Abstract
Despite wide spread vaccination, the public health burden of pertussis remains substantial. Current acellular pertussis vaccines comprise upto five Bordetella pertussis (Bp) antigens. Performing an ELISA to quantify antibody for each antigen is laborious and challenging to apply to pediatric samples where serum volume may be limited. We developed a microsphere based multiplex antibody capture assay (MMACA) to quantify antibodies to five pertussis antigens; pertussis toxin, pertactin, filamentous hemagglutinin and fimbrial antigens 2/3, and adenylate cyclase toxin in a single reaction (5-plex) with a calibrated reference standard, QC reagents and SAS® based data analysis program. The goodness of fit (R2) of the standard curves for five analytes was ≥0.99, LLOQ 0.04–0.15 IU or AU/mL, accuracy 1.9%–23.8% (%E), dilutional linearity slopes 0.93–1.02 and regression coefficients r2 = 0.91–0.99. MMACA had acceptable precision within a median CV of 16.0%−22.8%. Critical reagents, antigen conjugated microsphere and reporter antibody exhibited acceptable (< 12.3%) lot-lot variation. MMACA can be completed in < 3 h, requires low serum volume (5μL/multiplex assay) and has fast data turnaround time (< 1 min). MMACA has been successfully developed and validated as a sensitive, specific, robust and rugged method suitable for simultaneous quantification of anti-Bp antibodies in serum, plasma and DBS.
Keywords: Pertussis, Multiplex serology, Luminex, Assay validation, SAS
1. Introduction
Pertussis is an acute respiratory disease caused by Bordetella pertussis. This disease is also referred as ‘whooping cough’ due to its characteristic cough. Pertussis was a major cause of childhood morbidity and mortality in children during the first half of the 20th century until the introduction of whole cell pertussis vaccines, followed by replacement with acellular pertussis vaccines in many countries beginning in 1981 [1]. Even though there has been an over 90% decrease in the incidence of severe disease, pertussis incidence in the U.S. has increased steadily since the 1980s. Furthermore, epidemic cycles are reported every 2–3 or 5 years [2]. Despite enormous progress made in understanding the epidemiology, control and prevention of pertussis, the disease continues to be poorly controlled among infants [3]. In 2014, World Health Organization (WHO) 2014, estimated global pertussis cases in children < 5 years to be 24.1 million and 160,700 deaths annually despite 86% diphtheria-tetanus-pertussis (DTP) vaccine coverage [4]. In the United States, during 2013–2015, between 20,762 and 32,791 cases were reported which makes pertussis the most prominent emerging vaccine-preventable disease [5].
This resurgence of pertussis in the US warrants better understanding on the host-pathogen relationship. There have been several possible hypotheses suggested for the continued incidence or resurgence of pertussis, including antigenic changes in the organism, better or intensified diagnosis, and/or waning vaccine immunity from acellular pertussis vaccines [6,7]. Current acellular pertussis vaccines (aP) may comprise up to four Bp antigens; pertussis toxin (Pt), pertactin (Prn), filamentous hemagglutinin (Fha) and fimbrial antigen 2/3 (Fim2/3). Several additional vaccine candidate antigens might be considered to be combined with these antigens to increase the vaccine effectiveness. Even though most of these antigens induce a robust humoral immune response leading to the elaboration of IgG, IgM, and IgA, the combination and concentration of antibodies to confer immunity is poorly understood [8]. Lack of a confirmed immunological correlate of protection and standardized method of antibody response assessment have hampered the progress in understanding the aP vaccine response [9,10]. Although there is no definitive threshold of antibody response established for protection, it has been suggested that high levels of antibodies to pertussis toxin, pertactin, and fimbrial agglutinogens protect singly and synergize. In other words, having antibodies to any one of these antigens gives some protection and better protection is given by antibodies to 2 or all 3 of the antigens [11].
At present, antibody responses to pertussis vaccines are evaluated using sensitive and specific ELISAs for each antigen [12–15]. ELISA has several technical caveats especially when required to quantify the immune response to multivalent vaccines or natural infection with the pathogen presenting multiple virulence factors. In these situations, performing an ELISA for each antigen is time consuming and poses limitations when assessing immune response in children where the volume of serum samples may be limited. In vitro serological assays with the capacity to detect and quantify several analytes in a single reaction have broad acceptance and application [16–24]. With the increase in the number of multivalent vaccines and concurrent vaccinations, it is crucial to have serological techniques capable of quantifying the antigen specific immune response in as few reactions as plausible [14,22–24]. A major factor that challenges the use of serological antibody quantification assays is the inter-laboratory reproducibility. The assay formats, reportable values and interpretation often vary significantly between laboratories, leading to difficulties in data comparison between studies [25–27].
The objective of the present study was to address this variability by creating a standardized technology platform for the quantification of IgG antibodies to Bp antigens in a single reaction. The anti-Bp antigen specific IgG microsphere based multiplex antibody capture assay (MMACA) was developed and validated to a level of standardization that facilitates its use in a variety of laboratories, making this technology and critical reagents available thereby providing a framework for qualitative and quantitative comparison of pertussis vaccine responses and diagnostic tests. This article reports the development, performance characteristics and validation of MMACA for human serum in a robust and rugged format. Assay development experiments were described in section 2.1 and the summary of assay performance characteristics presented in Table 3. Section 2.2 describes assay validation experiments with the data summarized in Table 4. The validated MMACA was assessed for the serological screening of plasma and dried blood spot (DBS) as described in section 2.3 and 2.4.
Table 3.
Summary of MMACA assay performance characteristics.
| Parameter | Performance – Test reagent |
|||||
|---|---|---|---|---|---|---|
| Monitoring criteria | Act | Fha | Fim2/3 | Prn | Pt | |
|
| ||||||
| Accuracy | Median Absolute Percent Error | 17.87% | 13.67% | 16.55% | 14.36% | 24.77% |
| Goodness of Fit | Mean r2 | 0.9990 | 0.9989 | 0.9994 | 0.9996 | 0.9991 |
| Dilutional Linearity | n | 225 | 225 | 254 | 264 | 261 |
| r2 | 0.9887 | 0.9892 | 0.9894 | 0.9942 | 0.9909 | |
| Slope | 1.0196 | 1.0163 | 1.0141 | 1.0188 | 1.0993 | |
| Intercept | 0.0068 | −0.0720 | −0.0803 | −0.0622 | −0.2390 | |
| Intra-operator Precision | Median CV | 22.04%–29.38% | 13.50%–29.91% | 16.82%–24.81% | 14.14%–17.21% | 13.25%–24.78% |
| Inter-operator (Intermediate) Precision | Median CV | 26.63% | 25.80% | 23.16% | 20.52% | 26.75% |
Act – Adenylate cyclase; Fha – filamentous hemagglutinin; Fim2/3 - Fimbria; Prn – Pertactin; Pt – Pertussis toxin antigens.
Table 4.
MMACA upper and lower limit of quantification.
| Antigen | n | IgG concentration (IU or AU/mL) |
||||
|---|---|---|---|---|---|---|
| MDC | LOD | RDL | LOQ | ULOQ | ||
|
| ||||||
| Act | 219 | 0.013 | 0.127 | 0.024 | 0.239 | 4.24 |
| Fha | 198 | 0.006 | 0.059 | 0.011 | 0.109 | 3.47 |
| Fim2/3 | 218 | 0.003 | 0.027 | 0.005 | 0.048 | 1.97 |
| Prn | 218 | 0.003 | 0.034 | 0.006 | 0.063 | 2.89 |
| Pt | 234 | 0.013 | 0.127 | 0.022 | 0.221 | 2.51 |
MDC – Calculated minimum detectable concentration; LOD = Calculated lower limit of detection (MDC × initial serum dilution factor); RDL – Calculated reliable detection limit; LOQ – Calculated lower limit of quantification (RDL × initial serum dilution factor); ULOQ – Theoretical upper limit of quantification.
Act – Adenylate cyclase; Fha – filamentous hemagglutinin; Fim2/3 - Fimbria; Prn – Pertactin; Pt – Pertussis toxin antigens.
2. Materials and methods
2.1. Assay development and characterization
2.1.1. Bordetella pertussis antigens
Pertussis toxin (Pt), pertactin (Prn), filamentous hemagglutinin antigen (Fha), and fimbrial antigens (Fim2/3) were provided by Sanofi Pasteur (Swiftwater, PA) under a Materials Transfer Agreement (MTA#NCIRD-V116245–00). Bordetella pertussis Adenylate Cyclase Toxin/toxoid (Act) was purchased from Sigma (Cat#A0847, Sigma, St. Louis, MO), and List Biological Laboratories (Cat#188 & 189, List Biological Laboratories Inc. Act (MCR0022) was also purchased for this assay development from the University of Virginia, VA (UVA; Dr. Erik Hewlett). Antigens were stored in aliquots (50 μl) at 2–8 °C (Pt, Prn and Fha) or at −20 °C (Fim2/3 and Act) according to the manufacturer recommendation.
2.1.2. Human reference sera
The WHO International Standard Pertussis Antiserum (Human) WHO 06/140 was purchased from the National Institute for Biological Standards and Control (NIBSC, UK; NIBSC code: 06/140; http://www.nibsc.org/documents/ifu/06–140.pdf) and used as a calibrator for assay development. WHO 06/140 is a freeze-dried polyclonal anti-serum prepared from donor sera with respective IgG/IgA assignments for 3 Bp antigens, Pt (335/65 International Units (IU)/mL), Fha (130/65 IU/mL) and Prn (65/42 IU/mL). In the absence of international reference material with anti-Act IgG concentration assignment, an arbitrary concentration of 100 arbitrary units per milliliter (AU/mL) of anti-Act IgG was assigned. WHO 06/140 was calibrated to NIBSC reference reagent 89/530 (NIBSC, UK; NIBSC code: 89/530), a pooled human antiserum, for anti-fim 2/3 IgG concentrations (280 Units/mL) and expressed as IU/mL.
2.1.3. Quality control serum
Pertussis reference reagent WHO 06/142 purchased from the National Institute for Biological Standards and Control (NIBSC, UK; NIBSC code: 06/142; http://www.nibsc.org/documents/ifu/06–142.pdf) was used as the assay positive control throughout the assay development. A normal human serum depleted of total IgG, IgA and IgM (Sigma-Aldrich, St. Louis, MO) was used as the assay negative control and designated MCR0028. As a part of the preliminary assay development activities, QCs were repeatedly tested (2 analysts; n = 15/analyst) to capture their variability and establish the acceptance limits. Acceptable ranges for the QC point estimates were set as the geometric mean ± 2 standard deviations of the IgG concentration to each antigen.
2.1.4. Sera for assay development and performance characterization
A total of 57 human sera that were available in sufficient quantities (> 200 mL) were selected from the in-house human serum collection and screened for anti-Bp antibodies using MMACA. Human sera, 2012812612 and 2012789123 were selected based on their high IgG levels for the five Bp antigens, Pt, Prn, Fha, Fim2/3, and Act. A panel of 21 sera (MCR0001 – MCR0021) was constructed from three human sera, 2012812612, 2012789123 and WHO 06/142 for the assay development. Each of these three sera (neat) was spiked into MCR0028 and the serum panel was generated as indicated in Table 1. MMACA Diagnostic Sensitivity (DSN) and Specificity (DSP) was assessed using 119 clinical samples obtained from 71 clinically negative and 48 clinically positive patients.
Table 1.
Sera for MMACA assay development and characterization.
| Reagent ID | Formulation Dilution Factor | Expected IgG concentration (IU or AU/mL) |
||||
|---|---|---|---|---|---|---|
| Act | Fha | Fim2/3 | Prn | Pt | ||
|
| ||||||
| 2012812612a | Neat | 129.92 | 113.58 | 1153.47 | 1299.40 | 171.28 |
| MCR0001 | 2 | 64.96 | 56.79 | 576.74 | 649.70 | 85.64 |
| MCR0002 | 4 | 32.48 | 28.40 | 288.37 | 324.85 | 42.82 |
| MCR0003 | 16 | 8.12 | 7.10 | 72.09 | 81.21 | 10.71 |
| MCR0004 | 30 | 4.33 | 3.79 | 38.45 | 43.31 | 5.71 |
| MCR0005 | 120 | 1.08 | 0.95 | 9.61 | 10.83 | 1.43 |
| MCR0006 | 500 | 0.26 | 0.23 | 2.31 | 2.60 | 0.34 |
| MCR0007 | 2000 | 0.06 | 0.06 | 0.58 | 0.65 | 0.09 |
|
| ||||||
| 2012789123a | Neat | 264.62 | 1015.55 | 320.60 | 1771.89 | 45.86 |
| MCR0008 | 2 | 132.31 | 507.78 | 160.30 | 885.95 | 22.93 |
| MCR0009 | 4 | 66.16 | 253.89 | 80.15 | 442.97 | 11.47 |
| MCR0010 | 16 | 16.54 | 63.47 | 20.04 | 110.74 | 2.87 |
| MCR0011 | 30 | 8.82 | 33.85 | 10.69 | 59.06 | 1.53 |
| MCR0012 | 120 | 2.21 | 8.46 | 2.67 | 14.77 | 0.38 |
| MCR0013 | 500 | 0.53 | 2.03 | 0.64 | 3.54 | 0.09 |
| MCR0014 | 2000 | 0.13 | 0.51 | 0.16 | 0.89 | 0.02 |
|
| ||||||
| WHO06/142a | Neat | 64.65 | 107.11 | 38.51 | 29.50 | 82.88 |
| MCR0015 | 2 | 32.33 | 53.56 | 19.26 | 14.75 | 41.44 |
| MCR0016 | 4 | 16.16 | 26.78 | 9.63 | 7.38 | 20.72 |
| MCR0017 | 6 | 10.78 | 17.85 | 6.42 | 4.92 | 13.81 |
| MCR0018 | 12 | 5.39 | 8.93 | 3.21 | 2.46 | 6.91 |
| MCR0019 | 50 | 1.29 | 2.14 | 0.77 | 0.59 | 1.66 |
| MCR0020 | 200 | 0.32 | 0.54 | 0.19 | 0.15 | 0.41 |
| MCR0021 | 750 | 0.09 | 0.14 | 0.05 | 0.04 | 0.11 |
Act – Adenylate cyclase; Fha – filamentous hemagglutinin; Fim2/3 - Fimbria; Prn – Pertactin; Pt – Pertussis toxin antigens.
Human sera spiked into human serum depleted of total IgG, IgA and IgM (MCR0028) to formulate a serum panel (MCR0001 – MCR0021) for MMACA assay development and characterization.
2.1.5. Conjugation of Bp antigens to carboxylated microspheres
Bp antigens, Pt, Prn, Fha, Fim2/3, and Act, were individually and covalently conjugated to carboxylate modified 5 different luminex beads each having a distinct spectral signature using the carbodiimide reaction [28]. Microspheres (approximately 12.5 × 106 microsphere/mL) were activated in 10 μ$props_value{literPattern}/mL of sodium phosphate buffer (pH 6.2)(Cat# S0741, Sigma-Aldrich, St. Louis, MO) containing 2.5 mg of N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide (EDC, Cat# E7750, Sigma, St. Louis, MO) and 2.5 mg of N-Hydroxysuccinimide (NHS, Cat#130672, Sigma, St. Louis, MO). After 20 min incubation at ambient temperature (RT) with inversion and rotation in the dark, microspheres were harvested by centrifugation at 12,000×g for 5 min and resuspended in 500 μL 2-(N-Morpholino)ethanesulfonic acid hydrate (MES, Cat#M8250, Sigma, St. Louis, MO) containing the selected Bp antigen (Pt, Prn, Fha, Fim2/3 and Act: 5, 5, 12.5, 12.5 and 20 μg/mL respectively). After 2 h incubation at RT with inversion and rotation in the dark, microspheres were washed once with blocking buffer (PBS, CDC#0082 with 1% BSA (wt/vol), Cat#05470, Sigma-Aldrich, St. Louis, MO), resuspended in 500 μL of blocking buffer and incubated at RT for 30 min with inversion and rotation. Microspheres were washed twice with blocking buffer and stored in 1 mL storage buffer (PBS with 0.5% BSA (wt/vol)) at 4 °C.
2.1.6. Microsphere based multiplex antibody capture assay (MMACA) procedure
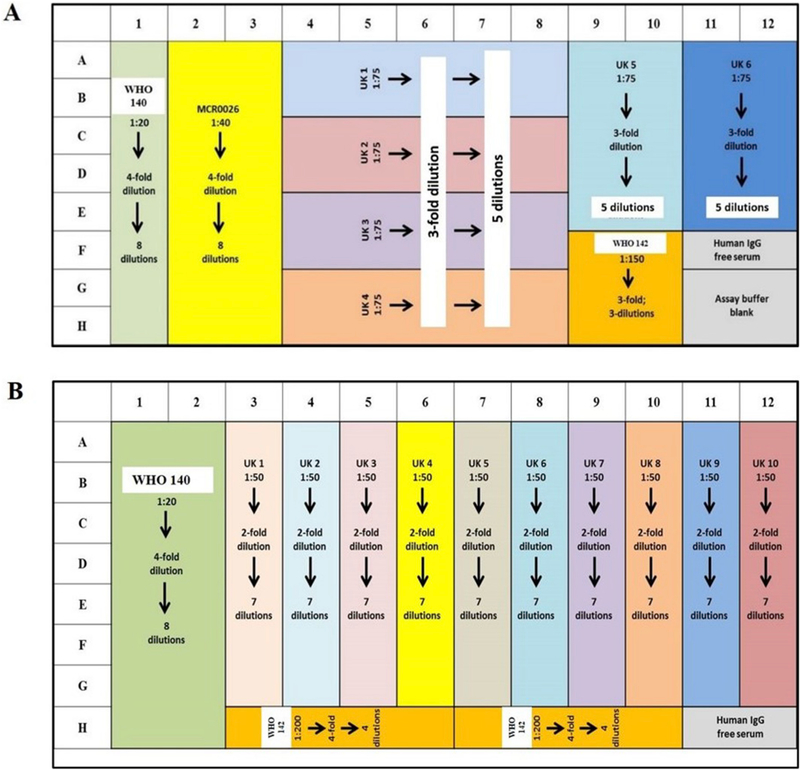
Fig. 2 provides the assay plate layout used during the assay development (Fig. 2A) and validation (Fig. 2B). Each assay plate (Fig. 2A) included a human standard reference serum, WHO 06/140, diluted 4-fold for 8 dilutions starting at 1/20; and an internal quality control (QC) serum, WHO 06/142 diluted 3-fold for 3 dilutions starting at 1/150. Test serum samples were diluted 2-fold for 7 dilutions starting at 1/50. IgG-free human serum (Sigma, St. Louis, MO, USA), and assay buffer blanks were included in each assay plate as reagent controls. Replicates were maintained for standard, QC serum and blanks. All QC, reference standard and sample dilutions were carried out in a 96-well round bottom titer plate (Dilution plate, CLS3799, Sigma, St. Louis, MO). A 96-well multiscreen HTS filter plate (MABVN1250, Millipore Corp, Billerica, MA) pre-wet with 100 μL assay buffer (0.1% BSA (wt/vol) in PBS) was aspirated and 25 μL/well of Bp antigen-conjugated microspheres (mono- or multi-plex; 2500 microspheres/region/well) were transferred. From the dilution plate, 25 μL reference standard, QCs and serum samples were transferred to the filter plate with microspheres and incubated in dark for 40 ± 10 min at RT with 150 RPM agitation in a horizontal orbital agitator. The plate was aspirated and was washed 3 times with 100 μL assay buffer. To each well, 50 μL of a 1/200 dilution of species specific reporter antibody coupled to Phycoerythrin (PE), R-PE Goat anti-human Fcγ specific IgG (GTIGF-001, Moss Inc. Pasadena, MD) in PBS was added and incubated in dark for 20 ± 10 min at RT with agitation. The plate was aspirated, washed 3 times with 100 μL assay buffer and the microspheres were resuspended in 130 μL assay buffer. The plate was read in a Luminex 200 reader (Luminex Corp, Houston, TX). Luminex 200 reader uses xMap technology where microspheres are populated into specific regions based on their spectral signature and the reporter signal strength (directly proportional to bead bound antibody density) is expressed as the median fluorescent intensity (MFI).
Fig. 2.
MMACA Assay Plate Layout. (A) Layout#1 and (B) Layout#2. (WHO 140 = WHO 06/140, MCR0026, in-house reference serum, UK = unknown samples and WHO 142 = WHO 06/142).
2.1.7. Data analysis
The reportable value (RV) of the assay is the mean of two independent tests and expressed as the serum concentration of anti-Bp antigens specific IgG in IU/mL (Pt, Prn, Fha and Fim2/3) or AU/mL (Act). The assay RV was calculated from a program developed by CDC that operates from the SAS system, version 9.0 or higher (SAS Institute, Cary, NC). The median fluorescence intensity (MFI) from each assay well for each antigen is used as the raw response variable. MFI are transformed as log10 (MFI+1) for model fitting. The program utilizes a four-parameter logistic-log (4-PL) robustly weighted function to model the test curves from the MFI of dilution points of the reference standard. Sample concentrations are calculated by interpolating the sample MFI to the reference standard curve. Standard curve fitting, sample concentrations and QC criteria are calculated independently for each antigen. All statistical analyses after endpoint calculation were done in programs written by the CDC using the SAS system. Summary procedures in SAS were used to capture the mean, standard deviation, and coefficient of variance (%CV) for five analytes in each test sample, standard reference serum, and the QC samples.
2.1.8. Assay acceptance criteria
Every assay plate is required to pass the ‘primary (plate) acceptance criteria’ for each antigen. Unknown samples from the plate that has passed the primary acceptance criteria will be required to pass ‘secondary (sample) acceptance criteria’. Any assay plate that did not pass these criteria was excluded from the analysis and the assay plate or specific samples were repeated. QC acceptance criteria are applied per antigen and it is possible for some antigens to fail while others from the same plate may pass.
2.1.8.1. Primary (plate) acceptance criteria.
The primary acceptance criteria for each assay plate check the goodness of fit of the reference standard curve, accuracy and precision of the positive QC sample. The reference standard must have no more than 2 censored wells and must have a model fit r2 ≥ 0.99, the positive QC sample must be within 2 standard deviations of its expected value. Wells with < 50 microspheres for each region will be censored. Additionally, the negative QC sample and buffer blanks must have a MFI < 20.
2.1.8.2. Secondary (sample) acceptance criteria.
Samples must have a concentration %CV between dilutions ≤25%. If the %CV is > 25%, outlying dilutions are censored to reduce the %CV. A sample was required to have at least three uncensored dilutions and no more than 3 censored dilutions to pass QC. Low concentration samples were required to have at least two uncensored dilutions in the readable range.
2.1.9. Accuracy
Accuracy is the closeness of agreement between the value which is accepted either as the conventional true value or an accepted reference value and the value determined. Accuracy is expressed as the percent error between the assay-determined value and the pre-determined value for a particular analyte (anti-Bp antigen-specific IgG) in that serum. Anti-Bp antigen specific IgG concentration was quantified in the serum to assess MMACA accuracy. MMACA accuracy was determined by repeated analysis of twenty one sera, MCR0001 - MCR0021. Each serum was tested at least 9 times by 3 operators. Accuracy expressed as percent error was calculated using the following formula; [(observed-expected)/expected] × 100. A percent error of < 25% guideline stipulated for traditional antibody capture assay such as ELISA was considered an acceptable level of accuracy for individual analytes (anti-Bp antigen specific IgG) in MMACA [29]. The overall accuracy of the assay is assessed separately for each antigen by taking the median absolute percent error of all tested samples for that antigen.
2.1.10. Goodness of fit
A 4-parameter logistic-log (4-PL) function was used to model the standard reference serum WHO 06/140. The ‘goodness of fit’ of the assay is an indication of how closely the 4-PL model fits the data points of the reference serum standard for an individual analyte (anti-Bp antigen specific IgG) in a multiplex assay. These data should exhibit a sigmoidal shape when plotted on an MFI vs. Log10 dilution scale. The goodness of fit was expressed as the regression coefficient (R2) of the standard curve. An R2 value that approaches 1.0 is indicative of a ‘good fit’ for the data to the curve. Goodness of fit was determined by averaging the R2 values of at least 100 independent standard reference curves for WHO 06/140.
2.1.11. Dilutional linearity
Linearity of an assay is the ability to elicit results that are directly, or by a well-defined mathematical transformation, proportional to the analyte specific IgG concentration in a test sample. Dilutional linearity was determined using constructed positive sera. These were tested by 3 operators on 3 separate assay days. Observed and expected values were log10 transformed for comparison. Dilutional linearity was assessed by least-squares regression of log10 transformed data in terms of regression coefficient (r2), slope and intercept. Performance targets were set as r2 > 0.9, slope between 0.9 and 1.1, and intercept between −0.25 and 0.25.
2.1.12. Lower limit of detection and lower limit of quantification
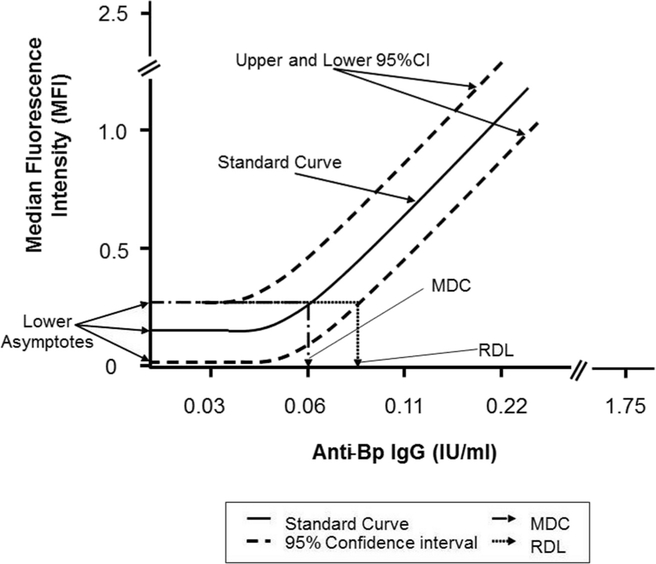
The theoretical (calculated) Minimum Detectable Concentration (MDC) of the anti-Bp antigen specific IgG and the Reliable Detection Limit (RDL) of the MMACA were derived from a 4-PL standard reference serum curve. The MDC is the lowest concentration of anti-Bp antigen specific IgG that can be detected in the assay titer plate well for a serum sample. The RDL is the lowest concentration of the anti-Bp antigen specific IgG that has the high probability of producing a response greater than the zero concentration (negative control). All of the development reference serum curves were used to determine a mean and 95% confidence intervals (95% CI (Fig. 1) [30]. The MDC and RDL were derived independently for each of the five analytes (anti-Bp antigen specific IgG) in this 5-plex assay. The product of MDC and initial serum sample dilution factor is the theoretical Lower Limit of Detection (LOD). The product of RDL and the initial serum sample dilution factor is the theoretical Lower Limit of Quantitation (LOQ).
Fig. 1.
Graphical representation of minimum detectable concentration (MDC) and reliable detection limit (RDL) in MMACA. The mathematical MDC is the concentration of anti-Bp antibody corresponding to the interpolated intersection of the lower asymptote of the upper 95% confidence interval (CI) with the 4-parameter logistic-log fit of the standard curve data. The RDL is the concentration of anti-Bp antibody corresponding to the interpolated intersection of the upper 95% confidence interval asymptote with the lower 95% confidence interval of the standard’s data.
The theoretical Upper Limit of Quantification (ULOQ) is the highest analyte concentration data point in the reference curve that can be used to calculate anti-Bp antigen specific IgG concentration in a serum sample. This has been calculated as the lower of either the first dilution of the reference standard or the point 5% below the reference standard upper asymptote. The interval between the LOQ and the ULOQ (inclusive) is the range of the assay for individual analyte. In practice, test sample analyte concentrations that exceed the ULOQ may be retested at a higher starting dilution to bring it into the range of the standard.
2.1.13. Assay precision
Precision is the measure of variance of the assay under normal operating conditions. Intra-operator precision was expressed for each operator as the mean %CV of anti-Bp antigen specific IgG concentration in serum samples. Intermediate precision was expressed as the mean %CV of anti-Bp antigens specific IgG concentration in serum samples tested by three operators. To test intra-operator and intermediate precision, each operator tested 21 samples (MCR0001 – MCR0021) over multiple days.
2.1.14. Assay ruggedness
Antigen and reporter lots are the two key assay components that were tested for their impact on the assay outcome. Eight lots of Act and four lots of Fha, Fim2/3, Prn and Pt were conjugated to microspheres as described. Lots were compared based on the anti-Bp antigen specific IgG concentration for 21 samples (MCR0001 – MCR0021). Two lots of reporter antibody were compared based on the anti-Bp antigen specific IgG concentration for 21 samples (MCR0001 – MCR0021), while holding the antigen conjugated microsphere lots constant. Samples were also tested in two different plate layouts (Fig. 2) to assess their impact on the outcome. As an additional index of assay ruggedness, the longterm performance of the QC WHO06/142 was assessed based on the %CV of anti-Bp antigen specific IgG concentration over 48 months.
2.1.15. Diagnostic Sensitivity (DSN) and specificity (DSP)
Diagnostic Sensitivity (DSN) is the ability of the assay to identify a true positive result in a test serum. The DSN was determined with sera that were clinically defined positives. The DSN of the assay was calculated as [TP/(TP + FN)] where TP = true positives and FN = false negatives. Diagnostic Specificity (DSP) measures the ability of the assay to identify a true negative result. The DSP was determined from a panel of serum samples from individuals with clinically confirmed negatives to pertussis. The DSP of the assay was calculated as [TN/(TN + FP)] where TN = true negatives and FP = false positives. Diagnostic Sensitivity (DSN) and Specificity (DSP) were assessed using 119 clinical samples obtained from 71 clinically negative and 48 clinically positive patients. The samples were tested by 2 operators, and the geometric mean concentration for each antigen was calculated as the final reportable results. DSN and DSP depend on which antigen(s) were used and what threshold was chosen – the threshold can be adjusted to favor specificity or sensitivity. Receiver Operator Characteristic (ROC) curves was generated using PROC LOGISTIC in SAS [31]All concentrations were log transformed using log (analyte+1) for use in the logistic model. For a single-analyte model, the diagnostic threshold was expressed as a simple concentration. For multi-analyte models, the threshold was expressed as the probability function calculated by the logistic model:
Where X1 – Xn are the measured effects (the log10 transformed antibody concentrations), α is the intercept and β1 – βn are the fit parameters for each effect. In the multi-analyte model, probability function predicts the concentration of individual antigens included in the analysis at the chosen DSN/DSP intersect.
2.2. Assay validation
The purpose of validation is to provide documented evidence that the assay will consistently meet its pre-determined specifications and quality attributes. For the purpose of validation, a panel of 15 sera (MCR259 – MCR286 and MCR298-MCR302) was constructed from three human sera, 2012812612, 2012789123 and WHO 06/142. Each of these three sera was spiked into MCR0028 and the serum panel was generated as indicated in Table 2.
Table 2.
Sera for MMACA assay validation.
| Reagent ID | Formulation dilution Factor | Expected IgG concentration (IU or AU/mL)M |
||||
|---|---|---|---|---|---|---|
| Act | Fha | Fim2/3 | Prn | Pt | ||
|
| ||||||
| 2012812612a | Neat | 129.92 | 113.58 | 1153.47 | 1299.40 | 171.28 |
| MCR277 | 3 | 43.31 | 37.86 | 384.49 | 433.13 | 57.09 |
| MCR278 | 8 | 16.24 | 14.20 | 144.18 | 162.43 | 21.41 |
| MCR279 | 20 | 6.50 | 5.68 | 57.67 | 64.97 | 8.56 |
| MCR280 | 40 | 3.25 | 2.84 | 28.84 | 32.49 | 4.28 |
| MCR281 | 80 | 1.62 | 1.42 | 14.42 | 16.24 | 2.14 |
|
| ||||||
| 2012789123a | Neat | 264.62 | 1015.55 | 320.60 | 1771.89 | 45.86 |
| MCR282 | 3 | 88.21 | 338.52 | 106.87 | 590.63 | 15.29 |
| MCR283 | 8 | 33.08 | 126.94 | 40.08 | 221.49 | 5.73 |
|
| ||||||
| WHO06/142a | Neat | 64.65 | 107.11 | 38.51 | 29.50 | 82.88 |
| MCR284 | 3 | 21.55 | 35.70 | 12.84 | 9.83 | 27.63 |
| MCR285 | 5 | 12.93 | 21.42 | 7.70 | 5.90 | 16.58 |
| MCR286 | 8 | 8.08 | 13.39 | 4.81 | 3.69 | 10.36 |
| MCR298 | 250 | 0.26 | 0.43 | 0.15 | 0.12 | 0.33 |
| MCR299 | 350 | 0.18 | 0.31 | 0.11 | 0.08 | 0.24 |
| MCR300 | 500 | 0.13 | 0.21 | 0.08 | 0.06 | 0.17 |
| MCR301 | 700 | 0.09 | 0.15 | 0.06 | 0.04 | 0.12 |
| MCR302 | 1000 | 0.06 | 0.11 | 0.04 | 0.03 | 0.08 |
Act – Adenylate cyclase; Fha – filamentous hemagglutinin; Fim2/3 - Fimbria; Prn – Pertactin; Pt – Pertussis toxin antigens.
Human sera spiked into human serum depleted of total IgG, IgA and IgM (MCR0028) to formulate a serum panel (MCR277 – MCR302) for MMACA assay validation.
A panel of validation sera (n = 15) was tested for five critical assay parameters, accuracy, intra-operator precision, intermediate precision, dilutional linearity and the limits of quantification. Each serum was tested at least 9 times by three operators. Plate layout#2 (Fig. 2B) was used for assay validation. Validation acceptance criteria are listed in Table 7.
Table 7.
MMACA assay Diagnostic Sensitivity (DSN) and Specificity (DSP).
| Proc Logistic Model | Antigen | Antigen concentration (IU or AU/ mL)/Probability cutoff (%) | DSN | DSP |
|---|---|---|---|---|
|
| ||||
| Single analyte | Pt | 17.10 | 85.42% | 81.69% |
| 22.35 | 83.33% | 84.51% | ||
| 37.41 | 79.17% | 95.78% | ||
| 53.20 | 77.08% | 97.18% | ||
|
| ||||
| Forward Selection | Fim2/3, Pt and Pt × Fim2/3 | 36.40% | 87.50% | 85.92% |
| 38.36% | 85.42% | 88.73% | ||
| 43.62% | 83.33% | 91.55% | ||
| 49.37% | 81.25% | 94.37% | ||
|
| ||||
| Forward Selection without Hierarchy | Fha, Act × Fha, Fim2/3, Fha × Pt and Act × Prn*Pt | 19.51% | 93.75% | 78.87% |
| 21.43% | 91.67% | 78.87% | ||
| 31.38% | 87.50% | 85.92% | ||
| 35.60% | 85.42% | 87.32% | ||
|
| ||||
| Stepwise Selection without Hierarchy | Fha, Fim2/3 and Fha × Pt | 25.69% | 89.58% | 81.69% |
| 27.00% | 87.50% | 83.10% | ||
| 35.22% | 85.42% | 91.55% | ||
| 38.85% | 83.33% | 91.55% | ||
| 57.81% | 77.08% | 98.59% | ||
|
| ||||
| Backwards Selection | Act, Fha, Fim2/3, Act × Fim2/3, Fha × Fim2/3, Prn, Act × Prn, Fim2/3 × Prn, Act × Fim2/3,*Prn, Pt, Act × Pt, Fha × Pt, Fim2/3 × Pt, and Act × Fim2/3 × Pt | 9.76% | 95.83% | 77.46% |
| 14.52% | 93.75% | 85.92% | ||
| 50.23% | 91.67% | 95.77% | ||
| 55.67% | 89.58% | 97.18% | ||
| 64.05% | 87.50% | 100.00% | ||
|
| ||||
| Backwards Selection without Hierarchy | Fha, Act × Fha*Fim2/3, Prn, Act × Prn, Fha × Prn, Act × Fha*Fim2/3 × Prn, Pt, Act × PT, Fim2/3 × Pt and Act × Fha*Fim2/3 × Prn*PT | 13.33% | 95.83% | 81.69% |
| 21.50% | 93.75% | 84.51% | ||
| 50.29% | 91.67% | 95.77% | ||
| 54.51% | 87.50% | 98.59% | ||
| 63.07% | 83.33% | 100.00% | ||
Act – Adenylate cyclase; Fha – filamentous hemagglutinin; Fim2/3 - Fimbria; Prn – Pertactin; Pt – Pertussis toxin antigens.
2.3. Plasma vs serum
A set of 15 paired human plasma/serum samples were tested for Bp antigen specific antibodies as described in section 2.6. The anti-Bp antibody concentration in paired plasma and serum samples were tested for agreement based on Deming regression analysis and Concordance Correlation Coefficient (CCC).
2.4. Dried blood spot vs serum
A set of 20 paired human dried blood spot (DBS)/serum samples were tested for Bp antigen specific antibodies. The DBS was eluted as described previously [32] with assay buffer for elution. The anti-Bp antibody concentration in paired DBS and serum samples were tested for agreement based on Deming regression analysis and Concordance Correlation Coefficient (CCC).
2.5. Statistical analysis
Data for each antigen were analyzed independently, including application of quality control criteria. The median fluorescence intensity (MFI) from a minimum of 50 beads was used as the input data for each sample well. All regression analyses were performed on log10 transformed data using log10(MFI+1). Reference standard data were fit to a 4 parameter logistic curve as:
Where A is the lower asymptote, B is the upper asymptote, C is the midpoint dilution, D is the slope parameter, and Dil is the reciprocal dilution of the sample. Curve fitting was first performed using an unweighted fit, then refined using robust weighting based on the Welsch weighting function (Holland and Welsch, 1977):
Where resid is the residual error of each point and c is a scale constant set as 2.1 times the total RMS error from the previous iteration.
The mean MFI of the duplicate wells of each sample was used for concentration calculations. Dilutions that were outside the useable range of the reference standard or that had a coefficient of variation (%CV) > 30% were censored. The mean concentration of the uncensored dilutions was calculated. If the CV of the concentrations was > 25%, the largest outlier was censored and the mean and CV were recalculated. A maximum of three dilutions could be censored with a minimum of 3 uncensored dilutions remaining in the useable range. Samples that still had a CV > 25% after censoring failed QC and were repeated. High concentration samples with fewer than three dilutions in the useable range were repeated at higher starting dilution.
Interpolated concentrations were log transformed for calculation of geometric mean for accuracy, CV for precision, and dilutional linearity. The CV of log-transformed data was calculated as
where σ is the standard deviation of the natural-log transformed concentrations.
3. Results
3.1. Assay performance characteristics
3.1.1. Accuracy
Twenty-one sera, MCR0001 – MCR0021, tested at least nine times by three operators, were used to assess the MMACA accuracy for five analytes (anti-Bp antigen specific IgG). Median absolute percent error for each antigen ranged from 13.67% to 24.77% for Act, Fha, Fim2/3, Prn, and Pt (Table 3).
3.1.2. Goodness of fit
Goodness of fit for the standards data for each anti-Bp antigen specific IgG analysis was determined by averaging the correlation coefficient (R2) values of 188–213 independent standard reference curves for WHO 06/140. Each plate generated five standard reference curves, one each for 5 anti-Bp IgG in WHO 06/140. Curves that failed the plate QC check for individual antigens were excluded. The goodness of fit was R2 ≥ 0.99 for all analyte standard curves (Table 3).
3.1.3. Dilutional linearity
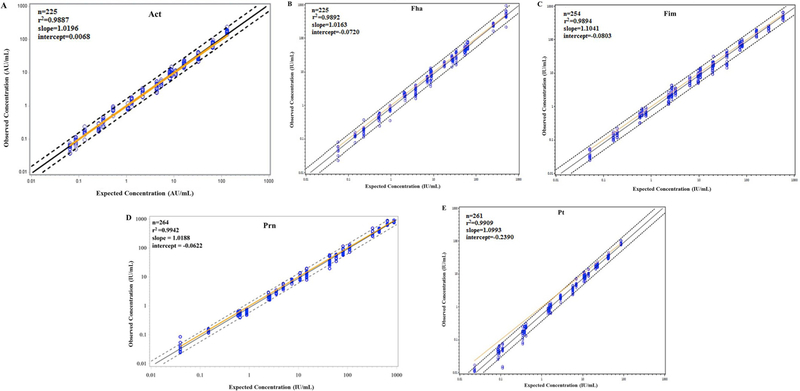
Regression analysis of log10 transformed observed concentration versus expected concentration for analytes (anti-Bp antigen specific IgG) was used to calculate the dilutional linearity. Each of the 21 test samples (MCR0001 – MCR0021) were tested a minimum of nine times. Regression analysis was done using CDC customized SAS program and r2, slope and intercept were calculated for all five analytes. Dilutional linearity r2 ranged between 0.9887 and 0.9942, slope of 1.0141–1.0993 and intercept of −0.2390–0.0068 (Fig. 3A–E; Table 3).
Fig. 3.
Dilutional linearity. (A) Act, (B) Fha, (C) Fim, (D) Prn, and (E) Pt. All results have been log10 transformed. Dotted lines are 95% confidence interval of individual observations. Solid black line is the best fit regression line, solid yellow line is where observed concentration = expected concentration. Slope, intercept and r2 are indicated. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.1.4. Lower limit of detection and lower limit of quantification
Minimum Detectable Concentration (MDC) for MMACA is 0.003–0.013 IU or AU/mL in the well for various analytes (anti-Bp antigen specific IgG) (Table 4). With a minimum starting dilution of 1/10 this produces an LOD of 0.027–0.127 IU or AU/mL for various analytes (anti-Bp antigen specific IgG) in the sample. Reliable Detection Limit (RDL) for MMACA is 0.005–0.024 IU or AU/mL in the well for various analytes (anti-Bp antigen specific IgG) (Table 4). With a minimum starting dilution of 1/10 this produces a LOQ of 0.048–0.239 IU or AU/mL for various analytes (anti-Bp antigen specific IgG) in the sample.
3.1.5. Assay precision
Intra-operator precision was calculated by taking the median %CV of anti-Bp antigen specific IgG concentration in 21 samples (MCR0001 – MCR0021) tested in at least 3 separate experiments by an operator. The range of median %CV for all operators was 13.25%–29.91% (Table 3). Inter-operator (intermediate) precision was determined by calculating the median %CV of anti-Bp antigen specific IgG concentration in 21 samples (MCR0001 – MCR0021) tested in 3 separate experiments by each operator. The range of median CV for all operators was 20.52%–26.75% for Bp analytes with the maximum %CV for Pt (26.75%) (Table 3).
3.1.6. Assay ruggedness
Antigens conjugated to microspheres and reporter antibodies are the key assay components whose variability was tested for their impact on the assay outcome. Different lots of microspheres were evaluated on percent difference from a normalized mean of 1.0. Different lots of reporter antibodies were evaluated by a mixed effects model. The model controlled for the variable nature of the assay by including a random effect for experiment, with variance components, covariance structure and used maximum likelihood estimation. This model also used the interaction of sample and antigen as a fixed effect. Operator was also included as a fixed effect for inter operator tendencies.
Individual antigen lots were evaluated by percent difference from 1.0. Each antigen lot was tested using twenty one samples. The concentration of each sample per antigen lot was normalized. This normalized value was calculated as the individual sample’s concentration over the mean of that sample from all antigen lots. The mean of the normalized values was then calculated for each antigen lot. Percent difference was calculated as the percent change of the normalized mean from 1.0. Percent difference for all the three variables included in this analysis ranged within −11.44% to 12.89% (Table 5). The mixed effects model was used to evaluate the effect of reporter antibody lots and operators. Reporter antibodies and operators are consistent across all 5 antigens. The results showed no statistical difference between conjugate lots (p = 0.8506). The effect of operators also showed no significant difference between three operators (p = 0.4371). The results from the positive QC sample WHO 06/142 on two different plate layouts indicated no significant impact on the results (data not shown).
Table 5.
Antigen lot comparison.
| Antigen | Vendor | Lot # | Normalized Mean | Percent Difference |
|---|---|---|---|---|
|
| ||||
| Act | List | MM0110 | 0.87113 | −12.89% |
| List | MM0109 | 0.90625 | −9.38% | |
| EH | MM0106 | 0.96349 | −3.65% | |
| EH | MM0105 | 0.98242 | −1.76% | |
| List BP | MM0103 | 0.99233 | −0.77% | |
| List BP | MM0104 | 1.06208 | 6.21% | |
| Sigma | MM0112 | 1.10789 | 10.79% | |
| Sigma | MM0111 | 1.11439 | 11.44% | |
|
| ||||
| Fha | List | MM0083 | 0.97715 | −2.29% |
| List | MM0084 | 1.00211 | 0.21% | |
| SP | MM0081 | 0.98069 | −1.93% | |
| SP | MM0082 | 1.04005 | 4.00% | |
|
| ||||
| Fim2/3 | List | MM0101 | 0.96868 | −3.13% |
| List | MM0102 | 0.98569 | −1.43% | |
| SP | MM0099 | 1.00195 | 0.19% | |
| SP | MM0100 | 1.04368 | 4.37% | |
|
| ||||
| Prn | List | MM0095 | 1.03112 | 3.11% |
| List | MM0096 | 1.05225 | 5.22% | |
| SP | MM0093 | 0.92874 | −7.13% | |
| SP | MM0094 | 0.98788 | −1.21% | |
|
| ||||
| Pt | List | MM0089 | 0.98973 | −0.77% |
| List | MM0090 | 0.99739 | 0.00% | |
| SP | MM0087 | 0.97409 | −2.34% | |
| SP | MM0088 | 1.03879 | 4.15% | |
List = List Biologies Inc; EH = Erik Hewlett; Sigma = Sigma-Aldrich; SP = Sanofi Pasteur.
Act – Adenylate cyclase; Fha – filamentous hemagglutinin; Fim2/3 - Fimbria; Prn – Pertactin; Pt – Pertussis toxin antigens.
Percent difference: Percent difference was calculated as the percent change of the normalized mean from 1.0 with the accepted range between ± 20%.
Long term performance (48 months) of QC sample WHO 06/142 is shown in Table 6. Highest fluctuation in the QC performance over time was observed for Act (%CV = 33.1) followed by Fha (%CV = 20.5). The other 3 Bp antigens, Fim2/3, Prn and Pt had %CV < 20.
Table 6.
WHO06/142 long term performance.
| Antigen | n | IgG Geometric Mean Concentration (lu or AU/mL) | CV |
|---|---|---|---|
|
| |||
| Act | 233 | 65.07 | 33.10% |
| Fha | 210 | 107.23 | 20.53% |
| Fim2/3 | 231 | 38.57 | 16.49% |
| Prn | 230 | 29.48 | 14.87% |
| Pt | 245 | 82.78 | 18.37% |
Act – Adenylate cyclase; Fha – filamentous hemagglutinin; Fim2/3 - Fimbria; Prn – Pertactin; Pt – Pertussis toxin antigens.
3.1.7. Diagnostic Sensitivity and specificity
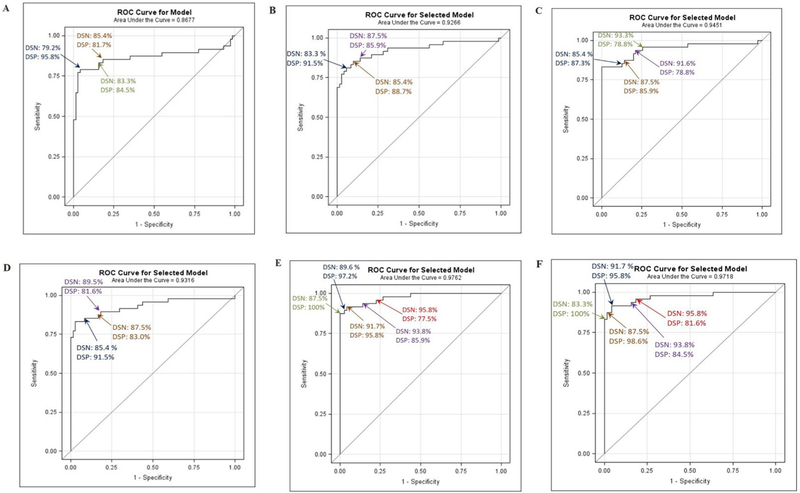
The model with Pt as the only effect indicated a modest Area Under the Curve (AUC) of 0.8677 and an optimum DSN/DSP of 83.3%/84.5% at 22.35 IU/mL (Table 7; Fig. 4 A). Forward selection method to evaluate multi-analyte models generated a 3-element model with AUC of 0.9266 and an optimal DSN/DSP of 87.5%/85.9% at the probability cutoff 36.40% (Fig. 4B and C). Stepwise selection model generated an AUC of 0.9316 and an optimal DSN/DSP of 85.4%/91.5% at the probability cutoff 35.22% (Fig. 4D). Backward selection method generated a 10-element model with an AUC of 0.9718 and DSN/DSP of 91.7%/95.8% with 50.23% & 50.29% cutoff (Fig. 4E and F).
Fig. 4.
DSN/DSP - Receiver Operator Curve (ROC). (A) Pt as only effect; (B) Forward selection or stepwise selection methods containing Pt, Fim2/3, and the interaction of Pt × Fim2/3 as effects; (C) Forward selection method without hierarchy containing Fha, Act × Fha, Fim2/3, Fha × Pt, and Act × Prn*Pt as effects; (D) Stepwise selection model without hierarchy containing Fha, Fim2/3, and Fha × Pt as effects; (E) Backwards selection method containing Act, Fha, Fim2/3, Act × Fim2/3, Fha × Fim2/3, Prn, Act × Prn, Fim2/3 × Prn, Act × Fim2/3 × Prn, Pt, Act × Pt, Fha × Pt, Fim2/3 × Pt, Act × Fim2/3 × Pt as effects; and (F) Backwards selection method without hierarchy containing Fha, Act × Fha*Fim2/3, Prn, Act × Prn, Fha × Prn, Act × Fha*Fim2/3 × Prn, Pt, Act × Pt, Fim2/3 × Pt, Act × Fha*Fim2/3 × Prn*Pt as effects.
3.2. Validation of MMACA
MMACA was validated with a panel of human serum mock samples (n = 15). Assay accuracy was analyzed and expressed as the median % Error for each antigen. Median absolute % Error for each antigen ranged from 1.93% to 23.84% Act, Fha, Fim2/3, Prn, and Pt. Dilutional linearity r2 ranged between 0.91 and 0.97, slope of 0.93–1.01 and intercept of −0.009–0.108 (Table 8). The validation data indicate that the LLOQ for MMACA was 0.04–0.15AU or IU/mL of anti-Bp antibodies (Table 8).
Table 8.
MMACA assay validation performance statistics.
| Parameter | Acceptance Criteria | Performance |
||||
|---|---|---|---|---|---|---|
| Act | Fha | Fim2/3 | Prn | Pt | ||
|
| ||||||
| Accuracy | Median %Error ≤25% | 23.84% | 10.97% | 5.97% | 19.76% | 1.93% |
| Goodness of Fit | Mean r2 | 0.9990 | 0.9989 | 0.9994 | 0.9996 | 0.9991 |
| Intra-operator Precision | Median %CV ≤40% | 18.10%–27.07% | 9.49%–22.96% | 9.16%–22.47% | 11.59%–15.92% | 12.63%–20.24% |
| Inter-operator (Intermediate) Precision | Median %CV ≤40% | 22.77% | 16.76% | 17.07% | 19.68% | 16.03% |
| Dilutional Linearity: | ||||||
| r2 | ≥0.9 | 0.91 | 0.99 | 0.99 | 0.98 | 0.97 |
| Slope | 0.9 to 1.1 | 0.93 | 1.00 | 1.02 | 0.98 | 1.01 |
| Intercept | −4.0 to 4.0 | −0.035 | 0.046 | −0.009 | 0.108 | −0.017 |
| Lower Limit of Quantification (LLOQ) | Median %Error ≤40% and %CV ≤40% | 0.09 AU/mL | 0.15 IU/mL | 0.06 AU/mL | 0.04 IU/mL | 0.08 IU/mL |
Act – Adenylate cyclase; Fha – filamentous hemagglutinin; Fim2/3 - Fimbria; Prn – Pertactin; Pt – Pertussis toxin antigens.
For validation, linearity of the assay was assessed by least-squares regression of log10 transformed data and was required to have the r2 > 0.9, and the slope between 0.9 and 1.1. Validation experiments have successfully satisfied these requirements with the r2 = 0.91–0.99 and slope between 0.93 and 1.02 (Table 8).
Intra-operator precision was determined by the repeated analysis (by 3 operators) of 5 validation sera representing a range (max-mid-min) of anti-Bp antibody concentration in the reference curve for individual analytes. The intra-operator precision (%CV) was in the range of 9.16%–27.07% for Bp analytes with the maximum %CV for Act (18.10%–27.07%). The intermediate precision (%CV) was in the range of 16.03%–22.77% for Bp analytes with the maximum %CV for Act (22.77%) (Table 8).
3.3. Plasma vs serum
The Deming regression of the median anti-Bp antigen specific antibody concentration values in plasma and serum produced a precision of 0.979–0.991, an accuracy of 0.992–0.999 and a CCC of 0.979–0.995 for five different Bp antigens (Table 9).
Table 9.
Correlation between the human plasma vs serum and DBS vs serum anti-Bp antibody concentrations.
| Specimens | Parameter | Antigen |
||||
|---|---|---|---|---|---|---|
| Act | Fha | Fim2/3 | Prn | Pt | ||
|
| ||||||
| Plasma vs Serum | Precision | 0.9798 | 0.9877 | 0.9954 | 0.9886 | 0.9918 |
| Accuracy | 0.9999 | 0.9924 | 0.9996 | 0.9986 | 0.9991 | |
| CCC | 0.9798 | 0.9802 | 0.9950 | 0.9872 | 0.9909 | |
|
| ||||||
| DBS vs Serum | Precision | 0.9690 | 0.9927 | 0.9927 | 0.9951 | 0.9945 |
| Accuracy | 0.9084 | 0.9989 | 0.9989 | 0.9959 | 0.9953 | |
| CCC | 0.8802 | 0.9915 | 0.9915 | 0.9910 | 0.9899 | |
CCC = Concordance Correlation Coefficient.
3.4. DBS vs serum
The Deming regression of the median anti-Bp antigen specific antibody concentration values in DBS and serum produced a precision of 0.992–0.995, an accuracy of 0.995–0.998 and a CCC of 0.989–0.991 for four Bp antigens, Fha, Fim, Prn and Pt (Table 9). A deviation in the CCC for Act from the acceptable limit of 0.95 to 0.88 was observed despite acceptable precision (0.969) and accuracy (0.908). Additional testing is in progress to address this deviation. Unless this deviation was addressed, MMACA will not be employed to quantify anti-Act antibodies in DBS.
4. Discussion
Quantification of Bp antigen specific antibodies in serum serves as an important tool to ascertain the vaccine immune response for acellular pertussis vaccines and pertussis diagnosis. Enzyme linked immunosorbent assay (ELISA) is the most common serological technique employed to quantify antibodies to pertussis vaccine antigens [28]. ELISA has several limitations in terms of time, resource, specimen volume and throughput. While multiplexed assays are being developed as viable alternatives, careful and meticulous characterization of the assay is warranted to ensure reproducible endpoints for all the analytes that were tested simultaneously in a single reaction. In addition, development of assay specific reagents and reference materials are crucial for the transfer of this assay to various laboratories and harmonize the outcome [27]. In this background, we have developed and characterized a multiplex immunoassay for the simultaneous quantification of IgG antibodies specific to five Bp antigens, Act, Pt, Fha, Prn and Fim2/3.
The MMACA is capable of quantifying antibodies to five different Bp antigens in a single reaction. All key assay parameters have been well characterized and the assay performance was subjected to rigorous testing. Importance of standardized assay for vaccine evaluation and product development has been reiterated but not accomplished frequently [33–36]. It is our objective to develop a multiplex serological assay for pertussis that can be transferred to any lab as a package that includes reference standard, QC materials, detailed protocols, procedures and customized data analysis software. In this context, we have standardized the MMACA for test reagents, test procedures, method of data reduction, and compatibility of critical reagents from various sources.
MMACA is a rugged, and robust method suitable for the simultaneous quantification of anti-Bp antigens (Pt, Prn, Fha, Fim2/3, and Act) specific antibodies in serum, plasma and DBS. Very low lot variations (< 12.30%) of the critical assay components that included antigen conjugated microsphere and reporter antibody demonstrate the ruggedness of the assay. Reference standard is the crucial reagent in a quantitative immunoassay as its analytical stability determines the reproducibility and reliability of the assay end point [37]. The goodness of fit (R2) describes how closely the reference standard data points fit the 4-PL model. The closer the R2 to 1.0, indicates a good fit for the data to the curve. The R2 measure of the standard curves for 5 analytes that range from 0.9989 to 0.9997, demonstrates MMACA as a reliable serological method to monitor and normalize the Bp immune response over time in a reliable manner.
Accuracy and precision are the two key characteristics of an immune assay. Validated assay with reproducible and robust performance characteristics ensures the transferability of an assay to other labs without any elaborate lab specific re-standardization requirements. Hence, several key parameters were assessed during the assay validation. MMACA is accurate with the assessed median %E of 1.93%–23.84% for antibodies to Bp antigens. Further, the assay has an acceptable precision within a median CV of 16.03%–22.77% between all operators. Dilutional linearity also demonstrated the ability of the assay to quantify anti-Bp antigens specific IgG in 5-plex format with precision. The r2 for dilutional linearity regression analyses ranged from 0.91 to 0.99, with slopes from 0.93 to 1.02 and intercept from −0.0009–0.108 for the five analytes. Validation data has clearly demonstrated the reproducibility and robustness of this assay where the critical assay parameters were consistent or even better than the performance characteristics established during the assay development.
Sensitivity of an immune assay determines its LLOD and LLOQ or vice versa [37]. The LLOQ (0.04–0.15 IU or AU/mL) indicates MMACA as highly sensitive compared to ELISAs [38]. While the LLOQ for Pt and Prn in ELISA was 2.0 IU/mL and 6.0 IU/mL respectively [39], MMACA was more sensitive with the LLOQ at 0.08 IU/mL and 0.15 IU/mL for Pt and Prn respectively. Van Gageldonk et al. have reported the development and validation of a pentaplex assay to quantify serum antibodies to pertussis (Pt, Fha and Prn), diphtheria and tetanus [22]. Unfortunately, this assay uses different reference materials (in-house reference serum) or test reagents and hence do not have any common denominator for comparison. LLOQs that extend below the ELISA LLOQ of 2–6 EU/mL for some Bp antigens may provide distinct discriminatory power when applying this assay for other uses such as pertussis diagnosis. Construction of specific algorithms and statistical modeling is in progress to adopt MMACA for pertussis diagnosis.
Serological tests such as anti-pertussis toxin ELISA (PT ELISA) is increasingly used as a confirmatory diagnostic tool for pertussis [39,40]. Even though the commercial pertussis sero-diagnostic kits measure antibodies against Pt or Fha, their diagnostic performance is questionable [41] partly due to a wide variations in the assay sensitivity (80%–100%) and specificity (51%–93%). We hypothesized that detection of IgG to Bp antigens apart from Pt especially non-vaccine antigen such as Act may improve pertussis diagnosis. For this purpose, logistic regression models using combinations of the 5 antigens were evaluated using PROC LOGISTIC in SAS®. The backward selection method with all 5 antigens gave the best model in terms of classification performance and DSN/DSP values (91.7%/95.8%). The Stepwise and Forward selection provided simpler models with improved DSN/DSP compared to Pt alone.
MMACA is the first validated multiplex assay capable of quantifying antibodies to 5 Bp antigens, Pt, Prn, Fha, Fim2/3, and Act in a single reaction in serum, plasma and DBS. It is a sensitive, accurate, precise, reproducible antigen and sample conserving serological assay with high throughput. Further, the multi analyte models demonstrate the potentials of MMACA in pertussis serodiagnosis. With the continued rise in pertussis disease burden worldwide despite high vaccination rates, MMACA can prove vital to peruse large-scale immune-surveillance studies.
Acknowledgements
The authors wish to thank Sanofi Pasteur for providing the aP vaccine antigens, and Dr. Erik Hewlett, University of Virginia, VA for providing Act for assay development. We also thank the CDC Pertussis laboratory for providing human sera for the initial assay development activities.
Funding
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 2005;18:326–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tan T, Dalby T, Forsyth K, Halperin SA, Heininger U, Hozbor D, et al. Pertussis across the globe: recent epidemiologic trends from 2000 to 2013. Pediatr Infect Dis J 2015;34:e222–32. [DOI] [PubMed] [Google Scholar]
- [3].Skoff TH, Kenyon C, Cocoros N, Liko J, Miller L, Kudish K, et al. Sources of infant pertussis infection in the United States. Pediatrics 2015;136:635–41. [DOI] [PubMed] [Google Scholar]
- [4].Yeung KHT, Duclos P, Nelson EAS, Hutubessy RCW. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis 2017;17:974–80. [DOI] [PubMed] [Google Scholar]
- [5].Notice to readers: final 2015 reports of nationally notifiable infectious diseases and conditions. MMWR Morb Mortal Wkly Rep 2016;65:1306–21. [DOI] [PubMed] [Google Scholar]
- [6].Bolotin S, Harvill ET, Crowcroft NS. What to do about pertussis vaccines? Linking what we know about pertussis vaccine effectiveness, immunology and disease transmission to create a better vaccine. Pathog Dis 2015;73. ftv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McGirr A, Fisman DN. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics 2015;135:331–43. [DOI] [PubMed] [Google Scholar]
- [8].Deen JL, Mink CA, Cherry JD, Christenson PD, Pineda EF, Lewis K, et al. Household contact study of Bordetella pertussis infections. Clin Infect Dis 1995;21:1211–9. [DOI] [PubMed] [Google Scholar]
- [9].Olin P, Hallander HO, Gustafsson L, Reizenstein E, Storsaeter J. How to make sense of pertussis immunogenicity data. Clin Infect Dis 2001;33(Suppl 4):S288–91. [DOI] [PubMed] [Google Scholar]
- [10].Plotkin SA. The pertussis problem. Clin Infect Dis 2014;58:830–3. [DOI] [PubMed] [Google Scholar]
- [11].Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis 2013;56:1458–65. [DOI] [PubMed] [Google Scholar]
- [12].Mahon BP, Brady MT, Mills KH. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J Infect Dis 2000;181:2087–91. [DOI] [PubMed] [Google Scholar]
- [13].Mielcarek N, Debrie AS, Raze D, Bertout J, Rouanet C, Younes AB, et al. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog 2006;2:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stenger RM, Smits M, Kuipers B, Kessen SF, Boog CJ, van Els CA. Fast, antigen-saving multiplex immunoassay to determine levels and avidity of mouse serum antibodies to pertussis, diphtheria, and tetanus antigens. Clin Vaccine Immunol 2011;18:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fadugba OO, Wang L, Chen Q, Halasa NB. Immune responses to pertussis antigens in infants and toddlers after immunization with multicomponent acellular pertussis vaccine. Clin Vaccine Immunol 2014;21:1613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen QR, Vansant G, Oades K, Pickering M, Wei JS, Song YK, et al. Diagnosis of the small round blue cell tumors using multiplex polymerase chain reaction. J Mol Diagn 2007;9:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lal G, Balmer P, Stanford E, Martin S, Warrington R, Borrow R. Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J Immunol Methods 2005;296:135–47. [DOI] [PubMed] [Google Scholar]
- [18].McCabe AF, Eliasson C, Prasath RA, Hernandez-Santana A, Stevenson L, Apple I, et al. SERRS labelled beads for multiplex detection. Faraday Discuss 2006;132:303–8. [DOI] [PubMed] [Google Scholar]
- [19].Pickering JW, Martins TB, Greer RW, Schroder MC, Astill ME, Litwin CM, et al. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am J Clin Pathol 2002;117:589–96. [DOI] [PubMed] [Google Scholar]
- [20].Pickering JW, Martins TB, Schroder MC, Hill HR. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for auantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae Type b. Clin Diagn Lab Immunol 2002;9:872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van Gageldonk PG, van Schaijk FG, van der Klis FR, Berbers GA. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods 2008;335:79–89. [DOI] [PubMed] [Google Scholar]
- [22].de Voer RM, van der Klis FR, Engels CW, Rijkers GT, Sanders EA, Berbers GA. Development of a fluorescent-bead-based multiplex immunoassay to determine immunoglobulin G subclass responses to Neisseria meningitidis serogroup A and C polysaccharides. Clin Vaccine Immunol 2008;15:1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Prince HE, Lape-Nixon M, Matud J. Evaluation of a tetraplex microsphere assay for Bordetella pertussis antibodies. Clin Vaccine Immunol 2006;13:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reder S, Riffelmann M, Becker C, Wirsing von Konig CH. Measuring immunoglobulin g antibodies to tetanus toxin, diphtheria toxin, and pertussis toxin with single-antigen enzyme-linked immunosorbent assays and a bead-based multiplex assay. Clin Vaccine Immunol 2008;15:744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Edwards KM. Review of the laboratory approaches to the detection of antibody and cell-mediated immunity to pertussis disease and vaccine. Expert Rev Vaccines 2014;13:1183–90. [DOI] [PubMed] [Google Scholar]
- [26].Barkoff AM, Grondahl-Yli-Hannuksela K, He Q. Seroprevalence studies of pertussis: what have we learned from different immunized populations. Pathog Dis 2015;73. [DOI] [PubMed] [Google Scholar]
- [27].Edwards KM. Unraveling the challenges of pertussis. Proc Natl Acad Sci U S A 2014;111:575–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Staros JV, Wright RW, Swingle DM. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem 1986;156:220–2. [DOI] [PubMed] [Google Scholar]
- [29].Findlay JW, Smith WC, Lee JW, Nordblom GD, Das I, DeSilva BS, et al. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharmaceut Biomed Anal 2000;21:1249–73. [DOI] [PubMed] [Google Scholar]
- [30].Quinn CP, Semenova VA, Elie CM, Romero-Steiner S, Greene C, Li H, et al. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg Infect Dis 2002;8:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].https://www.sas.com.
- [32].Schiffer JM, Maniatis P, Garza I, Steward-Clark E, Korman LT, Pittman PR, et al. Quantitative assessment of anthrax vaccine immunogenicity using the dried blood spot matrix. Biologicals 2013;41:98–103. [DOI] [PubMed] [Google Scholar]
- [33].Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, et al. Neisseria meningitidis group B correlates of protection and assay standardization–international meeting report vol. 24. Atlanta, Georgia, United States: Emory University; 2006. p. 5093–107. 16–17 March 2005. Vaccine. [DOI] [PubMed] [Google Scholar]
- [34].Plikaytis BD, Stella M, Boccadifuoco G, DeTora LM, Agnusdei M, Santini L, et al. Interlaboratory standardization of the sandwich enzyme-linked immunosorbent assay designed for MATS, a rapid, reproducible method for estimating the strain coverage of investigational vaccines. Clin Vaccine Immunol 2012;19:1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rymer JC, Sabatier R, Daver A, Bourleaud J, Assicot M, Bremond J, et al. A new approach for clinical biological assay comparison and standardization: application of principal component analysis to a multicenter study of twenty-one carcinoembryonic antigen immunoassay kits. Clin Chem 1999;45:869–81. [PubMed] [Google Scholar]
- [36].Li H, Soroka SD, Taylor TH Jr., Stamey KL, Stinson KW, Freeman AE, et al. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J Immunol Methods 2008;333:89–106. [DOI] [PubMed] [Google Scholar]
- [37].Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol 2005;12:959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Edwards KM, Meade BD, Decker MD, Reed GF, Rennels MB, Steinhoff MC, et al. Comparison of 13 acellular pertussis vaccines: overview and serologic response. Pediatrics 1995;96:548–57. [PubMed] [Google Scholar]
- [39].de Melker HE, Versteegh FG, Conyn-Van Spaendonck MA, Elvers LH, Berbers GA, van Der Zee A, et al. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J Clin Microbiol 2000;38:800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kapasi A, Meade BD, Plikaytis B, Pawloski L, Martin MD, Yoder S, et al. Comparative study of different sources of pertussis toxin (PT) as coating antigens in IgG anti-PT enzyme-linked immunosorbent assays. Clin Vaccine Immunol 2012;19:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Riffelmann M, Thiel K, Schmetz J, Wirsing von Koenig CH. Performance of commercial enzyme-linked immunosorbent assays for detection of antibodies to Bordetella pertussis. J Clin Microbiol 2010;48:4459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]