Abstract
Context
Single ZnT8 autoantibody (ZnT8A) positivity by standard radiobinding assay (RBA) is commonly seen in nondiabetes population-based screening and the risk of progression to type 1 diabetes (T1D) in subjects with single ZnT8A is unknown.
Objective
Identify the risk of progression to T1D in individuals positive only for ZnT8A.
Methods
We developed an electrochemiluminescence (ECL) assay to detect high-affinity ZnT8A and validated it in 3 populations: 302 patients newly diagnosed with T1D, 135 nondiabetic children positive for ZnT8A by RBA among 23 400 children screened by the Autoimmunity Screening for Kids (ASK) study, and 123 nondiabetic children multiple autoantibody positive or single ZnT8A positive by RBA participating in the Diabetes Autoimmunity Study in the Young (DAISY).
Results
In 302 patients with T1D at diagnosis, the positivity for ZnT8A was 62% both in RBA and ECL. Among ASK 135 participants positive for RBA-ZnT8A, 64 were detected ZnT8A as the only islet autoantibody. Of these 64, only 9 were confirmed by ECL-ZnT8A, found to be of high affinity with increased T1D risk. The overall positive predictive value of ECL-ZnT8A for T1D risk was 87.1%, significantly higher than that of RBA-ZnT8A (53.5%, P < .001). In DAISY, 11 of 2547 children who had no positivity previously detected for other islet autoantibodies were identified as single ZnT8A by RBA; of these, 3 were confirmed positive by ECL-ZnT8A and all 3 progressed to clinical T1D.
Conclusion
A large proportion of ZnT8A by RBA are single ZnT8A with low T1D risk, whereas ZnT8A by ECL was of high affinity and high prediction for T1D development.
Keywords: ZnT8 autoantibodies, type 1 diabetes, ECL assay, prediction, biomarker
Islet autoantibodies (IAbs) for insulin (IAA), glutamic acid decarboxylase (GADA), insulinoma-associated protein 2 (IA-2A), and zinc transporter 8 (ZnT8A) are currently used as the most reliable biomarkers for type 1 diabetes (T1D) prediction. Individuals who carry 2 or more IAbs with normoglycemia are classified as stage 1 T1D, an asymptomatic stage of the disease (1). Thus early identification of IAbs using a high-sensitivity and highly disease-specific assay at the presymptomatic stage of T1D is essential for the prediction, prevention, and clinical care of this disease. The progression of islet autoimmunity usually begins with a single IAb at initial seroconversion, then subsequently advances to 2 or more IAbs (2). Single IAb positivity represents the earlier stage of T1D autoimmunity, but individuals with a single IAb have been found to be at low risk overall (3). Multiple studies have demonstrated that individuals with low-affinity IAbs have less or no risk for T1D progression (4-8). Unlike individuals having multiple IAbs, a large proportion of individuals with single IAb, either relatives of T1D patients or the general population, were found to have low affinity autoantibodies (4, 8), and a majority of these antibodies disappear during follow-up, behaving as a “transient” and biologically “false” positive in terms of the disease. Recent evidence of our newly developed high-affinity electrochemiluminescence (ECL) assay has demonstrated its higher disease specificity than the current standard radiobinding assay (RBA) in multiple clinical studies (3, 6, 8), including TrialNet Pathway to Prevention, The Environmental Determinants of Diabetes in the Young (TEDDY), Autoimmunity Screening for Kids (ASK), and Diabetes Autoimmunity Study in the Young (DAISY). Our previous studies of ECL assay on GADA and IAA demonstrated that ECL assay is able to discriminate high-affinity, high-risk autoantibodies from low-affinity, low-risk autoantibodies generated by RBA (3-6, 8). Children positive for ZnT8A only, without other IAbs, were identified from 2 ongoing large population-based clinical studies: DAISY, which follows children at increased risk for T1D due to genetic markers or first-degree relatives, and ASK, a general population screening program for IAbs in children. The risk prediction of T1D development in children with single ZnT8A and their characteristics to date have not yet been studied. In the present study, we aim to differentiate the disease risk of these single ZnT8A detected by RBA in these 2 large cohort studies using a high-affinity ECL-ZnT8A assay. We analyzed the general predictive values of ZnT8A for risk of T1D development by RBA and ECL assay and their corresponding antibody affinity.
Materials and Methods
Participants
Serum samples from 302 Barbara Davis Center patients with T1D, age younger than 18 years with a median age of 11.1 years, and obtained within 3 months of diagnosis, were tested for ZnT8A by standard RBA and ECL assay. Normal controls, frequency matched for age and sex, were 2282 children negative for all IAbs, randomly selected from the ASK study. ASK screens general population Colorado children ages 1 to 17 years for presymptomatic T1D and celiac disease (9). Children found to be autoantibody positive at screening are retested for confirmation, and if confirmed positive, followed every 3 to 6 months. From December 2016 to December 2019, 23 400 children have been screened. Standard RBA and ECL assays for IAA, GADA, IA-2A, ZnT8A, and transglutaminase autoantibodies were performed, except for ECL-ZnT8A, which was not available initially. Retrospective analysis of ECL-ZnT8A was conducted on all ASK individuals positive for RBA-ZnT8A on initial screening (n = 135) and longitudinal follow-up samples.
DAISY (n = 2547) has followed for up to 27 years children at increased risk of T1D, including first-degree relatives of T1D patients and general population children with moderate to high T1D susceptibility HLA-DR/DQ genotypes (10). Currently 233 individuals have at least one persistent positive IAb, multiple IAbs in 128, and clinical T1D in 103. The ECL-ZnT8A assay was performed on sera obtained from 123 children: 112 who were multiple IAbs positive with available samples and all 11 children positive only for ZnT8A by RBA.
Characteristics of all study participants are shown in Table 1. All participants have provided written or electronic informed consent and all studies were approved by the University of Colorado Institutional Review Board.
Table 1.
Characteristics of all study participants
| Groups | ASK study | DAISY study | New-onset T1D | |||
|---|---|---|---|---|---|---|
| RBA-ZnT8A+ | All IAbs– | Single ZnT8A+ | Multiple IAbs+ | |||
| Single ZnT8A+ | Multiple IAbs+ | |||||
| No. | 64 | 71 | 1098 | 11 | 112 | 302 |
| Age at testing, y | 9.6 ± 4.3 | 9.0 ± 4.0 | 11.2 ± 4.7 | 7.6 ± 5.0 | 12.4 ± 9.1 | |
| Male sex, No. (%) | 31 (48.4%) | 33 (46.5%) | 426 (38.8%) | 62 (50.4%) | 162 (53.6%) | |
Abbreviations: ASK, Autoimmunity Screening for Kids; DAISY, the Diabetes Autoimmunity Study in the Young; IAbs, islet antibodies; RBA, radiobinding assay; T1D, type 1 diabetes; ZnT8A, zinc transporter 8 autoantibody.
Electrochemiluminescence Assay for Zinc Transporter 8 Autoantibody and Other Islet Autoantibodies
The human recombinant ZnT8 antigen protein used in the ECL assay is identical to the one used in current standard RBA and is a dimer of 2 intracellular domains of ZnT8, 1 with arginine at amino acid position 325 and 1 with tryptophan, linked with a human immunoglobulin hinge sequence to cover 2 major polymorphisms. The ZnT8 protein was produced and kindly provided by Dr Shaodong Dai at the University of Colorado, and its autoantibody binding activity was confirmed by complete absorption of ZnT8A in newly diagnosed patients with T1D by this unlabeled protein in RBA. The assay method of ZnT8A ECL assay is similar to the GADA ECL assay, which was published previously (11). In brief, 20 µL of 5× diluted serum was mixed with 20µL of ZnT8 antigen protein respectively labeled with Sulfo-TAG and biotin for overnight incubation at 4 °C. On the second day, overnight incubates were applied to a streptavidin-coated plate (Meso Scale Diagnostics), incubated at room temperature for 1 hour, and the plate was washed 3 times followed by the addition of 150 µL of reading buffer and then counted on an Imager SQ120 (Meso Scale Diagnostics). The optimal concentrations and best ratio of 2 labeled ZnT8 proteins were determined using a checkerboard assay as published previously (11). The upper limit of index 0.010 for assay cutoff was set at 99.8th percentile of 2282 normal control samples using a receiver operating characteristic curve among 302 T1D patients. The coefficiency of variation for intra-assay is 3.9% (n = 10) and interassay 5.0% (n = 10). The methods for the ECL assay for GADA, IA-2A, and IAA are similar and have been published previously (4-6). At the 2020 Islet Autoantibody Standardization Program (IASP) workshop, sensitivities and specificities of the ECL assay were 74% and 100% for ZnT8A, 78% and 100% for GADA, 66% and 99% for IAA, and 72% and 100% for IA-2A, respectively.
Zinc Transporter 8 Autoantibody Affinity Assay
Affinity measures the strength of interaction between an antibody and an antigen. It is defined by the same basic thermodynamic principles that govern any reversible biomolecular interaction. High-affinity antibodies will bind a greater amount of antigen at a lower concentration of antigen than low-affinity antibodies during the same period of time. The ZnT8A affinity assay was identical to that previously described for GADA or IAA (5, 6). For the present affinity study, 18 samples from individuals with persistent ZnT8A positivity by RBA in the ASK cohort were analyzed, including 10 participants positive for ECL-ZnT8A and 8 individuals negative by ECL-ZnT8A. To observe the dynamic changes of ZnT8A affinity with time, serum samples of the first positive ZnT8A at the initial screening and the latest positive at follow-up from all 18 participants with a mean follow-up time of 16.4 ± 8.2 months were analyzed for ZnT8A affinity. The ZnT8A affinity assay was performed using the standard ZnT8A RBA in which ZnT8A in serum samples were absorbed with a serial dilution of unlabeled ZnT8 antigen protein in 5 different concentrations (1.2 × 10–6–1.2 × 10–10 M). Inhibitions for 50% of signals at the concentrations of unlabeled ZnT8A were counted and the relative affinities were compared between antibodies. In addition, 5 samples positive with ECL-ZnT8A but negative with RBA-ZnT8A from the DAISY cohort were analyzed for ZnT8A affinity using the ZnT8A ECL assay.
Radiobinding Assay for Islet Autoantibodies
The methods of RBA assay for all 4 IAbs have been published previously (7, 12, 13). The cutoffs for all 4 RBAs were set at around the 99th percentile of normal controls. At the 2020 IASP (Islet Autoantibody Standardization Program) workshop, sensitivities and specificities of RBA assay were 62% and 99% for IAA, 78% and 99% for GADA, 72% and 100% for IA-2A, and 74% and 100% for ZnT8A, respectively.
Statistics
All statistical analyses were performed using GraphPad Prism software (version 8.0; GraphPad Software Institute) and SAS v9.4 (SAS Institute Inc). For continuous variables, the mean, median, range, and SD were presented. Frequency and its percentage were generated for categorical variables. Between-group comparisons were performed with the t test or Wilcoxon-Mann-Whitney test for continuous variables and with the chi-square or Fisher exact test for categorical variables as appropriate. Pearson correlation analysis was performed to access the strength of correlation between levels of ZnT8A in 2 assays. Kaplan-Meier survival curves were used to estimate cumulative risk for the development of clinical T1D in different groups and compared using the log-rank test. A P value of less than .05 at 2-sided test was considered statistically significant.
Results
Sensitivity and Specificity of Electrochemiluminescence–Zinc Transporter 8 Autoantibody in New-Onset Type 1 Diabetes Patients
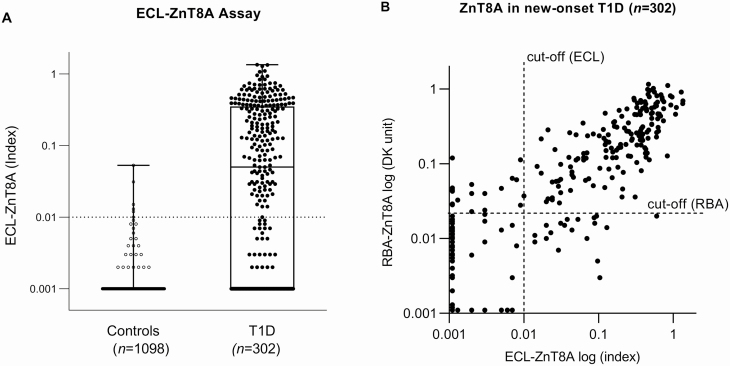
A total of 302 samples from randomly selected children with new-onset T1D within 3 months and 1098 age- and sex-matched healthy control children from the general population were tested for ECL-ZnT8A, and the results are illustrated in Fig. 1A. With the specificity set at 99.8% of 2282 normal controls for the ECL-ZnT8A assay and 99.0% for the RBA-ZnT8A assay (7), 62.3% (188 of 302) of new-onset T1D patients were found positive for ZnT8A by ECL compared to 61.9% (187/302) by RBA. In addition, ECL-ZnT8A and RBA-ZnT8A had the same sensitivity (74%) and specificity (100%) as the most recent 2020 IASP workshop, consisting of 50 new-onset T1D patients and 90 healthy controls. The levels of ECL-ZnT8A correlated well with RBA-ZnT8A among the samples positive with ZnT8A by ECL and/or RBA, as shown in Fig. 1B (r = 0.708, P < .001). Approximately 11% (34/302) of the samples were discordant between ECL assay and RBA at low levels.
Figure 1.
A, Boxplots of electrochemiluminescence (ECL)–zinc transporter 8 autoantibody (ZnT8A) in 2282 normal controls and 302 new-onset type 1 diabetes (T1D) patients. With a cutoff value of index 0.010 set at the 99.8th percentile of 2282 control samples, the positivity of new-onset T1D patients was 62.3% (188/302). B, ECL-ZnT8A and radiobinding assay (RBA)-ZnT8A were compared in levels among 302 new-onset T1D patients and 2 assays were well correlated (r = 0.779; P < .001). The dotted lines represented the cutoffs for 2 assays, respectively.
Positivity of Electrochemiluminescence–Zinc Transporter 8 Autoantibody in General Population Children
From 2017 to 2019, ASK screened 23 400 children from the general population aged 1 to 17 years for IAbs and celiac autoantibodies. At the initial screening by RBA, 3.4% (792/23 400) of the children were positive for at least one IAb, including 0.4% (98/23 400) for multiple IAbs and 3.0% (599/23 400) for a single IAb by RBA. Currently tested ZnT8A that bind to the C-terminal of the intracellular domain of the ZnT8 molecule usually appear later, often appearing with other IAbs during the prediabetes period. In the ASK cohort, 135 children were detected as ZnT8A positive in total and 47.4% (64/135) of these individuals were unexpectedly found as single ZnT8A without other IAbs. Of 64 children with single ZnT8A positivity, only 14.1% (9/64) were confirmed by ECL-ZnT8A, whereas 85.9% (61/71) of children who were ZnT8A positive with multiple IAbs were confirmed by ECL-ZnT8A (P < .001). Overall, 51.9% (70/135) of ZnT8A by RBA were detected positive by ECL assay, whereas 65 (48.1%) were negative by ECL assay. The majority of ZnT8A confirmed with the ECL assay (61/70, 87.1%) were found to be multiple IAbs positive while only 15.4% (10/65; P < .001) of ZnT8A not confirmed by ECL assay were found to have multiple IAbs.
The characteristics of the ASK children with positive or negative ECL-ZnT8A are summarized in Table 2. There were no differences in age at screening, sex, or family history of T1D between the 2 groups. Children who were confirmed positive by ECL-ZnT8A were more often multiple IAbs positive (P < .001) and more likely to develop clinical T1D (P < .001) during follow-up compared to those RBA-ZnT8A–positive children not confirmed by ECL-ZnT8A.
Table 2.
Demographic data of children from the Autoimmunity Screening for Kids study with zinc transporter 8 autoantibody
| Group | RBA + ECL+ | RBA + ECL– | P |
|---|---|---|---|
| No. | 70 | 65 | – |
| Age at screening, y | 8.7 ± 4.0 | 9.7 ± 4.5 | .21 |
| Male sex, % | 54.3 | 49.2 | .61 |
| Family history of T1D, % | 25.7 | 15.4 | .14 |
| RBA-ZnT8 RBA levels, DK units | |||
| Median | 0.150 | 0.055 | < .001 |
| IQR | 0.071 ~ 0.420 | 0.042 ~ 0.095 | |
| Multiple IAbs positive, % | 85.9 (61/70) | 15.6 (10/65) | < .001 |
| GADA positivity, No. (%) | 51 (72.9) | 4 (6.2) | < .001 |
| IAA positivity, No. (%) | 42 (60.0) | 3 (4.6) | < .001 |
| IA-2A positivity, No. (%) | 45 (64.3) | 8 (12.3) | < .001 |
| Clinical T1D at follow-up, No. | 16 | 1 | < .001 |
Abbreviations: ASK, Autoimmunity Screening for Kids; ECL, electrochemiluminescence; GADA, glutamic acid decarboxylase; IAA, insulin autoantibodies; IA-2A, insulinoma-associated protein 2; IAbs, islet antibodies; IQR, interquartile range; RBA, radiobinding assay; T1D, type 1 diabetes.
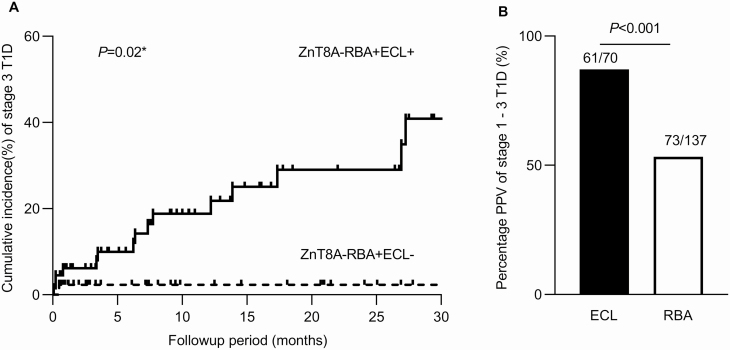
By the time of data analysis, 109 children among a total 135 positive ZnT8A, including 66 confirmed by ECL assay and 43 not confirmed by ECL assay, had follow-up data available with an average follow-up of 10.9 ± 10.4 months. In children with ZnT8A confirmed by ECL assay, 16 (16/66) children progressed to clinical T1D (all 16 children were multiple IAbs positive), whereas only one child (1/43, P < .0001) with a negative ECL-ZnT8A developed clinical T1D. This child quickly progressed to clinical T1D only 15 days after initial screening and was multiple IAbs positive with a low level of RBA-ZnT8A. The cumulative risk for clinical T1D development by 2 years was much higher in children with ZnT8A confirmed by ECL assay compared with children positive only by RBA (24% vs 2% respectively, log-rank test, P = .02) (Fig. 2A). The positive predictive values of overall ECL-ZnT8A at initial screening, in terms of T1D from stage 1 with multiple IAbs to stage 3 with clinical T1D, was 87.1% (61/70), significantly higher than overall RBA-ZnT8A (53.3%, 73/137; P < .001) as plotted in Fig. 2B. In addition, RBA-ZnT8A not confirmed by ECL assay was often lost over time behaving as “transient” positivity, with 27.9% (12/43) disappearing during 1 year follow-up vs 9.1% (6/66, P = .01) in those confirmed by ECL-ZnT8A. The majority of these ZnT8A lost during follow-up were those with a single ZnT8A without other IAbs.
Figure 2.
Electrochemiluminescence (ECL)–zinc transporter 8 autoantibody (ZnT8A) and radiobinding assay (RBA)-ZnT8A in the Autoimmunity Screening for Kids study. A, Comparison of cumulative incidence (%) of clinical type 1 diabetes (T1D) (stage 3 T1D) in 2 groups: RBA + ECL + and RBA + ECL–. Solid line: individuals with RBA-ZnT8A confirmed by ECL, dotted line: individuals with RBA-ZnT8A not confirmed by ECL. B, Positive predictive values (PPV) of stage 1 to stage 3 T1D in individuals with ECL-ZnT8A or RBA-ZnT8A.
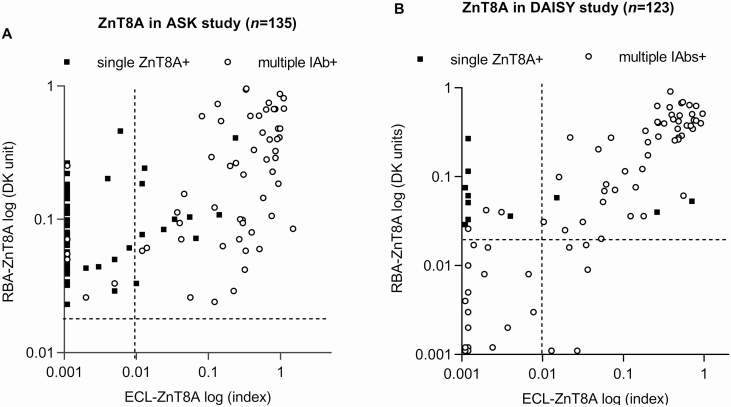
ECL-ZnT8A correlated in levels with RBA-ZnT8A (r = 0.588, P < .001) as illustrated in Fig. 3A. The levels of RBA-ZnT8A not confirmed by ECL assay (0.055, interquartile range [IQR] 0.042 ~ 0.095) were significantly lower than the levels of ZnT8A confirmed by ECL assay (0.150, IQR 0.071 ~ 0.420, P < .001). Similarly, the levels of RBA-ZnT8A in children with single ZnT8A (0.066, IQR 0.043 ~ 0.112) were also significantly lower than that in children with multiple IAbs (0.145, IQR 0.066 ~ 0.448, P < .001). However, absolute levels were not discriminatory for both cases.
Figure 3.
Levels of zinc transporter 8 autoantibody (ZnT8A) between radiobinding assay (RBA) and electrochemiluminescence (ECL) assay were compared in 135 children from A, the Autoimmunity Screening for Kids (ASK) study, and in 123 children from B, the Diabetes Autoimmunity Study in the Young (DAISY). The dotted lines represent cutoff values for ECL-ZnT8A and RBA-ZnT8A, respectively. Open circles: individuals with multiple islet autoantibodies (IAbs), solid squares: individuals with single ZnT8A.
Presentation of Electrochemiluminescence–Zinc Transporter 8 Autoantibody in the Diabetes Autoimmunity Study in the Young Cohort
In the DAISY cohort, the ECL-ZnT8A assay was performed on 112 out of 128 children who were multiple IAbs positive (65/112 were first-degree relatives of patients with T1D) and all 11 children (4/11 were first-degree relatives of patients with T1D) who were persistent single ZnT8A positive. Of 112 children with multiple IAbs, 49.1% (55/112) were detected to be ZnT8A positive by ECL assay and 47.3% (53/112) by RBA, whereas 50 were positive in both assays. The correlation of ECL-ZnT8A levels with RBA-ZnT8A for this group is illustrated in Fig. 3B. Similar to the other groups, ECL-ZnT8A was congruent with RBA in children at high risk with multiple IAbs. Of a total of 11 children who were persistent single ZnT8A positive, 8 children were consistently negative by ECL assay from their initial screening to the last follow-up, with a median follow-up of 7.2 years (range, 2.3 ~ 16.2 years). Of the 3 children who were confirmed positive for ECL-ZnT8A, all of them progressed to clinical T1D during the follow-up.
Zinc Transporter 8 Autoantibody by Radiobinding Assay not Confirmed by Electrochemiluminescence Assay Were of Low Affinity
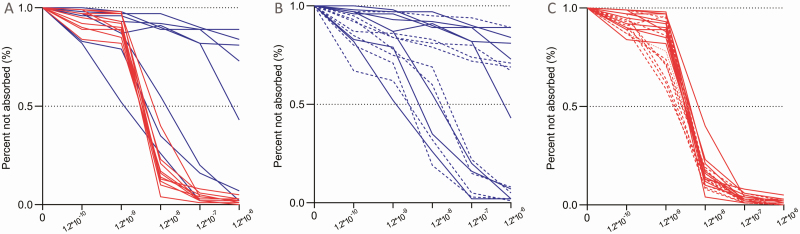
Affinity analysis for ZnT8A was performed on 18 children, including 10 positive ZnT8A in both assays and 8 positive in RBA only. As illustrated in Fig. 4A and Table 3, 6 of 8 ZnT8A not detectable by ECL assay required a 100- to 1000-fold higher concentration of unlabeled ZnT8 protein (10–9 to > 10– 6 [M]) for 50% inhibition of binding of ZnT8A to radiolabeled ZnT8 protein than for those 10 samples (< 10–8 [M]) positive by ECL-ZnT8A assay. At the ZnT8 concentration of 1.2 × 10–8 [M], 83% of ZnT8A on average were absorbed for RBA + ECL + samples compared with only 18% for RBA + ECL– samples (P = .004). At the ZnT8 concentration of 1.2 × 10–7 [M], nearly 100% of ZnT8A was absorbed for all RBA + ECL + samples compared with less than 50% for RBA + ECL– samples on average (P < .001). These results were consistent with our previous finding for ECL-IAA and ECL-GADA (8). The affinity of 5 RBA-ECL + samples was similar to RBA + ECL + samples and 50% inhibition of ZnT8A was at less than 1.2 × 10–9 [M] of ZnT8 protein on average. In addition, the affinities of ZnT8A from their initial positive screening samples and their last positive visit samples among these participants, with 16.4 ± 8.2 months of follow-up time, were compared to see the affinity changes with time. As shown in Fig. 4B for ZnT8A not detectable by ECL assay and in Fig. 4C for ZnT8A detectable by ECL assay, the affinities of ZnT8A from the same individuals were consistent over time and no converting events, either from low to high or high to low affinity, were seen.
Figure 4.
A, Affinity comparison of zinc transporter 8 autoantibody (ZnT8A) between 10 individuals with radiobinding assay (RBA) + electrochemiluminescence (ECL) + and 8 individuals with RBA + ECL– at initial screening, red lines: individuals with RBA + ECL + and blue lines: individuals with RBA + ECL–. B, Longitudinal follow-up of ZnT8A affinity in individuals with RBA + ECL–. C, Longitudinal follow-up of ZnT8A affinity in individuals with RBA + ECL+. Solid lines in B and C represent the screening samples and dotted lines represent samples from the last follow up.
Table 3.
Individuals with affinity analysis of zinc transporter 8 autoantibody
| RBA+/ECL+ | RBA+/ECL– | P | |
|---|---|---|---|
| No. | 10 | 8 | |
| Age, y | 10.4 ± 4.1 | 9.7 ± 3.9 | .91 |
| RBA-ZnT8A, DK unit | 0.18 ± 0.08 | 0.12 ± 0.06 | .48 |
| Multiple IAbs positive, No. | 9 | 0 | – |
| ZnT8 [M] at 50% inhibition | 1.7 × 10–9 | 1.3 × 10–6 | < .001 |
Abbreviations: ECL, electrochemiluminescence; IAbs, islet antibodies; RBA, radiobinding assay; ZnT8A, zinc transporter 8 autoantibody.
Discussion
Disease specificity of autoantibodies detected in population-based screening is critical for accurate disease-risk prediction. A large proportion of individuals with positive IAbs identified by current standard RBA from screening in either relatives of T1D patients or the general population are single IAb positive and have an overall risk for progression to clinical T1D of less than 15% in 10 years (1). Single IAb positivity is commonly seen with single IAA and single GADA, and most of these single IAbs were found to be low affinity with low risk (4, 5, 8, 14-18). Our recently developed ECL assay has been demonstrated for its remarkable higher disease specificity with discrimination of high-affinity, high risk from low-affinity, low risk IAA, and GADA in multiple clinical studies (8). In this ongoing ASK study of mass screening of children from the general population, overall positivity of ZnT8A by RBA was found in 135 children, whereas 64 children, nearly half of all ZnT8A-positive children (47.7%, 64/135) were unexpectedly found to be single ZnT8A positive without other IAbs. Similar to other IAbs, the majority of these single ZnT8As (85.9%, 55/64) generated by RBA were negative by ECL assay. Affinity analysis revealed that ZnT8A by RBA but not detectable by ECL assay were of low affinity, and many of them were quickly lost within a short time of follow-up. These findings were consistent with our previous studies on ECL-GADA and ECL-IAA. We believe the majority of these single ZnT8A positivity cases will disappear with longer follow-up time as we observed in our previous studies of single GADA or single IAA with low affinity (3-6, 8). We had hypothesized previously that these single IAb positivity with low-affinity cases might often result from the immunization of nonislet immunogens, but cross-react with islet proteins. Though this form of IAb can still be competed against with a native islet antigen protein and thus are not a “biochemical” false positive, in terms of biologic relevance those antibodies detected with only the RBA but negative with the ECL assay appear to predominantly be false positives relative to predicting disease status.
The ECL assay is a bivalent assay in which IAbs in serum link to both the Sulfo-tagged antigen and the biotinylated antigen, thereby potentially increasing specificity (6). In those with single IAb positivity, exclusion of those “low-risk autoantibodies” by using a more disease-specific assay, like a high-affinity ECL assay, will greatly enhance the predictive value of the IAb. The current ASK study, with its large-scale general population screening, provided an opportunity to apply an ECL assay in parallel with RBA for all 4 IAbs. Consistent with our previous studies on ECL-IAA and ECL-GADA (4-6, 8), all 4 ECL assays including for IAA, GADA, IA-2A, and ZnT8A were able to significantly discriminate high-risk autoantibodies with multiple IAbs from low-risk with single IAb (data for IAA, GADA, and IA-2A not shown). The ECL-ZnT8A assay showed excellent sensitivity, at least as high as RBA in the new-onset T1D patients studied, according to the IASP workshop and high-risk DAISY cohort with multiple IAbs, whereas many of them were followed to clinical T1D. Unlike RBA, which captures only immunoglobulin (Ig)G, the ECL assay is designed to grab all immunoglobulins (19), including IgG, IgM, IgA, etc. This may explain why the ECL assay is more sensitive than RBA in identifying the initial seroconversion of autoantibody earlier in young children followed from birth in the cohorts of DAISY (4, 6) and TEDDY study (unpublished data). In the ASK study, the cumulative risk for clinical T1D development by 2 years was 24% in children with RBA-ZnT8A confirmed by ECL assay compared to 2% in children positive by RBA-ZnT8A only. We believe that, by using a more disease-specific assay with high sensitivity like the ECL assay, the predictive value of IAbs will be greatly enhanced overall and, particularly, a single IAb identified in such a high-affinity assay will be expected as a reliable biomarker for early prediction and disease staging of T1D. This new methodology of ECL assay for IAbs has recently been implemented by several other laboratories with the results demonstrated at the 2020 IASP workshop (unpublished data) and a recent published study of the Latent Autoimmune Diabetes of Adults cohort (20). Several other IAb assay methods, in addition to RBA, like the enzyme-linked immunosorbent assay (21), antibody detection by agglutination–polymerase chain reaction (22), and the luciferase immunoprecipitation system (23) have been reported. However, their predictive values, compared with the current standard RBA, have not been evaluated in a large-scale screening of a nondiabetic population. A multiplex 3-screen enzyme-linked immunosorbent assay has been used in the Fr1da study (21), but the screening aimed only at detecting high-risk children with multiple IAbs while ignoring children with single IAb, whereas disease specificity of the assay is essential.
A limitation of this study is that the ECL-ZnT8A assay was developed more recently in our laboratory and was not available for prospective analysis at the time when either DAISY or ASK study started. The positivity of ECL-ZnT8A on entire cohorts of ASK and DAISY are not available. However, the results of the ECL assays on IAA, GADA, and IA2A, performed in parallel with RBA for the entirety of the ASK study cohort, demonstrate that the occurrence of a single ECL-positive but RBA-negative result was rare (data not shown), similar to our previous study (24). The positivity of IA2A and ZnT8A were usually similar, lower than IAA and GADA, in population-based screening. The occurrence of a single ECL-IA2A positive but RBA negative in the ASK cohort was seen in only 3 cases. This low incidence of single-ECL–positive but RBA-negative results may be due to high-affinity capture in the ECL assay. The follow-up duration of the ASK study is relatively short, and the data on T1D staging and clinical T1D progression are limited. Further study applying ECL assays on all 4 IAbs including the ECL-ZnT8A assay in ongoing ASK study and future large-scale screening studies are expected. With the multiplexed ECL assay platform we recently developed (25), the ECL-ZnT8A assay will join with IAA, GADA, and IA2A IAbs to form a complete panel of a 4-IAbs multiplex ECL assay. The multiplex ECL assay will provide a high-throughput screening tool with a low cost for future T1D screening in the general population.
Acknowledgments
We thank Dr Shaodong Dai at University of Colorado for providing an excellent ZnT8 antigen protein. Dr Liping Yu as guarantor takes full responsibility for this work as a whole.
Author Contributions: X.J. researched data and wrote the manuscript. L.H, D.M., K.W, C.G., and F.D. researched the data. A.K.S. and M.R. researched the data and reviewed/edited the manuscript. L.Y. designed the study and wrote/edited the manuscript. All authors had full access to the all the data in the study and accept responsibility for submitting it for publication.
Financial Support: This work was supported by the Diabetes Research Center (DRC grant No. P30DK116073), Juvenile Diabetes Research Foundation (JDRF) (grant Nos. 2-SRA-2018-533-S-B, 2-SRA-2020-965-S-B, and 1-SRA-2016-208-S-B), and the National Institutes of Health (NIH grant Nos. DK032493 and DK32083).
Glossary
Abbreviations
- ASK
Autoimmunity Screening for Kids
- DAISY
the Diabetes Autoimmunity Study in the Young
- ECL
electrochemiluminescence
- GADA
glutamic acid decarboxylase autoantibodies
- IA-2A
insulinoma-associated protein 2 autoantibody
- IAA
insulin autoantibodies
- IAbs
islet autoantibodies
- IASP
Islet Autoantibody Standardization Program
- IQR
interquartile range
- RBA
radiobinding assay
- T1D
type 1 diabetes
- ZnT8A
zinc transporter 8 autoantibodies
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices), study protocol, statistical analysis, plan, and analytic code will be available immediately and 36 months following publication. Investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose can access the data. Proposals should be directed to liping.yu@cuanschutz.edu to gain access; data requesters will need to sign a data access agreement.
References
- 1. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu L, Rewers M, Gianani R, et al. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab. 1996;81(12):4264-4267. [DOI] [PubMed] [Google Scholar]
- 3. Steck AK, Vehik K, Bonifacio E, et al. ; TEDDY Study Group . Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care. 2015;38(5):808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu L, Dong F, Miao D, Fouts AR, Wenzlau JM, Steck AK. Proinsulin/Insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity. Diabetes Care. 2013;36(8):2266-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miao D, Guyer KM, Dong F, et al. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes. 2013;62(12):4174-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu L, Miao D, Scrimgeour L, Johnson K, Rewers M, Eisenbarth GS. Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes. 2012;61(1):179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu L, Boulware DC, Beam CA, et al. ; Type 1 Diabetes TrialNet Study Group. Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care. 2012;35(6):1213-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steck AK, Fouts A, Miao D, et al. ; TrialNet Study Group. ECL-IAA and ECL-GADA can identify high-risk single autoantibody-positive relatives in the TrialNet Pathway to Prevention study. Diabetes Technol Ther. 2016;18(7):410-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McQueen RB, Rasmussen CG, Waugh K, et al. Cost and cost-effectiveness of large-scale screening for type 1 diabetes in colorado. Diabetes Care. 2020;43(7):1496-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia. 1996;39(7):807-812. [DOI] [PubMed] [Google Scholar]
- 11. Gu Y, Zhao Z, Miao D, High H, Yang T, Yu L. Electrochemiluminescence assays for human islet autoantibodies. J Vis Exp. 2018;( 133):57227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A. 2000;97(4):1701-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases Consortia. J Clin Endocrinol Metab. 2010;95(7):3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest. 2004;114(4):589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlosser M, Koczwara K, Kenk H, et al. In insulin-autoantibody-positive children from the general population, antibody affinity identifies those at high and low risk. Diabetologia. 2005;48(9):1830-1832. [DOI] [PubMed] [Google Scholar]
- 16. Mayr A, Schlosser M, Grober N, et al. GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes. 2007;56(6):1527-1533. [DOI] [PubMed] [Google Scholar]
- 17. Siljander H, Härkönen T, Hermann R, et al. Role of insulin autoantibody affinity as a predictive marker for type 1 diabetes in young children with HLA-conferred disease susceptibility. Diabetes Metab Res Rev. 2009;25(7):615-622. [DOI] [PubMed] [Google Scholar]
- 18. Curnock RM, Reed CR, Rokni S, Broadhurst JW, Bingley PJ, Williams AJ. Insulin autoantibody affinity measurement using a single concentration of unlabelled insulin competitor discriminates risk in relatives of patients with type 1 diabetes. Clin Exp Immunol. 2012;167(1):67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jia X, Gu Y, High H, Yu L. Islet autoantibodies in disease prediction and pathogenesis. Diabetol Int. 2020;11(1):6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu Y, Qian L, Liu Q, et al. Glutamic acid decarboxylase autoantibody detection by electrochemiluminescence assay identifies latent autoimmune diabetes in adults with poor islet function. Diabetes Metab J. 2020;44(2):260-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ziegler AG, Kick K, Bonifacio E, et al. ; Fr1da Study Group. Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA. 2020;323(4):339-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cortez FJ, Gebhart D, Robinson PV, et al. Sensitive detection of multiple islet autoantibodies in type 1 diabetes using small sample volumes by agglutination-PCR. PLoS One. 2020;15(11):e0242049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liberati D, Wyatt RC, Brigatti C, et al. A novel LIPS assay for insulin autoantibodies. Acta Diabetol. 2018;55(3):263-270. [DOI] [PubMed] [Google Scholar]
- 24. Dongmei M, Steck AK, Zhang L, et al. ; Type 1 Diabetes TrialNet Study Group. Electrochemiluminescence assays for insulin and glutamic acid decarboxylase autoantibodies improve prediction of type 1 diabetes risk. Diabetes Technol Ther. 2015;17(2):119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gu Y, Zhao Z, Waugh K, et al. High-throughput multiplexed autoantibody detection to screen type 1 diabetes and multiple autoimmune diseases simultaneously. EBioMedicine. 2019;47:365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices), study protocol, statistical analysis, plan, and analytic code will be available immediately and 36 months following publication. Investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose can access the data. Proposals should be directed to liping.yu@cuanschutz.edu to gain access; data requesters will need to sign a data access agreement.