Abstract
Background
Canadian-born children of South Asian [SA] ethnicity develop inflammatory bowel disease [IBD] at similar rates to those among Caucasian children. We evaluated the variation in phenotypic spectrum of IBD in SA and Caucasian children in a national paediatric inception cohort of new-onset IBD.
Methods
Patients aged <17 years, enrolled in a Canadian nationwide inception cohort study, were included. Baseline demographic and IBD phenotypic features were compared between SA and Caucasian children. Longitudinal outcomes through 18 months of follow-up were compared matched by propensity scores.
Results
Of 1156 children enrolled over 2014 to 2019, 623 were Caucasian [98% and 88% parents Canadian born] and 114 SA [79% Canadian born, 87% parents SA born]. Fewer SAs have a first-degree relative with IBD, 6% vs 19% in Caucasians, p = 0.002. SAs present at a younger age, median age 11.4 years (interquartile range [IQR] 9.2–14.3) vs 13 years [IQR 10.9‐15 years], p = 0.03 and more commonly with a UC/IBD-U [ulcerative colitis/IBD-unclassified] subtype [ratio of UC/IBD-U to CD 1.2:1 vs 1:1.8 for Caucasians, p <0.001]. Additionally, a greater proportion of SA CD patients present with colonic-only disease [colonic-only CD/UC/IBD-U in SAs 67% vs 57% for Caucasians, p = 0.001], and among those with CD, colonic CD in SAs 31% vs 23% in Caucasians, p = 0.20]. Perianal fistulising disease was also numerically more common in SAs (14 [27%] vs 64 [18%], p = 0.06]. Adjusting for differences in phenotypic presentation, anti-tumour necrosis factor [TNF] exposure, and time to initiation was similar, and two-thirds of children, whether anti-TNF exposed or naïve, were in corticosteroid-free clinical remission at 18 months irrespective of ethnicity.
Conclusions
The phenotypic spectrum of new-onset IBD in SA children differs from that of Caucasian children, but treatment and clinical course are similar within phenotypic subgroups.
Keywords: Inflammatory bowel disease, Crohn’s disease, ulcerative colitis, phenotype, South Asian, paediatric
1. Introduction
Traditionally inflammatory bowel disease [IBD] has been associated with Western industrialised countries, but recent epidemiological studies have highlighted the emerging global incidence and prevalence of IBD in Asian and Asian-Pacific countries.1,2 This marked increase in IBD is particularly notable in the Indian subcontinent, where the mean annual incidence of IBD in India is now 9.3/105.2 Interestingly, adult immigrants from South Asia to the UK were observed as early as two decades ago to have an increased incidence and prevalence of IBD following migration.3,4 The changing demographics of IBD in the USA have similarly been recognised.5
The incidence of childhood-onset IBD in Canada is among the highest globally, and its prevalence is projected to triple from 62 per 105 in 2008 to 159 per 105 in 20306; the highest rate of increase is observed in young children.6,7 Canada has a highly diverse population, with visible minorities comprising 22% of the total population and people of South Asian [SA] origin representing the largest group [25%].8 A study using linked health administrative and immigration data from Ontario, Canada’s most populous province, indicated that SA immigrants developed IBD at lower rates than non-immigrants after arrival in Canada, whereas incidence among Canadian-born children of SA immigrants is comparable to that observed among children from non-immigrant Caucasian families.9
Worldwide population-based studies have demonstrated ethnic variation in both phenotype and outcomes in IBD.10 Disease expression in both first- and second-generation SA adult IBD patients in the UK and North America differs from that in Caucasians and those of Northern European heritage. A greater prevalence of ulcerative colitis [UC], male predominance, more extensive colitis, and earlier age at presentation have been observed among SAs.11,12 Recent adult cohort studies have not demonstrated a difference in time from diagnosis to prescription of biologics or to surgical outcomes.13,14 However, retrospective single-centre paediatric studies from North America have suggested that SA children present with more severe and extensive colonic disease, and in Crohn’s disease [CD] with more complicated disease. They also had earlier time to immunomodulator or biologic therapy.15–17 The disparity in longitudinal outcomes may be attributed to difference in age, but nevertheless warrants further investigation.
The aim of this study was to compare the phenotypic spectrum at presentation and subsequent longitudinal outcomes of SA children with new-onset IBD with those of Caucasian children in a national, prospective, inception cohort.
2. Methods
2.1. Setting and participants
Children and adolescents aged 2–17 years, with new-onset IBD, are recruited into an inception cohort study as part of the Canadian Children Inflammatory Bowel Disease Network [CIDsCANN], a joint partnership of the Canadian Institutes of Health Research and the Children with Intestinal and Liver Disorders Foundation. Children were recruited from 12 participating academic paediatric IBD centres across Canada, where care is delivered until transition to adult services. As previously described,18 patients are evaluated via ileo-colonoscopy, upper endoscopy, and small bowel imaging. Comprehensive baseline and longitudinal phenotypic and demographic data, including ethnicity and family history of IBD, are recorded using standardised case report forms and are entered into a central database registry. Clinical site directors, all paediatric gastroenterologists with a clinical focus in IBD, are responsible for approving a diagnostic label of type of IBD as Crohn’s disease [CD], ulcerative colitis [UC], or IBD type unclassified [IBD-U], using conventional clinical, endoscopic, and histological criteria.
Disease phenotype at diagnosis is categorised according to the Paris modification of the Montreal classification,19 with location based on macroscopic findings observed via ileo-colonoscopy, upper endoscopy, and magnetic resonance imaging of the small bowel.19 Patients classified as IBD-U are combined with UC for analyses, unless CD is the diagnosis assigned at the latest visit. Disease activity at baseline is assessed using the weighted Paediatric Crohn’s Disease Activity Index [wPCDAI]20 for CD patients or Paediatric Ulcerative Colitis Activity Index [PUCAI]21 for UC/IBD-U patients. Physician global assessment [PGA] of disease activity at diagnosis is recorded as quiescent, mild, moderate, or severe. Endoscopic findings are recorded at each local site using the Mayo endoscopic score22 for UC/IBD-U and the simplified endoscopic severity score for CD [SES-CD].23 Heights and other anthropometric measures are converted to age- and gender-adjusted standard deviation scores [z scores] using Centres for Disease Control reference data. We determined time from symptom onset to diagnosis, [date of diagnostic colonoscopy]. We calculated diagnostic delay among the two ethnic groups, defined as time from symptom onset to diagnosis >75th percentile.
2.2. Study design
For this comparative study, SA and non-Jewish Caucasian patients with diagnosed IBD, <17 years old between April 1, 2014 and January 1, 2019, at seven of the 12 CIDsCANN sites, were included. CIDsCANN sites with no SA IBD patients were excluded. Baseline phenotypic characteristics, including IBD type, location, clinical and endoscopic severity, biomarkers, and longitudinal outcomes, including disease activity, medication use, IBD-related hospitalisations, and surgery during 18 months of follow-up, were compared between SA and non-Jewish Caucasian children.
2.3. Ethnicity assignments
Patients were assigned as South Asian if at least three out of four grandparents were identified by the patient or parents/guardians as being of Indian, Bangladeshi, Pakistani, Sri Lankan, or Nepalese ethnic or cultural origin. Caucasian patients were similarly identified, if three or more grandparents were Caucasian. To avoid a potential bias in our comparative observations, Jewish patients (who have an increased [2–4 times] risk of developing IBD) were excluded (Jewish [n = 56], 73% Ashkenazi Jews).24
2.4. Longitudinal outcome assessment
The primary outcome was sustained corticosteroid-free clinical remission between 12 and 18 months, defined as PGA of quiescent disease and wPCDAI ≤12.5 or PUCAI ≤10 and no corticosteroid usage during the defined period. Secondary outcomes included corticosteroid-free clinical remission [wPCDAI ≤12.5 or PUCAI ≤10 and no corticosteroids ≥4weeks] and biochemical remission (C-reactive protein [CRP] ≤5) at 18 months. We compared time to anti-tumour necrosis factor [TNF] therapy initiation and other medication use stratified by IBD phenotype. Non-induction corticosteroid use was defined as corticosteroid prescription for increased disease activity after the initial induction course. Other outcomes included IBD-related hospitalisations and luminal surgery [colectomy or intestinal resection] within 18 months of diagnosis.
Primary and secondary longitudinal outcomes were assessed within subgroups with similar phenotypes at baseline. Caucasian patients were matched with SA patients at a ratio of 2:1, using propensity score [PS] matching with PS caliper width of 0.25. The covariates included were selected a priori and included: age at diagnosis, sex, family history of first-degree relatives with IBD, IBD type [UC/IBD-U vs CD], baseline PGA, disease location/extent, and CIDsCANN site [to account for possible variation in care and treatment selection between sites]. For patients with CD, we further adjusted for choice of first therapy (corticosteroids vs exclusive enteral nutrition [EEN]), because choice of therapy is dictated by the patient and/or physician preference. Covariates included in the PS model had a standardised mean difference <0.1, indicating a negligible difference in mean or prevalence between the two groups [Supplementary Table 1C, available as Supplementary data at ECCO-JCC online].
2.5. Statistical analysis
We described normally distributed continuous variables as means and standard deviations [SD], and non-normally distributed continuous variables as medians and interquartile ranges [IQR]. Categorical variables were expressed as frequency and proportions. We compared continuous variables with either an independent sample t test, or a Mann‐Whitney U test, as appropriate. We compared categorical variables with the Pearson chi square test or Fisher’s exact test, as appropriate. Statistical significance was defined as two-tailed p-value of <0.05. We used mixed effects analyses to compare longitudinal, repeated measures of IBD activity [clinical activity scores, biomarkers, and anthropometrics] between PS-matched SA and Caucasian groups. All measurements available over the follow-up period were included in these analyses. We examined anti-TNF usage, post-induction corticosteroids, and IBD-related hospitalisations using univariable analyses [Kaplan‐Meier curves and log rank testing] and multivariable Cox proportional hazards regression or logistic regression. First, potential confounding variables were selected based on clinical relevance, and then we only included the variables in our final multivariable model based on at least a 10% change in the exposure [ethnicity variable] coefficient estimate. If there was evidence of collinearity [variance inflation factor >10], the variable that resulted in the greatest change was included in our final model [Supplementary Table 1A and B]. We also performed a sensitivity analysis restricted to colon-only IBD. Analyses were performed using SAS University Edition version 3.4 [SAS Institute, NC] and IBM®SPSS® Statistics Version 24 [IBM Corp., NY].
2.5.1.Ethical considerations
The study protocol was approved by the research ethics boards of each participating institution. Children and their parents or legal guardian provided informed assent and consent for enrolment.
3. Results
Of 1156 children presenting with new-onset IBD, 623 were non-Jewish Caucasian [98% born in Canada, 88% of parents born in Canada] and 114 were of South Asian ethnicity [78% born in Canada, 13% of parents born in Canada] [Supplementary Table 2, available as Supplementary data at ECCO-JCC online]. A total of 98 [86%] of South Asian parents were born in South Asia and 45 [39%] of SA patients were of Punjabi descent [Table 1]; 25 SA children had immigrated to Canada, the majority [76%] before the age of 5 years; the median age at immigration was 2.2 years [IQR 0.9‐5 years].
Table 1.
Birth country of parents of South Asian IBD patients.
| Maternal country of birth | Paternal country of birth | |
|---|---|---|
| Canada | 7 [6%] | 6 [5%] |
| UK | 3 [3%] | 1 [1%] |
| Indiaa | 63 [58%] | 63 [58%] |
| Pakistana | 18 [17%] | 17 [15%] |
| Bangladesha | 10 [9%] | 10 [9%] |
| Nepala | 1 [1%] | 1 [1%] |
| Sri Lankaa | 6 [5%] | 7 [7%] |
| UAE | - | 1 [1%] |
| Kenya | - | 3 [3%] |
| Uganda | 1 [1%] | - |
UAE< United Arab Emirates.
aSouth Asian countries missing data on country of birth for five parents.
In all, 451 children presented with new-onset CD (53 [12%] SA and 398 [88%] Caucasian), and 286 with UC or IBD-U (61 [21%] SA and 225 [79%] Caucasian). The ratio of UC/IBD-U to CD among SA [1.2:1] was significantly different from Caucasian children [1:1.8], where CD was the predominant IBD type [p <0.001]. Colon-only disease [composite of UC/IBD-U and colonic CD] predominated in SAs compared with Caucasians (76 [67%] vs 313 [50%], p = 0.001) [Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. Adjusted for age and sex, the odds of SAs presenting with colonic disease was 1.88 (95% confidence interval [CI]: 1.23–2.89) [Supplementary Table 3, available as Supplementary data at ECCO-JCC online].
Six [6%] of the SA cohort had a first-degree relative [parent and/or sibling] with IBD compared with 91 [19%] of Caucasians [p = 0.002]. The median time to diagnosis was 19.2 [IQR 9–41.6] weeks, and 21 [18%] South Asians and 161 [26%] Caucasians presented with diagnostic delay, p = 0.09 [Supplementary Table 4A, available as Supplementary data at ECCO-JCC online]. SA children with IBD were diagnosed at an earlier age (11.4 years [IQR 9.2–14.3] vs 13 years [IQR10.9‐15], p = 0.03] [Table 2]. Symptoms at presentation were similar between the two groups [Supplementary Table 4B].
Table 2.
Demographic and phenotypic features in children newly diagnosed with inflammatory bowel disease, comparing South Asian with Caucasian patients.
| Crohn’s disease | Ulcerative colitis/ IBD-U | |||||
|---|---|---|---|---|---|---|
| South Asian | Caucasian | p-value | South Asian | Caucasian | p-value | |
| Number with CD [% of total IBD] | 53 [46%] | 398 [64%] | <0.001 | |||
| Number UC/IBD-U [% of total IBD] | 61 [54%] | 225 [36%] | <0.001 | |||
| Age [y] | 11.4 [10.4–14.3] | 12.8 [10.9–14.8] | 0.05 | 11.6 [8.1–14.2] | 13.7 [10.6–15.3] | 0.02 |
| Sex [% male] | 36 [68%] | 238 [60%] | 0.30 | 33 [54%] | 117 [52%] | 0.77 |
| Disease location | ||||||
| L1 | 11 [22%] | 64 [17%] | 0.20 | |||
| L2 | 15 [31%] | 88 [23%] | ||||
| L3 | 23 [47%] | 232 [60%] | ||||
| Disease extent | ||||||
| E1 | 4 [7%] | 20 [9%] | 0.53 | |||
| E2 | 5 [8%] | 11 [5%] | ||||
| E3/E4 | 52 [85%] | 194 [86%] | ||||
| Any L4ab/L4b+ | 10 [23%] | 82 [26%] | 0.71 | |||
| Perianal fistulising disease | 14 [27%] | 64 [18%] | 0.06 | |||
| Isolated perianal fistulising disease | 3 [6.4%] | 2 [0.6%] | 0.01 | |||
| Inflammatory [B1] disease | 47 [89%] | 332 [83%] | 0.53 | |||
| Disease activity at presentation | ||||||
| wPCDAI | 55 [42–70] | 55 [32–78] | 0.93 | |||
| PUCAI | 50 [35–70] | 50 [35–70] | 0.95 | |||
| PGA mild | 9 [18%] | 89 [24%] | 0.32 | 20 [33%] | 51 [24%] | 0.37 |
| PGA moderate | 20 [40%] | 168 [45%] | 19 [32%] | 80 [39%] | ||
| PGA severe | 21 [42%] | 119 [32%] | 21 [35%] | 76 [37%] | ||
| Endoscopic score | ||||||
| Mild | 15 [30%] | 55 [17%] | 0.06 | 10 [18%] | 39 [19%] | 0.98 |
| Moderate | 17 [34%] | 119 [36%] | 26 [47%] | 95 [46%] | ||
| Severe | 18 [36%] | 158 [48%] | 19 [35%] | 74 [35%] | ||
| Anthropometrics at presentation | ||||||
| Height z-score | -0.15±1.22 | -0.16±1.13 | 0.96 | 0.26±1.16 | 0.09±1.12 | 0.29 |
| Weight z-score | -0.69±1.72 | -0.68±1.36 | 0.97 | -0.34±1.25 | -0.06±1.14 | 0.10 |
| BMI z-score | -1.12±2.22 | -0.89±1.76 | 0.41 | -0.89±1.80 | -0.21±1.25 | 0.01 |
| Laboratory parameters | ||||||
| CRP [mg/L] | 30 [11–83] | 25 [10–56] | 0.20 | 4.6 [0.8–11.2] | 6.6 [1.2–15] | 0.07 |
| ESR [mm/h] | 64 [39–88] | 36 [20–52] | <0.001 | 29 [17–47.5] | 23 [11–43] | 0.13 |
| Albumin [g/L] | 36 [30–39] | 34 [29–39] | 0.35 | 37 [31–42] | 38 [31–42] | 0.65 |
| Hb [g/L] | 107 [98–114] | 111 [99–121] | 0.06 | 106 [91–116] | 115 [94–127] | 0.006 |
| Platelets [109/L] | 441 [374–569] | 438 [353–550] | 0.89 | 413 [314–522] | 373 [300–479] | 0.15 |
Values are presented as n [%], mean ± standard deviation, or median [inter-quartile range], as appropriate. Magnetic resonance enterography not performed among children aged <6 years.
IBD-U, inflammatory bowel disease unclassified; CD, Crohn’s disease; UC, ulcerative colitis; y, years; BMI, body mass index; wPCDAI, weighted paediatric Crohn’s Disease Activity Index; PUCAI, Paediatric Ulcerative Colitis Activity Index; PGA, Physician’s Global Assessment; Endoscopic score in CD: mild [SES-CD 3–6]; moderate [SES-CD 7–15]; severe [SES-CD≥16]; UC/IBD-U Mayo endoscopic score: mild [Mayo 1]; moderate [Mayo 2]; severe [Mayo 3]; ESR; erythrocyte sedimentation rate; CRP; C-reactive protein; Hb; haemoglobin.
3.1. Phenotypic features of new-onset paediatric CD and UC/IBD-U
The phenotypic features in CD and UC/IBD-U are summarised by ethnicity in Table 2. A greater proportion of SAs with CD presented with colonic disease, but this did not reach statistical significance [L2]: (15 [31%] vs 88 [23%], p = 0.32). Perianal fistulising disease was more commonly observed among SA (14 [27%] vs 64 [18%], p = 0.06). In our exploratory analysis, perianal disease was more common in non-Punjabi SA compared with Punjabi SA children, (4 [20%] vs 11 [41%], p = 0.13) [Supplementary Table 11, available as Supplementary data at ECCO-JCC online]. Irrespective of ethnicity, children with UC/IBD-U presented predominantly with extensive [E3/E4] disease. Spectrum of disease severity at presentation was also similar, with the majority presenting with moderate-severe disease.
3.2. Longitudinal follow-up of South Asian children and adolescents with propensity-matched Caucasian children
Of the 114 SA children, 108 [50 CD; 58 UC/IBD-U] were propensity-matched with 204 Caucasian children [108 CD; 96 UC/IBD-U] [Supplementary Table 5A and B, available as Supplementary data at ECCO-JCC online]. All SA patients reported all four grandparents to be of SA ethnicity, and Caucasian ethnicity was present in all four grandparents in 201 [98%], and in three grandparents in the other three. All covariates had standardised errors of <0.25 after matching [Supplementary Figure 2, available as Supplementary data at ECCO-JCC online]. The primary outcome of sustained corticosteroid-free remission [12–18 months] was achieved in the majority of children with IBD (54 [66%] SA vs 87 [57%] Caucasians, p = 0.20). After adjusting for IBD type and infliximab exposure by 6 months, there was no significant difference in the likelihood of achieving remission in SAs compared with Caucasians (odds ratio [OR] 1.59, 95% CI: 0.89–2.81) [Table 3]. Mixed effects models over the 18-month period, demonstrated no difference in clinical disease activity [UC: PUCAI; CD: wPCDAI] between the ethnic groups [Supplementary Table 6, available as Supplementary data at ECCO-JCC online].
Table 3.
Clinical and biochemical outcomes in children and adolescents with an intact colon up to 18 months follow-up in the propensity score-matched cohort.
| South Asian | Caucasian | p-value | Adjusted OR [95% CI]b | |
|---|---|---|---|---|
| CD outcome in entire PS cohortc | ||||
| Sustained CSF remission 12-18 mo | 27 [75%] | 40 [58%] | 0.08 | 2.40 [0.99–5.84] |
| CSF remission 18 mo | 25 [83%] | 51 [76%] | 0.43 | 1.48 [0.48–4.56] |
| Biochemical remission 18 mo | 21 [75%] | 45 [78%] | 0.79 | 0.96 [0.33–2.82] |
| UC outcome in entire PS cohorta,c | ||||
| Sustained CSF remission 12‐18 mo | 27 [59%] | 39 [57%] | 0.82 | 1.14 [0.53–2.45] |
| CSF remission 18 mo | 34 [76%] | 50 [74%] | 0.81 | 1.12 [0.47–2.67] |
| Biochemical remission 18 mo | 31 [82%] | 41 [72%] | 0.28 | 1.79 [0.65–4.92] |
| UC outcome in patients on 5-ASA monotherapy only at 18 moa | ||||
| Sustained CSF remission 12‐18 mo | 10 [67%] | 16 [53%] | 0.39 | 1.75 [0.48–6.36] |
| CSF remission 18 mo | 12 [80%] | 23 [79%] | 0.96 | 1.04 [0.22–4.93] |
| Biochemical remission 18 mo | 10 [77%] | 15 [68%] | 0.58 | 1.56 [0.32–7.49] |
Corticosteroid free [CSF] clinical remission; wPCDAI≤12 or PUCAI <10 and no corticosteroids for ≥4 weeks. Biochemical remission; CSF clinical remission and CRP <5. Sustained CSF remission: physician global assessment score reported as quiescent and no use of corticosteroids during the interval period.
CD, Crohn’s disease; UC, ulcerative colitis; mo, months; wPCDAI, weighted paediatric Crohn’s Disease Activity Index; PUCAI, Paediatric Ulcerative Colitis Activity Index; OR, odds ratio; CI, confidence interval; PS, propensity score; CSF, corticosteroid-free; 5-ASA, 5-aminosalicylates; CRP, C-reactive protein.
aAnalysis was performed in those with an intact colon.
bOdds ratio comparing South Asian with Caucasian.
cOdds ratio comparing South Asian with Caucasian adjusted for infliximab exposure at 6 months.
The majority of children with IBD, irrespective of ethnicity, were not exposed to systemic steroids post-induction, resulting in 18-month steroid-free survival: in CD patients, SA 85% [n = 40] vs Caucasian 92% [n = 93], p = 0.19; and in UC patients, SA 39 [70%] and 63 [72%], p = 0.72 Table 4. In a multivariable survival analysis, we found no difference in time from induction to first post-induction steroid course among SA children relative to Caucasians in CD and UC [Supplementary Table 7, available as Supplementary data at ECCO-JCC online]. SA CD patients had a greater likelihood to be hospitalised at diagnosis (SA 23[46%] vs Caucasian 26 [24%], p = 0.006), adjusted for wPCDAI and SES-CD at diagnosis (adjusted OR [aOR] 3.30, 95% CI: 1.36–8.03) Table 4. In contrast, we found no difference in the likelihood of hospitalisation at diagnosis among SA UC patients (SA 22 [38%] vs 37 [39%], p = 0.94) relative to Caucasians, aOR 1.09 [95% CI: 0.51–2.3] [Supplementary Table 8, available as Supplementary data at ECCO-JCC online]. Most children [80%] after initial diagnosis were not re-hospitalised. In a multivariable survival model, we found no difference in time to IBD-related hospitalisation post-diagnosis among SA children relative to Caucasian children with CD or UC (hazard ratio [HR] in CD 1.30 [95% CI: 0.57–2.98] and in UC HR 0.80 [95% CI: 0.4–1.60]). Few UC/IBD-U children [n = 6], irrespective of ethnicity, underwent colectomy; the median time to colectomy was 18 weeks [IQR 7.8–46.5].
Table 4.
Hospitalisation and steroid usage among children and adolescents with IBD in PS matched cohort.
| South Asian | Caucasian | p-value | |
|---|---|---|---|
| Hospitalisation at diagnosis | |||
| Hospitalisation at diagnosis | |||
| CD | 23 [46%] | 26 [24%] | 0.006 |
| UC/IBD-U | 22 [38%] | 37 [39%] | 0.94 |
| Steroid use post-induction in CD | |||
| 18 mo event free survival | 40 [85%] | 93 [92%] | 0.19 |
| Time to post induction CS usagea | 33 [17–38] | 34 [18–57] | 0.13 |
| Steroid use post-induction in UC/IBD-U | |||
| 18 mo event free survival | 39 [70%] | 63 [72%] | 0.72 |
| Time to post induction CS usagea | 24 [15–43] | 39 [18–50] | 0.49 |
Values are presented as n [%].
CD, Crohn’s disease; UC, ulcerative colitis; IBD-U, inflammatory bowel disease unclassified; PS, propensity score; mo, months; CS, corticosteroid.
aMedian [interquartile range] in weeks.
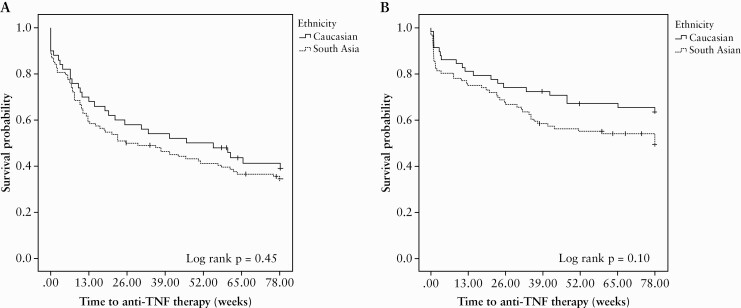
We demonstrated no difference in initial induction and first maintenance therapy between ethnic groups [Supplementary Table 9, available as Supplementary data at ECCO-JCC online]. By 18 months follow-up, two-thirds of all CD and 40–50% of UC/IBD-U children were exposed to anti-TNF therapy [Figure 1]. We found no difference in the risk of commencing anti-TNF therapy among SA and Caucasian CD patients, HR in SA 0.85 [95% CI: 0.53–1.30] or for UC patients, HR 0.65 [95% CI: 0.35–1.01].
Figure 1.
Kaplan‐Meier curve for time to [A] anti-TNF therapy in children and adolescents with Crohn’s disease; and [B] anti-TNF therapy in children and adolescents with ulcerative colitis/IBD-U in the propensity score-matched cohort. TNF, tumour necrosis factor; IBD-U, inflammatory bowel disease unclassified.
After controlling for haemoglobin and disease activity [PUCAI or wPCDAI], mixed effects modelling demonstrated an elevated erythrocytic sedimentation rate [ESR] in SA children relative to Caucasians, in both CD and UC patients. In SA UC patients only, haemoglobin levels were significantly lower at diagnosis and the difference persisted over follow-up time [Supplementary Table 6].
3.3. No differences in medication use and outcomes were observed among children with colon-only IBD
In the sensitivity analysis evaluating children with colon-only IBD [199 patients; SA:73, Caucasian:126], we found no differences between ethnic groups in medical therapy use, hospitalisation, or outcomes up to 18 months [Supplementary Table 10, available as Supplementary data at ECCO-JCC online].
4. Discussion
In this longitudinal, multi-centre, paediatric cohort study to evaluate the phenotypic spectrum and outcomes of IBD in SA children, we demonstrated greater predominance of colon-only IBD in SA children and a less frequent family history of IBD in first-degree relatives. SA children with CD presented at a younger age, and a higher proportion with perianal fistulising disease. Reassuringly, we did not demonstrate a difference in medical therapy use overall, and up to two-thirds of children were able to achieve corticosteroid-free remission at 18 months follow-up, irrespective of ethnicity.
In Canada, SAs predominately reside in metropolitan areas with the largest communities living in Toronto and Vancouver,8 mirroring our patient cohort. In the 2016 Canadian Census, SAs accounted for 12% and 6% of the population in Toronto and Vancouver, respectively.8 Approximately 9% of our IBD cohort were SA and 49% were non-Jewish Caucasians, a ratio proportional to the Census population statistics. Benchimol et al. found the incidence rate ratio [IRR] [1999–2008] of IBD among Ontario-born children with SA immigrant mothers to be comparable to non-immigrant children [IRR 0.83, 95% CI: 0.63–1.10].25 Interestingly, Pinsk et al. found the incidence of IBD [1996–2001] in a single-centre cohort study in British Columbia to be higher among the SA children, the majority born in Canada to parents from the Punjab with an incidence rate of 15.2/105 compared with 5.2/105 for non-SAs.15 These findings are consistent with recent adult studies from the UK: a nationwide cohort study reported IBD incidence rates in Indians of 25/105, Pakistani 14.9/105 and White Europeans 14.9/105.26 The variability in the comparative ethnic groups and the case definition of ‘South Asian’ among studies make it challenging to make a direct comparison.
In our study, the majority of Canadian-born SA children were second-generation immigrants, with parents immigrating from South Asia. The risk of IBD across South Asia varies, with higher risk observed in India, with a mean IBD incidence of 9.31 per 105 person-years, compared with Sri Lanka, with a reported incidence of 1.68 per 105 person-years.1,2 In our study, almost 60% of the South Asian children with IBD were of Indian descent, which may be reflective of immigration trends and the associated increased risk among Indians. Canadian population-based studies are required to further understand the evolving IBD epidemiological changes. Children born outside North America were largely under the age of 5 years at time of immigration. Benchimol et al. demonstrated that younger age at immigration to Canada was associated with an increased risk of IBD, with 14% increased risk per younger decade of life at immigration.25 Findings are consistent with a longitudinal birth cohort study of SAs born in the UK having an increased odds of developing IBD by age 26 [OR 6.10, 95% CI:2.14–17.3],12 and similarly SA IBD adult patients born in the USA or who migrated before the age of 5 years, presenting at a younger age [19 years].27 These findings are supportive of our study results, whereby SA children and adolescents with UC and CD presented at an earlier age. These findings further support the notion that the risk of IBD increases in certain migrant groups from countries with lower IBD prevalence once they arrive in higher prevalence countries such as Canada, the USA, and the UK.3–5,25 It has been speculated that early exposure to the Canadian environment modifies the risk in genetically susceptible individuals. Furthermore, the emergence of IBD in Asian and Asian-Pacific countries, aligned with globalisation and Westernisation, indicates an important role for environmental factors.1,2
Similarly, paediatric and adult studies from North America have demonstrated a lack of family history in first-degree relatives among SA.16,27 Contrary to our findings, these studies did not note a difference in the proportion with a family history among those with UC. The difference may be attributed to the differing study designs. The lack of family history may suggest the lack of interaction between risk alleles and environmental factors in India. Interestingly in our study, SA children irrespective of IBD type presented at an earlier age, and the difference remained apparent when restricted to those with colon-only IBD [UC/IBD-U and colonic CD]. We demonstrated no significant difference in diagnostic delay among the ethnic groups, which is encouraging. The lack of difference may be attributed to the Canadian health care system which is a publicly funded universal care system, whereby care is easily accessible.
The majority of studies to date support the predominance of UC/IBD-U among the SA IBD population.11,13,15,16 Indeed, adult population-based studies in North America and the UK have shown a greater incidence of UC in SAs compared with White Europeans, ranging from 2.9–17/105 versus 2.6‐7/105, respectively.4,25,26 Conversely, the reported CD incidence is higher in those of European ancestry [5.3–6.5/105] compared with SA ancestry [2.4–4.7/105].25,26,28 In paediatric IBD, CD is the predominant phenotype,18 accounting for two-thirds of IBD cases, and in our study only 46% of SA presented with CD compared with 64% of Caucasians. CD-associated polymorphisms in the NOD2/CARD15 gene are strongly associated with ileal CD,29 but the prevalence of these common risk alleles of European origin has not been widely observed in South Asians.30 The paucity of studies published to date warrants further phenotype-genotype association studies.
In our study we observed a numerical increase in the frequency of perianal fistulising among South Asian children with CD, and principally among the non-Punjabi SA children. A greater frequency has also been observed among South Asian adult patients,27 a phenotypic difference apparent also among both Asians and African Americans.10 These ethnic differences may be a result of differentially distributed single nucleotide polymorphisms. For example, in East Asians the TNFSF15 polymorphisms are common and associated with perianal fistulae and penetrating complications.31 The impact of the environment on the observed phenotypic ethnic variation to date remains elusive. Notably, in our study, we found no difference in complicating disease behaviour; the majority of children presented with inflammatory disease, consistent with a paediatric phenotype.18
SA UC patients presented with lower haemoglobin, and the difference persisted over the 18-month follow-up period. The difference may partly be attributed to a greater prevalence of the thalassaemia trait among South Asians,32 butr dietary variation should also be considered. We also found the ESR, irrespective of disease phenotype and following adjustment, was elevated in SAs. Racial differences in ESR have been observed, with higher ESR levels in South Asian ischaemic stroke patients compared with Chinese patients.33 This is a novel finding among South Asian children with IBD and warrants further study.
In the PS-matched analysis, which included phenotypic and clinical characteristics in the model, longitudinal follow-up comparison up to 18 months showed no significant difference in therapy use, rates of colectomy, or post-diagnosis hospitalisation among the two ethnic groups. Previous observational studies, predominately historical, have reported mixed results, with reduced surgical rates,11,13 earlier use of biologic therapy,16 and increased rates of hospitalisation in South Asian patients.34 The differences observed may lie in the methodological approaches undertaken. The strength of our study was the creation of a balanced cohort, removing potential confounders by undertaking PS matching. There may also be biologically distinct differences in adult and paediatric IBD patients, as well as variations in health care practices across the world. It is also becoming increasingly apparent that inequities lead to observed differences in health care use and outcomes, the predominant drivers being race, ethnicity, and socioeconomic status.35
The strengths of the study are its prospective longitudinal design and comprehensive data collection at centres where young patients are thoroughly and systematically evaluated. Matching by PS for distinctive phenotypic IBD features reduced the likelihood of confounding due to differences in phenotypic presentation and in evaluating longitudinal outcomes, improving precision of the comparative analyses. Limitations of the study include unmeasured confounders including diet and environmental factors that may differ between provinces and patients. Our study was not a population-based study, though practice patterns in Canada are such that the majority of paediatric IBD care is centralised to academic centres participating in the CiDSCaNN network. We also acknowledge the short follow-up time of 18 months.
In conclusion, SA children and adolescents with IBD in Canada present with a predominance of colon-only IBD. The earlier age of IBD at presentation and lower frequency of a family history among SA children suggest the importance of early life environmental exposures in Canada. SA children have a similar disease course, timing and use of IBD therapies, and outcomes compared with Caucasian children.
The data underlying this article were accessed from the Canadian Children Inflammatory Bowel Disease Network [CIDsCaNN]. The derived data generated in this research will be shared on reasonable request to the corresponding author.
Supplementary Material
Funding
This work was supported by grant 297862 from the Canadian Institutes of Health Research [CIHR] in partnership with the Children’s Intestinal and Liver Disease [Ch.I.L.D.] Foundation.
Conflict of Interest
MC; advisory board: Abbvie, Janssen. Jde B: advisory board: Abbvie, Janssen, Merck; speaker: Abbvie. SL: advisory board: Janssen; speaker Abbvie. MS: advisory board: Abbvie, Merck. WE-M: advisory board: Abbvie, Janssen, Merck; investigator-initiated research support: Janssen; speaker Abbvie. HB: advisory board: Abbvie, Janssen; speaker’s bureau: Nutricia; consultant: Nutricia; industry-sponsored drug trials: Alexion, Abbvie, Shire. PC: consultant: Abbvie, Ferring, Janssen, Merck; speaker: Abbvie; research support: Abbvie. EW: advisory board: Abbvie, Janssen, Nestle; speaker: Abbvie, Nestle. HH: advisory board: Abbvie, Janssen, Merck; research support: Janssen. TW: consultant: Abbvie, Ferring, Janssen, Merck; speaker: Abbvie, Ferring, Janssen, Merck, Nestle. AG:consultant: Abbvie, Merck, Janssen, Eli Lilly, Pfizer, Gilead, Roche, Takeda; speaker: Abbvie, Janssen, Shire; investigator-initiated research support: Abbvie. KJ: advisory board: Abbvie, Janssen, Merck; speaker’s bureau: Abbvie Janssen; investigator-initiated research support: Janssen.
Author Contributions
All authors contributed to acquisition of data and critical revision of the manuscript for important intellectual content, and approved the final manuscript as submitted. KJ, AMG, MC, and TDW contributed to study concept and design, interpretation of data, and drafting of the manuscript; JD contributed to study concept and design, draft of the manuscript and interpretation, and primarily performed the statistical analysis. AR also contributed to statistical analysis and interpretation of data. AMG and KJ provided study supervision.
References
- 1. Ng SC, Shi HY, Hamidi N, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 2. Ng SC, Kaplan GG, Tang W, et al. . Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am J Gastroenterol 2019;114:107–15. [DOI] [PubMed] [Google Scholar]
- 3. Probert CS, Jayanthi V, Hughes AO, Thompson JR, Wicks AC, Mayberry JF. Prevalence and family risk of ulcerative colitis and Crohn’s disease: an epidemiological study among Europeans and south Asians in Leicestershire. Gut 1993;34:1547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carr I, Mayberry JF. The effects of migration on ulcerative colitis: a three-year prospective study among Europeans and first- and second- generation South Asians in Leicester [1991-1994]. Am J Gastroenterol 1999;94:2918–22. [DOI] [PubMed] [Google Scholar]
- 5. Malhotra R, Turner K, Sonnenberg A, Genta RM. High prevalence of inflammatory bowel disease in United States residents of Indian ancestry. Clin Gastroenterol Hepatol 2015;13:683–9. [DOI] [PubMed] [Google Scholar]
- 6. Coward S, Clement F, Benchimol EI, et al. . Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 2019;156:1345–53.e4. [DOI] [PubMed] [Google Scholar]
- 7. Benchimol EI, Mack DR, Nguyen GC, et al. . Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology 2014;147:803–13.e7; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 8. Statistics Canada. Census Profile. 2016 Census.Ottawa: Statistics Canada; 2017
- 9. Benchimol EI, Manuel DG, To T, et al. . Asthma, type 1 and type 2 diabetes mellitus, and inflammatory bowel disease amongst South Asian immigrants to Canada and their children: a population-based cohort study. PLoS One 2015;10:e0123599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi HY, Levy AN, Trivedi HD, Chan FKL, Ng SC, Ananthakrishnan AN. Ethnicity influences phenotype and outcomes in inflammatory bowel disease: a systematic review and meta-analysis of population-based studies. Clin Gastroenterol Hepatol 2018;16:190–7.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walker DG, Williams HR, Kane SP, et al. . Differences in inflammatory bowel disease phenotype between South Asians and Northern Europeans living in North West London, UK. Am J Gastroenterol 2011;106:1281–9. [DOI] [PubMed] [Google Scholar]
- 12. Montgomery SM, Morris DL, Pounder RE, Wakefield AJ. Asian ethnic origin and the risk of inflammatory bowel disease. Eur J Gastroenterol Hepatol 1999;11:543–6. [DOI] [PubMed] [Google Scholar]
- 13. Bodiwala V, Marshall T, Das KM, Brant SR, Seril DN. Comparison of disease phenotypes and clinical characteristics among South Asian and White patients with inflammatory bowel disease at a tertiary referral center. Inflamm Bowel Dis 2020;26:1869–77. [DOI] [PubMed] [Google Scholar]
- 14. Misra R, Faiz O, Burisch JM, et al. . The epidemiology of IBD differs in South Asian migrants compared with caucasians; results from a systematic review and meta-analysis. Gastroenterology 2017;152:S979–80. [Google Scholar]
- 15. Pinsk V, Lemberg DA, Grewal K, Barker CC, Schreiber RA, Jacobson K. Inflammatory bowel disease in the South Asian pediatric population of British Columbia. Am J Gastroenterol 2007;102:1077–83. [DOI] [PubMed] [Google Scholar]
- 16. Carroll MW, Hamilton Z, Gill H, et al. . Pediatric inflammatory bowel disease among South Asians living in British Columbia, Canada: a distinct clinical phenotype. Inflamm Bowel Dis 2016;22:387–96. [DOI] [PubMed] [Google Scholar]
- 17. Li BH, Guan X, Vittinghoff E, Gupta N. Comparison of the presentation and course of pediatric inflammatory bowel disease in South Asians with Whites: a single center study in the United States. J Pediatr 2013;163:1211–3. [DOI] [PubMed] [Google Scholar]
- 18. Dhaliwal J, Walters TD, Mack DR, et al. . Phenotypic variation in paediatric IBD by age: a multi-centre prospective inception cohort study of the Canadian Children IBD Network. J Crohns Colitis 2019;14:445‐54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levine A, Griffiths A, Markowitz J, et al. . Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 20. Hyams JS, Ferry GD, Mandel FS, et al. . Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr 1991;12:439–47. [PubMed] [Google Scholar]
- 21. Turner D, Otley AR, Mack D, et al. . Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423–32. [DOI] [PubMed] [Google Scholar]
- 22. Mohammed Vashist N, Samaan M, Mosli MH, et al. . Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev 2018;1:CD011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khanna R, Nelson SA, Feagan BG, et al. . Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev 2016. PMID: 27501379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang H, McElree C, Roth MP, Shanahan F, Targan SR, Rotter JI. Familial empirical risks for inflammatory bowel disease: differences between Jews and non-Jews. Gut 1993;34:517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benchimol EI, Mack DR, Guttmann A, et al. . Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol 2015;110:553–63. [DOI] [PubMed] [Google Scholar]
- 26. Misra R, Limdi J, Cooney R, et al. . Ethnic differences in inflammatory bowel disease: results from the United Kingdom inception cohort epidemiology study. World J Gastroenterol 2019;25:6145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jangi S, Ruan A, Korzenik J, de Silva P. South Asian patients with inflammatory bowel disease in the United States demonstrate more fistulizing and perianal crohn phenotype. Inflamm Bowel Dis 2020;26:1933–42. [DOI] [PubMed] [Google Scholar]
- 28. Jayanthi V, Probert CS, Pinder D, Wicks AC, Mayberry JF. Epidemiology of Crohn’s disease in Indian migrants and the indigenous population in Leicestershire. Q J Med 1992;82:125–38. [PubMed] [Google Scholar]
- 29. Cleynen I, Boucher G, Jostins L, et al. ; International Inflammatory Bowel Disease Genetics Consortium. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet 2016;387:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ng SC, Tsoi KK, Kamm MA, et al. . Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis 2012;18:1164–76. [DOI] [PubMed] [Google Scholar]
- 31. Yang DH, Yang SK, Song K, et al. . TNFSF15 is an independent predictor for the development of Crohn’s disease-related complications in Koreans. J Crohns Colitis 2014;8:1315–26. [DOI] [PubMed] [Google Scholar]
- 32. Colah R, Gorakshakar A, Nadkarni A. Global burden, distribution and prevention of β-thalassemias and hemoglobin E disorders. Expert Rev Hematol 2010;3:103–17. [DOI] [PubMed] [Google Scholar]
- 33. De Silva DA, Woon FP, Chen C, Chang HM, Wong MC. Serum erythrocyte sedimentation rate is higher among ethnic South Asian compared with ethnic Chinese ischemic stroke patients. Is this attributable to metabolic syndrome or central obesity? J Neurol Sci 2009;276:126–9. [DOI] [PubMed] [Google Scholar]
- 34. Bhopal RS, Cezard G, Bansal N, Ward HJT, Bhala N; SHELS researchers . Ethnic variations in five lower gastrointestinal diseases: Scottish health and ethnicity linkage study. BMJ Open 2014;4:e006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sewell JL, Velayos FS. Systematic review: the role of race and socioeconomic factors on IBD healthcare delivery and effectiveness. Inflamm Bowel Dis 2013;19:627–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.