Abstract
Context
Experimental studies suggest that vitamin D receptor signaling may benefit the gut microbiome. In humans, whether vitamin D supplementation directly alters the gut microbiome is not well studied.
Objective
To determine whether correcting vitamin D deficiency with cholecalciferol (vitamin D3, D3) or calcifediol (25-hydroxyvitamin D3, 25(OH)D3) changes gut microbiome composition.
Methods
18 adults with vitamin D deficiency (25-hydroxyvitamin D [25(OH)D] <20 ng/mL) received 60 µg/day of D3 or 20 µg/day of 25(OH)D3 for 8 weeks. Changes in serum 25(OH)D, 1,25-diydroxyvitamin D (1,25(OH)2D), and 24,25-dihydroxyvitamin D (24,25(OH)2D) were assessed. We characterized composition of the fecal microbiota using 16S rRNA gene sequencing, and examined changes in α-diversity (Chao 1, Faith’s Phylogenetic Diversity, Shannon Index), β-diversity (DEICODE), and genus-level abundances (DESeq2).
Results
Vitamin D3 and 25(OH)D3 groups were similar. After 8 weeks of vitamin D3, mean 25(OH)D and 24,25(OH)2D increased significantly, but 1,25(OH)2D did not (25(OH)D: 17.8-30.1 ng/mL, P = .002; 24,25(OH)2D: 1.1 to 2.7 ng/mL, P =0.003; 1,25(OH)2D: 49.5-53.0 pg/mL, P = .9). After 8 weeks of 25(OH)D3, mean 25(OH)D, 24,25(OH)2D, and 1,25(OH)2D increased significantly (25(OH)D: 16.7-50.6 ng/mL, P < .0001; 24,25(OH)2D: 1.3-6.2 ng/mL, P = .0001; 1,25(OH)2D: 56.5-74.2 pg/mL, P = .05). Fecal microbial α-diversity and β-diversity did not change with D3 or 25D3 supplementation. Mean relative abundance of Firmicutes increased and mean relative abundance of Bacterioidetes decreased from baseline to 4 weeks, but returned to baseline by study completion. DESeq2 analysis did not confirm any statistically significant taxonomic changes.
Conclusion
In a small sample of healthy adults with vitamin D deficiency, restoration of vitamin D sufficiency with vitamin D3 or 25(OH)D3 did not lead to lasting changes in the fecal microbiota.
Keywords: Cholecalciferol, calcifediol, gut microbiome
The most recent NHANES data revealed that 18.3% of Americans are vitamin D deficient (serum 25-hydroxyvitamin D [25(OH)D] <20 ng/mL) (1). According to the Endocrine Society, these individuals are candidates for treatment with high-dose supplemental oral vitamin D, with a goal of sustaining a serum 25(OH)D level ≥30 ng/mL (2). Notably, vitamin D deficiency is 3.0, 2.2, and 2.5 times more prevalent in African, Latinx, and Asian vs White Americans, respectively (1). Because orally administered vitamin D is first encountered by the luminal gut microbiota, these ingested, lipid soluble agents may alter the balance of microbial inhabitants over the course of treatment.
The gut microbiome is increasingly recognized for its contributions to human health and disease. Composed of 1014 microbes (including ~500-1000 species of bacteria), the gut microbiome regulates nutrient acquisition, vitamin/hormone production, toxin degradation, gut immune system function, gut barrier integrity, and metabolism (3, 4). Several parameters characterize gut microbiome composition: α-diversity (diversity within an individual), β-diversity (diversity between individuals), and taxonomic profile (5). Microbial dysbiosis refers to unfavorable shifts in microbial composition, and can be associated with numerous disease states (eg, obesity (6), diabetes (7, 8), cardiovascular disease (9), dementia (10), and cancer (11)). The gut microbiome has thus been proposed as a therapeutic target for these conditions.
Vitamin D is consumed through the diet or produced by the skin from 7-dehyroxycholesterol following exposure to UV radiation. Approximately 75% of ingested vitamin D is absorbed in the jejunum and ileum, with transit time of unabsorbed vitamin D (including the sterol fraction undergoing enterohepatic circulation) to the large bowel and feces being 30 to 72 hours (12). Vitamin D is converted to its storage form, 25(OH)D, in the liver, primarily through CYP2R1 (2, 13-15). Circulating 25(OH)D is then converted to 1,25-dihydroxyvitamin D (1,25(OH)2D) via the 1-alpha hydroxylase (CYP27B1) in the kidney, as well as some extrarenal tissues (eg, innate immune cells, parathyroid gland. and placenta) (16). 1,25(OH)2D is the activated form of vitamin D that engages the vitamin D receptor (VDR) to transact gene expression (15, 17, 18). To prevent excess accumulation of storage and activated vitamin D, 1,25(OH)2D also induces CYP24A1 (24-hydroxylase) conversion of 25(OH)D and 1,25(OH)2D to their inactive metabolites, 24,25(OH)2D and 1,24,25(OH)2D, respectively (15, 17-19).
Beyond its classical role in promoting intestinal calcium absorption, circulating 1,25(OH)2D-driven, VDR-mediated bioactivity modulates gut barrier and immune function (factors that contribute to the gut microbiome) (20). Indeed, recent experimental and human studies demonstrate that VDR signaling may favorably impact gut microbiome composition. In murine systems, diminished VDR signaling (operationalized by restricting dietary vitamin D intake, or knocking out cyp27b1 or vdr) leads to unfavorable shifts in the fecal microbiota (21, 22). In humans, a large cross-sectional analysis of 567 older men reported that greater serum 1,25(OH) 2D was associated with greater microbial α-diversity (23). These data suggest that interventions that increase 1,25(OH) 2D synthesis may benefit gut microbiome composition.
Compared with cholecalciferol (vitamin D3), which is commonly used to treat vitamin D deficiency), calcifediol (pharmacologic 25-hydroxyvitamin D3 [25(OH)D3]) increases serum 25(OH)D and 1,25(OH)2D more quickly and robustly than parent vitamin D3, and is proposed to be a means of restoring 25(OH)D status to above normal (50 ng/mL) in a matter of days rather than weeks or months (24, 25). As noted above, it is likely that circulating, active vitamin D metabolites acting in an endocrine fashion have an effect on the gut microbiome (3). However, it is unknown whether oral ingestion of vitamin D compounds used to restore vitamin D sufficiency directly alter the gut microbiome. Accordingly, this pilot study examined the effects of vitamin D3 (60 µg [2400 IU] per day) vs 25(OH)D3 (20 µg per day) for 8 weeks on serum vitamin D metabolite concentrations, and composition of the gut microbiota in healthy adults with vitamin D deficiency.
We hypothesized that, compared with vitamin D3, 25(OH)D3 would (1) increase serum 25(OH)D levels to >50 ng/mL; (2) raise serum 1,25(OH) 2D more; and (3) lead to faster and more favorable shifts in the fecal microbiota. On an exploratory basis, we also examined whether oral supplementation with vitamin D3 or 25(OH)D3 would (1) reduce gut permeability, assessed noninvasively using serum markers of gut barrier dysfunction (fatty acid binding protein 2 [FABP2], produced by enterocytes and released into the circulation with gut epithelial cell damage), and/or (2) immune activation from gut microbial translocation (lipopolysaccharide binding protein [LBP], produced by hepatocytes in response to circulating lipopolysaccharide, a Gram-negative bacterial protein product) (26-28).
Materials and Methods
Study Participants
This study included 18 adults ≥18 years of age with a baseline 25(OH)D level <20 ng/mL. Participants were recruited from the University of California, Los Angeles (UCLA) student body and staff using an electronic flier. Medical history was ascertained by self-report. Exclusion criteria included history of hypercalcemia, hypercalciuria, nephrolithiasis, intestinal malabsorption, dysregulated vitamin D metabolism, or oral antibiotic use within the prior 3 months. Participants agreed to maintain their typical diet, and did not take self-prescribed vitamin D or food supplements during the study. All volunteers provided informed consent. This study was approved by the UCLA Institutional Review Board, and was listed on ClinicalTrials.gov using identifier NCT02091219.
Study Intervention
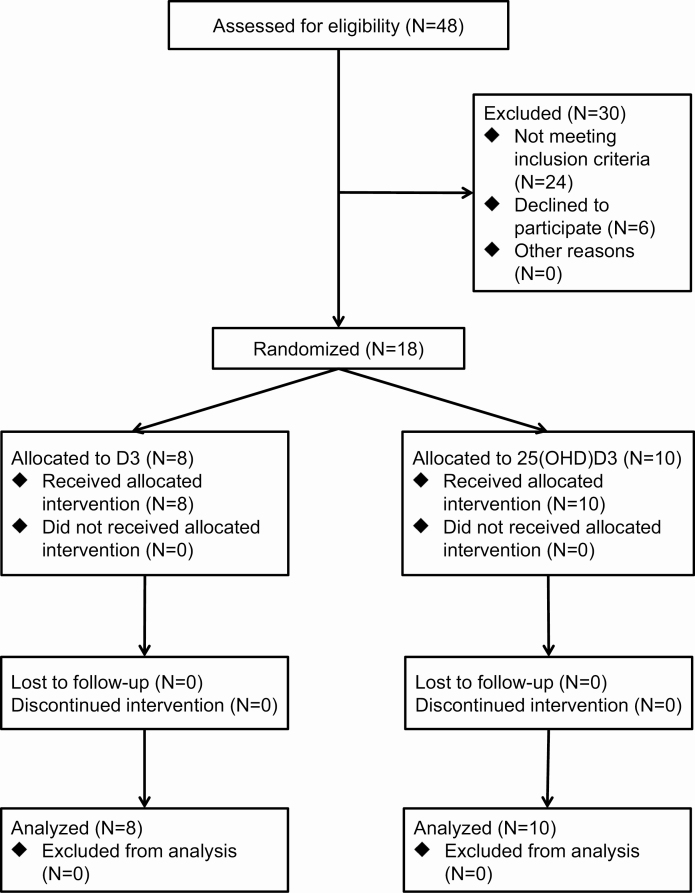
Participants were randomized 1:1 to receive either vitamin D3 (60 µg [2400 IU]/day) or 25-hydroxyvitamin D3 (20 µg/day) for 8 weeks (Fig. 1). A block randomization protocol stratified by race/ethnicity (Asian, Black, Latinx, White) was generated by a biostatistician. Blocks of 4 were utilized. Vitamin D3 and 25(OH)D3 was provided by DSM Nutritional Products (Heerlen, Netherlands) in powder form. The UCLA Investigational Pharmacy prepared capsules containing the appropriate doses of D3 or 25(OH)D3. D3 and 25(OH)D3 capsules were identical in appearance. After capsule preparation, the contents from 10 capsules each of D3 or 25(OH)D3 were pooled and assayed using liquid chromatography-mass spectrometry (Heartland Assays) to verify content. When a volunteer qualified for the study, a study coordinator provided the name and race/ethnicity to the investigational pharmacy. The investigational pharmacist then used the block randomization protocol to assign the participant to the appropriate intervention. All study investigators, coordinators, and volunteers were blinded to intervention allocation.
Figure 1.
Study flow chart.
Measurements
Study visits occurred at baseline, 4 weeks, and 8 weeks. At each study visit, blood and stool specimens were collected.
Vitamin D metabolite measurements
Blood specimens were assayed for 25(OH)D, 1,25(OH) 2D, and 24,25(OH) 2D using liquid chromatography-mass spectrometry (Heartland Assays). For 25(OH)D, intra- and interassay coefficient of variation (CV) were 4.0% and 5.0%, respectively. The intra- and interassay CV or 1,25(OH) 2D were 4.0% and 5.0%, respectively. For 24,25(OH) 2D, intra- and interassay CV were 3.0% and 6.0%, respectively. A vitamin D activation ratio was calculated as the ratio of 1,25(OH) 2D to 25(OH)D. Conversely, we computed the vitamin D catabolism ratio as 24,25(OH) 2D to 25(OH)D, multiplied by 100.
Gut permeability marker measurements
We noninvasively examined gut permeability using markers of gut barrier dysfunction (FABP2) and immune activation secondary to microbial translocation (LBP). FABP2 was measured using the Quantikine Human FABP2/I-FABP enzyme-linked immunosorbent assay (R&D Systems). The lower limit of detection for this assay is 6.21 pg/mL. The intra-assay CV was <4.1%. LBP was assayed using the Luminex platform with custom-made panels (R&D Systems). This platform uses microparticles that are precoated with analyte-specific antibodies and incubated with diluted plasma samples, followed by a biotin antibody, and lastly by a streptavidin–phycoreythin conjugate. The fluorescence intensity of each analyte’s microparticles are then quantified using a Bioplex 200 (Luminex) System Analyzer, and the data analyzed using Bioplex Manager software. The lower limit of detection for the LBP is 83.9 pg/mL, and the intra-assay CV was <2.9%.
Stool collection
Participants received stool collection kits for home sampling to be performed during the 3 days prior to study visits. Volunteers used a spoon to transfer freshly defecated feces from a toilet hat to a Para-Pak stool collection tube prefilled with 95% ethanol, and containing an autoclaved ball bearing. Participants homogenized the stool by shaking the collection tube, and stored the sample at room temperature until delivery to the study coordinator at each study visit. This method preserves DNA/RNA profiles for up to 2 weeks at room temperature prior to long-term storage at –80°C (29).
16S rRNA gene sequencing
We extracted DNA from ethanol preserved fecal samples using bead beating in conjunction with the ZymoBIOMICS DNA/RNA Miniprep Kit. We then sequenced the 254 base pair V4 region of 16S ribosomal DNA, as previously described, using the HiSeq 2500 in rapid run mode (2 × 250 bp) (30). Mean sequence depth was 57 305 reads, with a range from 17 931 to 110 865. DADA2 was used to perform quality filtering, merge paired end reads, and cluster sequences into exact amplicon sequence variants (31). Taxonomy was then assigned for amplicon sequence variants based on the SILVA database down to the level of family, genus, or species, depending on the depth of reliable classifier assignments (32).
Statistical Analyses
Our analyses examined the following questions: (1) What are the effects of vitamin D3 vs 25(OH)D3 supplementation on circulating vitamin D metabolite levels? (2) What are the effects of vitamin D3 vs 25(OH)D3 supplementation on fecal microbiota composition? (3) Are changes in serum vitamin D metabolite levels associated with changes in specific taxonomies? and (4) What are the effects of vitamin D3 or 25(OH)D3 supplementation on measures of gut permeability?
Change in vitamin D metabolites and gut permeability markers with D3 or 25(OH)D3 supplementation
We generated descriptive statistics of vitamin D metabolite and gut permeability marker levels, and confirmed their normal distributions. Within-individual changes in vitamin D metabolite and gut permeability marker levels from baseline to each follow-up visit were examined using the paired t-test. We assessed between-group differences in each variable, at each time point using the unpaired t-test.
Change in gut microbiome α/β-diversity and taxonomic abundances with D3 or 25(OH)D3 supplementation
We examined microbial α-diversity on datasets rarefied to equal sequencing depth (17 931) using the Chao1 index of richness, Faith’s phylogenetic diversity, and the Shannon index of evenness. Significance in differences was determined using analysis of variance (ANOVA). β-Diversity was calculated using the DEICODE plugin in QIIME 2, which employs a robust Aitchison distance metric, then visualized with principal coordinates analysis (33). The significance of differences in β-diversity was assessed using PERMANOVA adjusting for participant (34). Multilevel sparse partial least squares discriminant analysis (sPLS-DA) was utilized to visualize in a supervised manner the compositional differences attributable to study time point after adjusting for participant effects (35). Differential abundance of microbial genera was determined using multivariate negative binomial mixed models implemented in DESeq2 with participant as a covariate (36). Because analyses were adjusted for study participant, results for this set of analyses reflect within-individual differences in gut microbiota composition relative to study time point. Results were adjusted for multiple hypotheses testing with a significance threshold of false discovery rate <.05.
Associations of vitamin D metabolite levels with gut bacteria abundances
We constructed DESeq2 models to identify differences in microbes associated with differences in levels of vitamin D metabolites, after adjusting for study participant. Separate models were run for each vitamin D metabolite parameter. As analyses were adjusted for study participant, results for this set of analyses reflect within-individual differences in gut microbiota composition relative to vitamin D metabolite level.
Associations of vitamin D metabolite levels with gut permeability markers
We examined the associations with FABP2 or LBP as outcome variables, and 25(OH)D, 1,25(OH)2D, or 24,25(OH)2D as predictors using repeated measures, mixed effects, multivariable linear regression (each predictor outcome pair tested separately). Models were adjusted for age, body mass index (BMI), race/ethnicity, and sex.
Results
Participant Characteristics
A total of 18 volunteers were recruited, with 8 allocated to receive vitamin D3, and 10 randomized to 25(OH)D3. No participant reported chronic medical conditions. Clinical and biochemical variables were similar between groups (Table 1). Continuous variables were normally distributed. Among all participants (treatment groups pooled) at baseline, mean 25(OH)D was 17.2 ng/mL and mean age was 29 years of age. Thirty-nine percent of volunteers were Asian, 11% Black, 28% Latinx, and 22% White. Seventy-seven percent were women.
Table 1.
Participant characteristics at study baseline
| Cholecalciferol Group N = 8 | Calcifediol Group N = 10 | Combined N = 18 | |
|---|---|---|---|
| Age (years) | 30.6 (11.6) | 29.3 (7.6) | 29.8 (9.4) |
| Sex | |||
| Female | 7 (87) | 8 (80) | 14 (77) |
| Male | 1 (13) | 2 (20) | 4 (23) |
| Race/ethnicity | |||
| Asian | 4 (50) | 3 (30) | 7 (39) |
| Black | 1 (12) | 1 (10) | 2 (11) |
| Latinx | 2 (25) | 3 (30) | 5 (28) |
| White | 1 (13) | 3 (30) | 4 (23) |
| BMI (kg/m2) | 25.7 (5.5) | 27.2 (7.9) | 26.5 (6.3) |
| 25(OH)D (ng/mL) | 17.7 (5.6) | 16.7 (4.1) | 17.2 (4.6) |
| 1,25(OH)2D (pg/mL) | 49.5 (9.9) | 56.5 (12.2) | 53.4 (11.4) |
| 24,25(OH)2D (ng/mL) | 1.1 (0.5) | 1.3 (0.6) | 1.2 (0.6) |
| 1,25(OH)2D:25(OH)D (pg/ng) | 3.4 (3.7) | 3.2 (1.7) | 3.3 (2.7) |
| 100 × 24,25(OH)2D: 25(OH)D | 6.3 (2.2) | 7.5 (2.5) | 6.9 (2.4) |
| Calcium (mg/dL) | 9.4 (0.3) | 9.6 (0.3) | 9.5 (0.3) |
| FABP2 (pg/mL) | 1545 (581) | 1498 (175) | 1480 (330) |
| LBP (pg/mL) | 5663 (2924) | 5697 (1764) | 5684 (2161) |
Continuous variables presented as mean (SD), and categorical variables presented as count (percent).
Change in Vitamin D Metabolites With D3 or 25(OH)D3 Supplementation
Both D3 and 25(OH)D3 increased circulating 25(OH)D and 24,25(OH) 2D concentrations, with 25(OH)D3 increasing these metabolite levels more quickly and to a greater degree. 25(OH)D3 also raised serum 1,25(OH)2D levels, whereas D3 did not (Table 2). Following 8 weeks of D3 supplementation, 25(OH)D increased from a mean of 17.8 to 30.1 ng/mL (P = .002), and 24,25(OH)2D increased from 1.1 to 2.7 ng/mL (P = .003). Correspondingly, the vitamin D activation ratio (pg 1,25(OH)2D: ng 25(OH)D) decreased from 3.4 to 1.4, but this change did not reach statistical significance (P = .1). The vitamin D catabolism ratio (ng 24,25(OH)2D:ng 25(OH)D × 100) increased from 0.06 to 0.08 (P < .0001). After 8 weeks of 25(OH)D3 supplementation, 25(OH)D increased from 16.7 to 50.6 ng/mL (P < .0001), 1,25(OH)2D increased from 56.5 to 74.2 pg/mL (P = .05), and 24,25(OH)2D increased from 1.3 to 6.2 ng/mL (P = .0001). Over the 8-week study period, the vitamin D activation ratio (1,25(OH)2D:25(OH)D [pg/ng]) decreased from 3.3 to 1.6 (P = .01), and the vitamin D catabolism ratio increased from 0.07 to 0.12 (P < .00001).
Table 2.
Changes in vitamin D metabolites and markers of gut barrier dysfunction (fatty acid binding protein 2) and microbial translocation (lipopolysaccharide binding protein) with 8 weeks of supplementation with cholecalciferol or Calcifediol
| Week 0 | Week 4 | Week8 | |
|---|---|---|---|
| 25-hydroxyvitamin D (ng/mL) | |||
| Cholecalciferol (N = 8) | 17.8 (1.9) | 27.1 (3.1) | 30.1 (3.6) |
| P value (within-group vs week 0) | 0.004 | 0.002 | |
| Calcifediol (N = 10) | 16.7 (1.3) | 48.9 (3.4) | 50.6 (3.6) |
| P value (within-group vs week 0) | <.00001 | <.00001 | |
| P value (between-group at each time point) | .7 | .0003 | .001 |
| 1,25(OH) 2 D (pg/mL) | |||
| Cholecalciferol (N = 8) | 49.5 (3.8) | 48.2 (2.7) | 53.0 (4.2) |
| P value (within-group vs week 0) | .6 | .9 | |
| Calcifediol (N = 10) | 56.5 (4.0) | 71.8 (4.8) | 74.2 (6.4) |
| P value (within-group vs week 0) | .01 | .05 | |
| P value (between-group at each time point) | .2 | .001 | .02 |
| 24,25(OH)2D (ng/mL) | |||
| Cholecalciferol (N = 8) | 1.1 (0.2) | 2.1 (0.5) | 2.7 (0.5) |
| P value (within-group vs week 0) | 0.01 | 0.003 | |
| Calcifediol (N = 10) | 1.3 (0.2) | 4.5 (0.7) | 6.2 (0.8) |
| P value (within-group vs week 0) | .0003 | .0001 | |
| P value (between-group at each time point) | .5 | .01 | .002 |
| 1,25(OH) 2 D:25(OH)D (pg/ng) | |||
| Cholecalciferol (N = 8) | 3.4 (1.3) | 2.1 (0.4) | 1.7 (0.4) |
| P value (within-group vs week 0) | .2 | .1 | |
| Calcifediol (N = 10) | 3.3 (0.5) | 1.6 (0.2) | 1.6 (0.3) |
| P value (within-group vs week 0) | .001 | .01 | |
| P value (between-group at each time point) | .8 | .2 | .9 |
| 100*24,25(OH) 2 D: 25(OH)D | |||
| Cholecalciferol | 0.06 (0.01) | 0.07 (0.01) | 0.08 (0.01) |
| P value (within-group vs week 0) | .3 | <.0001 | |
| Calcifediol | 0.07 (0.01) | 0.09 (0.01) | 0.12 (0.01) |
| P value (within-group vs week 0) | .1 | <.00001 | |
| P value (between-group at each time point) | .2 | .1 | .02 |
| FABP2 (pg/mL) | |||
| Cholecalciferol | 1545 (581) | 1589 (624) | 1364 (332) |
| P value (within-group vs week 0) | .9 | .2 | |
| Calcifediol | 1498 (175) | 1550 (419) | 1486 (250) |
| P value (within-group vs week 0) | .7 | .6 | |
| P value (between-group at each time point) | .8 | .8 | .8 |
| LBP (pg/mL) | |||
| Cholecalciferol | 5663 (2924) | 5624 (1045) | 5156 (3355) |
| P value (within-group vs week 0) | .8 | .4 | |
| Calcifediol | 5697 (1764) | 5322 (1899) | 5294 (3211) |
| P value (within-group vs week 0) | .7 | .9 | |
| P value (between-group at each time point) | .9 | .4 | .8 |
Gut Microbiome Composition and D3 or 25(OH)D3 Supplementation
Neither supplementation regimen alone (vitamin D3 vs 25(OH)D3) led to specific changes in the gut microbiota profile (Figs 2 and 3). Thus, to maximize sample size, participants from both groups were pooled for subsequent analyses of the effect of vitamin D supplementation on the gut microbiota.
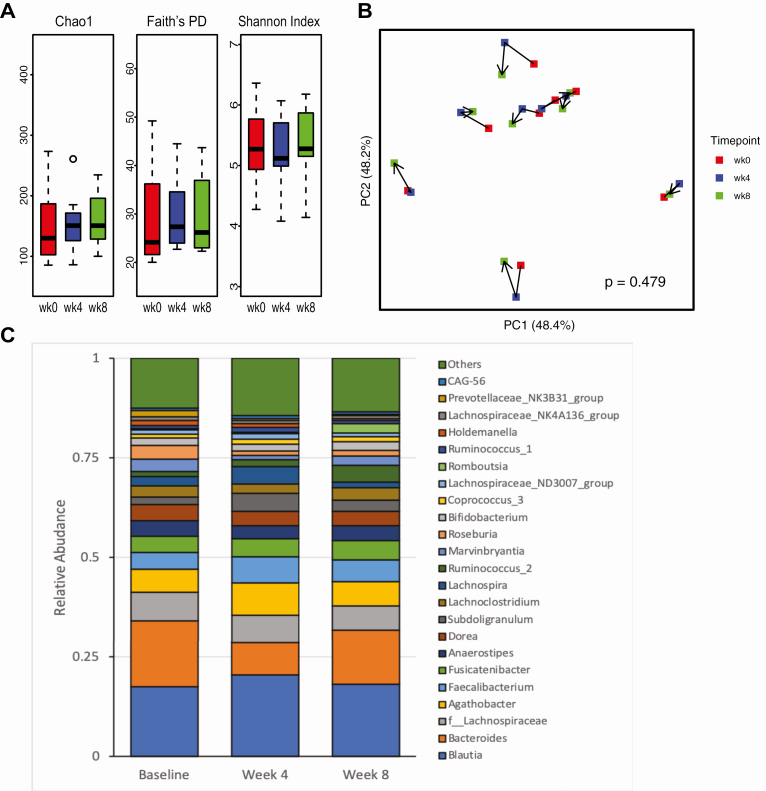
Figure 2.
Changes in fecal microbiota composition following 4 and 8 weeks of cholecalciferol (D3) supplementation. (A) α-Diversity measured by Chao1, Faith’s PD, and Shannon Index. (B) PCoA plot of DEICODE distances for the fecal microbiome. Significance of changes in β-diversity by timepoint was calculated using PERMANOVA adjusted for subject. (C) Genus level taxonomic profiles at baseline, week 4 and week 8 after D3 supplementation.
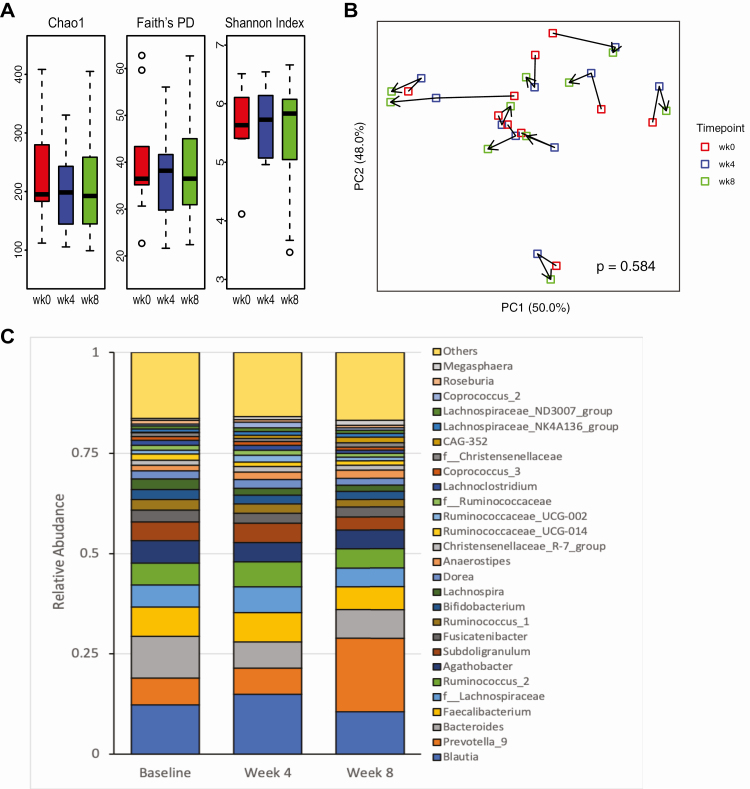
Figure 3.
Changes in fecal microbiota composition following 4 and 8 weeks of calcifediol (25D3) supplementation. (A) α-Diversity measured by Chao1, Faith’s PD, and Shannon Index. (B) PCoA plot of DEICODE distances for the fecal microbiome. Significance of changes in β-diversity by timepoint was calculated using PERMANOVA, adjusted for subject. (C) Genus level taxonomic profiles at baseline, week 4 and week 8 after 25D3 supplementation.
Change in gut microbiota diversity
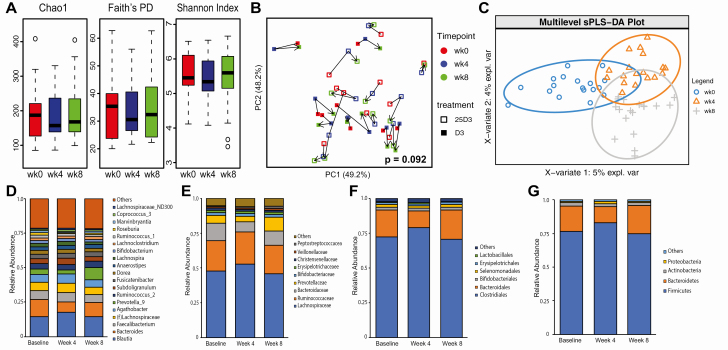
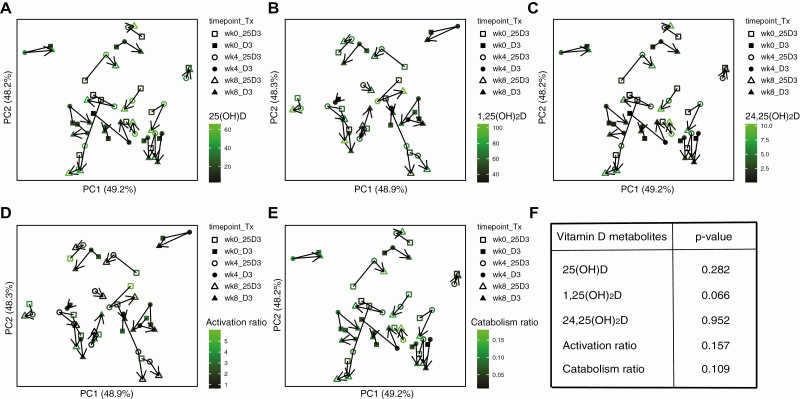
α-Diversity (community diversity within participants) did not change across the treatment time points with D3 or 25(OH)D3 supplementation (Fig. 4A). There was a trend toward change in β-diversity (community diversity between participants) across treatment time points using DEICODE (adjusted for interindividual differences), but this did not reach statistical significance (Fig. 4B, P = .092).
Figure 4.
Changes in fecal microbiota composition following 4 and 8 weeks of cholecalciferol (D3) or calcifediol (25D3) supplementation. (A) α-diversity measured by species richness (Chao1), phylogenetic diversity (Faith’s PD), and species evenness (Shannon Index). (B) Principle coordinates analysis (PCoA) plots of DEICODE distances for the fecal microbiome. The P value for differences in β-diversity by timepoint was calculated using PERMANOVA adjusted for participant. (C) Supervised multilevel analysis, adjusted for participant specific effects using sPLS-DA. (D) Genus, (E) family, (F) order, and (G) phylum level taxonomic profiles at baseline, week 4 and week 8 after 25D3 or D3 supplementation.
Taxonomic change in gut microbiota
The taxomonic effects of D3 or 25(OH)D3 supplementation were visualized using a supervised analysis with sPLS-DA (Fig. 4C). This analysis suggested distinct changes in microbiome profiles at weeks 4 and 8 of vitamin D supplementation. To ascertain changes in individual taxa with D3 or 25(OH)D3 repletion, we first visually examined the mean relative abundance of highly abundant taxa at each time point (Fig. 4D-4G). At the phylum level, Firmicutes increased (driven by changes in Lachnospiraceae and Ruminococcaceae) and Bacterioidetes decreased (driven by change in Bacteroidaceae) across participants from baseline to week 4 (Fig. 4E-4G). At the genus level, several high-abundance taxa were enriched (Blautia, unclassified Lachnospiraceae, Agathobacter, Subdoligranulum) or depleted (Bacteroides and Prevotella_9) (Fig. 4D). At week 8, the fecal microbiota profile returned to baseline.
To determine whether there were statistically significant within-individual taxonomic changes following D3 or 25(OH)D3 supplementation, we conducted a DESeq2 analysis at the genus level. Regardless of the supplementation regimen, during the first 4 weeks, several taxa increased (genera Blautia [P = 0.023, q = 0.760], Coprococcus_3 [P = 0.013, q = 0.760], Subdoligranulum [P = 0.021, q = 0.760], unclassified order Enterobacteriales [P = .021, q = 0.282]) or decreased (unclassified family Bacteroidaceae [P = .005, q = 0.285], genus Roseburia [P = .016, q = 0.760]). From week 4 to 8, unclassified Bacteroidales increased (P = 0.032, q = 0.453), and Subdoligranulum (P = .006, q = 0.449), and Streptococcus (P = .004, q = 0.449) decreased. However, these changes were no longer statistically significant after accounting for multiple testing (ie, P values were <.05, but q-values were not).
Vitamin D metabolites and gut microbiota diversity
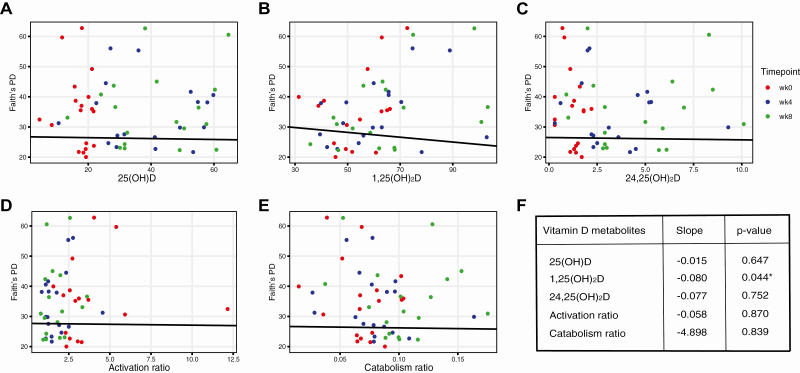
In multivariable linear regression, adjusted for study participant, 1 metric of α-diversity (Faith’s PD) decreased with increased 1,25(OH)2D (Fig. 5), but 2 other metrics (Chao1, Shannon index) did not show the same relation (data not shown). Change in β-diversity was not associated with any vitamin D metabolite (Fig. 6).
Figure 5.
Associations of vitamin D metabolite and metabolite ratios with microbial α-diversity measured by Faith’s PD. Associations assessed by multivariable linear regression adjusted for participant. Results reflect within-individual differences in Faith’s PD relative to vitamin D metabolite or vitamin D metabolite ratios.
Figure 6.
Associations of vitamin D metabolite and metabolite ratios with microbial β-diversity. Significance of associations was assessed by PERMANOVA adjusted for participant. Results reflect within-individual differences in β-diversity relative to vitamin D metabolite or vitamin D metabolite ratios.
Vitamin D metabolites and gut microbiota taxonomy
After adjusting for participant-related variation, increased abundance of genus Streptococcus was associated with increased 1,25(OH)2D levels (P = .0004, q = 0.058), but this association fell just short of significance after correcting for multiple testing. Change in no other individual taxa was associated with any vitamin D metabolite or metabolite ratio.
Gut Permeability Markers and D3 or 25(OH)D3 Supplementation
Change in gut permeability markers
Neither FABP2 nor LBP changed with D3 or 25(OH)D3 supplementation: D3, FABP2 from 1545 (581) (week 0) to 1364 (332) (week 8) pg/mL (P = .2) and LBP from 5663 (2924) (week 0) to 5156 (3355) (week 8) pg/mL (P = .4); 25(OH)D3, FABP2 from 1498 (175) (week 0) to 1486 (250) (week 8)) pg/mL (P = .6) and LBP from 5697 (1764) (week 0) to 5294 (3211) (week 8) pg/mL (P = .9).
Vitamin D metabolites and gut permeability markers
In mixed-effects, multivariable linear regression, adjusted for age, BMI, race/ethnicity, and sex, neither FABP2 nor LBP was associated with the vitamin D metabolites or vitamin D metabolite ratios.
Discussion
This pilot study examined the effects of 8 weeks of vitamin D3 and 25(OH)D3 on circulating vitamin D metabolite concentrations, and the composition of the gut microbiome. With respect to vitamin D metabolites, 25(OH)D3 administration increased 25(OH)D and 1,25(OH) 2D more quickly and to a greater degree than parent vitamin D3; the 8-week vitamin D3 and 25(OH)D3 treatment regimens met their targeted serum 25(OH)D levels, 30 ng/mL and 50 ng/mL, respectively. As for gut microbiome composition, supplementation with D3 or 25(OH)D3 (groups combined) did not alter α- or β-diversity. Across volunteers, the relative abundance of Firmicutes increased, and Bacterioidetes decreased from baseline to week 4, but these differences did not persist to 8 weeks. DESeq2 analysis did not reveal statistically significant, within-individual taxonomic changes at the genus level. Change in vitamin D metabolite and vitamin D metabolite ratios were not associated with any specific taxonomic shifts. Restoring serum 25(OH)2D levels to normal did not reduce gut barrier dysfunction (FABP2) or microbial translocation (LBP).
Vitamin D has been proposed to impact the gut microbiome through several mechanisms. First, although bacteria do not express the VDR (37), vitamin D metabolites generated by intestinal microbes can indirectly modulate the gut microbiome via action on host VDR-expressing enterocytes and inflammatory cells in the neighboring mucosa (38). CYP105A1 (expressed by Streptomyces griseolus) converts parent vitamin D to 1,25(OH)2D in 2 hydroxylation steps (39). Several taxa from the Firmicutes phylum express proteins that are homologous to CYP27A1 and CYP27B1 (40). A second mechanism by which vitamin D may affect the gut microbiome is by regulating the gut barrier. In experimental studies, increased VDR signaling promotes expression of gut epithelial junction proteins (eg, zonulin, claudin-1, claudin-2) that reduce gut permeability (41-43), whereas diminished VDR signaling leads to increased microbial translocation (44). Lastly, vitamin D may impact the gut microbiota through its actions on the innate immune system (15, 45-47).
A recent cross-sectional analysis of 576 older men from the MrOS cohort demonstrated that higher serum 1,25(OH)2D concentrations were associated with greater α- and β-diversity (23). In addition, men with higher 1,25(OH)2D levels and vitamin D activation ratios were more likely to harbor specific genera known to produce butyrate or provide substrate to bacteria that produce butyrate. While our study demonstrated that the relative abundance of Firmicutes increased and Bacterioidetes decreased after 4 weeks of vitamin D3 or 25(OH)D3 supplementation, these changes were not durable. After accounting for multiple testing and adjusting for participant-related variation, neither increased 1,25(OH) 2D nor 25(OH)D3 supplementation (which increased circulating 1,25(OH) 2D levels) were associated with specific taxonomic changes. One possible explanation for the discrepant findings between our study and the MrOS analysis is that the potentially beneficial effects of 1,25(OH) 2D-driven VDR signaling may only occur if there is underlying gut dysbiosis. Our young, healthy volunteers were unlikely to have substantial dysbiosis at baseline. In contrast, MrOS participants had several risk factors. For example, estradiol levels in men decrease with age (48), and loss of estrogen is associated with increased gut permeability (49, 50) and dysbiosis (51). Also, 7% of the MrOS sample had used antibiotics within the 30 days prior to their gut microbiome assessment (23), whereas we excluded volunteers who used antibiotics within 90 days of screening.
The premise that the effects of vitamin D on the gut microbiome may differ depending on whether there is underlying dysbiosis is also supported by previously published interventional studies. Vitamin D supplementation most consistently changes gut microbial composition in individuals with underlying immune dysfunction and gut dysbiosis (eg, Crohn’s disease, ulcerative colitis, and cystic fibrosis) (52-54). Notably, the studies of Crohn’s (52) and ulcerative colitis (53) patients also included disease-free controls; among controls, vitamin D supplementation did not lead to shifts in the fecal microbiota. In studies of healthy adults, results have been inconsistent, with some reporting taxonomic changes following vitamin D supplementation (55, 56), and others reporting no effect (57). However, in 1 of the studies reporting positive findings, average participant BMI was >30 kg/m2 (56), and obesity is linked to dysbiosis (58).
Several additional potential explanations for our negative findings warrant consideration. First, the optimum method for ascertaining vitamin D’s effects on the gut microbiome is uncertain. Approximately 75% of ingested vitamin D is absorbed in the jejunum and ileum, with transit time of unabsorbed vitamin D to the large bowel and feces lasting 30 to 72 hours (12). This suggests that not only does vitamin D likely impact the small intestinal microbiota, but also substantial amounts of vitamin D also reach the large intestine, where it can modulate the local microbial milieu. Moreover, if gut transit were normal, 1 would expect changes to the microbial profile incurred in the small intestine to be captured in the stool. However, in 1 of the above cited studies of healthy adults, vitamin D supplementation led to biopsy-confirmed changes in the gut microbiome of the upper gastrointestinal tract (as expected), but these changes were not reflected in stool analyses (which was unexpected) (57). Future studies should examine whether vitamin D has site-specific effects on the gut microbiome, and the optimal methods for detecting these effects. A second explanation is interindividual variability in the VDR expression at the level of gut intestinal epithelium in the host. For instance, 1 study demonstrated that human VDR polymorphisms were consistently associated with the genus Parabacterioides (phylum: Bacterioidetes) (59).
The principal weakness of this study is the small sample size. This likely limited our ability to detect significant taxonomic changes in the fecal microbiota in our DESeq2 analyses, after accounting for multiple testing. Nonetheless, we did observe an increase in relative abundance in Firmicutes, and decrease in relative abundance in Bacterioidetes across volunteers from baseline to at 4 weeks. This latter finding is hypothesis generating, and future investigations should examine whether it is possible to make this potential shift last beyond 4 weeks. The second weakness is that this study focuses on the effects of correcting vitamin D deficiency on the composition, but not function, of the fecal microbiota. Given the potential for certain bacterial taxa to metabolize vitamin D, whether those metabolites impact host enterocyte gene expression in a paracrine mode is of interest. Third, our analyses did not account for dietary patterns, which can impact gut permeability and the gut microbiota (60). However, this pilot study was relatively short in duration, and participants agreed to maintain their typical diets. Lastly, we indirectly assessed gut permeability using serum markers of gut barrier dysfunction (FABP2) and microbial translocation (LBP), instead of more direct approaches, such as histology or enteral administration of nondigestible carbohydrates. The latter approaches are not practical in larger human studies, and serum markers can detect physiologic changes in gut permeability (50).
To conclude, in a small sample of adults with baseline vitamin D deficiency (serum 25(OH)D levels <20 ng/mL), oral D3 and 25(OH)D3 did not produce lasting changes to the fecal microbiota profile, and changes in circulating vitamin D metabolite levels following supplementation were not associated with any specific taxonomic shifts or reduced gut permeability. In the future, more definitive studies should examine whether the presence of underlying gut dysbiosis is necessary to achieve vitamin D-mediated changes in the gut microbiome, and whether stool is the optimal medium through which to ascertain these potential shifts. Lastly, from a practical point of view for clinicians, the results here suggest that giving high doses of either oral vitamin D3 or 25(OH)D3 to young, healthy adults who are vitamin D deficient does not produce a long lasting alteration in the stool microbiome, and does not adversely impact the expected therapeutic outcome of restoring circulating 25(OH)D balance to normal.
Glossary
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25 (OH)D
25-hydroxyvitamin D
- 25(OH)D3
25-hydroxyvitamin D3
- ANOVA
analysis of variance
- BMI
body mass index
- CV
coefficient of variation
- FABP2
fatty acid binding protein 2
- LBP
lipopolysaccharide binding protein
- sPLS-DA
partial least squares discriminant analysis
- UCLA
University of California, Los Angeles
- VDR
vitamin D receptor
Acknowledgments
Financial Support: This work was funded by the National Institute of Health (NIH) (1R21AR073018), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (5R01AR063910), and the Veterans Affairs (VA) (5IK2CX001717).
Clinical Trial Information: ClinicalTrials.gov identifier NCT02091219 (registered March 19, 2014).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110(1):150-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. ; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 3. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620-625. [DOI] [PubMed] [Google Scholar]
- 4. Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227-238. [DOI] [PubMed] [Google Scholar]
- 5. Jovel J, Patterson J, Wang W, et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front Microbiol. 2016;7:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022-1023. [DOI] [PubMed] [Google Scholar]
- 7. Vatanen T, Franzosa EA, Schwager R, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562(7728):589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850-858. [DOI] [PubMed] [Google Scholar]
- 9. Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alkasir R, Li J, Li X, Jin M, Zhu B. Human gut microbiota: the links with dementia development. Protein Cell. 2017;8(2):90-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(24):1907-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bikle DD. Vitamin D insufficiency/deficiency in gastrointestinal disorders. J Bone Miner Res. 2007;22(Suppl 2):V50-V54. [DOI] [PubMed] [Google Scholar]
- 13. Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210(4466):203-205. [DOI] [PubMed] [Google Scholar]
- 14. Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68(5):882-887. [DOI] [PubMed] [Google Scholar]
- 15. Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95(2):471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3-5):316-321. [DOI] [PubMed] [Google Scholar]
- 17. Vanhooke JL, Prahl JM, Kimmel-Jehan C, et al. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proc Natl Acad Sci U S A. 2006;103(1):75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wacker M, Holick MF. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5(1):111-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tryfonidou MA, Oosterlaken-Dijksterhuis MA, Mol JA, van den Ingh TS, van den Brom WE, Hazewinkel HA. 24-Hydroxylase: potential key regulator in hypervitaminosis D3 in growing dogs. Am J Physiol Endocrinol Metab. 2003;284(3):E505-E513. [DOI] [PubMed] [Google Scholar]
- 20. Assa A, Vong L, Pinnell LJ, Avitzur N, Johnson-Henry KC, Sherman PM. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J Infect Dis. 2014;210(8):1296-1305. [DOI] [PubMed] [Google Scholar]
- 21. Jin D, Wu S, Zhang YG, et al. Lack of vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin Ther. 2015;37(5):996-1009.e7. [DOI] [PubMed] [Google Scholar]
- 22. Wu S, Zhang YG, Lu R, et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2015;64(7):1082-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas RL, Jiang L, Adams JS, et al. Vitamin D metabolites and the gut microbiome in older men. Nat Commun. 2020;11(1):5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shieh A, Ma C, Chun RF, et al. Effects of cholecalciferol vs calcifediol on total and free 25-hydroxyvitamin D and parathyroid hormone. J Clin Endocrinol Metab. 2017;102(4):1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, et al. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27(1):160-169. [DOI] [PubMed] [Google Scholar]
- 26. Stevens BR, Goel R, Seungbum K, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67(8):1555-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pugin J, Schürer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A. 1993;90(7):2744-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Opal SM, Scannon PJ, Vincent JL, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999;180(5):1584-1589. [DOI] [PubMed] [Google Scholar]
- 29. Song SJ, Amir A, Metcalf JL, et al. Preservation methods differ in fecal microbiome stability, affecting suitability for field studies. mSystems. Published online May 3, 2016;1(3). doi: 10.1128/mSystems.00021-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tong M, Jacobs JP, McHardy IH, Braun J. Sampling of intestinal microbiota and targeted amplification of bacterial 16S rRNA genes for microbial ecologic analysis. Curr Protoc Immunol. 2014;107:7.41.1-7.41.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue):D590-D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martino C, Morton JT, Marotz CA, et al. A novel sparse compositional technique reveals microbial perturbations. mSystems. Published online February 12, 2019;4(1). doi: 10.1128/mSystems.00016-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26(1):32-46. [Google Scholar]
- 35. Lê Cao KA, Boitard S, Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinformatics. 2011;12:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koivisto O, Hanel A, Carlberg C. Key vitamin D target genes with functions in the immune system. Nutrients. Published online April 19, 2020;12(4). doi: 10.3390/nu12041140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szaleniec M, Wojtkiewicz AM, Bernhardt R, Borowski T, Donova M. Bacterial steroid hydroxylases: enzyme classes, their functions and comparison of their catalytic mechanisms. Appl Microbiol Biotechnol. 2018;102(19):8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sugimoto H, Shinkyo R, Hayashi K, et al. Crystal structure of CYP105A1 (P450SU-1) in complex with 1alpha,25-dihydroxyvitamin D3. Biochemistry. 2008;47(13):4017-4027. [DOI] [PubMed] [Google Scholar]
- 40. Geer LY, Marchler-Bauer A, Geer RC, et al. The NCBI BioSystems database. Nucleic Acids Res. 2010;38(Database issue):D492-D496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G208-G216. [DOI] [PubMed] [Google Scholar]
- 42. Zhao H, Zhang H, Wu H, et al. Protective role of 1, 25 (OH) 2 vitamin D 3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang YG, Wu S, Lu R, et al. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci Rep. 2015;5:10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Assa A, Vong L, Pinnell LJ, et al. Vitamin D deficiency predisposes to adherent-invasive Escherichia coli-induced barrier dysfunction and experimental colonic injury. Inflamm Bowel Dis. 2015;21(2):297-306. [DOI] [PubMed] [Google Scholar]
- 45. Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3-5):316-321. [DOI] [PubMed] [Google Scholar]
- 46. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067-1077. [DOI] [PubMed] [Google Scholar]
- 47. Wang T-T, Nestel FP, Bourdeau V, et al. Cutting edge: 1, 25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909-2912. [DOI] [PubMed] [Google Scholar]
- 48. Lewerin C, Nilsson-Ehle H, Jacobsson S, et al. Serum estradiol associates with blood hemoglobin in elderly men: the MrOS Sweden study. J Clin Endocrinol Metab. 2014;99(7):2549-2556. [DOI] [PubMed] [Google Scholar]
- 49. Li JY, Chassaing B, Tyagi AM, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shieh A, Epeldegui M, Karlamangla AS, Greendale GA. Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight. Published online January 20, 2020;5(2). doi: 10.1172/jci.insight.134092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Becker SL, Manson JE. Menopause, the gut microbiome, and weight gain: correlation or causation? Menopause. 2020;28(3):327-331. [DOI] [PubMed] [Google Scholar]
- 52. Schäffler H, Herlemann DP, Klinitzke P, et al. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J Dig Dis. 2018;19(4):225-234. [DOI] [PubMed] [Google Scholar]
- 53. Garg M, Hendy P, Ding JN, Shaw S, Hold G, Hart A. The effect of vitamin D on intestinal inflammation and faecal microbiota in patients with ulcerative colitis. J Crohns Colitis. 2018;12(8):963-972. [DOI] [PubMed] [Google Scholar]
- 54. Kanhere M, He J, Chassaing B, et al. Bolus weekly vitamin D3 supplementation impacts gut and airway microbiota in adults with cystic fibrosis: a double-blind, randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2018;103(2):564-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Charoenngam N, Shirvani A, Kalajian TA, Song A, Holick MF. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: a randomized, double-blinded, dose-response study. Anticancer Res. 2020;40(1):551-556. [DOI] [PubMed] [Google Scholar]
- 56. Naderpoor N, Mousa A, Fernanda Gomez Arango L, Barrett HL, Dekker Nitert M, de Courten B. Effect of vitamin D supplementation on faecal microbiota: a randomised clinical trial. Nutrients. Published online November 27, 2019;11(12). doi: 10.3390/nu11122888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bashir M, Prietl B, Tauschmann M, et al. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur J Nutr. 2016;55(4):1479-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121(6):2126-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang J, Thingholm LB, Skiecevičienė J, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48(11):1396-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lam YY, Ha CW, Hoffmann JM, et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity (Silver Spring). 2015;23(7):1429-1439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.