Abstract
Synthetic cannabinoid receptor agonists (SCRAs) remain one the most prevalent classes of new psychoactive substances (NPS) worldwide, and examples are generally poorly characterised at the time of first detection. We have synthesised a systematic library of amino acid-derived indole-, indazole-, and 7-azaindole-3-carboxamides related to recently detected drugs ADB-BUTINACA, APP-BUTINACA and ADB-P7AICA, and characterised these ligands for in vitro binding and agonist activity at cannabinoid receptor subtypes 1 and 2 (CB1 and CB2), and in vivo cannabimimetic activity. All compounds showed high affinity for CB1 (Ki 0.299–538 nM) and most at CB2 (Ki = 0.912–2190 nM), and most functioned as high efficacy agonists of CB1 and CB2 in a fluorescence-based membrane potential assay and a βarr2 recruitment assay (NanoBiT®), with some compounds being partial agonists in the NanoBiT® assay. Key structure–activity relationships (SARs) were identified for CB1/CB2 binding and CB1/CB2 functional activities; (1) for a given core, affinities and potencies for tert-leucinamides (ADB-) > valinamides (AB-) ≫ phenylalaninamides (APP-); (2) for a given amino acid side-chain, affinities and potencies for indazoles > indoles ≫ 7-azaindoles. Radiobiotelemetric evaluation of ADB-BUTINACA, APP-BUTINACA and ADB-P7AICA in mice demonstrated that ADB-BUTINACA and ADB-P7AICA were cannabimimetic at 0.1 mg kg−1 and 10 mg kg−1 doses, respectively, as measured by pronounced decreases in core body temperature. APP-BUTINACA failed to elicit any hypothermic response up to the maximally tested 10 mg kg−1 dose, yielding an in vivo potency ranking of ADB-BUTINACA > ADB-P7AICA > APP-BUTINACA.
Synthetic cannabinoid receptor agonists (SCRAs) remain one the most prevalent classes of new psychoactive substances (NPS) worldwide, and examples are generally poorly characterised at the time of first detection.
Introduction
Synthetic cannabinoid receptor agonists (SCRAs) remain among the most structurally diverse and seized synthetic new psychoactive substances (NPS), accounting for 29% of the 1047 NPS identified between 2009 to 2019.1 Generally sold as herbal blends or incenses under product names like “Spice” and “K2”, SCRAs are generally consumed via smoking, much like cannabis itself, with the drug mixed with a plant-based vehicle and smoked.2–4 Designed to mimic the effects of (−)-trans-Δ9-tetrahydrocannabinol (1, Δ9-THC, Fig. 1), the primary psychoactive constituent of cannabis, SCRAs act as agonists of cannabinoid type 1 (CB1) and type 2 (CB2) receptors. Activation of central CB1 receptors is responsible for the principal psychoactive effects of cannabinoid receptor agonists like Δ9-THC and SCRAs; however, while Δ9-THC is a low efficacy agonist of CB1 receptors, SCRAs tend to function as potent, high efficacy CB1 agonists.5,6 Clinically significant impacts are commonly seen with patients reporting to emergency departments, and include seizure, psychosis, and acute kidney injury, as well as death.6–14
Fig. 1. Δ9-THC (1) and selected SCRAs JWH-018 (2), UR-144 (3), ADB-PICA (4), AB-PINACA (5), PX-1 (6), CUMYL-5F-P7AICA (7) and MDMB-FUBINACA (8).
One of the earliest SCRAs, JWH-018 (2), is an alkylated naphthoylindole derivative, detected as an NPS in Germany in late 2008.2 As JWH-018 and other early SCRA products were prohibited in Germany and other jurisdictions around the world, manufacturers introduced new SCRA analogues to avoid legislative controls. For example, the 2,2,3,3-tetracycloproanoyl analogue UR-144 (3), originally developed by Abbott Laboratories in 2006 as a selective CB2 agonist, was identified in a SCRA product in Korea in 2012.15,16 Shortly thereafter, amino acid-derived indole- and indazole-3-carboxamide SCRAs such as ADB-PICA (4) and AB-PINACA (5) were increasingly prevalent in NPS markets.17 Amino acid-derived SCRAs represent one of the most prevalent classes, and are notably more potent than earlier examples such as JWH-018 and UR-144.17,18 AB-PINACA was developed by Pfizer and disclosed in a 2009 patent family, and ADB-PICA and other amino acid-derived SCRAs fall broadly within the claims of these patents.19 Beyond l-valinamide (AB) and l-tert-leucinamide (ADB) SCRAs, analogues comprising l-phenylalaninamide (APP) have also been identified, such as PX-1 (6) and structural analogues.20,21 More recently, core-hopping SCRAs featuring 7-azaindole in place of indole or indazole have also been detected, exemplified by CUMYL-5F-P7AICA (7).22 MDMB-FUBINACA (8), featuring a l-tert-leucine methyl ester side-chain, was instrumental in resolving the first agonist-bound CB1–Gi signalling complex via cryo-EM.23
Recently, several amino acid-derived SCRAs featuring a truncated butyl group in place of the pentyl tail of 4 and 5, as well as a regioisomeric analogue of 5, were identified. To explore structure–activity relationships in this series of SCRAs, we have synthesised and characterised a systematic library (9–20, Fig. 2) based on the recent detection of ADB-BUTINACA (also known as ADB-BINACA, 13), APP-BUTINACA (also known as APP-BINACA, 14), and ADB-P7AICA (19). APP-BUTINACA (14) was the first of this series to be detected—in early 2019 in the EU, and mid-2019 the USA—with ADB-BUTINACA (13) being detected in late 2019 in the US.24–27 Early 2021, however, has brought the detection of ADB-P7AICA (19) in the USA, with no history of prior detection, and no pharmacological data regarding cannabinoid activity.28
Fig. 2. Structures of valinamide- (AB-), tert-leucinamide- (ADB-), and phenylalaninamide- (APP-)-derived indole, indazole and 7-azaindole SCRAs explored in this study.
While novel SCRAs entering the marketplace have been detected, chemically and metabolically characterised, virtually nothing is known about their pharmacology in vitro and in vivo, posing a significant unknown with regards to public health. As such, the synthesis and characterisation of newly detected SCRAs and related analogues are disclosed, with accompanying in vitro [3H]CP55,940 binding and functional activity determinations at both CB1 and CB2 receptors, as well as radiobiotelemetry experiments performed with selected compounds.
Results and discussion
The synthesis of 9–20 is shown in Fig. 3. Indoles 9–11 (Fig. 3A) were prepared by alkylating indole (21) with bromobutane in the presence of excess sodium hydride, followed by in situ trifluoroacetylation of the alkylated product. Aqueous quench gave the crude 1-butyl-3-(trifluoroacetyl)indole, which was air-dried overnight and refluxed in methanolic hydroxide to provide 1-butyl-1H-indole-3-carboxylic acid (22). Coupling of acid 22 with either l-valinamide, l-tert-leucinamide, or l-phenylalaninamide using EDC-HOBt afforded AB-BUTICA (9), ADB-BUTICA (10), and APP-BUTICA (11), respectively. All syntheses used the naturally occurring and abundant L-form of each amino acid amide to furnish the (S)-enantiomer of each product. Prior structure–activity studies on related SCRAs have confirmed that cannabinoid activity resides predominantly in the (S)-enantiomers in each case.29–32
Fig. 3. Syntheses of (A) indole-, (B) indazole-, and (C) 7-azaindole-derived SCRAs. Reagents and conditions: (a) (i) NaH, n-BuBr, DMF, 0 °C–rt, 1 h; (ii) (CF3CO)2O, 0 °C–rt, 2 h; (b) KOH, MeOH, PhMe, reflux, 2 h, 69% over 3 steps; (c) EDC·HCl, HOBt·H2O, amino acid hydrochloride, Et3N, DMF, rt, 24 h, 41–93%. (d) NaH, R(CH2)4Br, DMF, 0 °C–rt, 72 h, 51–86%; (e) 1 M aq. NaOH, MeOH, rt, 48 h, 86–90%.
Indazoles 12–14 (Fig. 3B) were generated by alkylating methyl 1H-indazole-3-carboxylate (23) with bromobutane to give methyl 1-butyl-1H-indazole-3-carboxylate (24), which was saponified to 1-butyl-1H-indazole-3-carboxylic acid (25). Coupling of acid 25 with the same amino acid amides above using analogous EDC-HOBt conditions afforded AB-BUTINACA (12), ADB-BUTINACA (13), and APP-BUTINACA (14). Similarly for 7-azaindoles 15–20 (Fig. 3C), methyl 1H-pyrrolo[2,3-b]pyridine-3-carboxylate (26) was alkylated with either bromobutane or bromopentane to give methyl 1-butyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylate (27) and methyl 1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylate (28), respectively, saponification of which gave the corresponding 1-substituted carboxylic acids 29 and 30. Acids 29 and 30 were each coupled with the same amino acid amide set using EDC-HOBt to furnish 1-butyl-substituted 7-azaindoles AB-BUT7AICA (15), ADB-BUT7AICA (16), and APP-BUT7AICA (17), and 1-pentyl-substituted AB-P7AICA (18), ADB-P7AICA (19), and APP-P7AICA (20). All materials were obtained in sufficient yield and purity for pharmacological evaluation.
In vitro binding data were acquired via a competitive radio binding assay, using radiolabelled [3H]CP55,940 and human CB1 and CB2 receptor membrane preparations of HEK293 cells, as previously described.33–35 The ability of the synthesised compounds to displace radioligands was measured and Ki was determined (Table 1). The concentration displacement curves are shown in Fig. S2 (see ESI†). All SCRAs (9–20) were sub-nanomolar to nanomolar CB1 ligands (Ki = 0.299–538 nM). The compounds 10 (Ki = 1.34 nM), 12 (Ki = 1.18 nM) and 13 (Ki = 1.34 nM) showed higher CB1 affinity than the common pharmacological tool molecule CP55,940 (Ki = 1.66 nM), of which two compounds have a ADB head group. Results were similar at CB2, with all compounds except for 17 (Ki = 2190 nM) displaying sub micromolar affinity at the receptor. Only compounds 10 (Ki = 1.32 nM) and 13 (Ki = 0.912 nM) showed higher affinity than CP55,940 (Ki = 1.99 nM). Across both receptors, the data showed that the ADB head group resulted in a higher binding affinity compared to the AB and APP head groups, in the respective indole (10 > 9 > 11), indazole (13 > 12 > 14), n-butyl-7-azaindole (16 > 15 > 17) or n-pentyl-7-azaindole (19 > 18 > 20) subgroups. At CB1, there was a general trend for indazoles (12–14) showing higher binding affinities than indoles (9–11), which were in turn better CB1 ligands than n-pentyl-7azaindoles (18–20), which were showing higher affinities over the n-butyl-7azaindoles (15–17). At CB2, indazoles (12–14) showed affinities than indoles (9–11), similarly to CB1, however the n-butyl-7azaindoles (15–17) displayed higher affinities than n-pentyl-7azaindoles (18–20). These trends confirm previous studies on other indole, indazole and 7-azaindole SCRAs.17,22,33
Binding affinities of compounds 9–20 at CB1 and CB2 receptors.
| Compound | CB1 | CB2 | ||
|---|---|---|---|---|
| pKi [M] ± SEM | K i (nM) | pKi [M] ± SEM | K i (nM) | |
| CP55,940 | 8.78 ± 0.060 | 1.66 | 8.70 ± 0.06 | 1.99 |
| JWH-018 (2) | 8.59 ± 0.094a | 2.56a | 8.02 ± 0.03 | 9.55 |
| AB-BUTICA (9) | 7.91 ± 0.55 | 12.2 | 7.11 ± 0.05 | 77.6 |
| ADB-BUTICA (10) | 8.87 ± 0.06 | 1.34 | 8.88 ± 0.11 | 1.32 |
| APP-BUTICA (11) | 6.51 ± 0.09 | 307 | 6.83 ± 0.07 | 148 |
| AB-BUTINACA (12) | 8.93 ± 0.07 | 1.18 | 8.19 ± 0.06 | 6.46 |
| ADB-BUTINACA (13) | 9.52 ± 0.05 | 0.299 | 9.04 ± 0.16 | 0.912 |
| APP-BUTINACA (14) | 7.60 ± 0.06 | 25.1 | 7.40 ± 0.09 | 39.8 |
| AB-BUT7AICA (15) | 6.30 ± 0.12 | 501 | 6.05 ± 0.09 | 891 |
| ADB-BUT7AICA (16) | 7.81 ± 0.07 | 15.5 | 7.39 ± 0.04 | 40.7 |
| APP-BUT7AICA (17) | 6.27 ± 0.24 | 538 | 5.66 ± 0.05 | 2190 |
| AB-P7AICA (18) | 7.31 ± 0.08 | 49.5 | 6.60 ± 0.06 | 251 |
| ADB-P7AICA (19) | 8.40 ± 0.13 | 3.94 | 7.69 ± 0.06 | 20.4 |
| APP-P7AICA (20) | 6.36 ± 0.11 | 437 | 6.10 ± 0.07 | 794 |
Data extracted from ref. 33.
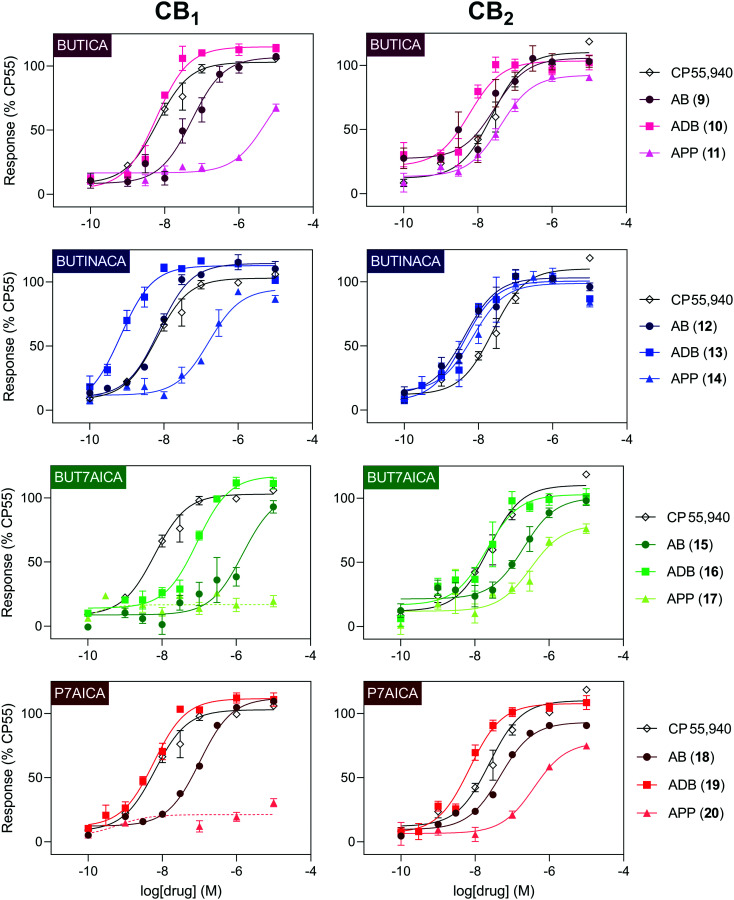
In vitro cannabinoid functional activity was determined using a fluorescence-based membrane potential assay in stably transfected AtT20 cells expressing CB1 or CB2 receptors, with receptor activation normalised to 1 μM CP55,940 (Table 2 and Fig. 4).
Functional activities of compounds 9–20 at CB1 and CB2 receptors using a G protein-mediated fluorescence assay.
| Compound | CB1 | CB2 | ||
|---|---|---|---|---|
| pEC50 [M] ± SEM (EC50, nM) | Max ± SEM (% CP55,940) | pEC50 [M] ± SEM (EC50, nM) | Max ± SEM (% CP55,940) | |
| CP55,940 | 8.20 ± 0.07 (6) | 103 ± 2 | 7.62 ± 0.07 (24) | 110 ± 2 |
| JWH-018 | 7.74 ± 0.16 (18)a | 116 ± 9a | 7.66 ± 0.16 (22)a | 87 ± 7a |
| AB-BUTICA (9) | 7.28 ± 0.09 (53) | 107 ± 4 | 7.55 ± 0.18 (28) | 106 ± 5 |
| ADB-BUTICA (10) | 8.23 ± 0.06 (6.0) | 115 ± 2 | 8.23 ± 0.15 (6.0) | 103 ± 4 |
| APP-BUTICA (11) | 5.29 ± 0.23 (5095) | 93 ± 15 | 7.4 ± 0.1 (40) | 92 ± 3 |
| AB-BUTINACA (12) | 8.13 ± 0.08 (7.4) | 114 ± 3 | 8.37 ± 0.1 (4.2) | 103 ± 3 |
| ADB-BUTINACA (13) | 9.17 ± 0.11 (0.67) | 113 ± 3 | 8.39 ± 0.12 (4.1) | 101 ± 3 |
| APP-BUTINACA (14) | 6.79 ± 0.09 (161) | 95 ± 3 | 8.17 ± 0.13 (6.8) | 99 ± 3 |
| AB-BUT7AICA (15) | 5.83 ± 0.19 (1493) | 104 ± 12 | 6.7 ± 0.13 (202) | 100 ± 5 |
| ADB-BUT7AICA (16) | 7.06 ± 0.08 (88) | 117 ± 3 | 7.68 ± 0.17 (21) | 103 ± 5 |
| APP-BUT7AICA (17) | NDb | 19 ± 4c | 6.5 ± 0.17 (319) | 79 ± 6 |
| AB-P7AICA (18) | 7.00 ± 0.08 (101) | 112 ± 3 | 7.33 ± 0.07 (47) | 93 ± 2 |
| ADB-P7AICA (19) | 8.24 ± 0.08 (5.8) | 112 ± 3 | 8.15 ± 0.08 (7.1) | 108 ± 3 |
| APP-P7AICA (20) | NDb | 30 ± 3c | 6.42 ± 0.14 (381) | 78 ± 4 |
Data extracted from ref. 36.
ND = not determined, <50% change in fluorescence at 10 μM.
Maximum response at 10 μM.
Fig. 4. Concentration–response curves for compounds 9–20 in a functional membrane receptor assay in AtT20 cells expressing either human CB1 (left) or CB2 (right). The reference compound CP55,940 has been plotted for reference (data is repeated between panels). A dashed line was used where a curve could not be fit.
All SCRAs (9–20) were efficacious agonists of CB2 (EC50 4.1–381 nM), with efficacies ranging from 78–106% relative to the tool compound CP55,940. At CB1, two compounds with the APP headgroup 17 (BUT7AICA) and 20 (P7AICA) had <50% activity at 10 μM, with the remaining compounds (9–16, 18–19) having sub-nanomolar to micromolar potencies (EC50 0.67–5095 nM) and 93–117% efficacy relative to CP55,940. Potencies were reflective of the binding affinities noted above, with ADB head group being more potent than AB and APP head groups, respectively, in each of the subgroups. The only exception to this was where ADB and AB indazole (13, 12) activity was almost identical at CB2. Again, potency followed the same trend between the subgroups, with indazoles (12–14) being generally more potent than indoles (9–11), n-pentyl-7-azaindoles (18–20), followed by n-butyl-7-azaindoles (15–17).
To offer insight into any distinctive ligand bias characteristics, thus helping to characterise the synthesised SCRAs more completely, functional activity was also determined using the NanoBiT® assay, a βarr2 recruitment assay in HEK293T cells stably expressing CB1 or CB2 receptors (Table 3 and Fig. 5). This assay has recently been utilised to evaluate a large panel of recently detected SCRAs, and comparatively evaluated against Gαi protein recruitment and [35S]GTPγS assays.37,38Emax values are relative to that of CP55,940, set at 100%.
Functional activities of compounds 9–20 at CB1 and CB2 using a βarr2 recruitment assaya.
| Compound | CB1 | CB2 | ||
|---|---|---|---|---|
| pEC50 [M] ± SEM (EC50, nM) | Max ± SEM (% CP55,940) | pEC50 [M] ± SEM (EC50, nM) | Max ± SEM (% CP55,940) | |
| CP55,940 | 8.63 ± 0.30 (2.36) | 100 ± 13 | 8.92 ± 0.27 (1.19) | 100 ± 11 |
| JWH-018 (2) | 7.45 ± 0.27 (35.5) | 310 ± 32 | 8.14 ± 0.32 (7.30) | 58 ± 7 |
| AB-BUTICA (9) | 5.48 ± 0.28 (3305) | 390 ± 49 | 6.55 ± 0.27 (283) | 94 ± 8 |
| ADB-BUTICA (10) | 6.86 ± 0.20 (139) | 756 ± 65 | 8.35 ± 0.25 (4.44) | 92 ± 5 |
| APP-BUTICA (11) | 4.81 ± 0.41 (15.5 × 103) | 50 ± 20 | 6.68 ± 0.21 (209) | 76 ± 6 |
| AB-BUTINACA (12) | 6.73 ± 0.24 (187) | 622 ± 58 | 7.86 ± 0.26 (13.7) | 91 ± 8 |
| ADB-BUTINACA (13) | 7.72 ± 0.23 (19.0) | 728 ± 64 | 8.75 ± 0.49 (1.79) | 83 ± 12 |
| APP-BUTINACA (14) | 5.93 ± 1.17 (1.17 × 103) | 216 ± 15 | 7.77 ± 0.43 (17.0) | 71 ± 12 |
| AB-BUT7AICA (15) | <4.22 (>60.8 × 103)b | 44b ± 2 | 5.34 ± 0.35 (4.62 × 103) | 88 ± 17 |
| ADB-BUT7AICA (16) | 5.54 ± 0.22 (2.89 × 103) | 406 ± 37 | 6.92 ± 0.43 (120) | 85 ± 10 |
| APP-BUT7AICA (17) | <5.98 (>1.05 × 103)b | <5b | 5.44 ± 0.37 (3.63 × 103) | 53 ± 9 |
| AB-P7AICA (18) | 5.38 ± 0.23 (4.15 × 103) | 278 ± 30 | 6.16 ± 0.40 (694) | 70 ± 10 |
| ADB-P7AICA (19) | 6.83 ± 0.14 (147) | 544 ± 57 | 7.65 ± 0.26 (22.2) | 102 ± 9 |
| APP-P7AICA (20) | NDc (>100 × 103)b | 6 ± 1b | 6.12 ± 0.39 (766) | 52 ± 8 |
Data represent mean values ± SEM from minimally 3 independent experiments.
No maximum reached at the highest tested concentration of 100 μM. EC50 values could not be determined accurately.
ND = not determined, EC50 value could not be calculated.
Fig. 5. Concentration–response curves obtained for the NanoBiT® CB1 (left) and CB2 (right) assay for compounds 9–20. CP55,940 is plotted for reference and JWH-018 is plotted for comparison (data is repeated between panels).
At CB1, a wide range of potencies was observed, with EC50 values ranging from 19 nM for ADB-BUTINACA (13) to an estimated >100 μM. The NanoBiT® data confirm the membrane potential assay data, showing that APP compounds 17 (BUT7AICA) and 20 (P7AICA) hardly had any (respectively <5% and 6%) activity at the highest concentration tested (100 μM). In addition, APP-BUTICA (11) was found to be a partial agonist, reaching a plateau of maximal activation at 50% relative to CP55,940. For compound 15 (AB-BUT7AICA), even a 100 μM concentration did not yield a plateau, with a maximal activation of 44%. All other compounds were full agonists at CB1, with Emax values ranging from 216–759% relative to CP55,940. In general, SCRAs with the ADB head group were more efficacious than their AB and APP counterparts.
In comparison to the membrane potential assay, the NanoBiT® assay yields a wider variety of Emax values, with some compounds exhibiting efficacies of over 700% in comparison with the reference CP55,940. As reported before, this can be due to the fact that the membrane potential assay is more prone to exhibiting a plateauing effect, as the maximal response is reached faster owing to signal amplification, as opposed to assays measuring events more proximal to the receptor (e.g., βarr2 recruitment).39 We previously demonstrated that this ‘super agonism’, as suggested by the NanoBiT® assay is solely attributable to receptor activation and is not subject to non-receptor mediated events that would indirectly lead to an increase in luminescence (manuscript submitted).
At CB2, Emax values were more clustered, varying from 52% to 102% in comparison to the reference CP55,940. The convergence towards 100% efficacy for CB2, as opposed to CB1, where ‘superagonism’ (reflected by efficacies exceeding 100%) is often observed, is in line with earlier comparative evaluations of CB1 and CB2 activation by SCRAs using the NanoBiT assay.40–42 At this point, we have no explanation for the large divergence in efficacies at CB1versus converging efficacies at CB2. Potencies ranging from the low nanomolar to micromolar range (1.79–4617 nM). The same general trend, as observed for CB1 and with the binding and membrane potential assays, is applicable here, with indazoles (12–14) showing greater potencies than the corresponding indoles (9–11), n-pentyl-7-azaindoles (18–20) and n-butyl-7-azaindoles (15–17), the latter being the least potent at CB2. Also, within the subgroups, the potencies followed the same trend as reported above, with SCRAs with an ADB head group generally being more potent than the AB and APP analogues.
The length of the n-alkyl chain in the tail region of the molecules has a consistent effect on affinity and potency at CB1, and potency at CB2 with comparison to the existing literature.42,43 Truncation of aliphatic n-butyl moiety from the n-pentyl generally worsens the ligand's CB1 profile, whilst the CB2 profile remains relatively intact, coinciding with not only recent work done around similar scaffolds, but indeed work performed around the older naphthoylindole pharmacophore.17,37,42,43 With regard to inter-assay variability between the fluorescence-based membrane potential assay and the NanoBiT® assay, general trends remain unchanged with regard to rank potencies of the ligands, and comparison between both the P7AICA (18–20) and BUT7AICA (15–17) scaffolds further confirms these trends in SAR. Inter-assay comparison continues to remain intact for the various head groups, with only the extremely high efficacies at CB1 of particular note. The functional in vivo implication of the observed excessive βarr2 activation in some of the compounds was not directly investigated in this study, and thus is currently unknown. Previous studies suggest that this type of activation may contribute towards toxicities and/or tolerance seen with SCRA consumption, and therefore suggests that this should be directly investigated in future.39,43–47
To rationalise observed SAR trends seen in vitro, ligands were docked to the agonist-bound cryo-EM structure of the CB1–Gi complex (PDB: 6N4B) using established techniques (induced fit docking) (Fig. 6).48 The predicted binding mode of the SCRAs in this study resembles CB1 agonists MDMB-FUBINACA (8, PBD: 6N4B) and AM11542 (PDB: 5XRA).23,49
Fig. 6. In silico analysis of 13 and CB1: A: molecular docking of 13 (blue) in CB1 receptor (6N4B); B: binding pose of 13 (blue) superimposed with 8 (yellow); C: binding pose of 13 (blue) superimposed with 17 (orange); D: interaction diagram between 13 and CB1 receptor (6N4B).
The heterocyclic core forms strong π–π interactions with aromatic residues PHE200 and PHE268, the amino group in the linker forms a H-bond interaction with SER383, and the carbonyl forming part of the terminal amide forms an H-bond interaction with HIE178 (the HIE code denotes protonation of the N-1 site). Hydrogen bonding interaction with SER383 was previously observed to be an important interaction for agonist binding and thus contributing to SCRA potency.49,50 Similar to the p-fluorobenzyl ring of 8, the alkyl chain of the SCRAs occupies the side pocket comprised of residues ILE271, TYR275, LEU276 and MET363 that was previously suggested as a conserved binding pocket for alkyl chains within lipid-binding receptors.23 Compared to the n-butyl-7-azaindoles (15–17), the stronger binding affinity of the n-pentyl-7-azaindoles (18–20) is likely due to the increased hydrophobic contacts between the ligand and the hydrophobic side pocket; these findings are in agreement with previous studies.49 The head group of the SCRAs makes hydrophobic interactions with PHE177, HIE178, PHE189 and LEU193. Compared to 9, 12, 15 and 18 with ADB head group, the higher binding affinity of 10, 13, 16 and 19 is likely attributable to the extended hydrophobic interactions of the AB head group with these residues. In contrast, the bulky APP head group is likely to result in steric repulsion with SER173, PHE177 and HIE178, which might explain the low binding affinities of 11, 14, 17 and 20 to the CB1 receptor.
In vivo pharmacological assessment of ADB-BUTINACA (13), APP-BUTINACA (14), and ADB-P7AICA (19)
Due to their recent detection within the SCRA landscape, compounds 13, 14, and 19 were assessed using radiobiotelemetry in mice to assess potential in vivo cannabimimetic activity. Radiobiotelemetric transmitters were surgically implanted as previously described, and core body temperature was measured following intraperitoneal (i.p.) injections of escalating doses of each compound.22,23,46,47,51–53 Compound 13 produced hypothermic responses at doses of 0.1 mg kg−1 and greater (Fig. 7; 0.1 mg kg−1 AUC: t(3) = 3.96, p = 0.04), while 19 required 10 mg kg−1 to elicit a clear response (10 mg kg−1 AUC: t(3) = 5.51, p = 0.04). Compound 14 did not produce any decrease in core body temperature up to the maximum tested dose of 10 mg kg−1.
Fig. 7. Change in core body temperature following i.p. injection of 13, 14, 19, and dose–response curves of peak reduction in body temperature for each drug. Vertical dash lines denote time of injection, and each data point represents the mean (± SEM) change in body temperature of four mice. Dose–response curves are normalised to the maximum observed peak reduction in body temperature of 13.
In vivo evaluation of cannabimimetic effects is standard in assessing potential brain penetrant ligands. Commonly, the cannabinoid “tetrad” – comprised of hypothermia, analgesia, hypolocomotion, and catalepsy – is used to evaluate centrally active CB1 agonists in rodent models.54,55 While the full tetrad was not used in this investigation, the marked, dose-dependent effects of 13, and to a lesser extent 19, provide a compelling case for central CB1 agonism. Radiobiotelemetry has been previously used to evaluate the central CB1 activity of emerging SCRAs in mice, because it provides additional information concerning drug time course and minimises the effects of experimenter handling and intervention.17,18,22,51–53,56–58 The lack of in vivo response to 14 is unsurprising given poor in vitro CB1 affinity, potency and efficacy, a result again consistent with other SCRAs lacking substantial CB1 agonistic activity such as 5F-PY-PICA and 5F-PY-PINACA.57 While the extremely small doses required to elicit an effect in vivo may not scale directly to humans, it is clear that 13 and 19, and potentially their analogues, represent extremely potent cannabinoid receptor agonists in these experiments. The full tetrad may be assessed in future experiments to exhaustively confirm central effects, as well as specific effects upon hypomotility, catalepsy and drug discrimination, as has been performed with other SCRAs.58
Conclusions
The recently detected SCRAs ADB-BUTINACA, APP-BUTINACA and ADB-P7AICA, as well as nine of their direct analogues were synthesised and underwent relevant chemical characterisation before undergoing both in vitro and in vivo pharmacological evaluation. Affinity at CB1 and CB2 was evaluated via a competitive radio binding assay utilising radiolabelled [3H]CP55,940, whilst functional activity at CB1 and CB2 was evaluated via both a membrane potential assay and βarr2 NanoBiT® assay to verify potency and efficacy. Potencies and efficacies showed similar trends between the assays, whilst efficacies in the βarr2 system were much higher than the membrane potential assay at CB1 for most ligands. In silico evaluation was then used to provide a rational explanation for the aforementioned SAR trends, with key interactions including core π–π stacking and head group-pocket hydrophobic contacts. In vivo evaluation was then carried out with the detected SCRAs, confirming ADB-BUTINACA and ADB-P7AICA as cannabimimetic in mice, with the former eliciting large, dose dependent drops in core body temperature. Pleasingly, APP-BUTINACA had no observable cannabimimetic properties-in vivo, in concert with the relevant in vitro and in silico data obtained. These results advance our understanding of recently detected SCRAs and pertinent SAR trends with contiguous analogues, and are important for informing clinicians, public health officials and other relevant authorities to minimise SCRA related harms and casualties.
General chemical synthesis details
All reactions were performed under an atmosphere of nitrogen unless otherwise specified. All reagents and reactants were obtained from Sigma-Aldrich (Castle Hill, NSW, Australia) and used as purchased. Deuterated solvents (CD3OD, CDCl3 and DMSO-d6) were purchased from Cambridge Isotope Laboratories (Tewksbury, MA, USA) and used as is. Other reagents were purchased and used as is from various commercial sources. Analytical thin-layer chromatography was performed using Merck aluminium-backed silica gel 60 F254 (0.2 mm) plates (Merck, Darmstadt, Germany), which were visualised using shortwave (254 nm) UV fluorescence. Flash chromatography was performed using a Biotage Isolera Spektra One and Biotage SNAP KP-Sil silica cartridges (Uppsala, Sweden), with gradient elution terminating at the solvent combination indicated for each compound (vide infra). Melting point ranges (m.p.) were measured in open capillaries using a Stuart SMP50 Automated melting point apparatus (Cole-Palmer, Staffordshire, UK) and are uncorrected. Nuclear magnetic resonance spectra were recorded at 298 K using an Agilent 400-MHz spectrometer (Santa Clara, CA, USA). The data are reported as chemical shift (δ ppm) relative to the residual protonated solvent resonance, relative integral, multiplicity (s = singlet, br s = broad singlet, d = doublet, t = triplet, q = quartet, quin. = quintet, m = multiplet), coupling constants (J Hz) and assignment. High resolution mass spectrometry (HRMS) data were collected using an Agilent LC 1260-QTOF/MS 6550 (Santa Clara, CA, US) or a Thermo Scientific Q Exactive HF-X Hybrid Quadrupole-Orbitrap mass spectrometer (Waltham, MA, USA). A methanolic extract of each pure standard was run using an electrospray ionisation source in automated MS/MS (information dependent acquisition) mode. Accurate mass for the parent ion and its corresponding mass error expressed in parts per million (ppm) are reported. Fourier-transform infrared spectroscopy (FTIR) spectra were collected using a Bruker Alpha II ATR FTIR spectrophotometer (Billerica, MA, USA), with the unit blanked before every run, and used the following parameters: 24 sample scans, resolution = 4, phase resolution = 32. Compounds were verified as greater than 95% purity via1H NMR analysis.
General procedure A: amidation of 1-alkylindole-3-carboxyic acids, 1-alkyl-1H-indazole-3-carboxylic acids and 1-alkyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid
To a solution of the appropriate 1-alkylindole-3-carboxyic acid (22, 1.00 mmol), 1-alkyl-1H-indazole-3-carboxylic acid (25, 1.00 mmol) or 1-alkyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid (29–30, 1.00 mmol), and the suitable amine reactant (1.10 mmol, 1.1 equiv.), HOBt·H2O (169 mg, 1.10 mmol, 1.1 equiv.) and EDC·HCl (288 mg, 1.50 mmol, 1.5 equiv.) in DMF (4 mL) was added. Et3N (490 μL, 3.5 mmol, 3.5 equiv.) was added dropwise and the mixture was stirred for 24 h. The mixture was poured onto H2O (100 mL), extracted with EtOAc (3 × 15 mL), and the combined organic layers were washed with H2O (2 × 10 mL), brine (1 × 10 mL), dried (MgSO4), and the solvent evaporated under reduced pressure. The products were obtained following purification by flash chromatography.
(S)-N-(1-Amino-3-methyl-1-oxobutan-2-yl)-1-butyl-1H-indole-3-carboxamide (9)
Subjecting 1-butyl-1H-indole-3-carboxylic acid (22, 87 mg, 0.40 mmol) was treated with (S)-2-amino-3-methylbutanamide hydrochloride (64 mg, 0.42 mmol) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH, 100 : 0 to 90 : 10), 9 as a white powder (63 mg, 50%). Rf 0.17 (hexane : EtOAc 50 : 50); m.p. 231–232 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.26 (s, 1H), 8.10 (dt, J = 7.8, 1.4, 0.7 Hz, 1H), 7.53 (d, 1H), 7.49 (d, J = 9.0 Hz, 1H), 7.47–7.45 (m, 1H), 7.22–7.17 (m, 1H), 7.16–7.11 (m, 1H), 7.06 (s, 1H), 4.34 (dd, J = 8.9, 7.1 Hz, 1H), 4.26–4.14 (m, 2H), 2.08 (sext., J = 6.8 Hz, 1H), 1.78 (quin., J = 7.6 Hz, 2H), 1.29 (sext., J = 7.7 Hz, 2H), 0.95–0.89 (m, 9H); 13C NMR (101 MHz, DMSO-d6) δ 173.6 ((CO)NH2), 164.1 (CO), 136.1 (quat.), 131.3 (CH), 126.5 (quat.), 121.9 (CH), 121.0 (CH), 120.6 (CH), 110.3 (quat.), 109.3 (CH), 57.4 (NHCH), 45.6 (NCH2), 31.7 (CH2), 30.4 (CH), 19.5 (d, J = 2.1 Hz, 2 × CH3), 18.5 (CH2), 13.6 (CH3); νmax (cm−1): 3369, 3291, 2955, 1625, 1512, 1467, 1380, 1293, 1236, 1205, 1154, 1135, 804, 780, 740, 666, 564; HRMS (ESI) C18H25N3O2 exact mass 315.1947, accurate mass 315.1955 (mass error 2.60 ppm).
(S)-N-(1-Amino-3,3-dimethyl-1-oxobutan-2-yl)-1-butyl-1H-indole-3-carboxamide (10)
Subjecting 1-butyl-1H-indole-3-carboxylic acid (22, 87 mg, 0.40 mmol) and (S)-2-amino-3,3-dimethylbutanamide hydrochloride (73 mg, 0.42 mmol) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH, 100 : 0 to 90 : 10), 10 as a white, crystalline solid (63 mg, 50%). Rf 0.23 (hexane : EtOAc 50 : 50); m.p. 96–97 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.30 (s, 1H), 8.08–8.05 (m, 1H), 7.54 (m, 2H), 7.23–7.12 (m, 3H), 7.10 (s, 1H), 4.47 (d, J = 9.5 Hz, 1H), 4.27–4.15 (m, 2H), 1.78 (quin., J = 7.5 Hz, 2H), 1.28 (sext., J = 7.5 Hz, 2H), 1.01 (s, 9H), 0.91 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 172.5 ((CO)NH2), 163.8 (CO), 136.1 (quat.), 131.5 (CH), 126.3 (quat.), 121.9 (CH), 120.8 (CH), 120.7 (CH), 110.45 (quat.), 109.3 (CH), 59.1 (NHCH), 45.6 (NCH2), 34.10 (quat.), 31.7 (CH2), 26.9 (3 × CH3), 19.5 (CH2), 13.6 (CH3); νmax (cm−1): 3186, 2957, 1613, 1534, 1464, 1394, 1235, 1195, 1123, 744, 536; HRMS (ESI) C19H27N3O2 exact mass 329.2103, accurate mass 329.2111 (mass error 2.32 ppm).
(S)-N-(1-Amino-1-oxo-3-phenylpropan-2-yl)-1-butyl-1H-indole-3-carboxamide (11)
Subjecting 1-butyl-1H-indole-3-carboxylic acid (22, 88 mg, 0.41 mmol) and (S)-2-amino-3-phenylpropanamide hydrochloride (84 mg, 0.42 mmol) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH, 100 : 0 to 90 : 10), 11 as a white powder (114 mg, 88%). Rf 0.14 (hexane : EtOAc 50 : 50); m.p. 139–140 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.13 (s, 1H), 8.02 (m, 1H), 7.86 (d, J = 8.5 Hz, 1H), 7.53–7.48 (m, 2H), 7.35–7.31 (m, 2H), 7.24 (t, J = 7.4 Hz, 2H), 7.19–7.14 (m, 2H), 7.14–7.05 (m, 2H), 4.70–4.63 (m, 1H), 4.25–4.13 (m, 2H), 3.10 (dd, J = 13.7, 4.6 Hz, 1H), 2.95 (dd, J = 13.8, 9.9 Hz, 1H), 1.77 (quin., J = 7.2 Hz, 2H), 1.28 (sext., J = 7.4 Hz, 2H), 0.91 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 173.8 ((CO)NH2), 164.0 (CO), 138.6 (quat.), 136.0 (quat.), 131.1 (CH), 129.2 (2 × CH), 128.0 (2 × CH), 126.5 (quat.), 126.1 (CH), 121.9 (CH), 121.01 (CH), 120.5 (CH), 110.3 (quat.), 109.3 (CH), 53.8 (NHCH), 45.6 (NCH2), 37.6 (CH2), 31.6 (CH2), 19.5 (CH2), 13.5 (CH3); νmax (cm−1): 3301, 1621, 1511, 1464, 1393, 1237, 1205, 1158, 1124, 740, 699, 658, 559; HRMS (ESI) C22H25N3O2 exact mass 363.1947, accurate mass 363.1953 (mass error 1.68 ppm).
(S)-N-(1-Amino-3-methyl-1-oxobutan-2-yl)-1-butyl-1H-indazole-3-carboxamide (12)
Subjecting 1-butyl-1H-indazole-3-carboxylic acid (25, 88 mg, 0.40 mmol) and (S)-2-amino-3-methylbutanamide hydrochloride (64 mg, 0.42 mmol) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH, 100 : 0 to 93 : 7), 12 as a white powder (55 mg, 43%). Rf 0.29 (hexane : EtOAc 50 : 50); m.p. 138–139 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.16 (dt, J = 8.2, 1.0 Hz, 1H), 7.78 (dt, J = 8.6, 0.9 Hz, 1H), 7.69 (d, J = 9.0 Hz, 1H), 7.66 (s, 1H), 7.49–7.43 (m, 1H), 7.30–7.25 (m, 1H), 7.23 (s, 1H), 4.51 (t, J = 7.1 Hz, 2H), 4.42 (dd, J = 9.0, 6.2 Hz, 1H), 2.10 (sext., J = 6.7 Hz, 1H), 1.85 (quin., J = 7.4 Hz, 2H), 1.28 (sext., J = 7.8 Hz, 2H), 0.94 (d, J = 6.8 Hz, 3H), 0.93–0.87 (m, 6H); 13C NMR (101 MHz, DMSO-d6) δ 172.6 ((CO)NH2), 161.3 (CO), 140.6 (quat.), 136.4 (quat.), 126.6 (CH), 122.5 (CH), 122.0 (quat.), 121.7 (CH), 110.5 (CH), 56.8 (NHCH), 48.5 (NCH2), 31.5 (CH), 31.3 (CH2), 19.4 (d, J = 2.8 Hz, 2 × CH3), 18.0 (CH2), 13.5 (CH3); νmax (cm−1): 3369, 2955, 1661, 1632, 1535, 1490, 1471, 1397, 1313, 1232, 1190, 1164, 1084, 775, 753, 653, 622, 601, 520; HRMS (ESI) C17H24N4O2 exact mass 316.1899, accurate mass 316.1906 (mass error 2.05 ppm).
(S)-N-(1-Amino-3,3-dimethyl-1-oxobutan-2-yl)-1-butyl-1H-indazole-3-carboxamide (13)
Subjecting 1-butyl-1H-indazole-3-carboxylic acid (25, 88 mg, 0.40 mmol) and (S)-2-amino-3,3-dimethylbutanamide hydrochloride (70 mg, 0.42 mmol, 1.05 equiv.) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH, 100 : 0 to 90 : 10), 13 as a white powder (69 mg, 52%). Rf 0.37 (hexane : EtOAc, 50 : 50); m.p. 148–149 °C; 1H NMR (400 MHz, CDCl3,) δ 8.30 1H NMR (400 MHz, DMSO-d6) δ 8.16 (dt, J = 8.2, 1.1 Hz, 1H), 7.79 (dt, J = 8.6, 0.9 Hz, 1H), 7.72 (s, 1H), 7.57 (d, J = 9.7 Hz, 1H), 7.50–7.43 (m, 1H), 7.31–7.24 (m, 2H), 4.51 (t, J = 7.1 Hz, 2H), 4.46 (d, J = 9.7 Hz, 1H), 1.84 (quin., J = 7.4 Hz, 2H), 1.28 (sext., J = 7.6 Hz, 2H), 0.99 (s, 9H), 0.90 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 171.8 (CO), 161.0 (CO), 140.7 (quat.), 136.3 (quat.), 126.7 (CH), 122.5 (CH), 121.9 (CH), 121.6 (quat.), 110.5 (CH), 58.6 (NCH), 48.5 (NCH2), 34.6 (quat.), 31.5 (CH2), 26.6 (3 × CH3), 19.4 (CH2), 13.5 (CH3); νmax (cm−1): 2958, 1694, 1647, 1529, 1488, 1365, 185, 828, 747, 592; HRMS (ESI) C18H26N4O2 exact mass 330.2056, accurate mass 330.2064 (mass error 2.43 ppm).
(S)-N-(1-Amino-1-oxo-3-phenylpropan-2-yl)-1-butyl-1H-indazole-3-carboxamide (14)
Subjecting 1-butyl-1H-indazole-3-carboxylic acid (25, 218 mg, 1.00 mmol) and methyl l-phenylalaninate hydrochloride (281 mg, 1.40 mmol, 1.40 equiv.) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH, 100 : 0 to 90 : 10), 14 as a white powder (337 mg, 93%). m.p. 91–93 °C; Rf 0.76 (hexane : EtOAc, 50 : 50); 1H NMR (400 MHz, CDCl3) δ 8.30 (dt, J = 8.2, 1.0 Hz, 1H), 7.51 (d, J = 7.9 Hz, 1H), 7.45–7.38 (m, 2H), 7.36–7.22 (m, 5H), 7.23–7.20 (m, 1H), 6.01 (s, 1H), 5.33 (s, 1H), 4.96–4.86 (m, 1H), 4.43–4.31 (m, 2H), 3.36–3.18 (m, 2H), 1.92 (quin., J = 7.2 Hz, 2H), 1.36 (sext., J = 7.7 Hz, 2H), 0.98 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 172.6 (CO), 161.3 (CO), 140.5 (quat.), 137.6 (quat.), 136.4 (quat.), 129.3 (2 × CH), 128.0 (2 × CH), 126.6 (CH), 126.3 (CH), 122.4 (CH), 122.0 (CH), 121.6 (quat.), 110.4 (CH), 53.2 (CH), 48.4 (CH2), 37.7 (CH2), 31.4 (CH2), 19.4 (CH2), 13.5 (CH3); νmax (cm−1): 3323, 2932, 1696, 1672, 1642, 1531, 1492, 1397, 1324, 1231, 1196, 1131, 909, 840, 780, 746, 698, 562; HRMS (ESI) C21H24N4O2 exact mass 364.1899, accurate mass 364.1907 (mass error 2.15 ppm).
(S)-N-(1-Amino-3-methyl-1-oxobutan-2-yl)-1-butyl-1H-pyrrolo[2,3-b]pyridine-3-carboxamide (15)
Subjecting 1-butyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid (29, 87 mg, 0.40 mmol) and (S)-2-amino-3-methylbutanamide hydrochloride (64 mg, 0.42 mmol) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH, 100 : 10 to 90 : 10), 15 as a white powder (90 mg, 71%). Rf 0.16 (hexane : EtOAc 20 : 80); m.p. 226–228 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.48 (s, 1H), 8.41 (dd, J = 7.9, 1.6 Hz, 1H), 8.31 (dd, J = 4.7, 1.6 Hz, 1H), 7.69 (d, J = 8.9 Hz, 1H), 7.47 (s, 1H), 7.20 (dd, J = 7.9, 4.7 Hz, 1H), 7.06 (s, 1H), 4.38–4.21 (m, 3H), 2.09 (sext., J = 6.8 Hz, 1H), 1.83 (quin., J = 7.5 Hz, 2H), 1.29 (sext., J = 7.4 Hz, 2H), 0.96–0.91 (m, 9H); 13C NMR (101 MHz, DMSO-d6) δ 173.4 ((CO)NH2), 163.5 (CO), 147.2 (quat.), 143.2 (CH), 131.2 (CH), 129.4 (CH), 118.9 (quat.), 117.1 (CH), 107.9 (quat.), 57.5 (NHCH), 43.8 (NCH2), 31.6 (CH2), 30.3 (CH), 19.5 (2 × CH2), 18.5 (CH2), 13.5 (CH3); νmax (cm−1): 3288, 2951, 1650, 1622, 1533, 1517, 1426, 1313, 1268, 1202, 1145, 799, 776, 753, 676, 571; HRMS (ESI) C17H24N4O2 exact mass 316.1899, accurate mass 316.1906 (mass error 2.04 ppm).
(S)-N-(1-Amino-3,3-dimethyl-1-oxobutan-2-yl)-1-butyl-1H-pyrrolo[2,3-b]pyridine-3-carboxamide (16)
Subjecting 1-butyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid (29, 87 mg, 0.40 mmol) and (S)-2-amino-3,3-dimethylbutanamide hydrochloride (70 mg, 0.42 mmol) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH, 100 : 0 to 90 : 10), 16 as a white powder (54 mg, 41%). Rf 0.24 (hexane : EtOAc 20 : 80); m.p. 90–91 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.55 (s, 1H), 8.39 (dd, J = 7.9, 1.6 Hz, 1H), 8.31 (dd, J = 4.7, 1.6 Hz, 1H), 7.55 (s, 1H), 7.40 (d, J = 9.5 Hz, 1H), 7.21 (dd, J = 7.9, 4.7 Hz, 1H), 7.10 (s, 1H), 4.47 (d, J = 9.5 Hz, 1H), 4.37–4.21 (m, 2H), 1.84 (quin., J = 7.2 Hz, 2H), 1.29 (sext., J = 7.6 Hz, 2H), 1.01 (s, 9H), 0.91 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 173.6 ((CO)NH2), 164.6 (CO), 147.7 (quat.), 143.9 (CH), 131.0 (CH), 128.9 (CH), 118.4 (quat.), 117.7 (CH), 108.8 (quat.), 59.9 (NHCH), 45.0 (CH2), 34.9 (quat.), 32.4 (CH2), 26.9 (3 × CH3), 20.1 (CH2), 13.8 (CH3); νmax (cm−1): 3183, 2957, 1673, 1618, 1534, 1426, 1397, 1259, 1195, 1141, 799, 774, 749, 559; HRMS (ESI) C18H26N4O2 exact mass 330.2056, accurate mass 330.2063 (mass error 2.06 ppm).
(S)-N-(1-Amino-1-oxo-3-phenylpropan-2-yl)-1-butyl-1H-pyrrolo[2,3-b]pyridine-3-carboxamide (17)
Subjecting 1-butyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid (29, 88 mg, 0.40 mmol) and (S)-2-amino-3-phenylpropanamide hydrochloride (86 mg, 0.42 mmol) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH, 100 : 0 to 90 : 10), 17 as a white powder (63 mg, 43%). Rf 0.14 (hexane : EtOAc 20 : 80); m.p. 187–189 °C; 1H NMR (400 MHz, CDCl3) δ 8.37 (dd, J = 4.8, 1.5 Hz, 1H), 8.18 (d, J = 8.6 Hz, 1H), 7.75 (s, 1H), 7.36–7.32 (m, 4H), 7.32–7.27 (m, 1H), 7.18 (dd, J = 8.0, 4.7 Hz, 1H), 6.58 (d, J = 6.7 Hz, 1H), 5.88 (s, 1H), 5.41 (s, 1H), 4.93 (td, J = 7.7, 5.9 Hz, 1H), 4.32 (t, J = 7.2 Hz, 2H), 3.38–3.13 (m, 2H), 1.86 (quin., J = 7.6 Hz, 2H), 1.35 (sext., J = 7.7 Hz, 2H), 0.94 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 173.7 ((CO)NH2), 163.5 (CO), 147.1 (quat.), 143.2 (CH), 138.6 (quat.), 131.1 (CH), 129.4 (CH), 129.2 (2 × CH), 128.0 (2 × CH), 126.2 (CH), 118.7 (quat.), 117.1 (CH), 107.9 (quat.), 53.8 (NHCH), 43.8 (NCH2), 37.6 (CH2), 31.6 (CH2), 19.4 (CH2), 13.5 (CH3); νmax (cm−1): 3207, 1677, 1572, 1538, 1453, 1423, 1397, 1309, 1272, 1201, 1143, 910, 807, 785, 751, 700, 581, 512; HRMS (ESI) C21H24N4O2 exact mass 364.1899, accurate mass 364.1906 (mass error 1.98 ppm).
(S)-N-(1-Amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxamide (18)
Subjecting 1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid (30, 92 mg, 0.40 mmol) and (S)-2-amino-3-methylbutanamide hydrochloride (64 mg, 0.42 mmol) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH 100 : 0 to 90 : 10), 18 as a white powder (58 mg, 44%). Rf 0.33 (hexane : EtOAc 20 : 80); m.p. 208–209 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.48 (s, 1H), 8.41 (dd, J = 7.9, 1.7 Hz, 1H), 8.31 (dd, J = 4.7, 1.6 Hz, 1H), 7.68 (d, J = 8.9 Hz, 1H), 7.48 (s, 1H), 7.20 (dd, J = 7.9, 4.7 Hz, 1H), 7.06 (s, 1H), 4.38–4.19 (m, 3H), 2.09 (sext., J = 6.8 Hz, 1H), 1.85 (quin., J = 7.2 Hz, 2H), 1.40–1.19 (m, 4H), 0.94 (dd, J = 6.8, 1.8 Hz, 6H), 0.85 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 173.4 ((CO)NH2), 163.5 (CO), 147.2 (quat.), 143.2 (CH), 131.2 (CH), 129.4 (CH), 118.9 (quat.), 117.1 (CH), 107.9 (quat.), 57.5 (NHCH), 44.1 (NCH2), 30.3 (CH), 29.2 (CH2), 28.3 (CH2), 22.7 (CH2), 19.5 (CH3), 18.5 (CH3), 13.8 (CH3); νmax (cm−1): 3293, 2933, 1648, 1620, 1516, 1447, 1425, 1313, 1267, 1233, 1186, 1143, 799, 777, 750, 668, 621, 570; HRMS (ESI) C18H26N4O2 exact mass 330.2056, accurate mass 330.2062 (mass error 1.92 ppm).
(S)-N-(1-Amino-3,3-dimethyl-1-oxobutan-2-yl)-1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxamide (19)
Subjecting 1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid (30, 93 mg, 0.40 mmol) and (S)-2-amino-3,3-dimethylbutanamide hydrochloride (72 mg, 0.42 mmol) to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH 100 : 0 to 90 : 10), 19 as a white, crystalline solid (96 mg, 69%). Rf 0.43 (hexane : EtOAc 20 : 80); m.p. 87–88 °C; 1H NMR (400 MHz, CDCl3) δ 8.43–8.32 (m, 2H), 7.83 (s, 1H), 7.22 (dd, J = 8.0, 4.8 Hz, 1H), 6.68 (d, J = 9.1 Hz, 1H), 6.22 (s, 1H), 5.68–5.64 (m, 1H), 4.65 (d, J = 9.2 Hz, 1H), 4.39–4.25 (m, 2H), 1.89 (quin., J = 7.4 Hz, 2H), 1.42–1.23 (m, 4H), 1.14 (s, 9H), 0.88 (t, J = 6.9 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 172.4 ((CO)NH2), 163.3 (CO), 147.2 (quat.), 143.2 (CH), 131.5 (CH), 129.2 (CH), 118.8 (quat.), 117.1 (CH), 107.8 (quat.), 59.3 (NHCH), 44.1 (NCH2), 34.1 (CH), 29.2 (CH2), 28.4 (CH2), 26.9 (3 × CH3), 21.7 (CH2), 13.8 (CH3); νmax (cm−1): 3186, 2955, 1674, 1617, 1534, 1426, 1398, 1367, 1260, 1187, 1140, 800, 775, 749, 610; HRMS (ESI) C19H28N4O2 exact mass 344.2212, accurate mass 344.2217 (mass error 1.52 ppm).
(S)-N-(1-Amino-1-oxo-3-phenylpropan-2-yl)-1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxamide (20)
Subjecting 1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid (30, 94 mg, 0.40 mmol) and (S)-2-amino-3-phenylpropanamide hydrochloride (85 mg, 0.42 mmol), to general procedure A gave, following purification by flash chromatography (CH2Cl2 : MeOH 100 : 0 to 90 : 10), 20 as a white, crystalline solid (123 mg, 81%). Rf 0.33 (hexane : EtOAc 20 : 80); m.p. 163–164 °C; 1H NMR (400 MHz, CDCl3) δ 8.38 (dd, J = 4.8, 1.5 Hz, 1H), 8.22 (d, J = 7.8 Hz, 1H), 7.76 (s, 1H), 7.36–7.32 (m, 4H), 7.30–7.27 (m, 1H), 7.20 (dd, J = 8.0, 4.8 Hz, 1H), 6.60 (d, J = 7.3 Hz, 1H), 5.84 (s, 1H), 5.40 (s, 1H), 4.98–4.88 (m, 1H), 4.34 (t, J = 7.3 Hz, 2H), 3.35 (dd, J = 13.8, 5.8 Hz, 1H), 3.16 (dd, J = 13.8, 8.0 Hz, 1H), 1.89 (quin., J = 7.4 Hz, 2H), 1.43–1.23 (m, 4H), 0.88 (t, J = 6.9 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 173.7 ((CO)NH2), 163.4 (CO), 147.1 (quat.), 143.2 (CH), 138.6 (quat.), 131.1 (CH), 129.4 (CH), 129.2 (2 × CH2), 128.0 (2 × CH2), 126.1 (CH), 118.7 (quat.), 117.1 (CH), 107.9 (quat.), 53.8 (NHCH), 44.0 (NCH2), 37.5 (CH2), 29.2 (CH2), 28.3 (CH2), 21.7 (CH2), 13.8 (CH3); νmax (cm−1): 3316, 3206, 2953, 1678, 1607, 1572, 1536, 1452, 1422, 1396, 1305, 1271, 1188, 1142, 912, 843, 807, 785, 750, 699, 577; HRMS (ESI) C22H26N4O2 exact mass 378.2056, accurate mass 378.2047 (mass error −0.90 ppm).
1-Butyl-1H-indole-3-carboxylic acid (22)
To a cooled (0 °C) mixture of NaH (2.41 g, 60.0 mmol, 2.0 equiv.) in DMF (40 mL), a solution of indole (13, 3.51 g, 30.0 mmol, 1.0 equiv.) in DMF (5 mL) was added dropwise. The mixture was warmed to ambient temperature, stirred for 0.5 h, cooled to 0 °C, and treated dropwise with the 1-bromobutane (3.4 mL, 32 mmol, 1.1 equiv.). The mixture was stirred for 1 h at ambient temperature, cooled to 0 °C, and treated portionwise with trifluoroacetic anhydride (10.4 mL, 75.0 mmol, 2.5 equiv.). The solution was stirred at ambient temperature for 2 h and quenched by pouring onto vigorously stirred ice–water (1 L). The precipitate was collected by vacuum filtration, washed with H2O (3 × 100 mL), and allowed to air dry for 24 h. The crude material was dissolved in PhMe (40 mL) and added dropwise to a refluxing solution of KOH (5.60 g, 100 mmol, 3.3 equiv.) in MeOH (20 mL). The solution was heated at reflux for 2 h, cooled to ambient temperature, and H2O (100 mL) was added. The layers were separated, and the organic layer was extracted with 1 M aq. KOH (3 × 20 mL). The combined aqueous layers were acidified to pH 1 with 10 M aq. HCl and extracted with EtOAc (3 × 100 mL). The combined organic layers were dried (Na2SO4) and solvent evaporated under reduced pressure. The crude solids were purified by recrystallisation from i-PrOH, affording 22 as a beige solid (4.47 g, 69%). 1H NMR (400 MHz, DMSO-d6) δ 8.06 (s, 1H), 8.04–8.01 (m, 1H), 7.56 (dt, J = 8.3, 0.9 Hz, 1H), 7.25–7.15 (m, 2H), 4.23 (t, J = 7.0 Hz, 2H), 1.75 (quin., J = 7.1 Hz, 2H), 1.23 (sext., J = 7.4 Hz, 2H), 0.87 (t, J = 7.4 Hz, 3H). All physical and spectral properties were consistent with those previously reported.51
General procedure B: alkylation of methyl indazole- and -7-azaindole-3-carboxylates
To a cooled (0 °C) solution of methyl indazole-3-carboxylate or methyl pyrrolo[2,3-b]pyridine-3-carboxylate (5.00 mmol, 1.0 equiv.) in DMF (10 mL), NaH (5.25 mmol, 1.05 equiv.) was added portionwise. The mixture was warmed to ambient temperature, stirred for 1 h, cooled to 0 °C, and treated dropwise with the appropriate alkyl bromide (5.25 mmol, 1.05 equiv.). The mixture was stirred at ambient temperature for 72 h and quenched by the addition of H2O (100 mL). The aqueous phase was extracted with EtOAc (3 × 50 mL), and the combined organic layers were dried (Na2SO4) and concentrated in vacuo. The crude material was purified by flash chromatography (hexane : EtOAc) and solvent removed in vacuo.
Methyl 1-butyl-1H-indazole-3-carboxylate (24)
Subjecting methyl 1H-indazole-3-carboxylate (2.63 g, 15.0 mmol) and 1-bromobutane (1.69 mL, 15.8 mmol, 1.05 equiv.) to general procedure B gave, following purification by flash chromatography (hexane : EtOAc, 100 : 0 to 10 : 90), 24 as a colourless oil (1.79 g, 51%). 1H NMR (400 MHz, CD3OD) δ 8.14 (d, J = 8.3 Hz, 1H), 7.67 (d, J = 8.6 Hz, 1H), 7.48 (t, J = 7.7 Hz, 1H), 7.33 (t, J = 7.6 Hz, 1H), 4.51 (t, J = 7.0 Hz, 2H), 4.01 (s, 3H), 1.93 (quin., J = 7.1 Hz, 2H), 1.32 (sext., J = 7.7 Hz, 3H), 0.94 (t, J = 7.4 Hz, 3H). All physical and spectral properties were consistent with those previously reported.51
Methyl 1-butyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylate (27)
Subjecting methyl 7-azaindole-3-carboxylate (2.64 g, 15.0 mmol) and 1-bromobutane (1.77 mL, 16.5 mmol, 1.10 equiv.) to general procedure B gave 27 as a colourless oil (1.23 g, 86%). 1H NMR (400 MHz, CD3OD) δ 8.40 (dd, J = 7.9, 1.6 Hz, 1H), 8.32 (dd, J = 4.8, 1.6 Hz, 1H), 8.15 (s, 1H), 7.26 (dd, J = 7.9, 4.8 Hz, 1H), 4.34 (t, J = 7.2 Hz, 2H), 3.89 (s, 3H), 1.86 (quin., J = 7.5 Hz, 2H), 1.33 (sext., J = 7.6 Hz, 2H), 0.94 (t, J = 7.4 Hz, 3H). All physical and spectral properties were consistent with those previously reported.33
Methyl 1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylate (28)
Subjecting methyl 7-azaindole-3-carboxylate (881 mg, 5.00 mmol) and 1-bromopentane (0.68 mL, 5.5 mmol, 1.1 equiv.) to general procedure B gave 28 as a colourless oil (1.03 g, 83%). 1H NMR (400 MHz, CDCl3) δ 8.43 (dd, J = 7.9, 1.6 Hz, 1H), 8.40–8.37 (m, 1H), 7.95 (s, 1H), 7.23 (dd, J = 7.9, 4.7 Hz, 1H), 4.33 (t, J = 7.3 Hz, 2H), 3.92 (s, 3H), 1.91 (quin., J = 7.4 Hz, 2H), 1.42–1.27 (m, 4H), 0.88 (t, J = 6.9 Hz, 3H). All physical and spectral properties were consistent with those previously reported.59
General procedure C: hydrolysis of 1-substituted methyl indazole- and -7-azaindole-3-carboxylates
A solution of the appropriate 1-substituted indazole- or pyrrolo[2,3-b]pyridine-3-carboxylate methyl ester (5.00 mmol, 1.0 equiv.) in MeOH (50 mL) was treated with NaOH (5.50 mmol, 1.1 equiv.) and the solution stirred for 48 h. The solvent was removed in vacuo and the crude product was dissolved in H2O (50 mL), pH adjusted to 2 using 1 M aq. HCl, extracted with EtOAc (3 × 50 mL). The organic layers were dried (Na2SO4), and the solvent removed in vacuo. The products were of sufficient purity for use in the subsequent steps.
1-Butyl-1H-indazole-3-carboxylic acid (25)
Subjecting methyl 1-butyl-1H-indazole-3-carboxylate (24, 1.60 g, 6.88 mmol) to general procedure C gave 25 as a white solid (1.34 g, 90%). 1H NMR (400 MHz, DMSO-d6) δ 12.99 (s, 1H), 8.08 (dt, J = 8.2, 0.9 Hz, 1H), 7.80 (d, J = 8.6 Hz, 1H), 7.46 (ddd, J = 8.5, 6.9, 1.1 Hz, 1H), 7.30 (ddd, J = 7.9, 6.9, 0.9 Hz, 1H), 4.50 (t, J = 7.0 Hz, 2H), 1.88–1.79 (m, 2H), 1.24 (sext., J = 7.4 Hz, 2H), 0.86 (d, J = 7.4 Hz, 3H). All physical and spectral properties were consistent with those previously reported.51
1-Butyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid (29)
Subjecting methyl 1-butyl-1H-[2,3-b]pyridine-3-carboxylate (27, 1.40 g, 6.02 mmol) to general procedure C gave 29 as a white solid (1.23 g, 86%). 1H NMR (400 MHz, DMSO-d6) δ 12.24 (s, 1H), 8.36–8.29 (m, 2H), 8.27 (s, 1H), 7.25 (dd, J = 7.9, 4.7 Hz, 1H), 4.31 (t, J = 7.1 Hz, 2H), 1.81 (quin., J = 7.3 Hz, 2H), 1.24 (sext., J = 7.4 Hz, 2H), 0.88 (t, J = 7.4 Hz, 3H). All physical and spectral properties were consistent with those previously reported.33
1-Pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid (30)
Subjecting methyl 1-pentyl-1H-[2,3-b]pyridine-3-carboxylate (28, 1.00 g, 4.04 mmol) to general procedure C gave 30 as a white solid (0.81 g, 86%). 1H NMR (400 MHz, DMSO-d6) δ 12.21 (s, 1H), 8.32–8.26 (m, 2H), 8.24 (s, 1H), 7.22 (dd, J = 7.9, 4.7 Hz, 1H), 4.27 (t, J = 7.1 Hz, 2H), 1.80 (quin., 2H), 1.31–1.12 (m, 4H), 0.79 (t, J = 7.2 Hz, 3H). All physical and spectral properties were consistent with those previously reported.60
In vitro CB1 receptor binding assays
A competitive ligand binding assay with [3H]CP55,940 was used to determine the receptor binding affinities to the human CB1 receptor for the test compounds and the reference compound CP55,940.33,35 Sample preparation was carried out on ice. The total assay volume was 200 μL and consisted of assay buffer (50 mM TRIS, 1 mg mL−1 BSA and 3 mM MgCl2 in deionised water (pH 7.4 adjusted with 10 M NaOH)), agonist dissolved in DMSO and serially diluted with deionised water/DMSO (99/1, v/v) at a final concentration range from 1 000 000 nM to 0.001 nM, [3H]CP55,940 radioligand (2 nM) and 8 μg hCB1 membrane protein (0.04 pmol hCB1 receptor) from HEK293-EBNA cells. Non-specific binding (NB, baseline) was measured with 100 μM CP55,940 and total binding (TB, maximal response) was assessed in the absence of a competing receptor agonist. Incubation was for 60 minutes at 37 °C. Reactions were stopped by transferring the samples to a MultiScreen™ filter plate (1.2 μM, Merck, Germany) and washed six times with ice-cold assay buffer using a vacuum manifold (MultiScreen™ 96-well vacuum manifold). The filters were then transferred to a scintillation vial, dried for 30 min at 50 °C and dissolved in liquid scintillation cocktail. Radioactivity was measured with a liquid scintillation counter (TriCarb 2100TR, Perkin Elmer).
Each concentration was tested in duplicates on three different days. Processing of raw data was performed using Excel VV and data was analysed using GraphPad Prism® (Version 8.0.2, GraphPad Software, Inc). IC50 values were determined at the turning point of the sigmoidal graph (semi logarithmic scale of the horizontal axis), which was generated using One site-fit Ki competitive binding function with KD specific for hCB1 membrane preparations (HEK293-EBNA, Perkin Elmer) with 0.05 nM. From the IC50 the respective Ki values were calculated using the Cheng–Prusoff equation.61
In vitro CB2 receptor binding assays
HEK293 cells expressing human CB2 receptor N-terminally tagged with 3HA were harvested in 5 mM EDTA in PBS. P2 membranes were prepared in sucrose buffer as previously described.62,63 Protein content was estimated using a BioRad (Hercules, CA) DC protein assay (modified Lowry assay). [3H]CP55,490 (1 nM, PerkinElmer, Waltham, MA, USA), non-radiolabelled drugs, and P2 membrane preparation (2 μg per point) were diluted in binding buffer (50 mM HEPES pH 7.4, 1 mM MgCl2, 1 mM CaCl2, 2 mg ml−1 NZ-origin BSA, MP Biomedicals, Santa Ana, CA, USA) and dispensed into 96-well, polypropylene V-well plates (Hangzhou Gene Era Biotech Co Ltd, Zhejiang, China) in a final reaction volume of 200 μL (membranes were dispensed last). When all components had been dispensed, the plate was sealed and incubated for 1 h at 30 °C. During the incubation, a 96 well harvest plate (GF/C filters, 1.2 μm pores) was treated with 0.1% w/v branched polyethyleneimine (PEI; Sigma Aldrich) in water. Immediately prior to termination, PEI was washed through the filters using a vacuum manifold (Pall Corporation, Port Washington, NY) and all wells were washed with ice cold wash buffer (50 mM HEPES pH 7.4, 500 mM NaCl, 1 mg ml−1 BSA). Equilibrated binding mixture was then transferred to the harvest plate under vacuum, and samples washed through. Binding wells were rinsed once with wash buffer and transferred to the harvest plate, and then wells were washed three more times with 200 μL of wash buffer. The plate was then removed, and filters allowed to dry overnight. The next day, the plate bottom was sealed, and 50 μL of Ultima Gold XR scintillation fluid (PerkinElmer) was dispensed to each well. The plate top was then sealed, and the plate was loaded into a 96 well “rigid” cassette and loaded into a Wallac MicroBeta2® TriLux Liquid Scintillation Counter (PerkinElmer). Scintillation was detected after a 30 min delay, for 2 min per well. Counts were corrected for detector efficiency. Data were then exported and analysed in GraphPad Prism v8 (GraphPad Software Inc., La Jolla, CA, USA). Ki was determined through fit of “Competition binding-One site fit Ki” in GraphPad prism using a Kd of 3 nM for the radioligand. log Ki were determined for at least three independent experiments and combined to determine mean pKi ± SEM reported in Table 1.
In vitro CB1 and CB2 membrane potential assays
Mouse AtT20 FlpIn pituitary tumour cells stably transfected with human CB1 or CB2 have been previously described, and were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U penicillin/streptomycin and 80 μg mL−1 of hygromycin.56,64,65 Cells were grown in 175 cm2 flasks at 37 °C and 5% CO2 and passaged as required at 80% confluency, or 90% confluency for assays. The day before the assay cells were detached from the flask with trypsin/EDTA (Sigma) and resuspended in Leibovitz's L-15 media supplemented with 1% FBS, 100 U penicillin/streptomycin and 15 mM glucose. The cells were plated in black walled, clear bottomed 96-well microplates (Corning), in 90 μL per well with a density of 100 000–130 000 cells per well and greater than 90% viability, as determined using a Countess II Automated Cell Counter (Thermo Fisher). Plates were incubated overnight at 37 °C in ambient CO2.
Membrane potential was measured using the FLIPR Membrane Potential Assay kit (blue) from Molecular Devices as described previously.66 The dye was reconstituted with assay buffer (145 mM NaCl, 22 mM HEPES, 0.338 mM Na2HPO4, 4.17 mM NaHCO3, 0.441 mM KH2PO4, 0.407 mM MgSO4, 0.493 mM MgCl2, 1.26 mM CaCl2, 5.56 mM glucose, pH 7.4, osmolarity 315 ± 5). Cells were loaded with 90 μL per well of the dye solution and incubated at 37 °C and ambient CO2 for 60 minutes. A FlexStation3 Microplate Reader (Molecular Devices) connected to SoftMax Pro 7 Software (Molecular Devices) was used to take fluorescence readings every 2 seconds (λ excitation = 530 nm, λ emission = 565 nm). Baseline readings were taken for 2 minutes, at which time either drug or vehicle was added in a volume of 20 μL. The background fluorescence of cells without dye or dye without cells was negligible. Changes in fluorescence were expressed as a percentage of baseline fluorescence after subtraction of the changes produced by vehicle (DMSO + BSA, 0.1% and 0.01% final concentration, respectively) addition. Data were analysed with Prism, using three-parameter nonlinear regression to fit concentration-response curves. CP55,940 (1 μM) was included in every column of every plate to facilitate comparisons between experiments via normalisation.
In vitro CB1 and CB2 β-arrestin2 recruitment assays
SCRAs were tested for CB1 and CB2 receptor activation using 2 separate live cell-based NanoBiT® reporter bioassays, based on the recruitment of a truncated β-arrestin2 to the activated receptor. The concept relies on the functional complementation of a split NanoLuc luciferase enzyme, of which both inactive subunits are fused to either the receptor (CB1 or CB2) or the transducer protein βarr2. Recruitment of βarr2 to the receptor upon activation by a ligand brings the 2 subunits in close proximity and results in the restoration of luciferase activity. In presence of the substrate furimazine, luminescence can be monitored using a luminometer. HEK293T cells stably expressing the CB1-βarr2 or CB2-βarr2 system, were cultivated at 37 °C and 5% CO2 under a humidified atmosphere in Dulbecco's modified Eagle's high glucose medium (DMEM, Thermo Fisher Scientific, Waltham, MA, USA) enriched with 10% heat-inactivated fetal bovine serum (Sigma Aldrich, Darmstadt, Germany), 2 mmol L−1 glutamine, 100 IU mL−1 penicillin, 100 μg mL−1 streptomycin and 0.25 μg mL−1 amphotericin B (all from Thermo Fisher Scientific). The development of the stable cell systems has been described before.67–69 On the day prior to the assay, cells were seeded on poly-d-lysine (Sigma Aldrich) coated 96-well plates (approximately 5 × 104 cells per well) and incubated overnight. On the day of the assays, the cells were washed twice with 150 μL Opti-MEM® I reduced serum medium (Thermo Fisher Scientific) before adding 100 μL of this medium to each well. After the addition of 25 μL of the 20-fold diluted Nano-Glo® Live Cell reagent (Promega, Madison, WI, USA) containing the substrate, luminescence was monitored for an equilibration period of 10 min using the CLARIOstar luminometer (BMG LABTECH GmbH, Ortenberg Germany). Upon stabilisation of the signal, 10 μL of 13.5× concentrated compound solutions were added to each well and luminescence was continuously monitored for 2 hours.
Stock solutions were made in methanol and were used to make working solutions by serial dilution in MeOH/Opti-MEM (50/50 v/v%). CP55,940 was included as a reference standard on each plate and solvent controls were taken along in all experiments. All test compounds were tested in duplicate in at least 3 independent experiments and JWH-018 was included in the set and taken along, as it has been used as a reference standard in previously published work on the NanoBiT® CB1 and CB2 assays. While we previously used a Tristar2 LB942 Multimode Reader luminometer (Berthold Technologies, Bad Wildbad, Germany), a CLARIOstar was used here, which yielded lower signals, explaining the somewhat higher variability in luminescence signals (as compared to previous work) and complicating a 1 : 1 comparison with our earlier published NanoBiT® data.
Raw luminescence data was first processed using Microsoft Excel 2019 and absolute luminescence signals were corrected for inter-well variability, after which the mean area under the curve (AUC) was calculated for the different concentrations. Curve fitting and statistical analysis was then performed using GraphPad Prism® (San Diego, CA, USA) and results were normalised to the maximum response of CP55,940, arbitrarily set at 100%. EC50 values were determined by fitting a non-linear regression model (four-parameter logistic fit) to the normalised data and concentration-response curves were generated. Results are represented as AUC ± standard error of mean (SEM).
In silico protein preparation
The cryo-EM structure of the CB1 receptor (PDB: 6N4B) was retrieved from RCSB PDB (https://www.rcsb.org/).23 The structure was prepared using Protein Preparation Wizard.70 The G proteins and cholesterol molecule were removed, leaving only the CB1 receptor and ligand MDMB-FUBINACA (8) in the active site. The preparation process involved assigning bond orders, adding hydrogens, creating zero-order bonds to metals, generating disulfide bonds, filling in missing side chains and loops using Prime, generating het states using Epik at pH 7.0 ± 2.0 and deleting water molecules beyond 5 Å from het groups.71 The hydrogen bond network was optimised, and water orientations were sampled. The pKa values of the protein were predicted using PROPKA and target pH value was set at 7.0.72 Lastly, the protein structure was minimised using the OPLS_2005 force field where RMSD of the atom displacement for terminating the minimisation was set as 0.3 Å.73
In silico ligand preparation
Ligands were prepared using LigPrep to generate energy minimised 3D structures. OPLS3e force field was used for minimisation. Epik was used to generate all possible ionised states at pH 7.0 ± 2.0.74 The desalt setting was used to remove any counter ions or water molecules. Tautomer and stereoisomers were generated (at most 32 per ligand) where specified chiralities were retained.
In silico induced fit docking
To generate docking poses, prepared ligands were docked against the prepared CB1 structure using induced fit docking.48 Extended sampling protocol and OPLS3e force field were used. MDMB-FUBINACA (8) was used to define the receptor. Core constraint was applied to restrict docking to MDMB-FUBINACA (8) with a tolerance of 1.0 Å; core atoms were determined based on the maximum common structure. Ring conformations sampling was performed with energy window set as 2.5 kcal mol−1; nonplanar conformation was penalised for amide bonds. Residues within 5.0 Å of ligand poses were refined using Prime.
In vivo radiobiotelemetric pharmacological assessments
All animal procedures were performed in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and approved by the Animal Ethics Committee of The University of Sydney. Three cohorts of four adult male C57BL/6J mice (Animal Resources Centre, Perth, Australia) were used for in vivo assessments of ADB-BUTINACA, APP-BUTINACA, and ADB-P7AICA. The mice weighed between 21 and 27 g on arrival, and were individually-housed in temperature controlled room (22 ± 1 °C) during testing on a 12 hour normal-phase light–dark cycle (lights on from 07:00 to 19:00), with water and standard rodent chow provided. Biotelemetric radiotransmitters (model TA-F10, Data Sciences International, St Paul, MN, USA) were surgically intraperitoneally implanted into the mice, per manufacturer instructions and as previously described.22,52,53,57 Mice were allowed 10 days to recover post-surgery before data collection commenced. Drug solutions were prepared in a vehicle solution of EtOH, polysorbate 80, and saline (5 : 5 : 90 v/v/v). Following a baseline vehicle injection (injection volume 10 μL g−1), escalating doses of each compound were injected i.p., starting at 0.03 mg kg−1 to a maximum of 10 mg kg−1, with doses administered every three days to minimise development of tolerance and carryover effects. Higher doses were not tested due to drug insolubility above 10 mg kg−1. For ADB-BUTINACA, the maximum tested dose was 3 mg kg−1 due to the severity of the drug effect at that dose. Raw body temperature data was gathered continuously at 1000 Hz and averaged into 15 min bins using Dataquest A.R.T. software (version 4.33, Data Sciences International). Using Prism (version 8.4.3), baseline (vehicle injection) data for each animal was subtracted to yield change in body temperature data, and the peak reduction in body temperature in the first 2.5 h post-injection was calculated. These peak values were normalised against the maximum observed reduction produced by any drug (in this case, ADB-BUTINACA) for ease of comparison between drugs, and dose–response curves were fitted via non-linear regression. Additionally, area under baseline curves (AUC) for the first 2.5 h was calculated for each drug dose using R (version 4.0.5) as a measure of total drug effect. For each drug dose, the mean AUC was compared to zero (i.e., no change from baseline) using Bonferroni-corrected one-sample t-tests.
Author contributions
E. S. synthesised and characterised all compounds under the supervision of S. D. B. E. A. C. and R. B. performed the in vitro membrane potential assays under the supervision of E. A. C. F. L. performed the ensemble docking experiments under the supervision of D. E. H. R. C. K. performed radiobiotelemetry experiments under the supervision of I. S. M. K. E. G. and S. C. performed the in vitro binding experiments under the supervision of V. A. and M. G., respectively. M. H. D. performed the NanoLuc experiments under the supervision of C. S. R. E. conducted high-resolution mass spectrometry under the supervision of R. R. G. E. S., E. A. C., R. C. K., L. M., D. E. H., V. A., M. G., C. S., and S. D. B. conceived the experiments, and E. S., E. A. C., R. C. K., F. L., K. E. G., M. H. D., C. S., and S. D. B. wrote the manuscript. All authors reviewed and edited drafts of the manuscript and approved the final version.
Conflicts of interest
None.
Supplementary Material
Acknowledgments
E. S., E. A. C., R. C. K., L. M., R. B., I. S. M., and S. D. B. gratefully acknowledge support from The Lambert Initiative for Cannabinoid Therapeutics, a philanthropic research program based at the Brain and Mind Centre, The University of Sydney. S. D. B. is supported by National Health and Medical Research Council Project Grant 1161571, and gratefully acknowledges support from a Brain and Mind Centre Research Development Grant from The University of Sydney. M. H. D. and C. S. acknowledge support from the Research Foundation Flanders (FWO) (grants no. 1S54521N, G069419N and G0B8817N). K. E. G. gratefully acknowledges the Swiss National Science Foundation (Fund No. SNF_P2BEP3_191780) for her postdoctoral fellowship. This research was facilitated by access to Sydney Mass Spectrometry, a core research facility at the University of Sydney. The authors from The University of Sydney would like to acknowledge the Gadigal people of the Eora Nation as the traditional custodians of the land on which they work, and where this research was conducted. These authors would like to pay their respects those who have cared and continue to care for Country, to elders past, present, and emerging.
Electronic supplementary information (ESI) available: A table of identifiers, 1H and 13C NMR spectra, and FTIR spectra are provided for all final compounds. Concentration-displacement curves for hCB1 binding are provided for screened compounds. Electrostatic potential maps are provided for selected compounds (10, 13, 16 and 19). See DOI: 10.1039/d1md00242b
References
- United Nations Office on Drugs and Crime (UNODC), World Drug Report 2021 (United Nations publication, Sales No. E.21.XI.8)
- Dresen S. Ferreiros N. Putz M. Westphal F. Zimmermann R. Auwarter V. J. Mass Spectrom. 2010;45:1186–1194. doi: 10.1002/jms.1811. [DOI] [PubMed] [Google Scholar]
- Brents L. K. Reichard E. E. Zimmerman S. M. Moran J. H. Fantegrossi W. E. Prather P. L. PLoS One. 2011;6:e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L. Fratta W. Front. Behav. Neurosci. 2011;5:60. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapoint J. James L. P. Moran C. L. Nelson L. S. Hoffman R. S. Moran J. H. Clin. Toxicol. 2011;49:760–764. doi: 10.3109/15563650.2011.609822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant S. Deshmukh A. Dholaria B. Kaur V. Ramavaram S. Ukor M. Teran G. A. Am. J. Med. Sci. 2012;344:67–68. doi: 10.1097/MAJ.0b013e31824cf5c2. [DOI] [PubMed] [Google Scholar]
- Schneir A. B. Baumbacher T. J. Med. Toxicol. 2012;8:62–64. doi: 10.1007/s13181-011-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi B. Lee J. D. J. Addict. Med. 2012;6:240–241. doi: 10.1097/ADM.0b013e3182619004. [DOI] [PubMed] [Google Scholar]
- Alhadi S. Tiwari A. Vohra R. Gerona R. Acharya J. Bilello K. J. Med. Toxicol. 2013;9:199–206. doi: 10.1007/s13181-013-0288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. R. Brown A. J. Emerg. Med. 2013;44:360–366. doi: 10.1016/j.jemermed.2012.07.061. [DOI] [PubMed] [Google Scholar]
- Thornton S. L. Wood C. Friesen M. W. Gerona R. R. Clin. Toxicol. 2013;51:189–190. doi: 10.3109/15563650.2013.770870. [DOI] [PubMed] [Google Scholar]
- Gugelmann H. Gerona R. Li C. Tsutaoka B. Olson K. R. Lung D. Clin. Toxicol. 2014;52:635–638. doi: 10.3109/15563650.2014.925562. [DOI] [PubMed] [Google Scholar]
- Louh I. K. Freeman W. D. Crit. Care. 2014;18:553. doi: 10.1186/s13054-014-0553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecki J. Gerona R. R. Schwartz M. D. N. Engl. J. Med. 2015;373:103–107. doi: 10.1056/NEJMp1505328. [DOI] [PubMed] [Google Scholar]
- Pace J. M., Tietje K., Dart M. J. and Meyer M. D., World Pat., WO2006069196A1, 2006
- Choi H. Heo S. Kim E. Hwang B. Y. Lee C. Lee J. Forensic Toxicol. 2013;31:86–92. doi: 10.1007/s11419-012-0170-5. [DOI] [Google Scholar]
- Banister S. D. Moir M. Stuart J. Kevin R. C. Wood K. E. Longworth M. Wilkinson S. M. Beinat C. Buchanan A. S. Glass M. Connor M. McGregor I. S. Kassiou M. ACS Chem. Neurosci. 2015;6:1546–1559. doi: 10.1021/acschemneuro.5b00112. [DOI] [PubMed] [Google Scholar]
- Banister S. D. Stuart J. Kevin R. C. Edington A. Longworth M. Wilkinson S. M. Beinat C. Buchanan A. S. Hibbs D. E. Glass M. Connor M. McGregor I. S. Kassiou M. ACS Chem. Neurosci. 2015;6:1445–1458. doi: 10.1021/acschemneuro.5b00107. [DOI] [PubMed] [Google Scholar]
- Buchler I. P., Hayes M. J., Hegde S. G., Hockerman S. L., Jones D. E., Kortum S. W., Rico J. G., Tenbrink R. E. and Wu K. K., World Pat., WO2009106980A2, 2009
- Hess C. Murach J. Krueger L. Scharrenbroch L. Unger M. Madea B. Sydow K. Drug Test. Anal. 2017;9:721–733. doi: 10.1002/dta.2030. [DOI] [PubMed] [Google Scholar]
- Lobo Vicente J. Chassaigne H. Holland M. V. Reniero F. Kolar K. Tirendi S. Vandecasteele I. Vinckier I. Guillou C. Forensic Sci. Int. 2016;265:107–115. doi: 10.1016/j.forsciint.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Banister S. D. Adams A. Kevin R. C. Macdonald C. Glass M. Boyd R. Connor M. McGregor I. S. Havel C. M. Bright S. J. Vilamala M. V. Lladanosa C. G. Barratt M. J. Gerona R. R. Drug Test. Anal. 2019;11:279–291. doi: 10.1002/dta.2491. [DOI] [PubMed] [Google Scholar]
- Krishna Kumar K. Shalev-Benami M. Robertson M. J. Hu H. Banister S. D. Hollingsworth S. A. Latorraca N. R. Kato H. E. Hilger D. Maeda S. Weis W. I. Farrens D. L. Dror R. O. Malhotra S. V. Kobilka B. K. Skiniotis G. Cell. 2019;176:448–458 e412. doi: 10.1016/j.cell.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Formal notification of N-(1-amino-1-oxo-3-phenylpropan-2-yl)-1-butyl-1H-indazole-3-carboxamide (APP-BINACA) by the United Kingdom as a new psychoactive substance under the terms of Regulation (EU) 2017/2101, Lisbon, Portugal, 2019 [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Formal notification of N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-butyl-1H-indazole-3-carboxamide (ADB-BUTINACA) by Sweden as a new psychoactive substance under the terms of Regulation (EU) 2017/2101, Lisbon, Portugal, 2019 [Google Scholar]
- Krotulski A. J. Mohr A. L. A. Diamond F. X. Logan B. K. Drug Test. Anal. 2020;12:136–144. doi: 10.1002/dta.2698. [DOI] [PubMed] [Google Scholar]
- Kavanagh P. Pechnikov A. Nikolaev I. Dowling G. Kolosova M. Grigoryev A. J. Anal. Toxicol. 2021 doi: 10.1093/jat/bkab088. [DOI] [PubMed] [Google Scholar]; , (in press)
- Gerona R., Ellison R., Sparkes E., Ametovski A., Chen S., Glass M., Banister S. and Trecki J., DEA-TOX: Announcement of a Newly Identified Synthetic Cannabinoid ADB-P7AICA, 2021, Available at: https://www.deadiversion.usdoj.gov/dea_tox/ADB-P7AICA.pdf (accessed 25 February 2021)
- Antonides L. H. Cannaert A. Norman C. Vives L. Harrison A. Costello A. Nic Daeid N. Stove C. P. Sutcliffe O. B. McKenzie C. Front. Chem. 2019;7:321. doi: 10.3389/fchem.2019.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ametovski A. Macdonald C. Manning J. J. Haneef S. A. S. Santiago M. Martin L. Sparkes E. Reckers A. Gerona R. R. Connor M. Glass M. Banister S. D. ACS Chem. Neurosci. 2020;11:3672–3682. doi: 10.1021/acschemneuro.0c00591. [DOI] [PubMed] [Google Scholar]
- Antonides L. H. Cannaert A. Norman C. NicDaeid N. Sutcliffe O. B. Stove C. P. McKenzie C. Drug Test. Anal. 2021;13:628–643. doi: 10.1002/dta.2965. [DOI] [PubMed] [Google Scholar]
- Doi T. Tagami T. Takeda A. Asada A. Sawabe Y. Forensic Toxicol. 2018;36:51–60. doi: 10.1007/s11419-017-0378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike E. Grafinger K. E. Cannaert A. Ametovski A. Luo J. L. Sparkes E. Cairns E. A. Ellison R. Gerona R. Stove C. P. Auwarter V. Banister S. D. Drug Test. Anal. 2021;13:1383–1401. doi: 10.1002/dta.3037. [DOI] [PubMed] [Google Scholar]
- Haschimi B. Grafinger K. E. Pulver B. Psychou E. Halter S. Huppertz L. M. Westphal F. Putz M. Auwarter V. Drug Test. Anal. 2021;13:1499–1515. doi: 10.1002/dta.3038. [DOI] [PubMed] [Google Scholar]
- Haschimi B. Giorgetti A. Mogler L. Nagy T. Z. Kramer S. Halter S. Boros S. Dobos A. Hidvegi E. Auwarter V. J. Anal. Toxicol. 2021;45:277–290. doi: 10.1093/jat/bkaa065. [DOI] [PubMed] [Google Scholar]
- Banister S. D. Moir M. Stuart J. Kevin R. C. Wood K. E. Longworth M. Wilkinson S. M. Beinat C. Buchanan A. S. Glass M. Connor M. McGregor I. S. Kassiou M. ACS Chem. Neurosci. 2015;6:1546–1559. doi: 10.1021/acschemneuro.5b00112. [DOI] [PubMed] [Google Scholar]
- Grafinger K. E. Cannaert A. Ametovski A. Sparkes E. Cairns E. Banister S. D. Auwärter V. Stove C. P. Drug Test. Anal. 2021;13:1402–1411. doi: 10.1002/dta.3035. [DOI] [PubMed] [Google Scholar]
- Grafinger K. E. Vandeputte M. M. Cannaert A. Ametovski A. Sparkes E. Cairns E. Juchli P. O. Haschimi B. Pulver B. Banister S. D. Stove C. P. Auwärter V. Drug Test. Anal. 2021;13:1412–1429. doi: 10.1002/dta.3054. [DOI] [PubMed] [Google Scholar]
- Wouters E. Walraed J. Banister S. D. Stove C. P. Biochem. Pharmacol. 2019;169:113623. doi: 10.1016/j.bcp.2019.08.025. [DOI] [PubMed] [Google Scholar]
- Cannaert A. Franz F. Auwarter V. Stove C. P. Anal. Chem. 2017;89:9527–9536. doi: 10.1021/acs.analchem.7b02552. [DOI] [PubMed] [Google Scholar]
- Noble C. Cannaert A. Linnet K. Stove C. P. Drug Test. Anal. 2019;11:501–511. doi: 10.1002/dta.2517. [DOI] [PubMed] [Google Scholar]
- Antonides L. H. Cannaert A. Norman C. Vives L. Harrison A. Costello A. Nic Daeid N. Stove C. P. Sutcliffe O. B. McKenzie C. Front. Chem. 2019;7:321. doi: 10.3389/fchem.2019.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. Finlay D. B. Glass M. Cell. Signalling. 2021;78:109865. doi: 10.1016/j.cellsig.2020.109865. [DOI] [PubMed] [Google Scholar]
- Manning J. J. Green H. M. Glass M. Finlay D. B. Neuropharmacology. 2021;193:108611. doi: 10.1016/j.neuropharm.2021.108611. [DOI] [PubMed] [Google Scholar]
- Ibsen M. S. Connor M. Glass M. Cannabis Cannabinoid Res. 2017;2:48–60. doi: 10.1089/can.2016.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D. B. Cawston E. E. Grimsey N. L. Hunter M. R. Korde A. Vemuri V. K. Makriyannis A. Glass M. Br. J. Pharmacol. 2017;174:2545–2562. doi: 10.1111/bph.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D. B. Manning J. J. Ibsen M. S. Macdonald C. E. Patel M. Javitch J. A. Banister S. D. Glass M. ACS Chem. Neurosci. 2019;10:4350–4360. doi: 10.1021/acschemneuro.9b00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman W. Day T. Jacobson M. P. Friesner R. A. Farid R. J. Med. Chem. 2006;49:534–553. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- Hua T. Vemuri K. Nikas S. P. Laprairie R. B. Wu Y. Qu L. Pu M. Korde A. Jiang S. Ho J.-H. Nature. 2017;547:468–471. doi: 10.1038/nature23272. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kapur A. Hurst D. P. Fleischer D. Whitnell R. Thakur G. A. Makriyannis A. Reggio P. H. Abood M. E. Mol. Pharmacol. 2007;71:1512–1524. doi: 10.1124/mol.107.034645. [DOI] [PubMed] [Google Scholar]
- Longworth M. Banister S. D. Boyd R. Kevin R. C. Connor M. McGregor I. S. Kassiou M. ACS Chem. Neurosci. 2017;8:2159–2167. doi: 10.1021/acschemneuro.7b00267. [DOI] [PubMed] [Google Scholar]
- Banister S. D. Olson A. Winchester M. Stuart J. Edington A. R. Kevin R. C. Longworth M. Herrera M. Connor M. McGregor I. S. Gerona R. R. Kassiou M. Drug Test. Anal. 2018;10:1099–1109. doi: 10.1002/dta.2362. [DOI] [PubMed] [Google Scholar]
- Kevin R. C. Anderson L. McGregor I. S. Boyd R. Manning J. J. Glass M. Connor M. Banister S. D. Front. Pharmacol. 2019;10:595. doi: 10.3389/fphar.2019.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R. Compton D. R. Thomas B. F. Prescott W. R. Little P. J. Razdan R. K. Johnson M. R. Melvin L. S. Mechoulam R. Susan W. Pharmacol., Biochem. Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Metna-Laurent M. Mondésir M. Grel A. Vallée M. Piazza P.-V. Curr. Protoc. Neurosci. 2017;80:9.59.51–59.59.10. doi: 10.1002/cpns.31. [DOI] [PubMed] [Google Scholar]
- Banister S. D. Longworth M. Kevin R. Sachdev S. Santiago M. Stuart J. Mack J. B. Glass M. McGregor I. S. Connor M. Kassiou M. ACS Chem. Neurosci. 2016;7:1241–1254. doi: 10.1021/acschemneuro.6b00137. [DOI] [PubMed] [Google Scholar]
- Banister S. D. Kevin R. C. Martin L. Adams A. Macdonald C. Manning J. J. Boyd R. Cunningham M. Stevens M. Y. McGregor I. S. Glass M. Connor M. Gerona R. R. Drug Test. Anal. 2019;11:976–989. doi: 10.1002/dta.2583. [DOI] [PubMed] [Google Scholar]
- Gamage T. F. Barrus D. G. Kevin R. C. Finlay D. B. Lefever T. W. Patel P. R. Grabenauer M. A. Glass M. McGregor I. S. Wiley J. L. Thomas B. F. Pharmacol., Biochem. Behav. 2020;193:172918. doi: 10.1016/j.pbb.2020.172918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth M. Reekie T. A. Blakey K. Boyd R. Connor M. Kassiou M. Forensic Toxicol. 2019;37:350–365. doi: 10.1007/s11419-019-00466-1. [DOI] [Google Scholar]
- Moir M. Lane S. Lai F. Connor M. Hibbs D. E. Kassiou M. Eur. J. Med. Chem. 2019;180:291–309. doi: 10.1016/j.ejmech.2019.07.036. [DOI] [PubMed] [Google Scholar]
- Yung-Chi C. Prusoff W. H. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Grimsey N. L. Goodfellow C. E. Dragunow M. Glass M. Biochim. Biophys. Acta. 2011;1813:1554–1560. doi: 10.1016/j.bbamcr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Finlay D. B. Cawston E. E. Grimsey N. L. Hunter M. R. Korde A. Vemuri V. K. Makriyannis A. Glass M. Br. J. Pharmacol. 2017;174:2545–2562. doi: 10.1111/bph.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Lai Y. Westenbroek R. Mitchell R. J. Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapman A. and Connor M., Fluorescence-Based, High-Throughput Assays for μ-Opioid Receptor Activation Using a Membrane Potential-Sensitive Dye, in Opioid Receptors. Methods in Molecular Biology (Methods and Protocols), ed. S. Spampinato, Humana Press, New York, NY, 2021, vol. 1230, Available at: 10.1007/978-1-4939-1708-2_14 (accessed 18 July) [DOI] [PubMed] [Google Scholar]
- Knapman A. Santiago M. Du Y. P. Bennallack P. R. Christie M. J. Connor M. J. Biomol. Screening. 2013;18:269–276. doi: 10.1177/1087057112461376. [DOI] [PubMed] [Google Scholar]
- Cannaert A. Storme J. Franz F. Auwarter V. Stove C. P. Anal. Chem. 2016;88:11476–11485. doi: 10.1021/acs.analchem.6b02600. [DOI] [PubMed] [Google Scholar]
- Cannaert A. Franz F. Auwarter V. Stove C. P. Anal. Chem. 2017;89:9527–9536. doi: 10.1021/acs.analchem.7b02552. [DOI] [PubMed] [Google Scholar]
- Cannaert A. Storme J. Hess C. Auwarter V. Wille S. M. R. Stove C. P. Clin. Chem. 2018;64:918–926. doi: 10.1373/clinchem.2017.285361. [DOI] [PubMed] [Google Scholar]
- Sastry G. M. Adzhigirey M. Day T. Annabhimoju R. Sherman W. J. Comput.-Aided Mol. Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Shelley J. C. Cholleti A. Frye L. L. Greenwood J. R. Timlin M. R. Uchimaya M. J. Comput.-Aided Mol. Des. 2007;21:681–691. doi: 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]
- Olsson M. H. Søndergaard C. R. Rostkowski M. Jensen J. H. J. Chem. Theory Comput. 2011;7:525–537. doi: 10.1021/ct100578z. [DOI] [PubMed] [Google Scholar]
- Jorgensen W. L. Maxwell D. S. Tirado-Rives J. J. Am. Chem. Soc. 1996;118:11225–11236. doi: 10.1021/ja9621760. [DOI] [Google Scholar]
- Schrödinger Release 2021–2, Schrödinger LLC, New York, NY, 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.