Abstract
Cosmeceuticals are a branch of cosmetic products that forms a bridge between cosmetic and drug products. It is a fast-growing branch of the cosmetic industry, especially after the introduction of novel formulation and manufacturing techniques such as lipid nanoparticles (LNPs). These LNPs-based cosmeceutical products offer several advantages such as enhanced bioavailability of cosmeceutical active ingredients (CAIs), improved aesthetic appeal, and stability of the final products. However, the use of these LNPs may raise some concerns about possible side effects of these LNPs and potential hazards to the customer’s health. Accordingly, an update that focuses on the use of this important branch of nanoparticles is necessary since most review papers are dealing with all types of nanocarriers in the same review with little focus on LNPs. Therefore, in the current review, a detailed analysis of the advantages and disadvantages of LNPs in this field was highlighted, to emphasize the LNPs-based cosmeceuticals on the market, as well as the potential risk posed by LNPs on exposure and recently introduced regulatory guidelines to address them. In addition, if these products can be a candidate as products that meet the sustainable development goals raised by the UN are discussed.
Keywords: Lipid nanoparticles, Cosmeceuticals characteristics, Applications, Sustainable, Bibliometrics
1. Introduction
The integumentary system is an essential system that composes the skin, hair, nails, and exocrine glands. The skin forms the outer barrier in our body that protects us from microorganisms, chemicals, diseases, UV radiation, mechanical shock as well as the regulation of body temperature (Zouboulis, 2014, Nicol, 2016). Like other organs of the body, skin must be well-nourished and moisturized to keep its normal physiological activity. This nourishment, if accompanied by the use of appropriate cosmetic products, would aid the skin in maintaining its normal physiological functions. However, it should be taken into account that these activities may be broken by internal and external factors such as certain systemic diseases, vitamin deficiencies as well as violations of the endocrine system (Schagen et al., 2014). In such cases, it is indispensable to intervene with the use of active ingredients (AIs) with the desired pharmacological activity. Therefore, it is difficult sometimes to establish the barriers between cosmetic and topical pharmaceutical products due to several borderlines between them. Nowadays, many consumers request a cosmetic product with specific therapeutic activity made it more difficult to classify the role of such topical preparations into pharmaceutical or cosmetic products.
U.S. Food and Drug Administration (FDA) and the European Union (EU) do not have the legal authority in charge of approving cosmetic products before they are placed on the market. However, these products should be effective and safe for consumers, and accurately labeled. Accordingly, it is the legal responsibility of companies and individuals who market these products to prove their safety and provide accurate proper labeling of their products (Administration, 2021, Union, 2009).
Recently, The term “cosmeceutical” was coined to describe a product category that fills the gap between cosmetic and pharmaceutical products (Fulekar, 2010).
Nowadays, this term is utilized in the professional skincare field to define cosmetics products that could affect the biology of the skin. So, cosmeceutical term is defined as cosmetic products that consist of active ingredients and showed medical benefits (Mukta and Adam, 2010, Lohani et al., 2014).
This term is not recognized by the FDA or the EU, but it is utilized by pharmacists, skin specialists, medical doctors, and skincare professionals, to promote the public to keep purchasing cosmetic products such as whitening, antiaging, and sunscreen products. Cosmeceuticals are a branch of the personal care industry that is the fastest-growing branch in this industry (Lohani et al., 2014).
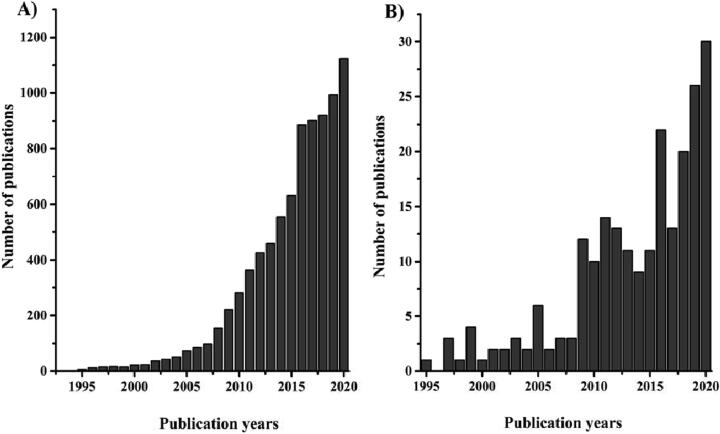
This segment is expanding to include not only skin body and hair products but also to include several topical products to treat conditions such as hyperpigmentation, photoaging, wrinkles, and hair loss which are all examples of signs of aging (Brandt et al., 2011, Lohani et al., 2014). The universal market of cosmeceutical products is offering a huge potential among many countries worldwide, especially with the inclusion of new technologies to develop and produce elegant and effective products (El Enshasy et al., 2018, Newton and Kaur, 2019). In this contest, cosmetic nanotechnology is finding a special place among the proposed technologies used to create novel, safe, effective, and elegant cosmeceuticals. Nanoparticles could be absorbed onto the skin due to their small size and deliver the active ingredient to repair the damaged part of the skin, so improve product efficacy (Bonifácio et al.,2014, Aziz et al., 2019). Accordingly, the purpose of including nanoparticles in the cosmeceutical field is to achieve products such as sunscreens with better skin protection, perfumes with long-lasting scent, high-quality antiaging creams with the better anti-aging outcome, as well as maintaining the efficient hydration of the skin by these newly formulated moisturizers (Aziz et al., 2019). These innovative nanotechnology products include nanoemulsions, nanocapsules, nano-pigments, liposomes, niosomes, nanocrystals, carbon nanomaterials, dendrimers, and lipid nanoparticles (LNPs) (Bugaj, 2015, Aziz et al., 2019). LNPs are colloidal lipophilic systems composed of a lipophilic core stabilized in a water phase using a monolayer of emulsifiers such as phospholipids, poly(ethylene glycol)-based (PEGylated) surfactants, etc. Recently, lipid nanoparticles were studied extensively by various research groups with an increment in the number of publications since 2008 (Fig. 1A). This was observed by searching in the web of science® database using the term “lipid nanoparticles” obtaining a total of 8413 publications until the end of 2020 with an obvious growth of publications above 100 in 2008 which is considered a very recent hot topic of research. Moreover, lipid nanoparticles were investigated and used in a variety of fields of research especially in pharmaceutical sciences with 48% of the number of publications. Regarding the cosmeceutical applications of LNPs, it can be observed that this application is very juvenile as the number of publications is 347 in total of lipid nanoparticles publications focused on cosmeceuticals. Moreover, the increment of publications began in the last ten years as shown in Fig. 1B. These data were extracted by typing in the search engine of the web of science® the terms “cosmetics”, “topical”, “dermal”, and skin. Then the obtained publications were refined to the desired cosmeceutical applications as shown in Fig. 1B.
Fig. 1.
The number of publications available on the web of science® (until the end of 2020) related to A) “lipid nanoparticles” and B) “lipid nanoparticles” applied in the cosmetics field.
Various types of LNPs are currently used in pharmaceutical and cosmetic technology. These include solid lipid nanoparticles (SLNs), lipid nano-emulsions (LNEs), and nanostructured lipid carriers (NSLCs). All these kinds of LNPs contain a lipophilic (lipid) phase in their formulation, but they can be identified by the structure of their core. Recently a novel generation called Lipid–polymer hybrid nanoparticles (LPHNPs) was developed and it is considered as next-generation core-shell nanostructures.
Most of LNPs used in the pharmaceutical and cosmeceutical fields exhibit a size range of 150–300 nm (Muller et al., 2011). Moreover, the majority of involved components in the composition of LNPs are approved by both EMA and FDA, with quasi-physiological lipophilic components molecules, being adapted to fit the encapsulated of active ingredients (AIs). Accordingly, these components are non-toxic and biodegradable (Desfrançois et al., 2018). Several advantages can be achieved using cosmeceuticals nanoparticles. These include (i) enhancement of the stability of sensitive cosmetic AIs; (ii) effective skin protection from the harmful effect of ultraviolet (UV) rays; (iii) obtaining aesthetically appealing final products; (iv) site targeting of AIs, and (v) modifying the release of AIs to the desired extent (Padamwar and Pokharkar, 2006, Mu and Sprando, 2010). Recently, sustainable development goals were raised and adopted by the United Nation. The integration of the specific rules of sustainable development into all processes of the product life cycle of a cosmeceutical product, starting from the design of the product and ending with the consumer use will be considered a very important goal for many cosmetic industries (Hitce et al., 2018).
The purpose of this manuscript is to review and update the scientific literature on the properties of LNPs for cosmeceutical aims. Moreover, several studies and applications of these types of lipid nanoparticles in the pharmaceutical/cosmetic industries are discussed.
2. Cosmeceuticals characteristics of LNPs
It is well recognized that the soft appearance of the skin usually reflects a person's health and well-being (Marcoux, 2000). Youth and teenagers are considered important consumers of a wide range of skincare and toiletry products that meet their cleansing, nourishing, and photo-protective requirements, while adults may focus on cosmeceuticals that retard aging. Accordingly, the cosmetic industry is interested in not only the elegance and the appearance of the product, which are essential characteristics for good advertising, but also in the functions of the cosmeceutical product that might be purchased. In this contest, LNPs play a crucial role due to their nano-sized and pearl-like appearance.
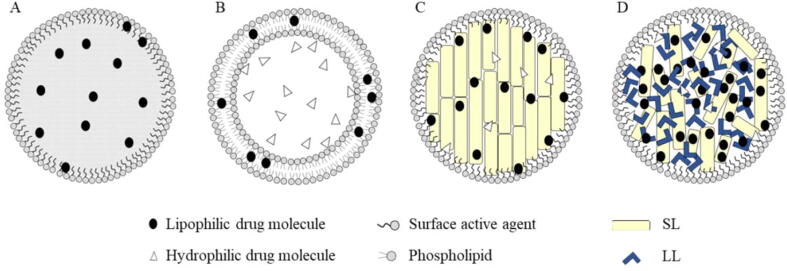
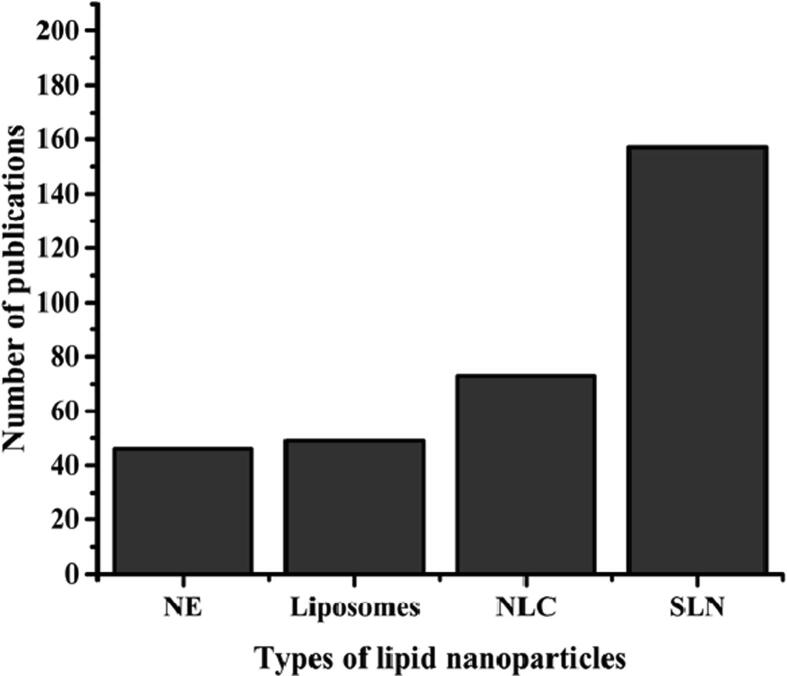
Solid lipid nanoparticles (SLNs) (Fig. 2) are commonly spherical nanosized colloidal particles with a diameter of less than 1000 nm. SLNs are composed of physiological lipids, dispersed in a water solution of surfactant (Sathali et al., 2012). SLNs are considered the most developed type of lipid nanoparticles applied in the cosmetics field with the highest number of publications of 157 of total 347 extracted from the web of science® database as shown in Fig. 3.
Fig. 2.
Various types of lipid nanoparticles. (A) Nanoemulsions; (B) Liposomes; (C) Solid Lipid Nanoparticles (SLNs) containing solid lipid (SL); and (D) Nanostructured Lipid Carriers (NLCs) containing solid and liquid lipids (LL). Reprinted with permission from (Haider et al., 2020). Copyright 2020, MDPI.
Fig. 3.
The number of publications extracted from the web of science® database until the end of 2020 of the different types of LNPs applied in cosmeceuticals.
Phospholipids are an essential component due to their numerous properties, such as biocompatibility, amphiphilic properties, and multifunctionality. Nonetheless, systems such as liposomes and lipospheres have several disadvantages due to their complex manufacturing method, their difficult large-scale production, and low percentage drug entrapment efficiency (Tekade et al., 2017, Duan et al., 2020). The key ingredients of SLNs are the solid lipid (SL), emulsifiers, and occasionally a blend of both, active pharmaceutical ingredients (APIs) and an appropriate solvent compartment (Fig. 2). These nanoparticles are popular in cosmeceuticals since they demonstrated various privileges including (i) they are made up of biodegradable low-toxicity physiological lipids; (ii) their small size ensures close adherence to the corneum layer of the skin which results in an improvement of the transdermal absorption of the cosmeceutical active ingredients (CAIs); (iii) the occlusive effect of SLNs increases skin hydration (Pardeike et al., 2009). However, SLNs showed some disadvantages such as the low loading capacity of hydrophilic drugs (Yoon et al., 2013), and the burst release of the drug (Mohammadi-Samani and Ghasemiyeh, 2018). Concerning Nanostructured Lipid Carriers (NSLCs), which are considered the second generation of the LNPs that consisted of a mixture of solid (SL) and liquid lipids (LL). Mainly, there are three types of NSLCs; imperfect, amorphous, and multiple, which can be achieved by changes in the composition of the formulation and manufacturing processes (Kaul et al., 2018). An increment of the scientific and commercial attention toward NSLC was observed during the last few years due to the higher safety as they showed a lower risk of systemic adverse effects which is considered the second type of LNPs that applied in cosmeceuticals with the number of publications of 73 articles as shown in Fig. 3. These carriers are formulated using biodegradable and physiological lipophilic ingredients which demonstrate limited toxicity (Purohit, 2016). Besides, NSLCs showed higher active-loading capacity than SLNs for entrapped CAIs due to their distorted structure which results in higher available space for loading. Moreover, these lipid carriers showed better stability than SLNs since they showed a reduction of drug expulsion during storage. However, some of the surfactants used in the NSLC preparation showed irritant effect (Jaiswal et al., 2016). Regarding, the cosmetic features of Nano Emulsions (NEs), these are thermodynamically stable colloidal dispersion of aqueous and oily phases in combination with a surfactant and cosurfactant. Their structure can be handled based on the used ingredients and method of preparation (Shah et al., 2010). Their liquid lipophilic core, which is covered by a layer of surfactants and cosurfactants, enables NEs to be more flexible and suitable for the delivery of hydrophobic molecules such as natural cosmeceutical ingredients. Moreover, these stable systems have overcome stability problems associated with macroemulsions. Problems associated with macroemulsions such as sedimentation, coalescence, creaming, and flocculation are not observed with NEs. In addition, NEs have a transparent or translucent appearance and suitable properties such as fluidity, high interfacial area, kinetic stability, and high solubilization capacity which are highly appreciated in a cosmeceutical final product (Patel and Joshi, 2012). In fact, NEs are widely used as a controlled delivery medium in various cosmeceuticals such as sunscreens, deodorants, lotions, hair serums, shampoos, and conditioners (Nakajima, 1997). In this contest, NEs provide hydration to the skin which promotes rapid penetration and active transport of CAIs. Other merits of NEs include the suitability of delivering both lipophilic and hydrophilic CAIs, and the ability to be formulated into various dosage forms such as foam, sprays, and creams (Sharma and Sarangdevot, 2012, Maali and Mosavian, 2013). However, nanoemulsion is the least type of LNPs that applied in cosmetics with 46 articles published in this field as shown in Fig. 3.
A new generation of LNPs is Lipid–polymer hybrid nanoparticles, (LPHNPs) were developed and now it is considered as next-generation core-shell nanostructures. This category is shared with both liposome and polymeric nanoparticles (PNPs), where the polymer core remains covered by a lipid layer. LPHNPs remaining not widely exploited or ubiquitous even though they have gathered significant interest by the scientific community worldwide. However, in recent years, a fundamental transition has occurred in their preparation and is characterized by adopting a one-step manufacturing strategy that involves asynchronous self-assembly of lipids and polymers. This approach is of special interest due to its two-in-one structure, which may suggest a combinatorial CAIs delivery platform in cosmeceuticals formulations (Meraj Anjum et al., 2016, Mukherjee et al., 2019, Castro et al., 2020).
2.1. Protective aspects of LNPs
Most of the commercial trade name cosmeceutical products available in the cosmetic market claim certain cosmeceutical efficacy, but such efficacy could never be proven scientifically. Nonetheless, if an appeal is done for a product's efficacy or activity, these properties should certainly be supported by well-designed scientific evidence. The protective role of SLNs on the skin is well reported in the literature and it is primarily concerned with their lipid components and the small size of the obtained SLNs (Souto et al., 2007, Souto and Müller, 2008, Müller et al., 2014, Suter et al., 2016).
2.2. Adherence, occlusion and skin hydration
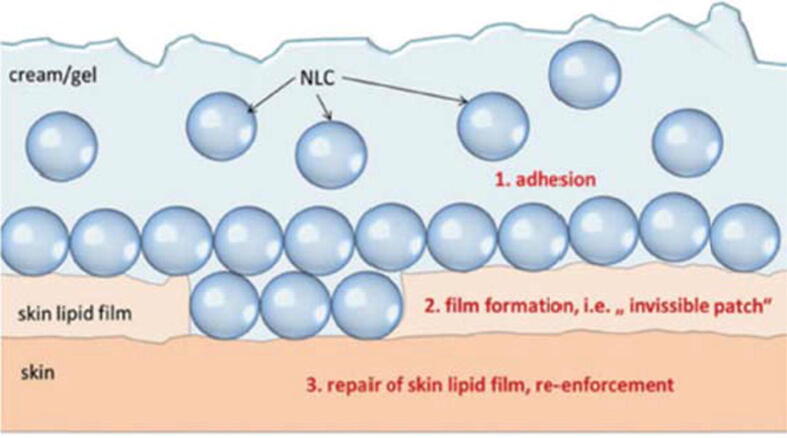
In general, nanoparticles show excellent adherence when coming in touch with the skin. This characteristic is due to a drop in the surface free energy by the adsorption of these particles on a skin which directly reduces the thermodynamic driving force of the obtained system (Felton, 2013). Polymeric nanoparticles and liposomes demonstrated good surface adhesiveness. Concerning SLNs, it has been published that approximately 4% of SLNs with about 200 nm size should form a single layer film when applying about 4 mg of formulated product on 1 cm2 (Wissing et al., 2001). Moreover, this mono-layered lipophilic film causing an occlusive impact on the skin delaying the loss of moisture caused by evaporation. The properties of the moisture barrier showed demonstrated various levels of occlusion depending on the particle size used (Wissing and Müller, 2003, Naseri et al., 2015). In vitro assessment of the occlusion factor can be carried out using de Vringer test. Shortly, the rate of water evaporation from a beaker covered with a cellulose acetate filter is measured, while another reference beaker covered with the same kind of filter paper is utilized as reference (De Vringer, 1997). An occlusion factor is equal to 0 if no occlusion occurs and, in this case, the evaporation of water from the sample and reference are equal, while an occlusion factor equals to 100 is achieved when the maximum occlusive effect takes place. It was demonstrated experimentally that the occlusion factor of microparticles having particle size higher than 1 µm was only 10%, while an occlusion factor approaching 50% was calculated when LNPs of approximately 200 nm were used (Müller and Dingler, 1998). Even though this kind of study does not completely simulate the natural moisture loss conditions, the smaller the particle size of the SLNs, the higher is the barrier against evaporation. In contrast, the greater the size is, the greater the amount of water that will be lost by evaporation. When lipid particles are applied to the skin, a thin film layer with a surface area that is particle size-dependent will be formed as shown in Fig. 4. When a layer of nano and microparticles is compared with each other, the dimensions of the air channels will be greatly reduced in the case of the nanoparticles; which causes a decrease in the hydrodynamic evaporation of water. On the other side, larger pores will aid the evaporation of water from the surface of the skin and accordingly the dryness of the skin.
Fig. 4.
The adherence of lipid nanoparticles on the skin surface forming a thin film. Reprinted with permission from (Müller et al., 2014). Copyright 2014, tks publisher.
However, an increase in the rate of moisture dissipation may be desired when the keratin layer of the skin becomes macerated (Müller and Dingler, 1998). Therefore, various effects can be obtained by the manipulation of the size of LNPs. The occlusive effect on skin obtained by applying conventional ointment products does not ensure quick moisturization, especially when the stratum corneum is extremely dry. In this special case, it is advisable to utilize a special preparation capable of supplying water to the skin. For this purpose, LNP suspensions are suitable since when placed onto the skin, a dense film is formed due to the pressure that caused a fusion of the particles. This fusion is endorsed by capillary forces involved during the evaporation of water (Wissing et al., 2001, López-García and Ganem-Rondero, 2015).
Usually, the dermis loses a significant amount of elasticity under aging conditions. Therefore, wrinkles are developed, when the skin is stretched by muscle movement and cannot return to its normal smoothness. Accordingly, it is reasonable to assume that LNPs may improve skin elasticity due to their hydration properties, and these nanoproducts can further be advertised as anti-aging products (Gupta et al., 2013, Iqbal et al., 2018, Kaul et al., 2018, Souto et al., 2020, Yeo et al., 2021). Moreover, the sunscreen properties of LNPs are suggested since these products show photo-protection properties (Villalobos-Hernández and Müller-Goymann, 2005, Jose and Netto, 2019).
2.3. Smoothness and lubrication
In some cases, it is demanded a cosmetic or cosmeceutical product has a lubricant property when applied between adjacent skin areas or into the skin surface that could scrub against cloths. In this contest, lipophilic vehicles may be used. However, these products have little acceptance by many customers due to their tacky feel. However, LNPs provide distinguished skin lubrication due to their spherical-like shape. The assessment of the lubricating effect could be carried out using rheological analysis. Viscoelastic characteristics of some colloidal dispersions can be assessed using oscillation tests, where the formulation is subject to sinusoidal stress. This should give information on its inter-particle and intermolecular forces. In this way, elastic and viscous products can be differentiated. Using this method, it was possible to figure out that the viscoelastic properties of LNPs are dependent on the level of lipid components (Souto et al., 2004a, Souto et al., 2004b). In practice, these results showed that LNP dispersions have sufficient viscosity or fluidity for easy application, and sufficient elasticity to adhere onto the skin (Garcês et al., 2018, de Souza et al., 2020). One of the purposes of cosmetic and toiletry products is to diminish the desire to grate that may cause skin damage. The mechanical barrier, as well as the lubricating effect of LNP formulations, provide protection and support to the skin, which is a very helpful allergic reaction and in skin sensitization and irritation. The accurate balance of the emolliency of LNP based cosmeceuticals is achieved by the level of hydration obtained with these nanoformulations (Üner et al., 2005). This could be adjusted by the accurate selection of surfactants and lipids used to produce the desired LNPs. These aspects should affect both the size of nanoparticles and their index of re-crystallinity.
Very pure lipids such as tristearin and tripalmitin are used to achieve highly crystalline LNPs which create a high degree of occlusiveness and thus emolliency. On the other side, supercooled melts can be achieved when using lipids of the low melting range such as trilaurin and tricaprin, that demonstrate low occlusive characteristics (Wissing and Müller, 2002a, Wissing and Müller, 2002b, Lambers et al., 2006, Duncan et al., 2013, Prakash et al., 2017, Blaak and Staib, 2018).
2.4. Osmotic effects and pH
Usually, the pH of the skin surface shows a slight but broad physiological acidic to neutral pH range. This range plays an important role in skin elasticity and suppliance. Accordingly, it is unlikely that any dermatological product causes a detrimental effect on the skin by producing a permanent deviation from the physiological pH range (Lambers et al., 2006, Duncan et al., 2013). Despite that, strongly acidic or alkaline products may cause skin irritation and, in those conditions, the pH clearly should be adjusted. Therefore, LNP dispersions offer the possibility to be formulated with the desired pH, and if necessary, suitably buffered and optimized formulation can be created for topical use. Also, even though the intact horny layer is almost tolerant to osmotic changes, the osmotic effects of topical preparations should be considered, since a skin irritation could happen if a great shift from isotonicity occurs. In this case, SLNs demonstrate appropriate isotonicity with the body fluids In fact, a marked hypertonic formulation may cause unwelcome stinging and may dissuade the customer from using it (Lambers et al., 2006, Jose and Netto, 2019).
2.5. Formulation aspects of LNPs
2.5.1. Depigmentation effects
A perfect whitening agent must include a powerful, fast bleaching activity without adverse effects and provide an irreversible elimination of the unwanted colored spots. The depigmentation issue of whitening agents can be obtained by proceeding with the melanization process of the skin. This process composes of various steps, thus whitening agents could interpose with one or more steps of this process, which are present in the viable skin tissue (Zaid and Al Ramahi, 2019). Accordingly, modern whitening preparations should facilitate the permeation of the whitening agent through the corneal layer to the viable tissue. In this contest, LNP dispersions are elegant candidates as skin whitening preparations since they play this role appropriately (Dingler et al., 1999). Accordingly, the incorporation of whitening active agents into LNPs may give the desired whitening effect, and represent a more appealing product from a marketing point of view.
2.5.2. Improvement of chemical stability of CAIs
Most of the ingredients used to formulate lipid nanoparticles have been approved by the FDA and the EMA since they are highly biocompatible and non-toxic (Doktorovova et al., 2016).
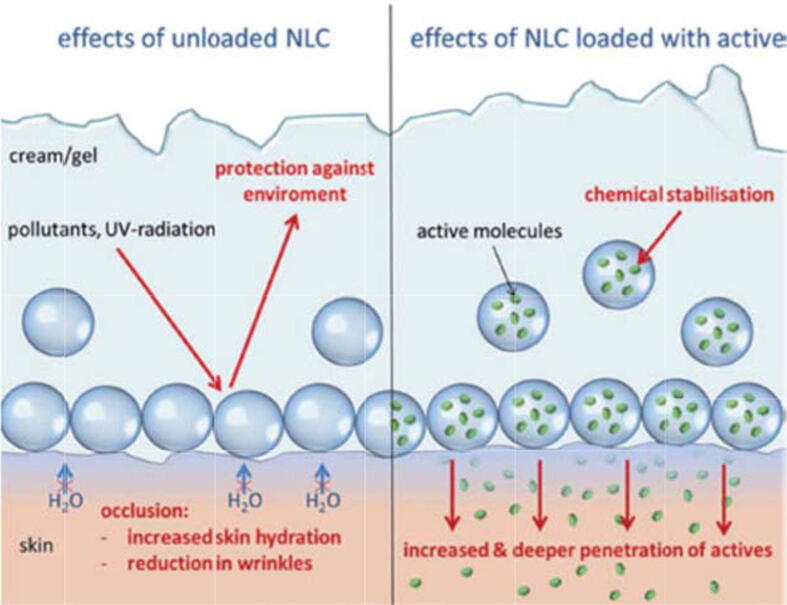
Fig. 5 showed that the lipophilic ingredients are supposed to play multiple roles such as protecting the CAIs which are entrapped inside the core of the LNPs from environmental (UV, pH) or physiological (immune system, enzymes) degradations, enhancing the solubility of the lipophilic ingredients and preventing the formation of the aggregates. However, various drawbacks regarding the stability of encapsulated ingredients may arise. Whereas significant leakage of active ingredients can be observed in the case of liquid-core LNPs, the crystalline core of SLNs can promote phase demixing and drug expulsion (Anton et al., 2008, Chauhan et al., 2016).
Fig. 5.
Lipid nanoparticles protect the skin from UV-radiation (left image), increase the stabilization and release of the active ingredient (right image). Reprinted with permission from (Müller et al., 2014). Copyright 2014, tks publisher.
The introduction of nanostructured lipid carriers (NLC) in the 2000s is capable to load a higher amount of active cosmeceutical ingredients, while better controlling their release due to the good blend of lipids (Wissing and Müller, 2002a, Wissing and Müller, 2002b, Mahant et al., 2018). The solid core of one of the SLN and NLC is an important characteristic that enables the stabilization of active ingredients which are chemically sensitive to other chemical species such as water or oxygen. The selection of the lipid plays an important role since the active ingredient must be solubilized and kept within the lipid core under the recommended storage condition and time. The improvement of chemical stability of many cosmeceutical active ingredients such as ascorbyl palmitate, retinoids, and coenzyme Q10 using this strategy has been published (Castro et al., 2009, Farboud et al., 2011, Carneiro et al.,2012, Jeon et al., 2013, Morales et al., 2015, Zhou et al., 2017, Alkhatib et al., 2021).
2.5.3. Skin effects
The application of cosmeceutical products on the skin plays protective passive rather than active aspects. Cosmeceutical products could be employed as simple toiletry and for protective purposes or may have some additional activity on the skin. Concerning the utilization of LNPs, which showed some privileges associated with their solid matrices such as the release of the CAIs and their transdermal absorption.
2.5.4. Release behavior
The release of CAIs from NLC and SLNs is an essential step to achieve the desired effect on the skin (Castro et al., 2007). The release profile of the CAI depends on several factors such as (i) the method of production of LNPs, (ii) their composition (i.e. type and concentration of surfactants), (iii) the solubilizing properties of these surfactants toward the incorporated AI, and the solubility and concentration of the CAI in the lipid matrix (oil/ water partition coefficient). The inner structure of the obtained LNPs is affected by these factors and therefore the release profile of incorporated materials can be changed from a slow to immediate release pattern (Souto et al., 2004a, Souto et al., 2004b, Üner et al., 2005, Pople and Singh, 2006, Khezri et al., 2021, Yeo et al., 2021).
2.5.5. Skin penetration
The majority of CAI are not intended for deep penetration and absorption through the skin, since they only perform a superficial action. Despite that, it is necessary to assess the activity of these topically applied cosmeceuticals in some detail. Transdermal absorption includes the penetration of the CAI and its absorption into the blood circulation. This may lead to a possible pharmacological response at a far site from the application area. Usually, CAIs are intended to perform a primarily local action, so formulating a cosmeceutical product that has limited absorption into the blood should be seriously considered. This could be achieved through LNPs dispersions which can control the rate of transdermal absorption of CAIs into the skin (Jain et al., 2005).
Transdermal absorption of the CAI after the application of common topical base ingredients is very limited, although such materials may become entrapped in the outer regions of the stratum corneum of the skin when massage is applied. Alteration of the release and active penetration into the various layers of the skin can be obtained for example through the creation of supersaturated systems (Wissing and Müller, 2001).
These systems could be achieved through the inclusion of LNPs into topical products such as emulsions, gels, and ointments. Increasing the saturated solubility causes an increase in the CAI's diffusion pressure into the skin. The CAI remains entangled in the lipid core during shelf life because the core matrix retains its polymorphic form. Following the application of a supersaturated formulation onto the skin, the lipid core matrix changes from a more unstable polymorph to a more ordered polymorph. This results in a significant release of CAI into a system already saturated with the same CAI, resulting in the desired supersaturation. Transdermal absorption studies using several interesting CAIs such as retinoids and molecular sunscreens have been conducted (Wissing and Müller, 2001, Wissing and Müller, 2002a, Wissing and Müller, 2002b, Charoenputtakun et al., 2014, Khameneh et al., 2015, Gilbert et al., 2016, Castleberry et al., 2017, Garcês et al., 2018). In certain cases, such as particulate and molecular ultraviolet (UV) sunscreens, a sustained release of the CAI with minimal penetration may be desired due to the negative effects that may occur if permeation into the skin takes place (Mariani et al., 1996, Newman et al., 2009, Netto MPharm and Jose, 2018, Jose and Netto, 2019, Liu and Bi, 2019).
3. Applications of LNPs in cosmeceuticals
A literature review of recent publications has been conducted to cover all recent cosmeceutical active ingredients that have been incorporated into the various types of LNPs (Table 1).
Table 1.
Recent examples of the different types of cosmeceutical active ingredients loaded in lipid nanoparticles.
| Application | CAI | Type of Nanoparticles | Reference |
|---|---|---|---|
| Whiting agents | Phenylethyl resorcinol | nanostructured lipid carriers | (Kim et al., 2017) |
| Glabridin | SLNs, NE | (Paliwal et al., 2020), (Liu et al., 2017) | |
| Kojic acid | LNPs | (Khezri et al., 2020) | |
| kojic acid dipalmitate | LNPs | (Mohammadi et al., 2020) | |
| Azelaic acid | Nanoemulsion | (Jacobus Berlitz et al., 2019) | |
| α-lipoic acid | LNPs | (Souto et al., 2005) | |
| Hydroquinone | LNPs | (Ghanbarzadeh et al., 2015) (Wu et al., 2017) |
|
| Antioxidants, Anti-aging, anti-wrinkle Agents |
Ellagic acid | Nanostructured lipid carriers | (Singh Hallan et al., 2020) |
| Coenzyme Q10 | NLC LNPs |
(Teeranachaideekul et al., 2007) (Gokce et al., 2012a, Gokce et al., 2012b, Lohan et al., 2015) |
|
| Ferulic acid | SLNs | (Gupta et al., 2020a, Gupta et al., 2020b) | |
| Resveratrol | LNPs LNC |
(Gokce et al., 2012a, Gokce et al., 2012b) (Sun et al., 2014) |
|
| green tea polyphenols | SLNs | (Dzulhi et al., 2018) | |
| Aloe vera | LNP | (Garcia-Orue et al., 2019, Rodrigues and Jose, 2020) |
|
| Curcumin | SLNs LNCs Lipid-based nanoparticles Nanostructured lipid carrier |
(Gonçalez et al., 2017, Shrotriya et al., 2018) (Waghule et al., 2020) (Caon et al., 2017) (Rapalli et al., 2020) |
|
| Peptides | Solid lipid nanoparticles | (Suter et al., 2016) | |
| Adenosine | Solid lipid nanoparticles | (Yeo et al., 2021) | |
| Anti-Acne | Retinoids Isotretinoin and α-tocopherol acetate |
SLNs LNPs |
(Castro et al., 2011, Raza et al., 2013, Morales et al., 2015, Gupta et al., 2020a, Gupta et al., 2020b) (Morales et al., 2015) |
| Adapalene adapalene + tea tree oil | SLNs LNE |
(Jain et al., 2014) (Najafi-Taher et al., 2018) |
|
| Cyproterone | LNPs | (Štecová et al., 2007) | |
| Benzoyl peroxide | SLNs | (Pokharkar et al., 2014) | |
| Neen oil | SLNs | [123] | |
| Dapsone | SLNs | .(Deshkar et al., 2019) | |
| Spironolactone | NSLC | (Kelidari et al., 2016) | |
| Roxithromycin | SLNs | (Krysztof and Wosick, 2015) | |
| Sunscreen | urucum oil + octyl methoxycinnamate |
SLNs |
(Andreo-Filho et al., 2018) |
| zinc oxide and octocrylene | SLNs | (Berkman and Yazan, 2012) | |
| Silymarin | SLNs | (Netto MPharm and Jose, 2018) | |
| Hair Loss and Alopecia | Minoxidil | SLNs | (Padois et al., 2011, Gomes et al., 2014) |
| Roxithromycin | SLNs | (Krysztof and Wosick, 2015) | |
| Otoba Wax | SLNs | (Rubiano et al., 2020) | |
| Flutamide | SLNs | (Hamishehkar et al., 2016) |
3.1. LNPs as a vehicle for whitening agents
Various whitening agents suffer from low water solubility, low stability, or insufficient skin penetration. For that reason, different research groups have developed whitening agents encapsulated in lipidic nanoparticles to improve the mentioned drawbacks. Ghanbarzadeh et al. have loaded hydroquinone in solid lipid nanoparticles (SLN) to enhance its stability and skin penetration. The obtained SLN developed by the hot melt homogenization technique showed high encapsulation efficiency with good physicochemical stability for five months. In vitro penetration analysis demonstrated three times higher drug accumulation in the skin and much less hydroquinone entrance to the systematic circulation (Ghanbarzadeh et al., 2015). In another study done by Wu et al., they prepared a nanostructured lipid carrier (NLC) loaded with hydroquinone to improve its light stability (Wu et al., 2017). They used the homogenization emulsification technique to prepare the nanoparticles obtaining a particle size of 393.30 ± 23 nm and loading efficiency of 22.13 ± 2.66%. These nanoparticles showed better skin permeability (125.1%) and better light stability after UVA/UVB irradiation with an inhibition rate (34.25%) in comparison to the blank (Wu et al., 2017).
Phenylethyl resorcinol (PR) is another new whitening agent that can inhibit tyrosinase activity, but it suffers from low light stability and poor solubility. A study was carried out by Kim et al. who loaded PR in nanostructured lipid carrier (NLC) using a hot-melted ultrasonic method. The particle size was 57.9 ± 1.3 nm, encapsulation efficiency, and loading capacity were 93.1 ± 4.2% and 8.5 ± 0.4%, respectively. The developed NLC showed high physicochemical and photo stabilities for at least three months at 4 ⁰C in the dark and 25 ⁰C under daylight. PR loaded NLC demonstrated significant inhibition of cellular tyrosinase enzyme which suggesting its possible utilization for effective delivery of skin whitening agent (Kim et al., 2017). Azelaic acid-loaded nanoemulsion with hyaluronic acid was conceived by Jacobus Berlitz et al. (Jacobus Berlitz et al., 2019). The oil in water nanoemulsion has a particle size of 419 ± 23 nm with an encapsulation efficiency of azelaic acid of 84.65%. The nanoemulsion showed a decrease in tyrosinase activity and good skin permeation and retention (Jacobus Berlitz et al., 2019). Another study was conducted by Khezri et al. who prepared Kojic acid (KA) loaded solid lipid nanoparticles (KA-SLNs) using high-speed homogenization followed by the sonication method to improve the Kojic acid skin penetration capability and tyrosinase inhibitory activity (Khezri et al., 2020). The obtained nanoparticles have a particle size of 156.97 ± 7.15 nm, encapsulation efficiency 59.02 ± 0.74%, drug loading 14.755 ± 1.63%, and stability for three months. The prepared nanoparticles showed more tyrosinase inhibition activity in comparison to the pure KA. The in vitro and ex-vivo percutaneous absorption study showed that KA-SLNs improved the percutaneous delivery of KA (Khezri et al., 2020). These developed lipid nanoparticles have improved the solubility, stability, and skin penetration of the mentioned whitening agents. Therefore, they have a great potential for the treatment of hyperpigmentation disorders.
3.2. LNPs loaded with antioxidants, anti-aging, and anti-wrinkle agents
The various molecules that are used as an antioxidant or as anti-aging suffer from different problems concerning its topical application. They could have low water solubility, low skin permeation, or poor stability. Encapsulating these molecules in various types of lipid nanoparticles could resolve the mentioned problems.
The production of reactive oxygen species (ROS) could cause damage to the skin. In 2012, Gokce et al. has utilized the antioxidant Resveratrol (RSV) to be loaded in solid lipid nanoparticles (SLN) and in nanostructured lipid carrier (NLC) to improve its skin absorption. The drug entrapment efficiency in the NLC was 18% higher than in the SLN. RSV loaded in NLC showed less ROS production in the cytofluorometric study. Moreover, the ex vivo skin study demonstrated that the RSV accumulation was in the epidermis in the case of the SLN, while more accumulated in the dermis in the case of the NLC (Gokce et al., 2012a, Gokce et al., 2012b). Another research conducted by Lohan et al. utilized coenzyme Q10 (CoQ10) as an antioxidant that was encapsulated in ultra-small lipid nanoparticles. The developed nanoparticles showed a good antioxidant activity after cellular exposure to UVA and UVB irradiation with a reduction of radical formation up to 23% in comparison to the control cells without loaded nanoparticles (Lohan et al., 2015). In 2016, Suter et al. have reported the utilization of solid lipid nanoparticles (SLN) as an effective vehicle to improve peptide delivery into the skin (Suter et al., 2016). They loaded a heptapeptide DEETGEF (P7) that could disrupt the Nrf2-Keap1 complex which would up-regulate the expression of various antioxidant enzymes. The particle size of the SLN-P7 was 173 nm, and entrapment efficiency of 90.8%. The ex-vivo study demonstrated the stimulation of NQO1 (NAD(P)H quinone oxidoreductase), HMOX1 (Heme oxygenase-1), and PRDX1 (Peroxiredoxin-1) genes which are cell-protecting enzymes. Moreover, the applied SLN-P7 showed good UV protection by inhibiting the depletion of Langerhans cells (LC) and also decreasing the DNA damage as confirmed by the percentage of DNA damage marker 8-hydroxy-2́-deoxyguanosine that decreased by 20% in comparison to the placebo.
A study conducted by Singh Hallan et al. to encapsulate Ellagic acid (EA) -antioxidant agent- in nanostructured lipid carriers to improve its water solubility for topical application (Singh Hallan et al., 2020). They created two kinds of formulas by combining two different lipid compositions, tristearin/tricaprylin (NLC-EA1) and tristearin/labrasol (NLC-EA2). The two formulas showed good stability for 40 days in the case of NLC-EA2 and two months in the case of NLC-EA1. Moreover, they showed antioxidant activity of around 60% and good skin penetration. Therefore, the developed NLC-EA formulas have demonstrated the capability to deliver Ellagic acid to the skin with high antioxidant activity.
Very recently, Lee et al. have reported the loading of adenosine (AD)- an agent used to treat wrinkles- in solid lipid nanoparticles for improving its skin permeation. Then, the AD-SLN was incorporated into the elastic artificial skin (Yeo et al., 2021). The SLN and the elastic artificial skin showed good biocompatibility. Moreover, the adenosine release profile showed a sustained release manner for 48 h. The release amount of adenosine from the elastic artificial skin and SLN was higher 5 times, and 10 times, respectively in comparison to adenosine solution alone. Therefore, the produced nanoparticles serve as a potential topical delivery system for skin wrinkles treatment.
3.3. LNPs loaded with Anti-acne agents
Isotretinoin (ITR) is considered the most commonly used therapy for various types of acne. However, it is topical administration could cause skin irritation, erythema, and peeling of the skin. Raza et al. have developed isotretinoin-loaded solid nanoparticles using the formulation by design (FbD) approach (Raza et al., 2013). The loaded nanoparticles showed high entrapment of 89.49 ± 4.1% with high stability and a particle size of 75.3 ± 2.4 nm. Moreover, the prepared nanoparticles exhibited good skin penetration with a high anti-acne activity without causing any inflammation to the skin in comparison to the marketed drug (Raza et al., 2013).
Adapalene (ADA) is another potent anti-acne drug that is considered the second generation of retinoid compounds with better acceptability. Agrawal and collaborators have loaded ADA in solid lipid nanoparticles (SLN) and investigate the topical delivery efficacy (Jain et al., 2014). The nano-formulated gel showed the potential to target the skin epidermal skin layer without systemic penetration. Therefore, reduce the possible adverse effects of the loaded drug.
Spironolactone (SP) is an anti-androgenic drug that proved to treat mild to moderate acne conditions. Nokhodchi and collaborators have reported the loading of spironolactone in nanostructured lipid carriers for effective topical delivery in the treatment of facial mild to moderate acne vulgaris (Kelidari et al., 2016). The formulated nanoparticles didn't show toxicity on fibroblast cells with good biocompatibility. Besides, they reduced the total mean number of lesions in the clinical treated patients with a significant increase in the water retention of the treated skin which suggested a good treatment behavior with skincare benefits.
3.4. Agents loaded LNPs for sunscreen application
The utilization of sunscreen products is highly recommended by various health agencies to protect the skin from any damage or sunburns. There are different types of chemical UV filters such as zinc oxide, titanium dioxide, or octyl methoxycinnamate (OMC) that are used as sunscreen protectors. However, they may cause some skin irritation or allergy. Therefore, the encapsulation of these compounds in lipid nanoparticles could reduce their side effects and minimize their photodegradation. Andréo-Filho et al. have loaded the OMC in solid colloidal nanocarriers composed of a lipid base and vegetable oils. They also developed another formula with the replacement of 20% of OMC amount with urucum oil to decrease the used amount of chemical UV filter and could reduce the oxidative stress or radiation-induced inflammation (Andreo-Filho et al., 2018). The designed two formulas didn't demonstrate any toxic effect on the skin. Moreover, there was no reduction of the skin protection factor (SPF) in the two formulas although there was a replacement of 20% of OMC by urucum oil as confirmed by in vitro studies. Therefore, this study suggests that the amount of OMC could be reduced to increase the safety of sunscreen products. Another study was conducted by Berkman and Yazan by incorporating zinc oxide as a physical blocker and octocrylene as a chemical absorber in solid lipid nanoparticles to obtain a UV blocking potential of the sunscreen product (Berkman and Yazan, 2012). The prepared nanoparticles showed good stability for 360 days with pH values between 5.4 and 5.9 which could be buffered by the skin. TransporeTM test proves the effectiveness of the developed formulas in the UV blocking potential. So, the lipid nanoparticles have the potential to enhance the activity of chemical UV filters as sunscreen products.
3.5. Drugs loaded LNPs for hair loss treatment
Various drugs were used for the treatment of hair loss. However, they showed several side effects due to the systematic absorption. Various research groups have developed lipid nanoparticles to deliver these drugs topically without their skin penetration into the circulation. Padois et al. have designed solid lipid nanoparticles loaded with 5% of minoxidil and were compared them with the commercially available minoxidil solution (Padois et al., 2011). It is known that the commercial solution causes skin irritation and redness as the formula contains high ethanol and propylene glycol content. The developed solid lipid nanoparticles showed the same efficiency in comparison to the commercial solution without any corrosive effect (Padois et al., 2011). Another study was conducted by Hamishehkar et al. who loaded flutamide (5α-reductase enzyme inhibitor) in solid lipid nanoparticles for selective follicular targeting as a treatment of androgenic alopecia (Hamishehkar et al., 2016). These nanoparticles showed stability for two months and higher skin deposition in comparison to flutamide hydroalcoholic solution which confirms the better skin localization of the drug. Moreover, the in vivo studies demonstrated more hair growth that could be explained by the higher accumulation and the sustained release of the flutamide from the SLN in comparison to flutamide hydroalcoholic solution (Hamishehkar et al., 2016). A study was done by Gomes et al. who encapsulated two anti-alopecia drugs (Minoxidil and finasteride) in nanostructured lipid carriers prepared by ultrasonication (Gomes et al., 2014). They achieved a high loading efficiency with controlled release profiles. Moreover, a low-level penetration of the pig ear skin for both drugs demonstrated the good suitability of the developed nanoparticles for the topical delivery of anti-alopecia drugs (Gomes et al., 2014).
4. Cosmeceutical products containing lipid nanoparticles on the market
From the previously mentioned privilege of the utilization of lipid nanoparticles in the cosmeceutical products. Various companies have raised and applied these LNPs on the market. The most common companies that developed such products are Dr. Rimpler (Germany), Chemisches Laboratorium Dr. Kurt Richter (CLR) (Germany), Amore Pacific (South Korea), Beate Johnen (Germany), La praire (Switzerland), and others. Table 2 showed examples of these cosmeceutical based lipid nanoparticles products on the market.
Table 2.
Examples of cosmeceutical based lipid nanoparticles products on the market.
| Product name | Company | Type of the used lipid nanoparticles | Uses |
|---|---|---|---|
| Clinicians Complex Liposome Face & Neck Lotion | Clinicians Complex | Liposome | Moisturizer & prevents photoaging |
| Mayu niosome base cream | Laon Cosmetics | Niosome | Moisturizing & antioxidant |
| Cutanova cream nanorepair Q10 | Dr. Rimpler | NLC | Antioxidant and antiaging |
| Cutanova Cream Nanovital Q10 | Dr. Rimpler | NLC | Energy-boosting, antioxidative, and antiaging |
| NanoLipid Q10 CLR | CLR | NLC | Antioxidant and antiaging |
| Iope Supervital Extra Moist Eye Cream | Amore Pacific | NLC | Treat wrinkles around the eye |
| NLC deep effect eye serum | Beate Johnen | NLC | Treat wrinkles around the eye |
| Swiss Cellular White Illuminating Eye Essence | La praire | NLC | Remove darkness under the eye |
| Olivenöl Anti Falten Pflegekonzentrat | Dr. Theiss/Medipharma Cosmetics | NLC | Remove wrinkles around the eye and mouth |
5. Smart and sustainable nanomaterials
Recently, several European and international entities such as the European Commission's new Action Plan for a Circular Economy, The European Green Deal, the new European Industrial Strategy, and the Chemicals Strategy for Sustainability are putting huge efforts to create a sustainable economy. Just in October 2020, they launched ambitious plans and policies to create a fair, sustainable, and inclusive European Union's economy. These policies are following the United Nations Sustainable Development Goals 2030, these policies demand that any novel substance or product should be functional, safe, cost-effective, and sustainable to ensure acceptance by consumers and compliance with regulation and guidelines. LNPs technology is a promising sector that could satisfy this ambitious green growth (Gottardo et al., 2021). LNPs are frequently reported among those technologies that could satisfy the requirements of green growth since they are often composed of edible natural, and biodegradable materials (Dahl et al., 2007, Varma, 2012, Gilbertson et al., 2015, Lu and Ozcan, 2015). Significant advances have been made in this field, for example by producing and using safe nano-ingredients such as nano-cellulose derivatives instead of synthetic nano-polymers or implementing green synthesis processes that involve the use of less hazardous and renewable reagents, solvents, and starting materials (Dahl et al., 2007, Lu and Ozcan, 2015, Oehlke et al., 2017). Even though significant advancements in the nanotechnology field in the last decade to understand the environmental and toxicological behavior of nanomaterials, opening various concerns regarding the safety and sustainability applications, especially those products designed for internal administration such as nano pharmaceutical products (Jantunen et al., 2018, Friedersdorf et al., 2019, Grieger et al., 2019). Recently, regulatory bodies have raised some concerns about assessing the risk and the governance of the type of nanomaterials that can be responded to external stimuli, which are also known as ‘smart nanomaterials’ (council, 2007). Recently, controlled delivery systems have flattened their manner from pharmaceutical products to cosmeceuticals (Kaul et al., 2018, Santos et al., 2019). A new class of stimuli-responsive LNPs products was developed and commercialized to enclose CAIs capable of treating various skin disorders such as contact dermatitis and skin photo-damage (Aziz et al., 2019). Thus, it is indispensable to encourage the development of novel smart nano-cosmeceuticals that are safe, effective, and sustainable. It is critical to ensure a sharp but flexible regulatory structure that can keep up with innovation without creating unwelcome barriers for industry, and in this contest, LNPs could be a suitable candidate because they meet the above-mentioned criteria.
6. Nanotoxicity and regulatory issues
Various researches have shown that nanoparticles with particle size less than 100 nm could penetrate the skin to the systematic circulation and cause various side effects (Khan et al., 2019). For that reason, in 2013, Keck and Mueller proposed a Nanopharmaceutical Classification System (NCS) to assess the toxicity of the nanomaterials based on their size (below or above 100 nm) and the biodegradability of the substances formed the nanomaterials (Keck and Müller, 2013). According to these two parameters, nanomaterials are classified into four classes (class I-IV). Class I describes the nanomaterials that have a particle size between 100 and 1000 nm (limiting access to cells) and composed of biodegradable materials. These nanomaterials are considered safe and tolerable. However, the nanomaterials that have a particles size less than 100 nm (can penetrates the cells) and consist of non-biodegradable materials are classified as class IV and are considered with a potential risk and toxicity. Regarding the most used lipid nanoparticles in cosmeceutical field have a particle size above 100 nm and consist of biodegradable substances. Therefore, they belong to the good tolerated class I.
Regarding the regulatory issues, as we mentioned previously in the introduction, the United State FDA doesn’t recognize the term cosmeceuticals and they have three classifications: cosmetics, drugs and OTC drugs and it depends on the claim of the product (Raj and Chandrul, 2016). However, other countries have exhibited specific definitions in their regulatory affairs. In Japan, they introduce “quasi-drug” concept to describe the cosmeceutical products. These products should preapproved by the authority before to release to the market (Lab, 2021). In Korea, the Korea Food and Drug Administration (KFDA) defined the cosmeceutical products under “functional cosmetics” concept and they provide the necessary regulations for their approval (safety, 2021). In the European Union (EU), they have regulations regarding cosmetics under Cosmetic Directive 76/768/EEC. However, they don’t have a special category of cosmeceuticals, but any claimed product by the company should have the specific approval especially for nanomaterials containing products. According to the EU cosmetic regulation, the company should mention a “nano” word in any product contains nanomaterials in their ingredients (nano). Finally, China Food and Drug Administration (CFDA) defined the concept “cosmetics for special use” to identify the cosmeceutical products. These products should have regulatory approval from the CFDA before the release to the market. Moreover, all required tests of safety and health quality should be provided (ceway). In conclusion, there still insufficient global regulations for the nanomaterials and the cosmeceutical products and there is a high demand for long term research to study the safety of the chronic use of such products.
7. Discussion and conclusion
LNPs are colloidal dispersed systems with reshaped characteristics of other nanoparticles such as nanosuspensions, liposomes, microemulsions, and polymeric nanoparticles which have recently attracted the attention of many researchers. The active ingredients may be susceptible to degradation in the production process especially when utilizing the hot homogenization technique due to the generation of heat and stress. Therefore, these nanosystems could provide a more chemical and physiological stables drug delivery system with fewer limitations. Moreover, nanostructured lipid carriers could overcome the gelation tendency if possibly occur. Therefore, it is necessary to select an adequate production method. Furthermore, factors such as particle size, the presence of colloidal forms, shape, and release pattern of the CAI from the lipid matrix should be considered. The bibliometric analysis showed the development and application of lipid nanoparticles in the field of cosmeceuticals is a recent topic of research as the research progress began in the 2010s with the most used nanoparticles are the solid lipid nanoparticles and the nanostructured lipid carriers. The various characteristics of the lipid nanoparticles applied in cosmeceutical application demonstrated their efficacy and effectiveness in improved the cosmeceutical products. They provide a good adherence on the skin surface which enhances the occlusion and the hydration of the skin without changing the skin pH. In addition, they are utilized as a good reservoir of cosmeceutical active ingredients to protect them from the external environment and improved their stability. These encapsulation properties enable to get a release profile of the CAIs with good skin penetration. Therefore, these interesting properties make the application of lipid nanoparticles in the field of cosmeceutical more beneficial and effective. They could be encapsulated with the adequate cosmeceutical active ingredient to provide a better solubility, stability, efficacy, and release profile which enable their application as sunscreen, antioxidants, hair loss treatment, anti-acne, and other applications were addressed. This technology is more promising with selective delivery as smart nanomaterials and with confidence sustainability. More research is needed to be achieved in the development of multi-purposed lipid nanoparticles in the cosmeceutical to provide a wider application. Moreover, the formation of targeted lipid nanoparticles with a controlled stimuli release of the CAIs could achieve even a better and specific delivery to the target with much fewer side effects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohyeddin Assali, Email: m.d.assali@najah.edu.
Abdel-Naser Zaid, Email: anzaid@najah.edu.
References
- Administration, U. S. F. a. D., 24/08/2020. Retrieved 09/03/2021, 2021, from https://www.fda.gov/cosmetics/cosmetics-labeling-regulations/summary-cosmetics-labeling-requirements.
- Alkhatib Y., Blume G., Thamm J., Steiniger F., Kralisch D., Fischer D. Overcoming the hydrophilicity of bacterial nanocellulose: Incorporation of the lipophilic coenzyme Q10 using lipid nanocarriers for dermal applications. Eur. J. Pharm. Biopharm. 2021;158:106–112. doi: 10.1016/j.ejpb.2020.10.021. [DOI] [PubMed] [Google Scholar]
- Andreo-Filho N., Bim A.V.K., Kaneko T.M., Kitice N.A., Haridass I.N., Abd E., Santos Lopes P., Thakur S.S., Parekh H.S., Roberts M.S., Grice J.E., Benson H.A.E., Leite-Silva V.R. Development and evaluation of lipid nanoparticles containing natural botanical oil for sun protection: characterization and in vitro and in vivo human skin permeation and toxicity. Skin Pharmacol. Physiol. 2018;31(1):1–9. doi: 10.1159/000481691. [DOI] [PubMed] [Google Scholar]
- Anton N., Benoit J.-P., Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates—a review. J. Control. Release. 2008;128(3):185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Aziz Z.A.A., Mohd-Nasir H., Ahmad A., Mohd. Setapar S.H., Peng W.L., Chuo S.C., Khatoon A., Umar K., Yaqoob A.A., Mohamad Ibrahim M.N. Role of nanotechnology for design and development of cosmeceutical: application in makeup and skin care. Front. Chem. 2019;7 doi: 10.3389/fchem.2019.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman M., Yazan Y. Solid lipid nanoparticles: A possible vehicle for zinc oxide and octocrylene. Pharmazie. 2012;67:202–208. [PubMed] [Google Scholar]
- Blaak J., Staib P. The relation of pH and skin cleansing. pH of the Skin: Issues and Challenges. C. Surber, C. Abels and H. Maibach, Karger. 2018;54:132–142. doi: 10.1159/000489527. [DOI] [PubMed] [Google Scholar]
- Bonifácio, B. V., da Silva, P.B., dos Santos Ramos, M.A., et al., 2014. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int. J. Nanomed. 9, 1. [DOI] [PMC free article] [PubMed]
- Brandt F.S., Cazzaniga A., Hann M. Cosmeceuticals: current trends and market analysis. Semin. Cutan. Med. Surg. 2011;30(3):141–143. doi: 10.1016/j.sder.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Bugaj A.M. Intradermal delivery of active cosmeceutical ingredients. Novel Delivery Syst. Transdermal Intradermal Drug Deliv. 2015;1:209–242. [Google Scholar]
- Caon T., Mazzarino L., Simões C.M.O., Senna E.L., Silva M.A.S. Lipid-and polymer-based nanostructures for cutaneous delivery of curcumin. AAPS PharmSciTech. 2017;18(3):920–925. doi: 10.1208/s12249-016-0554-7. [DOI] [PubMed] [Google Scholar]
- Carneiro, G., E. L. Silva, L. A. Pacheco, et al., 2012. Formation of ion pairing as an alternative to improve encapsulation and anticancer activity of all-trans retinoic acid loaded in solid lipid nanoparticles. Int. J. Nanomed. 7, 6011. [DOI] [PMC free article] [PubMed]
- Castleberry S.A., Quadir M.A., Sharkh M.A., Shopsowitz K.E., Hammond P.T. Polymer conjugated retinoids for controlled transdermal delivery. J. Control. Release. 2017;262:1–9. doi: 10.1016/j.jconrel.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro G.A., Oliveira C.A., Mahecha G.A.B., Ferreira L.A.M. Comedolytic effect and reduced skin irritation of a new formulation of all-trans retinoic acid-loaded solid lipid nanoparticles for topical treatment of acne. Arch. Dermatol. Res. 2011;303(7):513–520. doi: 10.1007/s00403-011-1130-3. [DOI] [PubMed] [Google Scholar]
- Castro G.A., Coelho A.L.L.R., Oliveira C.A., Mahecha G.A.B., Oréfice R.L., Ferreira L.A.M. Formation of ion pairing as an alternative to improve encapsulation and stability and to reduce skin irritation of retinoic acid loaded in solid lipid nanoparticles. Int. J. Pharm. 2009;381(1):77–83. doi: 10.1016/j.ijpharm.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Castro G.A., Oréfice R.L., Vilela J.M.C., Andrade M.S., Ferreira L.A.M. Development of a new solid lipid nanoparticle formulation containing retinoic acid for topical treatment of acne. J. Microencapsul. 2007;24(5):395–407. doi: 10.1080/02652040701288519. [DOI] [PubMed] [Google Scholar]
- Castro N.R., Pinto C.S.C., Santos E.P., Mansur C.R.E. Hybrid Vesicular Nanosystems Based on Lipids and Polymers Applied in Therapy, Theranostics, and Cosmetics. Crit. Rev. Therapeutic Drug Carrier Syst. 2020;37(3):271–303. doi: 10.1615/CritRevTherDrugCarrierSyst.2020030671. [DOI] [PubMed] [Google Scholar]
- ceway, 2021, from https://ceway.eu/international-services/china/.
- Charoenputtakun P., Pamornpathomkul B., Opanasopit P., et al. Terpene composited lipid nanoparticles for enhanced dermal delivery of all-trans-retinoic acids. Biol. Pharm. Bull. 2014:b14–00015. doi: 10.1248/bpb.b14-00015. [DOI] [PubMed] [Google Scholar]
- Chauhan H., Mohapatra S., Munt D.J., Chandratre S., Dash A. Physical-chemical characterization and formulation considerations for solid lipid nanoparticles. AAPS PharmSciTech. 2016;17(3):640–651. doi: 10.1208/s12249-015-0394-x. [DOI] [PubMed] [Google Scholar]
- Council, I.R.G., 2007. Nanotechnology Risk Governance: Recommendations for a global, coordinated approach to the governance of potential risks: 1-39.
- Dahl J.A., Maddux B.L.S., Hutchison J.E. Toward greener nanosynthesis. Chem. Rev. 2007;107(6):2228–2269. doi: 10.1021/cr050943k. [DOI] [PubMed] [Google Scholar]
- de Souza M.L., dos Santos W.M., de Sousa A.L.M.D., de Albuquerque Wanderley Sales V., Nóbrega F.P., de Oliveira M.V.G., Rolim-Neto P.J. Lipid Nanoparticles as a Skin Wound Healing Drug Delivery System: Discoveries and Advances. Curr. Pharm. Des. 2020;26(36):4536–4550. doi: 10.2174/1381612826666200417144530. [DOI] [PubMed] [Google Scholar]
- De Vringer, T., 1997. Topical preparation containing a suspension of solid lipid particles. Google Patents. EP0786251A3.
- Desfrançois C., Auzély R., Texier I. Lipid nanoparticles and their hydrogel composites for drug delivery: a review. Pharmaceuticals. 2018;11(4):118. doi: 10.3390/ph11040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshkar S.S., Bhalerao S.G., Jadhav M.S., Shirolkar S.V. Formulation and optimization of topical solid lipid nanoparticles based gel of Dapsone using design of experiment. Pharmaceut. Nanotechnol. 2018;6(4):264–275. doi: 10.2174/2211738506666181105141522. [DOI] [PubMed] [Google Scholar]
- Dingler A., Blum R., Niehus H., et al. Solid lipid nanoparticles (SLNTM/LipopearlsTM) a pharmaceutical and cosmetic carrier for the application of vitamin E in dermal products. J. Microencapsul. 1999;16:751–767. doi: 10.1080/026520499288690. [DOI] [PubMed] [Google Scholar]
- Doktorovova S., Kovačević A.B., Garcia M.L., Souto E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016;108:235–252. doi: 10.1016/j.ejpb.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Duan Y., Dhar A., Patel C., Khimani M., Neogi S., Sharma P., Siva Kumar N., Vekariya R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020;10(45):26777–26791. doi: 10.1039/d0ra03491f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C.N., Riley T.V., Carson K.C., Budgeon C.A., Siffleet J. The effect of an acidic cleanser versus soap on the skin pH and micro-flora of adult patients: a non-randomised two group crossover study in an intensive care unit. Intensive Crit. Care Nurs. 2013;29(5):291–296. doi: 10.1016/j.iccn.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Dzulhi S., Anwar E., Nurhayati T. Formulation, characterization and in vitro skin penetration of green tea (Camellia sinensis L.) leaves extract-loaded solid lipid nanoparticles. J. Appl. Pharmaceut. Sci. 2018;8:057–062. [Google Scholar]
- El Enshasy H.A., El Marzugi N.A., Elsayed E.A., Ling O.M., Malek R.A., Kepli A.N., Othman N.Z., Ramli S. In: Fungal Nanobionics: Principles and Applications. Prasad R., Kumar V., Kumar M., Wang S., editors. Springer; Singapore: 2018. Medical and cosmetic applications of fungal nanotechnology: production, characterization, and bioactivity; pp. 21–59. [DOI] [Google Scholar]
- Farboud E.S., Nasrollahi S.A., Tabbakhi Z. Novel formulation and evaluation of a Q10-loaded solid lipid nanoparticle cream: in vitro and in vivo studies. Int. J. Nanomed. 2011;6:611. doi: 10.2147/IJN.S16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton L. Pharmaceutical Press; 2013. Remington: Essentials of Pharmaceutics. [Google Scholar]
- Friedersdorf L.E., Bjorkland R., Klaper R.D., Sayes C.M., Wiesner M.R. Fifteen years of nanoEHS research advances science and fosters a vibrant community. Nat. Nanotechnol. 2019;14(11):996–998. doi: 10.1038/s41565-019-0574-z. [DOI] [PubMed] [Google Scholar]
- Fulekar M. IK International Pvt Ltd.; 2010. Nanotechnology: importance and applications. [Google Scholar]
- Garcês A., Amaral M.H., Sousa Lobo J.M., Silva A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018;112:159–167. doi: 10.1016/j.ejps.2017.11.023. [DOI] [PubMed] [Google Scholar]
- Garcia-Orue I., Gainza G., Garcia-Garcia P., Gutierrez F.B., Aguirre J.J., Hernandez R.M., Delgado A., Igartua M. Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing applications. Int. J. Pharm. 2019;556:320–329. doi: 10.1016/j.ijpharm.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Ghanbarzadeh S., Hariri R., Kouhsoltani M., Shokri J., Javadzadeh Y., Hamishehkar H. Enhanced stability and dermal delivery of hydroquinone using solid lipid nanoparticles. Colloids Surfaces B: Biointerfaces. 2015;136:1004–1010. doi: 10.1016/j.colsurfb.2015.10.041. [DOI] [PubMed] [Google Scholar]
- Gilbert E., Roussel L., Serre C., Sandouk R., Salmon D., Kirilov P., Haftek M., Falson F., Pirot F. Percutaneous absorption of benzophenone-3 loaded lipid nanoparticles and polymeric nanocapsules: A comparative study. Int. J. Pharm. 2016;504(1-2):48–58. doi: 10.1016/j.ijpharm.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Gilbertson L.M., Zimmerman J.B., Plata D.L., Hutchison J.E., Anastas P.T. Designing nanomaterials to maximize performance and minimize undesirable implications guided by the Principles of Green Chemistry. Chem. Soc. Rev. 2015;44(16):5758–5777. doi: 10.1039/c4cs00445k. [DOI] [PubMed] [Google Scholar]
- Gokce E.H., Korkmaz E., Dellera E., et al. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: evaluation of antioxidant potential for dermal applications. Int. J. Nanomed. 2012;7:1841. doi: 10.2147/IJN.S29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce E.H., Korkmaz E., Tuncay-Tanrıverdi S., et al. A comparative evaluation of coenzyme Q10-loaded liposomes and solid lipid nanoparticles as dermal antioxidant carriers. Int. J. Nanomed. 2012;7:5109. doi: 10.2147/IJN.S34921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M.J., Martins S., Ferreira D., et al. Lipid nanoparticles for topical and transdermal application for alopecia treatment: development, physicochemical characterization, and in vitro release and penetration studies. Int. J. Nanomed. 2014;9:1231. doi: 10.2147/IJN.S45561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalez M., Rigon R., Pereira-da-Silva M., et al. Curcumin-loaded cationic solid lipid nanoparticles as a potential platform for the treatment of skin disorders. Pharmazie. 2017;72:721–727. doi: 10.1691/ph.2017.7101. [DOI] [PubMed] [Google Scholar]
- Gottardo S., Mech A., Drbohlavová J., Małyska A., Bøwadt S., Riego Sintes J., Rauscher H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact. 2021;21:100297. doi: 10.1016/j.impact.2021.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger K., Jones J.L., Hansen S.F., Hendren C.O., Jensen K.A., Kuzma J., Baun A. Best practices from nano-risk analysis relevant for other emerging technologies. Nat. Nanotechnol. 2019;14(11):998–1001. doi: 10.1038/s41565-019-0572-1. [DOI] [PubMed] [Google Scholar]
- Gupta K.M., Das S., Chow P.S., Macbeath C. Encapsulation of ferulic acid in lipid nanoparticles as antioxidant for skin: Mechanistic understanding through experiment and molecular simulation. ACS Appl. Nano Mater. 2020;3(6):5351–5361. [Google Scholar]
- Gupta S., Gupta S., Jindal N., Jindal A., Bansal R. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013;4(4):267. doi: 10.4103/2229-5178.120635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Wairkar S., Bhatt L.K. Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel for the treatment of acne. J. Microencapsul. 2020;37(8):557–565. doi: 10.1080/02652048.2020.1823499. [DOI] [PubMed] [Google Scholar]
- Muller H., Shegokar R.R., Keck C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011;8:207–227. doi: 10.2174/157016311796799062. [DOI] [PubMed] [Google Scholar]
- Haider M., Abdin S.M., Kamal L., Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics. 2020;12(3):288. doi: 10.3390/pharmaceutics12030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamishehkar H., Ghanbarzadeh S., Sepehran S., Javadzadeh Y., Adib Z.M., Kouhsoltani M. Histological assessment of follicular delivery of flutamide by solid lipid nanoparticles: potential tool for the treatment of androgenic alopecia. Drug Dev. Ind. Pharm. 2016;42(6):846–853. doi: 10.3109/03639045.2015.1062896. [DOI] [PubMed] [Google Scholar]
- Hitce J., Xu J., Brossat M., Frantz M.-C., Dublanchet A.-C., Philippe M., Dalko-Csiba M. UN sustainable development goals: How can sustainable/green chemistry contribute? Green chemistry as a source of sustainable innovations in the cosmetic industry. Curr. Opin. Green Sustain. Chem. 2018;13:164–169. doi: 10.1016/j.cogsc.2018.06.019. [DOI] [Google Scholar]
- Iqbal B., Ali J., Baboota S. Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int. J. Dermatol. 2018;57(6):646–660. doi: 10.1111/ijd.13902. [DOI] [PubMed] [Google Scholar]
- Jacobus Berlitz S., De Villa D., Maschmann Inácio L.A., Davies S., Zatta K.C., Guterres S.S., Külkamp-Guerreiro I.C. Azelaic acid-loaded nanoemulsion with hyaluronic acid–a new strategy to treat hyperpigmentary skin disorders. Drug Dev. Ind. Pharm. 2019;45(4):642–650. doi: 10.1080/03639045.2019.1569032. [DOI] [PubMed] [Google Scholar]
- Jain A.K., Jain A., Garg N.K., Agarwal A., Jain A., Jain S.A., Tyagi R.K., Jain R.K., Agrawal H., Agrawal G.P. Adapalene loaded solid lipid nanoparticles gel: an effective approach for acne treatment. Colloids Surf., B. 2014;121:222–229. doi: 10.1016/j.colsurfb.2014.05.041. [DOI] [PubMed] [Google Scholar]
- Jain S.K., Chourasia M.K., Masuriha R., Soni V., Jain A., Jain N.K., Gupta Y. Solid lipid nanoparticles bearing flurbiprofen for transdermal delivery. Drug Delivery. 2005;12(4):207–215. doi: 10.1080/10717540590952591. [DOI] [PubMed] [Google Scholar]
- Jaiswal P., Gidwani B., Vyas A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2014;44(1):27–40. doi: 10.3109/21691401.2014.909822. [DOI] [PubMed] [Google Scholar]
- Jantunen A.P.K., Gottardo S., Rasmussen K., Crutzen H.P. An inventory of ready-to-use and publicly available tools for the safety assessment of nanomaterials. NanoImpact. 2018;12:18–28. doi: 10.1016/j.impact.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H.S., Seo J.E., Kim M.S., Kang M.H., Oh D.H., Jeon S.O., Seong Hoon Jeong, Choi Y.W., Lee S. A retinyl palmitate-loaded solid lipid nanoparticle system: effect of surface modification with dicetyl phosphate on skin permeation in vitro and anti-wrinkle effect in vivo. Int. J. Pharm. 2013;452(1-2):311–320. doi: 10.1016/j.ijpharm.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Jose J., Netto G. Role of solid lipid nanoparticles as photoprotective agents in cosmetics. J. Cosmet. Dermatol. 2019;18(1):315–321. doi: 10.1111/jocd.12504. [DOI] [PubMed] [Google Scholar]
- Kaul S., Gulati N., Verma D., Mukherjee S., Nagaich U. Role of nanotechnology in cosmeceuticals: a review of recent advances. J. Pharmaceut. 2018;2018:1–19. doi: 10.1155/2018/3420204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck C.M., Müller R.H. Nanotoxicological classification system (NCS) – A guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur. J. Pharm. Biopharm. 2013;84(3):445–448. doi: 10.1016/j.ejpb.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Kelidari H.R., Saeedi M., Hajheydari Z., Akbari J., Morteza-Semnani K., Akhtari J., Valizadeh H., Asare-Addo K., Nokhodchi A. Spironolactone loaded nanostructured lipid carrier gel for effective treatment of mild and moderate acne vulgaris: A randomized, double-blind, prospective trial. Colloids Surf., B. 2016;146:47–53. doi: 10.1016/j.colsurfb.2016.05.042. [DOI] [PubMed] [Google Scholar]
- Khameneh B., Halimi V., Jaafari M.R., et al. Safranal-loaded solid lipid nanoparticles: evaluation of sunscreen and moisturizing potential for topical applications. Iran. J. Basic Med. Sci. 2015;18:58. [PMC free article] [PubMed] [Google Scholar]
- Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. Arabian J. Chem. 2019;12(7):908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- Khezri K., Saeedi M., Morteza-Semnani K., Akbari J., Hedayatizadeh-Omran A. A promising and effective platform for delivering hydrophilic depigmenting agents in the treatment of cutaneous hyperpigmentation: kojic acid nanostructured lipid carrier. Artif. Cells Nanomed. Biotechnol. 2021;49(1):38–47. doi: 10.1080/21691401.2020.1865993. [DOI] [PubMed] [Google Scholar]
- Khezri K., Saeedi M., Morteza-Semnani K., Akbari J., Rostamkalaei S.S. An emerging technology in lipid research for targeting hydrophilic drugs to the skin in the treatment of hyperpigmentation disorders: kojic acid-solid lipid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2020;48(1):841–853. doi: 10.1080/21691401.2020.1770271. [DOI] [PubMed] [Google Scholar]
- Kim B.-S., Na Y.-G., Choi J.-H., Kim I., Lee E., Kim S.-Y., Lee J.-Y., Cho C.-W. The improvement of skin whitening of phenylethyl resorcinol by nanostructured lipid carriers. Nanomaterials. 2017;7(9):241. doi: 10.3390/nano7090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysztof, C. Wosicka, H., 2015. Solid lipid nanoparticles of roxithromycin for hair loss or acne. G. U. MEDYCZNY, Google Patents. WO/2014/077712.
- Lab, C.O., 2021. 2021, from https://oem-cosmetic.com/en/blog/quasi-drugs-oem-japan.
- Lambers H., Piessens S., Bloem A., Pronk H., Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006;28(5):359–370. doi: 10.1111/j.1467-2494.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- Liu C., Hu J., Sui H., Zhao Q., Zhang X., Wang W. Enhanced skin permeation of glabridin using eutectic mixture-based nanoemulsion. Drug Deliv. Translat. Res. 2017;7(2):325–332. doi: 10.1007/s13346-017-0359-6. [DOI] [PubMed] [Google Scholar]
- Liu D.i., Bi Y.-G. Controllable fabrication of hollow TiO2 spheres as sustained release drug carrier. Adv. Powder Technol. 2019;30(10):2169–2177. [Google Scholar]
- Lohan S.B., Bauersachs S., Ahlberg S., Baisaeng N., Keck C.M., Müller R.H., Witte E., Wolk K., Hackbarth S., Röder B., Lademann J., Meinke M.C. Ultra-small lipid nanoparticles promote the penetration of coenzyme Q10 in skin cells and counteract oxidative stress. Eur. J. Pharm. Biopharm. 2015;89:201–207. doi: 10.1016/j.ejpb.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Lohani A., Verma A., Joshi H., Yadav N., Karki N. Nanotechnology-Based Cosmeceuticals. Int. Schol. Res. Notices. 2014;2014:1–14. doi: 10.1155/2014/843687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-García R., Ganem-Rondero A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): occlusive effect and penetration enhancement ability. J. Cosmet. Dermatol. Sci. Appl. 2015;05(02):62–72. [Google Scholar]
- Lu Y., Ozcan S. Green nanomaterials: On track for a sustainable future. Nano Today. 2015;10(4):417–420. [Google Scholar]
- Maali A., Mosavian M.T.H. Preparation and application of nanoemulsions in the last decade (2000–2010) J. Dispersion Sci. Technol. 2013;34(1):92–105. [Google Scholar]
- Mahant S., Rao R., Nanda S. Design of Nanostructures for Versatile Therapeutic Applications. Elsevier; 2018. Nanostructured lipid carriers: Revolutionizing skin care and topical therapeutics; pp. 97–136. [Google Scholar]
- Marcoux D. Appearance, cosmetics, and body art in adolescents. Dermatol. Clin. 2000;18(4):667–673. doi: 10.1016/s0733-8635(05)70218-7. [DOI] [PubMed] [Google Scholar]
- Mariani E., Bargagna A., Longobardi M., et al. Synthesis of ethylammonium iodides of omega-dialkylaminoethyl ethers of 5-(arylmethylene)-1, 3, 3-trimethyl-2-oxabicyclo [2.2. 2.] octan-6-hydroxyimines as potential cosmetic ingredients. Boll. Chim. Farm. 1996;135:335–341. [PubMed] [Google Scholar]
- Meraj Anjum M.d., Kanoujia J., Parashar P., Arya M., K. Yadav A., A. Saraf S. Evaluation of a polymer-lipid-polymer system utilising hybrid nanoparticles of dapsone as a novel antiacne agent. Curr. Drug Therapy. 2016;11(2):86–100. [Google Scholar]
- Mohammadi-Samani S., Ghasemiyeh P. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res. Pharmaceut. Sci. 2018;13(4):288. doi: 10.4103/1735-5362.235156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi F., Giti R., Meibodi M.N., Ranjbar A.M., Bazooband A.R., Ramezani V. Preparation and evaluation of kojic acid dipalmitate solid lipid nanoparticles. J. Drug Delivery Sci. Technol. 2020;61:102183. doi: 10.1016/j.jddst.2020.102183. [DOI] [Google Scholar]
- Morales J.O., Valdés K., Morales J., Oyarzun-Ampuero F. Lipid nanoparticles for the topical delivery of retinoids and derivatives. Nanomedicine. 2015;10(2):253–269. doi: 10.2217/nnm.14.159. [DOI] [PubMed] [Google Scholar]
- Mu L.i., Sprando R.L. Application of nanotechnology in cosmetics. Pharm. Res. 2010;27(8):1746–1749. doi: 10.1007/s11095-010-0139-1. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Waters A.K., Kalyan P., et al. Lipid–polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019;14:1937. doi: 10.2147/IJN.S198353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukta S., Adam F. Cosmeceuticals in day-to-day clinical practice. J. Drugs Dermatol. 2010;9:s62–s66. [PubMed] [Google Scholar]
- Müller R., Dingler A. The next generation after the liposomes: solid lipid nanoparticles (SLN, Lipopearls) as dermal carrier in cosmetics. Eurocosmetics. 1998;7:19–26. [Google Scholar]
- Müller R.H., Staufenbiel S., Keck C. Lipid Nanoparticles (SLN, NLC) for innovative consumer care & household products. Household Personal Care Today. 2014;9:18–24. [Google Scholar]
- Najafi-Taher R., Ghaemi B., Amani A. Delivery of adapalene using a novel topical gel based on tea tree oil nano-emulsion: Permeation, antibacterial and safety assessments. Eur. J. Pharm. Sci. 2018;120:142–151. doi: 10.1016/j.ejps.2018.04.029. [DOI] [PubMed] [Google Scholar]
- Nakajima H. Microemulsions in cosmetics. Surfactant Sci. Ser. 1997;66:175–197. [Google Scholar]
- Naseri N., Valizadeh H., Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv. Pharmaceut. Bull. 2015;5(3):305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MPharm G., Jose J. Development, characterization, and evaluation of sunscreen cream containing solid lipid nanoparticles of silymarin. J. Cosmet. Dermatol. 2018;17(6):1073–1083. doi: 10.1111/jocd.12470. [DOI] [PubMed] [Google Scholar]
- Newman M.D., Stotland M., Ellis J.I. The safety of nanosized particles in titanium dioxide–and zinc oxide–based sunscreens. J. Am. Acad. Dermatol. 2009;61(4):685–692. doi: 10.1016/j.jaad.2009.02.051. [DOI] [PubMed] [Google Scholar]
- Newton A.M., Kaur S. Nanoarchitectonics Biomedicine. Elsevier; 2019. Solid lipid nanoparticles for skin and drug delivery: Methods of preparation and characterization techniques and applications; pp. 295–334. [Google Scholar]
- Nicol, N., 2016. Anatomy and physiology of the integumentary system. Dermatologic nursing essentials: a core curriculum. N. Nicol, Wolters Kluwer.
- Oehlke K., Behsnilian D., Mayer-Miebach E., Weidler P.G., Greiner R., Ceña V. Edible solid lipid nanoparticles (SLN) as carrier system for antioxidants of different lipophilicity. PLoS ONE. 2017;12(2):e0171662. doi: 10.1371/journal.pone.0171662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padamwar M., Pokharkar V. Development of vitamin loaded topical liposomal formulation using factorial design approach: drug deposition and stability. Int. J. Pharm. 2006;320(1-2):37–44. doi: 10.1016/j.ijpharm.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Padois K., Cantiéni C., Bertholle V., Bardel C., Pirot F., Falson F. Solid lipid nanoparticles suspension versus commercial solutions for dermal delivery of minoxidil. Int. J. Pharm. 2011 doi: 10.1016/j.ijpharm.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Paliwal R., Paliwal S.R., Kenwat R., Kurmi B.D., Sahu M.K. Solid lipid nanoparticles: a review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020;30(3):179–194. doi: 10.1080/13543776.2020.1720649. [DOI] [PubMed] [Google Scholar]
- Pardeike J., Hommoss A., Müller R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009;366(1-2):170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Patel R.P., Joshi J.R. An overview on nanoemulsion: a novel approach. Int. J. Pharmaceut. Sci. Res. 2012;3:4640. [Google Scholar]
- Pokharkar V.B., Mendiratta C., Kyadarkunte A.Y., Bhosale S.H., Barhate G.A. Skin delivery aspects of benzoyl peroxide-loaded solid lipid nanoparticles for acne treatment. Therapeutic Deliv. 2014;5(6):635–652. doi: 10.4155/tde.14.31. [DOI] [PubMed] [Google Scholar]
- Pople P.V., Singh K.K. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS Pharmscitech. 2006;7(4):E63–E69. doi: 10.1208/pt070491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash C., Bhargava P., Tiwari S., et al. Skin surface pH in acne vulgaris: insights from an observational study and review of the literature. J. Clin. Aesthetic Dermatol. 2017;10:33. [PMC free article] [PubMed] [Google Scholar]
- Purohit D.K. Nano-lipid carriers for topical application: Current scenario. Asian J. Pharmaceut. 2016;10:1–9. [Google Scholar]
- Raj R.K., Chandrul K.K. Regulatory Requirements for Cosmetics in Relation with Regulatory Authorities in India against US, Europe, Australia and Asean Countries. Int. J. Pharma Res. Health Sci. 2016;4:1332–1341. doi: 10.21276/ijprhs.2016.05.01. [DOI] [Google Scholar]
- Rapalli V.K., Kaul V., Waghule T., Gorantla S., Sharma S., Roy A., Dubey S.K., Singhvi G. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur. J. Pharm. Sci. 2020;152:105438. doi: 10.1016/j.ejps.2020.105438. [DOI] [PubMed] [Google Scholar]
- Raza K., Singh B., Singal P., Wadhwa S., Katare O.P. Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Colloids Surf., B. 2013;105:67–74. doi: 10.1016/j.colsurfb.2012.12.043. [DOI] [PubMed] [Google Scholar]
- Rodrigues L.R., Jose J. Exploring the photo protective potential of solid lipid nanoparticle-based sunscreen cream containing Aloe vera. Environ. Sci. Pollut. Res. 2020;27(17):20876–20888. doi: 10.1007/s11356-020-08543-4. [DOI] [PubMed] [Google Scholar]
- Rubiano S., Echeverri J.D., Salamanca C.H. Solid Lipid Nanoparticles (SLNs) with Potential as Cosmetic Hair Formulations Made from Otoba Wax and Ultrahigh Pressure Homogenization. Cosmetics. 2020;7(2):42. doi: 10.3390/cosmetics7020042. [DOI] [Google Scholar]
- safety, K. M. o. f. a. d. 2021. 2021, from https://www.mfds.go.kr/eng/wpge/m_24/de011014l001.do.
- Santos A.C., Morais F., Simões A., Pereira I., Sequeira J.A.D., Pereira-Silva M., Veiga F., Ribeiro A. Nanotechnology for the development of new cosmetic formulations. Expert Opinion Drug Deliv. 2019;16(4):313–330. doi: 10.1080/17425247.2019.1585426. [DOI] [PubMed] [Google Scholar]
- Sathali A., Ekambaram P., Priyanka K. Solid lipid nanoparticles: a review. Sci. Rev. Chem. Commun. 2012;2:80–102. [Google Scholar]
- Schagen S.K., Zampeli V.A., Makrantonaki E., Zouboulis C.C. Discovering the link between nutrition and skin aging. Dermato-Endocrinol. 2014;4(3):298–307. doi: 10.4161/derm.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P., Bhalodia D., Shelat P. Nanoemulsion: a pharmaceutical review. Syst Rev Pharm. 2010;1(1):24. doi: 10.4103/0975-8453.59509. [DOI] [Google Scholar]
- Sharma S., Sarangdevot K. Nanoemulsions for cosmetics. IJARPB. 2012;1:408–415. [Google Scholar]
- Shrotriya S., Ranpise N., Satpute P., Vidhate B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018;46(7):1471–1482. doi: 10.1080/21691401.2017.1373659. [DOI] [PubMed] [Google Scholar]
- Singh Hallan S., Sguizzato M., Pavoni G., Baldisserotto A., Drechsler M., Mariani P., Esposito E., Cortesi R. Ellagic acid containing nanostructured lipid carriers for topical application: A preliminary study. Molecules. 2020;25(6):1449. doi: 10.3390/molecules25061449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto E., Almeida A., Müller R. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: structure, protection and skin effects. J. Biomed. Nanotechnol. 2007;3:317–331. [Google Scholar]
- Souto E.B., Müller R.H. Cosmetic features and applications of lipid nanoparticles (SLN®, NLC®) Int. J. Cosmet. Sci. 2008;30(3):157–165. doi: 10.1111/j.1468-2494.2008.00433.x. [DOI] [PubMed] [Google Scholar]
- Souto E.B., Müller R.H., Gohla S. A novel approach based on lipid nanoparticles (SLN®) for topical delivery of α-lipoic acid. J. Microencapsul. 2005;22(6):581–592. doi: 10.1080/02652040500162378. [DOI] [PubMed] [Google Scholar]
- Souto E.B., Wissing S.A., Barbosa C.M., Müller R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004;278(1):71–77. doi: 10.1016/j.ijpharm.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Souto E.B., Wissing S.A., Barbosa C.M., Müller R.H. Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations. Eur. J. Pharm. Biopharm. 2004;58(1):83–90. doi: 10.1016/j.ejpb.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Souto E.B., Fernandes A.R., Martins-Gomes C., Coutinho T.E., Durazzo A., Lucarini M., Souto S.B., Silva A.M., Santini A. Nanomaterials for skin delivery of cosmeceuticals and pharmaceuticals. Appl. Sci. 2020;10(5):1594. doi: 10.3390/app10051594. [DOI] [Google Scholar]
- Štecová J., Mehnert W., Blaschke T., Kleuser B., Sivaramakrishnan R., Zouboulis C.C., Seltmann H., Korting H.C., Kramer K.D., Schäfer-Korting M. Cyproterone acetate loading to lipid nanoparticles for topical acne treatment: particle characterisation and skin uptake. Pharm. Res. 2007;24(5):991–1000. doi: 10.1007/s11095-006-9225-9. [DOI] [PubMed] [Google Scholar]
- Sun R., Zhao G., Ni S., Xia Q. Lipid based nanocarriers with different lipid compositions for topical delivery of resveratrol: comparative analysis of characteristics and performance. J. Drug Delivery Sci. Technol. 2014;24(6):591–600. [Google Scholar]
- Suter F., Schmid D., Wandrey F., Zülli F. Heptapeptide-loaded solid lipid nanoparticles for cosmetic anti-aging applications. Eur. J. Pharm. Biopharm. 2016;108:304–309. doi: 10.1016/j.ejpb.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Teeranachaideekul V., Souto E.B., Junyaprasert V.B., Müller R.H. Cetyl palmitate-based NLC for topical delivery of Coenzyme Q10–Development, physicochemical characterization and in vitro release studies. Eur. J. Pharm. Biopharm. 2007;67(1):141–148. doi: 10.1016/j.ejpb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Tekade R.K., Maheshwari R., Tekade M., Chougule M.B. Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes. Elsevier; 2017. Solid lipid nanoparticles for targeting and delivery of drugs and genes; pp. 256–286. [DOI] [Google Scholar]
- Üner M., Wissing S., Yener G., et al. Skin moisturizing effect and skin penetration of ascorbyl palmitate entrapped in solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) incorporated into hydrogel. Pharmazie. 2005;60:751–755. [PubMed] [Google Scholar]
- Union, O. J. o. t. E. 2009. REGULATION (EC) No 1223/2009 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on cosmetic products. 2021, from https://ec.europa.eu/health/sites/default/files/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf.
- Varma R.S. Greener approach to nanomaterials and their sustainable applications. Curr. Opin. Chem. Eng. 2012;1(2):123–128. [Google Scholar]
- Villalobos-Hernández J.R., Müller-Goymann C.C. Novel nanoparticulate carrier system based on carnauba wax and decyl oleate for the dispersion of inorganic sunscreens in aqueous media. Eur. J. Pharm. Biopharm. 2005;60(1):113–122. doi: 10.1016/j.ejpb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Waghule T., Gorantla S., Rapalli V.K., Shah P., Dubey S.K., Saha R.N., Singhvi G. Emerging trends in topical delivery of Curcumin through lipid nanocarriers: Effectiveness in skin disorders. AAPS PharmSciTech. 2020;21(7) doi: 10.1208/s12249-020-01831-9. [DOI] [PubMed] [Google Scholar]
- Wissing S., Lippacher A., Müller R. Investigations on the occlusive properties of solid lipid nanoparticles (SLN) J. Cosmet. Sci. 2001;52:313–324. [PubMed] [Google Scholar]
- Wissing S., Müller R. Solid lipid nanoparticles (SLN)–a novel carrier for UV blockers. Pharmazie. 2001;56:783–786. [PubMed] [Google Scholar]
- Wissing S.A., Müller R.H. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int. J. Pharm. 2002;242(1-2):377–379. doi: 10.1016/s0378-5173(02)00220-x. [DOI] [PubMed] [Google Scholar]
- Wissing S.A., Müller R.H. Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetration. J. Control. Release. 2002;81(3):225–233. doi: 10.1016/s0168-3659(02)00056-1. [DOI] [PubMed] [Google Scholar]
- Wissing S.A., Müller R.H. Cosmetic applications for solid lipid nanoparticles (SLN) Int. J. Pharm. 2003;254:65–68. doi: 10.1016/s0378-5173(02)00684-1. [DOI] [PubMed] [Google Scholar]
- Wu P.-S., Lin C.-H., Kuo Y.-C., Lin C.-C. Formulation and Characterization of Hydroquinone Nanostructured Lipid Carriers by Homogenization Emulsification Method. J. Nanomater. 2017;2017:1–7. [Google Scholar]
- Yeo S., Jung S., Cho H.K., Kim Y.H., Kim G.H., Kim D., Ko B.H., Lee J. Design and Characterization of Elastic Artificial Skin Containing Adenosine-Loaded Solid Lipid Nanoparticles for Treating Wrinkles. Pharmaceutics. 2021;13(1):33. doi: 10.3390/pharmaceutics13010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G., Park J.W., Yoon I.-S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): recent advances in drug delivery. J. Pharmaceut. Investig. 2013;43(5):353–362. doi: 10.1007/s40005-013-0087-y. [DOI] [Google Scholar]
- Zaid A.N., Al Ramahi R. Depigmentation and anti-aging treatment by natural molecules. Curr. Pharm. Des. 2019;25(20):2292–2312. doi: 10.2174/1381612825666190703153730. [DOI] [PubMed] [Google Scholar]
- Zhou M., Li X., Li Y., Yao Q., Ming Y., Li Z., Lu L., Shi S. Ascorbyl palmitate-incorporated paclitaxel-loaded composite nanoparticles for synergistic anti-tumoral therapy. Drug Deliv. 2017;24(1):1230–1242. doi: 10.1080/10717544.2017.1370619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis C.C. The skin as an endocrine organ. Dermato-Endocrinol. 2014;1(5):250–252. doi: 10.4161/derm.1.5.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]