Abstract
Context
Duodenopancreatic neuroendocrine tumors (dpNETs) frequently occur in patients with multiple endocrine neoplasia type 1 (MEN1), and metastatic dpNET is the primary cause of disease-related mortality. There is a need for biomarkers that can identify patients with MEN1-related dpNETs that are at high risk of developing distant metastasis. Polyamines have tumor-promoting roles in several cancer types.
Objective
We hypothesized that MEN1-dpNET–related disease progression is associated with elevated levels of circulating polyamines.
Methods
Through an international collaboration between The University of Texas MD Anderson Cancer Center, the National Institutes of Health, and the University Medical Center Utrecht, plasma polyamine levels were assessed using mass spectrometry in 84 patients with MEN1 (20 with distant metastatic dpNETs [patients] and 64 with either indolent dpNETs or no dpNETs [controls]). A mouse model of MEN1-pNET, Men1fl/flPdx1-CreTg, was used to test time-dependent changes in plasma polyamines associated with disease progression.
Results
A 3-marker plasma polyamine signature (3MP: N-acetylputrescine, acetylspermidine, and diacetylspermidine) distinguished patients with metastatic dpNETs from controls in an initial set of plasmas from the 3 participating centers. The fixed 3MP yielded an area under the curve of 0.84 (95% CI, 0.62-1.00) with 66.7% sensitivity at 95% specificity for distinguishing patients from controls in an independent test set from MDACC. In Men1fl/flPdx1-CreTg mice, the 3MP was elevated early and remained high during disease progression.
Conclusion
Our findings provide a basis for prospective testing of blood-based polyamines as a potential means for monitoring patients with MEN1 for harboring or developing aggressive disease.
Keywords: pancreatic neuroendocrine tumors, polyamines, biomarker
Multiple endocrine neoplasia type 1 (MEN1) is an inherited autosomal dominant disease that predisposes individuals to endocrine tumors caused by germline loss-of-function mutations in the MEN1 gene (1). Duodenopancreatic neuroendocrine tumors (dpNETs) are highly prevalent among patients with MEN1, with a penetrance of more than 80% by age 80 years (2, 3). MEN1-related dpNETs can be nonfunctioning or secrete excess hormones giving rise to a clinical syndrome. Nonfunctioning pancreatic NETs are the most common in MEN1, followed by (mostly duodenal) gastrinoma, pancreatic insulinomas, and other rare functioning tumors (4). Distant metastasis is the primary cause of disease-related death, occurring in approximately 15% to 25% of patients with MEN1-related dpNETs (5-8). A genetic diagnosis of MEN1 frequently precedes the development of dpNET or occurs at an early stage of disease progression. Currently, no preventive strategies are available for MEN1-related dpNET, and there is a need for biomarkers that can identify patients with MEN1-related dpNETs that are at high risk of developing distant metastasis.
Polyamines are naturally occurring polycationic alkylamines that have tumor-promoting roles in several cancer types (9-14). Tumor-derived polyamines are released into the circulation, and their levels have been linked to disease progression in pancreatic, breast, and ovarian cancers (15-17). We hypothesized that a plasma signature of elevated levels of polyamines would be associated with MEN1-dpNET–related disease progression. To test this hypothesis, we assessed polyamine levels in plasmas of patients with MEN1 and developed a 3-marker polyamine signature (3MP) that we tested in patients with MEN1 and distant metastatic dpNETs. We further examined time-dependent changes in plasma polyamine levels in an established mouse model of MEN1-pNET, Men1fl/flPdx1-CreTg (18, 19).
Materials and Methods
Human Participants
The study presented herein was organized through an international collaboration between The University of Texas MD Anderson Cancer Center (MDACC), the National Institutes of Health (NIH), and the University Medical Center Utrecht (UMCU). At each center, plasma and clinical data were collected under existing institutional review board (IRB)-approved protocols after written informed consent or the approval for waiver of informed consent was obtained as applicable. Under IRB-approved MDACC protocol PA19-0498, these biospecimens and associated retrospectively collected clinical data were used for the present study, with a waiver of informed consent. Coded samples and data were shared after appropriate material transfer agreements were in place.
For initial testing, EDTA plasmas were obtained from 14 case patients with MEN1 and liver metastases from a dpNET and 2 types of controls: patients with MEN1 and a nonmetastatic (distant or regional) indolent dpNET (n = 28; controls 1), and patients with MEN1 and without any NET (n = 14; controls 2) (Table 1). Associated clinical data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at MDACC (20).
Table 1.
Patient and tumor characteristics for test set 1 and test set 2 at time of sample collection
| Test set 1 | Test set 2 | ||||||
|---|---|---|---|---|---|---|---|
| Cases | Controls 1 | Controls 2 | Cases | Controls 1 | Controls 2 | Control NOS | |
| No. | 14 | 28 | 14 | 6 | 9 | 8 | 5 |
| Sex, No. (%) | |||||||
| Male | 6 (43) | 13 (46) | 7 (50) | 4 (67) | 5 (56) | 4 (50) | 1 (20) |
| Female | 8 (57) | 15 (54) | 7 (50) | 2 (33) | 4 (44) | 4 (50) | 4 (80) |
| MEN1 genotype | |||||||
| Positive in self | 13 (93) | 28 (100) | 14 (100) | 5 (83) | 8 (89) | 7 (88) | 5 (100%) |
| Positive in first-degree FM | 1 (7) | – | – | 1 (17) | 1 (11) | 1 (12) | – |
| Age (median, IQR) | 52.5 (41.8-60) | 39.5 (28.5-58) | 29.5 (22-38.5) | 67.5 (56.5-70) | 37 (21-52) | 38.5 (22-47.8) | 35 (20-46) |
| BMI (median, IQR) a | 26 (22.8-32.3) | 26 (23-36) | 23 (20.5-24.5) | 24 (20.4-39.8) | 26 (24-34.5) | 25 (23.2-29) | 31 (25.3-33.8) |
| Collection site, N (%) | |||||||
| MDACC | 5 (36) | 3 (11) | 1 (7) | 6 (100) | 9 (100) | 8 (100) | 5 (100) |
| NIH-NIDDK | 5 (36) | 0 (0) | 1 (7) | – | – | – | – |
| UMCU | 4 (29) | 25 (89) | 12 (86) | – | – | – | – |
| pNET | 13b | 28 | 0 | 4b | 9 | 0 | ambiguous |
| Prior dpNET surgery | 7 (50) | 7 (25) | 0 | 5 (83) | 3 (33) | 2 (25) | 2 (40) |
| Size pNET, No. (%), mm | |||||||
| < 20 | 11 (79) | 26 (93) | – | 2 (50) | 6 (67) | – | – |
| ≥ 20 | 2 (14) | 2 (7) | – | 2 (50) | 3 (33) | – | – |
| Size pNET median (range), mm | 12 (4-29) | 11 (6-23) | – | 26 (11-48) | 10 (8-24) | – | – |
| Unknown | – | – | – | – | – | ||
| Insulinoma, No. (%) c | |||||||
| No | 13 (93) | 27 (96) | 14 (100) | 6 (100) | 9 (100) | 8 (100) | 5 (100) |
| Yes | 1 (7) | – | – | – | – | – | – |
| Suspected | - | 1 (4%) | – | – | – | – | – |
| Gastrinoma, No. (%) d | |||||||
| No | 4 (29) | 26 (93) | 14 (100) | 4 (67) | 7 (78) | 8 (100) | 4 (80) |
| Yes | 7 (50) | – | – | 1 (11) | – | – | |
| Suspected | – | – | – | 1 (17) | 1 (11) | – | 1 (20) |
| Unknown | 3 (21) | 2 (7) | – | 1 (17) | - | – | – |
| Gastrin (median, IQR) e , pg/mL | 351 (151-1448) | 75 (55-145) | 57.5 (48.8-80) | 126 (24.5-201.5) | 27 (18-239) | 18 (13-37) | 39 (15-329) |
| Other functioning pNET f | 1 (VIP) | 0 | - | 0 | 0 | - | 0 |
| Liver metastasis, origin | |||||||
| pNET | 10 | 5 | |||||
| Gastrinoma | 2 | 0 | |||||
| pNET or gastrinoma | 2 | 1 | |||||
| LM | |||||||
| 1 | 6 (43%) | – | – | - | – | – | – |
| 2 or 3 | 3 (21%) | – | – | 1 (17%) | – | – | – |
| > 3 | 5 (36%) | – | – | 5 (83%) | – | – | – |
| Largest LM (median, IQR), mm | 11 (8.5-14) | – | – | 34.5 (9.8-107) | – | – | – |
| Distant metastases outside liver | 1 (7%) | – | – | 3 (50%) | – | – | – |
| Systemic or liver-directed therapy, No. (%) | – | – | – | – | |||
| None | 10 (71%) | 28 (100%) | 14 (100%) | 1 (17%) | 9 (100%) | 8 (100%) | 5 (100%) |
| Previous g | 3 (21%) | – | – | 5 (83%) | – | – | – |
| On active treatment h | 1 (7%) | – | – | 5 (83%) | – | – | – |
| Other MEN1 manifestations at time of sample collection | |||||||
| PHPT, No. (%) | |||||||
| No | 11 (78%) | 19 (68%) | 7 (50%) | 2 (33%) | 5 (55%) | 3 (38%) | 2 (40%) |
| Yes/Suspected | 3 (21%) | 9 (32%) | 7 (50%) | 4 (67%) | 4 (45%) | 4 (50%) | 3 (60%) |
| Unknown | – | – | – | – | – | 1 (13%) | |
| Hypoparathyroidism | 1 (7%) | 6 (21%) | 4 (28%) | 0 | 0 | 0 | 0 |
| Pituitary adenoma, No. (%) | |||||||
| No | 6 (43%) | 14 (50%) | 7 (50%) | 2 (33%) | 4 (44%) | 3 (38%) | 3 (60%) |
| Nonfunctioning | 1 (7%) | 8 (29%) | 2(14%) | 1 (17%) | 1 (11%) | 3 (38%) | 1 (20%) |
| Prolactinoma | 6 (43%) | 4 (14%) | 3 (21%) | 2 (33%) | 3 (33%) | 1 (13%) | 1 (20%) |
| Unknown | 1 (7%) | 2 (7%) | 2 (14%) | 1 (17%) | 1 (11%) | 1 (13%) | – |
| (Parial) pituitary insufficiency | 1 (7%) | 0 | 0 | 1 (17%) | 0 | 1 (13%) | 0 |
| Lung NET, No. (%) | |||||||
| No | 5 (36%) | 18 (64%) | 14 (100%) | – | 4 (44%) | 8 (100%) | 4 (80%) |
| Yes | 1 (7%) | 6 (21%) | – | 2 (22%) | – | – | |
| Lung nodules | 8 (57%) | 4 (14%) | 4 (67%) | 1 (11%) | – | – | |
| Unknown | – | – | – | 2 (33%) | 2 (22%) | – | 1 (20% |
| Adrenal, No. (%) | |||||||
| No | 8 (57%) | 21 (75%) | 14 (100%) | 4 (67%) | 7 (78%) | 8 (100%) | 4 (80%) |
| Nonfunctioning | 5 (36%) | 7 (25%) | 2 (33%) | 2 (22%) | – | 1 (20%) | |
| Unknown | 1 (7%) | – | – | – |
Abbreviations: BMI, body mass index; dpNET, duodenopancreatic neuroendocrine tumor; FM, family member; IQR, interquartile range; LM, liver metastasis; MDACC, The University of Texas MD Anderson Cancer Center; MEN1, multiple endocrine neoplasia type 1; NET, neuroendocrine tumor; NIH-NIDDK, National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases; NOS, not otherwise specified; ambiguous regarding presence of a dpNET; PHPT, primary hyperparathyroidism; pNet, pancreatic neuroendocrine tumor; SSA, somatostatin analogue; ULN, upper limit of normal; UMCU, University Medical Center Utrecht.
a BMI data were not available for 10 control individuals in test set 1 and 3 control individuals in independent test set 2.
b One patient and 2 patient in test set 1 and test set 2, respectively, had total or partial pancreatectomy but presented with dpNET-related liver metastasis at the time of blood collection.
c Insulinoma was defined as a positive supervised fast or symptoms confirmed by low plasma glucose, inappropriate insulin levels, and resolution of symptoms with ingestion of calories, not in a supervised fast setting.
d A gastrinoma diagnosis was made when one of the following criteria was met (1): gastrin more than 10 times the ULN; OR (2) gastrin more than 2 ULN twice consecutively in the absence of proton pump inhibitor use (no value < 2 ULN allowed in between) and not followed by 2 consecutive measurements less than 2 ULN without surgery or start of systemic antitumor therapy; OR (3) gastrin more than 5 ULN twice consecutively in the presence of proton pump inhibitor use (no value < 5 ULN allowed in between) and not followed by 2 consecutive measurements less than 5 ULN without surgery or start of systemic antitumor therapy; OR (4) positive secretin test; OR (5) there is dpNET or lymph nodes/liver metastases with positive immunohistochemistry for gastrin.
e Gastrin levels closest to sample collection within ± 12-month window (ULN 100 pg/mL). Gastrin levels were not available for 1 of the 14 patients and 1 of the 42 control individuals in test set 1 and 1 of the 6 patients and 2 of the 22 controls in independent test set 2.
f Other functioning tumors were defined as a clinical syndrome in conjunction with elevated hormone levels at least 2 times ULN.
g In test set 1, 3 patients were previously treated: one was treated with neoadjuvant chemotherapy and SSAs 1.2 years prior to blood draw, 1 with yttrium embolization of liver metastases 7 years prior to blood draw, and 1 with chemotherapy 12 years before sample collection and SSA up until 9 years before sample collection. None of these 3 individuals were on active treatment at the time of blood draw. In test set 2, 5 patients were previously treated: SSAs only (1); multiple lines of treatment including targeted therapy, liver-directed therapies, experimental therapies, and chemotherapy (4).
h In test set 1, 1 patient was on active treatment with SSAs. In test set 2, 5 patients were on active treatment: chemotherapy (3), SSAs (1), everolimus (1).
Individuals were considered to have MEN1 if they met any of the following criteria: (1) a confirmed germline MEN1 mutation; (2) 1 of the 3 major manifestations (parathyroid, pituitary, dpNET) and a first-degree family member with a confirmed MEN1 mutation; (3) to ensure inclusion of patients with MEN1 and avoid phenocopies in the event that no genetic testing was performed in the patient or first-degree family member, the patient and a first-degree family member had to have a clinical diagnosis of MEN1. This had to include a dpNET if genetic testing was never performed in the pedigree. Exclusion criteria were any active non-NET malignancy, an active thymus NET or active thymoma, rapidly progressive lung or gastric NET, and poorly differentiated neuroendocrine carcinoma.
For patients, liver metastases were confirmed either through histological examination or through consecutive positive imaging (magnetic resonance imaging, computed tomography, and/or somatostatin-receptor imaging). Patients were predominantly treatment naive (10 of the 14 cases). Three patients were previously treated: One was treated with neoadjuvant chemotherapy and somatostatin analogues 1.2 years prior to blood draw, one with yttrium embolization of liver metastases 7 years prior to blood draw, and one with chemotherapy 12 years before blood draw and somatostatin analogues up until 9 years before blood draw. None of these 3 patients were on active treatment at the time of blood draw. One patient was on active treatment with somatostatin analogues.
Selection criteria for controls 1 were a minimum of 3-year follow-up after diagnosis of dpNET and imaging (magnetic resonance imaging, computed tomography, and/or somatostatin-receptor imaging) taken 1 or more years after blood draw demonstrating absence of distant or regional metastasis. Criteria for controls 2 were that patients had no diagnosis of non-dpNET (ie, lung, thymus, or gastric NET), no prior diagnosis of dpNET, and that they were negative for dpNETs at the time of blood collection confirmed either by combined conventional and somatostatin-receptor imaging or through conventional imaging taken 6 or more months post blood draw.
Additional validation of findings was performed in an independent set of plasmas collected at MDACC under IRB-approved protocol PA15-0822 from 6 patients and 22 controls (9 controls 1; 8 controls 2; 5 control not otherwise specified because of ambiguous presence of a dpNET). MEN1 was defined as described previously. Five of 6 patients had received previous systemic therapy: somatostatin analogues only (1); multiple lines of treatment including targeted therapy, liver-directed therapies, experimental therapies and chemotherapy (4); and were on active treatment: chemotherapy (3); somatostatin analogues (1); everolimus (1). For controls 1, patients with regional disease were included. Individuals in the controls 2 group had no prior diagnosis of dpNET up until sample collection or had no evidence of disease after previous duodenopancreatic surgery.
Genetically Engineered Mouse Model of Multiple Endocrine Neoplasia Type 1–Pancreatic Neuroendocrine Tumor
Additional information is provided in the Supplementary appendix materials and methods section (21). All mouse experiments were performed in compliance with the NIH guidelines for animal research and approved by the MDACC Institutional Animal Care and Use Committee.
Men1 conditional knockout (stock No. 005109) and Pdx1-CreTg (stock No. 014647) mice were obtained from the Jackson Laboratory (19, 22). Men1fl/flPdx1-CreTg mice recapitulate MEN1 disease progression from hyperplastic islets (age 5-6 months) to insulinomas (age 10-12 months), thereby providing a powerful model to study tumorigenesis in a consistent genetic background and controlled environment (18). Moreover, tumors from these mice exhibit several features of human tumors, including hypervascularity and vascular endothelial growth factor expression (18). Morbidity is associated with hypoglycemia caused by high levels of insulin produced by the functional pancreatic neuroendocrine tumors (pNETs) (18).
Mice were maintained on a mixed background (C57Bl/6, FVB, 129), Cre-negative littermates (Men1fl/fl) were used as functionally wild-type controls, and male and female mice were both used in our analyses. Blood was collected once every 4 months through retro-orbital sampling under general anesthesia. Mice that developed other cancers during the course of this study were excluded from the analysis.
Metabolomics Analysis
Detailed information is provided in the Supplementary online appendix materials and methods section (21). Measurement of plasma polyamines was conducted using Waters Acquity ultraperformance liquid chromatography system with 2-dimensional column regeneration configuration (I class and H class) coupled to a Xevo G2-XS quadrupole time-of-flight mass spectrometer (MS) as previously described (15-17). Peak picking and retention time alignment of liquid chromatography–mass spectrometry data were performed using Progenesis QI software (Nonlinear, Waters). Data processing and peak annotations were performed using an in-house automated pipeline. Annotations for polyamines were determined by matching accurate mass and retention times using customized libraries created from authentic standards and by matching experimental tandem MS data against the NIST MSMS or HMDB v3 theoretical fragmentations. For mouse specimens, values are reported as relative area units. For human biospecimens, values are reported as ratios relative to the median of historical quality control reference samples run with every analytical batch for the given analyte.
Statistical Analyses
For human cohorts, we report areas under the receiver operating characteristic curves (AUCs); AUCs were generated using R statistical software (https://www.r-project.org/). The 95% CIs for individual biomarker performance were based on the bootstrap procedure in which we resampled with replacement 2000 bootstrap samples. Sensitivity and specificity at 95% specificity/sensitivity thresholds were determined using the pROC package. A 3MP consisting of N-acetyputrescine (NAcPut), acetylspermidine (AcSpmd), and diacetylspermidine (DiAcSpmd) was developed using logistic regression models; selected polyamines were chosen by odds ratios greater than 1. Model building was performed in the initial test set 1 and model validation using fixed coefficients performed in independent test set 2 (see Table 1).
A 2-way analysis of variance was used to determine statistical significance for association between plasma polyamines and disease progression in a mouse model of Men1-pNET. Here, our 2 variables of interest were group (Men1fl/flPdx1-CreTg/Men1fl/fl) effects and time of blood draw. We applied the 3MP using fixed coefficients developed in our human cohort to the Men1-pNET mouse model. As we hypothesize that an elevation in plasma polyamines would be associated with disease progression in Men1fl/flPdx1-CreTg mice, we report one-sided P values for group (Men1fl/flPdx1-CreTg/Men1fl/fl) effect. Cox proportional hazard models were performed using R statistical software. To test for the proportional hazards assumption of a Cox regression, we used the method of Grambsch and Therneau (23). Log rank statistic–based methods as described by Contal and O’Quigley (24) were used to determine optimal change points for plasma polyamines in predicting survival as previously described (16, 25). Figures were generated in GraphPad Prism v6 or R statistical software.
Results
Testing of Plasma Polyamines for Identifying Patients With Multiple Endocrine Neoplasia Type 1 With Distant Metastatic Duodenopancreatic Neuroendocrine Tumors and Model Development
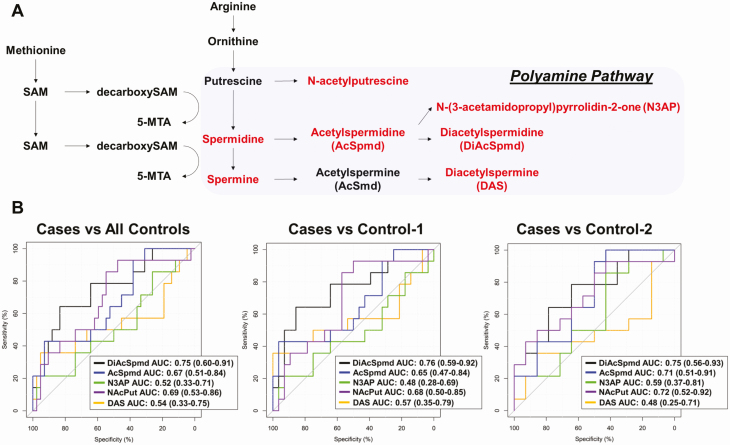
To determine the extent to which plasma polyamines may identify humans with distant metastatic MEN1-related dpNETs, we performed MS-based analyses on an initial set of plasmas (test set 1) from 14 patients (patients with MEN1 and with distant metastasis from a dpNET) and 42 controls (28 patients with MEN1 and with indolent dpNETs + 14 patients with MEN1 and without dpNETs) (see Table 1). Of the 5 quantifiable polyamines, NAcPut, AcSpmd, and DiAcSpmd were significantly elevated in plasma of patients compared to all controls with respective AUCs of 0.69 (95% CI, 0.53-0.86), 0.67 (95% CI, 0.51-0.84), and 0.75 (95% CI, 0.60-0.91) (Fig. 1A and 1B; Supplementary Fig. A1 and Table A1) (21). Spearman correlation analyses of plasma polyamines with age among controls yielded nonsignificant associations, suggesting that age is not a confounder in our analyses (data not shown). Subanalyses comparing the discrimination performance of NAcPut, AcSpmd, and DiAcSpmd for distinguishing cases from either control type yielded comparable findings (see Fig. 1B; Supplementary Table A1) (21). Notably, polyamines tended to be particularly elevated in plasmas of cases with a concurrent gastrinoma (Supplementary Fig. A2) (21).
Figure 1.
Predictive performance of plasma polyamines. A, Schematic of polyamine pathway. Polyamines highlighted in red represent those polyamines quantified in plasma of patients with multiple endocrine neoplasia type 1 (MEN1). B, Area under the receiver operating characteristic curve (AUC [95% CI]) of individual polyamines for distinguishing MEN1 patients with distant metastatic duodenopancreatic neuroendocrine tumors (dpNETs) (cases; n = 14) from MEN1 patients with indolent dpNETs and without distant or regional metastases (control 1; n = 28) as well as MEN1 patients without dpNETs (control 2; n = 14) in test set 1.
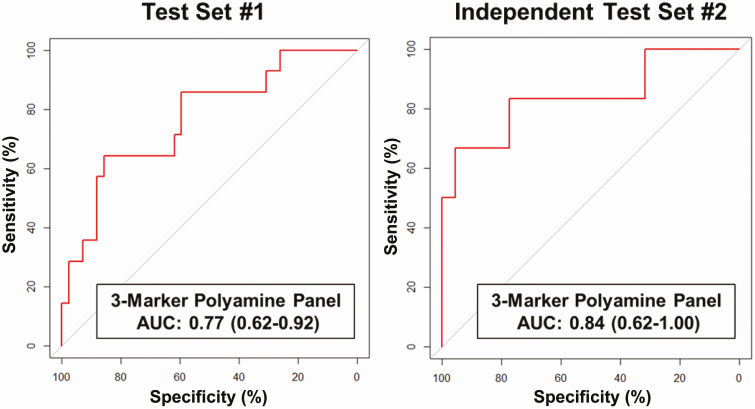
Using logistic regression models, we developed a 3MP consisting of NAcPut + AcSpmd + DiAcSpmd for distinguishing patients from controls. The resultant model yielded an AUC of 0.77 (95% CI, 0.62-0.92) with 28.6% sensitivity at 95% specificity (Fig. 2; see Supplementary Table A1) (21). Notably, in a subanalysis, the 3MP had an AUC of 0.74 (95% CI, 0.56-0.92) with 26.2% sensitivity at 95% specificity for distinguishing treatment-naive patients from controls.
Figure 2.
Predictive performance of a 3-marker polyamine panel. A, Area under the receiver operating characteristic curve (AUC [95% CI]) of a 3-marker polyamine panel consisting of N-acetylputrescine + acetylspermidine + diacetylspermidine developed in test set 1 for distinguishing cases (patients with multiple endocrine neoplasia type 1 [MEN1] with distant metastatic duodenopancreatic neuroendocrine tumors [dpNET]) from controls (patients with MEN1 with indolent dpNETs and without distant metastases as well as patients with MEN1 without dpNETs). B, AUC of the 3-marker polyamine panel using fixed coefficients from the logistic regression model developed in test set 1 for distinguishing cases (n = 6) from controls (n = 22) in independent test set 2.
The discrimination performance of individual polyamines as well as the fixed 3MP were further assessed in an independent test set (test set 2) of plasmas from 6 patients and 22 controls (see Table 1). The 3MP yielded an AUC of 0.84 (95% CI, 0.62-1.00) with 66.7% sensitivity at 95% specificity (see Fig. 2; Supplementary Fig. A3 and Table A2) (21). The AUC of the 3MP for distinguishing cases from patients with MEN1 with indolent dpNETs was 0.80 (95% CI, 0.53-1.00) with 50% sensitivity at 95% specificity. In a comparison of cases with patients with MEN1 without a dpNET, the 3MP yielded an AUC of 0.90 (95% CI, 0.71-1.00) with 66.7% sensitivity at 95% specificity (see Supplementary Table A2) (21).
Plasma Polyamines and Disease Progression in a Men1 Mouse Model of Pancreatic Neuroendocrine Tumors
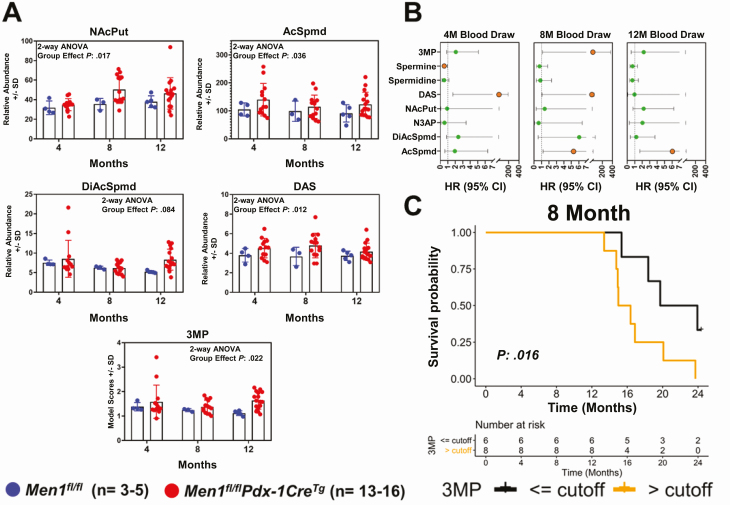
A cohort of Men1fl/flPdx1-CreTg mice was established to evaluate time-dependent changes in plasma polyamine level throughout Men1-pNET disease progression. Serial blood samples were collected at the following time points: age 4 months (limited hyperplasia), age 8 months (frequent islet hyperplasia), and age 12 months (extensive hyperplasia and pNET). The analyzed cohort had a median survival of 559 days (age 18 months) and, as expected, moribund animals had significantly reduced blood glucose levels, indicative of insulinomas (Supplementary Fig. A4) (21). Using MS, 7 distinct polyamines were detected and quantified in plasmas of Men1fl/flPdx1-CreTg mice and Men1fl/fl littermate controls (Fig. 3A; Supplementary Fig. A5) (21). In comparison to Men1fl/fl controls, time-dependent analyses revealed an initial rise in plasma levels of NAcPut, AcSpmd, and diacetylspermine (DAS) at the 4-month time point when Men1fl/flPdx1-CreTg mice typically begin developing hyperplastic islets (see Fig. 3A). Plasma levels of NAcPut, AcSpmd, and DAS remained elevated at 8- and 12-month time points when mice progressed to develop insulinomas (see Fig. 3A; Supplementary Fig. A5) (18, 21). A rise in plasma DiAcSpmd was additionally observed in Men1fl/flPdx1-CreTg mice at the 12-month time point compared to littermate controls (see Fig. 3A). We next applied the 3MP using fixed coefficients developed in our human cohort to the Men1-pNET mouse model. In comparison to Men1fl/fl controls, Men1fl/flPdx1-CreTg mice exhibited an initial increase in the 3MP starting at the 4-month blood draw and the 3MP remained elevated through disease progression (2-way analysis of variance group effect 1-sided P: .022) (see Fig. 3A).
Figure 3.
Association between plasma polyamines and disease progression in a mouse model of multiple endocrine neoplasia type 1–pancreatic neuroendocrine tumor (MEN1-pNET). A, Scatter plots illustrating relative abundance (area units ± SD) of plasma polyamines N-acetylputrescine (NAcPut), acetylspermidine (AcSpmd), diacetylspermidine (DiAcSpmd), diacetylspermine (DAS), and the polyamine signature at 4-, 8-, and 12-month blood collection time points in Men1fl/flPdx1-CreTg mice and littermate (Men1fl/fl) controls. The 3-marker plasma polyamine signature (3MP) consisting of AcSpmd + DiAcSpmd + NAcPut was developed in the human cohort of patients with MEN1 and applied, using fixed coefficients, to the MEN1-pNET mouse model. Statistical significance was determined by 2-way analysis of variance (ANOVA); 1-sided P values for group effect (Men1fl/flPdx1-CreTg/Men1fl/fl status) are reported. B, Time-dependent hazard ratios (HR; 95% CI) per unit log2 increase in relative abundance (area units) of individual polyamines as well as the 3MP for association with overall survival. Orange nodes indicate statistical significance (1-sided P < .05) C, Kaplan-Meier survival curve illustrating association between plasma polyamine signature scores at the 8-month blood collection time point and overall survival in Men1fl/flPdx1-CreTg mice. High (> cutoff; orange line) and low (≤ cutoff; black line) levels of the polyamine signature were defined based on an optimal changepoint value–derived log rank test statistics-based method as described by Contal and O’Quigley (24). Statistical significance between survival curves was determined by 1-sided log-rank (Mantel-Cox) test.
Using Cox proportional hazard models, we evaluated whether plasma polyamines and the polyamine signature were associated with overall survival in Men1fl/flPdx1-CreTg mice. Elevated polyamines were generally found to be associated with poor prognosis (Fig. 3B). At the 8-month time point, the 3MP was statistically significantly associated with poor overall survival among Men1fl/flPdx1-CreTg mice; Kaplan-Meier survival curves using an optimal changepoint for the 3MP at the 8-month time point demonstrated that an elevated 3MP was a prognostic predictor of poor overall survival in Men1fl/flPdx1-CreTg mice (median survival: 15.7 months vs 21.8 months; 1-sided log-rank (Mantel-Cox) test P = .016) (Fig. 3C). A similar result was obtained at the 12-month time point (Supplementary Fig. A5) (21). No differences were observed in blood glucose levels between Men1fl/flPdx1-CreTg mice that had poor survival compared to mice with longer survival at the 8-month time point (Supplementary Fig. A6) (21), indicating that plasma polyamines rise before onset of hypoglycemia.
Discussion
Herein we took advantage of our established international collaboration that enabled procurement of biospecimens with limited availability from well-annotated and characterized patient cohorts that included cases that were treatment naive to assess the predictive performance of polyamines for identifying patients with MEN1 at high risk of harboring distant metastatic dpNETs. We further leveraged a Men1fl/flPdx1-CreTg mouse model of MEN1-pNET to assess longitudinal trajectories of polyamines with disease progression.
In MEN1-related dpNET, surgery is the cornerstone of treatment aimed at curing any functional syndrome and preventing distant metastasis. Because of asynchronous multifocal primary tumor development and imprecise estimation of future risk of metastasis, the decision to proceed with duodenopancreatic surgery requires balancing the risk of future metastasis against the short- and longer-term risks of surgery (26). Currently, risk of future metastasis is predicted based on consideration of pNET size (> 2 cm), functionality, growth, and World Health Organization grade (27, 28). However, not all “high-risk” patients will develop distant metastasis. Moreover, distant metastasis can also occur among “low-risk” patients (3, 29). Consequently, there is a clinical need to develop biomarkers that can better predict which patients with MEN1 are likely to develop aggressive disease with distant metastasis, and therefore more accurately select patients for potentially curative surgical intervention and avoid unnecessary surgical intervention. To this end, tissue markers in clinical decision making have thus far been limited given the multifocal tumors and the need for invasive procedures. Clinically available biomarkers for MEN1-related and sporadic neuroendocrine neoplasms have been largely restricted to secreted hormones or amines that are primarily used as diagnostic and therapeutic markers with limited performance (30).
Our studies demonstrate a concordance between a mouse model and human MEN1 individuals with respect to an association between polyamines and dpNET progression. Compared to normal pancreatic tissues, activity of ODC1, a rate-limiting enzyme in the polyamine pathways, has been shown to be elevated in non-MEN1 NETs (11). Polyamine metabolizing enzymes, such as ODC1, are transcriptionally regulated by oncogenic MYC (31). A previous report demonstrated that loss of Men1 promotes upregulation of Myc in mouse-derived pancreatic cells (32). Myc has also been shown to be a key regulator of ductal-neuroendocrine lineage plasticity in pancreatic cancer (33).
Gastrinomas in MEN1 are associated with worse survival and a higher metastatic potential compared to patients with MEN1 with nongastrinoma dpNETs (8, 34). Elevated levels of gastrin (> 20-fold increase) have shown to be associated with poor prognosis among patients with MEN1-related gastrinoma (6, 34, 35). However, not all MEN1-related gastrinomas are aggressive and a primary challenge remains to identify those patients who harbor an aggressive MEN1-related gastrinoma (35). We found that plasma polyamines were particularly elevated in patients with MEN1 with distant metastasis and concurrent gastrinoma. We acknowledge that none of the controls in our test set 1 had a gastrinoma and that the presence of a concurrent gastrinoma among patients does not infer that the gastrinoma is the primary cause of the distant metastasis nor that the pNET is the source of elevated gastrin. However, our findings that polyamines are associated with disease progression and are prognostic of poor survival in mice with pNETs that do not have a gastrinoma favors the notion that polyamines are associated with aggressive disease. Moreover, polyamines where able to distinguish patients with MEN1 with metastatic dpNETs who do not have a concurrent gastrinoma from respective controls in independent test set 2.
There are some limitations to cohort studies of rare diseases like MEN1. Controls tend to be younger than patients, an aspect that is intrinsic to disease progression. However, Spearman correlation analyses between plasma polyamine levels and patient age did not reveal significant associations. Although controls were confirmed to be absent of metastatic disease within 1 year of sample collection, there is no certainty that these patients will not go on to develop future distant metastases. Systemic treatment may affect plasma polyamine levels. We emphasize that for test set 1, 72% of cases were treatment naive at the time of blood collection, and although cases in independent test set 2 had prior treatment or were on active treatment at the time of blood draw, the predictive performance of the 3MP yielded a similar AUC in both cohorts for distinguishing patients from controls. This would suggest that prior treatment is not a major confounding contributor to elevated polyamine levels. Whether plasma polyamines will be high in patients with MEN1 who harbor other non-dpNETs remains to be determined. Moreover, it is unclear if and how other manifestations of the disease, such as primary hyperparathyroidism, pituitary adenomas, and adrenal adenomas, may affect the polyamine signature. As patients in the present study had developed distant metastasis, it is not clear from the human data whether plasma polyamines provide sufficient lead time for risk of developing future metastasis. Given our finding that plasma polyamines are elevated during early stages of disease in Men1fl/flPdx1-CreTg mice, it is possible that a similar increase may occur early among individuals destined to develop distant metastasis. However, we note that insulinomas are the primary tumors found in mouse models with Men1 deficiency and morbidity is typically associated with insulin secretion and subsequent hypoglycemia rather than metastatic disease burden (18, 19, 36). Nevertheless, our investigations revealed that plasma polyamines are elevated in plasma of Men1fl/flPdx1-CreTg and that elevated polyamine levels are prognostic of poor overall survival in these mice. Prior studies have demonstrated a prognostic significance for polyamines. We previously reported that a plasma polyamine signature predicts future distant metastasis among women with triple-negative breast cancer (16). Future work will focus on elucidating the molecular changes that underlie the survival difference in Men1fl/flPdx1-CreTg as well as evaluating the predictive performance of polyamines for identifying people at high risk for developing future distant metastasis.
In conclusion, our study provides a basis for prospective testing of blood-based polyamines as a potential means for monitoring patients with MEN1 for harboring or developing aggressive disease. If confirmed in prospective studies, this polyamine signature may have utility for predicting progression and distant metastasis among individuals with MEN1.
Acknowledgements
We additionally thank Nikita Williams for technical assistance. We thank Sara Raymon for her important role in the collection of the human biospecimens.
Financial Support: This work was supported by The University of Texas MD Anderson Cancer Center Moon Shots Program, the McKee Early Career Investigator in Pancreatic Cancer Research, the Neuroendocrine Tumor Research Foundation, The National Institutes of Health and National Cancer Institute (NIH/NCI grant No. NIH/NCI R21 CA252426-01), and the intramural research program of the NIH/NIDDK and NIH/NCI. K.A.D. was partially supported by a Cancer Center Support Grant (NCI grant No. P30 CA016672), the NIH (grant Nos. UL1TR003167 and 5R01GM122775), and the prostate cancer Specialized Program of Research Excellence SPORE P50 CA140388, CPRIT grant (RP160693). We additionally thank Nikita Williams for technical assistance. We thank Sara Raymon for her important role in the collection of the human biospecimen.
Glossary
Abbreviations
- 3MP
3-marker plasma polyamine signature
- AcSpmd
acetylspermidine
- AUC
area under the curve
- DAS
diacetylspermine
- DiAcSpmd
diacetylspermidine
- dpNET
duodenopancreatic neuroendocrine tumor
- IRB
institutional review board
- MDACC
The University of Texas MD Anderson Cancer Center
- MEN1
multiple endocrine neoplasia type 1
- MS
mass spectrometry
- NAcPut
N-acetyputrescine
- NET
neuroendocrine tumor
- pNet
pancreatic neuroendocrine tumor
- UMCU
University Medical Center Utrecht.
Additional Information
Disclosures: D.M.H. has received research funding from AAA, Lexicon, Incyte, Genentech, and Tarveda. He has also had consulting roles with AAA, Lexicon, Ipsen, and Curium. J.B. is a full-time employee at AstraZeneca. The other authors have nothing to disclose.
Data Availability
Relevant data supporting the findings of this study are available within the article and supplementary information or are available from the authors on reasonable request.
References
- 1. Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276(5311):404-407. [DOI] [PubMed] [Google Scholar]
- 2. de Laat JM, van der Luijt RB, Pieterman CR, et al. MEN1 redefined, a clinical comparison of mutation-positive and mutation-negative patients. BMC Med. 2016;14(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Triponez F, Dosseh D, Goudet P, et al. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg. 2006;243(2):265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thakker RV, Newey PJ, Walls GV, et al. ; Endocrine Society . Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990-3011. [DOI] [PubMed] [Google Scholar]
- 5. Goudet P, Murat A, Binquet C, et al. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34(2):249-255. [DOI] [PubMed] [Google Scholar]
- 6. Ito T, Igarashi H, Uehara H, Berna MJ, Jensen RT. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger-Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine (Baltimore). 2013;92(3):135-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nell S, Verkooijen HM, Pieterman CRC, et al. Management of MEN1 related nonfunctioning pancreatic NETs: a shifting paradigm: results from the DutchMEN1 Study Group. Ann Surg. 2018;267(6):1155-1160. [DOI] [PubMed] [Google Scholar]
- 8. Vinault S, Mariet AS, Le Bras M, et al. Metastatic potential and survival of duodenal and pancreatic tumors in multiple endocrine neoplasia type 1: a GTE and AFCE Cohort Study (Groupe d’étude des Tumeurs Endocrines and Association Francophone de Chirurgie Endocrinienne). Ann Surg. 2020;272(6):1094-1101. [DOI] [PubMed] [Google Scholar]
- 9. Soda K. The mechanisms by which polyamines accelerate tumor spread. J Exp Clin Cancer Res. 2011;30(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerner EW, Meyskens FL Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4(10):781-792. [DOI] [PubMed] [Google Scholar]
- 11. Subhi AL, Tang B, Balsara BR, et al. Loss of methylthioadenosine phosphorylase and elevated ornithine decarboxylase is common in pancreatic cancer. Clin Cancer Res. 2004;10(21):7290-7296. [DOI] [PubMed] [Google Scholar]
- 12. Mastracci TL, Robertson MA, Mirmira RG, Anderson RM. Polyamine biosynthesis is critical for growth and differentiation of the pancreas. Sci Rep. 2015;5:13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Townsend CM Jr, Ishizuka J, Thompson JC. Studies of growth regulation in a neuroendocrine cell line. Acta Oncol. 1993;32(2):125-130. [DOI] [PubMed] [Google Scholar]
- 14. Evers BM, Hurlbut SC, Tyring SK, Townsend CM Jr, Uchida T, Thompson JC. Novel therapy for the treatment of human carcinoid. Ann Surg. 1991;213(5):411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fahrmann JF, Bantis LE, Capello M, et al. A plasma-derived protein-metabolite multiplexed panel for early-stage pancreatic cancer. J Natl Cancer Inst. 2019;111(4):372-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fahrmann JF, Vykoukal J, Fleury A, et al. Association between plasma diacetylspermine and tumor spermine synthase with outcome in triple-negative breast cancer. J Natl Cancer Inst. 2020;112(6):607-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fahrmann JF, Irajizad E, Kobayashi M, et al. A MYC-driven plasma polyamine signature for early detection of ovarian cancer. Cancers (Basel). 2021;13(4):913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen HCJ, He M, Powell A, et al. Recapitulation of pancreatic neuroendocrine tumors in human multiple endocrine neoplasia type I syndrome via Pdx1-directed inactivation of Men1. Cancer Res. 2009;69(5):1858-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crabtree JS, Scacheri PC, Ward JM, et al. Of mice and MEN1: insulinomas in a conditional mouse knockout. Mol Cell Biol. 2003;23(17):6075-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fahrmann J, Pieterman C, Wasylishen A.Supplementary data for: A blood-based polyamine signature associated with MEN1 duodenopancreatic neuroendocrine tumor progression. Figshare 2021. Uploaded May 5, 2021. 10.6084/m9.figshare.14639079.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437-450. [DOI] [PubMed] [Google Scholar]
- 23. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. [Google Scholar]
- 24. Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30(3):253-270. [Google Scholar]
- 25. Vykoukal J, Fahrmann JF, Gregg JR, et al. Caveolin-1-mediated sphingolipid oncometabolism underlies a metabolic vulnerability of prostate cancer. Nat Commun. 2020;11(1):4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pieterman CRC, Sadowski SM, Maxwell JE, et al. Hereditary endocrine tumours: current state-of-the-art and research opportunities: MEN1-related pancreatic NETs: identification of unmet clinical needs and future directives. Endocr Relat Cancer. 2020;27(8):T9-T25. [DOI] [PubMed] [Google Scholar]
- 27. Sadowski SM, Pieterman CRC, Perrier ND, Triponez F, Valk GD. Prognostic factors for the outcome of nonfunctioning pancreatic neuroendocrine tumors in MEN1: a systematic review of literature. Endocr Relat Cancer. 2020;27(6):R145-R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Beek DJ, Nell S, Verkooijen HM, Borel Rinkes IHM, Valk GD, Vriens MR; DutchMEN Study Group (DMSG); International MEN1 Insulinoma Study Group; DutchMEN Surgery Study Group . Prognosis after surgery for multiple endocrine neoplasia type 1-related pancreatic neuroendocrine tumors: functionality matters. Surgery. 2021;169(4):963-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pieterman CRC, de Laat JM, Twisk JWR, et al. Long-term natural course of small nonfunctional pancreatic neuroendocrine tumors in MEN1—results from the Dutch MEN1 Study Group. J Clin Endocrinol Metab. 2017;102(10):3795-3805. [DOI] [PubMed] [Google Scholar]
- 30. Herrera-Martínez AD, Hofland LJ, Gálvez Moreno MA, Castaño JP, de Herder WW, Feelders RA. Neuroendocrine neoplasms: current and potential diagnostic, predictive and prognostic markers. Endocr Relat Cancer. 2019;26(3): R157-R179. [DOI] [PubMed] [Google Scholar]
- 31. Bachmann AS, Geerts D. Polyamine synthesis as a target of MYC oncogenes. J Biol Chem. 2018;293(48):18757-18769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gurung B, Feng Z, Hua X. Menin directly represses Gli1 expression independent of canonical Hedgehog signaling. Mol Cancer Res. 2013;11(10):1215-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farrell AS, Joly MM, Allen-Petersen BL, et al. MYC regulates ductal-neuroendocrine lineage plasticity in pancreatic ductal adenocarcinoma associated with poor outcome and chemoresistance. Nat Commun. 2017;8(1):1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Beek DJ, Nell S, Pieterman CRC, et al. Prognostic factors and survival in MEN1 patients with gastrinomas: results from the DutchMEN Study Group (DMSG). J Surg Oncol. 2019;120(6):966-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gibril F, Venzon DJ, Ojeaburu JV, Bashir S, Jensen RT. Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab. 2001;86(11):5282-5293. [DOI] [PubMed] [Google Scholar]
- 36. Shen HCJ, Ylaya K, Pechhold K, et al. Multiple endocrine neoplasia type 1 deletion in pancreatic alpha-cells leads to development of insulinomas in mice. Endocrinology. 2010;151(8):4024-4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Relevant data supporting the findings of this study are available within the article and supplementary information or are available from the authors on reasonable request.