Key Points
Question
In patients with witnessed refractory out-of-hospital cardiac arrest, does early intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and invasive assessment and treatment improve outcomes compared with standard resuscitation?
Findings
In this randomized clinical trial that included 256 patients, survival with neurologically favorable outcome (Cerebral Performance Category 1-2) at 180 days occurred in 31.5% in the invasive strategy group and 22.0% in the standard resuscitation group, a difference that was not statistically significant.
Meaning
Among patients with refractory out-of-hospital cardiac arrest, the bundle of early intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and invasive assessment and treatment did not significantly improve survival with neurologically favorable outcome at 180 days compared with standard resuscitation, although the trial was possibly underpowered to detect a clinically relevant difference.
Abstract
Importance
Out-of-hospital cardiac arrest (OHCA) has poor outcome. Whether intra-arrest transport, extracorporeal cardiopulmonary resuscitation (ECPR), and immediate invasive assessment and treatment (invasive strategy) is beneficial in this setting remains uncertain.
Objective
To determine whether an early invasive approach in adults with refractory OHCA improves neurologically favorable survival.
Design, Setting, and Participants
Single-center, randomized clinical trial in Prague, Czech Republic, of adults with a witnessed OHCA of presumed cardiac origin without return of spontaneous circulation. A total of 256 participants, of a planned sample size of 285, were enrolled between March 2013 and October 2020. Patients were observed until death or day 180 (last patient follow-up ended on March 30, 2021).
Interventions
In the invasive strategy group (n = 124), mechanical compression was initiated, followed by intra-arrest transport to a cardiac center for ECPR and immediate invasive assessment and treatment. Regular advanced cardiac life support was continued on-site in the standard strategy group (n = 132).
Main Outcomes and Measures
The primary outcome was survival with a good neurologic outcome (defined as Cerebral Performance Category [CPC] 1-2) at 180 days after randomization. Secondary outcomes included neurologic recovery at 30 days (defined as CPC 1-2 at any time within the first 30 days) and cardiac recovery at 30 days (defined as no need for pharmacological or mechanical cardiac support for at least 24 hours).
Results
The trial was stopped at the recommendation of the data and safety monitoring board when prespecified criteria for futility were met. Among 256 patients (median age, 58 years; 44 [17%] women), 256 (100%) completed the trial. In the main analysis, 39 patients (31.5%) in the invasive strategy group and 29 (22.0%) in the standard strategy group survived to 180 days with good neurologic outcome (odds ratio [OR], 1.63 [95% CI, 0.93 to 2.85]; difference, 9.5% [95% CI, −1.3% to 20.1%]; P = .09). At 30 days, neurologic recovery had occurred in 38 patients (30.6%) in the invasive strategy group and in 24 (18.2%) in the standard strategy group (OR, 1.99 [95% CI, 1.11 to 3.57]; difference, 12.4% [95% CI, 1.9% to 22.7%]; P = .02), and cardiac recovery had occurred in 54 (43.5%) and 45 (34.1%) patients, respectively (OR, 1.49 [95% CI, 0.91 to 2.47]; difference, 9.4% [95% CI, −2.5% to 21%]; P = .12). Bleeding occurred more frequently in the invasive strategy vs standard strategy group (31% vs 15%, respectively).
Conclusions and Relevance
Among patients with refractory out-of-hospital cardiac arrest, the bundle of early intra-arrest transport, ECPR, and invasive assessment and treatment did not significantly improve survival with neurologically favorable outcome at 180 days compared with standard resuscitation. However, the trial was possibly underpowered to detect a clinically relevant difference.
Trial Registration
ClinicalTrials.gov Identifier: NCT01511666
This randomized clinical trial assesses whether an invasive resuscitation approach (early intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and invasive assessment and treatment), compared with standard advanced cardiac life support, improves survival with neurologically favorable outcomes among adults with refractory out-of-hospital cardiac arrest in Czech Republic.
Introduction
Out-of-hospital cardiac arrest (OHCA) is a significant socioeconomic burden to society.1 In a large trial, 50% of patients who attained stable return of spontaneous circulation (ROSC) during initial resuscitation and were transferred to the hospital for postresuscitation care achieved neurologically favorable survival.2 However, refractory cardiac arrest (ie, prolonged cardiac arrest and cardiac arrest without ROSC in the field) is associated with poor clinical outcomes.3 In patients without ROSC, the odds of survival are low when transport to the hospital occurs during ongoing cardiopulmonary resuscitation (CPR), usually less than 4%.4,5
Temporary replacement of a failing circulation by extracorporeal life support (ECLS), a method called extracorporeal cardiopulmonary resuscitation (ECPR), has been recognized as a potential approach to refractory cardiac arrest.6,7,8 Despite encouraging results of nonrandomized studies, a meta-analysis,9 and 1 recently published small randomized trial,10 the benefit of ECPR in refractory OHCA remains uncertain.11,12 Recent European Resuscitation Guidelines13 provide a weak recommendation for ECPR, which may be considered as a rescue method when conventional CPR is failing, with very low certainty of evidence.
The purpose of this randomized clinical trial was to compare the bundle of early intra-arrest transport to the hospital using mechanical CPR, ECPR, and immediate invasive assessment and treatment vs standard treatment in refractory OHCA for achieving survival with good neurologic outcome at 180 days.
Methods
Study Design
This randomized clinical trial was conducted at a single center in Prague, Czech Republic, from March 1, 2013, to October 25, 2020 (with final follow-up on March 30, 2021). The study protocol, including statistical analysis plan (Supplement 1), was published in detail prior to study initiation,14 and the study was approved by the institutional review board of the General University Hospital and First Faculty of Medicine, Charles University in Prague (192/11S-IV).
Each participant’s legal representative was informed of the participant’s study enrollment and was asked for written informed consent as soon as possible. All patients who regained normal neurologic function were asked to provide their written consent regarding the use of their data. Consent requirements were waived for patients who died at the scene and never reached the hospital and for participants without known legal representatives. As specified in the protocol, a data and safety monitoring board reviewed the data on patient outcome and complications every 6 months or after every 30 patients enrolled, whichever came first. An independent contract research organization verified and monitored the study data.
Participants
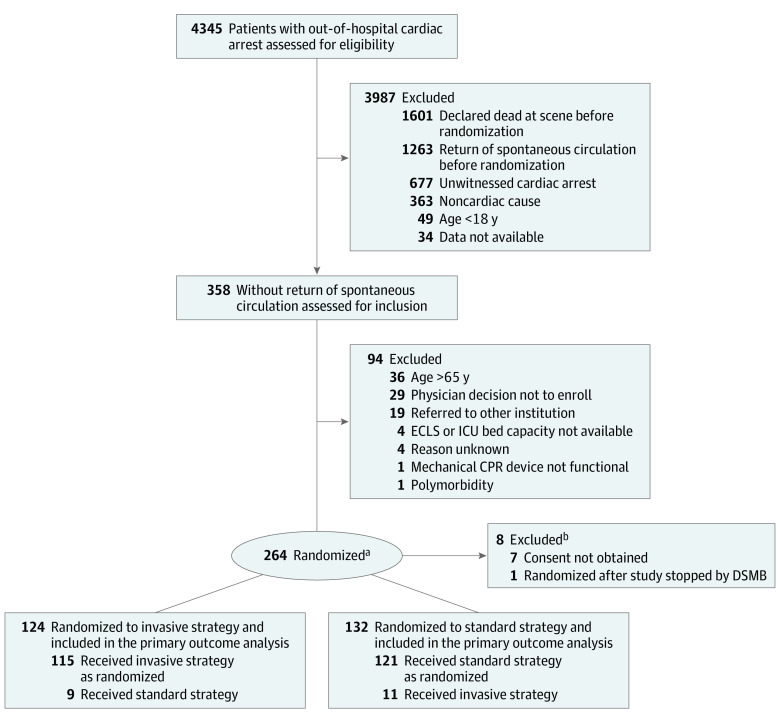
Adults aged 18 to 65 years receiving ongoing resuscitation for witnessed OHCA of presumed cardiac etiology were eligible for enrollment in the trial, given that they had received a minimum of 5 minutes of advanced cardiac life support without ROSC and when the ECPR team was available at the cardiac center. Patients who had unwitnessed cardiac arrest or presumed noncardiac cause, had suspected or confirmed pregnancy, attained ROSC within 5 minutes during initial resuscitation, regained consciousness, had obvious life-limiting comorbidities, bleeding diathesis, known do-not-resuscitate order, or known prearrest Cerebral Performance Category (CPC)15 3 or greater were excluded (Figure 1; eTable 1 in Supplement 2).
Figure 1. Prehospital Flow of Participants in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest.
DSMB indicates data and safety monitoring board; ECLS, extracorporeal life support; CPR, cardiopulmonary resuscitation; ICU, intensive care unit; OHCA, out-of-hospital cardiac arrest; ROSC, return of spontaneous circulation.
aRandomization into standard strategy and invasive strategy groups was based on 4 strata (men ≤45 years, men >45 years, women ≤45 years, women >45 years), with block size of 8.
bSeven patients were excluded after randomization because consent was refused and information was not available as to how many were randomized to each group for analysis.
Enrollment and Randomization
Enrollment was conducted with the close cooperation of the Prague Emergency Medical Service dispatch center. The study coordinator in the cardiac center was notified by an automatic Short Message Service alert on every occasion when the dispatch center initiated telephone-assisted bystander chest compressions and activated a rapid response vehicle for a witnessed collapse suspected to be cardiac arrest of presumed cardiac cause. A telephone connection was subsequently established during the ongoing chest compressions between the cardiac center coordinator and the physician or paramedic on scene (randomization call). The coordinator logged into a web-based secured randomization system that was available 24 hours per day and maintained by the Institute of Biostatistics and Analyses, Faculty of Medicine, Masaryk University, Brno. An assigned patient number and intervention group, ie, invasive group or standard group, was recorded. The log-in link was accessible from all computers within the cardiac center and from the smartphone of the coordinator.
For randomization, the patient’s estimated age and sex as well as confirmation of the inclusion/exclusion criteria were recorded (eTable 1 in Supplement 2). Randomization into the standard strategy or invasive strategy group was based on 4 strata (men ≤45 years, men >45 years, women ≤45 years, women >45 years), with block size of 8. The block size was not disclosed to research personnel.
Intervention
Patients randomized to the standard strategy group received continued advanced cardiac life support on site. The use of drugs, further defibrillations, or other interventions followed recommended guidelines.16,17 If ROSC was achieved (defined as a cardiac electrical activity with palpable pulse), transport to the hospital was initiated and an early invasive strategy (ie, coronary angiography) was encouraged.
A mechanical chest compression device (LUCAS, Lund University Cardiac Arrest System; Physio-Control Inc/Jolife AB, Lund, Sweden) was originally reserved for the invasive strategy group only; however, following the publication of a major trial on mechanical chest compression,18 the attachment of a mechanical chest compression device was left to the discretion of the emergency physician and was allowed for use at any point during CPR.
In the invasive strategy group, intra-arrest intranasal evaporative cooling via a RhinoChill device (BeneChill Inc) was initiated if feasible (this device became unavailable during the course of the study in 2016) and the patient was immediately transferred directly to the cardiac center catheterization laboratory during ongoing CPR with the intention of proceeding with ECPR if ROSC was not achieved en route or on admission. The use of drugs, further defibrillations en route, or other interventions during transport followed European Resuscitation Council guidelines.16,17 The team, including study coordinator, intensivist, perfusionist (a specialist responsible for an ECLS), interventional cardiologist, study data manager, and interventional and intensive care unit nurses simultaneously prepared all the necessary equipment. A dry-primed extracorporeal life support machine was ready to be used in the catheterization laboratory when needed.
On admission, the overall status, ROSC presence, and ECLS implantation inclusion/exclusion criteria (eTable 1 in Supplement 2) were evaluated. The ECLS cannulation was performed on the catheterization table during ongoing mechanical CPR using a femoro-femoral approach. After commencement of ECLS and following the completion of the invasive diagnostic and therapeutic procedures (ie, coronary, and eventually pulmonary or aortic angiography and percutaneous coronary intervention, if appropriate), an antegrade perfusion cannula was implanted in the cannulated limb under ultrasound guidance. Patients receiving ECLS were continuously anticoagulated with heparin unless contraindicated, with a target activated partial thromboplastin time of 50 to 70 seconds.
Postresuscitation care was standardized in both study groups. All patients admitted to the hospital had an immediate biochemical evaluation, an urgent bedside echocardiogram, and whole-body computed tomography if feasible and clinically indicated. In-hospital target temperature management to 33 °C was initiated as soon as possible either via ECLS heat exchanger or other routine measures (intravascular or surface feedback device cooling). Following the publication of a target temperature management trial,19 in cases with early awakening or complications of hypothermia, a strict temperature management to 36 °C was allowed instead of 33 °C. All other postarrest critical care management, including withdrawal of life-sustaining therapy, complied with European Resuscitation Council guidelines and other generally accepted approaches.16,17,18,20
A crossover from the standard strategy group to the invasive strategy group (and vice versa) was allowed in selected patients. In the standard to invasive strategy group, the decision was made based on the request of an emergency physician. At least 2 additional unsuccessful defibrillations were required after randomization before a crossover was accepted by the cardiac center coordinator. The crossover from invasive strategy to standard strategy was accepted when continuing care with invasive measures was deemed to be futile. The termination of resuscitation efforts followed the European Resuscitation Council guidelines,16,17 although the final decision was based on the discretion of the emergency physician or cardiac intensivist in charge.
Outcomes
Primary Outcome
Primary outcome was 180-day survival with favorable neurologic status defined as no or minimal neurologic impairment (CPC 1 or 2). The CPC schema ranges from 1 (defined as conscious, alert, able to work), 2 (conscious, sufficient cerebral function for independent activities of daily life, able to work in sheltered environment), 3 (conscious, dependent on others for daily support), 4 (comatous, vegetative state) to 5 (brain death).
Neurologic outcome was assessed by a neurologist in a blinded fashion.
Secondary Outcomes
Secondary outcomes included 30-day survival with cardiac recovery (no need for pharmacological or mechanical cardiac support for 24 hours) and neurologic recovery (CPC 1 or 2) at any point within the first 30 days after cardiac arrest.
Exploratory Analyses
Survival to 180 days was assessed as a post hoc outcome. Post hoc subgroup analyses for the primary outcome were performed in the following subgroups: older than 65 years vs 65 years or younger, sex, place of cardiac arrest, initial rhythm, pH below median value vs above, lactate level below median value vs above, and cause of cardiac arrest.
Complications
Bleeding complications were assessed based on Thrombolysis in Myocardial Infarction classification21 under “major” category, defined as any intracranial hemorrhage (excluding microhemorrhages <10 mm), fatal bleeding directly resulting in death within 7 days, or overt bleeding associated with a decrease in hemoglobin concentration of 5 g/dL or a 15% absolute decrease in hematocrit. Organ lacerations were assessed both by morphological examinations (mainly computed tomography) and during autopsies. Technical complications related to ECLS were gathered and reported by perfusionists.
Power Analysis and Sample Size Calculation
Sample size determination was computed for the statistical superiority of invasive strategy over standard strategy using a 2-tailed test with α = .05 and 90% power. A 10% 6-month survival with favorable neurologic outcome in the standard strategy group was expected. Three scenarios were suggested: 10% increase of primary outcome, with 571 patients expected to be enrolled; 15% increase, with 285 patients; and 20% increase, with 176 patients.14
Statistical Analysis
A complete case analysis, with no assumptions made for missing data, was performed for primary and secondary outcomes. In the main analysis, patient data were analyzed according to randomization group, and data from patients who crossed over were analyzed by original group assignment. A post hoc analysis pooled all patients treated with ECPR (both those allocated to the invasive strategy group and receiving ECPR and those allocated to the standard strategy group and receiving ECPR after crossover to the invasive strategy group).
Continuous data were evaluated for a normal distribution by Shapiro-Wilk test. Numeric variables are expressed as medians and IQRs. The 2-sided Mann-Whitney test was used to compare cardiac arrest times and laboratory values. Categorical values were compared using the 2-sided Fisher exact test (for 2 × 2 table) or χ2 test. The primary and secondary outcomes are reported by odds ratios and absolute differences with 95% confidence intervals.
The survival analysis was performed by the Kaplan-Meier analysis and log-rank test and considered patients alive at day 180 regardless of their neurologic status. A subgroup analysis was computed using logistic regression and analysis of interaction between given stratification and study group. Because of the potential for type I error due to multiple comparisons, findings for secondary outcomes and subgroup analyses should be interpreted as exploratory.
A 2-sided P < .05 was considered statistically significant. Statistical analyses were performed with MedCalc version 19.7 (MedCalc Software Ltd) and SPSS version 26.0.0.0 (IBM Corp).
Results
The study was terminated on October 25, 2020, at the recommendation of the data and safety monitoring board (Supplement 3) because the standardized test statistics for results of primary end point in the study intersected a prespecified stopping rule for futility at n = 256 (eFigure 1 in Supplement 2).
During the study enrollment period from March 1, 2013, to October 25, 2020, 4345 attended cardiac arrests occurred within the Prague region. After exclusion of those without presumed cardiac cause, those that lacked a witness, patients who achieved ROSC, or patients who died without consideration for study enrollment, 358 patients with arrest refractory to initial resuscitation efforts remained. Of these, 264 were eligible for the study enrollment and randomized. Later, 8 patients were withdrawn; for 7, consent was not obtained from the relatives, and 1 patient was erroneously randomized after the study was already stopped.
In total, 256 patients were analyzed, 124 allocated to the invasive strategy group and 132 to the standard strategy group. Overall, in 20 patients (7.6%), a crossover was accepted. There were 11 crossovers from the standard strategy group to the invasive strategy group (all except 1 involved patients with refractory ventricular fibrillation) and 9 crossovers from the invasive strategy group to the standard strategy group (Figure 1).
Patient and Cardiac Arrest Characteristics
Table 1 reports the main demographics of the study population. The median age was 59 years (IQR, 48-66) for the invasive strategy group and 57 years (IQR, 47-65) for the standard strategy group, and 44 of the 256 patients (17%) were women. Hypertension, diabetes, and coronary artery disease were prevailing comorbidities. The most frequent cause of cardiac arrest was acute coronary syndrome in both the invasive strategy group (64/124 [52%]) and the standard strategy group (63/132 [48%]).
Table 1. Baseline Demographics and Prehospital Resuscitation Characteristics of Included Patients in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest.
| Characteristics | No. (%) | |
|---|---|---|
| Invasive strategy (n = 124) | Standard strategy (n = 132) | |
| Age, median (IQR), y | 59 (48-66) | 57 (47-65) |
| Sex | ||
| Men | 102 (82) | 110 (83) |
| Women | 22 (18) | 22 (17) |
| Medical history, No./total (%)a | ||
| Hypertension | 47/108 (44) | 42/83 (51) |
| Diabetes | 19/104 (18) | 17/83 (21) |
| Coronary artery disease | 17/104 (16) | 17/83 (21) |
| Chronic heart failure | 11/106 (10) | 5/79 (6) |
| COPD | 8/105 (8) | 2/79 (3) |
| Chronic kidney disease | 3/104 (3) | 2/79 (3) |
| Implanted ICD | 3/121 (3) | 0/89 |
| Location of cardiac arrest | ||
| Public place | 44 (36) | 54 (41) |
| Home | 42 (34) | 34 (26) |
| EMS | 19 (15) | 17 (13) |
| Car | 8 (7) | 7 (5) |
| Workplace | 5 (4) | 14 (11) |
| Hotel | 4 (3) | 6 (5) |
| Health facility | 2 (2) | 0 |
| Initial rhythmb | ||
| Ventricular fibrillation | 72 (58) | 84 (64) |
| Asystole | 31 (25) | 24 (18) |
| Pulseless electrical activity | 21 (17) | 24 (18) |
| Bystander CPRc | 123 (99) | 129 (98) |
| Telephone-assisted bystander CPR | 96 (77) | 107 (81) |
| Time from collapse to EMS arrival, median (IQR), min | 8 (7-11) | 9 (7-11) |
| Time from collapse to ACLS, median (IQR), min | 10 (7-13) | 11 (8-14) |
| Time to telephone-assisted CPR, median (IQR), min | 3 (2-5) | 2 (1-4) |
| Time from collapse to randomization, median (IQR), min | 24 (21-30) | 26 (19-31) |
| No. of prehospital epinephrine doses, median (IQR), mg | 4 (2-5) | 5 (3-7) |
| No. of prehospital defibrillation attempts, median (IQR) | 4 (2-6) | 4 (2-7) |
| Mechanical CPRd | 114 (92) | 104 (79) |
| Intermittent ROSCe | 41 (33) | 45 (34) |
| Hypothermia initiated in fieldf | 21 (17) | 12 (9) |
Abbreviations: ACLS, advanced cardiac life support; COPD, chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation; EMS, emergency medical service; ICD, implantable cardioverter-defibrillator; ROSC, return of spontaneous circulation.
The information for several categories was obtained later during patient care from EMS, caregivers, relatives, and chart reviews and might not have been available to caregivers during initial treatment.
As determined by EMS.
High rate of bystander CPR consistent with generally high rate in Prague (>80%) as reported in a Eureca 2 study.27
Use of LUCAS device (Lund University Cardiac Arrest System; Physio-Control Inc/Jolife AB).
Defined as an unsustained palpable pulse with organized ECG rhythm.
Prehospital hypothermia provided by means of intranasal evaporative cooling was used in the invasive strategy group and those patients in the standard strategy group who crossed over to the invasive approach. This method became unavailable during the course of the study in 2016; therefore, the percentage of use is low.
Cardiac arrest occurred most commonly in a public place (44/124 patients [36%] in invasive strategy group, 54/132 [41%] in the standard strategy group). Ventricular fibrillation was the most common initial rhythm (72/124 patients [58%] in the invasive strategy group and 84/132 [64%] in the standard strategy group). Bystander CPR was performed in 123 of 124 cases (99%) in the invasive strategy group and in 129 of 132 (98%) in the standard strategy group, as well as telephone-assisted dispatch center CPR in 96 of 124 (77%) and 107 of 132 (81%), initiated within median of 3 (IQR, 2-5) and 2 (IQR, 1-4) minutes after the collapse in the respective groups. Patients were randomized within a median of 24 (IQR, 21-30) and 26 (IQR, 19-31) minutes after collapse for the invasive strategy and standard strategy groups, respectively.
Primary Outcome
Survival with favorable neurologic outcome at 180 days occurred in 39 of 124 patients (31.5%) in the invasive strategy group and 29 of 132 patients (22%) in the standard strategy group, a difference that was not statistically significant (odds ratio, 1.63 [95% CI, 0.93 to 2.85]; absolute difference, 9.5% [95% CI, −1.3% to 20.1%]; P = .09) (Table 2). There were no missing data for the primary outcome analysis.
Table 2. Primary and Secondary Outcomes in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest.
| No. (%) | Absolute difference, % (95% CI) | P value | ||
|---|---|---|---|---|
| Invasive strategy (n = 124) | Standard strategy (n = 132) | |||
| Primary outcome | ||||
| Survival with minimal or no neurologic impairment at 180 da | 39 (31.5) | 29 (22.0) | 9.5 (−1.3 to 20.1) | .09 |
| Secondary outcomes | ||||
| Survival with minimal or no neurologic impairment at 30 da | 38 (30.6) | 24 (18.2) | 12.4 (1.9 to 22.7) | .02 |
| Cardiac recovery at 30 db | 54 (43.5) | 45 (34.1) | 9.4 (−2.5 to 21) | .12 |
Defined as Cerebral Performance Category 1 or 2. The Cerebral Performance Category schema ranges from 1 (defined as conscious, alert, able to work), 2 (conscious, sufficient cerebral function for independent activities of daily life, able to work in sheltered environment), 3 (conscious, dependent on others for daily support), 4 (comatous, vegetative state) to 5 (defined as brain death). All patients observed to death or 180 days.
Defined as absence of both pharmacological and mechanical cardiac support for at least 24 hours.
Secondary Outcomes
Neurologic recovery at 30 days occurred in 38 of 124 patients (30.6%) in the invasive strategy group and 24 of 132 (18.2%) in the standard strategy group (odds ratio, 1.99 [95% CI, 1.11 to 3.57]; absolute difference, 12.4% [95% CI, 1.9% to 22.7%]; P = .02).
Cardiac recovery at 30 days occurred in 54 of 124 patients (43.5%) in the invasive strategy group and 45 of 132 (34.1%) in the standard strategy group (odds ratio, 1.49 [95% CI, 0.91 to 2.47]; absolute difference, 9.4% [95% CI, −2.5 to 21%]; P = .12).
Resuscitation and Hospitalization Procedures and Outcomes
In the invasive strategy group, a median of 4 (IQR, 2-5) epinephrine doses were used, compared with 5 (IQR, 3-7) in the standard strategy group (P = .002), while the number of prehospital defibrillations was median of 4 (IQR, 2-6) in the invasive strategy group vs 4 (IQR, 2-7) in the standard strategy group. Intermittent ROSC was identified in 41 of 124 patients (33%) in the invasive strategy group and 45 of 132 (34%) in the standard strategy group.
As Table 3 describes in detail, more patients in the invasive strategy group were admitted to the hospital after a shorter time of transport from the scene. The overall CPR time was longer in the invasive strategy group (median, 58 [IQR, 43-70] vs 46 [IQR, 33-68] minutes, P = .04), as every effort was made to bring the patient to the hospital catheterization laboratory for ECPR.
Table 3. Additional Outcomes Related to Transport, Hospitalization, and Intervention in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest.
| Prehospital and early hospital events | No. (%) | |
|---|---|---|
| Invasive strategy (n = 124) | Standard strategy (n = 132) | |
| Arrived to hospital | 123 (99) | 87 (66) |
| Time from collapse to hospital arrival, median (IQR), min | 49 (44-60) | 60 (50-69) |
| Transport time - time from randomization to admission, median (IQR), min | 26 (19-33) | 33 (25-42) |
| Prehospital declaration of death | 1 (1) | 45 (34) |
| Declaration of death within 1 h of hospital admission | 10 (8) | 19 (14) |
| Time of CPR (time to death/ROSC or ECLS), median (IQR), min | 58 (43-70) | 46 (33-68) |
| Duration of CPR, min | ||
| <30 | 14 (11) | 26 (20) |
| ≥30 and <45 | 19 (15) | 33 (25) |
| ≥45 | 91 (73) | 73 (55) |
| Sustained ROSC on admissiona | 34 (27) | 58 (44) |
| Hospitalization events | ||
| Target temperature management used, No./total (%)b | 117/123 (95) | 61/87 (70) |
| Extracorporeal life support | ||
| ECLS implanted | 82 (66) | 10 (8) |
| Time to ECLS, median (IQR), min | 61 (55-70) [n = 81] | 62 (51-73) [n = 10] |
| Time of implantation (door to ECLS), median (IQR), min | 12 (9-15) [n = 80] | 16 (11-17) [n = 10] |
| Invasive assessment, No./total (%) | ||
| Diagnostic angiography | 120/123 (98) | 67/87 (77) |
| Coronary angiography | 115/120 (96) | 66/67 (99) |
| Aortography | 28/120 (24) | 13/67 (19) |
| Left ventricle angiography | 26/120 (22) | 21/67 (31) |
| Pulmonary angiography | 22/120 (18) | 5/67 (8) |
| Emergency invasive interventions, No./total (%) | ||
| PCI (both for ACS and CAD)c | ||
| Successful | 56/62 (90) | 24/30 (80) |
| Unsuccessful | 6/62 (10) | 6/30 (20) |
| Balloon valvuloplasty | 0/120 | 3 (4) |
| Laboratory values on admission | ||
| pH [reference, 7.36-7.44], median (IQR) | 6.93 (6.8-7.1) | 7.03 (6.9-7.2) |
| Lactate [reference, 0.5-2.0], median (IQR), mmol/L | 12.5 (9.2-16) | 10.4 (7.5-13.5) |
| Cause of cardiac arrest (including autopsy findings) | ||
| Acute coronary syndrome | 64 (52) | 63 (48) |
| Coronary artery disease-chronic | 14 (11) | 18 (14) |
| Pulmonary embolism | 12 (10) | 12 (9) |
| Chronic heart failure | 8 (7) | 6 (5) |
| Myocarditis | 6 (5) | 2 (2) |
| Accidental hypothermia | 3 (2) | 1 (1) |
| Bleeding-other | 3 (2) | 0 |
| Cardiomyopathy | 3 (2) | 6 (5) |
| Unknown | 3 (2) | 12 (9) |
| Aortic stenosis | 2 (2) | 6 (5) |
| Aortic dissection type A | 2 (2) | 2 (2) |
| Pulmonary hypertension | 2 (2) | 0 |
| Intracranial hemorrhage | 1 (1) | 2 (2) |
| Other | 1 (1) | 1 (1) |
| Sepsis | 0 | 1 (1) |
| Cause of death | ||
| No. | 84 | 101 |
| Multiple organ failure | 35 (42) | 17 (17) |
| Brain death | 21 (25) | 9 (9) |
| Refractory arrest | 13 (16) | 67 (66) |
| Cardiogenic shock | 10 (12) | 4 (4) |
| Bleeding | 4 (5) | 0 |
| Unknown | 1 (1) | 4 (4) |
| Withdrawal of life-sustaining therapy | 21 (17) | 14 (11) |
| Evaluated for organ donationd | 21 (17) | 3 (2) |
| Accepted for organ donation | 13 (11) | 2 (2) |
| Complications/other events, No./total (%) | ||
| Bleeding—anye | 36/116 (31) | 10/69 (15) |
| Overt | 24/36 (67) | 8/10 (80) |
| Intracranial hemorrhage | 8/36 (22) | 2/10 (20) |
| Fatal | 4/36 (11) | 0/10 |
| Organ lacerations | 4/114 (4) | 3/103 (3) |
| Technicalf | 3/124 (2) | 0/132 |
Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; CPC, cerebral performance category; CPR, cardiopulmonary resuscitation; ECLS, extracorporeal life support; MOF, multiple organ failure syndrome; PCI, percutaneous coronary intervention; ROSC, return of spontaneous circulation.
Defined as a palpable pulse with organized ECG rhythm for at least 20 minutes.
Target temperature management indicates all cooling categories, including intravascular and surface feedback device cooling and ECLS heat exchanger cooling.
PCI was deemed successful if resulting in residual stenosis of less than 50% with Thrombolysis in Myocardial Infarction grade 2 or 3 flow.
Evaluation by the transplant center as a potential donor.
Bleeding complications were assessed based on Thrombolysis in Myocardial Infarction classification21 under “major” category, defined as any intracranial hemorrhage (excluding microhemorrhages <10 mm), fatal bleeding directly resulting in death within 7 days, or overt bleeding associated with a decrease in hemoglobin concentration of 5 g/dL or a 15% absolute decrease in hematocrit.
Any device failures during periresuscitation care, mainly focused on extracorporeal life support components.
Among patients admitted to the hospital, target temperature management was used in 117 of 123 patients (95%) in the invasive strategy group and 61 of 87 (70%) in the standard strategy group (P < .001). Those who did not receive temperature control (6 in the invasive strategy group and 26 in the standard strategy group) either had contraindications (mainly advanced hemodynamic instability) or died early, before reaching the intensive care unit (eTable 2 in Supplement 2).
An invasive assessment with diagnostic angiography was performed in 120 of 123 admitted patients (98%) in the invasive strategy group and 67 of 87 (77%) in the standard strategy group (P < .001), corresponding mainly to coronary angiography. Immediate PCI was performed successfully in 56 of 62 patients (90%) in the invasive strategy group and 24 of 30 (80%) in the standard strategy group (P = .20). Of note, in 3 patients, emergency balloon aortic valvuloplasty was performed. On admission, patients in invasive strategy vs standard strategy group had lower pH (median, 6.93 [IQR, 6.8-7.1] vs 7.03 [IQR, 6.9-7.2]; P = .001) and higher serum lactate levels (median, 12.5 [IQR, 9.2-16] mmol/L vs 10.4 [IQR, 7.5-13.5] mmol/L; P = .01).
Cause of death was different between the groups, with multiple organ failure syndrome being the most frequent cause in the invasive strategy group (35/84 [42%]) and refractory arrest in the standard strategy group (67/101 [66%]). Withdrawal of life-sustaining therapies occurred in 21 of 124 patients (17%) in the invasive strategy group and 14 of 132 (11%) in the standard strategy group. Organ donation, both considered and accepted, was more frequent in the invasive strategy group (Table 3).
In the invasive strategy group, 11 of 124 patients (9%) were declared dead on scene or during transport or died within 1 hour after admission, compared with 64 of 132 (49%) in the standard strategy group (P < .001). Thirty-four of 124 patients (27%) in the invasive strategy group and 58 of 132 (44%) in the standard strategy group achieved sustained ROSC (P = .01). For details of resuscitation outcomes, see Table 3 and eFigure 2 in Supplement 2.
Complications
In the invasive strategy group, more major bleeding events were observed (31% vs 15%), including fatal, intracranial, and overt bleeds (Table 3). By contrast, organ lacerations caused by CPR occurred in 4 patients (3.5%) in the invasive strategy group and 3 (2.9%) in the standard strategy group, and technical complications occurred in 3 patients (2.4%) in the invasive strategy group and 0 patients in the standard strategy group (eTables 3 and 4 in Supplement 2). Protocol deviations are described in eTable 5 in Supplement 2.
Additional Analyses
ECPR Outcomes and Crossover Groups
ECPR for ongoing refractory cardiac arrest at admission to the hospital was implemented in 10 patients in the standard strategy group, exclusively in those crossed over to the invasive strategy (10 of 11 crossovers; 1 reached sustained ROSC en route), and in 82 of 124 patients (66%) randomized to the invasive strategy group. Three patients in the invasive strategy group implanted with ECLS died within 1 hour after admission. Among those who ultimately received ECPR, survival with a favorable neurologic outcome at 180 days occurred in 4 of 10 (40%) of those crossed over from the standard strategy group to the invasive strategy group and in 16 of 82 (20%) who were randomized to the invasive group and received ECPR, corresponding to overall neurologically favorable outcome at 180 days of 22% (20/92 patients) when patients who received ECPR from both groups are pooled. All other patients in the standard strategy group who did not obtain stable ROSC and were not crossed over died.
While 5 of 11 patients (45%) who were randomized to the standard strategy and crossed over to the invasive approach had favorable neurologic outcome at 180 days, no patient who was randomized to the invasive strategy group and crossed over to standard resuscitation survived (n = 9).
Survival to 180 Days
Of the 256 participants, 68 (27%) survived to 180 days with favorable neurologic outcome. Comparison of 180-day Kaplan-Meier survival analysis in the entire invasive strategy and standard strategy groups is shown in eFigure 3 in Supplement 2.
Subgroup Analysis
Post hoc subgroup analysis is provided in Figure 2. Details of number of patients in different times of CPR subgroups with favorable neurologic outcome are reported in eFigure 4 in Supplement 2.
Figure 2. Post Hoc Analysis, Primary Outcome According to Subgroups in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest.
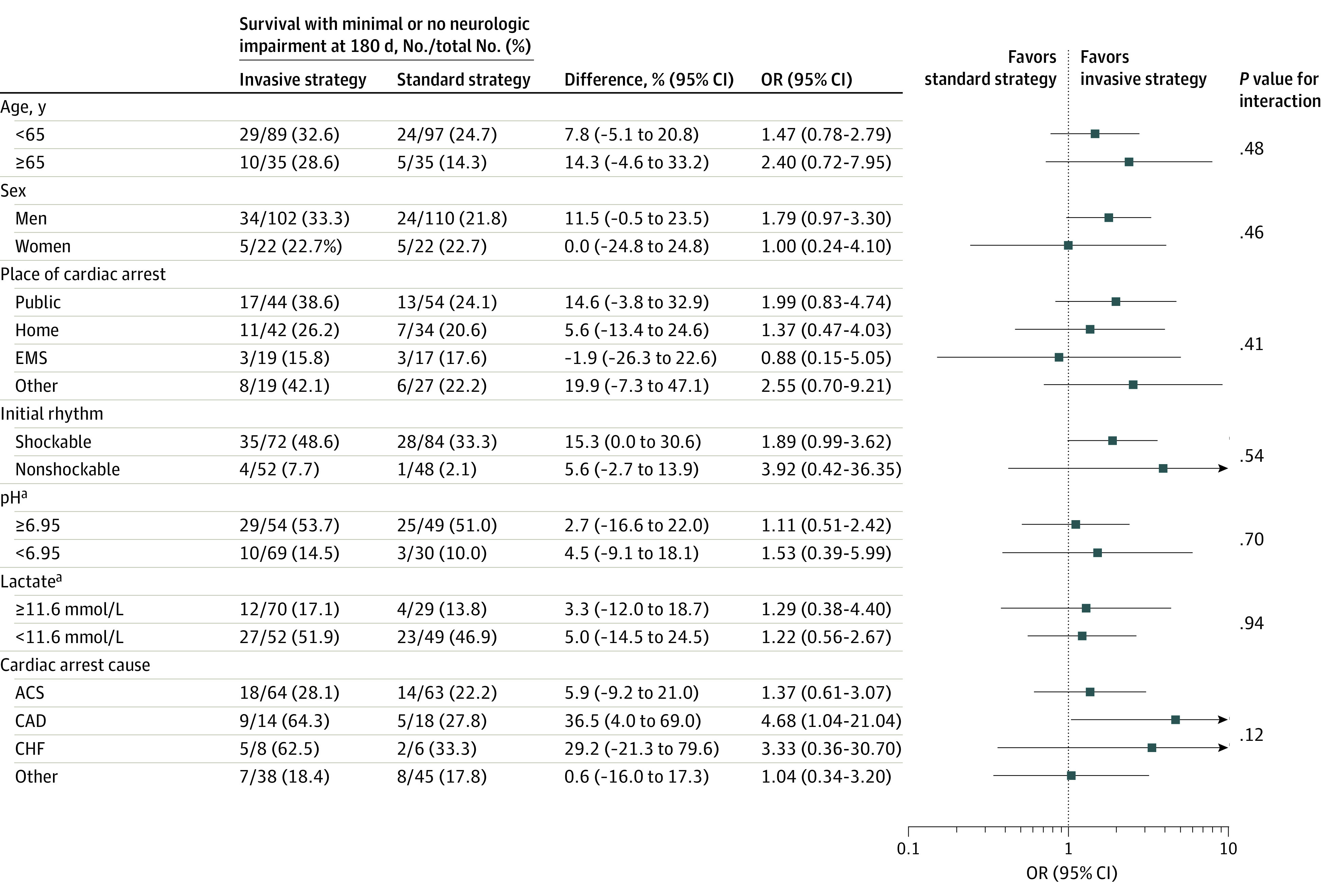
ACS indicates acute coronary syndrome; CAD, coronary artery disease; CHF, chronic heart failure; CPR, cardiopulmonary resuscitation; EMS, emergency medical service; OR, odds ratio; ROSC, return of spontaneous circulation.
aFor pH and lactate level, the first values after admission are used.
Discussion
In this single-center randomized clinical trial, an invasive strategy encompassing the bundle of early intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and invasive assessment in refractory out-of-hospital cardiac arrest of presumed cardiac origin did not significantly improve 180-day survival with favorable neurologic outcome compared with standard care. The study was terminated after enrolling 256 patients by the decision of the data and safety monitoring board, while reaching a stopping rule within prespecified scenarios. However, considering wide confidence intervals in the between-group difference for the primary outcome, the study may have been underpowered to detect a clinically important difference in favor of the invasive strategy group.
In the predefined secondary outcome analysis, a significantly improved 30-day neurologic recovery defined as CPC 1 or 2 was shown in favor of invasive strategy, in contrast to cardiac recovery, which was not statistically different between the groups. Invasive approach was associated with an increased risk of bleeding complications, an inherent complication of ECPR.23
Prague Emergency Medical Service is a single emergency service that covers the area of Prague, serving 1.25 million individuals, and operates with 1 dispatch center using a rapid response vehicle system with an emergency physician. Approximately 500 to 600 resuscitated cardiac arrests occur in Prague each year,24 and patients with presumed cardiac etiology who achieve ROSC are distributed to several cardiac centers. During the study period, randomized patients constituted 6% of all persons who experienced cardiac arrest and received CPR (Figure 1). This is comparable to the proportions in Vienna and other studies that have suggested 4% to 6% of OHCAs to be suitable for an intra-arrest transport approach.25,26 However, in these studies, potential candidates were evaluated retrospectively, whereas in this study, patients were evaluated during ongoing on-scene CPR. More than 90% of bystander CPR in this study affirms previously reported generally high percentage of bystander CPR in Prague,27 in line with more than 77% of patients receiving concurrently telephone-assisted CPR. Patients were randomized after a median of 24 (IQR, 21-30) and 26 (IQR, 19-31) minutes of ongoing cardiac arrest, thus including approximately 15 minutes of advanced cardiac life support. This is a reasonable time to consider rescue interventions such as ECPR followed by immediate coronary reperfusion.22,28 Patients experienced true refractory OHCAs, with many being resuscitated for more than 45 minutes in both groups while a still substantial proportion of patients ultimately achieved sustained ROSC.
Until now, to our knowledge, only 1 small, randomized study (ARREST) in refractory OHCA has been published.10 The study was prematurely stopped after 30 randomized patients based on a recommendation of the data and safety monitoring board because of superiority of early extracorporeal membrane oxygenation (ECMO)–facilitated resuscitation vs standard advanced cardiac life support treatment. The ARREST trial showed that ECMO-facilitated resuscitation for patients with OHCA and refractory ventricular fibrillation significantly improved survival to hospital discharge and functional status compared with patients receiving standard advanced cardiac life support (6/14 patients [43%] vs 1/15 [7%]; risk difference, 36.2% [95% CI, 3.7% to 59.2%]; posterior probability of ECMO superiority, 0.9861). Cumulative 6-month survival was also significantly better in the early ECMO group.10 The ARREST study differed from the present study mainly in 2 aspects: only patients presenting with shockable rhythms were considered, and patients were randomized after being transferred to the hospital, ie, after approximately 50 minutes of CPR. In contrast, the present study randomized patients during on-scene ongoing CPR, thus comparing different treatment scenarios to consider at the point of impending refractoriness, rather than ultimate rescue option after 50 minutes of unsuccessful CPR, when a standard approach has negligible chance for success.3,28,29
An ongoing question related to intra-arrest transport and early invasive treatment for refractory OHCA is the timing of when such an approach should be considered. In this study, the timeline that was adhered to matched the timeline as planned in the protocol and probably represents a realistic timeline in semicrowded urban areas using in-hospital ECPR for OHCA. Patients were admitted within a median of 49 (IQR, 44-60) minutes of collapse in the invasive strategy group, representing approximately 26 minutes of retrieval and transport from the scene to the hospital. The initial decision process to randomize patients after adequate time allowing to achieve ROSC prehospitally thus well correlates with the proposed 16 minutes of professional on-scene CPR22 and may be considered a satisfactory approach to select truly refractory cases, given that 64% of patients in this study experienced cardiac arrest longer than 45 minutes.
Still, converting on-scene CPR into intra-arrest transport eventually followed by ECPR may not improve outcome.3,26 Questions remain as to whether it is possible to identify patients early during CPR who may ultimately benefit from such an approach. Several studies have assessed the relationship between the length of cardiac arrest and ECPR treatment.28,29,30
To our knowledge, there have been no other studies in a cardiac arrest population that randomized patients online via a web-based randomization process during ongoing on-scene CPR. The overall pooled neurologically favorable survival at 180 days of 27% (31.5% in the invasive strategy group, 22% in the standard strategy group, 22% in the pooled ECPR group) is comparable to that in other nonrandomized studies evaluating ECPR (29%31 and 33%32).
If an early invasive approach is to be considered, it should be provided in a well-functioning prehospital system linked to a cooperating ECPR cardiac arrest center.33
Studies of refractory OHCA treated by ECPR inherently address potential organ donation34,35; potential donors were frequently considered, and organ donations occurred.
Limitations
This study has several limitations. First, the study had a single-center design and limited enrollment. Second, a priori scenarios of expected benefit provided by invasive approach were not reached, presumably because of higher-than-expected survival in the standard strategy group. Third, the study may have thus been underpowered to detect a statistically significant difference for the primary outcome. Fourth, the study design allowed crossover. The trial was designed to represent routine clinical care, and EMS crews thus decided to transport some patients receiving ongoing CPR for ECPR despite being originally randomized to the standard strategy group. For crossover from invasive to standard intervention, patients were apparently deemed not to be candidates for advanced therapies, but such determinations may contain a degree of subjectivity that could influence outcomes. Nonetheless, the rate of crossover was low (7.5%) compared with other studies.36,37
Conclusions
Among patients with refractory out-of-hospital cardiac arrest, the bundle of early intra-arrest transport, ECPR, and invasive assessment and treatment did not significantly improve survival with neurologically favorable outcome at 180 days compared with standard resuscitation. However, the trial was possibly underpowered to detect a clinically relevant difference.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Study Protocol and Summary of Changes
eTable 1. Study inclusion and Exclusion Criteria in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eFigure 1. Stopping Rule in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eFigure 2. Allocation and Resuscitation Outcomes Flow Chart in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eFigure 3. Kaplan-Meier Survival Analysis in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eFigure 4. Favorable Neurological Outcome After 180 Days in Time of CPR Subgroups in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eTable 2. Causes of Target Temperature Management Exclusion in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eTable 3. Organ Lacerations in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eTable 4. Technical Complications in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eTable 5. Summary of all Protocol Deviations in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
DSMB letter
Group Information: Prague OHCA Study Group
Data Sharing Statement
References
- 1.Perkins GD, Graesner JT, Semeraro F, et al. ; European Resuscitation Council Guideline Collaborators . European Resuscitation Council Guidelines 2021: executive summary. Resuscitation. 2021;161:1-60. doi: 10.1016/j.resuscitation.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 2.Dankiewicz J, Cronberg T, Lilja G, et al. ; TTM2 Trial Investigators . Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med. 2021;384(24):2283-2294. doi: 10.1056/NEJMoa2100591 [DOI] [PubMed] [Google Scholar]
- 3.Grunau B, Kime N, Leroux B, et al. Association of intra-arrest transport vs continued on-scene resuscitation with survival to hospital discharge among patients with out-of-hospital cardiac arrest. JAMA. 2020;324(11):1058-1067. doi: 10.1001/jama.2020.14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drennan IR, Lin S, Sidalak DE, Morrison LJ. Survival rates in out-of-hospital cardiac arrest patients transported without prehospital return of spontaneous circulation: an observational cohort study. Resuscitation. 2014;85(11):1488-1493. doi: 10.1016/j.resuscitation.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 5.de Graaf C, Beesems SG, Koster RW. Time of on-scene resuscitation in out of-hospital cardiac arrest patients transported without return of spontaneous circulation. Resuscitation. 2019;138:235-242. doi: 10.1016/j.resuscitation.2019.03.030 [DOI] [PubMed] [Google Scholar]
- 6.Schober A, Sterz F, Herkner H, et al. Emergency extracorporeal life support and ongoing resuscitation: a retrospective comparison for refractory out-of-hospital cardiac arrest. Emerg Med J. 2017;34(5):277-281. doi: 10.1136/emermed-2015-205232 [DOI] [PubMed] [Google Scholar]
- 7.Ortega-Deballon I, Hornby L, Shemie SD, Bhanji F, Guadagno E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: a systematic review of international practices and outcomes. Resuscitation. 2016;101:12-20. doi: 10.1016/j.resuscitation.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 8.Haas NL, Coute RA, Hsu CH, Cranford JA, Neumar RW. Descriptive analysis of extracorporeal cardiopulmonary resuscitation following out-of-hospital cardiac arrest—an ELSO registry study. Resuscitation. 2017;119:56-62. doi: 10.1016/j.resuscitation.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmberg MJ, Geri G, Wiberg S, et al. ; International Liaison Committee on Resuscitation’s (ILCOR) Advanced Life Support and Pediatric Task Forces . Extracorporeal cardiopulmonary resuscitation for cardiac arrest: a systematic review. Resuscitation. 2018;131:91-100. doi: 10.1016/j.resuscitation.2018.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396(10265):1807-1816. doi: 10.1016/S0140-6736(20)32338-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis M, Lal S, Forrest P, et al. In-depth extracorporeal cardiopulmonary resuscitation in adult out-of-hospital cardiac arrest. J Am Heart Assoc. 2020;9(10):e016521. doi: 10.1161/JAHA.120.016521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bougouin W, Dumas F, Lamhaut L, et al. ; Sudden Death Expertise Center investigators . Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. Eur Heart J. 2020;41(21):1961-1971. doi: 10.1093/eurheartj/ehz753 [DOI] [PubMed] [Google Scholar]
- 13.Lott C, Truhlář A, Alfonzo A, et al. ; ERC Special Circumstances Writing Group Collaborators . European Resuscitation Council Guidelines 2021: cardiac arrest in special circumstances. Resuscitation. 2021;161:152-219. doi: 10.1016/j.resuscitation.2021.02.011 [DOI] [PubMed] [Google Scholar]
- 14.Belohlavek J, Kucera K, Jarkovsky J, et al. Hyperinvasive approach to out-of-hospital cardiac arrest using mechanical chest compression device, prehospital intraarrest cooling, extracorporeal life support and early invasive assessment compared to standard of care: a randomized parallel groups comparative study proposal. J Transl Med. 2012;10:163. doi: 10.1186/1479-5876-10-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480-484. doi: 10.1016/S0140-6736(75)92830-5 [DOI] [PubMed] [Google Scholar]
- 16.Nolan JP, Soar J, Zideman DA, et al. ; ERC Guidelines Writing Group . European Resuscitation Council Guidelines for Resuscitation 2010: section 1: executive summary. Resuscitation. 2010;81(10):1219-1276. doi: 10.1016/j.resuscitation.2010.08.021 [DOI] [PubMed] [Google Scholar]
- 17.Monsieurs KG, Nolan JP, Bossaert LL, et al. ; ERC Guidelines 2015 Writing Group . European Resuscitation Council Guidelines for Resuscitation 2015: section 1: executive summary. Resuscitation. 2015;95:1-80. doi: 10.1016/j.resuscitation.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 18.Rubertsson S, Lindgren E, Smekal D, et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest: the LINC randomized trial. JAMA. 2014;311(1):53-61. doi: 10.1001/jama.2013.282538 [DOI] [PubMed] [Google Scholar]
- 19.Nielsen N, Wetterslev J, Cronberg T, et al. ; TTM Trial Investigators . Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197-2206. doi: 10.1056/NEJMoa1310519 [DOI] [PubMed] [Google Scholar]
- 20.Gaieski DF, Band RA, Abella BS, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418-424. doi: 10.1016/j.resuscitation.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 21.Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI) phase II trial. Ann Intern Med. 1991;115(4):256-265. doi: 10.7326/0003-4819-115-4-256 [DOI] [PubMed] [Google Scholar]
- 22.Grunau B, Reynolds J, Scheuermeyer F, et al. Relationship between time-to-ROSC and survival in out-of-hospital cardiac arrest ECPR candidates: when is the best time to consider transport to hospital? Prehosp Emerg Care. 2016;20(5):615-622. doi: 10.3109/10903127.2016.1149652 [DOI] [PubMed] [Google Scholar]
- 23.Thomas J, Kostousov V, Teruya J. Bleeding and thrombotic complications in the use of extracorporeal membrane oxygenation. Semin Thromb Hemost. 2018;44(1):20-29. doi: 10.1055/s-0037-1606179 [DOI] [PubMed] [Google Scholar]
- 24.Franěk O, Pokorná M, Sukupová P. Pre-hospital cardiac arrest in Prague, Czech Republic—the Utstein-style report. Resuscitation. 2010;81(7):831-835. doi: 10.1016/j.resuscitation.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 25.Poppe M, Weiser C, Holzer M, et al. The incidence of “load&go” out-of-hospital cardiac arrest candidates for emergency department utilization of emergency extracorporeal life support: a one-year review. Resuscitation. 2015;91:131-136. doi: 10.1016/j.resuscitation.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 26.Alm-Kruse K, Sørensen G, Osbakk SA, et al. Outcome in refractory out-of-hospital cardiac arrest before and after implementation of an ECPR protocol. Resuscitation. 2021;162:35-42. doi: 10.1016/j.resuscitation.2021.01.038 [DOI] [PubMed] [Google Scholar]
- 27.Gräsner JT, Wnent J, Herlitz J, et al. Survival after out-of-hospital cardiac arrest in Europe: results of the EuReCa TWO study. Resuscitation. 2020;148:218-226. doi: 10.1016/j.resuscitation.2019.12.042 [DOI] [PubMed] [Google Scholar]
- 28.Wengenmayer T, Rombach S, Ramshorn F, et al. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care. 2017;21(1):157. doi: 10.1186/s13054-017-1744-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartos JA, Carlson K, Carlson C, et al. Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: critical care and extracorporeal membrane oxygenation management. Resuscitation. 2018;132:47-55. doi: 10.1016/j.resuscitation.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 30.Leick J, Liebetrau C, Szardien S, et al. Door-to-implantation time of extracorporeal life support systems predicts mortality in patients with out-of-hospital cardiac arrest. Clin Res Cardiol. 2013;102(9):661-669. doi: 10.1007/s00392-013-0580-3 [DOI] [PubMed] [Google Scholar]
- 31.Maekawa K, Tanno K, Hase M, Mori K, Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med. 2013;41(5):1186-1196. doi: 10.1097/CCM.0b013e31827ca4c8 [DOI] [PubMed] [Google Scholar]
- 32.Bartos JA, Grunau B, Carlson C, et al. Improved survival with extracorporeal cardiopulmonary resuscitation despite progressive metabolic derangement associated with prolonged resuscitation. Circulation. 2020;141(11):877-886. doi: 10.1161/CIRCULATIONAHA.119.042173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinning C, Ahrens I, Cariou A, et al. The cardiac arrest centre for the treatment of sudden cardiac arrest due to presumed cardiac cause—aims, function and structure: position paper of the Association for Acute CardioVascular Care of the European Society of Cardiology (AVCV), European Association of Percutaneous Coronary Interventions (EAPCI), European Heart Rhythm Association (EHRA), European Resuscitation Council (ERC), European Society for Emergency Medicine (EUSEM) and European Society of Intensive Care Medicine (ESICM). Eur Heart J Acute Cardiovasc Care. 2020;9(4 suppl):S193-S202. doi: 10.1177/2048872620963492 [DOI] [PubMed] [Google Scholar]
- 34.Ortega-Deballon I, De La Plaza-Horche E. A comprehensive approach to refractory cardiac arrest: saving more lives one way or another. Heart Lung Vessel. 2014;6(3):149-151. [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega-Deballon I, Hornby L, Shemie SD. Protocols for uncontrolled donation after circulatory death: a systematic review of international guidelines, practices and transplant outcomes. Crit Care. 2015;19:268. doi: 10.1186/s13054-015-0985-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiele H, Akin I, Sandri M, et al. ; CULPRIT-SHOCK Investigators . PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419-2432. doi: 10.1056/NEJMoa1710261 [DOI] [PubMed] [Google Scholar]
- 37.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet . Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965-1975. doi: 10.1056/NEJMoa1800385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol and Summary of Changes
eTable 1. Study inclusion and Exclusion Criteria in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eFigure 1. Stopping Rule in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eFigure 2. Allocation and Resuscitation Outcomes Flow Chart in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eFigure 3. Kaplan-Meier Survival Analysis in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eFigure 4. Favorable Neurological Outcome After 180 Days in Time of CPR Subgroups in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eTable 2. Causes of Target Temperature Management Exclusion in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eTable 3. Organ Lacerations in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eTable 4. Technical Complications in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
eTable 5. Summary of all Protocol Deviations in a Study of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment in Refractory Out-of-Hospital Cardiac Arrest
DSMB letter
Group Information: Prague OHCA Study Group
Data Sharing Statement