Abstract
Context
Patients with congenital adrenal hyperplasia (CAH) are exposed to hyperandrogenism and supraphysiologic glucocorticoids, both of which can increase risk of metabolic morbidity.
Objective
Our aim was to evaluate cardiovascular and metabolic morbidity risk in a longitudinal study of patients with CAH spanning both childhood and adulthood.
Design and Setting
Patients with classic CAH followed for a minimum of 5 years during both childhood and adulthood (n = 57) at the National Institutes of Health were included and compared with the US general population using NHANES data.
Main outcome measures
Obesity, hypertension, insulin resistance, fasting hyperglycemia, and dyslipidemia.
Results
Compared to the US population, patients with CAH had higher (P < 0.001) prevalence of obesity, hypertension, insulin resistance, fasting hyperglycemia, and low high-density lipoprotein (HDL) during childhood and obesity (P = 0.024), hypertension (P<0.001), and insulin resistance (P < 0.001) during adulthood. In our cohort, obesity, hypertension, fasting hyperglycemia, and hypertriglyceridemia began prior to age 10. During childhood, increased mineralocorticoid dose was associated with hypertension (P = 0.0015) and low HDL (P = 0.0021). During adulthood, suppressed androstenedione was associated with hypertension (P = 0.002), and high low-density lipoprotein (P = 0.0039) whereas suppressed testosterone (P = 0.003) was associated with insulin resistance. Elevated 17-hydroxyprogesterone, possibly reflecting poor disease control, was protective against high cholesterol (P = 0.0049) in children. Children whose mothers were obese (maternal obesity) had increased risk of obesity during adulthood (P = 0.0021). Obesity, in turn, contributed to the development of hypertension, insulin resistance, and hypertriglyceridemia in adulthood.
Conclusion
Patients with CAH develop metabolic morbidity at a young age associated with treatment-related and familial factors. Judicious use of glucocorticoid and mineralocorticoid is warranted.
Keywords: metabolic syndrome, congenital adrenal hyperplasia, androgen, glucocorticoid, mineralocorticoid
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (21-OHD) is an autosomal recessive disorder characterized by multiple hormonal imbalances . Symptoms are caused by disease-related glucocorticoid and mineralocorticoid deficiencies as well as shunting of steroid precursors into androgen production[1]. Glucocorticoid and mineralocorticoid replacement is the mainstay of treatment for the classic form. Treatment-related comorbidities are also common as glucocorticoid doses often exceed physiological replacement to control androgen production; furthermore, oral replacement fails to mimic normal circadian rhythm (2).
Risk factors for cardiovascular disease (CVD) and metabolic morbidity have been reported in CAH patients with variable prevalence. A cross-sectional, population-based registry study of 588 21-OHD CAH patients in Sweden revealed an increased prevalence of hypertension, abnormal lipid profile, and venous thromboembolism compared to matched controls (3); however, the study did not investigate the role of androgens or glucocorticoid and mineralocorticoid therapy. A recent meta-analysis of 20 studies of patients with CAH confirmed the high prevalence of metabolic morbidity with increased risk of elevated blood pressure (BP), insulin resistance, and carotid intima thickness but found a high degree of bias and heterogeneity in the studies rendering quality of evidence low (4).
In a study from United Kingdom, obesity, hypercholesterolemia, and insulin resistance were commonly found among 203 adults with CAH (5). Risk factors for CVD and metabolic morbidity have also been reported in children with CAH (6-8), but prospective data are lacking. Furthermore, age at onset of risk factors is unknown, and correlations with disease control and glucocorticoid therapy have not been evaluated.
The aims of this study were to compare the prevalence of cardiovascular risk factors and metabolic morbidity in a cohort of patients with classic CAH to those in the US general population, to identify age of onset and describe changes in these outcomes over time, and to evaluate these cardiovascular risk factors in relation to glucocorticoid and mineralocorticoid dose, genotype, sex and other CAH-related characteristics.
Methods
Patients
Records of patients enrolled in the Natural History Study of patients with CAH at the National Institutes of Health Clinical Center (Bethesda, MD, USA; ClinicalTrials.gov Identifier no. NCT00250159) were reviewed. Criteria for inclusion were longitudinal enrollment for at least 5 years that spanned both childhood and adulthood and a diagnosis of classic 21-OHD CAH defined by clinical and hormonal criteria and confirmed by genotyping, as previously described (9). Pediatric patients were defined as age < 18 years. For descriptive purposes, patients under the age of 2 were referred to as young children, 2- to 5-years-old as preschoolers, 6-to 11-years-old as school-aged, 12- to 17-years-old as adolescents, 18- to 29-years old as young adults, and 30 or older as adults.
The study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Development Institutional Review Board and all patients (adults) or parents (children) provided written informed consent. Children at least 8 years old provided written assent.
Clinical data and definitions
Pediatric patients were seen every 6 months, and adults were seen annually. Clinical and biochemical assessments were done at each visit or more frequently if clinically indicated. Morning fasting blood samples were collected before taking medications. The main outcomes of interest were obesity, hypertension, insulin resistance, fasting hyperglycemia, dyslipidemia, and metabolic syndrome.
Height was measured by a stadiometer as an average of 3 measurements. Percentiles and SD score (SDS) for weight, height, and body mass index (BMI) were calculated during childhood based on National Health and Nutrition Examination Survey (NHANES) data. Obesity was defined as BMI ≥ 95th percentile during childhood or BMI ≥ 30 kg/m2 during adulthood. For patients under the age of 2 years, World Health Organization percentiles were used for BMI and, based on extrapolation of overweight status in the Centers for Disease Control and Prevention curves, ≥85th percentile was the cutoff for an abnormal weight (10). Maternal obesity during childhood was defined as maternal BMI ≥ 30 kg/m2 at first visit.
Blood pressure was measured while sitting using an automated measurement device and, if elevated, was repeated using the device and then finally by manual measurement. Classification of BP was done using the 2017 American Academy of Pediatrics guidelines for children (11) and the 2017 report of American College of Cardiology/American Heart Association Task Force for Adults (12). Fasting glucose was categorized as normal <100 mg/dL; fasting hyperglycemia ≥100 mg/dL. Insulin resistance was defined based on homeostasis model assessment of insulin resistance (HOMA-IR) as insulin (µU/mL) × glucose (mmol/L)/22.5 (13). HOMA-IR above 2.5 in adults or above 3.16 in adolescents (≥12 years) were considered abnormal (14).
Metabolic syndrome (MetS) was defined using the modified National Cholesterol Education Program Adult Treatment Panel III devised by the American Heart Association and National Heart, Lung, and Blood Institute (15). MetS was diagnosed if adults had any 3 of the following: fasting blood glucose ≥100 mg/dL, BP ≥130/85, high-density lipoprotein cholesterol (HDL) < 40 mg/dL in men or <50 mg/dL in women, triglyceride (TGL) ≥ 150 mg/dL, or waist circumference ≥102 cm (men) or ≥88 cm (women). For pediatric patients 4 years and older, MetS was defined using Weiss et al criteria (16) if they had 3 or more of the following: fasting blood glucose ≥100 mg/dL, BP >95th percentile, TGL > 95th percentile, HDL < 5th percentile, or BMI ≥ 95th percentile. Visits with insufficient data to assess MetS were excluded from applicable analysis.
NHANES data (2015-2016) (17) were used to interpret lipids based on age, race, and sex specific norms for pediatrics. Other lipids taken into consideration were total cholesterol and low-density lipoprotein cholesterol (LDL). For adults, elevated LDL was defined as >130mg/dL and elevated cholesterol was defined as >200 mg/dL (18). For children, both parameters were defined as elevated if >95th percentile.
17-Hydroxyprogesterone (17-OHP) was categorized as suppressed if below 100 ng/dL. Androstenedione, plasma renin activity, and total testosterone were all categorized as suppressed, normal, or high based on age-, tanner stage– and sex-specific normal ranges. Male hypogonadism was defined in adults as testosterone below the normal range. Prednisone and dexamethasone were represented as hydrocortisone equivalent: 5 times the prednisone dose and 80 times the dexamethasone dose (9). Glucocorticoid dose was calculated as total daily dose expressed in mg/m2/day and as percentage of daily glucocorticoid dose given at night.
Age- and sex-specific population control data were derived from NHANES’s 2015-2016 database. Controls were excluded if pregnant or if physical examination was not performed. Laboratory data were excluded if not obtained fasting. NHANES anthropometric data were available starting in infancy; BP was measured starting at age 8, and total cholesterol and HDL were measured after age 6 whereas TGL, insulin, and glucose were measured starting at age 12. For comparative purposes, prevalence of metabolic morbidity in children with CAH was calculated based on the first occurrence after applying age restrictions based on NHANES methodology. For adults, NHANES data was restricted to subjects under 40 years to make them comparable to our CAH cohort.
Statistical analysis
For each outcome of interest, the effect of age, sex, phenotype, genotype, maternal obesity, glucocorticoid dose, glucocorticoid type, mineralocorticoid dose, androstenedione, 17-OHP, and testosterone was studied by univariable and multivariable analyses. The effect of adult height SDS was also evaluated for hypertension and metabolic syndrome.
Data are reported as mean ± SD, median [interquartile range (IQR): 25th-75th percentile], odds ratios (OR), and 95% confidence intervals (CIs) or as frequencies and percentages. Distributional assumptions were assessed, and if appropriate, nonparametric tests were utilized. The prevalence of cardiovascular and metabolic risk factors were determined in CAH patients and NHANES healthy subjects and were compared using chi-square or Fisher’s exact tests, as appropriate; ORs and 95% CIs were also computed. Generalized linear models were used to assess the relation between various factors and cardiovascular and metabolic risk factor outcomes. Observation-level analyses accounted for repeated measures and correlated data by utilizing generalized estimating equations. The Bonferroni correction was applied to multiple comparisons involving multivariable analyses. Data were analyzed using SAS v9.4 (SAS Institute, Inc, Cary, NC, USA). Statistical evidence was determined based on data variability and measures of spread, and magnitude of effect (OR) with corresponding 95% CIs along with the actual P-values rather than establishing dichotomized statistical significance based on the arbitrary cutoff of 0.05.
Results
Patients
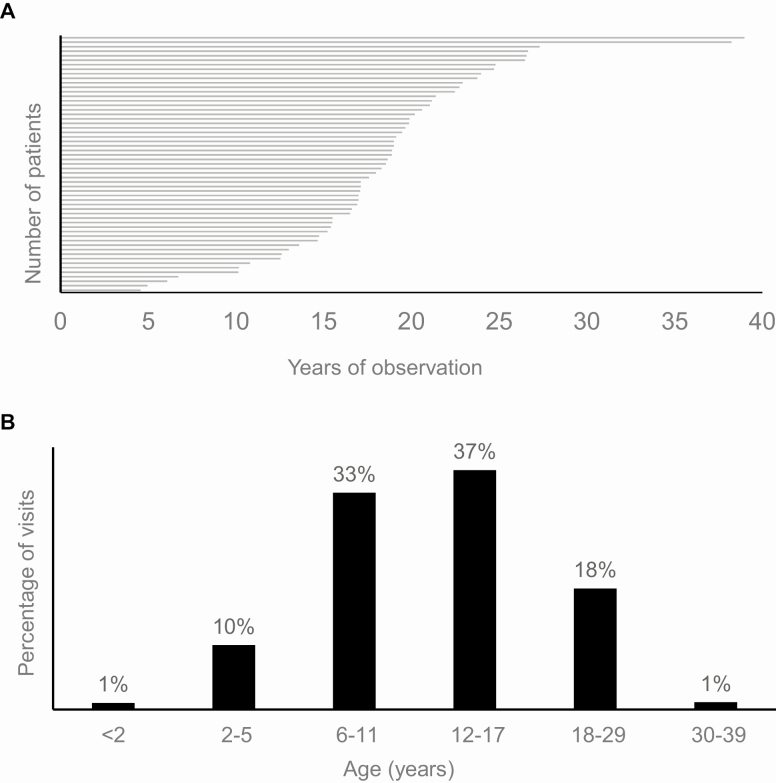
Our cohort of 57 patients (61.4% male; 84.2% white) consisted mostly (68.4%) of classic salt-wasting CAH who were evaluated over 1962 visits spanning childhood and adulthood. The median age at first visit was 5.3 years (IQR 3.0-7.9), with median follow-up of 18.6 years (IQR 15.4-21.2) (Fig. 1A).
Figure 1.
Cohort of patients with classic congenital adrenal hyperplasia. (A) Duration of follow-up per patient and (B) age distribution at visits are shown.
Prevalence of cardiovascular and metabolic risk factors
Compared to NHANES subjects, patients with CAH had higher (P < 0.0001) prevalence of obesity, hypertension, insulin resistance, fasting hyperglycemia, and low HDL during childhood and higher prevalence of obesity (P = 0.024), hypertension (P < 0.001), and insulin resistance (P < 0.001) during adulthood (Table 1).
Table 1.
Cardiovascular and metabolic risk factors in patients with congenital adrenal hyperplasia compared to age- and sex- specific NHANES population controls
| Pediatric | Adult | |||||||
|---|---|---|---|---|---|---|---|---|
| CAHa (n = 57), n (%) | NHANES/ Total n (%)a,b | OR (95% CI) | P-value | CAHa (n = 57), n (%) | NHANES/ Total n (%)a,b | OR (95% CI) | P-value | |
| Obesity | 32 (56.1) | 597 (15.9)/3754 | 6.8 (4.0-11.5) | <0.001 | 28 (49.1) | 706 (34.5)/2046 | 1.8 (1.1-3.1) | 0.024 |
| Hypertension (stage 1 and 2) | 50 (87.7) | 96 (5.5)/1750 | 123.0 (54.3- 278.6) | <0.001 | 36 (63.2) | 383 (19.2)/1992 | 6.7 (3.9-11.5) | <0.001 |
| Insulin resistance | 41 (71.9) | 117 (33.1)/353 | 5.2 (2.8-9.6) | <0.001 | 46 (80.7) | 330 (41.0)/805 | 6.0 (3.1-11.8) | <0.001 |
| Fasting hyperglycemia | 43 (75.4) | 106 (27.0)/392 | 8.3 (4.4-15.8) | <0.001 | 26 (45.6) | 324 (35.5)/912 | 1.5 (0.9-2.6) | 0.13 |
| High total Cholesterol | 4 (7.0) | 35 (5.7)/610 | 1.2 (0.4-3.6) | 0.69 | 8 (14.0) | 227 (23.8)/954 | 0.5 (0.2-1.1) | 0.095 |
| Low HDL | 24 (42.1) | 24 (3.9)/610 | 17.8 (9.1-34.6) | <0.001 | 23 (40.4) | 268 (29.5)/910 | 1.6 (0.9-2.8) | 0.084 |
| High TGL | 11 (19.3) | 48 (12.4)/388 | 1.7 (0.8-3.5) | 0.15 | 9 (15.8) | 172 (19.0)/907 | 0.8 (0.4-1.7) | 0.55 |
Abbreviations: CAH, congenital adrenal hyperplasia; CI, confidence interval; HDL, high-density lipoprotein; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; TGL, triglycerides.
a For purposes of comparability, data in CAH were restricted to match the data that were available in NHANES: anthropometric data were since birth, cholesterol and HDL for ≥ age 6, blood pressure ≥ age 8, TGL, insulin and blood glucose ≥ age 12. Thus, reported prevalences in this table may vary slightly from those reported in the text that were observed in CAH. NHANES adult data were further restricted to age <40 to be comparable to the CAH adults. NHANES denominators for each outcome vary due to the described age restrictions.
b Excluded if fasted <8 h or pregnant.
Treatment and biochemical disease control
At their first visit during childhood, 52 patients (91.2%) were being treated with hydrocortisone, while a variety of glucocorticoids were used during adulthood. Hydrocortisone equivalency dose was similar at the first (17.7 ± 9.2) and most recent (17.4 ± 6.5) visits; mineralocorticoid dose was also similar at the first (94.2 ± 58.3 mcg/day) and most recent (119.3 ± 65.6 mcg/day) visits.
Overall, androstenedione and 17-OHP were in a normal or acceptable range, respectively, each about 28% of the time, while testosterone was mostly in the normal range (74% of visits). Plasma renin activity was in the normal range at about half (49%) of the visits.
Cardiovascular and Metabolic Morbidity
Obesity
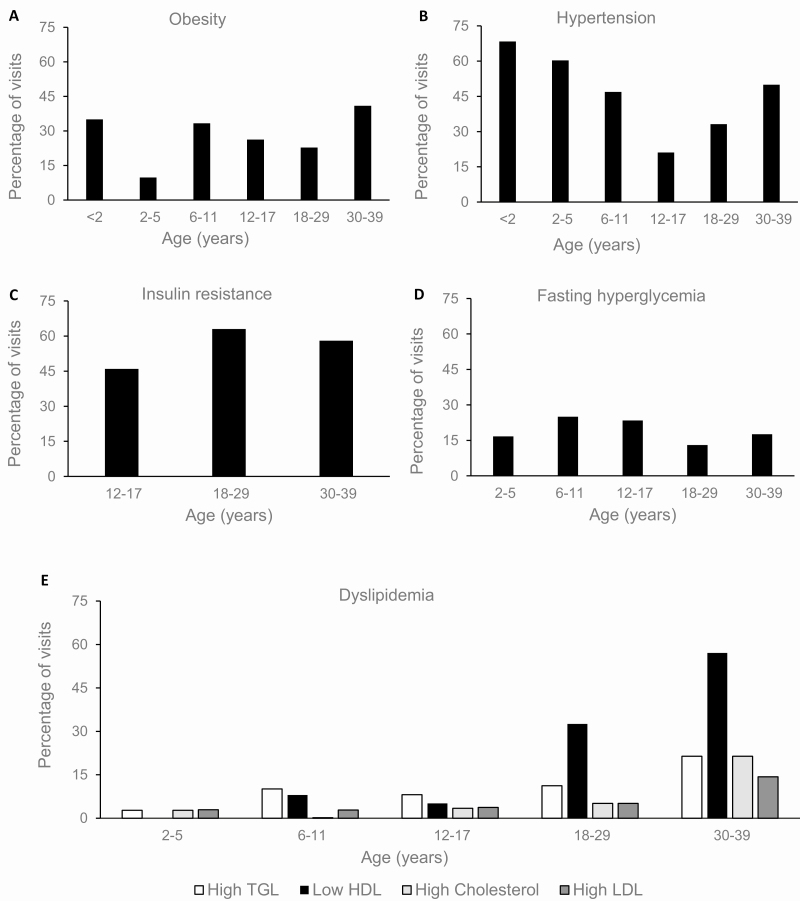
The median age of onset of obesity was 8.3 years (IQR 6.0-15.2). Obesity was observed in 40 (70.2%) patients at 1 or more visits. The 2 age groups with the highest obesity rates were young children (<2 year) and adults (>30 years) (Fig. 2A). An increase in the prevalence of obesity was seen from preschool to school-aged children followed by a downward trend as patients went through puberty and into young adulthood. Sex, glucocorticoid dose, type or percentage nighttime dose, and adrenal biomarkers were not independently associated with obesity during childhood or adulthood.
Figure 2.
Metabolic outcomes across age categories in a cohort of patients with classic congenital adrenal hyperplasia. Shown are (A) obesity, defined as BMI ≥ 85th percentile for <2 years, ≥95th percentile 2 to <18 years, or BMI ≥ 30 kg/m2 for ≥18 years; (B) hypertension (stage 1 and 2) based on the American Academy of Pediatrics’s 2017 guidelines for children and the American College of Cardiology/American Heart Association 2017 Task Force for Adults (11,12); (C) insulin resistance based on HOMA-IR: ≥2.5 in adults or ≥3.16 in adolescents (≥12 years) (13,14); (D) fasting hyperglycemia (blood glucose ≥100); (E) dyslipidemia (based on NHANES percentiles for age and sex in children and specific targets for adults based on sex) (17,18).
In multivariable analysis, associations between risk factors and obesity in childhood were not observed. In adults, maternal obesity during childhood (OR 7.43, 95% CI 2.07-26.69, P = 0.0021) was the only contributing factor for obesity.
Hypertension
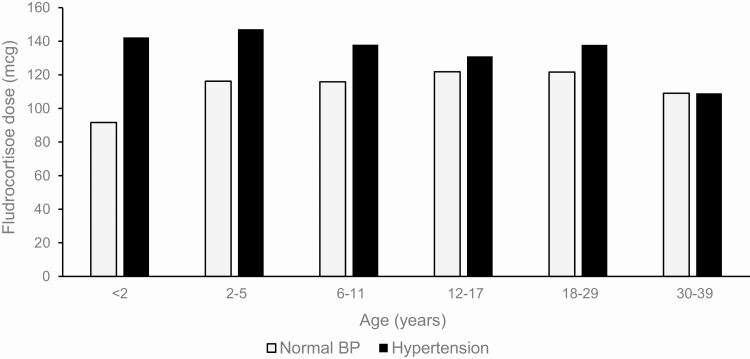
The median age of onset of hypertensive BP was 5.7 years (IQR 3.6-8.1). Hypertensive BP was observed in 53 (93.0%) patients at 1 or more visits. Hypertensive BP was most commonly observed in young children (<2 years) and then declined throughout childhood before increasing in adulthood (Fig. 2B). Males also had higher prevalence of hypertensive BP (39.5%) compared to females (25.4%) (P = 0.0120), even when adjusted for age (P = 0.0221). Obesity was independently associated with hypertensive BP (P = 0.0103) and when adjusted for age and sex (P = 0.0160). Higher mineralocorticoid doses were associated with hypertensive BP (P = 0.0194; P = 0.0139 when adjusted for age and sex) (Fig. 3) and was particularly striking in childhood (OR 1.0050 per mg, 95% CI 1.0019-1.0081, P = 0.0015). As expected, having suppressed plasma renin activity was associated with increased likelihood of hypertensive BP, which persisted after adjusting for age and sex (P < 0.0001). There was no association observed between adult height SDS and hypertension (P = 0.74).
Figure 3.
Fludrocortisone dose (mcg/day) in relation to age group among patients with and without hypertension.
Glucocorticoid dose, type, and percentage nighttime dose were not associated with hypertensive BP in any age group. However, suppressed androstenedione was associated with hypertensive BP during both childhood (OR 1.78, 95% CI 1.25-2.54, P = 0.0015) and adulthood (OR 2.60, 95% CI 1.46-4.61, P = 0.0011), and higher 17-OHP was protective against hypertensive BP (OR 0.58, 95% CI 0.42-0.81, P = 0.0014) during childhood.
Multivariable analysis confirmed that during childhood, hypertensive BP was associated with increasing fludrocortisone dose (OR 1.0042, 95% CI 1.0015-1.0070; P = 0.0027), while advancing age lowered the risk (OR 0.46, 95% CI 0.45-0.48; P < 0.0001). The contributing factors to hypertensive BP during adulthood were obesity (OR 2.49, 95% CI 1.35-4.61; P = 0.0035) and suppressed androstenedione (OR 1.99, 95% CI 1.83-7.26; P = 0.0002).
Insulin resistance
The median age of onset of insulin resistance was 13.4 years (IQR 12.4-17.2) (Table 2); however, evaluation began at age 12, when reference ranges were available. Insulin resistance was observed in 54 (94.7%) patients at 1 or more visits. The prevalence of insulin resistance increased as patients went into adulthood (Fig. 2C). Glucocorticoid dose and type were not associated with insulin resistance during childhood or adulthood, but percentage of daily glucocorticoid dose given at night was associated with insulin resistance (OR = 1.0116, 95% CI: 1.0012-1.0222, P = 0.0294), particularly in children (OR 1.024 95% CI 1.0073-1.0410, P = 0.0047).
Table 2.
Metabolic outcomes of interest at study enrollment during childhood and most recent visit during adulthood
| First assessment | Most recent assessment | Age of onset, years | |
|---|---|---|---|
| Age, years | 5.4 (3.0-7.9) | 23.3 (20.8-27.7) | |
| Obesitya | 18 (31.6) | 19 (33.3) | 8.3 (6.0-15.2) |
| Hypertensive blood pressure (stages 1 and 2)b | 24 (42.9) | 15 (26.3) | 5.7 (3.6-8.1) |
| Insulin resistancec | 0 | 24 (64.9) | 13.4 (12.4-17.2) |
| Fasting hyperglycemiad | 9 (17.0) | 8 (16.7) | 8.1 (5.3-11.2) |
| High total cholesterole | 1 (3.0) | 3 (7.5) | 15.4 (9.6-28.5) |
| High LDLf | 3 (16.7) | 4 (10.0) | 17.2 (9.1-23.6) |
| Low HDLg | 1 (5.0) | 13 (32.5) | 12.0 (9.6-18.1) |
| High TGLh | 3 (15.8) | 4 (10.0) | 9.3 (8.5-12.0) |
| Metabolic syndromei | 2 (8.0) | 3 (8.6) | 9.6 (8.1-12.0) |
Data are frequencies (percentages), mean ± SD or median (interquartile range: 25th-75th percentiles) Percentages are based on nonmissing values. Dose calculated for those on treatment.
Abbreviations: CAH, congenital adrenal hyperplasia; HDL, high-density lipoprotein; TGL, triglycerides.
a Obesity defined as BMI ≥ 85th percentile for age/sex if <2 years, ≥95th percentile for age/sex if 2 to 18 years, or BMI ≥ 30 kg/m2 for adults.
b Hypertension defined per the American Academy of Pediatrics’s 2017 guidelines for children and the American College of Cardiology/American Heart Association Task Force for Adults (11,12).
.c Insulin resistance defined as HOMA-IR > 2.5 in adults, >3.16 in adolescents (≥12 years).
d Defined as blood glucose ≥ 100; includes those with ‘impaired fasting glucose’ and those in diabetic range.
e High if >95th percentile for age/sex in pediatric patients or >200 mg/dL adults.
f High if >95th percentile for age/sex in pediatric patients or >130 mg/dL in adults.
g Low during childhood if <5th percentile for age/sex or during adulthood <40 mg/dL men or <50 mg/dL women.
h High during childhood if >95th percentile for age/sex or during adulthood >150 mg/dL in adults.
i Based on Weiss et al criteria in children and on the National Cholesterol Education Program criteria for adults (15).
Multivariable analysis showed that in children, obesity (OR 4.87, 95% CI 2.23-10.63, P < 0.0001) increased the risk of insulin resistance. In adulthood, obesity (OR 5.11, 95% CI 1.80-14.54 P = 0.0022) and suppressed testosterone (OR 3.41, 95% CI 1.75-6.68, P = 0.0003) were associated with insulin resistance.
Fasting hyperglycemia
The median age of onset of fasting hyperglycemia was 8.1 years (IQR 5.3-11.2) (Table 2). Fasting hyperglycemia was observed in 53 patients (93.0%) at 1 or more visits. Hyperglycemia increased in prevalence during school age and adolescence and dipped during young adulthood (Fig. 2D).
In multivariable analysis, associations between risk factors and hyperglycemia in childhood and adulthood were not observed.
Dyslipidemia
High TGL was first noted at median age 9.3 years (IQR 8.5-12.0) years, low HDL at 12.0 years (IQR 9.6-18.1), high total cholesterol at 15.4 years (IQR 9.6-28.5), and high LDL at 17.2 years (IQR 9.1-23.6).(Table 2). At 1 of more visits, high cholesterol was observed in 10 patients (17.5%), high LDL was observed in 16 patients (28.0%), low HDL was observed in 33 (57.9%) patients, and high TGL was observed in 24 patients (42.1%). Overall, dyslipidemia worsened with age, particularly low HDL, which had a large increase in prevalence in adulthood (Fig. 2E).
In univariable analysis, high 17-OHP was protective against high cholesterol in children (OR 0.16, 95% CI 0.04-0.57, P = 0.0049), but contrarily, it was a risk factor for low HDL (OR 1.69, 95% CI 1.34-2.13, P < 0.0001). Interestingly, mineralocorticoid dose was a risk factor for low HDL (OR 1.0069, 95% CI 1.0025-1.0113, P = 0.0021) in children. Suppressed androstenedione was a risk factor for high LDL (OR 11.23, 95% CI 2.17-58.10, P = 0.0039) in adults.
Multivariable analysis showed obesity as a risk factor for developing high TGL (OR 3.45, 95% CI 1.56-7.62, P = 0.0023) and mineralocorticoid dose as a risk factor for high LDL (OR 1.1241, 95% CI 1.0339-1.2221, P = 0.0061) in adults.
Metabolic syndrome
Fifty-eight percent of the data had sufficient criteria to assess presence of MetS. The median age of onset of MetS was 9.6 years (IQR 8.1-12.0) seen in 23 (40.4%) patients at 1 or more visits. Overall, no associations were observed in relation to glucocorticoid or mineralocorticoid dose, genotype, phenotype, sex, adult height SDS, maternal obesity, or androgens.
Discussion
In our natural history study of patients with CAH, we found that patients with classic CAH have metabolic morbidity starting at an early age, prior to puberty. Although the prevalence of cardiovascular and metabolic risk factors remained high during adulthood, the associated factors differed from those in childhood. Treatment-related variables influenced risk at all ages. During childhood, fludrocortisone dose strongly influenced the presence of hypertension and low HDL, while suppressed androstenedione, reflecting excess glucocorticoid therapy, was a risk factor for hypertension and high LDL during adulthood. Across all ages, excess adrenal steroids had a seemingly protective role. Maternal obesity during childhood was associated with obesity during adulthood, and obesity per se was associated with hypertension, insulin resistance, and hypertriglyceridemia. Among all visits, MetS was observed in 23 patients. The median age of onset was under 10 years, emphasizing the importance of intervention at young ages.
In this longitudinal study of cardiovascular risk factors in patients with classic CAH, the prevalence of obesity during childhood and adulthood was higher compared to the general population, and obesity was associated with hypertension and insulin resistance across all ages. Similarly, other studies of CAH patients have found increased rates of obesity in children (7,19,20), adults (21,22), or both (3). We found high prevalence of obesity in young children (<2 years), and although the definition of an abnormal BMI in young children is still exploratory, obesity at a young age is associated with being obese later in life (23). Our finding that maternal obesity at the first visit continued to be associated with obesity past childhood may reflect dietary and lifestyle choices of the family or genetic causes of obesity. Moreira et al (24) similarly found familial history of an adverse metabolic profile important in determining obesity and metabolic syndrome in CAH, but they only evaluated this effect in childhood. Overall, we observed an increase in the prevalence of obesity from preschool to school-aged children followed by a downward trend as patients went through puberty and into young adulthood, a time when physical activity and self-managed weight control are often increasing but also a time when medication compliance is frequently poor.
The characteristic hormonal imbalance of hyperandrogenism is important to consider in the pathophysiology of obesity and the development of CVD risk factors in CAH. Only about 28% of laboratory values were in target range demonstrating the challenges of attaining optimal disease control in CAH, especially in adolescents and young adults. Androgen excess in females has been associated with an adverse metabolic profile as seen in several studies of polycystic ovarian syndrome (25-27). It has been suggested that androgens of adrenal origin may have a greater adverse impact on metabolic profile (28). Interestingly, having elevated adrenal androgen precursors had a seemingly protective role against hypertension and abnormal cholesterol and LDL suggesting metabolic risk was greatest in patients maintaining tighter hormonal control and suggesting a possible glucocorticoid effect.
We found higher prevalence of hypertensive BP both in children and adults compared to the US general population, consistent with prior studies (4,29). Importantly, fludrocortisone dose was a major risk factor for hypertensive BP in childhood only, with the highest prevalence in young children, possibly due to the high doses of fludrocortisone sometimes used in infancy due to renal immaturity and variable capacity to reabsorb sodium. Reduction of fludrocortisone dose at approximately 6 to 12 months of age is key to preventing hypertension in the young child. As a research institute, patients in our study are enrolled through referrals (self and physician), and it is not uncommon for children on their first visit to our center to have elevated BP. Similarly, a multicenter study of children with CAH in Germany found higher prevalence of hypertension in younger children than adolescents, and fludrocortisone dose correlated with BP in children up to 8 years of age (30). Careful monitoring of BP and judicious use of fludrocortisone in children, especially young children, is recommended. We also found that suppressed androstenedione, a surrogate marker for excess glucocorticoid therapy, was a contributing factor for hypertension across all ages. In a cross-sectional study of 199 adults with CAH in the United Kingdom, short stature was associated with hypertension (31); however, we did not find a relationship between adult height and hypertension or metabolic syndrome in our cohort.
As expected, the prevalence of insulin resistance was higher in our cohort compared to the general population during both childhood and adulthood, and obesity was a risk factor for insulin resistance across all ages. This is consistent with previous studies (32,33) as well as the meta-analysis of CVD risk in CAH (4). In adults, having low or suppressed testosterone was a risk factor for insulin resistance, which was only observed in men. Hypogonadism is expected to result in metabolic stress in men (34), and in women with CAH, suppressed testosterone reflects overtreatment. It is our practice to use nighttime dosing of glucocorticoid in an attempt to suppress the overnight adrenocorticotropin surge with subsequent adrenal androgen production. Although we did not find a direct association between daily glucocorticoid dose and insulin resistance, we did find that higher percentage of daily dose given at bedtime increased the risk of insulin resistance.
In our cohort, the prevalence of hyperglycemia in childhood was nearly triple that of the general population. Conversely, Falhammar et al found hyperglycemia to only be prevalent in older adults (>40) (3); however, they used the diabetes ICD code to extract information from charts, while we measured fasting serum glucose in all patients and defined hyperglycemia as ≥100 mg/dL.
Prior studies evaluating lipids in children with CAH found no increased risk (29), but we found increased prevalence of low HDL in childhood. Falhammar et al (3) found dyslipidemia in adults with CAH over 40; it is not known, however, which lipid was abnormal. Dyslipidemia and reduced HDL have been described in patients with polycystic ovarian syndrome (35), hypercortisolemia, and adrenal adenoma and mild cortisol excess, as well as those on long-term supraphysiologic treatment with glucocorticoids (36). Thus, theoretically we would expect patients with CAH to be at risk for dyslipidemia if treated with supraphysiological doses of glucocorticoid.
Our study is the first longitudinal assessment of metabolic risk factors in a cohort of patients with CAH, an assessment that spanned childhood and adulthood. We assessed the major factors involved in metabolic comorbidity and risk of CVD. Having longitudinal data allowed us to describe the age of onset and changes over time and to investigate the effect of treatment as well as hormonal imbalances. Many of our findings implicate treatment-related metabolic risk. This may, in part, be due to our inability to mimic the circadian and ultradian rhythm of cortisol with current methods of glucocorticoid replacement (37,38). However, we also found mineralocorticoid-associated risk. Although other disease-related comorbidities were not evaluated in this study, patients with the lowest levels of adrenal steroids were at the most cardiometabolic risk, suggesting glucocorticoid dose should be minimized.
The limitations of this study are the retrospective analysis of data, potential bias of our cohort of patients that may not be representative of the general population of patients with CAH, and limited data on patients <2 and >30 years old. In addition, the methodology of BP assessment used at our institution is different than that used in NHANES where BP is always checked 3 times. American College of Cardiology proposed a new definition of elevated BP in 2017, and with this definition many patients considered hypertensive now would not have been considered to be hypertensive at the time of their visits. Furthermore, NHANES used the old definition of hypertension in interpreting its data. There is the possibility of overreporting of the metabolic outcomes of interest in children when compared to NHANES due to the age restrictions of NHANES pediatric data and the lack of waist circumference measurement in adult patient population, potentially causing a type II error when estimating the metabolic syndrome prevalence in adults. In addition, although we measured androgens at multiple visits, data on cumulative androgen exposure were lacking.
In conclusion, patients with classic CAH are at increased risk of metabolic morbidity starting at an early age. Some of this risk is directly related to obesity, but glucocorticoid and mineralocorticoid effects likely play an important role. Lifestyle assessment, evaluating family history, and careful monitoring and screening for risk factors are important to limit the development of metabolic comorbidities. Careful monitoring of symptoms and signs of excessive glucocorticoid and mineralocorticoid dosing and avoidance of suppression of adrenal hormones may mitigate the risk of metabolic morbidity in patients with CAH. Ultimately, this needs to be weighed against the risk of using lower-dose therapy, which has been associated with increased rates of adrenal crisis , tumor formation (39), and infertility (2). Our findings support the need for new treatment approaches for both glucocorticoid and mineralocorticoid replacement.
Acknowledgments
We are grateful to our patients for participating in this study. The authors thank the National Institutes of Health’s Biomedical Translational Research Information System staff for their contributions to the project, especially Ms. Andrea Beri.
Financial Support: This research was supported by the intramural research program of the National Institutes of Health.
Clinical Trials.gov Identifier no: NCT00250159.
Additional Information
Disclosures: D.P.M. received unrelated research funds from Diurnal Limited through the National Institutes of Health Cooperative Research and Development Agreement. All other authors report no potential conflicts of interest in this work.
Data Availability
The data sets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Merke DP, Auchus RJ. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 2020;383(13):1248-1261. [DOI] [PubMed] [Google Scholar]
- 2. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falhammar H, Frisén L, Hirschberg AL, et al. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Clin Endocrinol Metab. 2015;100(9):3520-3528. [DOI] [PubMed] [Google Scholar]
- 4. Tamhane S, Rodriguez-Gutierrez R, Iqbal AM, et al. Cardiovascular and metabolic outcomes in congenital adrenal hyperplasia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(11):4097-4103. [DOI] [PubMed] [Google Scholar]
- 5. Arlt W, Willis DS, Wild SH, et al. ; United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE) . Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95(11):5110-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams RM, Deeb A, Ong KK, et al. Insulin sensitivity and body composition in children with classical and nonclassical congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2010;72(2):155-160. [DOI] [PubMed] [Google Scholar]
- 7. Subbarayan A, Dattani MT, Peters CJ, Hindmarsh PC. Cardiovascular risk factors in children and adolescents with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2014;80(4):471-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ariyawatkul K, Tepmongkol S, Aroonparkmongkol S, Sahakitrungruang T. Cardio-metabolic risk factors in youth with classical 21-hydroxylase deficiency. Eur J Pediatr. 2017;176(4):537-545. [DOI] [PubMed] [Google Scholar]
- 9. Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(12):4429-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roy SM, Spivack JG, Faith MS, et al. Infant BMI or weight-for-length and obesity risk in early childhood. Pediatrics. 2016;137(5):e20153492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flynn JT, Kaelber DC, Baker-Smith CM, et al. ; Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. [DOI] [PubMed] [Google Scholar]
- 12. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. [DOI] [PubMed] [Google Scholar]
- 13. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. [DOI] [PubMed] [Google Scholar]
- 14. Rodden AM, Diaz VA, Mainous AG 3rd, Koopman RJ, Geesey ME. Insulin resistance in adolescents. J Pediatr. 2007;151(3):275-279. [DOI] [PubMed] [Google Scholar]
- 15. Grundy SM, Cleeman JI, Daniels SR, et al. ; American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735-2752. [DOI] [PubMed] [Google Scholar]
- 16. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362-2374. [DOI] [PubMed] [Google Scholar]
- 17. Hickman TB, Briefel RR, Carroll MD, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4-19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27(6):879-890. [DOI] [PubMed] [Google Scholar]
- 18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421. [PubMed] [Google Scholar]
- 19. Völkl TM, Simm D, Dötsch J, Rascher W, Dörr HG. Altered 24-hour blood pressure profiles in children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91(12):4888-4895. [DOI] [PubMed] [Google Scholar]
- 20. Amr NH, Ahmed AY, Ibrahim YA. Carotid intima media thickness and other cardiovascular risk factors in children with congenital adrenal hyperplasia. J Endocrinol Invest. 2014;37(10):1001-1008. [DOI] [PubMed] [Google Scholar]
- 21. Stikkelbroeck NM, Oyen WJ, van der Wilt GJ, Hermus AR, Otten BJ. Normal bone mineral density and lean body mass, but increased fat mass, in young adult patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2003;88(3):1036-1042. [DOI] [PubMed] [Google Scholar]
- 22. Harrington J, Peña AS, Gent R, Hirte C, Couper J. Adolescents with congenital adrenal hyperplasia because of 21-hydroxylase deficiency have vascular dysfunction. Clin Endocrinol (Oxf). 2012;76(6):837-842. [DOI] [PubMed] [Google Scholar]
- 23. Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869-873. [DOI] [PubMed] [Google Scholar]
- 24. Moreira RP, Villares SM, Madureira G, Mendonca BB, Bachega TA. Obesity and familial predisposition are significant determining factors of an adverse metabolic profile in young patients with congenital adrenal hyperplasia. Horm Res Paediatr. 2013;80(2):111-118. [DOI] [PubMed] [Google Scholar]
- 25. Diamanti-Kandarakis E, Piperi C, Kalofoutis A, Creatsas G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2005;62(1):37-43. [DOI] [PubMed] [Google Scholar]
- 26. Diamanti-Kandarakis E, Katsikis I, Piperi C, et al. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2008;69(4):634-641. [DOI] [PubMed] [Google Scholar]
- 27. Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95(5):2038-2049. [DOI] [PubMed] [Google Scholar]
- 28. Piontek U, Wallaschofski H, Kastenmüller G, et al. Sex-specific metabolic profiles of androgens and its main binding protein SHBG in a middle aged population without diabetes. Sci Rep. 2017;7(1):2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Improda N, Barbieri F, Ciccarelli GP, Capalbo D, Salerno M. Cardiovascular health in children and adolescents with congenital adrenal hyperplasia due to 21-hydroxilase deficiency. Front Endocrinol (Lausanne). 2019;10:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonfig W, Roehl FW, Riedl S, et al. ; AQUAPE CAH Study Group . Blood pressure in a large cohort of children and adolescents with classic adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency. Am J Hypertens. 2016;29(2):266-272. [DOI] [PubMed] [Google Scholar]
- 31. Han TS, Conway GS, Willis DS, et al. ; United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE) . Relationship between final height and health outcomes in adults with congenital adrenal hyperplasia: United Kingdom congenital adrenal hyperplasia adult study executive (CaHASE). J Clin Endocrinol Metab. 2014;99(8):E1547-E1555. [DOI] [PubMed] [Google Scholar]
- 32. Marra AM, Improda N, Capalbo D, et al. Cardiovascular abnormalities and impaired exercise performance in adolescents with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(2):644-652. [DOI] [PubMed] [Google Scholar]
- 33. Kim MS, Ryabets-Lienhard A, Dao-Tran A, et al. Increased abdominal adiposity in adolescents and young adults with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2015;100(8):E1153-E1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schiffer L, Kempegowda P, Arlt W, O’Reilly MW. Mechanisms in endocrinology: the sexually dimorphic role of androgens in human metabolic disease. Eur J Endocrinol. 2017;177(3):R125-R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Legro RS, Arslanian SA, Ehrmann DA, et al. ; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferraù F, Korbonits M. Metabolic comorbidities in Cushing’s syndrome. Eur J Endocrinol. 2015;173(4):M133-M157. [DOI] [PubMed] [Google Scholar]
- 37. Han TS, Stimson RH, Rees DA, et al. ; United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE) . Glucocorticoid treatment regimen and health outcomes in adults with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2013;78(2):197-203. [DOI] [PubMed] [Google Scholar]
- 38. Henley DE, Lightman SL. Cardio-metabolic consequences of glucocorticoid replacement: relevance of ultradian signalling. Clin Endocrinol (Oxf). 2014;80(5):621-628. [DOI] [PubMed] [Google Scholar]
- 39. Turcu AF, Mallappa A, Elman MS, et al. 11-oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2017;102(8):2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.