Abstract
The incidence of many hormone-dependent diseases, including testicular cancer, has sharply increased in all high-income countries during the 20th century. This is not fully explained by established risk factors. Concurrent, increasing exposure to antiandrogenic environmental endocrine disrupting chemicals (EDCs) in fetal life may partially explain this trend. This systematic review assessed available evidence regarding the association between environmental EDC exposure and risk of testicular cancer (seminomas and nonseminomas). Following PRISMA guidelines, a search of English peer-reviewed literature published prior to December 14, 2020 in the databases PubMed and Embase® was performed. Among the 279 identified records, 19 were eligible for quality assessment and 10 for further meta-analysis. The completeness of reporting was high across papers, but over 50% were considered subject to potential risk of bias. Mean age at diagnosis was 31.9 years. None considered effects of EDC multipollutant mixtures. The meta-analyses showed that maternal exposure to combined EDCs was associated with a higher risk of testicular cancer in male offspring [summary risk ratios: 2.16, (95% CI:1.78-2.62), 1.93 (95% CI:1.49-2.48), and 2.78 (95% CI:2.27-3.41) for all, seminoma, and nonseminoma, respectively]. Similarly, high maternal exposures to grouped organochlorines and organohalogens were associated with higher risk of seminoma and nonseminoma in the offspring. Summary estimates related to postnatal adult male EDC exposures were inconsistent. Maternal, but not postnatal adult male, EDC exposures were consistently associated with a higher risk of testicular cancer, particularly risk of nonseminomas. However, the quality of studies was mixed, and considering the fields complexity, more prospective studies of prenatal EDC multipollutant mixture exposures and testicular cancer are needed.
Keywords: endocrine disruption, xenobiotic chemicals, testicular cancer, systematic review, meta-analysis
Although rare in the general population, testicular cancer is the most common malignancy in men 15 to 44 years old worldwide, accounting for approximately 60% of all male cancers in this age group (1,2). Most testicular germ cell tumors (seminomas and nonseminomas) are derived from germ cell neoplasia in situ, which is thought to represent arrested fetal gonocytes that failed to mature to spermatogonia and subsequently transformed to malignant germ cells later in life (3,4). The incidence of testicular cancer, and many other hormone dependent diseases, has increased several folds across high income countries during the 20th century (5-9). The reasons for this remains largely unknown and are not fully explained by any secular changes in established risk factors, including cryptorchidism, Caucasian ethnicity, and family history of testicular cancer (10). Other risk factors in male offspring, such as inguinal hernia, maternal bleeding, low birthweight, small for gestational age, sibship size, and being the first-born child or a twin (11) also cannot explain this increased trend in testicular cancer incidence. In 2001, we proposed that testicular cancer was part of a syndrome that also comprises cryptorchidism, hypospadias, and poor semen quality, termed the testicular dysgenesis syndrome (TDS) (12). The TDS hypothesis proposes that insufficient prenatal androgen action including exposures to antiandrogenic environmental chemicals critically contribute to the etiology of testicular cancer.
Endocrine-disrupting chemicals (EDCs) are defined as an exogenous substance or mixture that alters the functions of the endocrine system and consequently causes adverse effects in an intact organism or its progeny (13). It has long been hypothesized that the rising incidence of testicular cancer is partially due to exposure to these ubiquitous chemicals (14). The impact of fetal environment on risk of testicular cancer is supported by the fact that male children of migrants moving from low-incidence to high-incidence countries acquire the testicular cancer risk of the high-incidence country, while fathers retain the incidence of their country of origin (15-18). Concurrent exponential increases in the production of EDCs has similarly led to speculations of a causal relationship. Most EDCs are extremely persistent with half-lives of up to 30 years and bioaccumulate in humans who are ubiquitously exposed via ingestion, inhalation, and skin absorption. Several EDCs can cross the placenta and enter fetal circulation (19-23), further supporting the TDS syndrome hypothesis, which is based on fetal exposure to chemicals impairing endogenous hormone production or action during critical developmental time windows (the fetal masculinization programming window).
Despite the tremendous scientific and public interest, there is still no consensus regarding the effects of individual EDCs on the risk of testicular cancer. More important, because of coexposure to multiple individual EDCs, there has been a shift from assessing the effects of a single chemical to a focus on grouping EDC combinations together, as recommended by the National Institute of Environmental Health Sciences (24,25). However, the combined effects of EDCs on testicular cancer risk is uncertain. The main objective of this systematic review was to determine the association between xenobiotic environmental EDC exposure and risk of testicular cancer. We hypothesized that multiple EDC exposures are associated with higher risk in varying degrees (additive, synergistic, antagonistic). The secondary objective was to elucidate life-stage dependency of exposure in relation to testicular cancer (maternal vs postnatal adult male exposure).
Materials and Methods: Review Strategy and Literature Search
Study Design
A systematic literature review and meta-analysis was performed in accordance with the PRISMA statement for reporting systematic reviews and meta-analyses of observational studies (26) (PRISMA checklist available in the Dryad Digital Repository (27)).
Protocol and Registration
A review protocol was approved by all authors and registered at PROSPERO.org with registration number CRD42020220065 prior to initiating the systematic literature review process on December 11, 2020.
Search Strategy
We conducted a systematic search to identify existing literature providing epidemiological evidence on the association between xenobiotic EDC exposures and the risk of testicular cancer in humans. We identified peer-reviewed original papers in English published prior to December 14, 2020 with search terms divided into 2 blocks; the first block covering the exposure (xenobiotic environmental EDCs) and the second block covering the outcome (testicular cancer). A combination of index (MeSH and Emtree) and free text search terms in the databases PubMed and Embase® were used and subsequently a hand search in reference lists of included papers was performed. PubMed and Embase® were selected as they cover literature within the health and biomedical field. The search was completed with a trained research librarian (University of Copenhagen Library) and the complete search specification including MeSH and Entrée terms is available in the Dryad Digital Repository (27).
Eligibility Criteria
Papers were considered if
Exposure was classified as xenobiotic EDCs, either as “high concern” for human or wildlife exposure (28), highly persistent, and/or with a high current production volume. Table 1 lists the characteristics of the specific chemical compounds included in the retrieved papers and the abbreviations that are referenced in this present review. Documentation of quantified environmental exposures to EDCs was required as direct measurements in biological samples or application of individual level models or proxies. Both maternal and postnatal adult male exposures were considered.
Outcome was classified as testicular cancer and histological subgroups (seminoma and nonseminoma) ascertained by medical standardized examination, medical records, reporting to health registries, or self-report.
Table 1.
Overview of the classification, properties and effects of the chemical compounds used in the included studies
| Compound group | Compound (abbreviation) | Period used | Main use and present routes of exposure | Half-life | Main effect in humans |
|---|---|---|---|---|---|
| Organochlorines | Dichlorodiphenyltrichloroethane (DDT) | ||||
| p,p’-dichloro-diphenyl-trichloroethane (p,p’-DDT) | 1940-1986 | Pesticides (ingestion, dermal exposure) | 7-8 years | Estrogenic | |
| o,p’-dichloro-diphenyl-trichloroethane (o,p’-DDT) | |||||
| p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE): metabolite of DDT | Antiandrogenic | ||||
| Cyclodienes | |||||
| Chlordanes | |||||
| cis-chlordane (α-chlordane) | 1948-1988 (all production was stopped in 1976) | Pesticide in agriculture and home building for termites. (ingestion, dermal exposure) | ≤30 years | Antiandrogenic | |
| trans-chlordane (β -chlordane) | |||||
| cis-heptachlordane [(+) − heptachlor] | |||||
| Heptachlor epoxide: metabolite of heptachlor | |||||
| Oxychlordane: metabolite of chlordane | |||||
| Dieldrin (an epoxy of aldrin) | 1948-1984 (an alternative to DDT). Still used in some developing countries | Insecticide in agriculture (ingestion, dermal exposure) | ≤30 years | Antiandrogenic | |
| Mirex (dechlorane, perchloropenta-cyclodecane) | 1959-1978 | Insecticide (ants), as a flame retardant in plastics, rubber, paint, paper, and electrical goods (ingestion, dermal exposure) | ≤30 years | Antiandrogenic | |
| Hexachlorobenzene or perchlorbenzene | 1945-1965 | Fungicide, in the manufacture of dyes, synthesis of organic chemicals, rubber, wood preservation and fireworks (ingestion, dermal exposure) | ≤6 years | Antiandrogenic | |
| Hexachlorocyclohexane (HCCH) | |||||
| β-HCCH | -late 1980’s | Insecticide (ingestion, dermal exposure) | ≤6 years | Human carcinogen | |
| α-HCCH | -late 1980’s | Insecticide (ingestion, dermal exposure) | |||
| γ-HCCH (or Lindane) | -late 1980’s | Insecticide (fruits) and to treat lead lice (ingestion, dermal exposure) | |||
| Organohalogens | Polychlorinated biphenyl congeners (6) | 1920-late 1970 | Electrical equipment, plasticizers in paints, coolants, flourescent lights. (ingestion, inhalation, dermal exposure) | ≤16 years | Variable effects depending on congener: estrogenic, antiestrogenic, antiandrogenic |
| Polybrominated diphenyl congeners | 1960-early 2000 | Flame-retardants in building materials, electronics, furnishings, motor vehicles, airplanes, plastics, polyurethane foams, and textiles (ingestion, inhalation, dermal exposure) | ≤12 years | Variable effects depending on congener: Lower brominated (up to hexa-BDEs) are estrogenic, higher brominated and some hydroxylated are antiestrogenic | |
| Per- and polyfluorinated alkyl substances (PFAS) | Perfluorooctanoic acid Perfluorooctane sulfonate (PFOS) |
Presently being phased out in favor of PFAS with shorter half-lives | Stain and water repellents (carpeting, upholstery, apparel), floor wax, firefighting foam, textiles and sealants. PFOS was a key ingredient in 3M Scotchgard. (ingestion, inhalation, dermal exposure) | ≤10 years | Mixed effects (antiandrogenic, antiestrogenic), experimental studies report that PFAS substances severely affect proliferation and function of Leydig cells in rats. |
| Plasticizers | Phthalates | 1920-partial restriction for many of the most potent phthalates (Di-n-butyl-phthalate, butylbenzyl phthalate BBzP, Di-(2-ethylhexyl) phthalate, and Di-isononyl phthalate) since 2010, and almost banned in EU since summer 2020 | Personal care products, plasticizers in polyvinyl chloride (PVC), construction materials, PVC consumer products (clothing, food packaging, toys, medical devices). (ingestion, inhalation, dermal exposure) | 12 h | Antiandrogenic. Exert their toxic action by inhibiting Leydig cell synthesis of testosterone, and there is consistent evidence of dose-related inverse associations between human blood levels and testosterone. |
| Bisphenols | Bisphenol A | 1958—restricted in 2019 | Polycarbonate plastics, epoxy resins, plastic toys, and water bottles (ingestion, dermal exposure) | 4-5 h | Estrogenic |
Exclusion Criteria
Experimental studies (in vitro and in vivo), case reports, reviews, conference proceedings/abstracts, letters, editorials, and comments were excluded. In addition, papers addressing mechanisms and other outcomes related to endocrine disruption (eg, effects on sexual hormone tissue levels) and papers repeating risk estimates reported in previous publications (eg, risk according to gene polymorphisms for specific compounds) were also excluded.
Selection of Relevant Papers
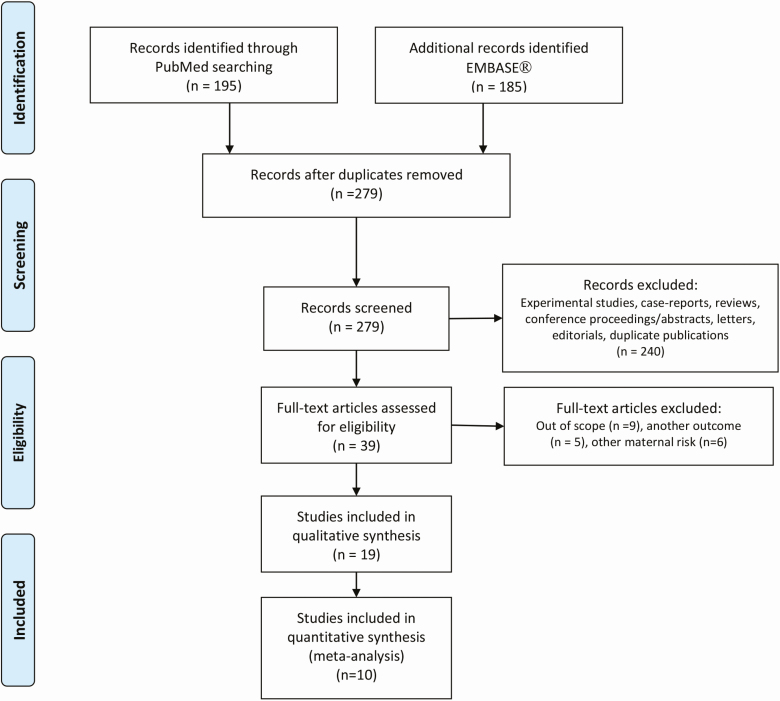
We obtained 279 search hits after removal of duplicates (Fig. 1). Two authors (E.V.B. and T.K.) screened titles and abstracts independently to assess eligibility based on the above exclusion criteria and retrieved 39 papers for full text reading. Among these, 20 were beyond the context of the eligibility criteria specified in the previous discussion and were ultimately excluded. Specific reasons for exclusion are detailed in Figure 1. Hand searches of the bibliographies of retrieved primary reports and reviews did not capture additional papers. We selected 19 papers, based on 13 different populations [Dryad Digital Repository (27)] that provided at least 1 risk estimate for a testicular cancer according to at least 1 xenobiotic classified as an EDC. No attempt was made to retrieve papers from the unpublished literature as these are not peer-reviewed with the same rigor as published papers. We assessed the quality (completeness of reporting and risk of bias/confounding) of all 19 papers and 10 papers (all measuring EDCs directly in serum) were further included in the meta-analyses. The papers that used proxy exposures to EDC were excluded from our meta-analyses because of the heterogeneity in ascertainment of exposures (varied occupations, location, and data collected) rendering these data unsuitable.
Figure 1.
Flow diagram of identified English articles published before December 14, 2020.
Data Extraction
Descriptive information [Table 2; also see Supplemental Table 1 in (27)] was recorded from each publication using a standardized data extraction template constructed prior to the collection process. Risk estimates with 95% CIs were extracted for each measured compound and groups of compounds (if provided). When risk according to several levels of exposure was reported, the highest level of exposure vs the reference category was chosen.
Table 2.
Testicular Cancer Systematic Review 2021: key papers (individual measurement of exposure level) - study characteristics
| Author, year | Location | Population | Study design | Exposure | Exposure contrast | Xenobiotic | All TGCT | Nonseminoma | Seminoma | Included confounders | CRa | Biasb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCases/controls | RR/OR | 95% CI | RR/OR | 95% CI | RR/OR | 95% CI | ||||||||||
| Maternal exposure | ||||||||||||||||
| Biospecimens | ||||||||||||||||
| Cohn et al (38) | USA, California | The Child Health and Development Studies | Nested case control | Early postpartum maternal serumc | IQR for controls | p,p’-DDT | 15/45 | 0.70 | 0.26-1.64 | Sons race and age (by design) | 8 | 1 | ||||
| p,p’-DDE | 0.19 | 0.04-0.62 | 8 | 1 | ||||||||||||
| o,p’-DDT | 0.77 | 0.37-1.33 | 8 | 1 | ||||||||||||
| Adjusted for p,p’-DDE | 4.81 | 0.92-48.62 | 8 | 1 | ||||||||||||
| Ratio of DDT to DDE | 3.56 | 1.34-11.88 | 8 | 1 | ||||||||||||
| Hardell et al (39) | Sweden | Hospital departments in 5 Swedish cities | Case referent | Maternal serum at sons diagnostic | Dichotomized (cutoff median for controls) | Sum PCB | 44/45 | 3.8 | 1.4-10 | 4.3 | 1.3-14 | 3.1 | 0.7-14 | Maternal age and BMI at the time of sampling | 9 | 0 |
| HCB | 4.4 | 1.7-12 | 9.0 | 2.4-33 | 2.1 | 0.6-8.2 | 9 | 0 | ||||||||
| p,p’-DDE | 1.3 | 0.5-3.0 | 1.4 | 0.5-4.0 | 1.0 | 0.3-3.7 | 9 | 0 | ||||||||
| cis-Heptachlordane | 2.1 | 0.8-5.0 | 1.8 | 0.7-4.7 | 3.2 | 0.8-13 | 9 | 0 | ||||||||
| cis-Chlordane | 2.5 | 1.0-6.1 | 2.1 | 0.7-5.7 | 4.3 | 1.1-17 | 9 | 0 | ||||||||
| Oxychlordane | 2.6 | 0.9-7.1 | 2.5 | 0.8-7.9 | 3.3 | 0.7-16 | 9 | 0 | ||||||||
| MC6 | 1.3 | 0.5-3.2 | 1.3 | 0.5-3.6 | 1.3 | 0.4-5.0 | 9 | 0 | ||||||||
| trans-Nonachlordane | 4.1 | 1.5-11 | 5.6 | 1.7-19 | 1.9 | 0.5-7.5 | 9 | 0 | ||||||||
| cis-Nonachlordane | 3.1 | 1.2-7.8 | 2.8 | 1.0-7.8 | 4.1 | 1.0-18 | 9 | 0 | ||||||||
| Sum chlordane | 1.9 | 0.7-5.0 | 2.4 | 0.8-7.3 | 1.2 | 0.3-4.8 | 9 | 0 | ||||||||
| Hardell et al (40) | Sweden | Hospital departments in 5 Swedish cities | Case referent | Maternal serum at sons diagnostic | Dichotomized (cutoff median for controls) | PCB 74 | 44/45 | 3.0 | 1.2-7.6 | 2.8 | 1.0-7.9 | 3.3 | 0.8-14 | Maternal age and BMI at the time of sampling | 9 | 0 |
| PCB 99 | 2.4 | 1.0-5.7 | 2.6 | 0.9-7.2 | 1.6 | 0.5-6.0 | 9 | 0 | ||||||||
| PCB 114 | 1.7 | 0.7-4.2 | 1.7 | 0.6-4.5 | 2.0 | 0.5-7.6 | 9 | 0 | ||||||||
| PCB 105 | 1.8 | 0.7-4.3 | 2.2 | 0.8-6.1 | 0.9 | 0.3-3.3 | 9 | 0 | ||||||||
| PCB 153 | 2.7 | 1.1-6.8 | 3.5 | 1.2-10 | 1.4 | 0.4-5.3 | 9 | 0 | ||||||||
| PCB 138 | 2.8 | 1.1-7.1 | 4.0 | 1.3-12 | 1.5 | 0.4-5.3 | 9 | 0 | ||||||||
| PCB 128/167 | 3.8 | 1.5-9.8 | 3.4 | 1.2-9.8 | 5.7 | 1.1-29 | 9 | 0 | ||||||||
| PCB 156 | 3.8 | 1.4-9.9 | 4.2 | 1.3-13 | 3.7 | 0.9-16 | 9 | 0 | ||||||||
| PCB 178 | 2.9 | 1.1-7.7 | 3.9 | 1.2-13 | 1.7 | 0.4-7.4 | 9 | 0 | ||||||||
| PCB 182/187 | 2.3 | 0.9-5.8 | 2.9 | 1.0-8.5 | 1.5 | 0.4-5.4 | 9 | 0 | ||||||||
| PCB 183 | 2.5 | 1.0-6.2 | 3.1 | 1.1-8.8 | 1.7 | 0.5-5.9 | 9 | 0 | ||||||||
| PCB 174 | 2.0 | 0.8-5.0 | 2.7 | 0.9-7.5 | 1.1 | 0.3-3.9 | 9 | 0 | ||||||||
| PCB 177 | 2.0 | 0.8-5.0 | 3.8 | 1.2-12 | 0.7 | 0.2-2.4 | 9 | 0 | ||||||||
| PCB 180 | 2.5 | 1.0-6.3 | 2.7 | 0.9-9.6 | 2.1 | 0.5-7.9 | 9 | 0 | ||||||||
| PCB 170/190 | 3.1 | 1.2-8.2 | 4.0 | 1.3-12 | 1.9 | 0.5-7.8 | 9 | 0 | ||||||||
| PCB 189 | 3.3 | 1.3-8.4 | 4.7 | 1.5-14 | 2.1 | 0.6-7.6 | 9 | 0 | ||||||||
| PCB 208 | 3.4 | 1.3-8.6 | 2.9 | 1.0-8.1 | 5.7 | 1.2-27 | 9 | 0 | ||||||||
| PCB 207 | 3.0 | 1.2-7.5 | 2.9 | 1.0-8.1 | 3.7 | 0.9-15 | 9 | 0 | ||||||||
| PCB 209 | 1.4 | 0.6-3.4 | 1.5 | 0.5-4.1 | 1.4 | 0.4-5.1 | 9 | 0 | ||||||||
| Estrogenic PCBs | 30/20 | 2.4 | 1.0-6.0 | 2.4 | 0.8-6.8 | 2.3 | 0.6-8.9 | 9 | 0 | |||||||
| Hardell et al (37) | Sweden | Hospital departments in 5 Swedish cities | Case referent | Maternal serum at son’s diagnostic | Dichotomized (cutoff median for controls) | PBDE (47, 99, 153) | 44/45 | 2.5 | 1.0-6.0 | 2.9 | 1.0-8.2 | 1.8 | 0.5-6.5 | Maternal age and BMI at the time of sampling | 9 | 0 |
| Proxy exposures | ||||||||||||||||
| Ghazarian et al (44) | USA, Silver Spring | US Servicemen Testicular Tumor Environment Endocrine Determinant (STEED). | Case control | Maternal prenatal personal care products | Dichotomous >1 week (ref >=1 week) | Face lotion | 527/562 | 1.42 | 1.08-1.86 | Age (diagnosis), TGCT family history, race, cryptorchidism, maternal (age at delivery, pregnancy weight gain) | 8 | 0 | ||||
| Perfume | 0.89 | 0.65-1.20 | 8 | 0 | ||||||||||||
| Hairspray | 1.17 | 0.89-1.55 | 8 | 0 | ||||||||||||
| Nail polish | 1.11 | 0.62-2.01 | 8 | 0 | ||||||||||||
| Body lotion | 1.27 | 0.97-1.67 | 8 | 0 | ||||||||||||
| Deodorant | 1.80 | 0.94-3.44 | 8 | 0 | ||||||||||||
| Sunscreen | 0.74 | 0.37-1.49 | 8 | 0 | ||||||||||||
| Kristensen et al (45) | Norway | Registry | Cohort | JEM Maternal prenatal exposure | Dichotomous (y/n) | Pesticides | 188680 /323359 | 0.89 | 0.60-1.32 | Age and calendar year | 5 | 0 | ||||
| Horticulture | 0.79 | 0.41-1.49 | 5 | 0 | ||||||||||||
| Le Cornet et al (46, 47) | DK, Finland, Sweden, and Norway | NORD-TEST study | Case control (nested in a birth cohort) | JEM prenatal exposure | Dichotomous (y/n) | Pesticides | 8443/28752 | 0.83 | 0.56-1.23 | 0.72 | 0.43-1.21 | 1.03 | 0.57-1.88 | Date and age at diagnosis, cryptorchidism, hypospadias, TGCT family history | 8 | 0 |
| ARHC sum | 8112/26264 | 1.23 | 0.97-1.55 | 8 | 0 | |||||||||||
| Benzene | 1.18 | 0.91-1.52 | 8 | 0 | ||||||||||||
| Toulene | 1.22 | 0.88-1.68 | 8 | 0 | ||||||||||||
| CHC sum | 1.05 | 0.84-1.31 | 8 | 0 | ||||||||||||
| Methylene chloride | 1.34 | 0.97-1.85 | 8 | 0 | ||||||||||||
| Perchloroethylene | 1.10 | 0.77-1.57 | 8 | 0 | ||||||||||||
| Trichloroethylene | 0.92 | 0.69-1.24 | 8 | 0 | ||||||||||||
| 1,1,1,-trichloroethane | 1.03 | 0.80-1.32 | 8 | 0 | ||||||||||||
| Nori et al (48) | Italy | Hospitals in Rome | Case referent | JEM Prenatal exposure | Dichotomous (y/n) | EDC | 63/123 | 0.97 | 0.23-4.07 | 1.13 | 0.19-6.86 | 0.99 | 0.16-6.07 | Age, education, cryptorchidism, hypospadias | 8 | 1 |
| Rural (pesticides) | 103/215 | 1.35 | 0.49-3.71 | 1.29 | 0.34-4.94 | 1.54 | 0.44-5.35 | |||||||||
| Paoli et al (49) | Italy | Hospitals in Rome | Case referent | JEM Prenatal exposure | Dichotomous (y/n) | Pesticide | 125/103 | 1.97 | 0.36-10.66 | Age, education | 7 | 1 | ||||
| PVC | 1.00 | 0.57-17.57 | 7 | 1 | ||||||||||||
| Phthalates | 1.03 | 0.26-4.11 | 7 | 1 | ||||||||||||
| Alkyl phenolic | 1.54 | 0.49-4.80 | 7 | 1 | ||||||||||||
| Postnatal adult male exposure | ||||||||||||||||
| Biospecimens | ||||||||||||||||
| Barry et al (34) | USA, Mid-Ohio Valley | C8 Health Project survey/Dupont worker cohort | Cohort | Serum (estimated cumulative) | Highest quartile vs lowest | PFOA | 17/32254 | 3.17 | 0.75-13.45 | Smoking, alcohol, education (age by design) | 8 | 1 | ||||
| Biggs et al (41) | USA, Washington State | The Adult Testicular Lifestyle and blood Specimen (ATLAS) Study | Case control | Serum after diagnosis | 85th vs <50th percentile | β-HCCH | 246/630 | 0.92 | 0.51–1.64 | Age, race, BMI change, serum lipid | 10 | 1 | ||||
| α-HCCH | 1.36 | 0.75–2.46 | 10 | 1 | ||||||||||||
| Dieldrin | 0.79 | 0.44–1.41 | 10 | 1 | ||||||||||||
| HCB | 0.85 | 0.37–1.96 | 10 | 1 | ||||||||||||
| HCE | 0.67 | 0.35-1.29 | 10 | 1 | ||||||||||||
| Mirex | 0.87 | 0.50–1.53 | 10 | 1 | ||||||||||||
| p,p’-DDT | 1.17 | 0.68–2.00 | 10 | 1 | ||||||||||||
| o,p-DDT | 1.30 | 0.67–2.53 | 10 | 1 | ||||||||||||
| p,p’-DDE | 0.61 | 0.32–1.14 | 10 | 1 | ||||||||||||
| oxychlordane | 0.93 | 0.50-1.73 | 10 | 1 | ||||||||||||
| trans-chlordane | 0.89 | 0.49-1.61 | 10 | 1 | ||||||||||||
| Total chlordanes | 0.93 | 0.51–1.68 | 10 | 1 | ||||||||||||
| Cheng et al (50) | USA, Connecticut/Massachusetts | Population based | Population-based case control | Serum after diagnosis | Highest quartile vs lowest | Sum PCB | 308/323 | 1.0 | 0.4-2.1 | 1.0 | 0.3-2.7 | 0.8 | 0.3-2.2 | Age, race, BMI, center, previous cancer/testes injury, education, BTW, cryptorchidism | 11 | 0 |
| Sum estrogenic PCBs | 2.5 | 1.3-4.7 | 2.4 | 1.1-5.4 | 2.2 | 1.0-4.7 | 11 | 0 | ||||||||
| Sum Anti-estrogenic PCBs | 0.8 | 0.4-1.8 | 0.8 | 0.3-2.1 | 0.9 | 0.4-2.3 | 11 | 0 | ||||||||
| Sum enzyme inducing PCBs | 0.5 | 0.2-1.2 | 0.5 | 0.2-1.5 | 0.5 | 0.2-1.4 | 11 | 0 | ||||||||
| Giannandrea et al (42) | Italy, Rome | Case control | Serum | Dichotomized (cutoff 0.2 ng/mL) (LOD) | p,p´-DDT + HCB | 50/48 | 3.34 | 1.09-10.19 | Education, maternal (age at birth, parity) | 8 | 1 | |||||
| p,p’-DDE | 3.21 | 0.77-13.30 | 8 | 1 | ||||||||||||
| Hardell et al (39) | Sweden | Case-referent | Serum at diagnosis | Dichotomized (cutoff median for controls) | Sum PCB | 58/61 | 1.1 | 0.5-2.6 | 1.1 | 0.4-3.0 | 1.1 | 0.4-3.5 | Age, BMI | 9 | 0 | |
| HCB | 1.7 | 0.8-3.6 | 1.8 | 0.7-4.4 | 1.6 | 0.6-4.5 | 9 | 0 | ||||||||
| p,p’-DDE | 1.7 | 0.8-3.7 | 1.9 | 0.8-4.7 | 1.5 | 0.5-4.5 | 9 | 0 | ||||||||
| cis-Heptachlordane | 1.6 | 0.8-3.4 | 2.1 | 0.9-5.1 | 1.4 | 0.5-3.7 | 9 | 0 | ||||||||
| cis-Chlordane | 1.2 | 0.6-2.6 | 1.9 | 0.8-4.7 | 0.7 | 0.2-1.9 | 9 | 0 | ||||||||
| Oxychlordane | 1.4 | 0.7-2.9 | 1.9 | 0.8-4.7 | 1.0 | 0.4-2.8 | 9 | 0 | ||||||||
| MC6 | 1.3 | 0.6-2.9 | 1.8 | 0.7-4.9 | 0.9 | 0.3-2.7 | 9 | 0 | ||||||||
| trans-Nonachlordane | 1.0 | 0.4-2.1 | 1.2 | 0.4-2.9 | 0.7 | 0.2-2.1 | 9 | 0 | ||||||||
| cis-Nonachlordane | 2.6 | 1.2-5.7 | 2.0 | 0.8-4.7 | 4.8 | 1.4-16 | 9 | 0 | ||||||||
| Sum chlordane | 1.3 | 0.6-2.8 | 1.8 | 0.7-4.4 | 0.8 | 0.3-2.4 | 9 | 0 | ||||||||
| Hardell et al (40) | Hospital-based study of TC cases and healthy controls Hospital departments in 5 Swedish cities |
Hospital departments in 5 Swedish cities | Case referent | Serum at diagnosis | Dichotomized (cutoff median for controls) | Estrogenic PCBs | 29/30 | 1.3 | 0.5-3.0 | 1.5 | 0.5-4.1 | 1.0 | 0.3-3.5 | Age, BMI | 9 | 0 |
| Enzyme inducing PCBs | 1.2 | 0.5-2.8 | 1.1 | 0.4-3.1 | 1.4 | 0.5-4.6 | 9 | 0 | ||||||||
| Toxic equivalents | 1.4 | 0.6-3.2 | 1.6 | 0.6-4.3 | 1.1 | 0.3-3.5 | 9 | 0 | ||||||||
| McGlynn et al (36) | USA | US Servicemen’s Testicular Tumor Environmental and Endocrine Determinants | Case referent | Serum | Highest quartile vs lowest | Cis-Nonachlor | 739/915 | 1.56 | 1.11-2.18 | 1.32 | 0.86-2.03 | 1.93 | 1.27-2.93 | Age and race (by match design), cryptorchidism, TGCT family history, height, age at serum draw, BMI | 11 | 0 |
| trans-Nonachlor | 1.46 | 1.07-2.00 | 1.39 | 0.96-2.00 | 1.72 | 1.11-2.67 | 11 | 0 | ||||||||
| Oxychlordane | 1.27 | 0.92-1.76 | 1.11 | 0.75-1.63 | 1.64 | 1.04-2.60 | 11 | 0 | ||||||||
| p,p’-DDE | 1.71 | 1.23-2.38 | 1.63 | 1.10-2.42 | 1.91 | 1.22-2.99 | 11 | 0 | ||||||||
| p,p’-DDT | 1.13 | 0.71-1.82 | 0.94 | 0.50-1.77 | 1.30 | 0.73-2.30 | 11 | 0 | ||||||||
| β-HCCH | 0.90 | 0.65-1.24 | 0.85 | 0.57-1.26 | 0.97 | 0.63-1.49 | 11 | 0 | ||||||||
| Mirex | 1.24 | 0.90-1.74 | 1.24 | 0.82-1.88 | 1.15 | 0.75-1.77 | 11 | 0 | ||||||||
| Total chlordane | 1.51 | 1.09-2.10 | 1.37 | 0.93-2.02 | 1.9 | 1.20-3.0 | 11 | 0 | ||||||||
| McGlynn et al (10) | USA | US Servicemen’s Testicular Tumor Environmental and Endocrine Determinants | Case referent | Serum | Highest quartile vs lowest | Sum PCB | 736/913 | 0.61 | 0.43-0.86 | 0.55 | 0.37-0.83 | 1.9 | 1.20-3.0 | Age, race, cryptorchidism, TGCT family history, height, age at serum draw, BMI | 11 | 0 |
| PCB 99 | 0.80 | 0.57-1.13 | 0.64 | 0.41-1.02 | 11 | 0 | ||||||||||
| PCB 101 | 1.01 | 0.74-1.38 | 0.91 | 0.62-1.33 | 1.12 | 0.74-1.70 | 11 | 0 | ||||||||
| PCB 118 | 0.55 | 0.40-0.76 | 0.45 | 0.31-0.66 | 0.72 | 0.47-1.12 | 11 | 0 | ||||||||
| PCB 138 | 0.46 | 0.32-0.66 | 0.42 | 0.27-0.65 | 0.52 | 0.31-0.86 | 11 | 0 | ||||||||
| PCB 153 | 0.45 | 0.31-0.66 | 0.40 | 0.26-0.63 | 0.52 | 0.31-0.87 | 11 | 0 | ||||||||
| PCB 156 | 0.57 | 0.40-0.81 | 0.58 | 0.37-0.91 | 0.54 | 0.34-0.86 | 11 | 0 | ||||||||
| PCB 163 | 0.59 | 0.42-0.83 | 0.57 | 0.37-0.86 | 0.58 | 0.37-0.92 | 11 | 0 | ||||||||
| PCB 170 | 0.56 | 0.39-0.80 | 0.55 | 0.36-0.85 | 0.56 | 0.35-0.91 | 11 | 0 | ||||||||
| PCB 180 | 0.56 | 0.38-0.82 | 0.51 | 0.32-0.81 | 0.67 | 0.39-1.13 | 11 | 0 | ||||||||
| PCB 183 | 0.86 | 0.58-1.29 | 0.92 | 0.56-1.52 | 0.77 | 0.46-1.29 | 11 | 0 | ||||||||
| PCB 187 | 0.60 | 0.42-0.86 | 0.48 | 0.31-0.75 | 0.75 | 0.47-1.20 | 11 | 0 | ||||||||
| Estrogenic PCBs | 0.65 | 0.45-0.93 | 0.55 | 0.36-0.84 | 0.80 | 0.49-1.29 | 11 | 0 | ||||||||
| Anti-estrogenic (non-/mono-ortho) | 0.42 | 0.29-0.59 | 0.38 | 0.25-0.58 | 0.44 | 0.27-0.72 | 11 | 0 | ||||||||
| Anti-estrogenic (di-ortho) | 0.46 | 0.31-0.67 | 0.43 | 0.27-0.68 | 0.49 | 0.29-0.83 | 11 | 0 | ||||||||
| Purdue et al (43) | Norway | The Janus Serum Bank (The Cancer Registry of Norway, Oslo, Norway) | Nested case control | Serum | highest tertile vs lowest | o,p’-DDT | 34/34 | 1.4 | 0.4-4.5 | 2.2 | 0.50-8.7 | None | 7 | 1 | ||
| p,p’-DDT | 2.1 | 0.6-7.2 | 7 | 1 | ||||||||||||
| p,p’-DDE | 2.2 | 0.7-6.5 | 7 | 1 | ||||||||||||
| HCE | 2.4 | 0.6-9.1 | 7 | 1 | ||||||||||||
| Oxychlordane | 3.2 | 0.6-16.8 | 5.1 | 0.7-36.8 | 7 | 1 | ||||||||||
| t-Nonachlor | 2.6 | 0.7-8.9 | 1.6 | 0.4-6.0 | 7 | 1 | ||||||||||
| Total chlordane | 2.3 | 0.6-7.2 | 1.6 | 0.4-6.6 | 7 | 1 | ||||||||||
| β-HCCH | 1.8 | 0.5-6.1 | 7 | 1 | ||||||||||||
| α-HCCH | 1.1 | 0.2-5.0 | 7 | 1 | ||||||||||||
| Dieldrin | 2.1 | 0.5-9.5 | 7 | 1 | ||||||||||||
| HCB | 2.9 | 0.5-15.2 | 7 | 1 | ||||||||||||
| Mirex | 1.2 | 0.4-3.0 | 7 | 1 | ||||||||||||
| PCB 44 | 0.6 | 0.1-3.8 | 0.2 | 0.01-2.0 | 7 | 1 | ||||||||||
| PCB 49 | 1.2 | 0.2-7.6 | 0.3 | 0.02-4.7 | 7 | 1 | ||||||||||
| PCB52 | 1.0 | 0.3-3.5 | 0.4 | 0.07-2.3 | 7 | 1 | ||||||||||
| PCB 99 | 2.2 | 0.8-5.9 | 4.4 | 1.0-20.5 | 7 | 1 | ||||||||||
| PCB 138 | 1.8 | 0.6-5.1 | 2.1 | 0.6-7.2 | 7 | 1 | ||||||||||
| PCB 153 | 1.2 | 0.4-3.4 | 1.2 | 0.4-4.3 | 7 | 1 | ||||||||||
| PCB 167 | 4.4 | 1.0-19.8 | 6.7 | 1.1-42.9 | 7 | 1 | ||||||||||
| PCB 183 | 1.3 | 0.5-3.5 | 2.9 | 0.6-13.7 | 7 | 1 | ||||||||||
| PCB 195 | 1.7 | 0.6-4.6 | 3 | 0.8-11.7 | 7 | 1 | ||||||||||
| Total PCB | 1.3 | 0.5-3.8 | 1.2 | 0.4-4.1 | 7 | 1 | ||||||||||
| High degree of PCB chlorination | 1.4 | 0.6-3.3 | 7 | 1 | ||||||||||||
| Proxy exposures | ||||||||||||||||
| Giannandrea et al. 2011 (42) | Italy, Rome | Hospital cases and controls | Case referent | Survey | Dichotomized (cutoff 0.2 ng/mL) (LOD) | Home use pesticide | 50/48 | 4.8 | 0.91-25.3 | Education, maternal (age at birth, parity) | 8 | 1 | ||||
| Household insecticide | 3.21 | 1.15-9.11 | 8 | 1 | ||||||||||||
| Hardell et al (51) | Sweden, middle to north | TC patients (registry) and population Registry (controls) | Case control | JEM | None/low-grade and high-grade exposure (days) | PVC (phthalates) | 148/315 | 6.6 | 1.4-32 | - | - | 5.6 | 1.1-196 | None | 7 | 1 |
| Styrene | 0.6 | 0.2-2.0 | 1 | 0.2-6.4 | 0.5 | 0.2-2.3 | 7 | 1 | ||||||||
| Urethane | 1.5 | 0.4-5.6 | 3.2 | 0.3-37 | 1 | 0.2-5.5 | 7 | 1 | ||||||||
| Acrylate | 3.2 | 0.3-37 | 3.2 | 0.3-37 | - | - | 7 | 1 | ||||||||
| Plastic unspecified | 4.3 | 0.8-24 | 6 | 0.6-58 | 2.5 | 0.2-40 | 7 | 1 | ||||||||
| Helmfrid et al (52) | Sweden | Registry | Case referent | Address | Dichotomous | PCB contaminated site | 7/35 | 2.46 | 0.99-5.08 | None | 6 | 1 | ||||
| Vieira et al (53) | USA, Mid-Ohio Valley | C8 Health Project survey and Dupont worker cohort | Case control | Estimated | Highest quintile vs lowest | PFOA | 134/25107 | 2.8 | 0.8-9.2 | Age, race, sex, smoking, year of diagnosis, insurance provider | 8 | 1 |
Bold text: statistically significant estimates and CI.
Abbreviations: ARHC, aromatic hydrocarbon solvents; BMI, body mass index; CHC, chlorinated hydrocarbon solvents; CI, confidence interval; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; HCB, hexachlorobenzene; HCCH, hexachlorocyclohexane; HCE, heptachlor epoxide; IQR, interquartile range; JEM, job-exposure matrix; LOD, limit of detection; OR, Odds ratio; PBDE, polybrominated diphenyl congeners; PCB, polychlorinated biphenyl congeners; PFOA, perfluorooctanoic acid; PVC, polyvinyl chloride; RR, risk ratio; TGCT, testicular germ cell tumors.
aCompleteness of reporting (CR): a sum of ≥8 (or ≥7 with indirect measures of exposure) was considered sufficient.
bBias and confounding: 1 = potential risk of bias. 0 = lower risk of bias.
cThe authors state that the postpartum serum strongly correlated with prenatal maternal serum, however the prenatal serum was not available for all mothers.
Assessment of Quality
To determine the quality of the identified papers, all were evaluated for completeness of reporting and bias (including confounding) using a standardized form adapted from Bonzini et al (29) and Shamliyan et al (30). Two authors (E.V.B. and T.K.) completed the forms independently, and any potential discrepancies in ratings were settled through a consensus discussion.
Completeness of reporting was assessed in the following 11 steps: (1) study design, (2) sampling frame and procedures, (3) inclusion and exclusion criteria, (4) population characteristics of exposed/unexposed or cases/referents, (5) response rates/study numbers reported or given implicitly, (6) methods for exposure assessment, (7) methods for outcome ascertainment, (8) external quality assurance program of biochemical analyses, (9) detection level and precision for biological samples, (10) statistical analysis, and (11) measures of association with 95% CIs. The 11 items were assigned equal weights with a value of 1 given for adequate reporting. A sum of completeness of reporting score of ≥8 (or ≥7 with indirect measures of exposure) was considered sufficient. Completeness of reporting is not considered a direct measure of quality but is used to adequately evaluate bias and confounding.
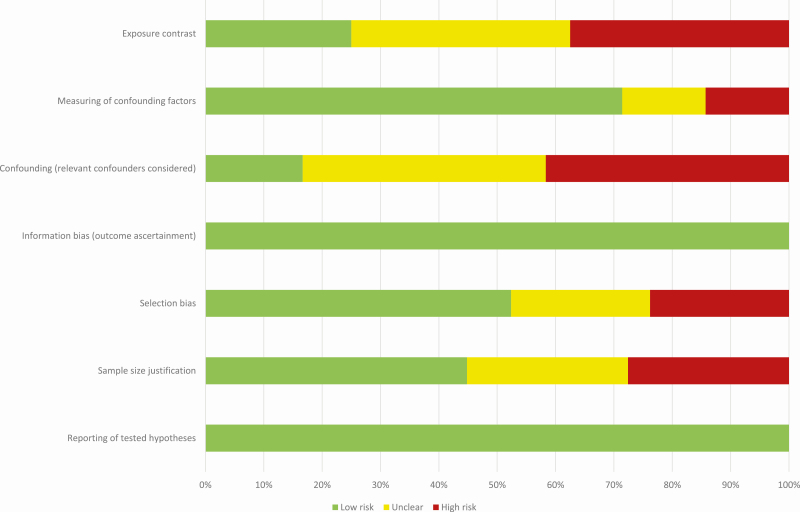
Potential sources of bias and confounding were evaluated in 7 zones (27) comprising (1) reporting of tested hypotheses, (2) sample size justification, (3) selection bias from loss to follow-up or lack of representativeness in a population sample, (4) information bias related to outcome ascertainment, (5) accounting for confounding, (6) measuring of confounding factors, and (7) reasonable exposure contrasts. Each of the zones were either rated as high risk, uncertain risk, or low risk. Zones 1, 3, 4, and 5 were considered the most important to evaluate potential sources of bias. Papers with 2 or more of these zones rated as high risk were considered of higher risk of bias. The assigned quality assessment scores for completeness of reporting, bias, and confounding for individual papers are presented in Table 2. A risk of bias graph for each individual study is also available in Figure 2.
Figure 2.
Risk of bias: the proportion of the included studies with each of the judgments (low risk, uncertain risk, high risk).
Random Effects Meta-Analysis
The following associations were analyzed separately for all maternal and postnatal adult male exposures:
The specific individual EDCs and testicular cancer (including histological sub-types).
The specific EDC compound groups and testicular cancer (including histological sub-types) under the assumption of specific within group mechanisms.
The association between combinations of all EDCs and testicular cancer (including histological subtypes).
In the first analysis we only included exposure-outcome estimates for individual EDCs in the meta-analysis if 3 or more risk estimates were available, leading to a narrow scope. The last 2 analyses were broader in scope and attempt to address the effects of groups and multiple combined EDC compounds.
We pooled evidence of the associations by a random effect meta-analysis and computed a common summary risk estimate by weighing the risk ratio (RR) or equivalent odds ratio (OR) with the inverse variance (within study and between studies) computed from the provided confidence limits. This is justified because testicular cancer is a rare outcome (prevalence < 0.5%). Between-study variance was estimated using restricted maximum likelihood (31). Due to the inherent heterogeneity of the studies owing to differences of study designs and patient characteristics, random effects meta-analysis Wald-type CIs were used. Heterogeneity between studies was assessed by I2 statistics. Funnel plots were constructed to identify the presence of publication and disclosure bias and the Begg’s adjusted rank correlation test was performed to test symmetry in the funnel plots (P < 0.05 suggesting asymmetry and potential publication bias) (32,33). All statistical analyses were performed using R (version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria) and a significance level of 0.05. This software was also used to create funnel plots of the SE of the logarithm of the hazard ratio for all maternal and postnatal adult male EDCs exposures separately.
Results
Selected characteristics of all 19 papers reporting on the associations between EDC exposure and testicular cancer (and histological subgroups, n = 8 papers) are presented in Table 2 and Supplemental Table 1 (27).
Eighteen papers were case-control and one was a cohort study. The majority (12 papers, 63%) were conducted in Europe and the remaining were conducted in United States or Canada (27)). The exposure measurements covered 5 different EDC compound groups, of which most (15 papers) addressed persistent EDCs (organochlorine pesticides, organohalogens), while only 2 addressed the more rapidly metabolized phthalates and bisphenol A (both using proxy measurements), and 2 addressed per- and polyfluorinated alkyl substances. In total, 65 different individual compound exposures or proxy exposures were assessed (Table 2; also see Supplemental Table 1 (27)) and a total of 291 associations were reported. Of the included papers, the majority (79%) were published within the past 15 years.
Assessment of testicular cancer predominantly relied on clearly defined diagnoses from standardized clinical examinations or validated registers. But 1 retrospective study used self-reported cancer diagnoses (with a recall period of up to almost 60 years) potentially introducing errors of misclassification and a survivorship bias (34). Almost all of the included papers applied multiple comparisons, and only 1 group of authors addressed the risk of chance findings due to these multiple comparisons, using a Bonferroni correction (35,36).
Ten papers reported associations between EDCs and testicular cancer based on direct EDC exposure biomarkers ascertained in serum including: One based on maternal serum sampled at the time of the son’s diagnosis (37): 1 in early postpartum serum (38); 2 based on measures of both maternal and son’s serum at the time of the son’s diagnosis (39,40), and 6 in postnatal adult male serum at or after diagnosis (34-36,41-43). These 10 were used in the quantitative meta-analysis. The remaining 9 reported on indirect exposure (maternal/postnatal adult men: 6/3) either occupationally or through other sources and were excluded from meta-analyses (see Selection of Relevant Papers, Figure 1).
Assessment of Quality
The completeness of reporting was generally high and only three papers scored below the sufficiency limit (Table 2). However, 53% of the investigations (10 papers) were considered subject to potential risk of bias according to our predefined criteria. Potential issues with residual confounding were the main concern in these papers, and there was a large variation in included confounders/covariates (Table 2; Fig. 2). A full overview of the available covariates/confounders is presented in Table 2.
Overall, there were no consistent indications of a statistically significant association between exposure to individual EDCs and testicular cancer among the risk estimates extracted from each of the 19 studies, mainly because most of these were limited by low statistical power with wide CIs crossing unity (Table 2). None of the included individual papers considered multipollutant mixture analyses of the effect of exposures, whereas 5 assessed the effect of compound groups [chlordanes and/or polychlorinated biphenyls (PCBs)] (35,36,39,41,43).
Random Effects Meta-analyses
We included 10 papers in our quantitative meta-analysis, corresponding to a population of 1234 (maternal/postnatal adult male: 133/1176) testicular cancer cases. All 10 applied EDC exposure biomarkers directly measured in serum.
EDC Combinations
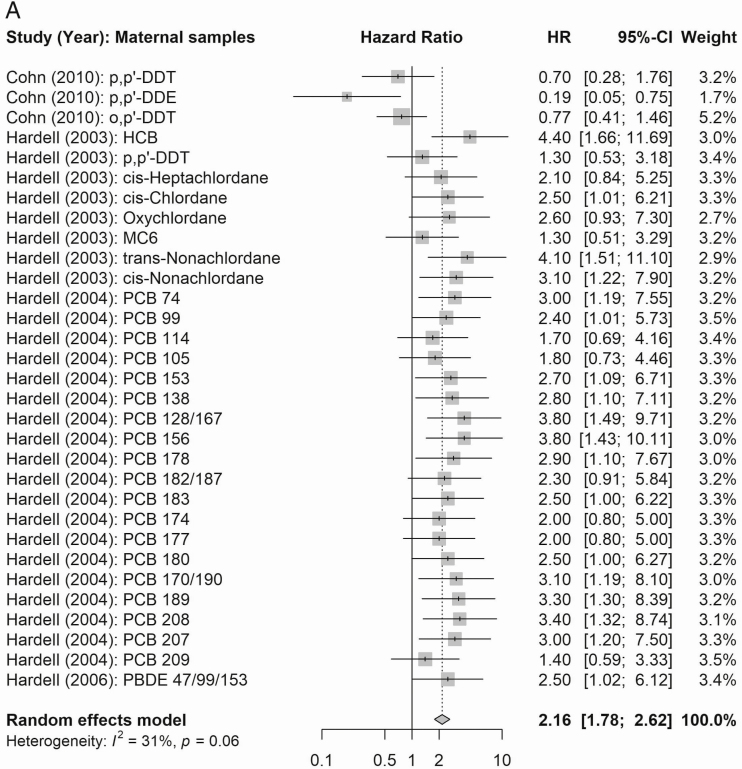
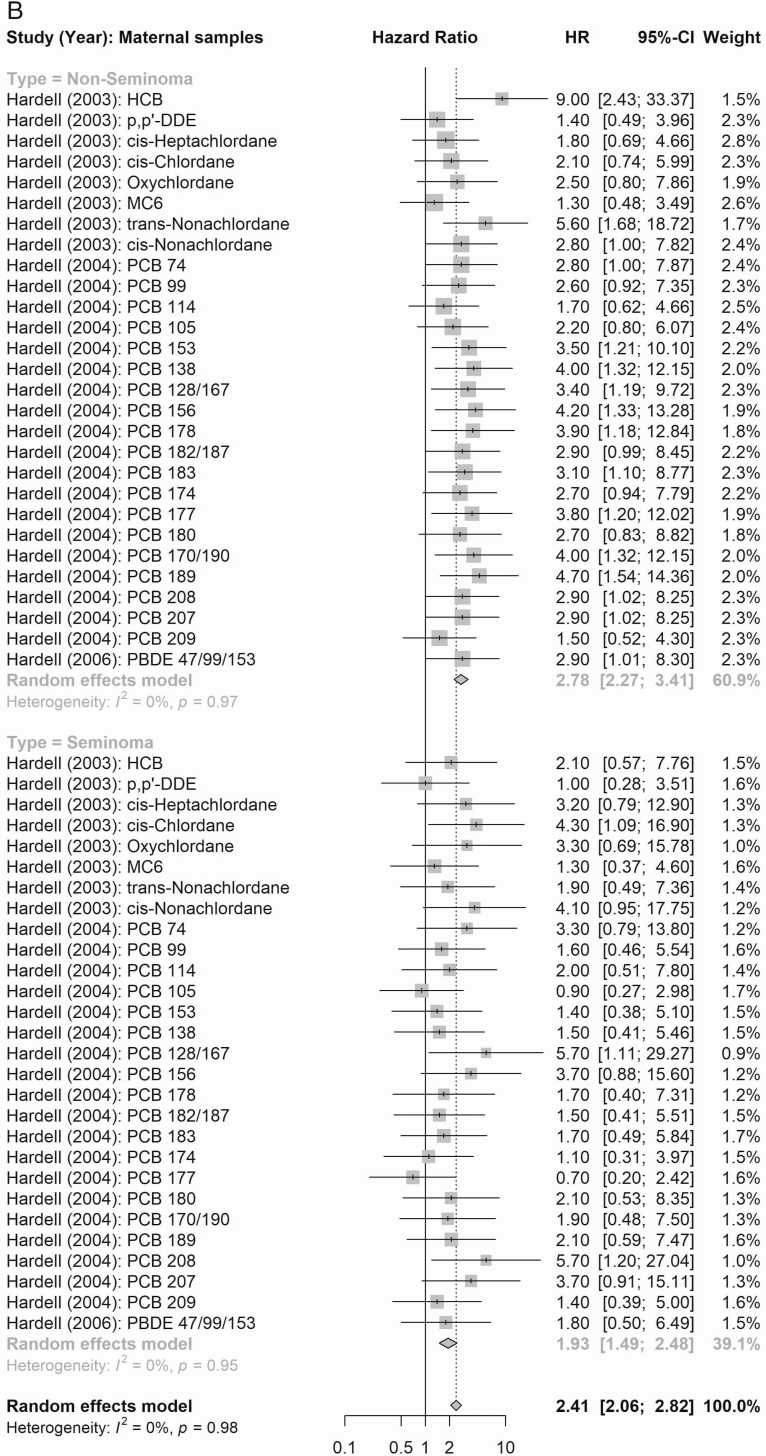
The summary estimates of the meta-analysis of the eligible estimates for overall combined multipollutant according to maternal EDC exposures are presented in Figure 3. In these analyses, maternal mixed exposures were associated with a summary risk estimate of 2.16 (95% CI 1.78-2.62), 2.78 (95% CI 2.27-3.41), and 1.93 (95% CI 1.49-2.48) for all, nonseminoma, and seminoma testicular cancers, respectively. Postnatal adult male exposure (see Supplemental Figure 1A in (27)] showed an overall risk of 1.05 (95% CI 0.92-1.20) and similarly summary estimates for risk of histological subtypes of testicular cancer according to EDC exposures were not statistically significant [summary risk estimate of 0.91 (95% CI:0.74-1.13) and 1.06 (95% CI:0.88-1.27) for non-seminoma and seminoma, respectively] [see Supplemental Figure 1B in (27)].
Figure 3.
(A) Summary estimates of the meta-analyses [n cases: Cohn et al (38), 15; Hardell et al (39) 44; Hardell et al (40), 44; Hardell et al (37), 44] of all EDC compound groups according to maternal EDC exposures (n = 31) and all testicular cancer. (B) Summary estimates of the meta-analyses [n cases: Cohn et al (38), 15; Hardell et al (39) 44; Hardell et al (40), 44; Hardell et al (37), 44] of all EDC compound groups according to maternal EDC exposures (n = 28) and testicular cancer subtypes.
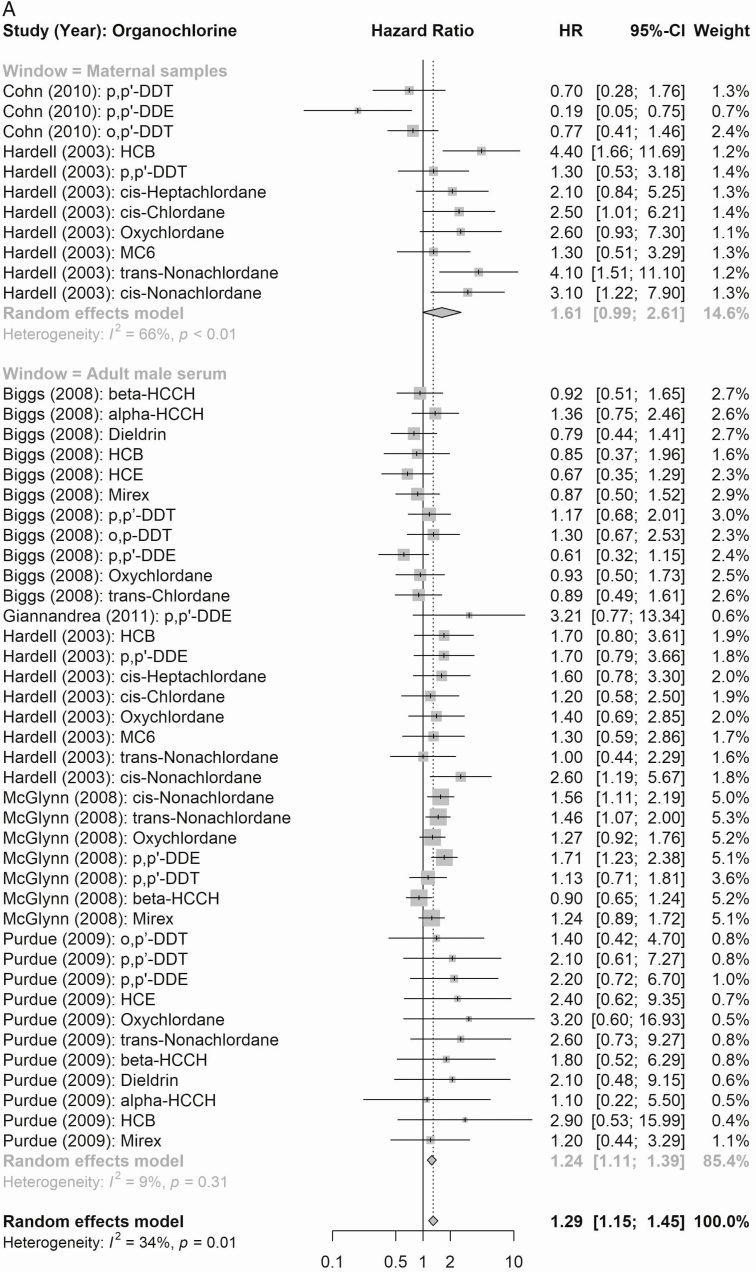
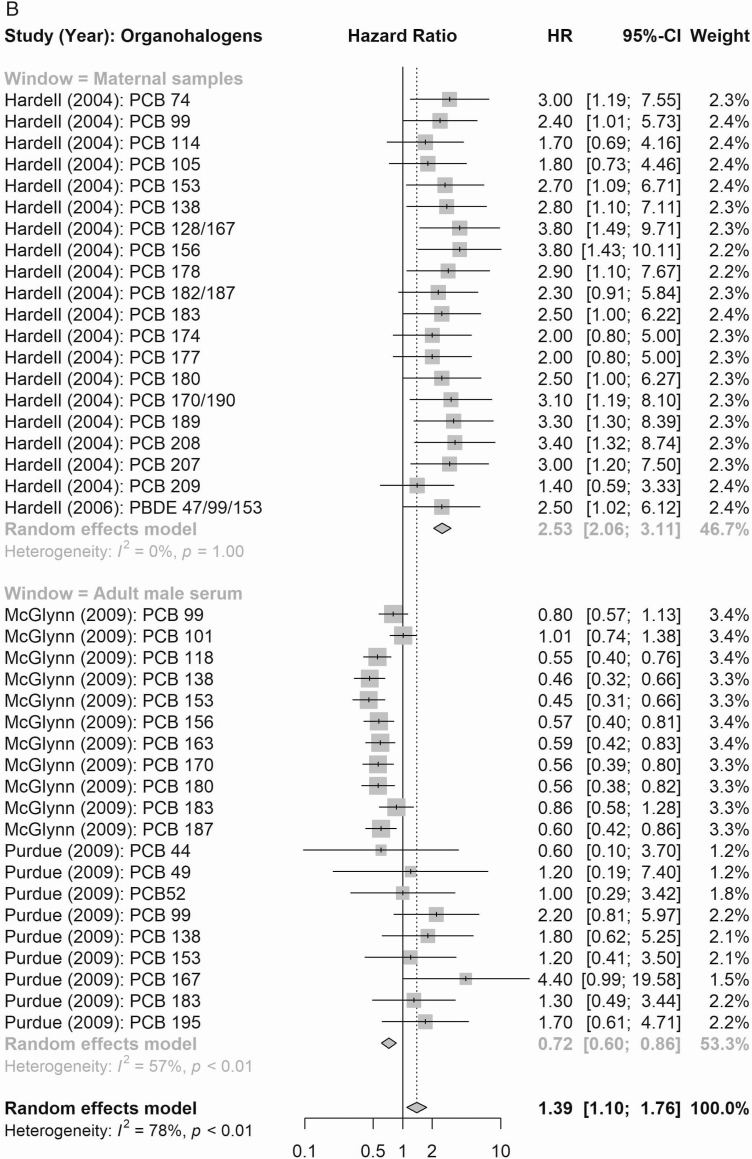
Compound Groups
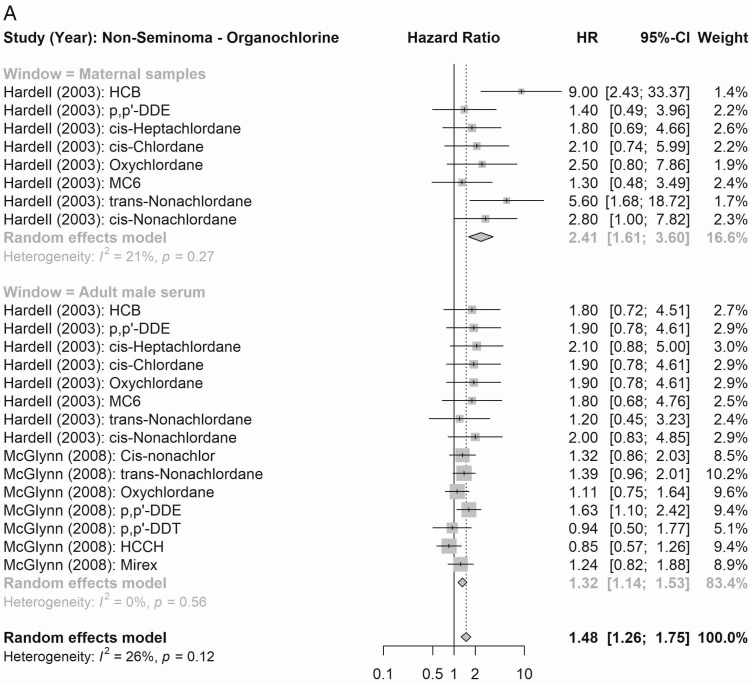
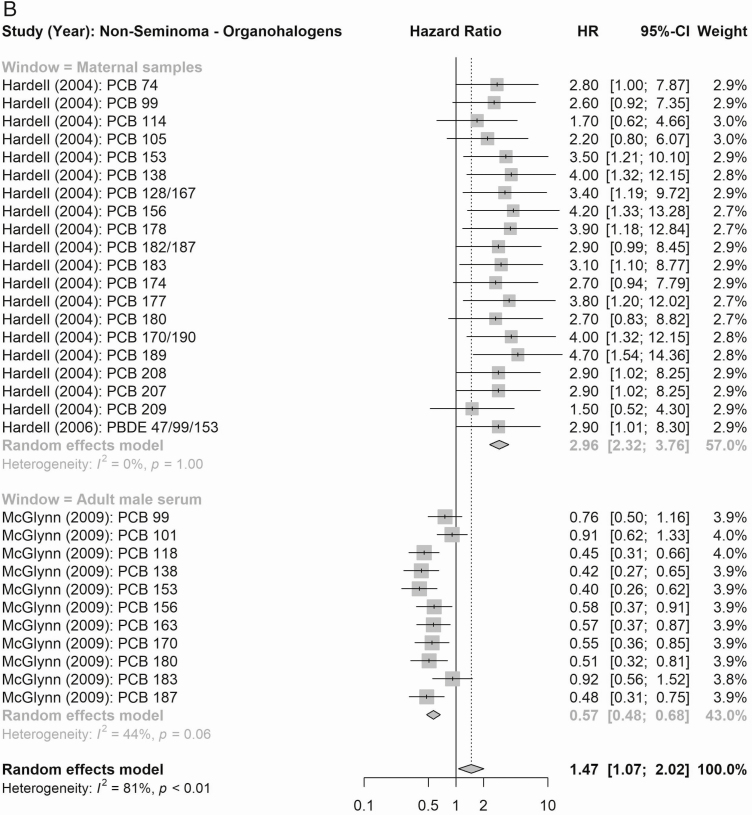
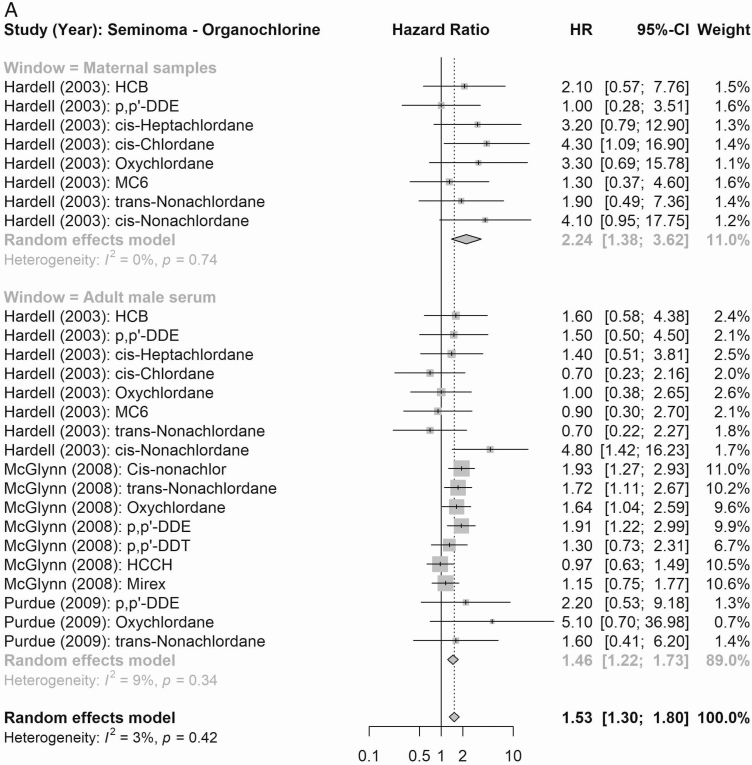
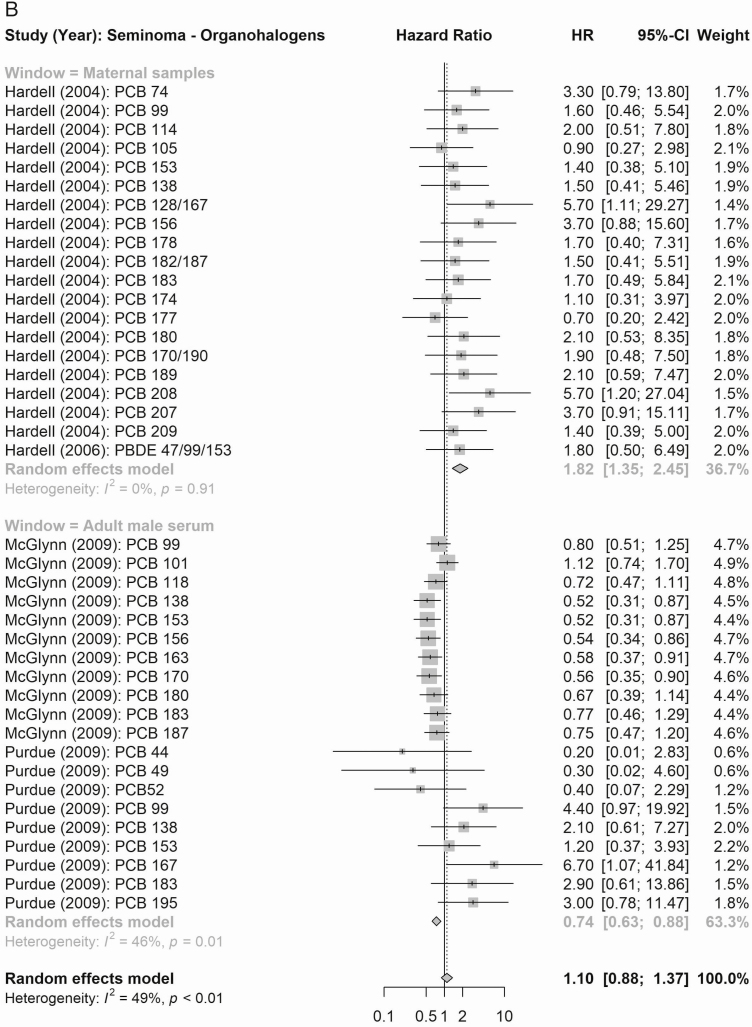
According to maternal exposure, the summary effect estimates for all organochlorines (1.61, 95% CI 0.99-2.61) and for all organohalogens (PCBs and polybrominated diphenyl congeners; 2.53, 95% CI 2.06-3.11) were associated with elevated testicular cancer risk (Fig. 4). When considering risk of testicular cancer subtypes (Figs. 5 and 6), maternal exposure to organochlorines was likewise associated with significant higher risk of nonseminoma (2.41, 95% CI 1.61-3.61) and seminoma (2.24, 95% CI 1.38-3.62) testicular cancer. Maternal exposure to organohalogens was associated with elevated risk of nonseminoma (2.96, 95% CI 2.32-3.76) and seminoma (1.82, 95% CI 1.35-2.45) testicular cancer.
Figure 4.
Summary risk ratios (RR; all testicular cancer) of the meta-analyses of maternal and adult male exposure to the EDC compound groups. (A) Organochlorines (top: maternal; bottom: adult male). (B) Organohalogens (top panel window: maternal, bottom: adult male).
Figure 5.
Summary RR (nonseminoma testicular cancer) of the meta-analyses of maternal and adult male exposure to the EDC compound groups. (A) Organochlorines (top: maternal; bottom: adult male). (B) Organohalogens (top panel window: maternal, bottom: adult male).
Figure 6.
Summary RR (seminoma testicular cancer) of the meta-analyses of maternal and adult male exposure to the EDC compound groups. (A) Organochlorines (top: maternal, bottom: adult male). (B) Organohalogens (top: maternal, bottom: adult male).
The summary estimates according to postnatal adult male exposure to compound groups were inconsistent, and while exposure to all organochlorines was associated with a significantly higher risk of testicular cancer (1.24, 95% CI 1.11-1.39), the association according to summed organohalogens was protective (0.72, 95% CI 0.60-0.86) (Fig. 4). Similar inconsistent effects were detected for risk of the histological subgroups (Figs. 5 and 6), postnatal adult male exposure to organochlorines was associated with significant higher risk of nonseminoma (1.32, 95% CI 1.14-1.53) and seminoma (1.46, 95% CI 1.22-1.73) testicular cancer. Meanwhile, postnatal adult male exposure to organohalogens was associated with decreased risk of nonseminoma (0.57, 95% CI 0.48-0.68 and seminoma (0.77, 95% CI 0.63-0.95) testicular cancer.
Individual Exposures
The summary estimates of the meta-analysis of the 12 eligible individual compounds (≥3 available estimates) regardless of time window are presented in Supplemental Figure S2 in (27). Statistically significant elevated risk estimates were only detected according to chlordane exposures [cis-nonachlordane, 2.00 (95% CI:1.27-3.15); oxychlordane 1.30 (95% CI:1.01-1.68), and trans-chlordane 1.75 (95% CI:1.03-2.99)]. The pooled estimates of the other organochlorines (hexachlorobenzene, mirex, dichlorodiphenyltrichloroethane, dichlorodiphenyldichloroethylene) and PCB congeners (#138, 153, 183, and 99) showed statistically nonsignificant positive risk estimates with respective ranges of 1.07 to 1.91 and 1.06 to 1.45.
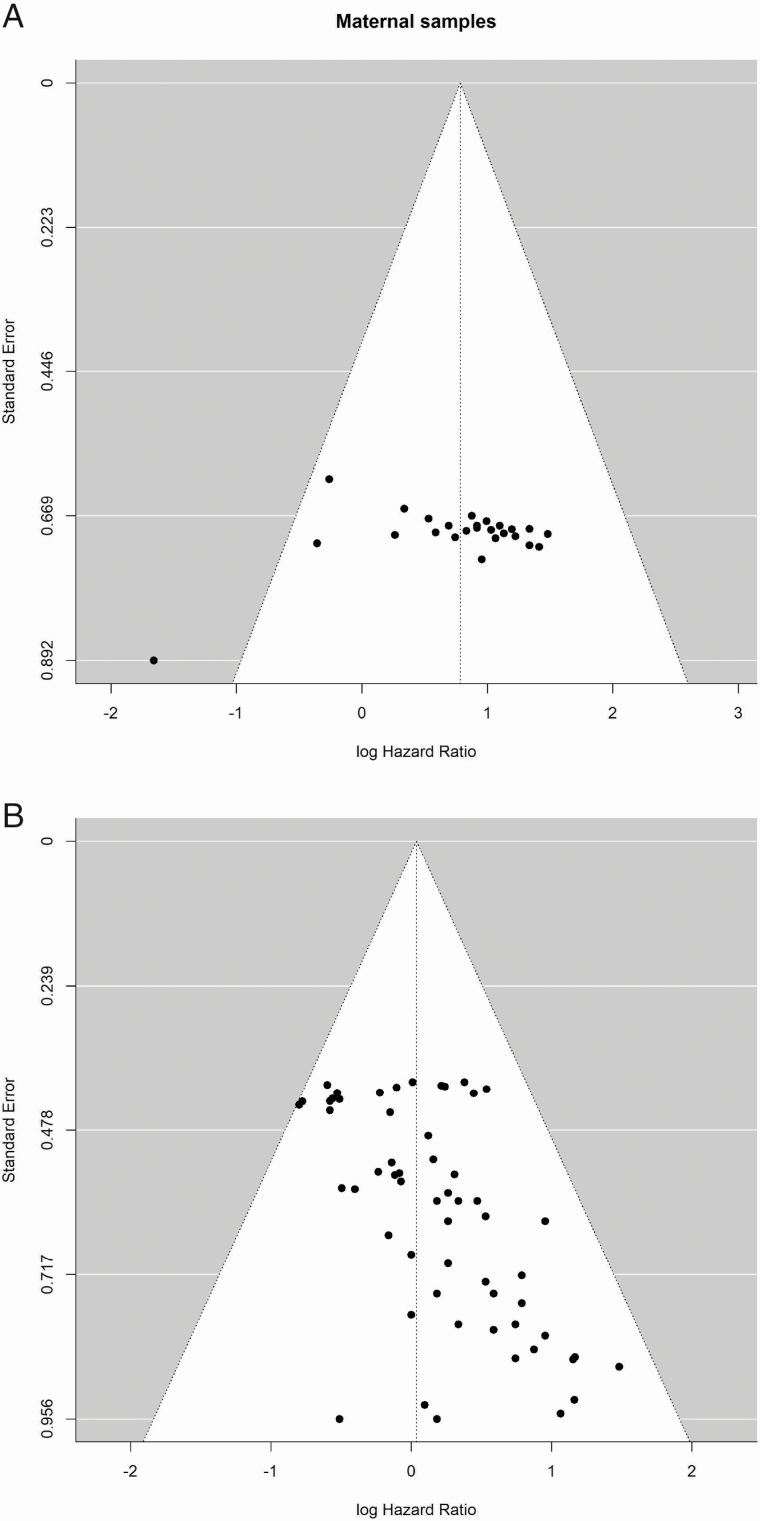
A funnel plot of the studies included in the meta-analyses assessing maternal EDC exposures revealed no indication of publication bias (PBegg’s Rank Correlation = 0.14) (Fig. 7A). Some publication bias (PBeggs Rank Correlation < 0.0001) was indicated in the funnel plot assessing postnatal adult male exposures (Fig. 7B).
Figure 7.
(A) Funnel plot of all risk estimates from the studies included in the meta-analysis: association between maternal exposure to EDC and testicular cancer (PBeggs = 0.14, indicating symmetry). (B) Funnel plot of all risk estimates from the studies included in the meta-analysis: association between postnatal adult male exposure to EDC and testicular cancer (PBeggs = 0.0001 indicating asymmetry).
Of the 9 papers excluded from the meta-analyses, only 3 case-control studies reported statistically significant risk estimates for single compounds (Table 2). The first of these three was a case-control study carried out with the US Servicemen Testicular Tumor Environment Endocrine Determinant (STEED) and reported on risk associated with use of specific personal care products (face/skin lotions, sunscreen, nail polish, perfume; recognized to contain EDCs such as phthalates, bisphenol A, perfluorinated chemicals, triclosan, parabens). The authors reported a higher risk of testicular cancer associated with prenatal maternal use of face lotion (RR 1.41, 95% CI 1.08-1.86) but not with other personal care products (including body lotions and sunscreen expected to be similar in EDC composition) (44). The second reported an increased risk associated with postnatal adult male self-report of home insecticide use (RR 3.21, 95% CI 1.15-9.11) but not postnatal adult male self-report of pesticide use (42). The third used a job exposure matrix and detected a 6-fold increased risk of testicular cancer (RR 6.6, 95% CI 1.4-32) associated with polyvinyl chloride postnatal adult occupational exposure (proxy for phthalates) but not associated with other plastics (styrene, urethane, acrylate) (51). Multiple testing in these 3 studies may have led to chance findings and CIs were wide.
Discussion
This systematic review pooled available human epidemiological evidence on the association between exposure to EDCs and testicular cancer, assessing study quality and providing summary estimates of associations. On balance, an association was consistently observed in maternal but not postnatal adult male exposures. The higher risk of testicular cancer according to maternal exposure remained according to specific compound groups, and the risk of nonseminomas was higher than that of seminomas. In adult men, postnatal exposure to organochlorines was associated with higher risk, and organohalogens were associated with lower risk of all testicular cancer and of histological subgroups. Completeness of reporting was high, but over 50% of the included papers were at potential risk of bias. None of the included individual papers considered multipollutant mixture analyses of the effect of exposures.
Given the strong scientific premise regarding the relevance of fetal exposure to EDCs (12), the consistent associations with maternal exposure to EDCs (combinations and compound groups) detected in the summary estimates of meta-analyses are biologically plausible and support the TDS hypothesis. Similarly, the antiandrogenic mode of action of both the organochlorides and organohalogens further supports the detected association. However, very limited data were available on maternal exposures, which mandates a degree of caution when interpreting the results.
Both seminoma and nonseminoma start as neoplasia in situ (3,4). Although etiology is similar and fetal origins are suspected for both groups, it is noteworthy from this present review that the overall estimated effects of EDC exposures on nonseminomas was apparently higher than that of seminomas. The mechanisms underlying this are unclear and may be due to chance. However, the median age for occurrence of seminomas is around 10 years older (54). So, if a maternal blood sample was taken at the time of her son’s tumor diagnosis, there would be a 10-year time difference between seminoma and nonsemonima cases. Within that 10-year time window the mother could have further metabolized the EDCs relevant to her son’s prenatal exposure, which could potentially attenuate RR estimates of seminomas, potentially accounting for the observed differences in summary estimates for the seminomas and nonseminomas. In addition, the second tumor of metachronous contralateral testicular tumors are most often seminomas (55). Thus if environmental EDC exposures result in a lower prevalence of contralateral testicular tumors, this could also partially explain our finding.
Interestingly, the summary meta-analysis estimate results related to postnatal adult male exposure to EDC multipollutant combinations and compound groups were inconsistent while exposure to organochlorines increased risk and exposure to organohalogens lowered risk consistently for all cancers and cancer subtypes. We are unable to explain this inconsistency that is in direct contrast to our hypothesis but believe this may be an important finding that requires corroboration from further investigations. Some inconsistency in our meta-analyses in risk of testicular cancer according to EDC levels in adult men could be attributed to the fact that EDC levels measured in men in adulthood may not reflect exposures during the etiologically relevant prenatal period. Nondifferential exposure measurement error of this type would attenuate RR estimates toward the null. There are presently no published data on the correlation between fetal and adult EDC concentrations, and the long-time span (up to 40 years from prenatal exposure to diagnosis) is likely to profoundly affect circulating EDC concentrations due to lifetime metabolization and clearing of prenatal EDC exposures as well as accumulation of new lifetime exposure to EDC. Two studies included adult postdiagnostic blood samples and thus could not exclude the possibility that EDC levels in cases were affected by the cancer itself or its treatment (41,42) as both chemo- and radiation therapy (56,57) and changes in body weight (58, 59) are reported to influence blood EDC levels. These unmeasured effects could potentially result in either differential or nondifferential exposure misclassification attenuating the risk estimates in either direction.
Quality of the Included Studies
The included studies measured and assessed testicular cancer in a homogeneous way by using either standardized clinical examinations or validated registers. Many of the identified studies also included histological subtypes. All of the 10 studies included in our meta-analyzes based exposure assessments on serum (either maternal or the postnatal adult male) considered the gold standard with minor risks of exposure misclassification. The timing of sampling was a limitation in all 4 studies utilizing maternal samples, as serum was not sampled during pregnancy and levels may have changed throughout the life course of the mother.
Exposure contrasts used were heterogenous, among these 10 studies applying direct exposure assessment in serum, 6 used exposure contrasts on continuous scales (tertiles or quartiles, low risk of misclassifying measurements), and 4 used a dichotomized approach (likely resulting in some exposure misclassifications and less robust estimates). On the other hand, the 9 studies only appraised for quality (not included in the meta-analysis) all applied indirect exposure proxies (occupation, questionnaire, address) using a dichotomous approach (yes/no). These 9 studies are at risk of exposure misclassification due to differential recall of exposure and the dichotomous approach only relates to the probability of exposure and not a continuous scale, also increasing the risk of exposure dose misclassification.
While the majority of studies accounted for the potential confounding effects of age and body mass index in their analyses, the inclusion of additional factors (race, cryptorchidism, breastfeeding, family history, education, etc) varied immensely despite their documented importance. Therefore, bias from residual confounding was a major concern across half of the included studies.
Nonresponse and low participation rates were not a concern in most of the studies (35-37,39-41,44,46,47,53), but several studies did not report participation rates, potentially resulting in unknown selection bias (38,43,49,52). Selection bias is of particular concern in 1 study (34), in which participation depended upon a cancer diagnosis (including testicular cancer), thus having cancer may have affected the motivation for participation in that study.
None of the included studies considered multipollutant EDC mixture analyses of the effect of exposures, even though EDC exposure is ubiquitous and testicular cancer risks may not be entirely attributable to single compounds and rather depend on multipollutant EDC mixtures. In our meta-analyses based on 10 of the studies, we attempted to address the combined joint effects within and between different classes of EDC multipollutants. We acknowledge that the analysis may be oversimplified. Correlations between individual compounds with similar modes of action would probably vary according to lifestyle, and potencies of individual chemicals are also varying. Based on our meta-analyses, we are unable to provide any information on potentially additive or synergistic interactions between these EDCs.
Strengths and Limitations of the Present Review
The main strength of this systematic review was the extensive and transparent literature search followed by application of predefined eligibility and quality assessment tools. Although 19 studies were included, unfortunately, the available data on 9 of them were for indirect exposure ascertainment and were not considered suitable for the quantitative meta-analysis.
Exclusion of non-English and unpublished studies may have introduced some reporting bias in the present review.
As the present review focused on chemical measurements in serum, it is most likely to include chemicals for which serum is the preferred matrix (hence more persistent chemicals), and we acknowledge that the more nonpersistent chemicals are generally more difficult to investigate is original studies of testicular cancer as the timing of exposure measurement in relation to expected period of etiology are more critical.
Until 2016, “seminoma” was used for both germ cell neoplasia in situ–derived seminoma (the classical) and the rare type spermatocytic seminoma, which are now called “spermatocytic tumor” according to WHO 2016 classification. Likewise nonseminomas (embryonic tumors) in newborns should be distinguished from nonseminomas in adults (3). Although the majority of studies included in the present systematic review were published prior to 2016, this potential misclassification should be considered when comparing future studies to older studies in which subgroups were ascertained prior to 2016.
All systematic reviews may miss eligible important hits that are published after the date of their respective literature research completion. Likewise, we received a PubMed alert in March 2021, about a new relevant peer-review publication that would be eligible for inclusion in the present systematic review (50). This new large case-control study assessed testicular cancer risk associated with PCBs ascertained in postnatal adult male serum. Because of the scarcity of available studies, that paper was post hoc rated for quality and included in the main tables of this review. Applying a high completeness of reporting and low potential risk of bias, the authors of that study report a higher risk of testicular cancer predominately associated with PCBs with an estrogenic mode of action.
Wider Implications and Perspectives
For several decades it has been hypothesized that our increasing ubiquitous exposure to environmental EDCs play an important role in the development of testicular cancer. Our finding of associations to maternal exposures and not to exposures of the young men with testicular cancer is another piece of evidence in support of the hypothesis that testicular cancer, although occurring in adulthood, is of developmental origin. This hypothesis is further strengthened by the already-established strong links between testicular cancer and cryptorchidism, hypospadias; and disorders of sex development. The increasing trends in incidence of testicular cancer therefore strongly emphasizes the importance of measures to improve fetal reproductive health, with measures such as education of pregnant women regarding sources of EDCs so they are able to avoid unnecessary EDC exposures. However, the overall findings from this is review are subject to the varying quality of the included studies as well as general difficulties in interpreting associations with full certainty, due to the timing of the biosample collection (for mothers this was performed at the time of the son’s diagnosis rather than during pregnancy in all but 1 study, and for postnatal adult men, in some studies this was performed after diagnosis) and some evidence of publication bias in the studies assessing the impact of postnatal adult male EDC exposure.
Future studies on the associations between EDCs and risk of testicular cancer should focus on prenatal maternal exposure (potentially also paternal) to EDCs based on ascertainment in biospecimens. A combined lifetime effect of pre- and postnatal, adolescent or adulthood exposures would also be valuable although logistically complex and expensive to perform. Multipollutant mixture effects (additive, synergistic, antagonistic) of EDCs remain unexplored, and separating true contributions from individual compounds is difficult. In future research, the development of methods to account for overall chemical mixture body burden to supplement individual EDC measurements is recommended. In addition, due to the continuing phase-out/replacement of several EDCs, more research effort relating to ascertainment of the potential effects of new compounds on testicular cancer risk is also relevant. Although EDC proxy exposures such as reports of domestic, environmental, and occupational exposures provide important objective evidence, these should be carefully considered in the future as they are recognized to be associated with exposure misclassification bias. To overcome problems linked to low statistical power, an issue prevalent in all the studies included in our meta-analysis (direct exposure assessment), we recommend the use of more standardized approaches (confounder control, exposure assessment, statistical analysis, compound groupings) to allow easy comparison of studies and to strengthen the overarching epidemiological evidence.
In this systematic review, we detected greater than a 2-fold higher risk of testicular cancer associated with maternal exposure to combined EDCs but no consistent evidence associated with postnatal adult male exposures. There is a current gap in knowledge apparent in relation to studies assessing prenatal exposure and assessment of the combined effects of EDC multipollutant mixtures. Large, high-quality epidemiological studies are needed to further elucidate the association between prenatal exposure to EDC and risk of testicular cancer in adult life.
Acknowledgments
We are extremely grateful to the Susie Rimborg, librarian at the University of Copenhagen Library, for her assistance with the systematic literature search protocol in PubMed and Embase®.
Financial Support: The salaries of E.V.B., R.H., B.A.C., A.J., C.S.U., A-M.A., and J.H.P. were partially supported by a grant from the National Institute of Health (grant no. 1R01CA236816-01A1). E.V.B was also partially supported by grants from the Danish Health Foundation (Helsefonden, grant no. 18-B-0016, Aase and Ejnar Danielsen, grant no. 10-002122, Svend Andersen Fonden, grant no 81A-01 and Familien Erichsens Mindefond, grant no. 6000073). M.K.P. and T.K. were funded by a grant from the Danish Cancer Research Association (Kræftens Bekæmpelse, grant no. R204-A12636). L.S.G. is funded by a grant from the Danish Environmental Protection Agency (Miljøstyrelsen, grant no. MST-611-00012). M.H. is funded by an NHMRC Practitioner Fellowship (grant no. 1193838).
Author Contributions: E.V.B. performed the systematic literature review and drafted the manuscript. E.V.B. and T.K. screened all papers and assessed completeness and bias. Y.H.L. performed meta-analyses. All authors contributed to manuscript revisions and the final draft of the manuscript.
Additional Information
Disclosure Statement: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5(3):210-222. [DOI] [PubMed] [Google Scholar]
- 2. Ulbright TM. Germ cell neoplasms of the testis. Am J Surg Pathol. 1993;17(11):1075-1091. [DOI] [PubMed] [Google Scholar]
- 3. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70(1):93-105. [DOI] [PubMed] [Google Scholar]
- 4. Skakkebaek NE, Berthelsen JG, Giwercman A, Müller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987;10(1):19-28. [DOI] [PubMed] [Google Scholar]
- 5. Znaor A, Lortet-Tieulent J, Jemal A, Bray F. International variations and trends in testicular cancer incidence and mortality. Eur Urol. 2014;65(6):1095-1106. [DOI] [PubMed] [Google Scholar]
- 6. Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Møller H. Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. Int J Cancer. 2006;118(12):3099-3111. [DOI] [PubMed] [Google Scholar]
- 7. Le Cornet C, Lortet-Tieulent J, Forman D, et al. . Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur J Cancer. 2014;50(4):831-839. [DOI] [PubMed] [Google Scholar]
- 8. Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, et al. . Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev. 2016;96(1):55-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghazarian AA, Trabert B, Devesa SS, McGlynn KA. Recent trends in the incidence of testicular germ cell tumors in the United States. Andrology. 2015;3(1):13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGlynn KA, Cook MB. Etiologic factors in testicular germ-cell tumors. Future Oncol. 2009;5(9):1389-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cook MB, Akre O, Forman D, Madigan MP, Richiardi L, McGlynn KA. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer—experiences of the son. Int J Epidemiol. 2010;39(6):1605-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16(5):972-978. [DOI] [PubMed] [Google Scholar]
- 13.Environment and Climate Research Programme of DG XII of the European Commission. European Workshop on the Impact of Endocrine Disrupters on Human Health and Wildlife: Report of proceedings (Weybridge, UK, 2-4 December 1996). Report no. EUR 17549. European Commission;1996. [Google Scholar]
- 14. Toppari J. Environmental endocrine disrupters. Sex Dev. 2008;2(4-5):260-267. [DOI] [PubMed] [Google Scholar]
- 15. Schmiedel S, Schüz J, Skakkebaek NE, Johansen C. Testicular germ cell cancer incidence in an immigration perspective, Denmark, 1978 to 2003. J Urol. 2010;183(4):1378-1382. [DOI] [PubMed] [Google Scholar]
- 16. Myrup C, Westergaard T, Schnack T, et al. . Testicular cancer risk in first- and second-generation immigrants to Denmark. J Natl Cancer Inst. 2008;100(1):41-47. [DOI] [PubMed] [Google Scholar]
- 17. Hemminki K, Li X. Cancer risks in second-generation immigrants to Sweden. Int J Cancer. 2002;99(2):229-237. [DOI] [PubMed] [Google Scholar]
- 18. Levine H, Afek A, Shamiss A, et al. . Risk of germ cell testicular cancer according to origin: a migrant cohort study in 1 100 000 Israeli men. Int J Cancer. 2013;132(8):1878-1885. [DOI] [PubMed] [Google Scholar]
- 19. Mose T, Mortensen GK, Hedegaard M, Knudsen LE. Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod Toxicol. 2007;23(1):83-91. [DOI] [PubMed] [Google Scholar]
- 20. Frederiksen M, Vorkamp K, Mathiesen L, Mose T, Knudsen LE. Placental transfer of the polybrominated diphenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: an experimental study. Environ Health. 2010;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mørck TJ, Sorda G, Bechi N, et al. . Placental transport and in vitro effects of Bisphenol A. Reprod Toxicol. 2010;30(1):131-137. [DOI] [PubMed] [Google Scholar]
- 22. Bradman A, Barr DB, Claus Henn BG, Drumheller T, Curry C, Eskenazi B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ Health Perspect. 2003;111(14):1779-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Main KM, Kiviranta H, Virtanen HE, et al. . Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect. 2007;115(10):1519-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Philippat C, Wolff MS, Calafat AM, et al. . Prenatal exposure to environmental phenols: concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environ Health Perspect. 2013;121(10):1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Braun JM, Gennings C, Hauser R, Webster TF. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect. 2016;124(1):A6-A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hutton B, Salanti G, Caldwell DM, et al. . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. [DOI] [PubMed] [Google Scholar]
- 27. Bräuner E, Lim Y-H, Koch T, et al. Supplemental material for: Endocrine disrupting chemicals and risk of testicular cancer: a systematic review and meta-analysis. Deposited June 24, 2021. 10.5061/dryad.w6m905qpw [DOI] [PMC free article] [PubMed]
- 28. McCarthy M. European Chemical Agency (ECHA). List of pre-registered substances. Published online 2011. https://www.cairn.info/revue-responsabilite-et-environnement1-2013-3-page-5.htm
- 29. Bonzini M, Coggon D, Palmer KT. Risk of prematurity, low birthweight and pre-eclampsia in relation to working hours and physical activities: a systematic review. Occup Environ Med. 2007;64(4):228-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shamliyan T, Kane RL, Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol. 2010;63(10):1061-1070. [DOI] [PubMed] [Google Scholar]
- 31. Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30(3):261-293. [Google Scholar]
- 32. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. [PubMed] [Google Scholar]
- 33. Viechtbauer W. Conducting Meta-analysis in R with the metafor package. J Stat Softw. 2010;36(3). doi:10.18637/jss.v036.i03 [Google Scholar]
- 34. Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013;121(11-12):1313-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Polychlorinated biphenyls and risk of testicular germ cell tumors. Cancer Res. 2009;69(5):1901-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J Natl Cancer Inst. 2008;100(9):663-671. [DOI] [PubMed] [Google Scholar]
- 37. Hardell L, Bavel B, Lindström G, Eriksson M, Carlberg M. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int J Androl. 2006;29(1):228-234. [DOI] [PubMed] [Google Scholar]
- 38. Cohn BA, Cirillo PM, Christianson RE. Prenatal DDT exposure and testicular cancer: a nested case-control study. Arch Environ Occup Health. 2010;65(3):127-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hardell L, van Bavel B, Lindström G, et al. . Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ Health Perspect. 2003;111(7):930-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hardell L, Van Bavel B, Lindström G, et al. . Concentrations of polychlorinated biphenyls in blood and the risk for testicular cancer. Int J Androl. 2004;27(5):282-290. [DOI] [PubMed] [Google Scholar]
- 41. Biggs ML, Davis MD, Eaton DL, et al. . Serum organochlorine pesticide residues and risk of testicular germ cell carcinoma: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2012-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giannandrea F, Gandini L, Paoli D, Turci R, Figà-Talamanca I. Pesticide exposure and serum organochlorine residuals among testicular cancer patients and healthy controls. J Environ Sci Health B. 2011;46(8):780-787. [DOI] [PubMed] [Google Scholar]
- 43. Purdue MP, Engel LS, Langseth H, et al. . Prediagnostic serum concentrations of organochlorine compounds and risk of testicular germ cell tumors. Environ Health Perspect. 2009;117(10):1514-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghazarian AA, Trabert B, Robien K, Graubard BI, McGlynn KA. Maternal use of personal care products during pregnancy and risk of testicular germ cell tumors in sons. Environ Res. 2018;164:109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kristensen P, Andersen A, Irgens LM, Bye AS, Sundheim L. Cancer in offspring of parents engaged in agricultural activities in Norway: incidence and risk factors in the farm environment. Int J Cancer. 1996;65(1):39-50. [DOI] [PubMed] [Google Scholar]
- 46. Le Cornet C, Fervers B, Dalton SO, et al. . Testicular germ cell tumours and parental occupational exposure to pesticides: a register-based case-control study in the Nordic countries (NORD-TEST study). Occup Environ Med. 2015;72(11):805-811. [DOI] [PubMed] [Google Scholar]
- 47. Le Cornet C, Fervers B, Pukkala E, et al. . Parental occupational exposure to organic solvents and testicular germ cell tumors in their offspring: NORD-TEST Study. Environ Health Perspect. 2017;125(6):067023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nori F, Carbone P, Giordano F, Osborn J, Figa-Talamanca I. Endocrine-disrupting chemicals and testicular cancer: a case-control study. Arch Environ Occup Health. 2006;61(2):87-95. [DOI] [PubMed] [Google Scholar]
- 49. Paoli D, Giannandrea F, Gallo M, et al. . Exposure to polychlorinated biphenyls and hexachlorobenzene, semen quality and testicular cancer risk. J Endocrinol Invest. 2015;38(7):745-752. [DOI] [PubMed] [Google Scholar]
- 50. Cheng Z, Zhang X, Bassig B, et al. . Serum polychlorinated biphenyl (PCB) levels and risk of testicular germ cell tumors: a population-based case-control study in Connecticut and Massachusetts. Environ Pollut. 2021;273:116458. [DOI] [PubMed] [Google Scholar]
- 51. Hardell L, Ohlson CG, Fredrikson M. Occupational exposure to polyvinyl chloride as a risk factor for testicular cancer evaluated in a case-control study. Int J Cancer. 1997;73(6):828-830. [DOI] [PubMed] [Google Scholar]
- 52. Helmfrid I, Berglund M, Löfman O, Wingren G. Health effects and exposure to polychlorinated biphenyls (PCBs) and metals in a contaminated community. Environ Int. 2012;44:53-58. [DOI] [PubMed] [Google Scholar]
- 53. Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013;121(3):318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rothermundt C, Thurneysen C, Cathomas R, et al. . Baseline characteristics and patterns of care in testicular cancer patients: first data from the Swiss Austrian German Testicular Cancer Cohort Study (SAG TCCS). Swiss Med Wkly. 2018;148:w14640. [DOI] [PubMed] [Google Scholar]
- 55. Schaapveld M, AW van den B-D, Gietema JA, et al. . Risk and prognostic significance of metachronous contralateral testicular germ cell tumours. BrJ Cancer. 2012;107(7):1637-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gammon MD, Wolff MS, Neugut AI, et al. . Treatment for breast cancer and blood levels of chlorinated hydrocarbons. Cancer Epidemiol Biomarkers Prev. 1996;5(6):467-471. [PubMed] [Google Scholar]
- 57. Baris D, Kwak LW, Rothman N, et al. . Blood levels of organochlorines before and after chemotherapy among non-Hodgkin’s lymphoma patients. Cancer Epidemiol Biomarkers Prev. 2000;9(2):193-197. [PubMed] [Google Scholar]
- 58. Hagmar L, Wallin E, Vessby B, Jönsson BA, Bergman A, Rylander L. Intra-individual variations and time trends 1991-2001 in human serum levels of PCB, DDE and hexachlorobenzene. Chemosphere. 2006;64(9):1507-1513. [DOI] [PubMed] [Google Scholar]
- 59. Charlier CJ, Foidart JM. Comparative study of dichlorodiphenyldichloroethylene in blood and semen of two young male populations: lack of relationship to infertility, but evidence of high exposure of the mothers. Reprod Toxicol. 2005;20(2):215-220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.