Key Points
Pooled safety data from zanubrutinib monotherapy studies in B-cell malignancies are consistent with the toxicity profile of BTK inhibitors.
Zanubrutinib exhibits a lower incidence of atrial fibrillation and hypertension than previously reported with ibrutinib.
Visual Abstract
Abstract
Zanubrutinib is a selective Bruton tyrosine kinase (BTK) inhibitor evaluated in multiple B-cell malignancy studies. We constructed a pooled safety analysis to better understand zanubrutinib-associated treatment-emergent adverse events (TEAEs) and identify treatment-limiting toxicities. Data were pooled from 6 studies (N = 779). Assessments included type, incidence, severity, and outcome of TEAEs. Median age was 65 years; 20% were ≥75 years old. Most patients had Waldenström macroglobulinemia (33%), chronic lymphocytic leukemia/small lymphocytic lymphoma (29%), or mantle-cell lymphoma (19%). Median treatment duration was 26 months (range, 0.1-65); 16% of patients were treated for ≥3 years. Common nonhematologic TEAEs were upper respiratory tract infection (URI, 39%), rash (27%), bruising (25%), musculoskeletal pain (24%), diarrhea (23%), cough (21%), pneumonia (21%), urinary tract infection (UTI), and fatigue (15% each). Most common grade ≥3 TEAEs were pneumonia (11%), hypertension (5%), URI, UTI, sepsis, diarrhea, and musculoskeletal pain (2% each). Atrial fibrillation and major hemorrhage occurred in 3% and 4% of patients, respectively. Atrial fibrillation, hypertension, and diarrhea occurred at lower rates than those reported historically for ibrutinib. Grade ≥3 adverse events included neutropenia (23%), thrombocytopenia (8%), and anemia (8%). Serious TEAEs included pneumonia (11%), sepsis (2%), and pyrexia (2%).Treatment discontinuations and dose reductions for adverse events occurred in 10% and 8% of patients, respectively. Thirty-nine patients (4%) had fatal TEAEs, including pneumonia (n = 9), sepsis (n = 4), unspecified cause (n = 4), and multiple organ dysfunction syndrome (n = 5). This analysis demonstrates that zanubrutinib is generally well tolerated with a safety profile consistent with known BTK inhibitor toxicities; these were manageable and mostly reversible.
Introduction
B-cell receptor signaling is essential for normal B-cell development but is also implicated in survival and proliferation of malignant B cells.1-3 Bruton tyrosine kinase (BTK), an intermediary in the B-cell receptor signaling pathway, has been validated as a therapeutic target based on clinical data generated from BTK inhibitor-treated patients with various B-cell malignancies.4-11 Ibrutinib, the first-in-class BTK inhibitor, is health authority-approved in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), Waldenström macroglobulinemia (WM), relapsed/refractory (R/R) mantle-cell lymphoma (MCL), and marginal zone lymphoma. Despite clinical benefits, ibrutinib-associated toxicities, such as diarrhea, hypertension, hemorrhage, musculoskeletal pain, and atrial fibrillation, can be treatment limiting.12 These may be due to off-target inhibition of kinases structurally related to BTK, including epidermal growth factor receptor, tyrosine kinase, expressed in hepatocellular carcinoma (TEC), Src family kinases, and others. Acalabrutinib, a second-generation, selective BTK inhibitor, is approved for the treatment of R/R MCL and CLL/SLL.
Zanubrutinib (BRUKINSA) is a selective BTK inhibitor that exhibits less inhibition of off-target kinases than ibrutinib.13 In phase 1 studies conducted in China (BGB-3111-1002) and other countries (BGB-3111-AU-003), zanubrutinib demonstrated tolerability without dose-limiting toxicities at daily doses up to 320 mg. The recommended phase 2 dose was 320 mg administered either once daily or 160 mg twice daily, based on pharmacokinetic (PK), pharmacodynamic (PD), safety, and efficacy results in patients with B-cell malignancies.13 The efficacy and safety of zanubrutinib were further investigated in additional studies at 160 mg twice daily. In 2019, zanubrutinib was health authority approved for the treatment of R/R MCL.
Here, we report a pooled safety analysis compiled from zanubrutinib studies to generate a comprehensive toxicity profile from a large population of patients with a variety of B-cell malignancies.
Methods
Studies and patients
Safety data were pooled from 6 multicenter studies that collectively enrolled 779 patients receiving zanubrutinib at doses from 40 to 320 mg/d (Table 1). These included a phase 1/2 dose escalation and cohort expansion study investigating the PK, PD, safety, and preliminary antitumor activity in patients with various B-cell malignancies (BGB-3111-AU-003)5,13; a phase 1 dose-comparison study of PK, PD, and safety in patients with R/R B-cell malignancies (BGB-3111-1002); 3 phase 2 efficacy and safety studies in patients with R/R CLL/SLL (BGB-3111-205),14 MCL (BGB-3111-206),15 and WM (BGB-3111-210); and 1 phase 3, randomized comparative study of ibrutinib and zanubrutinib in patients with MYD88MUT WM,11 which included a single-arm substudy of zanubrutinib in patients with WM and MYD88WT disease (BGB-3111-302).16 Studies BGB-1002, BGB-205, BGB-206, and BGB-210 were conducted exclusively in China. The AU-003 study was conducted in Australia, New Zealand, South Korea, United States, United Kingdom, and Italy; BGB-3111-302 was conducted at 58 trial sites in the United States, Australia, and 10 European countries. AU-003 allowed enrollment of patients with R/R or treatment-naïve (TN) CLL/SLL or MCL; study 302 enrolled patients with R/R disease or TN unfit for standard frontline therapies. All others enrolled patients with R/R disease exclusively. In all studies except 302 (which included an ibrutinib comparator arm), concurrent administration of warfarin was not exclusionary.
Table 1.
Individual clinical trial details for integrated safety analysis*
| Clinical trial | Study no. | Phase | Zanubrutinib dose (n) | B-cell malignancies | Patients, no. | Enrollment dates |
|---|---|---|---|---|---|---|
| NCT0234312 | AU-003 (first in humans) | 1/2 | 160 mg BID (n = 278) 40 mg QD (n = 3)† 80 mg QD (n = 4)† 160 mg QD (n = 5)† 320 mg QD (n = 95) | CLL/SLL, DLBCL, FL, HCL, MCL, MZL, RT, or WM | 385 | August 2014-June 2019 |
| NCT03189524 | 1002 | 1 | 160 mg BID (n = 34) 320 mg QD (n = 10) | CLL/SLL, MCL, WM/LPL, FL, MZL, HCL, or non-GCB DLBCL | 44 | July 2016-November 2017 |
| NCT03206918 | 205 | 2 | 160 mg BID (n = 91) | CLL/SLL | 91 | March-December 2017 |
| NCT03206970 | 206 | 2 | 160 mg BID (n = 86) | MCL | 86 | March-September 2017 |
| NCT03332173 | 210 | 2 | 160 mg BID (n = 44) | WM | 44 | August 2017-May 2018 |
| NCT03053440 | 302‡ | 3 | 160 mg BID (n = 129) | WM | 129 | January 2017-July 2018 |
| Total | 779 |
BID, twice daily; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HCL, hairy cell leukemia; LPL, lymphoplasmacytic lymphoma; MCL, mast-cell lymphoma; MZL, marginal zone lymphoma; non-GCB, non-germinal center B-cell type; QD, every day; RT, Richter transformation.
Data cutoff date: 31 March 2020 for all studies.
Patients assigned to daily doses of 40 to 160 mg were enrolled to the dose escalation component (part 1) of AU-003.
Studies were approved by the independent ethics committee or institutional review board at each participating institution and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent. Data analyses were performed by biostatisticians at BeiGene USA and BeiGene (Beijing) China.
Assessments
For all studies, safety was evaluated based on the type, frequency, causality, severity, and seriousness of treatment-emergent adverse events (TEAEs). Adverse events (AEs) leading to dose reduction, treatment interruption, discontinuation, or death are also summarized. Serious AEs (SAEs) included those requiring or prolonging hospitalization and/or were life-threatening or resulted in death. All verbatim AE descriptions were coded to Medical Dictionary for Regulatory Activities standardized terminology. AEs of interest (AEIs) based on the known toxicity profile for the class of BTK inhibitors were summarized based on prespecified criteria defining each AEI category (supplemental Table 1). Categories of AEIs include bleeding, major hemorrhage (any non-central nervous system event that was serious or grade ≥3 or central nervous system hemorrhage of any grade), atrial fibrillation or flutter, hypertension, second primary malignancies (including skin cancers), infections (including opportunistic infections [OIs]), neutropenia, anemia, thrombocytopenia, and tumor lysis syndrome. Treatment-emergent neutropenia, thrombocytopenia, and anemia are summarized as clinical AEs; grade ≥3 cytopenias are also summarized based on absolute neutrophil counts (ANC), platelet counts, and hemoglobin concentrations, respectively. Cytopenias were graded for severity using the International Workshop on CLL Severity Grading Scale in patients with CLL/SLL only.17 For all other patients, hematologic and nonhematologic toxicities were graded using National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.03.
Statistical analyses
Data were summarized using descriptive statistics. All-grade and grade ≥3 TEAEs, AEIs, and events that led to dose reduction, treatment discontinuation, and death are summarized as crude incidence rates. Exposure-adjusted incidence rates for AEIs (reported as events/100 person-months) were calculated as the quotient of the number of patients having the event of interest and time from first dose date to first occurrence of the event or exposure time in the absence of the event (censored at the data cutoff date for patients continuing treatment). Clinically relevant differences in demographics, baseline disease characteristics or incidence/severity of toxicities as a function of geography (China vs ex-China) are also reported.
Distributions for times to first occurrence of AEIs are summarized using the Kaplan-Meier methodology. Associations between specific AEIs and potential covariates (eg, hemorrhage reported in association with thrombocytopenia, antiplatelet, or anticoagulant medication use; hypertension incidence/worsening in patients with a history of hypertension) were assessed. Cox proportional hazard regressions were performed to test for significance of an association with the covariates of interest.
All patients receiving ≥1 dose of zanubrutinib were included. The cutoff date was 31 March 2020.
Results
Patient characteristics
Approximately one-third of patients were enrolled at trial sites within China; the remainder were enrolled outside of China, including 34% from Australia. Across all studies, median patient age was 65 years (range, 20-90); 52% were ≥65 years, and 20% were ≥75 years of age. Almost 70% were male (Table 2). Chinese patients were generally younger, with a median age of 60 years vs 68 years among those ex-China. Most patients (88%) had R/R disease; median number of prior regimens for previously treated patients was 2 (range, 1-12). Eighty-one percent of patients had WM (33%), CLL/SLL (29%), or MCL (19%). Twenty-one percent had a history of or ongoing cardiac disorder, including atrial fibrillation/flutter in 7% (primarily ex-China). Approximately 20% (n = 151) had serologic evidence of prior hepatitis B virus (HBV) infection/reactivation; two-thirds (n = 97) were Chinese. Eighty-five (56%) patients were prescribed HBV prophylaxis while receiving zanubrutinib. Twenty-six percent had a history of neoplasia, most commonly nonmelanoma skin cancers, with the majority in patients from Australia and New Zealand.
Table 2.
Baseline demographic and disease characteristics*
| N = 779 | |
|---|---|
| Age | |
| Years, median (range) | 65 (20-90) |
| <65, n (%) | 375 (48) |
| ≥65-75, n (%) | 252 (32) |
| ≥75, n (%) | 152 (20) |
| Sex | |
| Male, n (%) | 528 (68) |
| ECOG performance status, n (%) | |
| 0 | 364 (47) |
| 1 | 367 (47) |
| 2 | 48 (6) |
| Prior treatment status, n (%) | |
| Relapsed/refractory | 688 (88) |
| Prior lines of therapy, median (range) | 2 (1-12) |
| ≥3 prior lines, n (%) | 232 (30) |
| Prior hematopoietic stem cell transplant | 37 (5) |
| Treatment-naïve | 91 (12) |
| Race, n (%) | |
| Asian | 322 (41) |
| White | 417 (54) |
| Other | 20 (3) |
| Black or African American | 5 (0.6) |
| Native Hawaiian or Pacific Islander | 2 (0.3) |
| Multiple | 1 (0.1) |
| Missing/not reported/unknown | 12 (2) |
| Region, n (%) | |
| Asia | 305 (39)† |
| Australia/New Zealand | 301 (39) |
| European Union | 99 (13) |
| North America | 74 (10) |
| Diagnosis, n (%) | |
| Non-Hodgkin lymphoma | 276 (35) |
| Mantle-cell lymphoma | 145 (19) |
| Follicular lymphoma | 59 (8) |
| Marginal zone lymphoma | 25 (3) |
| Diffuse large B-cell lymphoma | 45 (6) |
| Other‡ | 2 (0.3) |
| CLL/SLL | 225 (29) |
| WM | 253 (33) |
| Other (hairy cell leukemia, n = 12; Richter transformation, n = 13) | 25 (3) |
| History of atrial fibrillation | 55 (7) |
| History of hypertension | 270 (35) |
| History of skin cancers§ | 83 (11) |
Percentages may not always add to 100 because of rounding.
Includes 265 (34%) patients enrolled at study sites within China.
Includes 1 patient with “B lineage lymphoma” and 1 patient with “indolent lymphoma.”
Includes basal cell carcinoma (n = 40), squamous cell carcinoma (n = 20), skin cancer, unspecified (n = 16), squamous cell carcinoma of the skin (n = 12), malignant melanoma (n = 10), and Bowen disease (n = 5). Some patients had multiple skin cancers.
Treatment and study status
In all, 662 (85%) patients received zanubrutinib 160 mg twice daily and 105 (13%) received 320 mg once daily (Table 1). Relative treatment intensities were high for both regimens. Median exposure durations were similar among Chinese (26.4 months; interquartile range, 21.8) and ex-China patients (25.6 months; interquartile range, 21.6); there was a slight downward trend in median exposures by age group (Table 3). Collectively, 426 (55%) patients were treated for ≥2 years, including 125 (16%) treated ≥3 years and 43 (6%) treated ≥4 years. At data cutoff, 43% of patients had discontinued zanubrutinib, most commonly for progressive disease (27%).
Table 3.
Zanubrutinib exposure and treatment status
| N = 779 | |
|---|---|
| Months, median (25th-75th percentile) | 25.8 (11-32) |
| <24, n (%) | 353 (45) |
| 24 to <36, n (%) | 301 (39) |
| 36 to >48, n (%) | 82 (11) |
| ≥48, n (%) | 43 (6) |
| Exposure by age group, y | |
| <65 | 27.6 (11-32) |
| 65 to <75 | 25.1 (11-31) |
| ≥75 | 23 (12-31) |
| Relative treatment intensity, median % (25th-75th percentile) * | |
| Overall | 99 (96-100) |
| 160 mg BID† | 99 (96-100) |
| 320 mg QD† | 99 (97-100) |
| Dose reductions, n (%) | |
| All patients with at least 1 dose reduction‡ | 66 (8) |
| Number of dose reductions | |
| 1 | 47 (6) |
| 2 | 13 (2) |
| ≥3 | 6 (1) |
| Treatment interruptions, n (%) | |
| All patients with at least 1 treatment interruption§ | 282 (38) |
| Number of treatment interruptions | |
| 1 | 173 (24) |
| 2 | 68 (9) |
| ≥3 | 41 (6) |
| Treatment discontinuations, n (%) | |
| Patients discontinued from treatment | 335 (43) |
| Progressive disease | 208 (27) |
| Adverse event | 80 (10) |
| Withdrawal by patient | 18 (2) |
| Investigator’s discretion | 16 (2) |
| Other | 11 (1) |
| Protocol deviation | 1 (0.1) |
| Patients remaining on treatment | 444 (57) |
Defined as the actual cumulative dose divided by the intended cumulative dose over the duration of the treatment period.
Based on the number of patients assigned to each dosing regimen at treatment onset.
Includes all patients with at least 1 dose reduction. Fifty-three patients had 1 or more dose reduction for AEs; for the remaining, dose reduction was based on investigator discretion or other reason.
Includes treatment interruptions from AEs only for all studies except BGB-3111-1002 (n = 735).
The median (25th-75th percentile) follow-up time on study was 28.6 months (22-34). A total of 223 (29%) patients discontinued study participation in almost equal proportions among Chinese (29%) and ex-China (28%) patients. Primary reasons for study discontinuation were death (18%) and voluntary withdrawal (7%).
Overview of AEs
Almost all patients (98%) reported at ≥1 TEAE. Nonhematologic toxicities reported in ≥10% of the study population were upper respiratory tract infection (URI, 39%), rash (27%), bruising (25%), musculoskeletal pain (24%), diarrhea (23%), cough and pneumonia (each 21%), urinary tract infection (UTI), fatigue (each 15%), hematuria, constipation (each 14%), headache, pyrexia (each 13%), hypertension (12%), and nausea (11%) (Figure 1). Respiratory infections (including pneumonias) and hematuria were more common among Chinese patients. Gastrointestinal TEAEs (diarrhea, nausea, vomiting, and constipation), fatigue, bruising or purpura, headache, dyspnea, and musculoskeletal pain were more common among ex-China patients. At least 1 grade ≥3 TEAE was reported in 66% of patients, including 37% with treatment-related events. Grade ≥3 nonhematologic TEAEs reported in ≥2% of patients were pneumonia (11%), hypertension (5%), URI, UTI, sepsis, diarrhea, and musculoskeletal pain (each 2%) (Figure 1). Grade ≥3 pneumonia occurred more commonly in Chinese (16%) vs ex-China (7%) patients. At least 1 SAE was reported in 356 (46%) patients, including 130 (17%) with treatment-related events. SAEs were primarily infections, including pneumonia (11%), cellulitis, sepsis, UTI, URI, pyrexia (each 2%), and febrile neutropenia (1%) (supplemental Table 2). A lower proportion of Chinese patients compared with those ex-China reported SAEs (39% and 49%, respectively), although there was no difference in incidence of grade ≥3 TEAEs (67% and 66%, respectively). Patients ≥75 years of age experienced higher frequencies of both grade ≥3 TEAEs (76%) and SAEs (61%) than patients 65 to <75 (64% and 48%, respectively) and <65 years old (63% and 38%, respectively). There were no clear trends for the frequencies of specific SAEs as a function of age group.
Figure 1.
All grade, nonhematologic, TEAEs reported in ≥10% and grade ≥3 events reported in ≥2% of the integrated safety population (n = 779). *Events include multiple preferred terms (PTs) within the Medical Dictionary for Regulatory Activities.
Eighty (10%) patients discontinued zanubrutinib for TEAEs (Table 3), including treatment-related events in 35 (5%); events leading to discontinuation were most commonly pneumonias (n = 13, 2%) and hemorrhage (n = 7, 1%) (supplemental Table 3). Forty-three (54%) and 57 (71%) patients discontinued within the first 6 and 12 months, respectively. Treatment discontinuation for TEAEs occurred in 11% and 10% of Chinese and ex-China patients, respectively and was twice as frequent in patients ≥75 and 65 to <75 (each 14%) than in patients <65 years old (7%). TEAEs resulting in treatment discontinuation occurred at similar rates between disease subtypes (10%, 13%, and 10% of patients with WM, MCL, and CLL/SLL, respectively). Sixty-six (8%) patients had at ≥1 dose reduction (Table 3). TEAEs leading to dose reduction were most commonly neutropenia (n = 10, 1%), diarrhea (n = 8, 1%), and pneumonia (n = 8, 1%) (supplemental Table 4). Dose reductions were more common among patients ≥75 years of age (11%) compared with those <65 (6%) and 65 to <75 (8%) years. Thirty-eight percent of patients had at ≥1 treatment interruption for TEAEs (Table 3). Of the total number of TEAE-related interruptions (n = 464), 208 (45%) lasted ≤7 days, 109 (23%) lasted 8 to 14 days, and 147 (32%) were ≥15 days. Pneumonia, HBV infection/reactivation, and neutropenia were most commonly associated with treatment interruptions lasting ≥15 days (supplemental Table 5).
AEIs
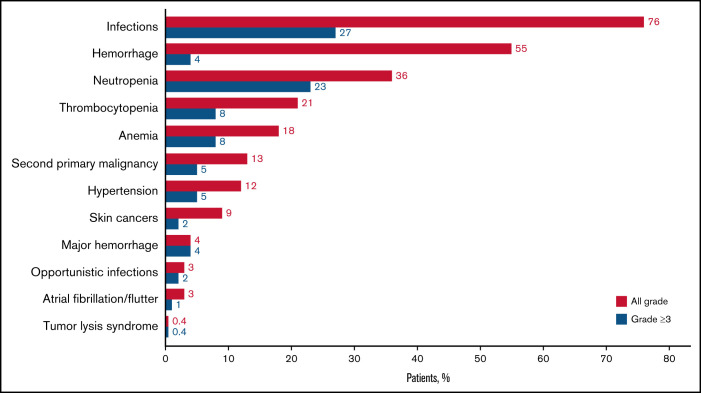
Ninety-two percent of patients reported at ≥1 AEI, most commonly intrinsic to the categories of infection, bleeding, or cytopenias.
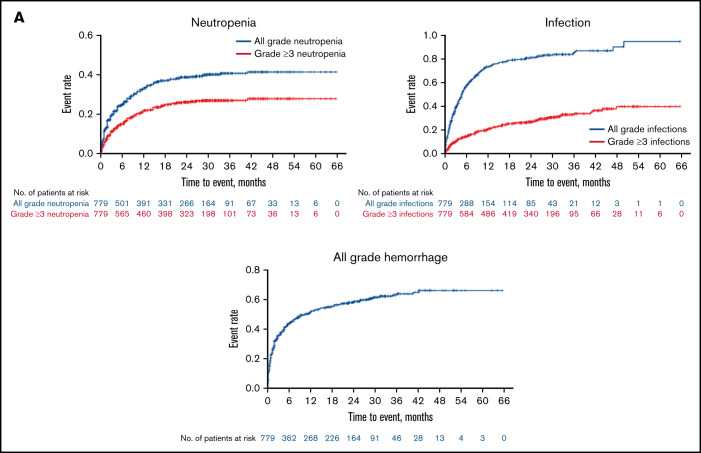
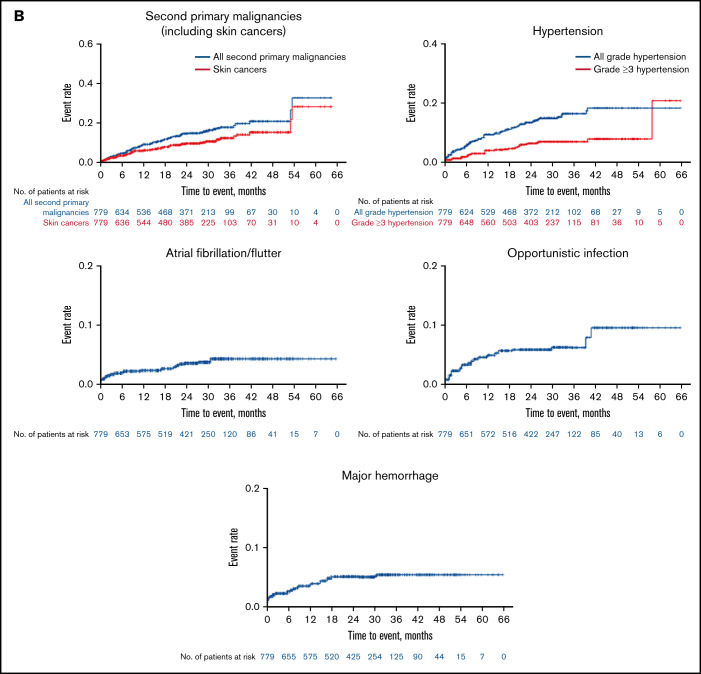
All-grade and grade ≥3 infections were reported in 76% and 27% of patients, respectively (Figure 2; Table 4). All-grade infections were reported in 75% and 76% of Chinese and ex-China patients, respectively. Grade ≥3 infections were more prevalent among Chinese patients (32% and 25%, respectively). The median time (25th-75th percentile) to first infection was 89 days (29-199); 57% of patients with infection reported a first occurrence within the initial 6 months of zanubrutinib exposure (Figure 3A). Mucosal infections of the sinopulmonary and urinary tracts (URI, pneumonia, nasopharyngitis, sinusitis, UTI), mostly grade 1 or 2, were most common. Infections were more commonly reported in patients with CLL/SLL (87%) and WM (80%) than in those with MCL (69%) but occurred with equal frequency among patients <65, ≥65-75, and ≥75 years of age (supplemental Table 6). One or more OIs were reported in 23 (3%) patients (8 WM, 2 MCL, 10 CLL/SLL, 2 hairy cell leukemia, and 1 follicular lymphoma). Grade ≥3 OIs were reported in 15 patients (1 fatality, Scedosporium infection, in a patient with WM). OIs were most commonly caused by Aspergillus (bronchopulmonary, cerebral aspergillosis), Cryptococcus (meningitis, pneumonia, fungemia), unspecified fungi (pneumonia, encephalitis), herpes zoster, and Mycobacterium (supplemental Table 7). Seventeen patients were reported as having received a systemic antimycotic agent before zanubrutinib initiation; however, no patient with a treatment-emergent fungal infection was receiving antifungal prophylaxis. Infections leading to death and treatment discontinuation were reported in 16 (2%) and 25 (3%) patients, respectively; pneumonia and sepsis were the most common of these (supplemental Table 8), whereas those leading to treatment discontinuation were most commonly pneumonia, sepsis, and HBV (supplemental Table 3).
Figure 2.
AEIs are reported by category and severity (all grade and grade ≥3). See supplemental Table 1 for a description of criteria that define each category of AEI. Neutropenia includes the PTs neutropenia (n = 104), neutrophil count decreased (n = 184), febrile neutropenia (n = 15), and neutropenic sepsis (n = 1); anemia includes the PTs anemia (n = 131) and hemoglobin decreased (n = 6); and thrombocytopenia includes the PTs thrombocytopenia (n = 65) and platelet count decreased (n = 107). Hypertension includes the PTs hypertension (n = 91) and blood pressure increased (n = 6). Hemorrhage is inclusive of major hemorrhagic events (also reported separately); second primary malignancies include skin cancers (reported separately); and infections include opportunistic infections (reported separately).
Table 4.
Exposure-adjusted incidence rates for AEIs by category*
| Category | All grades | All patients with eventn (%) | Grade ≥3 | All patients with eventn (%) |
|---|---|---|---|---|
| Infections | 9.6 | 590 (76) | 1.4 | 214 (27) |
| Opportunistic infections | 0.1 | 23 (3) | 0.1 | 15 (2) |
| Hemorrhage† | 4.8 | 428 (55) | 0.2 | 28 (4) |
| Major hemorrhage | 0.2 | 31 (4) | 0.2 | 28 (4) |
| Neutropenia‡ | 2.1 | 277 (36) | 1.2 | 183 (23) |
| Thrombocytopenia§ | 1.1 | 167 (21) | 0.3 | 61 (8) |
| Anemiaǁ | 0.8 | 137 (18) | 0.04 | 63 (8) |
| Second primary malignancies¶ | 0.6 | 102 (13)ǁ | 0.2 | 40 (5) |
| Skin cancers | 0.4 | 69 (9) | 0.1 | 13 (2) |
| Hypertension | 0.6 | 95 (12) | 0.2 | 41 (5) |
| Atrial fibrillation and flutter | 0.1 | 22 (3) | 0.03 | 6 (1) |
| Tumor lysis syndrome# | 0.02 | 3 (0.4) | 0.02 | 3 (0.4) |
Exposure-adjusted incidence rate is calculated as the first occurrence of each adverse event of interest per 100 person- months of zanubrutinib exposure.
Inclusive of major hemorrhage.
Includes clinical AEs reported under the preferred terms (PTs) neutropenia (n = 97), neutrophil count decreased (n = 178), febrile neutropenia (n = 14), and neutropenic sepsis (n = 1).
Includes clinical AEs reported under the PTs thrombocytopenia (n = 58) and platelet count decreased (n = 97).
Includes clinical AEs reported under the PTs anemia (n = 125) and hemoglobin decreased (n = 6).
Inclusive of skin cancers.
#Two cases of tumor lysis syndrome occurred >30 d after discontinuation of zanubrutinib for disease progression; both were assessed as grade ≥3 and serious. In 1 patient, the event occurred in association with venetoclax exposure, a known precipitant of tumor lysis syndrome.35 A third patient experienced an event with onset 9 d after discontinuation of zanubrutinib for progression of MCL on study day 150, which was unresponsive to medical management. The patient died 3 d after onset from complications of acute kidney injury.
Figure 3.
Kaplan-Meier curves illustrating the temporal relationship between selected AEIs and zanubrutinib exposure for (A) common AEIs (neutropenia, infection, and all-grade hemorrhage) and (B) less common or rare events (second primary malignancies [predominantly skin cancers], hypertension, atrial fibrillation/flutter, opportunistic infection, and major hemorrhage). Major hemorrhage is defined as any grade ≥3 or serious hemorrhage or any central nervous hemorrhage of any grade. The y-axis is the cumulative proportion of patients reporting a first event over the duration of study treatment (x-axis).
Twenty-one (3%) patients reported HBV events; 6 were seronegative for both anti-hemoglobin C (HBc) and anti-hemoglobin S (HBs) at baseline. HBV events were reported in 14 (5.3%) Chinese patients, 9 of whom had anti-HBc and/or anti-HBs at baseline compared with 7 (1.4%) ex-China patients, 5 of whom were anti-HBc or anti-HBs seropositive at baseline; 1 patient was receiving prophylactic lamivudine at event onset. Grade ≥3 events were reported in 14 (2%) patients; 5 events were serious, including 1 fatal event in a patient without baseline serologic evidence of prior HBV infection who died of complications of multiple organ dysfunction syndrome in the setting of HBV infection after approximately 300 days of zanubrutinib exposure. Three (0.4%) HBV events resulted in zanubrutinib discontinuation. Nineteen patients received antiviral therapies (entecavir, lamivudine, and/or tenofovir) for HBV events.
Bleeding or bruising events were reported in 428 (55%) patients (Figure 2; Table 4), with slightly higher incidence among ex-China (58%) vs Chinese patients (50%). Most were minor, involving the skin (petechiae, purpura, or contusion, 32%), urinary tract (hematuria, 14%; blood urine present, 3%), or other mucosal surfaces (eg, epistaxis, 8%). Bleeding events were reported more frequently in patients ≥75 years of age (65%; supplemental Table 6) and in patients with CLL/SLL (72%) compared with those with MCL (47%) or WM (54%). The median (25th-75th percentile) time to a first bleeding event of any grade was 52 (15-167) days (Figure 3A). Thirty-one (4%) patients reported major hemorrhages (Figure 2; Table 4) with similar proportions among Chinese (3%) and ex-China (5%) patients. Approximately one-half of major hemorrhages (14/31) occurred in the first 6 months of zanubrutinib exposure (Figure 3B). Major hemorrhages were slightly more frequent among patients with MCL (5%) and WM (6%) compared with those with CLL/SLL (2%) and were more common in patients ≥75 years (9%; supplemental Table 6). Major hemorrhages reported in >1 patient included gastrointestinal hemorrhage in 6 patients, including 3 at a site of MCL recurrence and in 1 patient with CLL coincident with newly diagnosed colon cancer; hematuria (n = 4); purpura (n = 3); hemothorax, periorbital hematoma after a fall, intracranial hemorrhage, and subdural hemorrhage (each in 2 patients). Treatment discontinuation from major hemorrhage was reported in 10 (1.0%) patients. The only bleeding-related fatality occurred in a 70-year-old male with R/R MCL, blastic histology, and extensive nodal and extranodal tumor burden but without other risk factors for bleeding, who died of complications of a left occipital lobe hemorrhage on study day 7.
In all, 222 (28%) patients received ≥1 antithrombotic medication while on study, primarily aspirin/other antiplatelet agents, heparin, and factor Xa inhibitor; only 4 received concurrent warfarin. There was a weak association between the concurrent use of antiplatelet agents and risk of grade ≥3 hemorrhage (hazard ratio [HR], 2.0; 95% confidence interval [CI], 0.9-4.8; P = .1; supplemental Table 9). There was a statistically significant relationship between the risk of grade ≥3 hemorrhage and anticoagulant medication use up to and including the day of hemorrhage (HR, 10.1; 95% CI, 4.6-21.9; P < .0001); most occurred in association with factor Xa inhibitors (supplemental Table 9). There was no association with concurrent thrombocytopenia (HR, 1.3; 95% CI, 0.5-3.6; P = .6).
A total of 102 (13%) patients reported a second primary malignancy (Figure 2; Table 4), consisting primarily of nonmelanoma skin cancers, including basal cell carcinomas (n = 37, 5%), squamous cell carcinomas (n = 24, 3%), Bowen disease (n = 7, 1%), and unspecified skin cancers (n = 7, 1%). Three (0.4%) and 10 (1.0%) patients reported lentigo maligna and malignant melanomas, respectively. No skin cancers were reported among Chinese patients; most (81%) were reported in patients from Australia and New Zealand, where 55 (18%) patients had a history of 1 or more skin cancers. Recurrent, metastatic squamous cell skin carcinoma led to treatment discontinuation in 1 patient. Other second malignancies reported in >1 patient include prostate cancer (n = 6), breast cancer or invasive ductal carcinoma (n = 4), gastric adenocarcinoma (n = 3), follicular lymphoma, colon cancer, malignant lung neoplasm, “external ear malignant neoplasm,” unspecified squamous cell carcinoma, and parotid gland squamous cell carcinoma (each n = 2). Four patients died of complications of second primary malignancies; 3 with WM died of complications of acute myeloid leukemia, gastric adenocarcinoma, and transformation to aggressive lymphoma; the fourth patient with CLL/SLL died of recurrent, metastatic cutaneous squamous cell carcinoma.
All-grade and grade ≥3 hypertension was reported in 95 (12%) and 41 (5%) patients, respectively (Figure 2; Table 4). Hypertension was equally frequent among Chinese and ex-China patients. The median (25th-75th percentile) time to onset of hypertension was 227 days (68-521) (Figure 3B). Fifteen percent (41/273) and 11% (54/506) of patients with and without a history of hypertension, respectively, developed treatment-emergent hypertension. The HR for treatment-emergent hypertension in patients with a history of hypertension was 1.5 (95% CI, 0.97-2.37; P = .07), suggesting that the risk of hypertension might be higher in patients with a history of hypertension. No patients required either dose reduction or treatment discontinuation for hypertension.
Atrial fibrillation or flutter was reported in 22 (3%) patients (Figure 2; Table 4), most of whom had ≥1 risk factor (supplemental Table 10); 6 events were grade ≥3. The median (25th-75th percentile) time to atrial fibrillation onset was 183 (36-622) days (Figure 3B). One occurrence of atrial fibrillation was reported in a Chinese patient compared with a 4% incidence among patients ex-China, where a history of atrial fibrillation was more prevalent (1% and 10%, respectively). As expected, atrial fibrillation was more prevalent in older patients (9% in patients ≥75, 3% in patients ≥65 to 75, and 0.3% among patients <65 years old; supplemental Table 6). Eight (1%) patients, including 4 Chinese patients, experienced ventricular tachyarrhythmias (5 ventricular extrasystoles; 3 ventricular arrhythmia); 3 additional patients (all ex-China) reported the occurrence of “extrasystoles.” One event (ventricular arrhythmia) was grade 3 and 1 (ventricular extrasystoles) was serious and grade 3. None led to treatment discontinuation or death; 1 led to treatment interruption. An 84-year-old patient with WM and a history of cardiomegaly, hypertension, and valvular and ischemic heart disease experienced sudden cardiac arrest after a plasmapheresis procedure 3 months after initiating zanubrutinib.
Eighteen percent of patients were neutropenic at baseline, and 277 (36%) reported ≥1 occurrence of treatment-emergent neutropenia (Figure 2; Table 4). One or more grade ≥3 events were reported in 23% of patients (including febrile neutropenia in 2%) and in 28% based on longitudinal assessments of ANC. Grade ≥3 ANC abnormalities were more prevalent among Chinese patients than ex-China patients (33% and 26%, respectively). The incidence of neutropenia was highest in patients with CLL/SLL (45.0%), comparable among patients with WM (32%) and MCL (34%) and higher in patients <65 years old (45%; supplemental Table 6). Fifty-five percent of patients with neutropenia reported a first occurrence within the initial 3 months of zanubrutinib exposure (Figure 3A) and 140 (51%) received myeloid growth factor support with granulocyte- or granulocyte macrophage-colony-stimulating factor within 30 days after onset. Factors associated with a heightened risk of treatment-emergent neutropenia were baseline neutropenia (odds ratio [OR], 2.420; P < .0001); any baseline peripheral blood cytopenia (OR, 1.752; P = .005); number of prior lines of therapy (OR, 1.480; P = .005 for 0, 1 vs ≥2 prior therapies); and a ≤24-month interval between last therapy and study enrollment (OR for ≤24 vs >24 months, 2.227; P < .0001). Twenty-three percent of patients were thrombocytopenic at baseline. Any -grade and grade ≥3 treatment-emergent thrombocytopenia were reported in 167 (21%) and 61 (8%) patients, respectively (Figure 2; Table 4). Grade ≥3 thrombocytopenia based on longitudinal assessments of platelet counts was reported in 12% of patients. The incidence of thrombocytopenia was comparable among patients with CLL/SLL (27%) and MCL (30%) but lower among patients with WM (15%). Fifty-six percent of patients with thrombocytopenia reported a first occurrence within the first 3 months of zanubrutinib, and 15 (9.0%) received platelet transfusions within 30 days after onset. Forty-seven percent of patients had baseline anemia; 137 (18%) and 63 (8%) reported ≥1 occurrence of any -grade and grade ≥3, treatment-emergent anemia, respectively (Figure 2; Table 4). Grade ≥3 anemia was observed in 9% of patients based on longitudinal assessments of hemoglobin concentrations. Fifty-nine percent of patients with anemia reported a first occurrence within the first 3 months of zanubrutinib and 50 (37%) received red blood cell transfusions within 30 days after onset. As expected, neutropenia, thrombocytopenia, and anemia were reported more frequently in R/R than TN patients (38% vs 14%; 23% vs 0%; and 18% vs 14%, respectively). Anemia, thrombocytopenia, and neutropenia led to treatment discontinuation in 2, 2, and 1 patient and dose reductions in 2, 2, and 10 patients, respectively.
Temporal relationships between selected AEIs and zanubrutinib exposure
Kaplan-Meier curves for the distributions of neutropenia, hemorrhage, and infection showed disproportionately high hazards during the first 6 to 18 months of therapy with a relative plateauing of risk thereafter (Figure 3A). In contrast, the distributions for second primary malignancies (predominantly skin cancers) and hypertension follow a more linear pattern whereby the hazard appears relatively constant up to approximately 30 to 36 months of exposure (Figure 3B). The hazard of OIs and major hemorrhage appears highest in the first 18 months of exposure, whereas that for atrial fibrillation/flutter shows no clear temporal relationship to zanubrutinib exposure, although the total number of events is small (Figure 3B).
Survival status
A total of 144 (18%) patients have died, most from complications of progressive disease (n = 79) and most (n = 93) >30 days after last zanubrutinib exposure. Thirty-nine (5%) patients had TEAEs that led to death, including 13 treatment related. These were most commonly from complications of pneumonia (n = 9), sepsis (n = 4), unspecified cause (n = 4), and multiple organ dysfunction syndrome (n = 5) (supplemental Table 8). Among patients with infection-related deaths, 2 were receiving prophylactic antibiotics. Grade 5 events were evenly distributed among patients within and ex-China (each 5%) and were higher in patients ≥75 (10%) than those 65 to <75 (5%) and <65 years old (3%). In at least 7 patients, grade 5 events occurred in the setting of progressive disease.
Discussion
This pooled analysis offers the most comprehensive evaluation of safety to date for zanubrutinib monotherapy. The dataset is noteworthy for its geographic and ethnic diversity, variety of B-cell malignancies, proportion of patients exposed to treatment ≥24 months, and substantial representation of elderly (≥75 years) patients.
The spectrum of TEAEs reported is generally consistent with that observed in previous studies of zanubrutinib and other BTK inhibitors.11,13-16,18 Infections (particularly of the respiratory tract) were the most common category of TEAEs and likely the greatest source of morbidity and mortality4-10,19; 41% of all-grade 5 TEAEs (16/39) and 31% (25/80) of treatment discontinuations were infection related. Contributions to the risk of infection from BTK inhibitors include neutropenia (an on-target effect of BTK inhibition) and depletion/dysfunction of normal B cells.20,21 Grade ≥3 infection incidence associated with zanubrutinib (27%) is similar to that reported in a pooled analysis from 1476 ibrutinib-exposed patients (21%)22 but higher than reported for acalabrutinib (18%).23 OIs similar to those reported here have also been reported in association with ibrutinib, acalabrutinib, and other kinase inhibitors (eg, idelalisib).9,20,24,25 Contributions to OI risk include prior immunochemotherapy exposure and immunodeficiency associated with advanced disease. The occurrence of HBV events with zanubrutinib and other BTK inhibitors highlights the importance of prophylactic suppression in at-risk patients.
BTK inhibitor-associated primary hemostatic defects are mediated through effects on both BTK and TEC platelets. Additionally, the activity C-type lectin-like receptor 2, which mediates thrombus stability after platelet adhesion, is impaired by BTK and TEC inhibition. Patients with X-linked agammaglobulinemia have mildly diminished collagen-mediated platelet activation without bleeding diathesis, suggesting that TEC activity can compensate for the absent BTK.26,27 The vast majority of BTK inhibitor-associated bleeding episodes involve skin or mucous membranes, are relatively minor, and not treatment limiting; a small proportion are potentially life-threatening and, on occasion, fatal. In the randomized BGB-3111-302 trial of patients with WM, the rate of major hemorrhage was lower for zanubrutinib than ibrutinib (0.3 vs 0.6 events/100 person-months, respectively).11 In the current analysis, ∼4% of patients reported major hemorrhages, comparable to that reported for both ibrutinib (4%) and acalabrutinib (4%).22,23 Although concurrent warfarin use was not prohibited in all except 1 trial, only 4 patients received concurrent warfarin while on zanubrutinib, 1 of whom experienced major hemorrhage (supplemental Table 9). Results from regression analyses suggest there may be an increased risk of major hemorrhage among zanubrutinib recipients receiving concurrent antiplatelet or anticoagulant therapy but must be interpreted cautiously, given the small number of major bleeding events.
One important observation supported by our analysis is confirmation of a lower risk of atrial fibrillation among zanubrutinib- vs ibrutinib-treated patients; when considering only patients enrolled to single-arm trials in our series, grade ≥3 atrial fibrillation/flutter was reported in 6 (1%) patients compared with a 4% incidence in a large pool of ibrutinib-treated patients.22 Because risk factors for the pooled ibrutinib analysis were not reported, whether they are similar to those in the present zanubrutinib population is unknown. The lower rate of ≥3 atrial fibrillation events for zanubrutinib relative to ibrutinib, however, is consistent with results from the randomized, phase 3, BGB-3111-302 study comparing the efficacy and safety of ibrutinib and zanubrutinib in patients with WM. In this study, all-grade and grade ≥3 atrial fibrillation occurred in 18% and 4% of ibrutinib-treated patients, respectively, compared with 4% and 0% zanubrutinib-treated patients,11 (similar to that in the general population). Risk factors of age, history of atrial fibrillation, and history of hypertension were comparable between arms.
A meta-analysis reported a pooled incidence rate (95% CI) for atrial fibrillation of 3.3 events (2.5-4.1) per 100 person-years among ibrutinib recipients (compared with 1.3 events/100 person-years in our series), which was substantially higher than that in clinical trial patients receiving non-ibrutinib therapy in 4 randomized trials (0.84 [0.32-1.6] per 100 person-years) as well as the general population of men and women aged 65 to 74 years (1.8 and 1.0 per 100 person-years, respectively).28,29 Less grade ≥3 atrial fibrillation/flutter was also recently reported in a pooled analysis of 1040 acalabrutinib-exposed patients (1.3%).23 A lower atrial fibrillation risk has implications not only for cardiovascular morbidity but also the risk of bleeding insofar as atrial fibrillation patients typically require stroke prophylaxis with antiplatelet agents or anticoagulants.26,30 No occurrences of atrial fibrillation were reported among Chinese patients, likely because of a lower incidence of cardiovascular risk factors and younger age compared with ex-China patients. Other cardiovascular toxicities reported in recipients of ibrutinib include rare but potentially serious ventricular tachyarrhythmias, some leading to sudden cardiac arrest and death.22,31 Rare occurrences of ventricular arrhythmias were also documented in our series. None led to zanubrutinib discontinuation or death, although ventricular tachyarrhythmia as a preterminal event cannot be excluded in the elderly patient with WM and preexisting heart disease who experienced sudden death. Finally, all grade and grade ≥3 hypertension were reported in 10% and 4%, respectively, in our series compared with 19% and 8% in a large pool of ibrutinib recipients.22
Cytopenias were relatively frequent, usually not serious, and typically managed with supportive care and/or treatment interruption. The 23% incidence of grade ≥3 neutropenia in our series is identical to the incidence of grade 3 or 4 neutropenia reported in ibrutinib-exposed patients.22 The incidence of grade ≥3 neutropenia was higher among Chinese patients than those ex-China. One possible explanation is that all Chinese patients had R/R disease, whereas ex-China patients were a mix of both R/R and treatment-naïve patients (∼12%). This may also have had a role in the higher incidence of grade ≥3 infections noted among Chinese patients. Treatment discontinuations and dose reductions for management of cytopenias were uncommon.
Diarrhea, although generally not life-threatening, has quality-of-life implications for patients receiving BTK inhibitors and may be treatment limiting. The 22% incidence of all-grade diarrhea reported here is lower than that reported in association with ibrutinib (39%)32 and acalabrutinib (37%).23 With 2 (0.3%) and 8 (1%) patients requiring treatment discontinuation and dose reduction, respectively, diarrhea was an infrequent cause of treatment limitation in our series.
Our analysis has several limitations, including the small number of Black patients (1% of the total population), and those with severe renal and hepatic impairment, who are often excluded from trial participation on safety grounds. Because our analysis includes only patients enrolled in clinical trials, the frequencies of toxicities reported herein may underestimate those encountered in a real-world setting.12,33 In addition, safety results need to be interpreted in the context of efficacy, which was not examined in this analysis. Notably, however, head-to-head studies with zanubrutinib vs ibrutinib reported higher response rates with zanubrutinib. In a study of zanubrutinib vs ibrutinib in patients with R/R CLL (BGB-3111-305), overall response rates by investigator assessment were significantly higher with zanubrutinib.11,34 Finally, the previously cited comparisons between zanubrutinib and other BTK inhibitors for specific toxicities, although derived from large data pools, are limited by variable treatment and follow-up duration for each and are not a substitute for randomized, controlled studies.
In summary, this comprehensive analysis indicates that zanubrutinib is generally well tolerated and exhibits a safety profile consistent with known toxicities for the BTK inhibitor class and those intrinsic to B-cell malignancies. These could be monitored with conventional safety assessments, were manageable, and mostly reversible. For toxicities such as all-grade diarrhea, atrial fibrillation/flutter, and hypertension, the incidence among zanubrutinib-treated patients was lower than for ibrutinib. These observations are consistent with findings from a head-to head trial comparing ibrutinib and zanubrutinib, BGB-3111-302. That study reported AEIs among zanubrutinib-treated patients similar to those reported here (Table 4), with lower rates of atrial fibrillation/flutter, major hemorrhage, and hypertension than in the ibrutinib arm.11 Thus, zanubrutinib may offer the potential for improved safety and tolerability in patients with B-cell malignancies relative to existing treatment options.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients and their families for participating in clinical studies cited herein. Writing and editorial support was provided by Gordon Bray, and funded by BeiGene, San Mateo, CA, USA.
This work was supported by funding from BeiGene USA, Inc.
Authorship
Contribution: All the investigators and their research teams collected data; the sponsor (A.C., W.N., J.H.) provided the conception and planning for this work, confirmed the accuracy of the data, and compiled the data for analysis; BeiGene biostatistician (H.Z.) was responsible for all data summaries and analyses; all the authors contributed to data interpretation, reviewed the manuscript, made the decision to submit for publication, and vouch for the accuracy and completeness of the data, analyses, and adherence to the trial protocols; and, together with BeiGene, authors (C.D., M.J., L.Z., H.G., H.Z., W.Y.C., A.C., W.N., J.H.) and external authors (C.S.T., J.T., S.O., A.W.R., P.M., and J.M.) further contributed to data interpretation and analysis.
Conflict-of-interest disclosure: C.S.T. reports honoraria from Janssen, AbbVie, and BeiGene and research funding from Janssen and AbbVie. M.D. reports honoraria from Amgen, Takeda, Bristol Myers Squibb, Celgene, Janssen, and BeiGene. R.G.-S. reports honoraria from Takeda, Roche, Janssen, and Novartis; consulting or advisor for Takeda, Janssen, and Gilead; travel accommodations or expenses from Takeda, Roche, Janssen, Novartis, and Gilead; and clinical trials relationships with many companies. J.T. reports research funding from BeiGene, Celgene, Roche, Takeda, Pharmacyclics, and Janssen. S.O. reports honoraria from Roche, Janssen, AbbVie, Celgene, Takeda, Merck, Gilead, Mundipharma, and AstraZeneca; consulting or advisor for Gilead, Mundipharma, AstraZeneca, and CSL Behring; research funding from BeiGene, Roche, Janssen, AbbVie, Takeda, Merck, Gilead, Epizyme, and AstraZeneca; and travel and accommodations from Roche. A.W.R. reports research funding from AbbVie and Janssen and patents, royalties, and other intellectual property from Walter and Eliza Hall Institute and Genentech. R.O. reports honoraria from BeiGene, Janssen, Celgene, and AstraZeneca, and consulting or advisor for BeiGene and Janssen. S.D. reports consulting or advisor for Sanofi; research funding from Janssen and BeiGene; speakers’ bureau for Janssen; and travel accommodations from Sanofi. W.J. reports consulting or advisor for BeiGene, Janssen, and AstraZeneca, and research funding from BeiGene, Janssen, AstraZeneca, and Loxo Oncology. G.C. reports honoraria from Roche; research funding from BeiGene and AstraZeneca; and travel accommodations from Roche and Glycomimetics. P.M. reports consulting or advisor for BeiGene, Janssen, AstraZeneca, AbbVie, Roche, Astellas, Novartis, and Gilead. D.G. reports honoraria from Novartis P/L, and research funding from Hematologix P/L. J.M. reports consulting or advisor for Pharmacyclics, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, Bristol Myers Squibb, Kyowa, Alexion, BeiGene, Fosun Kite, Innovent, and Seattle Genetics; research funding from Bayer, Gilead, Kite Pharma, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, Millennium; and speakers’ bureau for Gilead, Kite Pharma, Kyowa, Bayer, Pharmacyclics, Janssen, Seattle Genetics, Acrotech, AurobinAdo, BeiGene, Verastem, AstraZeneca, Celgene/Bristol Myers Squibb, Genentech/Roche, and AbbVie. T.P. reports consulting or advisor for AbbVie, BeiGene, Genmab, Incyte, Kite, Bristol Myers Squibb, Bayer, and Gilead, and research funding from Bayer, AbbVie, and Bristol Myers Squibb. C.D. reports employment and stock ownership at BeiGene. M.J. and L.Z. report employment, stock ownership, and honoraria at BeiGene (Beijing) Co., Ltd. H.G. reports employment and stock ownership at BeiGene. H.Z. reports employment and stock ownership at BeiGene and Janssen (spouse). W.Y.C. reports employment and stock ownership at BeiGene, and stock ownership at Bristol Myers Squibb. A.C. and W.N. report employment and stock ownership at BeiGene. J.H. reports employment, leadership, and stock ownership at BeiGene. A.T. reports consulting or advisor for AbbVie, AstraZeneca, Janssen, and BeiGene, and speakers’ bureau for AbbVie, AstraZeneca, Janssen, and BeiGene. Y.S., W.X., J.Z., J.L., and L.Q. declare no competing financial interests.
Correspondence: Constantine S. Tam, Peter MacCallum Cancer Center, 305 Grattan St, Melbourne, VIC 3000, Australia; e-mail: constantine.tam@petermac.org.
References
- 1.Bernard S, Danglade D, Gardano L, et al. Inhibitors of BCR signalling interrupt the survival signal mediated by the micro-environment in mantle cell lymphoma. Int J Cancer. 2015;136(12):2761-2774. [DOI] [PubMed] [Google Scholar]
- 2.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590-2594. [DOI] [PubMed] [Google Scholar]
- 3.Ponader S, Burger JA. Bruton’s tyrosine kinase: from X-linked agammaglobulinemia toward targeted therapy for B-cell malignancies. J Clin Oncol. 2014;32(17):1830-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trotman J, Opat S, Gottlieb D, et al. Zanubrutinib for the treatment of patients with Waldenström macroglobulinemia: 3 years of follow-up [published correction appears in Blood. 2021;137(8):1131]. Blood. 2020;136(18):2027-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen RG, McCarthy H, Rule S, et al. Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: a single-arm, multicentre, phase 2 study. Lancet Haematol. 2020;7(2):e112-e121. [DOI] [PubMed] [Google Scholar]
- 8.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372(15):1430-1440. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391(10121):659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126(6):739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tam CS, Opat S, D’Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136(18):2038-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Weerdt I, Koopmans SM, Kater AP, van Gelder M. Incidence and management of toxicity associated with ibrutinib and idelalisib: a practical approach. Haematologica. 2017;102(10):1629-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam CSL, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu W, Yang S, Zhou K, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;13(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y, Zhou K, Zou D, et al. Treatment of patients with relapsed or refractory mantle-cell lymphoma with zanubrutinib, a selective inhibitor of Bruton’s tyrosine kinase. Clin Cancer Res. 2020;26(16):4216-4224. [DOI] [PubMed] [Google Scholar]
- 16.Dimopoulos M, Sanz RG, Lee H-P, et al. Zanubrutinib for the treatment of MYD88 wild-type Waldenström macroglobulinemia: a substudy of the phase 3 ASPEN trial. Blood Adv. 2020;4(23):6009-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallek M, Cheson BD, Catovsky D, et al. ; International Workshop on Chronic Lymphocytic Leukemia . Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trotman J, Opat S, Gottlieb DJ, et al. Zanubrutinib for the treatment of patients with Waldenstrom macroglobulinemia: three years of follow-up [published correction appears in Blood. 2021;137(8):1131]. Blood. 2020;136(18):2027-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam CS, Robak T, Ghia P, et al. Zanubrutinib monotherapy for patients with treatment naïve chronic lymphocytic leukemia and 17p deletion. Haematologica. 2020;106(9):2354-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilal T, Gea-Banacloche JC, Leis JF. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: linking mechanisms with infections. Blood Rev. 2018;32(5):387-399. [DOI] [PubMed] [Google Scholar]
- 21.Fiedler K, Sindrilaru A, Terszowski G, et al. Neutrophil development and function critically depend on Bruton tyrosine kinase in a mouse model of X-linked agammaglobulinemia. Blood. 2011;117(4):1329-1339. [DOI] [PubMed] [Google Scholar]
- 22.Imbruvica (ibrutinib) [package insert]. Sunnyvale, CA: Pharmacyclics, LLC; 2015. [Google Scholar]
- 23.Furman RR, Byrd JC, Owen RG, et al. Safety of acalabrutinib (Acala) monotherapy in hematologic malignancies: pooled analysis from clinical trials. J Clin Oncol. 2020;38(15 suppl):8064. [Google Scholar]
- 24.Calquence (R) [package insert]. Gaithersburg, MD: AstraZeneca; 2019. [Google Scholar]
- 25.Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis. 2018;66(1):140-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Series J, Garcia C, Levade M, et al. Differences and similarities in the effects of ibrutinib and acalabrutinib on platelet functions. Haematologica. 2019;104(11):2292-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shatzel JJ, Olson SR, Tao DL, McCarty OJT, Danilov AV, DeLoughery TG. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J Thromb Haemost. 2017;15(5):835-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140. [DOI] [PubMed] [Google Scholar]
- 29.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiczer TE, Levine LB, Brumbaugh J, et al. Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv. 2017;1(20):1739-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampson BL, Yu L, Glynn RJ, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129(18):2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien S, Hillmen P, Coutre S, et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18(10):648-657.e15. [DOI] [PubMed] [Google Scholar]
- 33.UK CLL Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillmen P, Eichhorst B, Brown J. First interim analysis of ALP INE study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Paper presented at European Hematology Association Annual Meeting (virtual). 11 June 2021.
- 35.AbbVie. Venclexta (Venetoclax). Tablets full prescribing information; 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.