Abstract
The landscape of HIV medicine dramatically changed with the advent of contemporary antiretroviral therapies, which has allowed persons with HIV (PWH) to achieve good virologic control, essentially eliminating HIV-related complications and increasing life expectancy. As PWH are living longer, noncommunicable diseases, such as cardiovascular disease (CVD), have become a leading cause of morbidity and mortality in PWH with rates that are 50% to 100% higher than in well-matched persons without HIV. In this review, we focus on disease of the coronary microvasculature and myocardium in HIV. We highlight a key hormonal system important to cardiovascular endocrinology, the renin-angiotensin-aldosterone system (RAAS), as a potential mediator of inflammatory driven-vascular and myocardial injury and consider RAAS blockade as a physiologically targeted strategy to reduce CVD in HIV.
Keywords: HIV, renin-angiotensin-aldosterone system, coronary flow reserve, myocardial dysfunction, eplerenone, sacubitril/valsartan
The landscape of HIV medicine dramatically changed with the advent of contemporary antiretroviral therapies (ART) which has allowed persons with HIV (PWH) to achieve good virologic control, essentially eliminating HIV-related complications and increasing life expectancy. As PWH are living longer, noncommunicable diseases, such as cardiovascular disease (CVD), have become a leading cause of morbidity and mortality in PWH with rates that are 50% to 100% higher than in well-matched persons without HIV (PWOH) (1,2). In this review, we focus on disease of the coronary microvasculature and myocardium in HIV. We highlight a key hormonal system important to cardiovascular endocrinology, the renin-angiotensin-aldosterone system (RAAS), as a potential mediator of inflammatory driven-vascular and myocardial injury and consider RAAS blockade as a physiologically targeted strategy to reduce CVD in HIV.
Microvascular and Myocardial Dysfunction in HIV
An estimated 38 million people were living with HIV globally in 2019. The introduction of effective ART regimens has shepherded a decline in new infections and AIDS-related deaths by 40% and 60% since their respective peaks in 1998 and 2004 (3). As lifespan increases, a paradigm shift has occurred from a reduction in deaths due to communicable illnesses to an increase in deaths mirroring causes found in the aging general population (4). CVD is increasing in prevalence in HIV, particularly with increased morbidity and mortality from atherosclerosis and heart failure (HF ) (4-6). Investigations of coronary plaque have been the primary focus of CVD in HIV, and less is known about emerging pathology related to the structure and function of the microvasculature and myocardium.
Whereas the increased risk of myocardial infarction and atherosclerotic disease is well established in PWH, the coronary microcirculation is not as well studied as its counterpart, the epicardial coronary circulation (4). Coronary microvascular dysfunction (CMD) is believed to be mediated by an interplay of factors such as abnormal vascular remodeling, chronic inflammation and endothelial dysfunction (7), and can lead to abnormalities in cardiac structure and function. Coronary flow reserve (CFR) is an integrated measure of the overall functioning of the coronary vasculature, including epicardial coronary arteries, resistance arteries, and capillaries. Indeed, impaired CFR is associated with increased mortality in studies of individuals with suspected CVD (8). In the absence of large vessel disease, reductions in CFR are thought to represent CMD. In patients without known coronary artery disease, impairments in CFR still predict cardiac mortality (9). In the general population, all-cause mortality and cardiovascular (CV) morbidity are increased in select groups with CMD, particularly those with an underlying cardiomyopathy or angina (7). Invasive (coronary vasoreactivity testing) and noninvasive [positron emission tomography (PET), magnetic resonance imaging, and doppler echocardiography] methods to evaluate CMD are used to determine CFR, the ratio of maximal to resting coronary blood flow during vasodilatation (8,10). Limited studies evaluating CMD in PWH reveal conflicting results and have not yet provided clear evidence for a mechanism for CMD (7).
Myocardial dysfunction among PWH is increased approximately 2-fold as compared to PWOH and also manifests at an earlier age, by 1 to 2 decades (11-13). Notably, left ventricular (LV) diastolic rather than systolic dysfunction is the predominating phenotype in the current era in which PWH achieve better virological control and fewer AIDS-defining illnesses (14,15). In the post-ART era, HF presenting as an overt severe dilated cardiomyopathy is becoming exceedingly rare (16). More commonly, myocardial disease manifests as subclinical systolic or diastolic dysfunction, estimated at 12.3% and 29.3%, respectively, in a recent meta-analysis (17). Modern diagnostic procedures have detected ultrastructural abnormalities of the myocardium in PWH. Elevated intramyocardial triglyceride content (18-21), inflammation and fibrosis (19,20,22) imaged by cardiac magnetic resonance (CMR) are found to be associated with diastolic dysfunction, a precursor of HF with a preserved as well as reduced ejection fraction (HFpEF and HFrEF, respectively) (18). Strain imaging by CMR or speckle tracking echocardiography highlight reduced systolic and diastolic strain patterns in predominantly asymptomatic PWH vs controls (19,20,22-25).
Our understanding of the pathophysiology of myocardial dysfunction in HIV is evolving. HF can be independent of atherosclerotic disease (12) and may be secondary to a constellation of other unique mediators. Traditional CV risk factors (hypertension, diabetes, dyslipidemia, and smoking) have been found to be increased in PWH (1,4). Causes of myocardial dysfunction specific to HIV include myocarditis directly caused by HIV-1 or other cardiotropic viruses, cardiac autoimmunity, micronutrient deficiency particularly selenium, drug toxicity, and myopericardial infections (14). Furthermore, chronic inflammation and fibrosis that persist despite virologic control and immune dysregulation appear to be intricately intertwined with the aforementioned etiologic changes (7). Chronic inflammation is hypothesized to result from gut microbial translocation, persistent HIV-1 viral reservoirs, other viral co-infections, and immune dysregulation (26,27).
Role of the RAAS in Inflammation and Heart Disease
Hormonal cross-talk of the RAAS plays an important role in the regulation of the CV system. In this regard, excess activation and/or dysfunction of the RAAS pathways may contribute to the development of CVD. RAAS hormones are intimately involved with control of blood volume and sodium balance by activating the mineralocorticoid receptor (MR) in renal epithelial tissues. Emerging data suggest that RAAS activation may also contribute to the development of vascular and cardiac injury principally through stimulatory effects on inflammation and fibrosis (28,29) (Fig. 1). Vascular dysfunction, abnormal collagen deposition, vascular and myocardial inflammation, and increased oxidative stress may result from excess MR signaling (31). Indeed, aldosterone-induced vascular injury leads to an increase in inflammatory and vascular markers, such as monocyte chemoattractant protein-1 (MCP-1), interleukin 6 (IL-6), plasminogen activator inhibitor-1, and intercellular adhesion molecule (30,32). Aldosterone-induced injury has been simulated in rodents through angiotensin II (Ang II) infusion or renin/angiotensinogen double transgenic rats (30,33). In these rodent models, reducing aldosterone activity through MR blockade or adrenalectomy effectively reduces vascular damage and myocardial inflammation. In addition, MR activation by aldosterone or deoxycorticosterone acetate promotes cardiac fibrosis and adverse remodeling of the coronary vasculature (34). Aldosterone-mediated myocardial fibrosis can increase LV hypertrophy, reduce vascular compliance, and impair diastolic dysfunction. In contrast, application of MR blockade can attenuate Ang-II stimulated changes in myocardial inflammation and necrosis and plasminogen activator inhibitor-1 expression in coronary endothelial cells (30,32). Indeed, the effects of MR blockade to improve CVD were independent of blood pressure (BP) changes (32). Overall, these data suggest aberrant RAAS signaling may promote CVD, and, in contrast, blockade of abnormal RAAS signaling could mitigate CV-related injury.
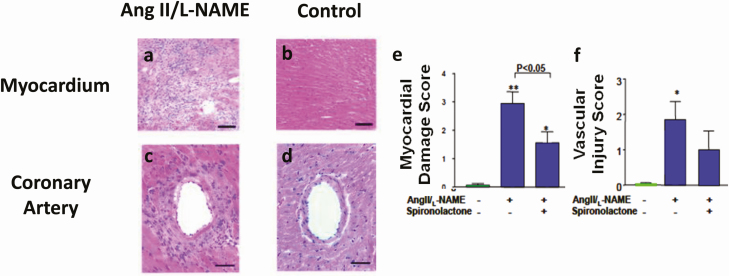
Figure 1.
Representative myocardial tissue sections stained with hematoxylin and eosin from (A) a mouse treated with Ang II/L-NAME and (B) a control mouse. Ang II/L-NAME-treated mice show areas of organizing myocardial necrosis with granulation tissue. Representative micrographs of a coronary artery section stained with hematoxylin and eosin from (C) a mouse treated with Ang II/L-NAME and (D) a control mouse. Coronary arteries from the Ang II/L-NAME-treated mouse show fibrinoid necrosis of the vessel wall with intimal thickening, a mixed inflammatory response. Effect of spironolactone on (E) myocardial damage and (F) vascular injury in mice treated with Ang II/L-NAME, Ang II/L-NAME/spironolactone (50 mg·100 g−1 day−1), or control group. *P < 0.05, **P < 0.005 vs control. Adapted from Oestreicher et al (30).
Evidence for RAAS Activation in HIV
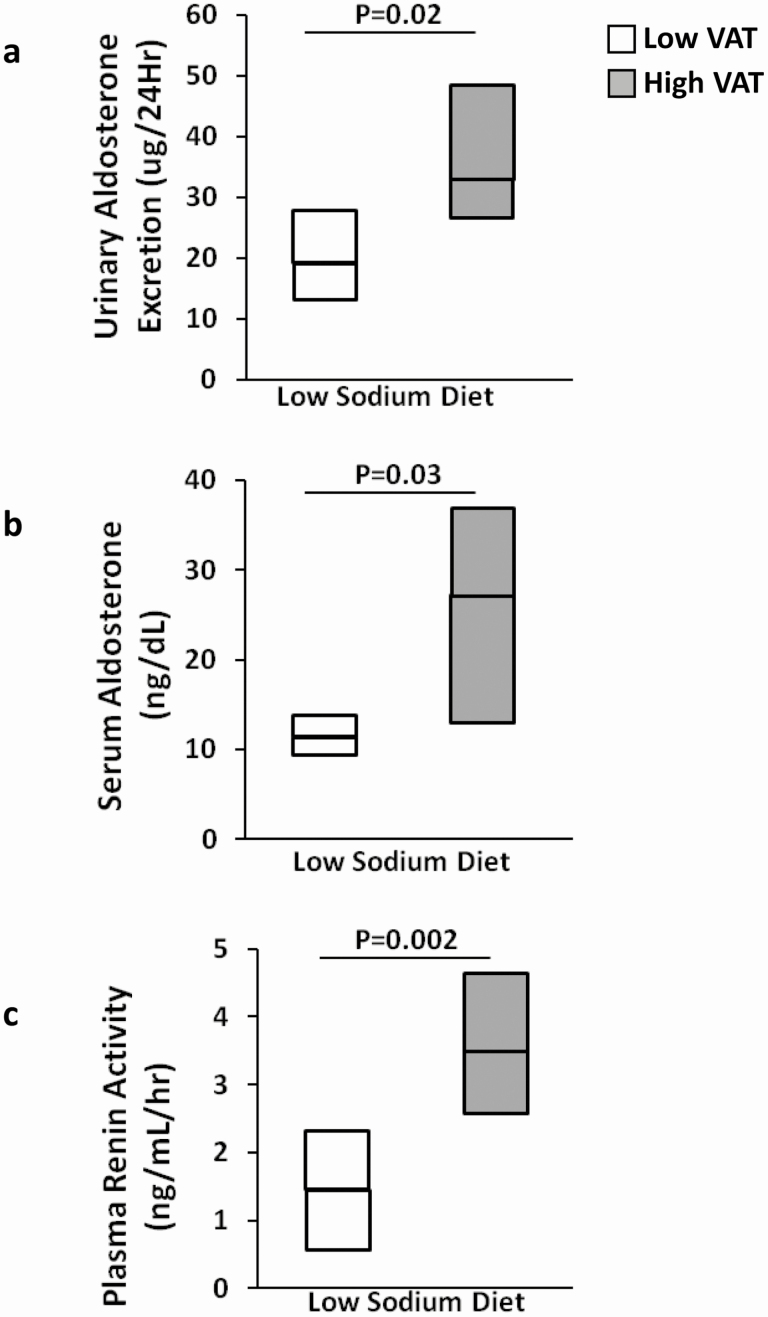
Given the role of the RAAS as a potential mediator of inflammation and CVD, it would be important to understand the physiology of this hormonal system in the HIV population, in whom chronic inflammation can lead to CVD-related sequelae. Indeed, emerging data demonstrate unique RAAS physiology among PWH and provide compelling evidence for a hormone-mediated mechanism for metabolic dysfunction in this population. Data related to the RAAS needs to be interpreted carefully in the context of other commonly used medications among PWH, which may have an effect of RAAS physiology directly, or contribute to metabolic disease themselves, such as ART, glucocorticoids, diuretics, etc. Initial studies were performed in a group of age and body mass index (BMI)-matched women with HIV; 24-h urinary aldosterone excretion (UAE) was highest among women with HIV with excess visceral adipose tissue (VAT), followed by women with HIV without excess VAT, and lowest among women without HIV (35). In addition, 24-h UAE was significantly associated with metabolic parameters, including systolic BP, BMI, total body fat, VAT, and hemoglobin A1c. Further modeling, controlling for basic demographics, ART class, and anti-hypertensive medications, showed that VAT and hemoglobin A1c both remained independently related to 24-h UAE. More detailed physiologic studies investigated RAAS activation among both men and women with HIV compared to men and women without HIV under well-controlled nutrient and posture conditions. RAAS parameters were obtained after participants were asked to lay supine and fast overnight following a 6-day standardized low-sodium diet, critical conditions to achieve precise measurements of the RAAS. Data from these studies were consistent with prior findings and demonstrated increased serum aldosterone among PWH vs PWOH (36). In addition, PWH with above-median VAT demonstrated increased 24-h UAE, serum aldosterone, and plasma renin activity compared to PWH with below-median VAT during RAAS activated conditions (Fig. 2). Upon stratification by serostatus and median VAT, a significant trend was seen, such that serum aldosterone during RAAS activated conditions was highest among PWH with above-median VAT, followed by PWH with below-median VAT, then PWOH with above-median VAT, and finally lowest among PWOH with below-median VAT. In the HIV group, RAAS parameters including plasma renin activity, Ang II, and serum aldosterone were all highly correlated with homeostatic model assessment for insulin resistance, and serum aldosterone was independently related to homeostatic model assessment for insulin resistance after controlling for age, sex, ART class, and VAT (36). These data are consistent with studies of uninfected individuals, which demonstrate a relationship of adverse body composition (such as obesity and increased BMI) to increased aldosterone (37,38). These insights are particularly relevant to HIV, a model of fat redistribution with increased visceral adiposity.
Figure 2.
Comparison of RAAS parameters (A) urinary aldosterone excretion (mcg/24 h), (B) serum aldosterone (ng/dL), and (C) plasma renin activity (ng/mL/h) during the low-sodium diet (simulating RAAS activation) among persons with HIV with low VAT vs high VAT. Adapted from Srinivasa et al (36) with permission from the Endocrine Society.
RAAS activation has also been linked to inflammation in HIV. Comparing a RAAS-activated state (simulated by controlled low sodium diet) vs RAAS-suppressed state (simulated by liberal sodium controlled diet), high-sensitivity C-reactive protein (hsCRP) and IL-6 were increased and adiponectin decreased among PWH during RAAS activation (36). Persistent and chronic immune activation is a hallmark of well-controlled HIV infection. Both generalized inflammatory markers and markers of monocyte and macrophage activation are related to increased mortality in HIV (39). Markers of monocyte and macrophage activation have traditionally related to indices of CVD, such as arterial inflammation (40) and high-risk plaque morphology (41) as well as to indices of metabolic dysregulation, including insulin resistance (42). There are data to suggest MR can be found on immunoregulatory cells (43), and thus RAAS activation could potentiate inflammatory signaling. In this regard, MCP-1 and sCD163 significantly increase with RAAS activation (simulated by a low sodium controlled diet) among PWH (44).
Parallel studies evaluating cardiac biomarkers have similarly demonstrated an adverse cardiometabolic association with RAAS activation in HIV. Studies have suggested that aldosterone-mediated effects on cardiac remodeling may be related to the secretory release of lipocalin-2 from immune type cells. Lipocalin-2 may be a target gene of the MR (45). Among PWH, lipocalcin-2 increased during RAAS activation compared to RAAS suppression. Lipocalin-2 was also related to markers of inflammation (tumor necrosis factor α, sCD163, MCP-1) and cardiac injury [B-type natriuretic peptide (BNP), N-terminal prohormone BNP) during a RAAS activated state (46).
The natriuretic peptide (NP) system has important hormonal feedback interactions with the RAAS system with suppressive effects of NP on the RAAS. PWH appear to demonstrate a relative NP deficiency, similar to the obese population, with fat accumulation potentially contributing to metabolic disease by this mechanism. A relative NP deficiency may contribute to uninhibited RAAS activation through reduced suppression of the RAAS. In contrast, in primary hyperaldosteronism, a model of autonomous aldosterone secretion, NPs are elevated in association with increased aldosterone as a protective mechanism (47,48). Under a controlled RAAS activated state, BNP was significantly lower and atrial natriuretic peptide tended to be lower among PWH vs PWOH (49). Reduced BNP was additionally associated with body composition. Stratification by overweight BMI (BMI < 25 kg/m2 or ≥25 kg/m2) demonstrated lowest BNP among PWH with BMI ≥ 25 kg/m2, with an increasing trend across the following groups, PWH with BMI < 25 kg/m2, PWOH with BMI ≥ 25 kg/m2, and highest in PWOH with BMI < 25 kg/m2 (49). These physiologic studies of increased RAAS activation, coupled with low BNP among PWH support a potential unique link between fat accumulation/visceral adiposity, inflammation, chronic immune activation, and cardiac injury in HIV.
Parallelisms of HIV and SARS-CoV2 Viral Infections
Even with the timely development of vaccines to provide immunity and reduce transmission, new-onset SARS-CoV2 infections may persist endemically, and there may be long-term metabolic implications for those already infected—a paradigm similar to HIV infection. SARS-CoV2 and HIV infections both have known interactions with a key hormonal axis, the RAAS; are hallmarked by cytokine dysregulation; and increase susceptibility to CVD. Furthermore, there may be mechanistic implications for both SARS-CoV2 and HIV on inflammatory-mediated comorbidities focused on angiotensin converting enzyme 2 (ACE2) biology (50). Viral entry of SARS-CoV2 is dependent on the ACE2 receptor, and subsequent interaction leads to internalization of ACE2 and a loss of circulating ACE2. ACE2 is thought to divert the RAAS toward an anti-inflammatory pathway by converting Ang II to Ang 1-7, thereby shunting away from aldosterone (51). In this way, states of relative ACE2 deficiency (typically associated with metabolic phenotypes) may face a higher burden on inflammatory-mediated sequalae from SARS-CoV2 (52,53).
Traditional/Non-Traditional Use of RAAS Blocking Agents in Heart Disease
Prior studies have investigated the potential impact of MR blockade on coronary microvascular function in other metabolic populations, such as patients with type 2 diabetes mellitus (DM), who demonstrate reduced CFR. In this regard, spironolactone or eplerenone added onto standard angiotensin converting enzyme inhibitor (ACEi) therapy improved global CFR when compared to hydrochlorothiazide among those with DM (54,55). A substudy of the Women’s Ischemia Syndrome Evaluation investigated ACEi among women with known nonobstructive ischemia and showed an improvement in CFR compared to placebo (56). In contrast, when eplerenone was added on to ACEi therapy in an ancillary study of the Women’s Ischemia Syndrome Evaluation cohort, there was no additional improvement (57). When compared to a calcium channel blocker, losartan use was shown to improve CFR among hypertensive patients (58). In addition, augmentation of coronary blood flow was more effective in patients treated with lisinopril vs losartan in hypertensive patients with LV hypertrophy (59).
Large randomized controlled trials investigating spironolactone (RALES) and eplerenone (EMPHASIS-HF) have been completed and provide strong evidence for the addition of MR blockers to traditional therapies to improve CV morbidity and mortality risk in HFrEF (60,61). The addition of an MR blocker in HFrEF results in a 30% relative reduction in the risk of death. The TOPCAT study was a large international randomized clinical trial assessing spironolactone in HFpEF (62). Although there was no overall reduction in CV morbidity and mortality, a subanalysis limited to the Americas showed compelling CV benefits (63). The Aldo-DHF investigation demonstrated that spironolactone improved other cardiac endpoints, such as diastolic dysfunction and remodeling of the left ventricle, among those with HFpEF (64). Sacubitril/valsartan is a newer combination medication with dual angiotensin receptor blockade and neprilysin inhibition and has been shown to decrease CV and all-cause mortality in HFrEF when compared to ACEi in the PARADIGM-HF trial (65). There was a higher incidence of hypotension and lower incidence of hyperkalemia and renal dysfunction in the sacubitril/valsartan treated group. Recent investigations of sacubitril/valsartan in a HFpEF population in the PARAGON-HF trial did not show a significant reduction in CV mortality when compared to angiotensin II receptor blocker (ARB) alone (66). In general, ACEi or ARB use alone have not shown CV mortality benefit in HFpEF (67-69).
There may be even broader utility of MR blockade in HIV, related to key mediators of CV pathology—vascular dysfunction, inflammation, and fibrosis. Clinically, MR antagonists are primarily used in treatment of HFrEF, resistant hypertension, and primary aldosteronism. If MR blockade were shown to improve coronary vascular function and treatment of myocardial dysfunction in HIV, these findings would extend the use of a clinically accepted therapy. Data from the PARADIGM-HF and PARAGON-HF studies did not specifically assess those with HIV, and this may be a particular subgroup that would benefit from sacubitril/valsartan as a cardioprotecive agent as well.
Evidence of RAAS and MR Blockade in HIV
Limited preclinical data suggest additional utility of RAAS blocking medications through HIV-specific mechanisms. It has been postulated that the HIV protease may have similar properties to renin and have the ability to produce angiotensin I (70,71), which could lead to increased aldosterone. Moreover, adipocytes incubated with ritonavir boosted protease inhibitors (lopinavir or atazanavir) overexpress Ang II type 1 receptor protein and angiotensin messenger RNA and amplify Ang II signaling (72). Subsequent application of ARBs (irbesartan or telmisartan) to adipocytes prevented protease inhibitor–induced RAAS activation, as well as adipocyte dedifferentiation and other adverse sequelae including insulin resistance, inflammation, and oxidative stress. Irbesartan has a similar mechanism of action to telmisartan, augmented by partial peroxisome proliferation-activated receptor gamma activity (73). Spironolactone prevents HIV-1 and HIV-2 infection of both Jurkat and primary CD4+ T cell lines, specifically through inhibition of Tat-dependent transcription (74). In addition, MR activation upregulates SGK1 expression, which boosts Th17 differentiation, a subset of T cells that influences viral persistence in HIV (75). Parallel to this, MR blockade can reduce T helper 17 upregulation (76). Taken together, these data suggest dual viral and ART-mediated effects on the RAAS. Data on more contemporary ART are lacking.
Metabolic patterns differ with respect to specific medications affecting the RAAS. ACEi are not engaged in significant CYP450-mediated interactions, whereas ARBs and MR blockers are involved with some CYP450 mediated interactions. Eplerenone is metabolized primarily by CYP3A4, and CYP3A inhibitors will increase drug concentrations. Of the ARBs, telmisartan and valsartan are notably not metabolized by the CYP450 system. Bioactivation of losartan is dependent on CYP2C9, and some nonnucleoside reverse transcriptase inhibitors (eg, efavirenz or etravirine) inhibit the CYP2C9 enzyme and could result in reduced efficacy of losartan. Losartan is also metabolized by CYP3A4, and protease inhibitors are known to inhibit the CYP3A4 enzyme (77).
Medications acting along RAAS pathway have recently been assessed in the HIV population (Table 1). Some data are available investigating ARB and MR blockade to modify metabolic disease in HIV. Use of telmisartan, a combination ARB and partial PPAR γ agonist, was initially reported in a PWH with insulin-dependent type 2 DM to evaluate its action as an antihypertensive. The patient’s unexpected clinical course included hypoglycemic episodes prompting the discontinuation of insulin, which raised the question as to whether the potential insulin-sensitizing effect of the telmisartan could be useful among PWH who are prone to multiple metabolic disorders (84). An observational study followed, evaluating the effect of telmisartan on metabolic outcomes in HIV. Results demonstrated an expected decrease in systolic BP and diastolic BP, as well as reductions in insulin resistance, lipids, endothelin-1, and interleukin 18 during the course of the 6-month study (85).
Table 1.
Completed and active clinical trials investigating medications acting along the RAAS pathway in persons with HIV
| Study name | NCT number | Class | Treatment arms | Clinical indication | Primary endpoint | Location | Status/ publication |
|---|---|---|---|---|---|---|---|
| ACE Inhibitors to Decrease Lymphoid Fibrosis in Antiretroviral-Treated, HIV Infected Patients: A Pilot Study | 01535235 | Angiotensin converting enzyme inhibitor | Lisinopril Placebo |
HIV infection | Change in HIV RNA measured in gut-associated lymphoid tissue | San Francisco General Hospital, San Francisco, CA, USA | Completed |
| Cardiovascular Prevention for Persons With HIV | 00982189 | Angiotensin-converting enzyme inhibitor | Lisinopril Pravastatin |
HIV infection Cardiovascular disease risk | Number participants with self-reported side effects Number participants >90% pill count compliance Change in Framingham risk score |
Hennepin County Medical Center, Minneapolis, MN, USA | Completed Baker et al, 2012 (78) |
| Optimal Management of HIV Infected Adults at Risk for Kidney Complications in Nigeria | 03201939 | Angiotensin-converting enzyme inhibitor | Lisinopril Placebo |
HIV infection/AIDS Albuminuria Kidney diseases Genetic predisposition |
Reduction/improvement in degree/grade of albuminuria Progression/worsening in degree/grade of albuminuria Mean change in urinary albumin excretion |
Aminu Kano Teaching Hospital, Kano, Nigeria | Not yet recruiting |
| Effects of Losartan and Antiretroviral Regimen Containing Raltegravir in Fibrosis Inflammation Mediators, Cardiovascular Risk and Neurocognitive Disorders in HIV Infected Patients Previously Effectively Treated | 01529749 | Angiotensin II receptor blocker | EFV/FTC/TDF+ losartan EFV/FTC/TDF FTC/TDF + MK-0518 FTC/TDF+ MK-0518 + losartan |
HIV infection | Proportion of patients with 50% reduction of fibrosis in lymphatic tissue | Hospital Clinic, Barcelona, Spain | Completed |
| Losartan to Reduce Inflammation and Fibrosis Endpoints in HIV Trial (LIFE HIV) | 02049307 | Angiotensin II receptor blocker | Losartan Placebo |
HIV infection Inflammation Fibrosis |
Change in IL-6 plasma levels | UCSF, San Francisco, CA, USA NIH Clinical Center, Bethesda, MD, USA Allina Health, Minneapolis, MN, USA Hennepin County Medical Center, Minneapolis, MN, USA |
Completed |
| Reversing Tissue Fibrosis to Improve Immune Reconstitution in HIV | 01852942 | Angiotensin II receptor blocker | Losartan Placebo |
HIV infection | The impact of losartan treatment on lymphoid tissue fibrosis | University of Minnesota, Division of Infectious Diseases, MN, USA | Completed |
| Effects of Sacubitril/Valsartan on Subclinical Heart Failure in HIV (The ENCHANTMENT HIV Study) | 04153136 | Angiotensin II receptor blocker | Sacubitril-valsartan Placebo |
HIV/AIDS Subclinical heart failure with preserved ejection fraction |
Myocardial inflammation/fibrosis Left atrial volume index |
Massachusetts General Hospital, Boston, MA, USA | Recruiting |
| Analysis of Telmisartan Administered With Antiretroviral Therapy (ART) in Patients With Acute HIV Infection | 02170246 | Angiotensin II receptor blocker | Telmisartan + ART ART alone |
Acute HIV infection | Change in level of neopterin in the cerebrospinal fluid | Chulalongkorn University Hospital, Bangkok, Thailand Thai Red Cross AIDS Research Centre, Bangkok, Thailand | Completed |
| A Clinical Trial for People HIV + Age > 50 at Risk for Heart Disease | 01578772 | Angiotensin II receptor blocker | Telmisartan | HIV infection Endothelial dysfunction |
Change in diameter, flow-mediated dilatation (FMD) and maximum relative FMD of the Brachial Artery | UCLA CARE Center, Los Angeles, CA, USA | Completed Lake et al, 2016 (79) |
| HIV and Fat Accumulation (MATH) | 01088295 | Angiotensin II receptor blocker | Telmisartan | HIV infection | Median change in VAT volume | UCLA CARE Center, Los Angeles, CA, USA | Completed Lake et al, 2013 (80) |
| Telmisartan to Reduce AIDS-Related Fibrotic and Inflammatory Contributors (TRAFIC Study) | 01928927 | Angiotensin II receptor blocker | Telmisartan | HIV infection | Change in collagen I deposition on lymph node and subcutaneous abdominal adipose tissue pathology | AIDS Clinical Trials Group Sites (12) including UCLA CARE Center CRS (601), Los Angeles, CA, USA | Completed Utay et al, 2018 (81) |
| Using Telmisartan With ART During Acute HIV Infection to Reduce the CNS Reservoirs of HIV and Lymph Node Fibrosis | 02750059 | Angiotensin II receptor blocker | TDF/3TC/ EFV + Telmisartan TDF/3TC/ EFV |
Acute HIV infection HIV CNS involvement |
Difference in CSF neopterin | SEARCH Thailand Bangkok, Thailand | Completed |
| Effects of Eplerenone on Cardiovascular Disease in HIV (MIRACLE HIV Study) | 02740179 | Mineralocorticoid receptor antagonist | Eplerenone Placebo |
HIV infection | Coronary flow reserve Myocardial inflammation |
Massachusetts General Hospital, Boston, MA, USA | Recruiting |
| Eplerenone in HIV Associated Abdominal Fat Accumulation | 01405456 | Mineralocorticoid receptor antagonist | Eplerenone Placebo |
HIV infection | Insulin stimulated glucose uptake | Massachusetts General Hospital, Boston, MA, USA | Completed Srinivasa et al, 2018 (82) |
| Evaluating Drug Interactions Between Doravirine With Estradiol and Spironolactone in Healthy Transgender Women | 04283656 | Mineralocorticoid receptor antagonist | Doravirine/Lamivudine/tenofovir Spironolactone Estradiol Placebo |
HIV infection Transgender women Gender dysphoria |
Doravirine, tenofovir and estradiol plasma concentration area under the curve, maximum and trough concentrations | Clinical Research Unit at Thomas Jefferson University, Philadelphia, PA, USA | Recruiting |
| Gender-Affirmative Transgender Care to Improve the HIV Treatment Cascade | 03757117 | Mineralocorticoid receptor antagonist | Estradiol valerate Spironolactone |
HIV infection | HIV viral suppression Knowledge of HIV serostatus |
Epicentro, Lima, Peru | Completed Lama et al, 2019 (83) |
| Transgender Youth and PrEP: PK, Safety, Uptake & Adherence - PK Study | 03652623 | Mineralocorticoid receptor antagonist | TDF/FTC + estradiol +/− spironolactone or testosterone | HIV infection/AIDS Transgender men and women | Change in tenofovir, estradiol and testosterone levels | University of Colorado-Denver, Aurora, CO, USA | Recruiting |
Abbreviations: CNS, central nervous system; EFV, efavirenz; FMD, flow-mediated dilatation; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate.
More recent investigations of telmisartan include an open-label study of PWH over 24 weeks in the MATH trial. Prior data among PWOH demonstrated some beneficial effects on VAT reduction and improvement in inflammatory and metabolic indices (86,87), suggesting that this agent could have a significant clinical impact on the HIV population. Results from the MATH trial demonstrated modest decreases in subcutaneous fat (median −2.7%) as well as waist circumference and waist-to-hip ratio (80). A follow-up study using a high dose of telmisartan among PWH with history of ≥1 traditional CVD risk factors demonstrated significant improvements of BP and a treatment effect on flow mediated vasodilation (88). Additional pilot data highlight an increase in vascular reparative capacity through quantification of endothelial progenitor cells after telmisartan use for 12 weeks among PWH (79). Further exploration of telmisartan among PWH showed neutral effects on lymph node and adipose tissue fibrosis in a 48-week randomized controlled trial (81).
Further studies have investigated other agents to modulate the RAAS in PWH. One study utilized a 2 × 2 factor feasibility study with randomization to low-dose lisinopril and/or pravastatin or matching placebos among well-treated PWH selected for Framingham Risk Score ≥3% to assess the effects of these medications to lower BP, lipids, and inflammatory parameters. Overall, lisinopril-treated patients demonstrated BP and inflammatory lowering effects, with a significant reduction in diastolic BP (−3.3 mmHg), hsCRP, and tumor necrosis factor α. In contrast, the study did not show lipid lowering effects of lisinopril, even in combination with pravastatin (78). While few side effects were reported in the lisinopril arm, reduced pill adherence was noted.
In the first randomized clinical trial of MR blockade in HIV, 46 PWH with increased waist circumference and abnormal glucose homeostasis were treated with either eplerenone or placebo for 6 months. While there was no benefit of MR blockade on the primary endpoint, insulin sensitivity as measured by euglycemic hyperinsulinemic clamp, there were favorable effects of eplerenone to reduce MCP-1 and intramyocellular lipid and to increase high-density lipoprotein (82). Interestingly, preclinical data show that MCP-1 is elevated 7-fold among obese db/db mice, and treatment with eplerenone reduces MCP-1 levels to that of healthy lean mice (89). These animal data and the human physiology/treatment studies of HIV are complementary, both showing that MCP-1 is elevated in metabolic phenotypes and can be dampened with MR blockade (44,82,89) (Fig. 3). Among PWH in the aforementioned clinical trial, the eplerenone-treated group also tended to have decreases in IL-6 and hsCRP. These data suggest that eplerenone may have some anti-inflammatory properties and may modulate ectopic fat accumulation in the HIV population predisposed to metabolic dysregulation. In addition, these data also provide some initial evidence that there may be broader applications of MR blockade to improve CVD indices in PWH. Taken together, these initial studies of RAAS medications have demonstrated safety and tolerability among PWH and generated enthusiasm for the continued explorations of these medications on inflammatory-mediated pathways of CVD. As there are no currently US Food and Drug Administration–approved treatment strategies to reduce CVD risk in HIV, manipulation of the RAAS system offers significant promise, and more detailed studies are needed to assess the full potential of ACEi, ARBs, and MR blockers in HIV.
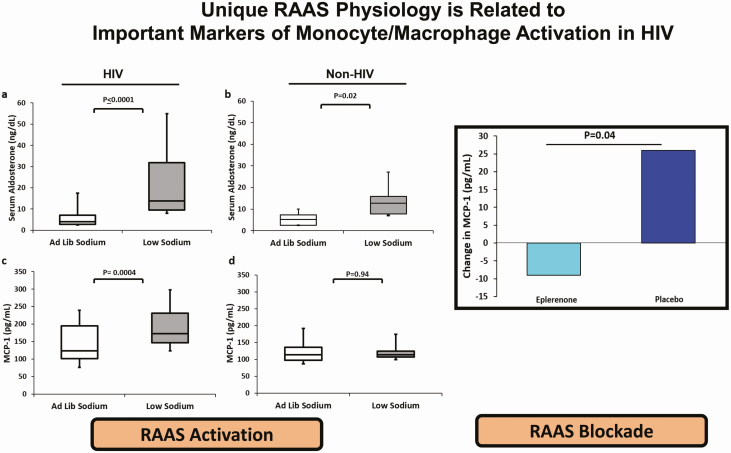
Figure 3.
Left figure: Study of RAAS activation. Comparison of the serum aldosterone (ng/dL) and MCP-1 (pg/mL), marker of immune activation during ad libitum and low-sodium diets in persons with (A and C) and persons without (B and D) HIV. Box plot represents the 25th and 75th percentiles, and lines within the boxes represents medians. Adapted from Srinivasa et al (44). Right figure: Study of RAAS blockade. Absolute between-group change of MCP-1 (pg/mL) after 6 months treatment of eplerenone vs placebo among PWH. Adapted from Srinivasa et al (82) with permission from the Endocrine Society.
While this review has predominantly focused on the interplay between the RAAS and microvascular and myocardial dysfunction in HIV, there may also be similar implications of the RAAS on kidney disease in HIV. PWH are at risk for chronic kidney disease, the most prevalent type characterized as HIV-associated nephropathy (HIVAN) which could be exacerbated by RAAS activation (90,91). In addition, tenofovir disoproxil fumarate, a type of nucleoside reverse transcriptase inhibitor, has been implicated in renal disease in association with upregulation of Ang II in murine models (92). Preclinical studies of HIVAN have suggest that RAAS blockade could attenuate podocyte injury and glomerular scarring (93) and provide mortality benefit (94). Few human studies have evaluated the RAAS in HIVAN and have demonstrated potential benefit with use of RAAS blockade (95,96). Use of captopril and fosinopril have been associated with improved renal survival among those with biopsy-proven HIVAN (97,98). Treatment with ACEi also been linked to reduced proteinuria in HIVAN (99), and in general, use of telmisartan among PWH with mild hypertensive disorder reduced microalbuminuria (100).
Other Therapeutics Strategies (Non-RAAS) for Microvascular and Myocardial Dysfunction in HIV
In general, consistent use of ART to reduce viral load and improve immune function leads to improved CVD rates (101). However, CVD rates remain increased even among well-treated PWH, suggesting the need for supplementary strategies specifically targeting coronary microvascular and myocardial dysfunction and other aspects of CVD in HIV. To date, limited studies with small sample size and inconsistent endpoints have been initiated to treat CMD in PWH (102). Lifestyle modifications should be encouraged; smoking cessation and weight loss are shown to improve endothelial function, and cardiometabolic comorbidities should be addressed (103). Given the lack of HIV-specific data, it is recommended that these strategies be used in accordance with approved consensus guidelines for the general population (14).
To date, there are no approved drugs targeting CFR or HFpEF in PWH (104). Statins show promise in improving endothelial function and inflammation (4,10,102,103, 105,106) while beta blockers improve symptoms and delay progression of systolic dysfunction (10,14,103). Use of metformin is compelling in PWH with diabetes, and perhaps those without diabetes, for its pleiotropic effects, including reduction in inflammation and HIV-1 reservoirs, improvement of endothelial function, and modulation of gut dysbiosis (4,10,107,108). Advanced HF should be treated with transplant and LV assist devices; recent studies report similar outcomes to PWOH (14). Conflicting evidence exists for L-arginine and ranolazine in improving CFR (10,105,109). Nitrates play no role in improving CMD and may be harmful (110). Aspirin has not been conclusively shown to reduce inflammation, and its use as primary prevention should adhere to published guidelines (106). A number of experimental agents have not been shown to improve outcomes pertaining to CMD and myocardial dysfunction, including pentoxifylline (111), pioglitazone (112), methotrexate (113), and canakinumab (107).
Future Directions
The role of the RAAS in CVD is well-recognized in the general population. Moreover, there are compelling data to suggest that RAAS activation may play a broad role in inflammation and metabolic dysfunction, which may be particularly deleterious in PWH (Fig. 4). In this regard, strategies are now being investigated to target specific RAAS activation pathways and improve microvascular and cardiac function indices of CVD in HIV. The MIRACLE HIV (MIneralocorticoid Receptor Antagonism for CardiovascuLar HEalth in HIV, #NCT02740179) study is a 12-month clinical trial investigating the effects of eplerenone vs. placebo on coronary flow reserve using 13N coronary PET, myocardial extracellular volume using CMR, and coronary atherosclerosis using coronary computed tomography angiography in HIV. The MIRABELLA HIV (MIneralocorticoid Receptor Antagonism By EpLerenone to Lower Arterial Inflammation in HIV) study is a substudy of the MIRACLE HIV study and separately will evaluate the effects of eplerenone on arterial inflammation using 18fluoro-2-deoxy-d-glucose-PET/computed tomography. Leveraging interactions between the RAAS and natriuretic peptide systems, the ENCHANTMENT HIV (ENding subClinical Heart failure using an Aldosterone and Natriuretic peptide Targeted treatMENT in HIV, #NCT04153136) study is a 6-month clinical trial evaluating the effects of sacubitril/valsartan on subclinical myocardial dysfunction in HIV using CMR and cardiac transthoracic echocardiography.
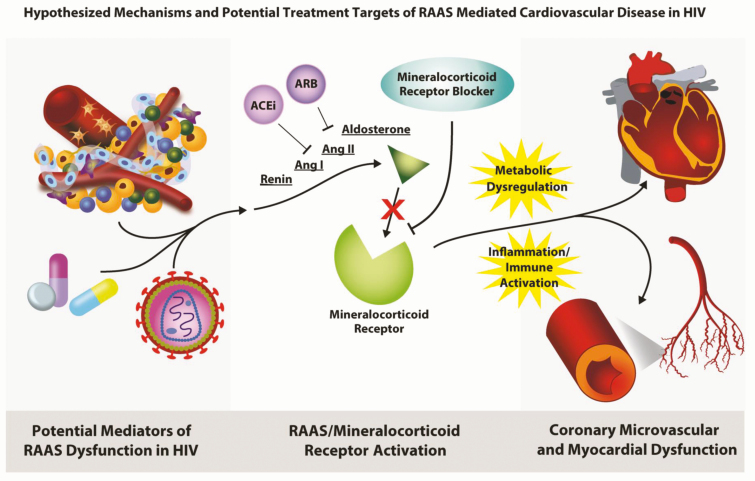
Figure 4.
Hypothesized mechanisms and potential treatment targets of RAAS-mediated cardiovascular disease in HIV. Potential mediators of RAAS dysfunction may include direct viral effects, antiretroviral therapy effects, or the adipose depot. Increased RAAS activation activates the mineralocorticoid receptor, which may potentiate metabolic dysregulation, increased inflammation, and immune activation. Adverse cardiovascular sequalae of metabolic disease and inflammation may include coronary microvascular and myocardial dysfunction. US Food and Drug Administration–approved medications acting along the RAAS pathway, such as ACEi, ARB, and mineralocorticoid receptor blockers, are physiologically based treatment targets, which may be useful for CVD risk reduction based on strong data that RAAS blockade may reduce BP, decrease inflammation, and improve metabolic indices in PWH.
Conclusion
Well-treated PWH are at increased risk for cardiovascular complications of the coronaries and the myocardium. Compelling, physiologic-based evidence suggests RAAS activation among PWH with metabolic abnormalities, including increased visceral adiposity and insulin resistance, and that this unique physiology may contribute to a metabolic phenotype that is pro-inflammatory and profibrotic and prone to cardiac injury. Studies currently underway will advance our knowledge of RAAS blockade as a treatment strategy in HIV, a population for whom no tailored strategies as of yet exist to reduce CVD risk. Medications acting along the RAAS pathway could offer a biologically targeted approach for CVD risk reduction based on strong data that RAAS blockade may reduce BP, decrease inflammation, and improve metabolic indices in PWH.
Acknowledgments
The authors would like to thank Tom DiCesare for his technical assistance with the graphic design in Fig. 4.
Financial Support: Funding was provided by National Institutes of Health (NIH) R01 DK49302 to SKG; NIH K23 HL136262, NIH R01 HL151293 to SS; NIH K24 HL103845 to GKA; and NIH P30 DK040561, Nutrition and Obesity Research Center at Harvard. Funding sources had no role in the design of the study, data analysis, or writing of the manuscript.
Additional Information
Disclosures: TST and MNF have nothing to declare. SS was the recipient of a Gilead Sciences Research Scholars award. GKA has received consulting fees from Pfizer. SKG has received research funding from KOWA, Gilead/ViiV, and Theratechnologies and received consulting fees from Theratechnologies and ViiV. All disclosures are unrelated to this manuscript.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freiberg MS, Chang CC, Kuller LH, et al. . HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. Global HIV & AIDS statistics: 2020 fact sheet. 2020. https://www.unaids.org/en/resources/fact-sheet. Accessed October 14, 2020. [Google Scholar]
- 4. Sinha A, Feinstein M. Epidemiology, pathophysiology, and prevention of heart failure in people with HIV. Prog Cardiovasc Dis. 2020;63(2):134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alvi RM, Afshar M, Neilan AM, et al. . Heart failure and adverse heart failure outcomes among persons living with HIV in a US tertiary medical center. Am Heart J. 2019;210:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feinstein MJ, Bahiru E, Achenbach C, et al. . Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117(2):214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rethy L, Feinstein MJ, Sinha A, Achenbach C, Shah SJ. Coronary microvascular dysfunction in HIV: a review. J Am Heart Assoc. 2020;9(1):e014018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gdowski MA, Murthy VL, Doering M, Monroy-Gonzalez AG, Slart R, Brown DL. Association of isolated coronary microvascular dysfunction with mortality and major adverse cardiac events: a systematic review and meta-analysis of aggregate data. J Am Heart Assoc. 2020;9(9):e014954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murthy VL, Naya M, Foster CR, et al. . Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126(15):1858-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen C, Wei J, AlBadri A, Zarrini P, Bairey Merz CN. Coronary microvascular dysfunction: epidemiology, pathogenesis, prognosis, diagnosis, risk factors and therapy. Circ J. 2016;81(1):3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaplan RC, Hanna DB, Kizer JR. Recent insights into cardiovascular disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep. 2016;13(1):44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freiberg MS, Chang CH, Skanderson M, et al. . Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the veterans aging cohort study. JAMA Cardiol. 2017;2(5):536-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feinstein MJ, Steverson AB, Ning H, et al. . Adjudicated heart failure in HIV-infected and uninfected men and women. J Am Heart Assoc. 2018;7(21):e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lumsden RH, Bloomfield GS. The causes of HIV-associated cardiomyopathy: a tale of two worlds. Biomed Res Int. 2016;2016:8196560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cerrato E, D’Ascenzo F, Biondi-Zoccai G, et al. . Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34(19):1432-1436. [DOI] [PubMed] [Google Scholar]
- 16. Laurence J, Elhadad S, Ahamed J. HIV-associated cardiovascular disease: importance of platelet activation and cardiac fibrosis in the setting of specific antiretroviral therapies. Open Heart. 2018;5(2):e000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erqou S, Lodebo BT, Masri A, et al. . Cardiac dysfunction among people living with HIV: a systematic review and meta-analysis. JACC Heart Fail. 2019;7(2):98-108. [DOI] [PubMed] [Google Scholar]
- 18. Neilan TG, Nguyen KL, Zaha VG, et al. . Myocardial steatosis among antiretroviral therapy-treated people with human immunodeficiency virus participating in the REPRIEVE trial. J Infect Dis. 2020;222(Suppl 1):S63-S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holloway CJ, Ntusi N, Suttie J, et al. . Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128(8):814-822. [DOI] [PubMed] [Google Scholar]
- 20. Thiara DK, Liu CY, Raman F, et al. . Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV-infected adults. J Infect Dis. 2015;212(10):1544-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei J, Nelson MD, Szczepaniak EW, et al. . Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol. 2016;310(1):H14-H19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luetkens JA, Doerner J, Schwarze-Zander C, et al. . Cardiac magnetic resonance reveals signs of subclinical myocardial inflammation in asymptomatic HIV-infected patients. Circ Cardiovasc Imaging. 2016;9(3):e004091. [DOI] [PubMed] [Google Scholar]
- 23. Sims A, Frank L, Cross R, et al. . Abnormal cardiac strain in children and young adults with HIV acquired in early life. J Am Soc Echocardiogr. 2012;25(7):741-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cetin S, Gündüz A, Şabablı Çetin A, et al. . Evaluation of subtle left ventricular systolic dysfunction by longitudinal systolic strain in patients with human immunodeficiency virus. Acta Cardiol Sin. 2018;34(4):321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodrigues RC, Azevedo KML, Moscavitch SD, Setubal S, Mesquita CT. The use of two-dimensional strain measured by speckle tracking in the identification of incipient ventricular dysfunction in HIV-infected patients on antiretroviral therapy, untreated HIV patients and healthy controls. Arq Bras Cardiol. 2019;113(4):737-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Butler J, Kalogeropoulos AP, Anstrom KJ, et al. . Diastolic dysfunction in individuals with human immunodeficiency virus infection: literature review, rationale and design of the characterizing heart function on antiretroviral therapy (CHART) study. J Card Fail. 2018;24(4):255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(Suppl 3):S375-S382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caprio M, Newfell BG, la Sala A, et al. . Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102(11):1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rocha R, Stier CT Jr, Kifor I, et al. . Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141(10):3871-3878. [DOI] [PubMed] [Google Scholar]
- 30. Oestreicher EM, Martinez-Vasquez D, Stone JR, et al. . Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-L-arginine methyl ester-induced myocardial injury. Circulation. 2003;108(20):2517-2523. [DOI] [PubMed] [Google Scholar]
- 31. Markowitz M, Messineo F, Coplan NL. Aldosterone receptor antagonists in cardiovascular disease: a review of the recent literature and insight into potential future indications. Clin Cardiol. 2012;35(10):605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rocha R, Rudolph AE, Frierdich GE, et al. . Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283(5):H1802-H1810. [DOI] [PubMed] [Google Scholar]
- 33. Fiebeler A, Schmidt F, Müller DN, et al. . Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension. 2001;37(2 Pt 2):787-793. [DOI] [PubMed] [Google Scholar]
- 34. Young M, Head G, Funder J. Determinants of cardiac fibrosis in experimental hypermineralocorticoid states. Am J Physiol. 1995;269(4 Pt 1):E657-E662. [DOI] [PubMed] [Google Scholar]
- 35. Lo J, Looby SE, Wei J, Adler GK, Grinspoon SK. Increased aldosterone among HIV-infected women with visceral fat accumulation. AIDS. 2009;23(17):2366-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Srinivasa S, Fitch KV, Wong K, et al. . RAAS activation is associated with visceral adiposity and insulin resistance among HIV-infected patients. J Clin Endocrinol Metab. 2015;100(8):2873-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bentley-Lewis R, Adler GK, Perlstein T, et al. . Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92(11):4472-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7(4):355-362. [DOI] [PubMed] [Google Scholar]
- 39. Hunt PW, Sinclair E, Rodriguez B, et al. . Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210(8):1228-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Subramanian S, Tawakol A, Burdo TH, et al. . Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fitch KV, Srinivasa S, Abbara S, et al. . Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208(11):1737-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shikuma CM, Chow DC, Gangcuangco LM, et al. . Monocytes expand with immune dysregulation and is associated with insulin resistance in older individuals with chronic HIV. PLoS One. 2014;9(2):e90330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bene NC, Alcaide P, Wortis HH, Jaffe IZ. Mineralocorticoid receptors in immune cells: emerging role in cardiovascular disease. Steroids. 2014;91:38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Srinivasa S, Burdo TH, Williams KC, et al. . Effects of sodium restriction on activation of the renin-angiotensin-aldosterone system and immune indices during HIV infection. J Infect Dis. 2016;214(9):1336-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Latouche C, El Moghrabi S, Messaoudi S, et al. . Neutrophil gelatinase-associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension. 2012;59(5):966-972. [DOI] [PubMed] [Google Scholar]
- 46. Bogorodskaya M, Fitch KV, Burdo TH, et al. . Serum lipocalin 2 (neutrophil gelatinase-associated lipocalin) in relation to biomarkers of inflammation and cardiac stretch during activation of the renin-angiotensin-aldosterone system in human immunodeficiency virus. J Infect Dis. 2019;220(9):1420-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kelly TM, Nelson DH. Sodium excretion and atrial natriuretic peptide levels during mineralocorticoid administration: a mechanism for the escape from hyperaldosteronism. Endocr Res. 1987;13(4):363-383. [DOI] [PubMed] [Google Scholar]
- 48. Hu W, Zhou PH, Zhang XB, Xu CG, Wang W. Pathophysiological functions of adrenomedullin and natriuretic peptides in patients with primary aldosteronism. Endocrine. 2015;48(2):661-668. [DOI] [PubMed] [Google Scholar]
- 49. Murphy CA, Fitch KV, Feldpausch M, et al. . Excessive adiposity and metabolic dysfunction relate to reduced natriuretic peptide during RAAS activation in HIV. J Clin Endocrinol Metab. 2018;103(4):1558-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuba K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6(3):271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383(Pt 1):45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen D, Li X, Song Q, et al. . Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3(6):e2011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomas MC, Pickering RJ, Tsorotes D, et al. . Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res. 2010;107(7):888-897. [DOI] [PubMed] [Google Scholar]
- 54. Garg R, Rao AD, Baimas-George M, et al. . Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes. 2015;64(1):236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Joffe HV, Kwong RY, Gerhard-Herman MD, Rice C, Feldman K, Adler GK. Beneficial effects of eplerenone versus hydrochlorothiazide on coronary circulatory function in patients with diabetes mellitus. J Clin Endocrinol Metab. 2007;92(7):2552-2558. [DOI] [PubMed] [Google Scholar]
- 56. Pauly DF, Johnson BD, Anderson RD, et al. . In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162(4):678-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bavry AA, Handberg EM, Huo T, et al. . Aldosterone inhibition and coronary endothelial function in women without obstructive coronary artery disease: an ancillary study of the national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation. Am Heart J. 2014;167(6):826-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kamezaki F, Tasaki H, Yamashita K, et al. . Angiotensin receptor blocker improves coronary flow velocity reserve in hypertensive patients: comparison with calcium channel blocker. Hypertens Res. 2007;30(8):699-706. [DOI] [PubMed] [Google Scholar]
- 59. Akinboboye OO, Chou RL, Bergmann SR. Augmentation of myocardial blood flow in hypertensive heart disease by angiotensin antagonists: a comparison of lisinopril and losartan. J Am Coll Cardiol. 2002;40(4):703-709. [DOI] [PubMed] [Google Scholar]
- 60. Pitt B, Zannad F, Remme WJ, et al. . Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709-717. [DOI] [PubMed] [Google Scholar]
- 61. Zannad F, McMurray JJ, Krum H, et al. ; EMPHASIS-HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11-21. [DOI] [PubMed] [Google Scholar]
- 62. Pitt B, Pfeffer MA, Assmann SF, et al. ; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392. [DOI] [PubMed] [Google Scholar]
- 63. Pfeffer MA, Claggett B, Assmann SF, et al. . Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation. 2015;131(1):34-42. [DOI] [PubMed] [Google Scholar]
- 64. Edelmann F, Wachter R, Schmidt AG, et al. ; Aldo-DHF Investigators . Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791. [DOI] [PubMed] [Google Scholar]
- 65. McMurray JJ, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. [DOI] [PubMed] [Google Scholar]
- 66. Solomon SD, McMurray JJV, Anand IS, et al. ; PARAGON-HF Investigators and Committees . Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609-1620. [DOI] [PubMed] [Google Scholar]
- 67. Yusuf S, Pfeffer MA, Swedberg K, et al. ; CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777-781. [DOI] [PubMed] [Google Scholar]
- 68. Massie BM, Carson PE, McMurray JJ, et al. ; I-PRESERVE Investigators . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456-2467. [DOI] [PubMed] [Google Scholar]
- 69. Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J; PEP-CHF Investigators . The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338-2345. [DOI] [PubMed] [Google Scholar]
- 70. Goldberg B, Stricker RB. HIV protease and the pathogenesis of AIDS. Res Virol. 1996;147(6):375-379. [DOI] [PubMed] [Google Scholar]
- 71. Sharma SK, Evans DB, Hui JO, Heinrikson RL. Could angiotensin I be produced from a renin substrate by the HIV-1 protease? Anal Biochem. 1991;198(2):363-367. [DOI] [PubMed] [Google Scholar]
- 72. Boccara F, Auclair M, Cohen A, et al. . HIV protease inhibitors activate the adipocyte renin angiotensin system. Antivir Ther. 2010;15(3):363-375. [DOI] [PubMed] [Google Scholar]
- 73. Di Filippo C, Lampa E, Tufariello E, et al. . Effects of irbesartan on the growth and differentiation of adipocytes in obese Zucker rats. Obes Res. 2005;13(11):1909-1914. [DOI] [PubMed] [Google Scholar]
- 74. Lacombe B, Morel M, Margottin-Goguet F, Ramirez BC. Specific inhibition of HIV infection by the action of spironolactone in T cells. J Virol. 2016;90(23):10972-10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wacleche VS, Goulet JP, Gosselin A, et al. . New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology. 2016;13(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Amador CA, Barrientos V, Peña J, et al. . Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension. 2014;63(4):797-803. [DOI] [PubMed] [Google Scholar]
- 77. Peyriere H, Eiden C, Macia JC, Reynes J. Antihypertensive drugs in patients treated with antiretrovirals. Ann Pharmacother. 2012;46(5):703-709. [DOI] [PubMed] [Google Scholar]
- 78. Baker JV, Huppler Hullsiek K, Prosser R, et al. . Angiotensin converting enzyme inhibitor and HMG-CoA reductase inhibitor as adjunct treatment for persons with HIV infection: a feasibility randomized trial. PLoS One. 2012;7(10):e46894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lake JE, Seang S, Kelesidis T, Currier JS, Yang OO. Telmisartan increases vascular reparative capacity in older HIV-infected adults: a pilot study. HIV Clin Trials. 2016;17(6):225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lake JE, Tseng CH, Currier JS. A pilot study of telmisartan for visceral adiposity in HIV infection: the metabolic abnormalities, telmisartan, and HIV infection (MATH) trial. PLoS One. 2013;8(3):e58135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Utay NS, Kitch DW, Yeh E, et al. ; A5317 AIDS Clinical Trials Group Team . Telmisartan therapy does not improve lymph node or adipose tissue fibrosis more than continued antiretroviral therapy alone. J Infect Dis. 2018;217(11):1770-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Srinivasa S, Fitch KV, Wong K, et al. . Randomized, placebo-controlled trial to evaluate effects of eplerenone on metabolic and inflammatory indices in HIV. J Clin Endocrinol Metab. 2018;103(6):2376-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lama JR, Mayer KH, Perez-Brumer AG, et al. . Integration of Gender-Affirming Primary Care and Peer Navigation With HIV Prevention and Treatment Services to Improve the Health of Transgender Women: Protocol for a Prospective Longitudinal Cohort Study. JMIR Res Protoc. 2019;8(6):e14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ucciferri C, Mancino P, Vecchiet J, Falasca K. Beneficial effects of telmisartan in an HIV+ diabetic insulin-dependent patient. Int J Immunopathol Pharmacol. 2009;22(3):853-857. [DOI] [PubMed] [Google Scholar]
- 85. Vecchiet J, Ucciferri C, Falasca K, Mancino P, Di Iorio A, De Caterina R. Antihypertensive and metabolic effects of telmisartan in hypertensive HIV-positive patients. Antivir Ther. 2011;16(5):639-645. [DOI] [PubMed] [Google Scholar]
- 86. Shimabukuro M, Tanaka H, Shimabukuro T. Effects of telmisartan on fat distribution in individuals with the metabolic syndrome. J Hypertens. 2007;25(4):841-848. [DOI] [PubMed] [Google Scholar]
- 87. Chujo D, Yagi K, Asano A, et al. . Telmisartan treatment decreases visceral fat accumulation and improves serum levels of adiponectin and vascular inflammation markers in Japanese hypertensive patients. Hypertens Res. 2007;30(12):1205-1210. [DOI] [PubMed] [Google Scholar]
- 88. Lake JE, Seang S, Kelesidis T, et al. . Telmisartan to reduce cardiovascular risk in older HIV-infected adults: a pilot study. HIV Clin Trials. 2015;16(5):197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Guo C, Ricchiuti V, Lian BQ, et al. . Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117(17):2253-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mikulak J, Singhal PC. HIV-1 and kidney cells: better understanding of viral interaction. Nephron Exp Nephrol. 2010;115(2):e15-e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ideura H, Hiromura K, Hiramatsu N, et al. . Angiotensin II provokes podocyte injury in murine model of HIV-associated nephropathy. Am J Physiol Renal Physiol. 2007;293(4):F1214-F1221. [DOI] [PubMed] [Google Scholar]
- 92. Canale D, de Bragança AC, Gonçalves JG, et al. . Vitamin D deficiency aggravates nephrotoxicity, hypertension and dyslipidemia caused by tenofovir: role of oxidative stress and renin-angiotensin system. PLoS One. 2014;9(7):e103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shimizu A, Zhong J, Miyazaki Y, Hosoya T, Ichikawa I, Matsusaka T. ARB protects podocytes from HIV-1 nephropathy independently of podocyte AT1. Nephrol Dial Transplant. 2012;27(8):3169-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bird JE, Durham SK, Giancarli MR, et al. . Captopril prevents nephropathy in HIV-transgenic mice. J Am Soc Nephrol. 1998;9(8):1441-1447. [DOI] [PubMed] [Google Scholar]
- 95. Mohan S, Herlitz LC, Tan J, et al. . The changing pattern of glomerular disease in HIV and hepatitis C co-infected patients in the era of HAART. Clin Nephrol. 2013;79(4):285-291. [DOI] [PubMed] [Google Scholar]
- 96. Menez S, Hanouneh M, McMahon BA, Fine DM, Atta MG. Pharmacotherapy and treatment options for HIV-associated nephropathy. Expert Opin Pharmacother. 2018;19(1):39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kimmel PL, Mishkin GJ, Umana WO. Captopril and renal survival in patients with human immunodeficiency virus nephropathy. Am J Kidney Dis. 1996;28(2):202-208. [DOI] [PubMed] [Google Scholar]
- 98. Wei A, Burns GC, Williams BA, Mohammed NB, Visintainer P, Sivak SL. Long-term renal survival in HIV-associated nephropathy with angiotensin-converting enzyme inhibition. Kidney Int. 2003;64(4):1462-1471. [DOI] [PubMed] [Google Scholar]
- 99. Burns GC, Paul SK, Toth IR, Sivak SL. Effect of angiotensin-converting enzyme inhibition in HIV-associated nephropathy. J Am Soc Nephrol. 1997;8(7):1140-1146. [DOI] [PubMed] [Google Scholar]
- 100. Ucciferri C, Falasca K, Mancino P, Di Iorio A, Vecchiet J. Microalbuminuria and hypertension in HIV-infected patients: a preliminary study of telmisartan. Eur Rev Med Pharmacol Sci. 2012;16(4):491-498. [PubMed] [Google Scholar]
- 101. El-Sadr WM, Lundgren J, Neaton JD, et al. Strategies for Management of Antiretroviral Therapy Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283-2296. [DOI] [PubMed] [Google Scholar]
- 102. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(21):2625-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35(17):1101-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Patel RB, Shah SJ. Drug targets for heart failure with preserved ejection fraction: a mechanistic approach and review of contemporary clinical trials. Annu Rev Pharmacol Toxicol. 2019;59:41-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;8(2):210-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Triant VA, Grinspoon SK. Epidemiology of ischemic heart disease in HIV. Curr Opin HIV AIDS. 2017;12(6):540-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jacob M, Holloway CJ. Cardiac steatosis in HIV: a marker or mediator of disease? Front Endocrinol (Lausanne). 2018;9:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ouyang J, Isnard S, Lin J, et al. . Metformin effect on gut microbiota: insights for HIV-related inflammation. AIDS Res Ther. 2020;17(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kaski JC, Crea F, Gersh BJ, Camici PG. Reappraisal of ischemic heart disease. Circulation. 2018;138(14):1463-1480. [DOI] [PubMed] [Google Scholar]
- 110. Ford TJ, Berry C. How to diagnose and manage angina without obstructive coronary artery disease: lessons from the British Heart Foundation CorMicA trial. Interv Cardiol. 2019;14(2):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gupta SK, Dubé MP, Stein JH, Clauss MA, Liu Z. A pilot trial of pentoxifylline on endothelial function and inflammation in HIV-infected patients initiating antiretroviral therapy. AIDS. 2016;30(13):2139-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cade WT, Reeds DN, Overton ET, et al. . Pilot study of pioglitazone and exercise training effects on basal myocardial substrate metabolism and left ventricular function in HIV-positive individuals with metabolic complications. HIV Clin Trials. 2013;14(6):303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ridker PM, Everett BM, Pradhan A, et al. ; CIRT Investigators . Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.