.1) |
| Other typesc | 37 (13.7) | 61 (34.7) | 36 (24.2) |
| Negative | 215 (79.6) | 60 (34.1) | 22 (14.8) |
Determined by Pap cytology.
Determined by histologic analysis of biopsy specimen.
Positive in L1 consensus PCR, negative in all type-specific assays.
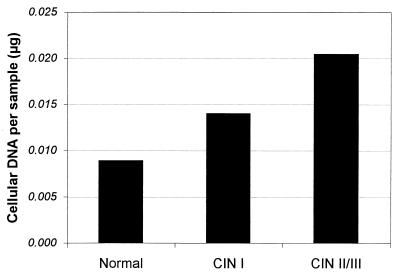
The amount of human cellular DNA per sample varied widely, ranging from 4 × 10−4 to 2 × 103 μg per sample. As shown in Fig. 1, cellular yield varied with cervical disease status; there was a modest but statistically significant increase in DNA content as cervical disease status changed from normal through CIN II-III (Kruskal-Wallis test, P = 0.0004). To minimize the effect of cellularity on HPV levels, HPV copy number was normalized to cellular DNA and expressed as copies per microgram of cellular DNA.
FIG. 1.
Sample cellular DNA content. Shown are sample cellular DNA amounts (micrograms), calculated from β-globin signals, stratified by disease grade.
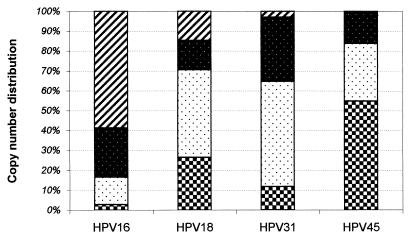
The quantity of HPV DNA in each sample covered a range of 12 logs (102 to 1014 copies). Replicate assays were generally within 10%. The HPV copy number per microgram varied greatly with HPV type. Most (55%) of the HPV16-positive samples contained more than 108 copies/μg whereas most (55%) of the HPV45 specimens contained 104 copies/μg or less (Fig. 2). To facilitate comparisons and statistical analysis, log[HPV copy number per microgram] was used. Because of the range of copies encountered, comparisons between groups of samples were best achieved by looking at medians of the log[HPV copy number per microgram of cellular DNA]. The median values for types 16, 18, 31, and 45 were 5.0 × 108, 1.5 × 105, 2.7 × 105, and 6.9 × 103 copies/μg, respectively (includes both single and multiple infections).
FIG. 2.
High-risk HPV copy number distribution in patient specimens. The copy number ranges were selected to maximize the number of patients in each category. The segments (striped, reverse-stippled, stippled, and checkered boxes) represent numbers of patient specimens with copy numbers of >108, >106, >104, and >0 per μg, respectively, for each of the HPV types.
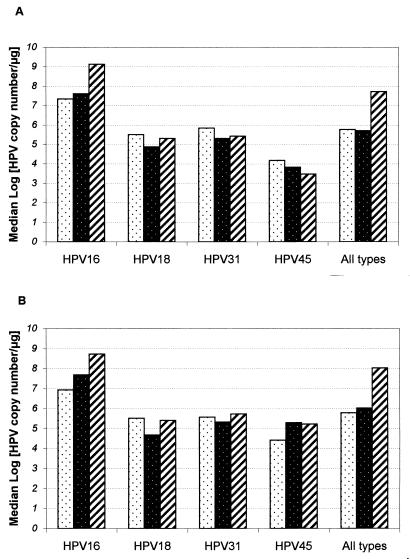
Stratification of the medians of the log[HPV copy number per microgram] by cervical disease status and HPV type is shown in Fig. 3; Fig. 3A includes samples with multiple infections, whereas Fig. 3B includes only single infections. Within each stratification, the amount of HPV DNA varied significantly, ranging over 7 logs. HPV16 is distinguished from the other types by having the highest copy numbers and by showing a statistically significant increase in copy number per microgram with increasing severity of cervical disease (Fig. 3A, P = 0.028; Fig. 3B, P = 0.030). Because of the high prevalence of HPV16 in the patient samples, this effect was also seen when the data for all four HPV types was combined (Fig. 3). For cervical samples without detected abnormality (normals), there was no significant HPV type-specific difference in the viral load (median of log HPV DNA per microgram), whereas for CIN I and CIN II-III, HPV type-specific differences in viral load were seen (P = 0.0002 and P = 0.0001, respectively).
FIG. 3.
High-risk HPV copy number per microgram stratified by HPV type and sample histology. The median HPV copy numbers per microgram were calculated for each of the three disease categories. The stippled, reverse-stippled, and striped bars indicate median (log [copy numbers per microgram of cellular DNA]) in normal, CIN I, and CIN II-III samples, respectively. (A) All HPV-positive samples (including multiple infections). A Kruskal-Wallis test of the significance of the copy number differences among normal, CIN I, and CIN II-III samples for the four HPV types returned the following P values: HPV16, P = 0.03; HPV18, P = 0.45; HPV31, P = 0.97; and HPV45, P = 0.30. (B) Singly infected samples. P values were as follows: HPV16, P = 0.03; HPV18, P = 0.12; HPV31, P = 0.73; and HPV45, P = 0.94.
DISCUSSION
This report describes the quantitative measurement of viral DNA amounts for the four most common high-risk HPV types in cervical specimens. Two primary results were obtained. First, the amount of HPV DNA differed by orders of magnitude among high-risk HPV types; patients with CIN II-III who were HPV16 positive had a median HPV DNA amount that was 4,000 to 6,000 times that seen in HPV18-, HPV31-, or HPV45-positive patients with the same disease. Second, the amount of HPV DNA for type 16, but not for type 18, 31, or 45, increased by orders of magnitude with increasing disease grade. Women with CIN II-III had a median amount of HPV16 DNA, which was more than 30 times higher than that of HPV16-infected women with CIN I and more than 60 times higher than that of HPV16-positive, cytologically normal women. This effect was seen both in women infected with a single HPV type (Fig. 3B) and in those infected with multiple types (Fig. 3A). Approximately 45% of the L1 consensus, HPV-positive samples consisted of types not included in the fluorogenic assay; thus some of the samples reported as single infections may in fact be mixed infections including types not assayed. The percentage of samples containing multiples of HPV type 16, 18, 31, or 45 increased with grade of disease.
The large dynamic range of the fluorogenic PCR assay used in this study allowed the 12-log range of HPV copy number in cervical samples to be clearly documented. Only one previous report of quantitative PCR for HPV16 DNA in cervical samples demonstrated a range of viral detection similar to the mean values found in this study (19), and quantitation of other types was not examined. While our findings, in agreement with others (7, 14), clearly demonstrate that only HPV16 shows a significant change in copy number with increasing dysplasia, combining results for all HPV types does demonstrate an overall correlation with disease (Fig. 3, all types) because of the high prevalence of HPV16 in our population. Other studies suggesting that high viral load could be clinically useful as a predictor of cervical dysplasia used the Hybrid Capture assay (12). Hybrid Capture, the current commercially available, Food and Drug Administration-approved HPV test, groups HPV types and includes no normalization for input cellular DNA. The sensitivity is much lower than that of the fluorogenic assay (5,000 versus 100 copies per assay), a factor increasing the observed prevalence of HPV16 because low-copy viruses would be below the level of detection. In addition, since sample DNA content increases with increasing cervical disease (Fig. 1), the lack of normalization for cellular DNA would also favor observing a trend for increase in HPV copy number with increased cervical disease. In this study, the range of viral DNA copies per microgram of cellular DNA, even when stratified by disease status and viral type, was quite large. Because of the marked variation, a cutoff value for “high” copy numbers that would allow accurate prediction of high-grade cervical disease was not possible. This, as well as the fact that only HPV16 copy number changes significantly with cervical disease, suggests that the usefulness of HPV quantitation requires careful assessment and is assay dependent.
The observation that yield of cellular DNA increased with degree of cervical abnormality was somewhat unexpected and cannot be entirely explained. Dysplasia is known to be associated with decreased intercellular adhesion, and actual increases in the yield of cells in the sample may explain the trend that we observed. Dysplasia is also associated with changes in ploidy, but because of the small contribution of dysplastic cells to the total normal background population in the sample, we feel that ploidy changes are less likely to contribute significant changes in the overall yield of cellular DNA.
Assuming that the endocervical sample is equally representative of all HPV-associated lesions, HPV16 appears to be different from HPV types 18, 31, and 45; it is present in higher copy numbers and varies with disease. The quantitative differences in HPV DNA may be the result of type-specific differences in HPV replication in the cervical epithelium. Since HPV DNA replication is a complex, multiprotein reaction, relatively small differences in the interactions between molecules involved in replication could be amplified into the large differences in HPV DNA observed here (1, 21). Type-specific differences in the immune response may also influence copy number.
The significance of the relatively high copy number of HPV DNA in samples from women with no identifiable lesions cannot be addressed in this cross-sectional design. The HPV DNA is presumed to be attributable to latently infected cells, but sampling errors cannot be excluded. Prospective studies of cytologically normal women using a quantitative analysis of HPV DNA will help to determine the natural history and biology of latent infection.
ACKNOWLEDGMENT
We are sincerely grateful to Diane Pardi for helpful discussions.
REFERENCES
- 1.Banks L, Edmonds C, Vousden K H. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene. 1990;5:1383–1389. [PubMed] [Google Scholar]
- 2.Bosch F X, Manos M M, Muõz N, Sherman M, Jansen A M, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) study group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 3.Cavuslu S, Mant C, Starkey W G, Bible J M, Biswas C, Kell B, Rice P, Best J M, Cason J. Analytic sensitivities of hybrid-capture, consensus and type-specific polymerase chain reactions for the detection of human papillomavirus type 16 DNA. J Med Virol. 1996;49:319–324. doi: 10.1002/(SICI)1096-9071(199608)49:4<319::AID-JMV10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Chang D Y, Chen R J, Lee S C, Huang S C. Prevalence of single and multiple infection with human papillomaviruses in various grades of cervical neoplasia. J Med Microbiol. 1997;46:54–60. doi: 10.1099/00222615-46-1-54. [DOI] [PubMed] [Google Scholar]
- 5.Chang D Y, Hsieh C Y, Chen R J, Lee S C, Huang S C. Comparison of detection of human papillomavirus 16 DNA in cervical carcinoma tissues by Southern blot hybridisation and nested polymerase chain reaction. J Med Microbiol. 1995;43:430–435. doi: 10.1099/00222615-43-6-430. [DOI] [PubMed] [Google Scholar]
- 6.Cullen A P, Reid R, Campion M, Lörincz A T. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991;65:606–612. doi: 10.1128/jvi.65.2.606-612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuzick J, Terry G, Ho L, Hollingworth T, Anderson M. Type-specific human papillomavirus DNA in abnormal smears as a predictor of high-grade cervical intraepithelial neoplasia. Br J Cancer. 1994;69:167–171. doi: 10.1038/bjc.1994.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das B C, Sharma J K, Gopalakrishna V, Luthra U K. Analysis by polymerase chain reaction of the physical state of human papillomavirus type 16 DNA in cervical preneoplastic and neoplastic lesions. J Gen Virol. 1992;73:2327–2336. doi: 10.1099/0022-1317-73-9-2327. [DOI] [PubMed] [Google Scholar]
- 9.Förster V T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann Phys. 1948;2:55–75. [Google Scholar]
- 10.Kaufman R H, Adam E, Icenogle J, Lawson H, Lee N, Reeves K O, Irwin J, Simon T, Press M, Uhler R, Entman C, Reeves W C. Relevance of human papillomavirus screening in management of cervical intraepithelial neoplasia. Am J Obstet Gynecol. 1997;176:87–92. doi: 10.1016/s0002-9378(97)80017-8. [DOI] [PubMed] [Google Scholar]
- 11.Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol. 1996;175:1099–1104. doi: 10.1016/s0002-9378(96)70011-x. [DOI] [PubMed] [Google Scholar]
- 12.Lörincz A. Hybrid capture method for detection of human papillomavirus DNA in clinical specimens. Papillomavirus Rep. 1998;7:1–5. doi: 10.1111/j.1447-0756.1996.tb01081.x. [DOI] [PubMed] [Google Scholar]
- 13.Manos M M, Ting Y, Wright D K, Lewis A J, Broker T R, Wolinsky S M. Use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cells. 1989;7:209–214. [Google Scholar]
- 14.Mansell M E, Ho L, Terry G, Singer A, Cuzick J. Semi-quantitative human papillomavirus DNA detection in the management of women with minor cytological abnormality. Br J Obstet Gynaecol. 1994;101:807–809. doi: 10.1111/j.1471-0528.1994.tb11952.x. [DOI] [PubMed] [Google Scholar]
- 15.Mun̈oz N, Bosch F X, de Sanjose S, Tafur L, Izarzugaza I, Gili M, Viladiu P, Navarro C, Martos C, Ascunce N, et al. The causal link between human papillomavirus and invasive cervical cancer: a population-based case-control study in Colombia and Spain. Int J Cancer. 1992;52:743–749. doi: 10.1002/ijc.2910520513. [DOI] [PubMed] [Google Scholar]
- 16.Poletti P A, Halfon A, Marti M C. Papillomavirus and anal carcinoma. Int J Colorectal Dis. 1998;13:108–111. doi: 10.1007/s003840050145. [DOI] [PubMed] [Google Scholar]
- 17.Stoler M H, Rhodes C R, Whitbeck A, Wolinsky S M, Chow L T, Broker T R. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992;23:117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- 18.Swan D C, Tucker R A, Holloway B P, Icenogle J P. A sensitive, type-specific, fluorogenic probe assay for detection of human papillomavirus DNA. J Clin Microbiol. 1997;35:886–891. doi: 10.1128/jcm.35.4.886-891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terry G, Ho L, Jenkins D, Hills M, Singer A, Mansell B, Beverley E. Definition of human papillomavirus type 16 DNA levels in low and high grade cervical lesions by a simple polymerase chain reaction technique. Arch Virol. 1993;128:123–133. doi: 10.1007/BF01309793. [DOI] [PubMed] [Google Scholar]
- 20.Vernon S D, Unger E R, Miller D L, Lee D R, Reeves W C. Association of human papillomavirus type 16 integration in the E2 gene with poor disease-free survival from cervical cancer. Int J Cancer. 1997;74:50–56. doi: 10.1002/(sici)1097-0215(19970220)74:1<50::aid-ijc9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Vousden K. Interactions of human papillomavirus transforming proteins with the products of tumor suppressor genes. FASEB J. 1993;7:872–879. doi: 10.1096/fasebj.7.10.8393818. [DOI] [PubMed] [Google Scholar]