Key Points
Question
Is sublingual dexmedetomidine effective in treating acute agitation associated with bipolar disorder?
Findings
In this randomized clinical trial that included 380 adults with bipolar disorder and mild to moderate agitation, treatment with sublingual dexmedetomidine 180 μg, 120 μg, or placebo resulted in a mean change in the Positive and Negative Syndrome Scale-Excited Component (PEC) total score at 2 hours after treatment of −10.4, −9.0, and −4.9, respectively (possible PEC total score range, 5-35). The differences between each dose and placebo were statistically significant.
Meaning
Sublingual dexmedetomidine at a dose of 180 μg or 120 μg reduced mild to moderate agitation in patients with bipolar disorder.
Abstract
Importance
Acute agitation is common in patients with bipolar disorder and requires urgent management to relieve distress and to prevent escalation to aggressive behavior.
Objective
To evaluate the effect of orally absorbed, sublingual dexmedetomidine, a selective α2A-adrenergic receptor agonist on symptoms of acute agitation in patients with bipolar disorder.
Design, Setting, and Participants
Phase 3, randomized, double-blind, placebo-controlled trial conducted in 15 sites in the US with enrollment between February 24, 2020, and April 27, 2020, and final follow-up on May 21, 2020. A total of 380 adults with bipolar I or II disorder were randomized and 362 completed the study.
Interventions
Participants were randomized to 3 groups: sublingual dexmedetomidine 180 μg (n = 127), sublingual dexmedetomidine 120 μg (n = 127), or placebo (n = 126).
Main Outcomes and Measures
The primary efficacy end point was the mean change from baseline at 2 hours for the Positive and Negative Syndrome Scale-Excited Component (PEC) total score. The range of possible total scores is 5 (absence of agitation) to 35 (extremely severe). The secondary end point was the earliest time of a statistically significant change in PEC total score from baseline for the drug vs placebo. On the primary efficacy end point, to account for multiplicity associated with comparing 2 sublingual dexmedetomidine doses with placebo, the 2-sided significance level for each dose vs placebo was set at .025.
Results
Of 380 patients randomized (mean age, 45.6 years; 54.8% women; and 56.1% Black individuals), 378 (99.5%) self-administered the study medication and completed the study. Baseline agitation was mild to moderate, with an overall mean PEC total score of 18.0. Two hours after taking the medication, the mean changes from baseline in PEC total score were −10.4 for sublingual dexmedetomidine 180 μg, −9.0 for sublingual dexmedetomidine 120 μg, and −4.9 for placebo. Least-square mean differences from placebo in the sublingual dexmedetomidine groups at 2 hours were −5.4 (97.5% CI, −6.6 to −4.2) for 180 μg and −4.1 (97.5% CI, −5.3 to −2.9) for 120 μg (both doses P < .001 vs placebo). Treatment effects began 20 minutes after taking the medication among patients in the sublingual dexmedetomidine groups (least-square mean difference for 180 μg, −1.1 [97.5% CI, −2.0 to −0.2]; P = .007; for 120 μg, −1.0 [97.5% CI, −1.9 to −0.1]; P = .009). Adverse events occurred in 35.7% of patients taking 180 μg of dexmedetomidine, 34.9% taking 120 μg, and 17.5% taking placebo. The most common adverse events (≥5%) in the respective 180 μg, 120 μg, and placebo groups were somnolence (21.4% and 20.6% vs 4.8%); dry mouth (4.8% and 7.1% vs 0.8%); hypotension (6.3% and 4.8% vs 0%); and dizziness (5.6% and 5.6% vs 0.8%).
Conclusions and Relevance
Among patients with mild to moderate agitation associated with bipolar disorder, treatment with a sublingual film formulation of dexmedetomidine 120 μg or 180 μg, compared with placebo, resulted in significantly greater reduction in the agitation score at 2 hours. Further research is needed to understand the spectrum of patients for whom this treatment would be effective and feasible and to better understand the clinical importance of the observed effect size.
Trial Registration
ClinicalTrials.gov Identifier: NCT04276883
This randomized clinical trial evaluates whether single dose of sublingual dexmedetomidine reduces symptoms of acute agitation associated with bipolar disorder.
Introduction
Patients with acute agitation are commonly encountered by physicians and other health care personnel across disciplines and settings. Agitation is characterized by a range of motor, emotional, behavioral, and ideational symptoms and can be associated with neurological, psychiatric, and general medical conditions.1 A prospective observational study in an urban trauma center that screened more than 43 000 patients found a prevalence of agitation in the emergency department of 2.6%.2 According to the National Hospital Ambulatory Medical Care Survey, there were an estimated 215 000 emergency department visits for bipolar disorder in 2018 and episodes of agitation associated with bipolar disorder are common.3 Symptoms can range from mild (uneasiness, restlessness) to severe (aggression, violence) and escalation can occur quickly.1,4,5,6 Patients presenting with agitation require prompt medical attention to facilitate evaluation and to prevent escalation and injury to themselves and medical personnel.1,3,5,6
Recent guidance for the management of agitation6 recommends patient-centered approaches, in which verbal and nonverbal deescalation techniques are used and less invasive treatments are preferred when possible.3,7,8,9
When pharmacotherapy is needed, antipsychotics, benzodiazepines, and ketamine are commonly used,10 although only intramuscular olanzapine and inhaled loxapine are formally indicated for the treatment of agitation associated with bipolar disorder.3,8,9 The goal of pharmacological treatment should be to induce calm without oversedation, which can prevent timely assessment and triage.3
Sublingual dexmedetomidine is a rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dexmedetomidine is an α2-adrenergic receptor agonist approved in intravenous form for procedural sedation and anesthesia.11 The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.12 This formulation has demonstrated dose-dependent exposure and a plasma half-life between 2 and 3 hours.13 The objective of this study was to determine if a single dose of sublingual dexmedetomidine reduces symptoms of acute agitation associated with bipolar disorder.
Methods
This study was conducted in accordance with the principles of the Guidelines for Good Clinical Practice, the Declaration of Helsinki, and all applicable local regulations. The protocol was approved by independent ethics committees and/or institutional review boards at each study center and by a central institutional review board (Advarra Inc). Participants provided written informed consent before any study procedures were undertaken. The study protocol is available in Supplement 1 and the statistical analysis plan is available in Supplement 2.
Study Design
This randomized, double-blind, placebo-controlled study, which was conducted between February and May 2020 at 15 sites in the US (eTable 2 in Supplement 3), evaluated the safety and efficacy of sublingual dexmedetomidine in the treatment of acute agitation in adults with bipolar disorder. The study included a screening visit, treatment visit (day 1), follow-up visit (day 2), discharge (day 3), and end of study visit (day 7). Patients were confined to a clinical research setting or hospitalized under medical supervision while undergoing screening procedures, and they had to remain in the clinical unit until at least the morning of day 3.
Patient Population
Patients aged 18 through 75 years were eligible if they had acute agitation associated with bipolar I or II disorder (Diagnostic and Statistical Manual of Mental Disorders [Fifth Edition]), regardless of polarity (manic, mixed features, or depressed). The Mini-International Neuropsychiatric Interview was administered at screening.14 Patients were identified in outpatient clinics; mental health, psychiatric, or medical emergency services including medical or psychiatric observation units; or as patients newly admitted to a hospital setting for acute agitation or already hospitalized for chronic underlying conditions. Patients had a total score of 14 or higher on the 5 items of the Positive and Negative Syndrome Scale (PANSS) excited component (PEC)15 scale at screening and baseline, and a score of 4 or higher on at least 1 of the 5 PEC items at baseline. Detailed eligibility criteria are available in the study protocol (Supplement 1).
During intake, participants self-reported race from among fixed categories (African American or Black, American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, White, other, or multiple) and ethnicity from fixed categories (Hispanic or Latino and not Hispanic or Latino). Self-identified race and ethnicity are included in Table 1, along with age, sex, and other characteristics of the study sample to inform the assessment of generalizability of the results.
Table 1. Demographics and Baseline Characteristics.
| No. (%) | |||
|---|---|---|---|
| Sublingual dexmedetomidine | Placebo (n = 126) | ||
| 180 μg (n = 126) | 120 μg (n = 126) | ||
| Age, y | |||
| Mean (SD) | 45.9 (11.3) | 46.1 (11.5) | 44.8 (12.1) |
| Median (range) | 47 (18-69) | 49 (19-70) | 48 (18-67) |
| Women | 67 (53.2) | 67 (53.2) | 73 (57.9) |
| Men | 59 (46.8) | 59 (46.8) | 53 (42.1) |
| Race | |||
| Asian | 1 (0.8) | 0 | 2 (1.6) |
| Black or African American | 72 (57.1) | 68 (54.0) | 72 (57.1) |
| Multiple | 3 (2.4) | 1 (0.8) | 1 (0.8) |
| White | 49 (38.9) | 56 (44.4) | 50 (39.7) |
| Othera | 1. (0.8) | 1 (0.8) | 1 (0.8) |
| Hispanic or Latino | 15 (11.9) | 12 (9.5) | 11 (8.7) |
| Body weight, kg | |||
| Mean (SD) | 96.8 (26.0) | 91.8 (25.9) | 92.0 (20.7) |
| Median (range) | 93.8 (54.8-200.1) | 87.6 (49.9-274.0) | 90.7 (48.0-150.8) |
| BMI | |||
| Mean (SD) | 33.3 (8.7) | 31.6 (8.0) | 32.5 (7.4) |
| Median (range) | 31.7 (19.0-66.9) | 29.8 (18.0-78.4) | 31.8 (16.8-59.6) |
| Diagnosis | |||
| Mania | 59 (46.8) | 58 (46.0) | 63 (50.0) |
| Depressed | 28 (22.2) | 20 (15.9) | 26 (20.6) |
| Mixed episodes | 30 (23.8) | 27 (21.4) | 22 (17.5) |
| Hypomania | 5 (4.0) | 14 (11.1) | 10 (7.9) |
| Unspecified | 4 (3.2) | 7 (5.6) | 5 (4.0) |
| Current agitation episode, d | |||
| Mean (SD) | 25.1 (74.3) | 21.8 (31.3) | 15.7 (21.8) |
| Median (range) | 9 (1-730) | 7 (1-192) | 7 (1-132) |
| No. of hospitalizations | |||
| Mean (SD) | 2.8 (4.4) | 3.5 (4.7) | 2.8 (3.7) |
| Median (range) | 1 (0-20) | 2 (0-22) | 2 (0-20) |
| Hours of sleep/night this wk | |||
| Mean (SD) | 5.1 (1.5) | 5.3 (1.6) | 5.1 (1.5) |
| Median (range) | 5 (1-9) | 5 (2-10) | 5 (1-10) |
| Current smoker | 78 (61.9) | 97 (77.0) | 83 (65.9) |
| PEC scoreb | |||
| Mean (SD)c | 18.0 (3.0) | 18.0 (2.7) | 17.9 (2.9) |
| Median (range) | 17.5 (14-28) | 17.0 (14-27) | 17.0 (14-30) |
| CGI-Sd | |||
| Mean (SD)c | 4.1 (0.7) | 4.1 (0.5) | 4.1 (0.6) |
| Median (range) | 4 (2-6) | 4 (3-5) | 4 (1-6) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CGI-S, Clinical Global Impressions–Severity of Illness; PEC, Positive and Negative Syndrome Scale-Excited Component.
Participants self-identified race and ethnicity. No participant identified themselves as American Indian or Alaska Native, Native Hawaiian, or Other Pacific Islander. A participant could select the category “other” if none of the other categories was appropriate.
Comprises 5 items with a range of 5 (absence of agitation) to 35 (extremely severe).
The mean baseline PEC score in these data corresponds to the CGI-S score of moderately ill. A moderately ill individual would have PEC subscale items of moderate severity (poor impulse control, tension, hostility, uncooperativeness, and excitement).
Scores are rated on a 7-point scale, with a range of responses from 1 (normal) to 7 (among the most severely ill patients).
Randomization and Blinding
Patients were randomized (1:1:1) to sublingual dexmedetomidine 180 μg, sublingual dexmedetomidine 120 μg, or matching placebo film. Randomization was computer generated based on a permuted block design and stratified by age (<65, ≥65 years) with a block size of 6. Patients, investigators, and study staff were blinded to the identity of the assigned treatment.
Intervention
Patients were instructed on the appropriate method of self-administering sublingual dexmedetomidine (BXCL501, BioXcel Therapeutics). The study drug was self-administered under the supervision of a staff member. In the event of persistent or recurrent agitation, a repeat dose of 90 μg or 60 μg could be given 2 hours after the first dose, at the investigators’ discretion, if the change from baseline on the PEC scale was less than 40% and if there were no safety concerns (eg, blood pressure drop or sedation). The maximum number of repeat doses per patient was 2 during the 12 hours after the first dose.
Outcomes
The primary efficacy end point was the absolute change from baseline in the PEC total score at 2 hours after taking the medication. The PEC comprises 5 items associated with agitation—poor impulse control, tension, hostility, uncooperativeness, and excitement—with each item rated from 1 (minimum) to 7 (maximum). The PEC total score is the sum of these 5 items and ranges from 5 (absence of agitation) to 35 (extremely severe). The PEC was administered at screening, within 15 minutes of administering the dose and at 10, 20, 30, 45 minutes, and 1, 1.5, 2, 4, 6, 8, and 24 hours after taking the dose; 6 and 24 hours after taking the dose, the PEC was given before the Positive and Negative Syndrome Scale interview. The PEC was done prior to any other assessments.
The secondary end point was the earliest time at which the absolute change from baseline in the PEC total score statistically significantly separated from placebo.
There were several prospectively defined exploratory end points. Overall clinical improvement after treatment was evaluated at 30 minutes, 1, 2, and 4 hours after taking the dose using the Clinical Global Impression–Improvement (CGI-I)9 scale, with possible scores ranging from 1 (very much improved) to 7 (very much worse). Overall agitation and sedation were assessed before (within 15 minutes of dosing), and at 2, 4, and 8 hours after using the Agitation-Calmness Evaluation Scale (ACES), a single-item rating scale where 1 indicates marked agitation and 9, unarousable. The change from baseline in total PEC score from 10 minutes through 24 hours after dosing and PEC response rate, defined as a 40% or more reduction in total score from 10 minutes through 2 hours after dosing were assessed. The percent change from baseline was calculated as 100 times the change from predose score divided by (baseline −5); by subtracting 5 from the item scores, a score of 0 was associated with values of “not present.” The choice of a 40% or more change in PEC from baseline to define response was based on an external validation of the PEC,15 which found that a change in PEC of 38% corresponded to a CGI-I rating of “much improved.” The CGI-I response rate, defined as a score of 1 (very much improved) or 2 (much improved) 2 hours after dosing, was evaluated with the CGI-I scale. Time to rescue medication from baseline through 24 hours and the number of patients requiring rescue medication from 4 to 24 hours after taking the medication were used to assess patient need for rescue medication. The change from baseline for PEC total score at 4, 6, 8, and 24 hours was used to evaluate the duration of calming effect.
Other assessments included spontaneously reported adverse events; clinical laboratory tests (chemistry, hematology, urinalysis); electrocardiogram (ECG); pulse oximetry; and vital signs (systolic blood pressure [SBP], diastolic blood pressure [DBP], and heart rate). All ECG measurements were evaluated for abnormality by machine-derived algorithms and overread by blinded cardiologists. The criteria for ECG abnormality are included in eTable 3 of Supplement 3. All adverse events were characterized by type, severity, seriousness, and relationship to treatment and coded by preferred term and system organ class using the Medical Dictionary for Regulatory Activities, version 23.0. A buccal examination was performed 30 minutes and 2, 4, and 24 hours after taking the medication for local irritation. The Columbia-Suicide Severity Rating Scale16 was administered at screening, baseline, 24 hours, and discharge.
Sample Size Calculation
The sample size for this study was estimated using information from the phase 1b clinical trial of sublingual dexmedetomidine (BXCL501-102) regarding the standard deviation of the change from baseline in the PEC score and the expected magnitude of this difference.17 For a 2-sided significance level of .025 and a randomization ratio of 1:1:1, 125 patients per treatment group were needed to provide 90% statistical power for the sublingual dexmedetomidine–placebo pairwise comparison on the primary efficacy end point, of a difference of 2 or more in the change from baseline in the PEC total score (Supplement 2).17
Statistical Analysis
For the efficacy analysis, the null and alternate hypotheses were tested using a mixed-model repeated measures.18 On the primary efficacy end point, to account for multiplicity associated with comparing 2 sublingual dexmedetomidine doses with placebo, the 2-sided significance level for each dose vs placebo was determined using the Bonferroni correction and set at .025. The outcome variable for the mixed-model repeated measures was the change from baseline in the PEC score at 10, 20, 30, 45, 60, 90, and 120 minutes after taking the medication. Covariates were treatment group, baseline PEC, visit, baseline PEC × visit interaction term, age stratum, study site (as a fixed effect), and treatment group × visit interaction term. A post hoc analysis of the primary efficacy end point was also conducted with site as a random effect. The difference in the mean change from baseline in each sublingual dexmedetomidine group relative to placebo, as well as the significance levels associated with the null hypotheses, were obtained from differences in least-squares means at each time point.
To control for multiple comparisons on secondary end points and assess the onset of treatment effect, a hierarchical testing procedure and a prespecified sequence of comparisons was used; starting at 90 minutes after dosing, each earlier end point was tested in succession until a comparison with placebo failed to achieve statistical significance at the .025 level. According to the gatekeeping rules of the hierarchy, no tests were performed on end points following a comparison that failed to demonstrate a treatment effect.
The study was not designed with sufficient power to assess safety end points; adverse events were analyzed for all patients who received a dose of the study drug and data were analyzed according to participant randomization group.
Exploratory outcomes were analyzed using a variety of techniques depending on the outcome and including 2-sample t tests, Fisher exact test, and Kaplan-Meier curves for time-to-event outcomes and descriptive methods, such as box plots, frequency distributions, and shift tables. Because of the potential for type I error due to multiple comparisons, findings for exploratory end points should be interpreted as exploratory.
A sensitivity analysis was conducted to assess the effect of any missing data. Control-based, multiple imputation was used to impute missing values based on the placebo group experience. Participants with missing data were considered nonresponders. There were no missing values in the primary or secondary outcome data. For efficacy analyses, all values collected after the use of rescue treatment or withdrawal from the study were censored and considered nonresponders.
Data were analyzed with SAS/STAT software, version 9.4 (SAS Institute Inc).
Results
Participants
From February 24, 2020, to April 27, 2020, 380 patients were enrolled at 15 hospitals and research units in the US. Of the 380 patients randomized (sublingual dexmedetomidine 180 μg [n = 127], sublingual dexmedetomidine 120 μg [n = 127], placebo [n = 126]), 362 (95.3%) completed the study (Figure 1). One patient in each of the sublingual dexmedetomidine groups was randomized in error and did not receive study drug, leaving 378 efficacy-evaluable patients. Sixteen patients (4.7%) discontinued the study for voluntary withdrawal, adverse events, or loss to follow-up. The majority of patients received 1 dose of the study medication; 10.3% (13 of 126) of patients in the sublingual dexmedetomidine 180 μg group, 23.8% (30 of 126) patients in the sublingual dexmedetomidine 120 μg group, and 46.0% (58 of 126) of patients in the placebo group received at least 2 doses. Rescue medication for persistent agitation was required in 3 patients (2.4%) in the sublingual dexmedetomidine 180 μg group and 2 (1.6%) in both of the sublingual dexmedetomidine 120 μg and placebo groups.
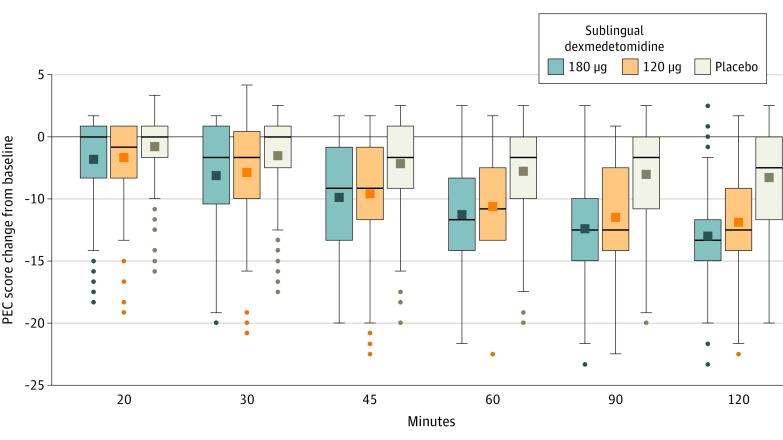
Figure 1. Recruitment, Randomization, and Follow-up of Patientsa.
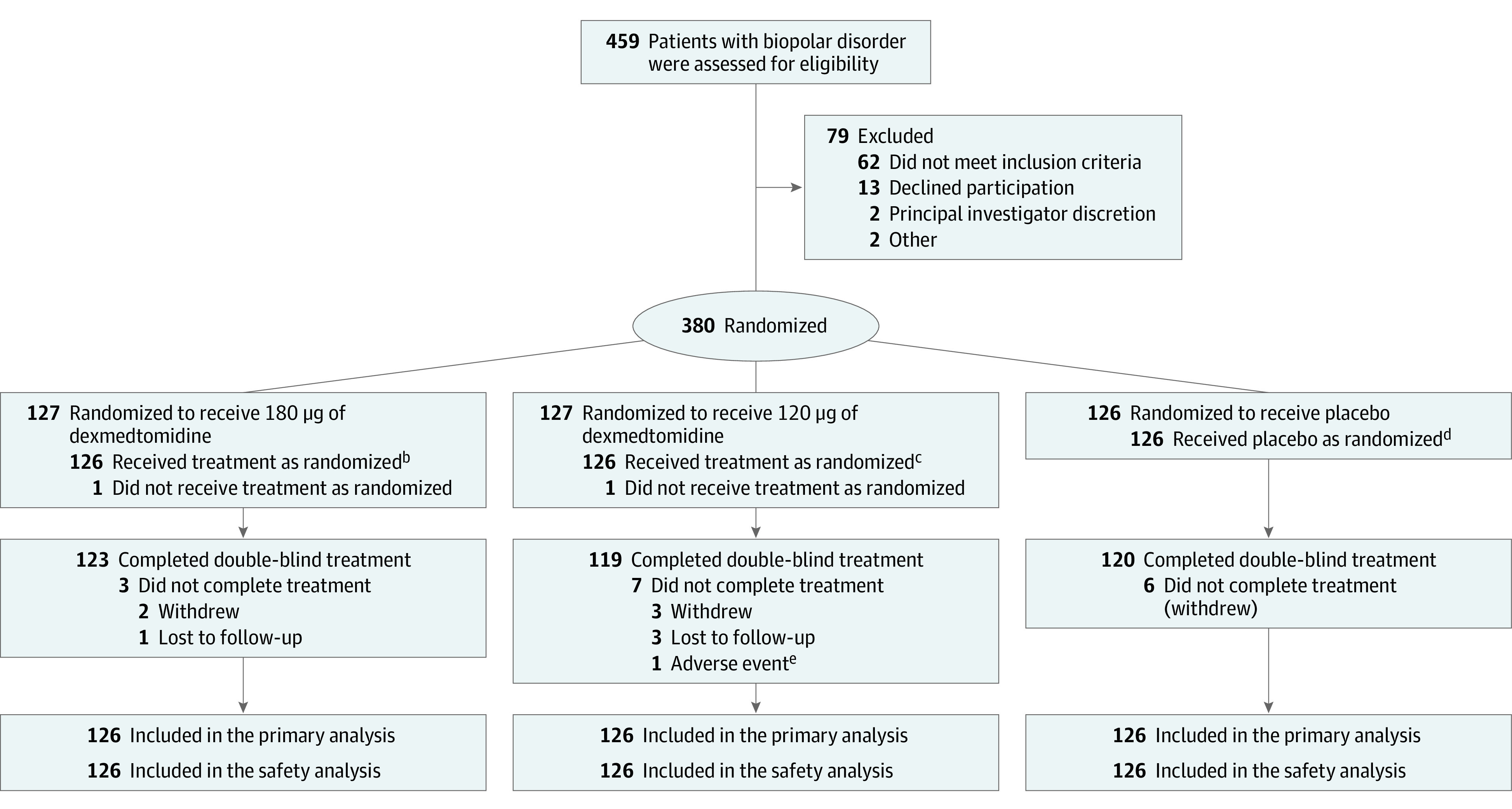
aThis was a single-dose study, and the safety, full analysis, and per-protocol data sets all contained 378 patients—126 in each group. There were no missing data for the primary or secondary outcome measures. One patient each from 180 μg and 120 μg groups did not receive a dose of medication, but all other discontinuations occurred after dosing and primary and secondary end point assessments.
bOne hundred thirteen patients (89.7%) received only 1 dose; 5 (4%), 2 doses; and 8 (6.3%), 3 doses.
cNinety-six patients (76.2%) received only 1 dose; 18 (14.7%), 2 doses; and 12 (9.5%), 3 doses.
dSixty-eight patients (54%) received only 1 dose; 29 (23.0%), 2 doses; and 29 (23%), 3 doses.
eDue to acute agitation on day 7 that was judged to be unrelated to study drug.
Baseline characteristics were comparable between treatment groups, except for a higher number of agitation days experienced by patients assigned to the sublingual dexmedetomidine 180-μg group (Table 1). The study population had a mean (SD) age of 45.6 (11.6) years, 54.8% identified as women, and 56.1% identified as Black or African American individuals. The mean (SD) body mass index was 32.5 (8.0), calculated as weight in kilograms divided by height in meters squared. At screening, 47.6% of patients were diagnosed with mania, 20.9% with mixed episodes, and 19.6% with depressed mood. Eight patients were 65 years or older (sublingual dexmedetomidine 180 μg [n = 3], sublingual dexmedetomidine 120 μg [n = 3], and placebo [n = 2]). Concomitant medications and enrollment by study site are presented in eTable 1 and 2 in Supplement 3.
Primary End Point
The mean (SD) changes from baseline in PEC total score 2 hours after taking the medication were −10.4 (4.4) for sublingual dexmedetomidine 180 μg, −9.0 (5.3) for sublingual dexmedetomidine 120 μg, and −4.9 (4.7) for placebo (Figure 2). The least-squares mean differences from placebo were −5.4 (97.5% CI, −6.6 to −4.2; P < .001) for sublingual dexmedetomidine 180 μg and −4.1 (97.5% CI, −5.3 to −2.9; P < .001) for sublingual dexmedetomidine 120 μg. Results of a post hoc analysis of the primary end point with site as a random effect are presented in eTable 5 in Supplement 3.
Figure 2. Mean Change From Baseline in the Positive and Negative Syndrome Scale-Excited Component Total Score Through 2 Hours .
Dark squares in boxes indicate the mean; the top box lines, upper quartile; the bottom box line, lower quartile; bars in boxes, median; whiskers, data falling within 1.5 times the interquartile range; dots, values outside the range indicated by the whiskers; and PEC, Positive and Negative Syndrome Scale-Excited Component.
Secondary End Points
Treatment effects were first observed 20 minutes after patients were treated with sublingual dexmedetomidine 180 μg (least-squares mean difference, −1.1 [97.5% CI, −2.0 to −0.2]; P = .007) and sublingual dexmedetomidine 120 μg (least-squares mean difference, −1.0 [97.5% CI, −1.9 to −0.1]; P = .009). As shown in Figure 2, patients in both sublingual dexmedetomidine treatment groups showed greater improvements in the PEC total score than did patients in the placebo group at all subsequent time points through 90 minutes after treatment.
Exploratory End Points
The percentage of participants with a PEC response at 2 hours, defined by a reduction of at least 40% from baseline, was 90.5% with sublingual dexmedetomidine 180 μg and 77.0% with sublingual dexmedetomidine 120 μg, and 46.0% with placebo (Table 2 and eFigure 1 in Supplement 3).
Table 2. Exploratory Efficacy End Points for Positive and Negative Syndrome Scale-Excited Component, Clinical Global Impressions–Improvement, and Acceptability.
| No. (%) | |||
|---|---|---|---|
| Sublingual dexmedetomidine | Placebo (n = 126) | ||
| 180 μg (n = 126) | 120 μg (n = 126) | ||
| PEC respondersa | |||
| 20 min | 29 (23.0) | 26 (20.6) | 16 (12.7) |
| Difference (95% CI)b | 10.3 (0.2 to 20.5) | 7.9 (−2.0 to 17.9) | |
| P valuec | .048 | .13 | |
| 30 min | 47 (37.3) | 48 (38.1) | 28 (22.2) |
| Difference (95% CI)b | 15.1 (3.2 to 27.0) | 15.9 (3.9 to 27.8) | |
| P valuec | .01 | .009 | |
| 45 min | 72 (57.1) | 68 (54.0) | 36 (28.6) |
| Difference (95% CI)b | 28.6 (16.1 to 41.1) | 25.4 (12.9 to 37.9) | |
| P valuec | <.001 | <.001 | |
| 60 min | 92 (73.0) | 85 (67.5) | 47 (37.3) |
| Difference (95% CI)b | 35.7 (23.5 to 48.0) | 30.2 (17.6 to 42.7) | |
| P valuec | <.001 | <.001 | |
| 90 min | 107 (84.9) | 90 (71.4) | 52 (41.3) |
| Difference (95% CI)b | 43.7 (32.2 to 55.1) | 30.2 (17.7 to 42.6) | |
| P valuec | <.001 | <.001 | |
| 120 min | 114 (90.5) | 97 (77.0) | 58 (46.0) |
| Difference (95% CI)b | 44.4 (33.6 to 55.3) | 31.0 (18.8 to 43.1) | |
| P valuec | <.001 | <.001 | |
| CGI-I respondersd | |||
| 30 min | |||
| No./total (%) | 37/125 (29.6) | 38/126 (30.2) | 24 (19.0) |
| Difference (95% CI)b | 10.6 (−0.8 to 21.9) | 11.1 (−0.2 to 22.5) | |
| P valuec | .06 | .06 | |
| 1 h | 89 (70.6) | 75 (59.5) | 37 (29.4) |
| Difference (95% CI)b | 41.3 (29.2 to 53.3) | 30.2 (17.7 to 42.6) | |
| P valuec | <.001 | <.001 | |
| 2 h | 109 (86.5) | 88 (69.8) | 48 (38.1) |
| Difference (95% CI)b | 48.4 (37.3 to 59.6) | 31.7 (19.3 to 44.2) | |
| P valuec | <.001 | <.001 | |
| 4 h | |||
| No./total (%) | 103/114 (90.4) | 82/108 (75.9) | 43/76 (56.6) |
| Difference (95% CI)b | 33.8 (20.3 to 47.3) | 19.3 (4.5 to 34.2) | |
| P valuec | <.001 | .007 | |
| Resolution of agitatione | |||
| 2 h | 108 (85.7) | 89 (70.6) | 47 (37.3) |
| 4 h, No./total (%) | 101/115 (87.8) | 82/108 (75.9) | 43/76 (56.6) |
| 8 h, No./total (%) | 92/123 (74.8) | 74/116 (63.8) | 50/118 (42.4) |
| Overall acceptancef | 101 (80.2) | 100 (79.4) | 100 (79.4) |
| Flavorf | |||
| Liked | 82 (65.1) | 75 (59.6) | 80 (63.5) |
| Neutralf | 25 (19.8) | 44 (34.9) | 39 (31.0) |
| Satisfied with time to dissolveg | 108 (87.8) | 112 (90.3) | 113 (93.4) |
| No unpleasant smellh | 125 (99.2) | 125 (99.2) | 126 (100.0) |
| No unpleasant aftertasteh | 107 (84.9) | 116 (92.1) | 119 (94.4) |
Abbreviations: CGI-I, Clinical Global Impressions–Improvement with possible scores ranging from 1 (very much improved) to 7 (very much worse); PEC, Positive and Negative Syndrome Scale-Excited Component, comprising 5 items with a range of 5 (absence of agitation) to 35 (extremely severe).
Defined by 40% or higher reduction from the baseline score.
Difference (percentage, dexmedetomidine vs placebo) and associated 2-sided 95% CI based on the Wald method with a continuity correction.
P value based on a Fisher exact test.
Defined by a score of 1 (very much improved) or 2 (much improved).
Defined by an Agitation-Calmness Evaluation Scale (ACES) score of 4 or higher. ACES is a single-item measure for which 1 indicates marked agitation and 9, unarousable.
Based on responses to 5-item scale that included strongly agree, agree, neither agree nor disagree, disagree, and strongly disagree taken 20 minutes after dosing.
Based on yes or no responses 20 minutes after dosing.
Based on yes or no responses 20 minutes after dosing.
One hour after taking sublingual dexmedetomidine, CGI ratings improved to a mean score of 2.0 (95% CI, 1.8-2.20) for the 180-μg dose group and to 2.2 (95% CI, 2.03-2.43) for the 120-μg dose group. The mean 2-hour scores were 1.6 (95% CI, 1.40-1.71) for the 180-μg dose group and 1.9 (95% CI, 1.73-2.13) for the 120-μg dose group. The mean 4-hour scores were 1.5 (95% CI, 1.36-1.65) for the 180-μg dose group and 1.8 (95% CI, 1.6-1.96) for the 120-μg dose group (eTable 4 and eFigure 2 in Supplement 3). The mean CGI-I values for placebo were 3.0 (95% CI, 2.79-3.18) at 1 hour, 2.8 (95% CI, 2.62-3.04) at 2 hours, and 2.3 (95% CI, 2.04-2.57) at 4 hours. The percentage of participants with a CGI-I response, defined by a score of 1 (very much improved) or 2 (much improved), was 29.6% at 30 minutes, 70.6% at 1 hour, 86.5% at 2 hours, and 90.4% at 4 hours with sublingual dexmedetomidine 180 μg; 30.2% at 30 minutes, 59.5% at 1 hour, 69.8% at 2 hours, and 75.9% at 4 hours with sublingual dexmedetomidine 120 μg; and 19.0% at 30 minutes, 29.4% at 1 hour, 38.1% at 2 hours, and 56.6% at 4 hours with placebo (Table 2 and eFigure 3 in Supplement 3).
The mean ACES scores 2 hours after taking sublingual dexmedetomidine were 5.6 (95% CI, 5.34-5.95) for the 180-μg dose group, 5.0 (95% CI, 4.71-5.39) for the 120-μg dose group, and 3.3 (95% CI, 3.05-3.57) for the placebo group. Mean ACES scores remained at 4 (normal behavior) or higher at 4 and 8 hours in both sublingual dexmedetomidine groups (eTable 4 in Supplement 3). The percentage of participants with resolution of agitation on the ACES score, defined by 4 or higher for the 180-μg sublingual dexmedetomidine dose were 85.7% at 2 hours, 87.8% at 4 hours, and 74.8% at 8 hours; for the 120-μg dose group, 70.6% at 2 hours, 75.9% at 4 hours, and 63.8% at 8 hours; and for the placebo group, 37.3% at 2 hours, 56.6% at 4 hours, and 42.4% at 8 hours for placebo (Table 2 and eFigure 4 in Supplement 3).
Participant ratings of medication acceptability at 20 minutes after taking the medication (flavor, odor, dissolve time, and overall acceptance) were positive across all groups (Table 2).
Adverse Events
The incidence of adverse events with sublingual dexmedetomidine 180 μg and 120 μg was 35.7% and 34.9%, respectively, and 17.5% with placebo (Table 3). No treatment–related serious or severe adverse events were reported. The most common adverse events with sublingual dexmedetomidine were somnolence, dry mouth, hypotension, and dizziness. Of 53 patients reporting somnolence with sublingual dexmedetomidine, the adverse event was mild in 64% and moderate in 36%, as judged by the investigator. One patient in the each of the sublingual dexmedetomidine groups reported suicidal ideation that lasted for 1 day, both of whom completed the study; no patient in the placebo group experienced this adverse event. No clinically meaningful changes in laboratory values were observed.
Table 3. Incidence of Adverse Events Occurring in at Least 2% of Patients (Safety Population)a.
| Patients, No. (%) | |||
|---|---|---|---|
| Sublingual dexmedetomidine | Placebo (n = 126) | ||
| 180 μg (n = 126) | 120 μg (n = 126) | ||
| Serious adverse event | 0 | 1 (0.8)b | 0 |
| Any treatment-emergent adverse event | 45 (35.7) | 44 (34.9) | 22 (17.5) |
| Any treatment-related adverse eventc | 39 (31.0) | 41 (32.5) | 15 (11.9) |
| Discontinuation for adverse event | 0 | 1 (0.8) | 0 |
| Somnolence | 27 (21.4) | 26 (20.6) | 6 (4.8) |
| Hypotension | 8 (6.3) | 6 (4.8) | 0 |
| Dizziness | 7 (5.6) | 7 (5.6) | 1 (0.8) |
| Dry mouth | 6 (4.8) | 9 (7.1) | 1 (0.8) |
| Orthostatic hypotension | 6 (4.8) | 5 (4.0) | 1 (0.8) |
| Hypoesthesia oral | 5 (4.0) | 2 (1.6) | 1 (0 .8) |
| Nausea | 5 (4.0) | 3 (2.4) | 3 (2.4) |
| Bradycardia | 3 (2.4) | 2 (1.6) | 0 |
| Paresthesia oral | 3 (2.4) | 2 (1.6) | 0 |
Each adverse event reported was assessed by the investigator for severity (mild, moderate, severe) and relationship to study drug (not related, unlikely or remotely related, possibly related, probably related, definitely related).
Judged by investigator to be unrelated to study drug.
Adverse events, clinical laboratory tests, electrocardiogram with rhythm strip, pulse oximetry, and vital signs were monitored for tolerability assessment. All observed and volunteered. Adverse events were recorded. The relationship of adverse events to the study drug were graded as not related, unlikely or remotely related, possibly related, probably related or definitely related by the investigators. Vital signs including systolic blood pressure, diastolic blood pressure, and heart rate were monitored.
The proportion of patients who experienced hypotension, orthostatic hypotension, or bradycardia was comparable between both sublingual dexmedetomidine groups (Table 3). Bradycardia was reported for 3 patients in the 180-μg dose group, 1 of which was considered clinically meaningful, and 2 in the 120-μg dose group, both of which were considered clinically meaningful. Sinus bradycardia was reported for 2 patients in the 180-μg dose group, 1 of which was considered clinically meaningful and 1 in the 120-μg dose group that was considered clinically meaningful, 1 case of sinus bradycardia was reported that was considered clinically meaningful. Based on examination by an external cardiologist, 4 of 5 cases of bradycardia were rated as mild and 1 as moderate and all cases of sinus bradycardia were rated as mild.
Two hours after treatment with sublingual dexmedetomidine, patients in the 180-μg dose group had a mean (SD) decrease in SBP from baseline for 1 minute of standing of −18.1 mm Hg (16.3 mm Hg), in DBP of −11.5 mm Hg (11.1 mm Hg); and in heart rate of −9.2 bpm (11.7 bpm); patients in the 120-μg dose group had a mean (SD) decrease in SBP of −14.8 mm Hg (15.6 mm Hg), in DBP of −8.8 mm Hg (11.0 mm Hg), and in heart rate of −7.9 bpm (13.0 bpm).
No clinically important mean changes from baseline at 2 hours or 24 hours after treatment were observed for PR interval, QRS duration, and QTcF. No patients experienced an adverse event related to ECG parameters or had a clinically significant abnormal ECG result at screening or at 2 or 24 hours after treatment. Local buccal irritation, assessed at 30 minutes and 2, 4, and 24 hours, was reported in 1 patient in the sublingual dexmedetomidine 180-μg group and 2 patients in the 120-μg group at 30 minutes but at no other time points.
Discussion
In this multicenter, double-blind, randomized, clinical trial, a single 180-μg or 120-μg dose of sublingual dexmedetomidine reduced the severity of agitation in participants with mild to moderate agitation associated with bipolar I or II disorder after self-administering the medication. Sublingual dexmedetomidine produced improvement on the primary end point of change in PEC score at 2 hours compared with placebo and treatment effects began as early as 20 minutes for both doses.
This study involved individuals with bipolar disorder because such patients are at elevated risk of acute agitation, during manic, depressed, or mixed mood states, all of which were included in this study.1 For example, a European survey4 of 583 individuals with bipolar disorder or schizophrenia reported that respondents had on average 2.7 episodes of agitation requiring hospital trips in the prior 12 months. The mean baseline PEC score in these data corresponds to the CGI–Severity of Illness score of moderately ill. A moderately ill individual in this study would have PEC subscale items of moderate severity (poor impulse control, tension, hostility, uncooperativeness, and excitement).
Verbal and nonverbal deescalation strategies are the preferred first-line treatment to reduce agitation in a collaborative and noncoercive manner.6 When pharmacotherapy is necessary, the goal is to calm rather than sedate19,20 so that assessment can proceed safely for patient and physician. Currently available treatments for acute agitation associated with bipolar disorder include oral, parenteral, and inhaled medications.5,9,21,22,23,24 However, none of these is a panacea, and each has limitations. Based on this study, self-administered dexmedetomidine may be an additional option for patients with bipolar disorder who are mildly to moderately agitated with an onset of effect beginning within 20 minutes. Although in this study, dexmedetomidine did not cause extrapyramidal adverse effects, unarousable sedation, or QTc prolongation, the study was not powered to assess these end points.
Limitations
This study has several limitations. First, it assessed efficacy and tolerability of sublingual dexmedetomidine following only a single episode of agitation. Second, because patients had mild to moderate agitation based on their baseline PEC score, the level of cooperation required to administer a medication sublingually limits the generalizability of these results to patients who are able or willing to self-administer this treatment. Third, participants were excluded for acute alcohol intoxication, but it was not possible to determine if drug or alcohol withdrawal contributed to agitation. Fourth, there is no consensus on the change in PEC score that represents the minimal clinically important difference. Fifth, a large placebo effect was observed in the trial supporting the recommended use of nonpharmacological techniques as part of the management of agitation.
Conclusions
Among patients with mild to moderate agitation associated with bipolar disorder, treatment with an investigational sublingual film formulation of dexmedetomidine 180 μg or 120 μg, compared with placebo, resulted in significantly greater reduction in the agitation score at 2 hours. Further research is needed to understand the spectrum of patients for whom this treatment would be effective and feasible and to better understand the clinical importance of the observed effect size.
Trial Protocol
Statistical Analysis Plan
eMethods
eResults. Exploratory endpoints
eTable 1. Concomitant medication
eTable 2. Enrollment by study site
eTable 3. Electrocardiogram parameters notable abnormalities criteria
eTable 4. Exploratory efficacy endpoints: CGI-ACES
eTable 5. Post hoc analysis of PEC change from baseline 20 minutes to 120 minutes postdose with site as random effect
eFigure 1. Percentage of patients with a response on the PEC total score
eFigure 2. Mean (standard deviation) on the Clinical Global Impression-Improvement scale
eFigure 2. Percentage of patients with a response on the Clinical Global Impression-Improvement scale
eFigure 4. Percentage of patients with resolution of agitation on the Agitation-Calmness Evaluation scale
eFigure 5. Mean (standard deviation) change from baseline on the Agitation-Calmness Evaluation scale
Data Sharing Statement
References
- 1.Pompili M, Ducci G, Galluzzo A, Rosso G, Palumbo C, De Berardis D. The management of psychomotor agitation associated with schizophrenia or bipolar disorder: a brief review. Int J Environ Res Public Health. 2021;18(8):4368. doi: 10.3390/ijerph18084368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361-370. doi: 10.1016/j.annemergmed.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 3.Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatry. 2016;17(2):86-128. doi: 10.3109/15622975.2015.1132007 [DOI] [PubMed] [Google Scholar]
- 4.Roberts J, Gracia Canales A, Blanthorn-Hazell S, Craciun Boldeanu A, Judge D. Characterizing the experience of agitation in patients with bipolar disorder and schizophrenia. BMC Psychiatry. 2018;18(1):104. doi: 10.1186/s12888-018-1673-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi: 10.5811/westjem.2015.12.28763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holloman GH Jr, Zeller SL. Overview of project BETA: Best Practices in Evaluation and Treatment of Agitation. West J Emerg Med. 2012;13(1):1-2. doi: 10.5811/westjem.2011.9.6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA de-escalation workgroup. West J Emerg Med. 2012;13(1):17-25. doi: 10.5811/westjem.2011.9.6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel MX, Sethi FN, Barnes TR, et al. Joint BAP NAPICU evidence-based consensus guidelines for the clinical management of acute disturbance: de-escalation and rapid tranquillisation. J Psychopharmacol. 2018;32(6):601-640. doi: 10.1177/0269881118776738 [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Raga J, Amore M, Di Sciascio G, et al. 1st International Experts’ Meeting on Agitation: conclusions regarding the current and ideal management paradigm of agitation. Front Psychiatry. 2018;9:54. doi: 10.3389/fpsyt.2018.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HK, Leonard JB, Corwell BN, Connors NJ. Safety and efficacy of pharmacologic agents used for rapid tranquilization of emergency department patients with acute agitation or excited delirium. Expert Opin Drug Saf. 2021;20(2):123-138. doi: 10.1080/14740338.2021.1865911 [DOI] [PubMed] [Google Scholar]
- 11.Zhou LJ, Fang XZ, Gao J, Zhangm Y, Tao LJ. Safety and efficacy of dexmedetomidine as a sedative agent for performing awake intubation: a meta-analysis. Am J Ther. 2016;23(6):e1788-e1800. doi: 10.1097/MJT.0000000000000319 [DOI] [PubMed] [Google Scholar]
- 12.Yocca F, DeVivo M, Seth S, Sharma S. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented: at the 58th Annual meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL. [Google Scholar]
- 13.Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: the 23rd Annual Conference of the International Society for Bipolar Disorders (S17); May 13-15, 2021. Accessed May 27, 2021.
- 14.Sheehan DV, Lecrubier Y, Harnett Sheehan K, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry. 1997;12(5):232-241. doi: 10.1016/S0924-9338(97)83297-X [DOI] [Google Scholar]
- 15.Montoya A, Valladares A, Lizán L, San L, Escobar R, Paz S. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi: 10.1186/1477-7525-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preskorn S, Risinger R, Kakar R, Ereshefsky L, Yocca F. Double-blind, placebo-controlled, single ascending dose, study to determine the efficacy, safety, and pharmacokinetics of a BXCL 501 (sublingual dexmedetomidine) in agitation associated with schizophrenia or related disorders (M72). Neuropsychopharmacology. 2019;44(1):78-229.31801976 [Google Scholar]
- 18.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61(3):310-317. doi: 10.1001/archpsyc.61.3.310 [DOI] [PubMed] [Google Scholar]
- 19.Battaglia J. Pharmacological management of acute agitation. Drugs. 2005;65(9):1207-1222. doi: 10.2165/00003495-200565090-00003 [DOI] [PubMed] [Google Scholar]
- 20.Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project BETA psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi: 10.5811/westjem.2011.9.6866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward K, Citrome L. The treatment of acute agitation associated with schizophrenia or bipolar disorder: investigational drugs in early stages of their clinical development, and their clinical context and potential place in therapy. Expert Opin Investig Drugs. 2020;29(3):245-257. doi: 10.1080/13543784.2020.1727884 [DOI] [PubMed] [Google Scholar]
- 22.Hankin CS, Bronstone A, Koran LM. Agitation in the inpatient psychiatric setting: a review of clinical presentation, burden, and treatment. J Psychiatr Pract. 2011;17(3):170-185. doi: 10.1097/01.pra.0000398410.21374.7d [DOI] [PubMed] [Google Scholar]
- 23.Bak M, Weltens I, Bervoets C, et al. The pharmacological management of agitated and aggressive behaviour: a systematic review and meta-analysis. Eur Psychiatry. 2019;57:78-100. doi: 10.1016/j.eurpsy.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 24.Roppolo LP, Morris DW, Khan F, et al. Improving the management of acutely agitated patients in the emergency department through implementation of project BETA (Best Practices in the Evaluation and Treatment of Agitation). J Am Coll Emerg Physicians Open. 2020;1(5):898-907. doi: 10.1002/emp2.12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods
eResults. Exploratory endpoints
eTable 1. Concomitant medication
eTable 2. Enrollment by study site
eTable 3. Electrocardiogram parameters notable abnormalities criteria
eTable 4. Exploratory efficacy endpoints: CGI-ACES
eTable 5. Post hoc analysis of PEC change from baseline 20 minutes to 120 minutes postdose with site as random effect
eFigure 1. Percentage of patients with a response on the PEC total score
eFigure 2. Mean (standard deviation) on the Clinical Global Impression-Improvement scale
eFigure 2. Percentage of patients with a response on the Clinical Global Impression-Improvement scale
eFigure 4. Percentage of patients with resolution of agitation on the Agitation-Calmness Evaluation scale
eFigure 5. Mean (standard deviation) change from baseline on the Agitation-Calmness Evaluation scale
Data Sharing Statement