Abstract
Although the Behavioral Inhibition System (BIS) is associated with threat-sensitivity, little is known about its neurofunctional correlates during cognitive control over task-irrelevant threat distractors. Thirty non-clinical participants, who ranged in BIS sensitivity, completed an attentional control paradigm during fMRI. The paradigm varied in cognitive demand with low perceptual load comprising identical target letters and high perceptual load comprising a target letter in a mixed letter string; each superimposed on threatening and neutral face distractors. Whole-brain results indicated that individuals with higher, relative to lower BIS sensitivity, exhibited enhanced dorsolateral prefrontal cortex activation to angry (vs. neutral) and enhanced dorsal anterior cingulate cortex activation to fearful (vs. neutral) face distractors under low load whereas no differences in activation were observed under high load. These findings are consistent with literature indicating that the BIS is involved in conflict processing, including between cognitive and emotional or motivational goals.
Keywords: Behavioral inhibition system (BIS), Cognitive control, fMRI, Neural activation, Cognitive demand
Cognitive control is a process wherein top-down resources are allocated to goals, when salient, task-irrelevant stimuli compete for neural representation (MacDonald, Cohen, Stenger, & Carter, 2000; Pessoa, Kastner, & Ungerleider, 2002). This process reflects a balance between aims to accomplish goals while maintaining sensitivity to ‘bottom-up’ information (Whalen et al., 2006). Prior findings indicate frontal regions implicated in cognitive control (e.g., anterior cingulate cortex [ACC], medial prefrontal cortex [MPFC], dorsolateral PFC [DLPFC)]) are recruited to resolve the conflict that arises when different streams of information compete for processing resources (e.g., emotional conflict resolution) (Bishop, Duncan, Brett, & Lawrence, 2004; Duncan & Owen, 2000; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Kanske & Kotz, 2010; MacDonald et al., 2000; Vuilleumier & Driver, 2007). Individual differences in neurofunctional activity in these regions may be related to the behavioral inhibition system (BIS), which is associated with conflict processing in the presence of threat.
The BIS is part of an architecture of defensive systems, which involve the Behavioral Activation System (BAS), the BIS, and the Fight/Flight/Freeze System (FFFS). According to the most recent formulation of the Reinforcement Sensitivity Theory, specific aspects of the defensive system rest on functional distinctions between behaviors (McNaughton & Corr, 2004). For example, behaviors that remove an organism from a source of danger (e.g., flight, fight, or freezing); a function governed by the FFFS, are different from those that allow it to assess a source of potential danger to determine and engage in an appropriate response; a function governed by the BIS (McNaughton & Corr, 2004; McNaughton & Gray, 2000). Specifically, the BIS is a conflict detecting, monitoring, and resolving system that functions as a comparator of inputs (McNaughton & Gray, 2000) to determine course of action (McNaughton & Corr, 2004; McNaughton & Gray, 2000). As per the Theory (McNaughton & Corr, 2004; McNaughton & Gray, 2000) if the BIS receives input from only one highly activated goal, it monitors this fact but produces no functional output. If and when a second goal becomes similarly activated, summation of these activities may pass a threshold that warrants production of output from the BIS (McNaughton & Gray, 2000). When more than one goal is such activated, the BIS produces output that includes inhibition of current responses (aimed at the competing goals) and increase of gain of any negatively affective associations with competing goals. This process continues recursively and incrementally either until a specific goal becomes predominant or until exploratory behavior yields new, affectively significant information which causes some response (not necessarily one of those originally in conflict) to become predominant. Accordingly, although termed the ‘behavioral inhibition system’, the BIS both inhibits pre-potent behavior and generates additional outputs of attention and arousal to support exploratory behavior designed to resolve conflict (McNaughton & Corr, 2004). Therefore, a function of the BIS is making assessments in situations involving approach-avoidance, approach-approach, and avoidance–avoidance conflicts (McNaughton & Corr, 2004; McNaughton & Gray, 2000).
Approach and avoidance motivations purportedly interact with cognitive control to guide goal-directed behavior (Gray & Braver, 2002). Yet, despite the pertinence of the BIS to cognitive control, the relation between the two has been surprisingly neglected. Neuroimaging studies of threat sensitivity have largely focused on anxiety − a relevant but not synonymous phenomenon − as non-clinical and clinically anxious individuals exhibit an automatic, preferential attention to threat (i.e., attentional bias) (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & Van Ijzendoorn, 2007). In this aspect, there is overlap between individuals with BIS hyperactivity and excessive anxiety, as BIS hyperactivity may result in excessive focus on environmental threat and, as an indirect consequence, increased focus on threatening associations with previous stimuli (McNaughton & Gray, 2000). Indeed, symptoms of clinical anxiety are associated with high activity of the broader defense system of which the BIS is part and the syndrome of clinical anxiety is associated with hyper-reactivity of such a system (Corr et al., 2012). Despite this overlap, as noted, the response of the defense system of which the BIS is part is context- or state-dependent. In contrast, individuals with elevated levels of anxiety stably perceive the environment as dangerous; as such, elevated trait anxiety is associated with negative schema, hyper-vigilance to threatening information, and at the memory level, hyper-recall of threatening information (Gidron, 2013).
Given general absence of research on the association between the BIS and cognitive control (but see below for exceptions) but the relevance of the BIS to anxiety, drawing on the anxiety literature may prove helpful in generating hypotheses about neurofunctional correlates of the relation between the BIS and cognitive control over emotional distractors. In studies of anxiety, accumulating evidence indicates an association between attenuated frontal recruitment in the face of threat distractors and attentional bias to threat, particularly when perceptual load on cognitive control mechanisms is low (Bishop, Jenkins, & Lawrence, 2007; Wheaton, Fitzgerald, Phan, & Klumpp, 2014). Specifically, data obtained across studies suggest that when cognitive goals are relatively easy to execute, leftover resources are available to process motivationally-relevant (but task-irrelevant) distractors. For example, Bishop and colleagues (Bishop et al., 2007) observed a negative association between activation in the anterior cingulate cortex (ACC) to threat distractors and trait anxiety but only when load on cognitive control processes was nominal (i.e., low perceptual load) in non-clinical adults. Conversely, no relationship was observed between activation in higher-order functions to threat distractors and trait anxiety when load on cognitive control processes were maximized (i.e., high perceptual load). Results are consistent with the notion that emotional interference is greater when demands on processing resources are minimal (i.e., low load). Interestingly, at the behavioral level, high and low trait anxious participants performed similarly in the low, but not high, perceptual load condition suggesting accuracy and reaction time did not track neurofunctional activity (Bishop et al., 2007).
In a separate study, our group found less ACC activation to task-irrelevant threatening faces under low perceptual load in clinically anxious participants relative to healthy controls. Yet, under high load, ACC activity to threat distractors was greater in the anxious group, potentially indicating a compensatory mechanism. In support of a compensatory function, no group effects were observed in behavioral performance (Wheaton et al., 2014).
Although these data implicate attenuated or compensatory frontal activation in excessive, selective attention to threat in anxiety, far less is known about the neurofunctional correlates of the BIS. To date, limited research in non-clinical individuals indicate a positive association between BIS and conflict detection. Relevant findings include an association between BIS and the N2 (inhibition related to ‘No-Go’) and error-related negativity (ERN) evoked response potential (ERP) components (both of which reflect enhanced conflict detection-related ACC activity) (Amodio, Master, Taylore, Yee, & Taylor, 2008) and associations between heightened childhood BIS and enhanced ERN (McDermott et al., 2009) as well as temperamental shyness and an enhanced N2 (Henderson, 2010) to errors in non-affective conflict detection paradigms. The findings of the only pertinent fMRI study indicate that adults with heightened childhood BIS2 relative to adults without heightened BIS sensitivity, exhibited enhanced dorsomedial PFC (DMPFC) activation to conflict detection (Jarcho et al., 2012). Notably, across these studies, behavioral performance was not associated with BIS (Amodio et al., 2008) and group differences between high and low BIS individuals, based on median split, were not observed in behavioral performance (Jarcho et al., 2012; McDermott et al., 2009). These results suggest neural measures, relative to behavioral ones, may be more sensitive to BIS effects.
Collectively, ERP and fMRI data support the function of the BIS in conflict detection, monitoring, and resolution (McNaughton & Corr, 2004; McNaughton & Gray, 2000), to engage response processes to align performance with goals (Gratton, Coles, & Donchin, 1992; Schneider & Shiffrin, 1977). Extending this work, Dennis and Chen (2007) examined the impact of BIS on attentional control over threat distractors with a modified3 version of the Attention Network Test (ANT) (Fan, McCandliss, Sommer, Raz, & Posner, 2002). The ANT is an experimental paradigm of alerting and orienting (associated with automatic attentional systems) and executive attention (associated with voluntary attentional systems related to the ACC) (Derryberry & Rothbart, 1997). In the modified ANT, task-irrelevant fearful, sad, and happy faces were presented before the task, therefore, emotional interference was relatively mild. Results revealed participants with low, relative to high BIS, had reduced cogntive control to fearful face distractors signified by an enhanced N200 response during executive attention. Again, no BIS group differences emerged at the behavioral level.
Together, theory and empirical findings indicating a positive association between the BIS and conflict detection support conceptualization of the BIS as not only inhibiting prepotent behavior but also generating additional outputs of attention and arousal to support exploratory behavior to resolve conflict (McNaughton & Corr, 2004). However, it is unknown whether the enhanced top-down functioning exhibited by individuals with elevated BIS is maintained when cognitive goals directly compete with threat distractors.
Accordingly, the aim of the present study was to examine the association between BIS sensitivity and neural activation to cognitive control during a validated threat-interference paradigm that varied in perceptual load. Threat distractors consisted of angry and fearful faces and were examined separately for the following reasons. First, although both anger and fear are related to threat, there are considerable differences between the two, including with regard to behavioral and communicative function as well as underlying neural processing circuitry. Regarding the former, anger and fear signals are different as far as elicited behaviors in the observer: anger, in contrast to fear, is a more interactive signal (e.g., indicative of interpersonal aggression) often displayed to alter the behavior of the addressed agent. On the other hand, the source of threat related to fear is more ambiguous thus requiring additional contextual information on part of the addressed agent (Pichon, de Gelder, & Grèzes, 2009). With increasing ambiguity, there is greater activation of neural circuitry associated with increasing attention and vigilance towards environmental stimuli via the modulation of response thresholds of sensory cortical neurons (Kapp, Supple, & Whalen, 1994; Weinberger et al., 1990; Whalen, 1998), so as to increase the likelihood of acquiring additional information (about the source of threat). Accordingly, there is a body of work indicating that anger and fear differentially perturb emotion processing circuitry (Adolphs, Tranel, Damasio, & Damasio, 1994; Adolphs, Tranel, Damasio, & Damasio, 1995; Broks et al., 1998; Calder et al., 1996; Whalen et al., 2001). For example, in a meta-analytic study of emotional face processing, angry (vs. neutral) faces engaged insula and inferior occipital gyrus whereas fearful (vs. neutral) faces recruited parahippocampal gyrus, fusiform gyrus, and medial frontal gyrus (Fusar-Poli et al., 2009). Secondly, differential neural response to angry and fearful faces has also been observed in individuals with excessive anxiety when these faces serve as task-irrelevant stimuli (e.g., anterior cingulate cortex, inferior temporal gyrus) suggesting interactions between anxiety and type of threat distractor (Klumpp et al., 2011; Wheaton et al., 2014). These findings are consistent with evidence indicating that factors such as stimulus content (i.e., stimulus specificity) play a role in selective attention when processing resources are limited (Yiend, 2010). In light of these data and the possibility that the BIS may interact with stimulus content, we evaluated cognitive control in the context of angry and fearful faces distractors.
Study hypotheses were formulated based on conceptualization of the BIS and prior findings (Dennis & Chen, 2007). As per the Reinforcement Sensitivity Theory, the presence of more than one highly relevant goal engages the BIS (McNaughton & Corr, 2004; McNaughton & Gray, 2000); however, BIS engagement may not be necessary in the presence of only one highly relevant goal. The implication is that under low perceptual load, the cognitive, task-relevant goal and the emotional, task-irrelevant distractor are inputs into the BIS with similar activation or weight. As such, they − so to say − are two equally highly activated “goals”. This is consistent with Reinforcement Sensitivity Theory in that goals are neither a stimulus nor a response but a stimulus (or stimuli) with an associated action tendency (McNaughton & Gray, 2000). Conversely, under high perceptual load, the cognitive, task-relevant goal and the emotional, task-irrelevant distractor are inputs into the BIS with dissimilar activation or weight; the cognitive goal, given its difficulty, may be a more highly activated goal than the emotional distractor. As such, there is no need for the BIS to engage in or continue its engagement in its comparator process. Namely, it is unnecessary to inhibit current responses and increase gain of negatively affective associations of the competing goals until a specific goal becomes predominant (or exploratory behavior yields new information to cause some response to become predominant), in which case the need for neural compensation is also obviated. Accordingly, when demands on cognitive resources are low, we expected individuals with elevated BIS to exhibit greater ACC and/or frontal activity (e.g., medial prefrontal cortex) than individual with low BIS. Conversely, when demands on cognitive resources are high, we did not expect to find differences between individuals with elevated relative to individuals with low BIS.
1. Method
1.1. Participants
Participants were 30 healthy volunteers between the ages of 18 and 65 who were recruited via community advertisements. Participants were free of major medical or neurologic illness as confirmed by a Board Certified physician and all participants completed the Structured Clinical Interview for DSM-IV (SCID-IV) (First, Spitzer, Gibbon, & Williams, 1995). Exclusion criteria included psychoactive medications, history of any Axis I diagnosis, and contraindications to magnetic resonance imaging (e.g., claustrophobia, pregnancy, non-removable ferrous objects). No participants tested positive for illicit substances. BIS was evaluated with the subscale of the Behavioral Inhibition/Behavioral Activation Scale (BIS/BAS Scale), a 24-item self-report questionnaire shown to have good test-retest reliability and validity (Carver & White, 1994). Of note, although there are several measures to assess functioning of the BAS, BIS, and FFFS, many have limitations with regard to content and/or psychometric properties. One common limitation is no differentiation among the BAS factors and lack of corresponding subscales (Corr, 2016). The BIS/BAS Scale is one of the few of the measures with acceptable content (including differentiation of the BAS factors) and psychometric properties. Therefore, the BIS subscale of the BIS/BAS Scale was used in the current study. Consistent with prior studies wherein individual characteristics related to cognitive processes (Braem, Duthoo, & Notebaert, 2013; Cavanagh, Frank, & Allen, 2011; Robinson, Letkiewicz, Overstreet, Ernst, & Grillon, 2011), including cognitive control over emotional interference (Bishop et al., 2007; Dennis & Chen, 2007) were examined, a median split was used to assign participants into high and low BIS groups. All participants provided written informed consent and this research was approved by the Institutional Review Board at the University of Illinois at Chicago (UIC). All procedures were conducted in compliance with the Helsinki Declaration. Participants were compensated $15/hour for their time.
1.2. fMRI task
During fMRI, participants completed an emotional faces interference task (EFIT) that varied in perceptual load (Bishop et al., 2007; Klumpp et al., 2016; Wheaton et al., 2014). In the task, participants were presented with a string of six letters that are superimposed on task-irrelevant face distractors (angry, fearful, or neutral) and were instructed to identify target letters (N or X). In trials involving low perceptual load, the string of letters comprised target letters only. In trials involving high perceptual load, the string of letters included a single target letter and five non-target letters (H, K, M, W, Z), arranged in randomized order (see Fig. 1 for a diagram of the experimental design and paradigm). Participants were asked to respond by button press as accurately and quickly as possible. The face distractors were part of a standardized set of photographs. Angry, fearful, and neutral expressions were exhibited by eight different individuals (Ekman & Friesen, 1976).
Fig. 1.

Diagram of experimental design and paradigm.
The experimental paradigm consisted of two image acquisition runs, each including 12 blocks of five trials. A mixed block/event-related design was used wherein perceptual load (high vs. low) varied across blocks and facial expression (angry, fearful, and neutral) and varied within blocks on a trial-by-trial basis. Images were presented for 200 msec and followed by a fixation cross presented for 1800 msec. Within blocks, trials were separated by a jittered interstimulus interval lasting two-six secs; trials between blocks were separated by four-eight secs.
1.3. fMRI data acquisition and preprocessing
Functional imaging was performed with blood-oxygen-level-dependent (BOLD) sensitive whole-brain fMRI using GE software (LX 8.3, Neuro-optimized gradients) on a 3.0 T GE Signa System (General Electric;Waukesha, WI) using a 4-channel GE Quadrature sending and receiving head coil. Images were acquired with 30 axial, 5-mm-thick slices using a standard T2*-sensitive gradient echo reverse spiral acquisition sequence (2 s repetition time; 25 msec echo time; 64 × 64 matrix; 24 cm field of view; flip angle 77°; 3.75 × 3.75 × 5 mm final voxel size). A high-resolution, T1-weighted volumetric anatomical scan was also acquired in the axial plane (9 msec repetition time, 1.8 msec echo time; 256 × 256 matrix; 256 mm field of view; flip angle 15°; 124 slices; 1.2 mm slice thickness) at the same position as the functional images for anatomical localization.
Data from all participants met criteria for quality with minimal motion correction (movements were < 3 mm and < 3 ° rotation in any one direction) and the first 4 vols from each run were discarded to allow for T1 equilibration effects. Conventional preprocessing steps were used in Statistical Parametric Mapping (SPM8) software package (Wellcome Trust Centre for Neuroimaging, London www.fil.ion.ucl.ac. uk/spm). Briefly, images were temporally corrected to account for differences in slice time collection, spatially realigned to the first image of the first run, normalized to a Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm3 voxels, and smoothed with an 8 mm isotropic Gaussian kernel.
A general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128 s high-pass filter. Blocks of low and high perceptual load were modeled separately based on task-irrelevant face type (angry, fearful, neutral) resulting in six regressors (angry low, angry high, fearful low, fearful high, neutral low, neutral high), the effects of which were estimated for each voxel for each participant and taken to the second level for random effects analysis.
In SPM8, a 2 (Group: high/low BIS) × 2 (Perceptual Load: high/low) analysis of variance (ANOVA) was performed for angry distractors (i.e., angry>neutral) and a separate ANOVA was conducted for fearful distractors (i.e., fearful>neutral). To correct for multiple comparisons, 3dClustSim was applied (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) to a priori anatomy-based masks (e.g., ACC, DLPFC, DMPFC) via Monte Carlo simulations (10,000 iterations) and to second-level statistical results for a corrected p< 0.05 alpha level. Results revealed the most conservative minimum cluster size for significant effects under low or high load for angry (vs. neutral) faces or fearful (vs. neutral) faces was 47 contiguous voxels for a priori regions of interest (ROIs).
For exploratory purposes, whole-brain significance was set at p< 0.005 (uncorrected) with a cluster extent threshold of at least 20 contiguous voxels (volume ≥160 mm3). This type of joint intensity and cluster size threshold is within the recommended threshold to attain a balance between Type I and Type II errors (Lieberman & Cunningham, 2009).
Subsequently, parameter estimates of peak activation for significant a priori findings (β weights, arbitrary units [a.u.]) were extracted from spherical (10-mm diameter) ROIs from each participant and submitted to simple-effects analyses (e.g., independent t-tests) and bivariate (Pearson) correlation analyses in the Statistical Package for the Social Sciences (SPSS) (Chicago, IL version 22) to illustrate the direction and magnitude of the observed effects. All analyses were two-tailed with an alpha level of 0.05.
2. Results
2.1. Participants
Participants (33.3% male) had an average age of 25.67 (SD = 6.98) and educational level of 15.78 years (SD = 2.48, range 12–24 years). Regarding ethnicity/race, 46.7% self-identified as Caucasian, 30.0% as Asian, 16.7% as African American, and 6.7% as biracial or multiracial. Self-reported BIS scores ranged from 9 to 24, with a M = 16.43 and SD = 3.82, comparable to the BIS scores of Carver and White’s (Carver & White, 1994) sample. The BIS median value was 16; thus, 16 participants were assigned to the low BIS group and 14 to the high BIS group. Groups were similar in age, gender distribution, and ethnicity/race (all ps>0.08).
2.2. Behavioral results
A 2 (Perceptual Load: high/low) × 2 (Group: high/low BIS) × 3 (Distractor Type: angry, fearful, neutral) mixed repeated measures ANOVA for accuracy revealed a difference given perceptual load F(1, 168) = 57.34, p< 0.001; accuracy for angry, fearful, and neutral distractors for low load was higher than that for high load) but not group (p= 0.263) or distractor type (p =0.799).
The same ANOVA performed for reaction time (RT) in accurate trials indicated a difference given perceptual load F(1, 168) = 89.86, p< 0.001; RT for angry, fearful, and neutral distractors for low load was lower than that for high load) but not group (p = 0.635) or distractor type (p = 0.524).
2.3. Functional MRI
Angry (vs. Neutral) Distractors.
Whole-brain voxel-wise ANOVA results that survived correction for multiple comparisons revealed a 2 (Group: high/low BIS) × 2 (Perceptual Load: high/low) interaction in the left DLPFC ([−8, 30, 50], z = 3.17, k = 26, volume = 208 mm3) (Fig. 2a).
Fig. 2.
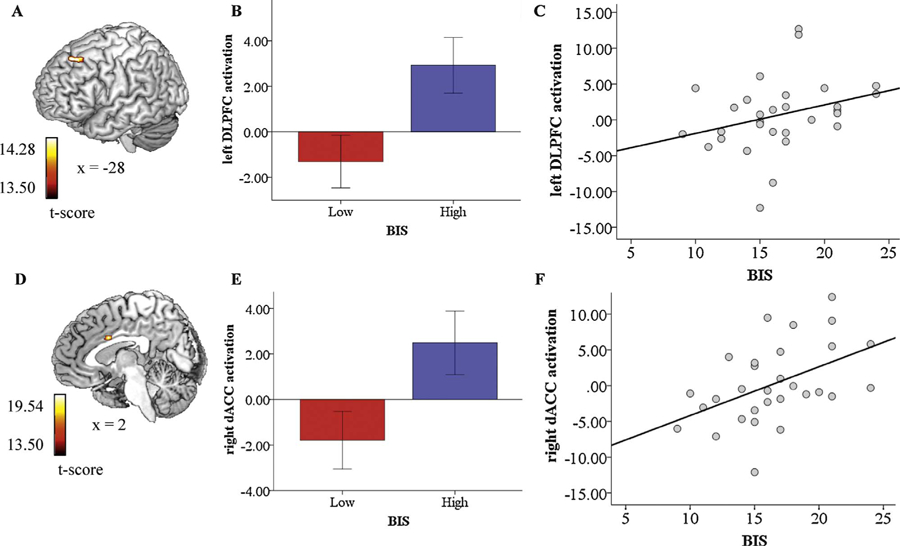
Interaction effects between BIS and perceptual load following angry distractors on activation in the left DLPFC and following fearful distractors on activation in the right dACC (B and E, respectively); bar graphs depicting left DLPFC activation following angry distractors and right dACC activation following fearful distractors for individuals with low and high BIS scores (A and D, respectively); correlation between BIS and left DLPFC activation following angry distractors and BIS and right dACC activation following fearful distractors (C and F, respectively).
To probe significant interactions, regions of interest (ROI) were extracted for angry vs. neutral faces under high load and angry vs. neutral faces under low load and exported into SPSS. The model was not significant for the high-load comparison (p =0.087) but was significant for the low-load comparison. Specifically, there was a difference between individuals high and low on BIS under low perceptual load in response to angry vs. neutral faces, F(1, 28) = 6.308, p = 0.018.
A follow-up independent-samples t-test indicated individuals high on BIS (M= 2.925, SD= 4.57) exhibited more DLPFC activation than individuals low on BIS (M= −1.307, SD = 4.62) in response to angry vs. neutral faces, t(16) = −2.512, p = 0.018 (Fig. 2b). To examine whether the observed relationships were driven solely by the dichotomous conceptualization of BIS, bivariate correlation coefficients were computed between DLPFC activation in response to angry vs. neutral faces under low perceptual load and continuous BIS scores. Results indicate the observed relationships hold with a continuous conceptualization of BIS, in that DLPFC activation was positively associated with BIS (r =0.31, 95% CI =[.092; 0.510]) (a medium-sized effect) (see Fig. 2c).
Regarding exploratory whole-brain findings (p < 0.005), uncorrected for multiple comparisons, no significant Group × Load interaction or main effects for Group or Load emerged.
For completeness, we report whole-brain findings (p < 0.005), uncorrected for multiple comparisons, which revealed a Group × Load interaction in bilateral angular gyrus (Table 1).
Table 1.
Flexible Factorial Analyses of Variance of the Effects of BIS Status and Perceptual Load on Neural Activation.
| Coordinates | z | k | volume | Automated Anatomical Labeling region |
|---|---|---|---|---|
| Angry vs. neutral | ||||
| −64,−58,28 | 3.35 | 17 | 136 | Left angular gyrus |
| 56,−56,28 | 3.30 | 35 | 280 | Right angular gyrus |
| −28,30,50 | 3.19 | 26 | 208 | Left middle frontal gyrus |
| Fearful vs. neutral | ||||
| 2,8,30 | 3.36 | 39 | 312 | Right anterior cingulate gyrus |
Fearful (vs. Neutral) Distractors.
Whole-brain voxel-wise ANOVA results that survived correction for multiple comparisons showed a 2 (Group: high/low BIS) × 2 (Perceptual Load: high/low) interaction in the right dorsal ACC (DACC) ([2, 8, 30], z = 3.36, k = 39, volume = 312 mm3) (Fig. 2d).
Similar to the results obtained in relation to angry (vs. neutral) distractions, the results of a one-way ANOVA conducted to examine differences in each fearful distractor and perceptual load-pairing between individuals high and low on BIS indicated the model was not significant for the high-load comparison (p = 0.737) but was significant for the low-load comparison. Specifically, there was a difference between individuals high and low on BIS under low perceptual load in response to fearful vs. neutral faces, F(1, 28) = 5.167, p = 0.031.
A follow-up independent-samples t-test indicated individuals high on BIS (M =2.489, SD = 5.22) exhibited more DACC activation than individuals low on BIS (M= −1.786, SD = 5.06) in response to fearful vs. neutral faces, t(16) = −2.273, p = 0.031 (Fig. 2e). The results of bivariate correlation analyses indicate the observed relationships hold with a continuous conceptualization of BIS, in that DACC activation was positively associated with BIS (r = 0.47, 95% CI =[.226; 0.668]) (a medium-large-sized effect) (Fig. 2f).
Regarding exploratory whole-brain findings (p< 0.005), uncorrected for multiple comparisons, no significant Group × Load interaction or main effects for Group or Load emerged.
3. Discussion
As hypothesized, individuals with higher relative to individuals with lower BIS sensitivity exhibited more frontal activity to threatening (vs. neutral) face distractors under low perceptual load. Specifically, whole-brain Group × Load interactions revealed higher BIS level was associated with more dorsolateral prefrontal cortex (DLPFC) activity to angry (vs. neutral) face distractors and with more dorsal anterior cingulate cortex (DACC) activity to fearful (vs. neutral) face distractors under low load. Also as hypothesized, no relation was observed between BIS sensitivity and neural activation under high perceptual load. Consistent with previous neurophysiological or neuroimaging studies of the BIS (Dennis & Chen, 2007; Jarcho et al., 2012; McDermott et al., 2009) behavioral performance was similar between high and low BIS groups.
Evidence indicating that BIS sensitivity and type of threat distractor modulated frontal activity when demands on processing resources were minimal (i.e., low perceptual load) is consistent with the notion that stimulus content figures into attentional bias to salient cues (Yiend, 2010) From an evolutionary perspective, an angry face looking directly at someone (as in our paradigm) conveys more of a clear and direct threat than a fearful face looking at someone (Biehl, Matsumoto, & Ekman, 1997; Ewbank et al., 2009; Fox, Mathews, Calder, & Yiend, 2007; Whalen, 1998). Therefore, neural response suggestive of stimulus specificity may relate to varied functions that sub-serve cognitive control. For example, while both DLPFC and DACC are implicated in a conflict-control loop, the DLPFC is primarily involved in conflict resolution whereas the DACC is involved in conflict detection and monitoring (Carter & Van Veen, 2007). Potentially, BIS modulated DLPFC activity when a cognitive goal competed with a task-irrelevant stimulus of danger with direct signal of interpersonal aggression against self. On the other hand, BIS modulated DACC activity when a cognitive goal competed with a task-irrelevant ambiguous signal of danger. It will be important to examine the influence of (threat) distractor type on the relation between cognitive control and BIS sensitivity in future studies, as our paradigm did not allow for a disassociation of these less vs. more ambiguous threat signals or between conflict resolution vs. detection.
Alternatively, as findings were observed when task-relevant resources were available (i.e., ‘left over’) to process threat distractors (Lavie, Lin, Zokaei, & Thoma, 2009) differential effects may reflect emotion processing. There is continued discussion as to the automaticity (e.g., encoding) of salient stimuli when demands are placed on attentional resources (e.g., bottleneck in early selective attention) (Dolan & Vuilleumier, 2003; Pessoa et al., 2002; Vuilleumier, Armony, Driver, & Dolan, 2001) and as noted earlier, angry and fearful faces have differential effects on emotion processing circuitry (Fusar-Poli et al., 2009). Although the rapid presentation of threat distractors in the current task provides an element of cognitive demand (i.e., constraint on resources), even in the low perceptual load condition, we cannot rule out the potential that results may involve the encoding of ‘bottom-up’ distractors.
Nonetheless, our data warrant the conclusion that an association between enhanced BIS sensitivity and greater neural activation during cognitive control over emotional distracters is congruent with conceptualization of the BIS as a system that processes conflict between competing inputs and goals (McNaughton & Gray, 2000). Of note, these data should be interpreted in the context of similar behavioral performance across BIS groups, potentially reflecting distinct mechanisms at play. One such mechanism may be compensatory engagement of a comparator system in the service of determining adaptive course of action (McNaughton & Gray, 2000) (McNaughton & Corr, 2004). In the current case, results may reflect effectively avoiding or minimizing effects of threat distractors (McNaughton & Gray, 2000). This is a testable hypothesis for authors of future studies to examine.
The compensatory engagement conceptualization is relevant to our hypotheses and null findings in the high perceptual load condition. Although it may appear counterintuitive that there is enhanced activation under low but not high load, as we predicted following the Reinforcement Sensitivity Theory (McNaughton & Corr, 2004; McNaughton & Gray, 2000), it is likely the case that under low perceptual load, the cognitive goal and the emotional distractor are BIS inputs with similar weight and thus resemble two comparably activated “goals”. Conversely, we predicted that under high perceptual load, the cognitive goal and the emotional distractor are BIS inputs with dissimilar weight, with the cognitive goal, given its difficulty, being a more highly activated goal. With the differences in activation levels, the need for comparison across goals to make one predominant by the BIS diminishes, also obviating the need for neural compensation.
For completeness we reported whole-brain findings that did not survive correction for multiple comparisons, which was limited to a BIS Group × Perceptual Load interaction in the bilateral angular gyrus in the context of angry face distractors. We hesitate to interpret this finding as the angular gyrus was not an a priori region of interest and is not conceptually related to the BIS.
The current findings have implications both with regard to research on psychopathology and with regard to theoretical interpretations of the BIS. First, with regard to implications for research on psychopathology, others (Blackford & Pine, 2012) have proposed that in addition to examining neural bases of anxiety, an important task for the next generation of research is to study temperamental dimensions that confer risk for pathological anxiety. The BIS may be one of the more important of such temperamental dimensions as prior evidence suggests it is heritable (Robinson, Kagan, Reznick, & Corley, 1992), emerges in infancy (Calkins, Fox, & Marshall, 1996; Kagan, Snidman, & Arcus, 1998), and its hyperactivity is associated with excessive focus on environmental threat (McNaughton & Gray, 2000). Combined with prior findings of an association between trait anxiety and attenuated frontal activation when cognitive demands are low during cognitive control (Bishop et al., 2007; Wheaton et al., 2014), our findings of association between BIS and enhanced frontal activation when cognitive demands are low during cognitive control underscore that trait anxiety and BIS are not synonymous and indicate that the two are differentially related to cognitive control. It may be through an interaction between trait anxiety and BIS sensitivity on conflict processing that heightened BIS sensitivity confers risk for pathological anxiety (Corr et al., 2012). Nevertheless, to adequately address the differences between neurofunctional correlates of the BIS and anxiety in cognitive control, future work should directly compare the variance explained by the BIS vs. trait anxiety on neural activation to attentional and cognitive control, as well as other, relevant tasks.
It is important to note here, given that the BIS and anxiety are often conflated, that the BIS and anxiety are − as discussed earlier − separate constructs that may or may not vary independently. Anxiety, in the most basic sense of the term (Corr & McNaughton, 2012), is a state that results from conflict between two incompatible goals, such as approach and avoidance (McNaughton & Corr, 2004). Anxiety in this sense is different from both trait (i.e., “the stable tendency to attend to, experience, and report negative emotions such as anxiety, fears, and worries across situations”; Gidron, 2013; p. 1989) and clinical anxiety. Symptoms of clinical anxiety are associated with high activity of the defense system and the syndrome of clinical anxiety is associated with hyper-reactivity of such system (Corr et al., 2012). Clinical anxiety disorders are, in ethological terms, disorders of fear (e.g., panic, phobia) and of anxiety (e.g., agoraphobia, social anxiety) (Corr et al., 2012). Conversely, anxiety, as most relevant to the BIS, is a state that results from conflict that is detected, monitored, and resolved by the BIS, a comparator system (McNaughton & Gray, 2000). Importantly and as noted, although termed the ‘behavioral inhibition system’, the BIS both inhibits prepotent behavior and generates additional outputs of attention and arousal so as to support exploratory behavior designed to resolve conflict (McNaughton & Corr, 2004); in this sense, the inhibition of approach by (e.g., approach–avoidance) conflict − the primary output of the BIS, is neurally distinct from avoidance (Gray, 1977) − a hallmark feature of trait and clinical anxiety. In further support of this point, the correlation between BIS and trait anxiety on the State-Trait Anxiety Inventory in the present sample was r= 0.387, p= 0.035. Although statistically significant, the correlation coefficient indicates a relationship of medium magnitude that is not so large as to indicate isomorphism.
Our results have further inferences with regard to theoretical interpretations of the BIS. The term “inhibition” is used to refer to distinct behavioral and psychological processes in different literatures. This inconsistent use of the term may have contributed to obscurities in conceptual models of the BIS. As noted, ‘behavioral inhibition’, if it means a reduction in behavior, is not necessarily dependent on the BIS (Corr et al., 2012). Although our study was not designed to test this, our current findings are consistent with prior EEG and fMRI data indicating that the BIS is related to conflict detection and resolution.
3.1. Limitations
Although no prior theory or data related to the association between BIS sensitivity and attentional control in the context of high perceptual demands is available, our relatively small sample may have limited power to detect group differences in neural reactivity to threat under high perceptual load. Second, our paradigm was not designed to dissociate activation implicated in the detection of conflict and resolution of conflict. Lastly, results are based on healthy individuals and, therefore, may not generalize to psychiatric populations, including anxious populations. Although our task was not directly relevant for activating the amygdala and anterior insula, others have employed emotional distractors to activate such regions (Etkin et al., 2006). That we observed no task-effects in these structures and no behavioral effect of distractor type limits confidence in the effectiveness of the stimuli used to create emotional conflict. Yet, others note that some of the properties typically linked to automaticity (e.g., detecting fearful faces among distractors) may not depend on the amygdala (Pessoa & Adolphs, 2010), suggesting that the absence of an observed activation in the named regions may not be as problematic for the effectiveness of the stimuli used as may prima facie appear.
Notwithstanding limitations, to the best of our knowledge this is the first study wherein the neural correlates of BIS were examined in the context of threat distractors with a well-validated (Bishop et al., 2007; Klumpp et al., 2016; Wheaton et al., 2014) attentional control paradigm. We observed higher BIS level corresponded with more activation in DLPFC and DACC to threatening face distractors when demands on cognitive control processes were low. BIS is a temperamental characteristic and conflict processing in the presence of threat is a key aspect of BIS sensitivity. Potentially, increased frontal activation over task-irrelevant threat cues reflects greater engagement of a preparatory response system to disrupt or minimize the processing of sensory-driven threat signals.
Acknowledgements
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K23MH093679 (HK), the Center for Clinical and Translational Science (CCTS) UL1RR029879, and the National Institutes of Health under Award Number 1S10RR028898.
Footnotes
Assessed at ages four, 24, and 48 months and indexed by emotional and motor reactivity to novel auditory, olfactory, and visual stimuli; inhibited behavior in response to novel auditory and visual stimuli; and socially reticent behavior when confronted with unfamiliar peers, respectively.
Task-irrelevant face distractors were included in the task.
References
- Adolphs R, Tranel D, Damasio H, & Damasio A (1994). Impared recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372(6507), 669–672. 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, & Damasio AR (1995). Fear and the human amygdala. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 15(9), 5879–5891. 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Master SL, Taylore SE, Yee CM, & Taylor SE (2008). Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories ofself-regulation. Psychophysiology, 45, 11–19. 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & Van Ijzendoorn MH (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1. [DOI] [PubMed] [Google Scholar]
- Biehl M, Matsumoto D, & Ekman P (1997). Matsumoto and Ekman’s Japanese and Caucasian facial expressions of emotion (JACFEE): Reliability data and cross-national differences. Journal of Nonverbal Behavior, 21, 3–21. [Google Scholar]
- Bishop SJ, Duncan J, Brett M, & Lawrence AD (2004). Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature Neuroscience, 7(2), 184–188. 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Jenkins R, & Lawrence AD (2007). Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cerebral Cortex, 17(7), 1595–1603. 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Blackford J, & Pine D (2012). Neural substrates of childhood anxiety disorders a review of neuroimaging findings. Child and Adolescent Psychiatric Clinics, 21(3), 501–525. 10.1016/j.chc.2012.05.002 [Neural.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braem S, Duthoo W, & Notebaert W (2013). Punishment sensitivity predicts the impact of punishment on cognitive control. Public Library Of Science, 8(9), 10.1371/journal.pone.0074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broks P, Young AW, Maratos EJ, Coffey PJ, Calder AJ, Isaac CL, … Hadley D (1998). Face processing impairments after encephalitis: Amygdala damage and recognition of fear. Neuropsychologia, 36(1), 59–70. 10.1016/S0028-3932(97)00105-X. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW, Rowland D, Perrett DI, Hodges JR, & Etcoff NL (1996). Facial emotion recognition after bilateral amygdala damage: Differentially severe impairment of fear. Cognitive Neuropsychology, 13(5), 699–745. 10.1080/026432996381890. [DOI] [Google Scholar]
- Calkins SD, Fox NA, & Marshall TR (1996). Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development, 67(2), 523–540. [PubMed] [Google Scholar]
- Carter CS, & Van Veen V (2007). Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience, 7(4), 367–379. 10.3758/CABN.7.4.367. [DOI] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67(2), 319. [Google Scholar]
- Cavanagh JF, Frank MJ, & Allen JJB (2011). Social stress reactivity alters reward and punishment learning. Social Cognitive and Affective Neuroscience, 6(3), 311–320. 10.1093/scan/nsq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr PJ, & McNaughton N (2012). Neuroscience and approach/avoidance personality traits: A two stage (valuation-motivation) approach. Neuroscience & Biobehavioral Reviews, 36, 2339–2354. [DOI] [PubMed] [Google Scholar]
- Corr PJ (2016). Reinforcement sensitivity theory of personality questionnaires: Structural survey with recommendations. Personality and Individual Differences, 89, 60–64. 10.1016/j.paid.2015.09.045. [DOI] [Google Scholar]
- Dennis TA, & Chen CC (2007). Neurophysiological mechanisms in the emotional modulation of attention: The interplay between threat sensitivity and attentional control. Biological Psychology, 76(1–2), 1–10. 10.1016/j.biopsycho.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, & Rothbart MK (1997). Reactive and effortful processes in the organization of temperament. Development and Psychopathology, 9(4), 633–652. 10.1017/S0954579497001375. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, & Vuilleumier P (2003). Amygdala automaticity in emotional processing. Annals of the New York Academy of Sciences, 985, 348–355. 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Duncan J, & Owen AM (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences, 23(10), 475–483. [DOI] [PubMed] [Google Scholar]
- Ekman P, & Friesen WV (1976). Measuring facial movement. Environmental Psychology and Nonverbal Behavior, 1, 56–75. [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, & Hirsch J (2006). Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51(6), 871–882. 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Lawrence AD, Passamonti L, Keane J, Peers PV, & Calder AJ (2009). Anxiety predicts a differential neural response to attended and unattended facial signals of anger and fear. Neuroimage, 44(3), 1144–1151. 10.1016/j.neuroimage.2008.09.056. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, & Posner MI (2002). Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14(3), 340–347. 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, & Williams J (1995). St ructured clinical interview for DSM-IV axis I disorders, (SCID-P), version 2 (patient ed.). New York, NY: Biometrics Research. [Google Scholar]
- Fox E, Mathews A, Calder AJ, & Yiend J (2007). Anxiety and sensitivity to gaze direction in emotionally expressive faces. Emotion (Washington, D.C.), 7(3), 478–486. 10.1037/1528-3542.7.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, … Politi P (2009). Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience, 34(6), 418–432. 10.1016/S1180-4882(09)50077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidron Y (2013). Trait anxiety. In Gellman MD, & Turner JR (Eds.), Encyclopedia of behavioral medicine New York, NY: Springer, New York. 10.1007/978-1-4419-1005-9_1539 [p. 1989]. [DOI] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1992). Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General, 121, 480–506. [DOI] [PubMed] [Google Scholar]
- Gray JR, & Braver TS (2002). Integration of emotion and cognitive control: A neurocomputational hypothesis of dynamic goal regulation. Emotional cognition: From brain to behaviour, Philadelphia, PA: John Benjamins Publishing Company, 289–316. [Google Scholar]
- Gray JA (1977). Drug effects on fear and frustration: Possible limbic site of action of minor tranquilizers. In Iversen LL, Iversen SD, & Snyder SH (Eds.), Handbook of psychopharmacology (pp. 433–529). New York, NY: Plenum Press. [Google Scholar]
- Henderson HA (2010). Electrophysiological correlates of cognitive control and the regulation of shyness in children. Developmental Neuropsychology, 35, 177–193. 10.1080/87565640903526538. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Benson BE, Plate RC, Guyer AE, Detloff AM, Pine DS, … Ernst M (2012). Developmental effects of decision-making on sensitivity to reward: An fMRI study. Developmental Cognitive Neuroscience, 2(4), 437–447. 10.1016/j.dcn.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Snidman N, & Arcus D (1998). Childhood derivatives of high and low reactivity in infancy. Child Development, 69(6), 1483–1493. [PubMed] [Google Scholar]
- Kanske P, & Kotz SA (2010). Modulation of early conflict processing: N200 responses to emotional words in a flanker task. Neuropsychologia, 48(12), 3661–3664. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Supple WF, & Whalen PJ (1994). Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behavioral Neuroscience, 108(1), 81–93. 10.1037/0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Ho SS, Taylor SF, Phan KL, Abelson JL, & Liberzon I (2011). Trait anxiety modulates anterior cingulate activation to threat interference. Depression and Anxiety, 28(3), 194–201. 10.1002/da.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald DA, Piejko K, Roberts J, Kennedy AE, & Phan KL (2016). Prefrontal control and predictors of cognitive behavioral therapy response in social anxiety disorder. Social Cognitive Affective Neuroscience, 11, 630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N, Lin Z, Zokaei N, & Thoma V (2009). The role of perceptual load in object recognition. Journal of Experimental Psychology: Human Perception and Performance, 35, 1346–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, & Cunningham WA (2009). Type I and Type II error concerns in fMRI research: Re-balancing the scale. Social Cognitive and Affective Neuroscience, 4(4), 423–428. 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AM, Cohen JD, Stenger VA, & Carter CS (2000). Dissociating the role of the dorsolateral prefrontal cortex and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838. 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, & Fox NA (2009). A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry, 65(5), 445–448. 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, & Corr PJ (2004). A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neuroscience & Biobehavioral Reviews, 28, 285–305. [DOI] [PubMed] [Google Scholar]
- McNaughton N, & Gray JA (2000). Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. Journal of Affective Disorders, 61(3), 161–176. 10.1016/S0165-0327(00)00344-X. [DOI] [PubMed] [Google Scholar]
- Pessoa L, & Adolphs R (2010). Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience, 11(11), 773–783. 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, & Ungerleider LG (2002). Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Research, 15(1), 31–45. [DOI] [PubMed] [Google Scholar]
- Pichon S, de Gelder B, & Grèzes J (2009). Two different faces of threat. Comparing the neural systems for recognizing fear and anger in dynamic body expressions. Neuroimage, 47(4), 1873–1883. 10.1016/j.neuroimage.2009.03.084. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Kagan J, Reznick JS, & Corley R (1992). The heritability of inhibited and uninhibited behavior: A twin study. Developmental Psychology, 28(6), 1030–1037. 10.1037/0012-1649.28.6.1030. [DOI] [Google Scholar]
- Robinson OJ, Letkiewicz AM, Overstreet C, Ernst M, & Grillon C (2011). The effect of induced anxiety on cognition: Threat of shock enhances aversive processing in healthy individuals. Cognitive, Affective & Behavioral Neuroscience, 11(2), 217–227. 10.3758/s13415-011-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, & Shiffrin RM (1977). Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review, 84(1), 1–66. 10.1037/0033-295X.84.1.1. [DOI] [Google Scholar]
- Vuilleumier P, & Driver J (2007). Modulation of visual processing by attention and emotion: Windows on causal interactions between human brain regions. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 362(1481), 837–855. 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, & Dolan RJ (2001). Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron, 30(3), 829–841. 10.1016/S0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin JS, … Cassady JM (1990). Neural adaptive information processing: A preliminary model of receptive-field plasticity in auditory cortex during Pavlovian conditioning. In Gabrial M, & Moore J (Eds.), Learning and computational neuroscience: Foundations of adaptive networks (pp. 91–138). Camridge MA: MIT Press. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, & Rauch SL (2001). A functional MRI study of human amygdala responses to facial expressions offear versus anger. Emotion, 1, 70–83. [DOI] [PubMed] [Google Scholar]
- Whalen PJ (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7(6), 177–188. 10.1111/1467-8721.ep10836912. [DOI] [Google Scholar]
- Wheaton MG, Fitzgerald DA, Phan KL, & Klumpp H (2014). Perceptual load modulates anterior cingulate cortex response to threat distractors in generalized social anxiety disorder. Biological Psychology, 101(1), 13–17. 10.1016/j.biopsycho.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiend J (2010). The effects of emotion on attention: A review of attentional processing of emotional information. Cognition & Emotion, 24(1), 3–47. 10.1080/02699930903205698 [Pii 916460670]. [DOI] [Google Scholar]


