Key Points
Clinically actionable thrombotic microangiopathy (CA-TMA) occurring in the absence of aGVHD occurs earlier than day 100.
aGVHD is a key and consistent risk factor for late-onset CA-TMA.
Visual Abstract
Abstract
Hematopoietic cell transplantation–associated thrombotic microangiopathy (TMA) is a complication associated with higher nonrelapse mortality (NRM) in patients who undergo allogeneic transplant (HCT). Current classification criteria are not generally agreed on or validated, and the presence of confounding factors after transplant contribute to underdiagnosis or delayed diagnosis of TMA. We studied risk factors, incidence, and biomarkers of TMA in 119 adult allogeneic HCT recipients. Twenty-seven patients developed a clinically actionable phenotype of TMA (CA-TMA) and the incidence of CA-TMA was 22% by day 180. Among the 27 patients who developed CA-TMA, 10 developed it before the onset of acute graft-versus-host disease (aGVHD), and 17 patients developed it after the onset of aGVHD. We report for the first time that age >50 years, BK hemorrhagic cystitis, and other viral infections (CMV, HHV-6, or adenovirus) are risk factors for adult CA-TMA. Even after adjustment for aGVHD, CA-TMA was independently associated with significantly higher NRM. These data illustrate relationships between CA-TMA and aGVHD, describe new risk factors for CA-TMA and emphasizes the need to develop validated set of criteria for timely diagnosis.
Introduction
Transplant-associated thrombotic microangiopathy (TMA) is an increasingly recognized complication of hematopoietic cell transplants (HCTs) and has a significant association with morbidity and mortality.1,2 Clinical manifestations include anemia, thrombocytopenia, and renal dysfunction, although multiple organ systems can be involved.3 In addition, El-Bietar et al4 reported the histologic findings of intestinal TMA and Wall et al5 reported a higher incidence of TMA in steroid-refractory graft-versus-host disease (GVHD) than in steroid-responsive GVHD. Studies of TMA in pediatric subjects have shown serositis (with pericardial effusions) and pulmonary hypertension in TMA.6
Previously reported risk factors of TMA include HLA mismatch, conditioning regimens, and GVHD prophylaxis.7 In addition, posttransplant complications have been associated with increased risk of TMA, including viral infection and GVHD.8 Evidence suggests a strong link between the development of GVHD, endothelial injury, and TMA development.9-11 Whereas the associated general clinical manifestations and risk factors of TMA have begun to become well established, a lack of a clear standard for diagnostic criteria and absence of uniform screening strategies has led to a wide margin of reported incidences of TMA, ranging from 0.5% to 76%.12
The variability in reported frequency of TMA can be attributed in part to the disease’s potential to manifest in multiple systems, making significant comparisons between published reports more difficult and placing greater emphasis on a need for standardized criteria. In response, both the Blood and Marrow Transplantation Clinical Trials Network (BMT-CTN) and the International Working Group (IWG) have published diagnostic criteria.13,14 However, inconsistencies in diagnosis remain and may be a consequence of our currently incomplete understanding of the pathogenesis of TMA.15-18 Current criteria for TMA include anemia, thrombocytopenia, and renal dysfunction, but there is a spectrum of these abnormalities ranging from mild to severe. In this study, we focused on manifestations that were severe (required transfusions or dialysis) or resulted in a change in treatment (ie, discontinuation of tacrolimus, change to sirolimus, or use of eculizumab). We categorize this phenotype as a clinically actionable form of TMA (CA-TMA).
Plasma biomarkers of complement pathway activation have been evaluated in diagnosing TMA.19,20 Recent data have shown evidence of the value of using plasma biomarkers for diagnosis of complications, such as acute GVHD (aGVHD), as well as for predicting eventual severity.21,22
Because aGVHD is a risk factor for TMA, we hypothesized that patients with higher grades of aGVHD would be at a higher risk of presenting with CA-TMA. In this report, we describe the relationships between CA-TMA and aGVHD, describe new risk factors for CA-TMA and emphasize need to develop validated set of criteria for timely diagnosis.
Methods
Study design
Patients provided informed consent for blood collection and analysis, and analysis of clinical data under a protocol approved by The Ohio State University (OSU) Wexner Medical Center Institutional Review Board. Consecutive eligible patients receiving an allogeneic HCT at OSU from January 2017 through September 2018 were accrued, and patient follow-up was maintained up through February 2019. A total of 119 patients had samples collected at the pre-HCT and day +30 time points. A standard operating procedure was written to diagnose and treat CA-TMA. Patient with acute kidney injury and transfusion-dependent anemia or thrombocytopenia underwent laboratory testing for hemolysis (schistocytes, lactate dehydrogenase, and haptoglobin) and biopsy when feasible. TMA diagnoses were based on the criteria of Cho et al.23 We defined CA-TMA as one that was associated with higher resource utilization, such as anemia or thrombocytopenia requiring transfusions or acute renal failure requiring dialysis or necessitating change in calcineurin inhibitors, or addition of eculizumab.
Plasma complement marker analysis
All plasma samples collected at the pre-HCT and day +30 time points were analyzed in a Clinical Laboratory Improvement Amendments (CLIA)–approved laboratory using enzyme-linked immunosorbent assay (ELISA) kits. The terminal pathway membrane attack complex (sC5b-9), alternate pathway (BBPlus), and classic pathway (C4d) complement pathways were analyzed using the Quidel produced MicroVue sC5b-9 Plus EIA, MicroVue BB Plus EIA, and MicroVue C4d EIA kits, respectively. Samples were analyzed for C5a using BD Biosciences Human C5a ELISA Kit II.23 Samples were analyzed for ST2 based on previously published methods at Cincinnati Children’s Hospital Medical Center.24
Statistical analysis
Medians, ranges, frequencies, and percentages were used to describe patient characteristics. Complement data at day +30 were summarized using medians and ranges, and compared by the Wilcoxon rank sum test as well as box-and-whisker plots by CA-TMA status by day 30. ST2 pretransplant concentrations as well as ST2 at day 30 after transplant were summarized with medians and ranges, and compared between aGVHD or TMA groups by Wilcoxon rank sum test. Time to CA-TMA was defined as number of days from transplantation to CA-TMA onset, censoring those without CA-TMA at last follow-up date and treating death as a competing risk. The cumulative incidence rate of CA-TMA was estimated and the Fine and Gray regression models accounting for competing risks were used to examine the association between potential risk factors and risk of CA-TMA. The outcomes of aGVHD were similarly analyzed except the competing risks for aGVHD were relapse and death. Overall survival (OS) was defined from the date of transplantation to the date of death, censoring those alive at the last follow-up date. Cox proportional hazard models were used to evaluate the associations with risk of death. Viral infections after transplantation included cytomegalovirus (CMV) reactivation, adenovirus disease, Epstein-Barr virus, human herpes virus-6, and BK hemorrhagic cystitis . For both the Fine and Gray regression model and Cox proportional hazard model, risk factors considered for modeling included patient age, sex, sex match, race, disease, CMV status of both donor and recipient, stem cell source (peripheral blood stem cells or bone marrow, conditioning regimens (myeloablation vs reduced intensity), remission status before transplant, use of antithymocyte globulin (ATG), comorbidity index score, and Karnofsky performance status. Onset of aGVHD and different types of infections were treated as time-dependent covariates in the model for risk of CA-TMA, whereas in the model of evaluating the risk of CA-TMA and aGVHD on non-relapse mortality (NRM) and OS, CA-TMA and aGVHD were treated as time-dependent covariates. Risk factors with a significance level of P < .20 from univariable analyses were further evaluated in a multivariable analysis using a stepwise selection procedure, retaining those with P < .05 in the final model. The association between complement levels at day 30 after transplantation, changes from baseline to day 30, ST2 concentrations before transplant, as well as ST2 at day 30 after transplant, and clinical outcomes were analyzed similarly. The significance level was set at P < .05 and Stata 14 (College Station, TX) was used for all analyses.
Results
Patient characteristics
The analysis included 119 patients. Patient characteristics are described in Table 1. Median age of patients was 60 years (range, 19-76 years), and 43% of patients were women. RIC was used in 60 patients (50.4%). Preparative regimen included ATG in 48 (40.3%) and Post-Cy in 36 (30.3%) patients receiving GVHD prophylaxis. A total of 86 patients (72.3%) received peripheral blood stem cells and 83 (69.8%) of all stem cell sources were from an unrelated donor.
Table 1.
Patient demographic and clinical characteristics among the 119 HCT recipients
| n | % | |
|---|---|---|
| Age at transplant | ||
| Median, range, y | 60 | 19-76 |
| Race | ||
| Black | 5 | 4.2 |
| Caucasian | 114 | 95.8 |
| Sex | ||
| Female | 51 | 42.86 |
| Male | 68 | 57.14 |
| ABO mismatch | ||
| Yes | 49 | 41.18 |
| No | 69 | 57.98 |
| Unknown | 1 | 0.84 |
| Disease | ||
| Myeloid | 81 | 68.07 |
| Lymphoid | 35 | 29.41 |
| Myeloma | 3 | 2.52 |
| Comorbidity index | ||
| 0 | 18 | 15.13 |
| 1-2 | 44 | 36.97 |
| 3-4 | 41 | 34.45 |
| >4 | 16 | 13.45 |
| Stem cell source | ||
| Cord Blood | 1 | 0.84 |
| Haplo donor | 18 | 15.13 |
| Related | 17 | 14.29 |
| Unrelated | 83 | 69.75 |
| Stem cell product | ||
| Bone marrow | 32 | 26.89 |
| Cord blood | 1 | 0.84 |
| Peripheral blood stem cells | 86 | 72.27 |
| HLA match 8/8 | ||
| No | 22 | 18 |
| Yes | 97 | 82 |
| HLA match 10/10 | ||
| No | 24 | 20 |
| Yes | 95 | 80 |
| Donor/recipient CMV | ||
| Donor positive, recipient positive | 33 | 27.73 |
| Donor negative, recipient positive | 35 | 29.41 |
| Donor positive, recipient negative | 10 | 8.4 |
| Donor negative, recipient negative | 41 | 34.45 |
| Conditioning regimen | ||
| Myeloablative | 59 | 49.58 |
| RIC | 60 | 50.42 |
| GVHD prophylaxis | ||
| FK containing regimens | 104 | 87.39 |
| Siro containing regimens | 3 | 2.52 |
| FK and Siro containing regimens | 7 | 5.88 |
| T-cell depletion | 3 | 2.52 |
| Post-cy alone | 2 | 1.68 |
Incidence of CA-TMA, GVHD and Survival post-transplant
Twenty-seven patients developed CA-TMA during study, and time of onset of CA-TMA was 10-269 days from HCT. The cumulative incidence rates of CA-TMA by day +100 and day +180 after HCT were 18.5% (95% confidence interval [CI] 12.1-25.9) and 21.9% (95% CI, 15.1-30.0), respectively. Of the 27 patients, only 11 were alive at 1 year after transplant. Of those 11, all had tacrolimus doses either lowered or discontinued early because of acute kidney injury. Fourteen patients required dialysis and proceeded to multiorgan failure and death. The remaining 13 patients had transfusion dependence for platelets and packed red blood cells, and renal dysfunction improved after discontinuation of tacrolimus. In the cohort of 14 patients, there was a high prevalence of treatment-resistant aGVHD and active viral infections and were not considered to be candidates for eculizumab. Some of these patients were in clinical trials for virus-specific T cells and could not receive eculizumab. Two of the 27 patients received eculizumab as outpatients, with full recovery of platelet counts and hemoglobin.
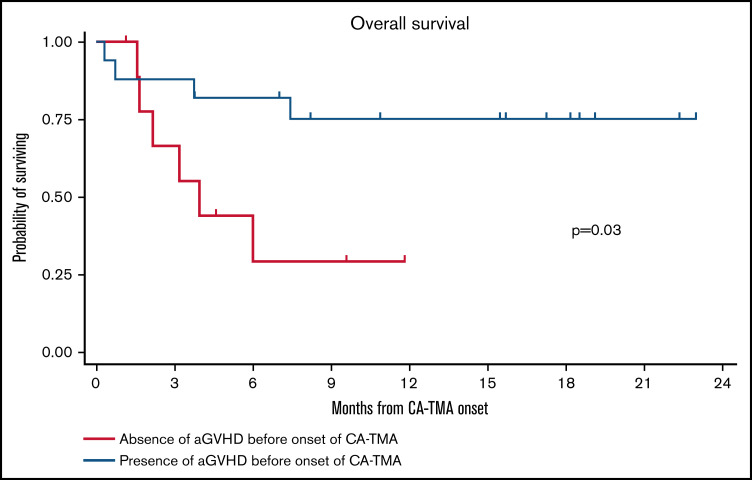
Seventy-seven patients developed aGVHD, among which 54 patients (70%) had grades 1 or 2 aGVHD, and 23 (30%) had grades 3 or 4 aGVHD. Eleven aGVHD cases (14.3%) were steroid refractory. A total of 21 patients (17.6%) relapsed and 30 patients (25.2%) died. The OS rates by years 1 and 2 were 74.7% (95% CI, 65.1-82.0) and 68.0% (95% CI, 56.1-77.4), respectively. We then analyzed OS starting from the onset of CA-TMA in patients stratified by the onset of CA-TMA before and after aGVHD. The 6-month OS rates from the onset of CA-TMA in patients who developed CA-TMA before aGVHD (n = 10) and CA-TMA after aGVHD (n = 17) were 44.4% (95% CI, 13.6-71.9) and 82.4% (95% CI, 54.7-93.9), respectively. (Figure 1). The incidence of NRM in the overall population was 6.8% (95% CI, 3.2-12.2) at day 100, 10.3% (95% CI, 5.6-16.5) at day 180, and 13.5% (95% CI, 7.9-20.6) at day 365. Among the 10 patients who developed CA-TMA before the onset of aGVHD, the incidence of NRM from onset of CA-TMA was 44.4% (95% CI, 13.6-71.9) at day 100, 55.6% (95% CI, 20.4-80.5) at day 180, and 70.4% (95% CI, 27.6-90.9) at day 365. For the 17 patients who developed CA-TMA after the onset of aGVHD, the incidence of NRM was 11.8% (95% CI, 1.96-31.2) at day 100, 17.6% (95% CI, 4.3-38.3) at day 180, and 24.5% (95% CI, 7.5-46.6) at day 365.
Figure 1.
Overall survival in patients who according to the presence or absence of aGVHD before the onset of aGVHD. Log-rank test was used for statistical comparison.
Incidence of CA-TMA is higher in aGVHD grade 3-4
Given that aGVHD is a consistent reported trigger for TMA we sought to evaluate the association between aGVHD and CA-TMA. Among the 17 patients who developed CA-TMA after onset of aGVHD, 7 patients had aGVHD grade 2, and 10 patients had aGVHD grade 3 or 4 (Figure 2A). We show the temporal relationships between aGVHD and CA-TMA in the patients who developed CA-TMA (Figure 2B).
Figure 2.
The distribution of aGVHD grading among patients with CA TMA onset. (A) After aGVHD. (B) The temporal progression of TMA development in patients stratified by aGVHD grading.
Biomarkers and CA-TMA
ST2 is a known biomarker for high-risk aGVHD. We measured ST2 levels at the pre-HCT and day +30 time points in 65 of the 119 patients from whom samples were available at both time points. Pre-HCT ST2 levels correlated with the incidence of CA-TMA by day +30 (40.9 vs 13.0, P = .0038) and did not correlate with the development of aGVHD by day +30 (P = .71). Day +30 ST2 levels were significantly associated with development of aGVHD (grades 2-4) by day +100 (P = .004) and CA-TMA by day +30 (P = .0125). We then evaluated the association between levels of complement markers at day 30 with presence or absence of CA-TMA by day 30. Day +30 complement markers were not associated with development of CA-TMA by day 30 (Figure 3).
Figure 3.
Complement markers at day 30 by CA-TMA status by day 30.
Risk factors for CA-TMA
We conducted a multivariable analysis looking at demographics, aGVHD, GVHD prophylaxis and viral infections (Table 2). On multivariable analysis, age (≥50 years), grade 3 and 4 aGVHD, viral infections (CMV, HHV-6, and adenovirus) and BK hemorrhagic cystitis were strongly correlated with development of CA-TMA. After controlling for aGVHD and BK hemorrhagic cystitis, patients aged >50 years were at increased risk of developing CA-TMA (hazard ratio [HR], 5.17; 95% CI, 1.70-15.69; P = .004). HLA matches of 8/8 or 10/10 were not significantly associated with risk of CA-TMA (HR, 0.59; 95% CI, 0.25-1.40; P = .231; and HR, 0.67; 95% CI, 0.28-1.60; P = .371, respectively). Among patients younger than 50 years old, BK was not significantly associated with risk of CA-TMA (HR, 7.4; 95% CI, 0.1-517.3), after controlling for age and aGVHD. However, 35 of the patients <50 years old and had only 4 CA-TMA events. The study was underpowered, and that is reflected in the wide CIs.
Table 2.
Multivariable fine and gray model for risk of developing CA-TMA
| HR | 95% CI | P | TMA events, n | ||
|---|---|---|---|---|---|
| age≥50 y | 5.17 | 1.70 | 15.69 | .004 | 23 |
| aGVHD grade 0-1 | Reference | 4 | |||
| aGVHD grade 2 | 2.50 | 0.92 | 6.76 | .072 | 10 |
| aGVHD grade 3-4 | 11.28 | 4.43 | 28.70 | <.001 | 13 |
| BK virus hemorrhagic cystitis* | 2.55 | 1.06 | 6.17 | .038 | 9 |
| Other viral† | 3.07 | 1.03 | 9.18 | .045 | 14 |
BK hemorrhagic cystitis (n = 3).
Other viral infections included CMV reactivation (n = 1), parainfluenza (n =1), HHV-6 encephalitis (n = 2), and adenovirus colitis (n = 2).
Discussion
A clinically actionable phenotype of TMA occurred in 21% of adult patients in this single-center study. We showed that CA-TMA occurring in the absence of aGVHD occurs earlier than day 100 and that aGVHD is a key and consistent risk factor for late-onset CA-TMA. In addition to aGVHD as the most important risk factor, age, BK hemorrhagic cystitis, and other viral infections are also associated with higher incidence of CA-TMA. Age >50 years was associated with a fivefold elevation of risk for CA-TMA, and BK hemorrhagic cystitis conferred a 2.5-fold increased risk, suggesting the need for targeted screening for CA-TMA and testing of prophylactic strategies in patients in these groups.
There is a broad range of incidence of TMA, varying from 3% in CIBMTR studies,25 1.4% in a single-center study using post-cyclophosphamide,7 to 8% in a large single center study,26 to 40% in children receiving uniform prospective screening.3 Lack of systematic uniform screening is an important factor, allowing for either a delayed diagnosis or lack of diagnosis of TMA. Our data differ from these previous reports in showing a higher incidence of CA-TMA, which we believe is related to a policy that promotes screening and heightened awareness of CA-TMA. Higher incidence has been reported in pediatric subjects who underwent prospective screening for TMA.3
Our data show that aGVHD with concomitant CA-TMA has higher NRM than aGVHD alone. In addition, there are 2 distinct temporal patterns of occurrence of CA-TMA (1) occurring early after HCT, most likely caused by direct endothelial damage from conditioning chemotherapy, cell lysis, and exposure to F-actin and neutrophil extracellular traps (NETS), and (2) a second peak that occurs after aGVHD.27 Because immunosuppression taper typically begins after day +100, we sought to evaluate the incidences of aGVHD and of CA-TMA triggered by aGVHD. Beyond day +100, CA-TMA alone in the absence of prior aGVHD was not observed. We propose that aGVHD occurring with concomitant CA-TMA reflects a more severe phenotype of aGVHD.28 Whether this implies that treatment of CA-TMA will affect NRM associated with aGVHD should be evaluated in clinical trials.
Given the reported higher incidence of mortality in aGVHD associated with TMA, we evaluated whether markers associated with high-risk aGVHD were associated with CA-TMA.29 We observed that both pre-HCT ST2 and day-30 ST2 were significantly associated with a higher incidence of CA-TMA occurring by day 30. This finding is similar to those reported in pediatric subjects by Rotz et al.30 ST2 has been shown to be a marker for treatment-resistant GVHD and early death.29 Whether the patients with elevated pre-HCT ST2 levels go on to develop early-onset CA-TMA must be evaluated in larger data sets. If confirmed, this may allow for investigation for prophylactic strategies for CA-TMA or high-risk aGVHD in patients with elevated pre-HCT ST2 levels. The findings from our study and from Rotz et al30 suggest evaluating whether ST2-dependent risk stratification of aGVHD along with screening and early treatment of TMA could mitigate poor survival in high-grade aGVHD. Elevated C5b-9, a complement marker has been shown to be a high-risk marker that has negative prognostic value in TMA in pediatric patients. Our data differ from previous reports in that we did not see correlation between day 30 C5b-9 levels and CA-TMA. Patients with elevated C5b-9 along with the clinical manifestations such as anemia, thrombocytopenia, elevated LDH, schistocytosis, and low haptoglobin would benefit from a therapeutic trial of complement inhibition. Whether elevated C5b-9 alone in the absence of clinical features merits complement inhibition should be evaluated in a prospective research study.
Regarding risk factors, we show for the first time in adults of age >50 years, viral infections (CMV, HHV-6, and adenovirus) and BK hemorrhagic cystitis are significantly associated with CA-TMA. BK hemorrhagic cystitis has been shown to be associated with TMA in pediatric subjects.3 Aging has been associated with endothelial dysfunction.24,31 Given that endothelial damage and complement activation seems to be at least 1 mechanism for the pathogenesis of TMA, larger multicenter studies would have to evaluate the impact of age on incidence of TMA. In patients aged <50 years without aGVHD or viral infections, there were no instances of CA-TMA.
There are several pitfalls when considering TMA diagnostic criteria, and this lack of clarity is an important contributor to confusion regarding the true incidence of TMA. Current grading systems for schistocytes vary by institution and the 1+ grading system is not uniformly available. In addition, biopsy-proven thrombotic microangiopathy without concomitant schistocytosis has been reported.32 Cho et al analyzed 672 patients to evaluate incidence of TMA based on BMT-CTN and IWG criteria23 and showed that 66% of cases of TMA defined by CTN criteria did not have schistocytosis by IWG criteria. Conversely, 185 of cases of TMA by IWG criteria did not have renal or neurologic manifestations. Recent data show that if inciting factors for TMA continue to be active, TMA can become a chronic condition with progressive deterioration of renal function.33 Prospective screening using lactate dehydrogenase, haptoglobin or schistocytes as performed in our study is increasingly used in centers.7 Because the diagnosis is challenging and plagued by several confounding factors, treatment with complement inhibition is likely initiated at later stages of the disease, while awaiting improvement with reduction in calcineurin inhibitors. Li et al showed that TMA does not reverse with a reduction of immunosuppressant medication. GVHD triggered by discontinuation of calcineurin inhibitors likely worsens TMA. Another limitation in this study was the use of a backward selection model, which presents potential problems when evaluating conditions that occur in a collinear fashion. The degree of correlation between the predictor variables affected the frequency with which predictor variables found their way into the final model. In conclusion, unlike pediatric population, CA-TMA in adults occurs early after HCT and later exclusively in association with aGVHD. Given the higher incidence of NRM in patients with CA-TMA, with or without aGVHD, it is imperative to recognize and manage CA-TMA as a distinct entity even when patients have active aGVHD. Given the higher incidence of CA-TMA in grade 3 and 4 aGVHD, we suggest prospective screening for CA-TMA in patients with high-grade aGVHD. Therapeutic options for CA-TMA outside of clinical trials are not broadly available and are limited by cost, which may be mitigated as data from ongoing trials evaluating complement inhibitors or defibrotide becomes available. Further research is necessary to validate diagnostic criteria, and in survivors, develop an understanding of the chronic, irreversible manifestations of TMA. As the therapeutic landscape with complement inhibitors increases, we will have options to effectively treat TMA. We and other groups are conducting prospective studies to evaluate and screen for TMA. These studies will give real-world data on the phenotypic spectrum of TMA, identify phenotypes of TMA that occur with and without aGVHD and lead to identification of novel biomarkers for early diagnosis of TMA.
Authorship
Contribution: S.V. conceived and designed the study; M.B. collected and assembled the data; Q.Z., S.V., and M.B. analyzed and interpreted the data; S.V., M.B., and N.S. wrote and edited the manuscript; and all authors provided scientific input and critical comments.
Conflict-of-interest disclosure: S.V. served on the Advisory Board of Omeros Inc. The remaining authors declare no competing financial interests.
Correspondence: Sumithira Vasu, Division of Hematology, The Ohio State University Wexner Medical Center, 1110 H Lincoln Tower, 1800 Cannon Dr, Columbus, OH 43210; e-mail: Sumithira.Vasu@osumc.edu
References
- 1.Martinez MT, Bucher C, Stussi G, et al. Transplant-associated microangiopathy (TAM) in recipients of allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant. 2005;36(11):993-1000. [DOI] [PubMed] [Google Scholar]
- 2.Jodele S, Licht C, Goebel J, et al. Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic angiopathy [plenary paper]. Blood. 2013;122(12):2003-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124(4):645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Bietar J, Warren M, Dandoy C, et al. Histologic features of intestinal thrombotic microangiopathy in pediatric and young adult patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(11):1994-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wall SA, Zhao Q, Yearsley M, et al. Complement-mediated thrombotic microangiopathy as a link between endothelial damage and steroid-refractory GVHD. Blood Adv. 2018;2(20):2619-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dandoy CE, Hirsch R, Chima R, Davies SM, Jodele S. Pulmonary hypertension after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(11):1546-1556. [DOI] [PubMed] [Google Scholar]
- 7.Imus PH, Tsai HL, DeZern AE, et al. Thrombotic Microangiopathy after post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2020;26(12):2306-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epperla N, Hemauer K, Hamadani M, Friedman KD, Kreuziger LB. Impact of treatment and outcomes for patients with posttransplant drug-associated thrombotic microangiopathy. Transfusion. 2017;57(11):2775-2781. [DOI] [PubMed] [Google Scholar]
- 9.Changsirikulchai S, Myerson D, Guthrie KA, McDonald GB, Alpers CE, Hingorani SR. Renal thrombotic microangiopathy after hematopoietic cell transplant: role of GVHD in pathogenesis. Clin J Am Soc Nephrol. 2009;4(2):345-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inamoto Y, Ito M, Suzuki R, et al. ; Nagoya Blood and Marrow Transplantation Group . Clinicopathological manifestations and treatment of intestinal transplant-associated microangiopathy. Bone Marrow Transplant. 2009;44(1):43-49. [DOI] [PubMed] [Google Scholar]
- 11.McDonald GB, Tabellini L, Storer BE, Lawler RL, Martin PJ, Hansen JA. Plasma biomarkers of acute GVHD and nonrelapse mortality: predictive value of measurements before GVHD onset and treatment. Blood. 2015;126(1):113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi CM, Schmaier AH, Snell MR, Lazarus HM. Thrombotic microangiopathy in haematopoietic stem cell transplantation: diagnosis and treatment [review]. Drugs. 2009;69(2):183-198. [DOI] [PubMed] [Google Scholar]
- 13.Ruutu T, Barosi G, Benjamin RJ, et al. ; European LeukemiaNet . Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92(1):95-100. [DOI] [PubMed] [Google Scholar]
- 14.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571-575. [DOI] [PubMed] [Google Scholar]
- 15.Chapin J, Shore T, Forsberg P, Desman G, Van Besien K, Laurence J. Hematopoietic transplant-associated thrombotic microangiopathy: case report and review of diagnosis and treatments. Clin Adv Hematol Oncol. 2014;12(9):565-573. [PubMed] [Google Scholar]
- 16.Gavriilaki E, Sakellari I, Anagnostopoulos A, Brodsky RA. Transplant-associated thrombotic microangiopathy: opening Pandora’s box. Bone Marrow Transplant. 2017;52(10):1355-1360. [DOI] [PubMed] [Google Scholar]
- 17.Batts ED, Lazarus HM. Diagnosis and treatment of transplantation-associated thrombotic microangiopathy: real progress or are we still waiting? [Review] Bone Marrow Transplant. 2007;40(8):709-719. [DOI] [PubMed] [Google Scholar]
- 18.Cutler C, Henry NL, Magee C, et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):551-557. [DOI] [PubMed] [Google Scholar]
- 19.Horvath O, Kallay K, Csuka D, et al. Early increase in complement terminal pathway activation marker sC5b-9 is predictive for the development of thrombotic microangiopathy after stem cell transplantation. Biol Blood Marrow Transplant. 2018;24(5):989-996. [DOI] [PubMed] [Google Scholar]
- 20.Qi J, Wang J, Chen J, et al. Plasma levels of complement activation fragments C3b and sC5b-9 significantly increased in patients with thrombotic microangiopathy after allogeneic stem cell transplantation. Ann Hematol. 2017;96(11):1849-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113(2):273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turcotte LM, DeFor TE, Newell LF, et al. Donor and recipient plasma follistatin levels are associated with acute GvHD in Blood and Marrow Transplant Clinical Trials Network 0402. Bone Marrow Transplant. 2018;53(1):64-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90(8):918-926. [DOI] [PubMed] [Google Scholar]
- 24.Rotz SJ, Dandoy CE, Davies SM. ST2 and endothelial injury as a link between GVHD and microangiopathy. N Engl J Med. 2017;376(12):1189-1190. [DOI] [PubMed] [Google Scholar]
- 25.Epperla N, Li A, Logan B, et al. Incidence, risk factors for and outcomes of transplant-associated thrombotic microangiopathy. Br J Haematol. 2020;189(6):1171-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li A, Wu Q, Davis C, et al. Transplant-associated thrombotic microangiopathy is a multifactorial disease unresponsive to immunosuppressant withdrawal. Biol Blood Marrow Transplant. 2019;25(3):570-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luebbering N, Abdullah S, Lounder D, et al. Endothelial injury, F-actin and vitamin-D binding protein after hematopoietic stem cell transplant and association with clinical outcomes. Haematologica. 2020;106(5):1321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtan SG, MacMillan ML. A risk-adapted approach to acute GVHD treatment: are we there yet? Bone Marrow Transplant. 2016;51(2):172-175. [DOI] [PubMed] [Google Scholar]
- 29.Vander Lugt MT, Braun TM, Hanash S, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369(6):529-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther. 2012;133(2):159-176. [DOI] [PubMed] [Google Scholar]
- 31.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond). 2011;120(9):357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphree CR, Nguyen NN, Shatzel JJ, et al. Biopsy-proven thrombotic microangiopathy without schistocytosis on peripheral blood smear: a cautionary tale. Am J Hematol. 2019;94(9):E234-E237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ang Li M, Shang H, Gupta R, et al. Thrombotic microangiopathy increases the risk of chronic kidney disease but not overall mortality in long-term survivors [abstract]. Blood. 2020;136(suppl 1):47-48. [Google Scholar]