Abstract
Objective
To examine the effectiveness of physical activity interventions delivered or prompted by primary care health professionals for increasing moderate to vigorous intensity physical activity (MVPA) in adult patients.
Design
Systematic review and meta-analysis of randomised controlled trials.
Data sources
Databases (Medline and Medline in progress, Embase, PsycINFO, CINAHL, SPORTDiscus, Sports Medicine and Education Index, ASSIA, PEDro, Bibliomap, Science Citation Index, Conference Proceedings Citation Index), trial registries (Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, TRoPHI), and grey literature (OpenGrey) sources were searched (from inception to September 2020).
Eligibility criteria for selecting studies
Randomised controlled trials of aerobic based physical activity interventions delivered or prompted by health professionals in primary care with a usual care control group or another control group that did not involve physical activity.
Study selection and analysis
Two independent reviewers screened the search results, extracted data from eligible trials and assessed the risk of bias using the Cochrane risk of bias tool (version 2). Inverse variance meta-analyses using random effects models examined the primary outcome of difference between the groups in MVPA (min/week) from baseline to final follow-up. The odds of meeting the guidelines for MVPA at follow-up were also analysed.
Results
14 566 unique reports were identified and 46 randomised controlled trials with a range of follow-ups (3-60 months) were included in the meta-analysis (n=16 198 participants). Physical activity interventions delivered or prompted by health professionals in primary care increased MVPA by 14 min/week (95% confidence interval 4.2 to 24.6, P=0.006). Heterogeneity was substantial (I2=91%, P<0.001). Limiting analyses to trials that used a device to measure physical activity showed no significant group difference in MVPA (mean difference 4.1 min/week, 95% confidence interval −1.7 to 9.9, P=0.17; I2=56%, P=0.008). Trials that used self-report measures showed that intervention participants achieved 24 min/week more MVPA than controls (95% confidence interval 6.3 to 41.8, P=0.008; I2=72%, P<0.001). Additionally, interventions increased the odds of patients meeting guidelines for MVPA by 33% (95% confidence interval 1.17 to 1.50, P<0.001; I2=25%, P=0.11) versus controls. 14 of 46 studies were at high risk of bias but sensitivity analyses excluding these studies did not alter the results.
Conclusions
Physical activity interventions delivered or prompted by health professionals in primary care appear effective at increasing participation in self-reported MVPA. Such interventions should be considered for routine implementation to increase levels of physical activity and improve health outcomes in the population.
Systematic review registration
PROSPERO CRD42021209484.
Introduction
Physical inactivity is a leading global risk factor for mortality and morbidity.1 The World Health Organization updated their physical activity guidelines in 2020 and now state that adults should undertake at least 150-300 minutes of moderate intensity physical activity, or 75-150 minutes of vigorous intensity physical activity, or an equivalent combination of aerobic based physical activity each week.2 Current national physical activity programmes have been ineffective in most countries,3 with one in four adults insufficiently physically active and no improvement in participation rates evident over the past two decades.4 The World Health Assembly has set a target to reduce physical inactivity by 15% by 2030.5 This target includes a recommendation for all countries to integrate physical activity counselling programmes into primary healthcare. On average, 70-80% of adults visit their general practice at least once each year.6 Therefore, health professionals in primary care have a unique opportunity to routinely prompt and provide physical activity interventions to patients through the millions of health consultations that take place worldwide each week.
Previous reviews have investigated the effectiveness of physical activity interventions delivered in primary care settings and some of these have reported small to moderate effects depending on the inclusion criteria used.7 8 9 10 11 12 13 14 15 However, these reviews have not been able to offer definitive conclusions to guide implementation or health policy on this question for several reasons: they were narrative reports,7 11 12 included non-randomised trials,7 8 10 12 recruited specific clinical populations,11 13 the findings were based solely on self-report measures of physical activity,8 10 11 13 15 or they included interventions not delivered or prompted by health professionals in primary care.7 9 11 14 More recent reviews have only investigated outcomes such as energy expenditure13 and total amounts of physical activity,14 15 making it unclear how effective primary care delivered physical activity interventions are for increasing moderate to vigorous intensity physical activity (MVPA), the required intensity to meet WHO physical activity guidelines.2 This systematic review and meta-analysis aimed to robustly and comprehensively synthesise evidence from randomised controlled trials on whether physical activity interventions delivered or prompted by health professionals in primary care are effective in increasing MVPA in their patients.
Methods
This systematic review and meta-analysis has been reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA).16 The protocol was registered with the international prospective register of systematic reviews (PROSPERO) on 1 February 2021 (CRD42021209484).
Eligibility criteria
Randomised controlled trials were eligible when adult participants or clusters were randomly allocated to a physical activity intervention or a usual care control group, or another control group that did not involve physical activity. No other restrictions were applied relating to personal characteristics. Any type of predominately aerobic based physical activity intervention delivered or prompted (referred to here as delivered) by a health professional in a primary care setting was eligible. Prompted refers to interventions where the primary care health professional was involved in the intervention but an additional interventionalist (eg, a physical activity counsellor) was also involved in intervention delivery. Delivered refers to interventions delivered solely by primary care health professionals. Interventions aimed entirely at body conditioning (eg, yoga, tai-chi) were excluded because these types of activities do not involve an aerobic component and are unlikely to increase levels of MVPA. All primary care settings were included, broadly defined as the first point of contact in the healthcare system providing accessible, continued, comprehensive, and coordinated care, which focuses on people’s long term health rather than short disease durations.17 Trials were included when at least one interaction took place between health professionals in primary care and patients.
We excluded trials evaluating exercise referral schemes because primary care health professionals would only be acting as referral mechanisms rather than being directly involved in delivering interventions. Rehabilitation trials were excluded because patients might have limited capability to perform MVPA. Trials that assessed interventions lasting four weeks or longer were eligible and were required to have at least one follow-up beyond baseline.
Trials were required to report data (in continuous or dichotomous units) related to participation in MVPA from baseline to final follow-up or provide data that allowed this to be calculated. Studies measuring MVPA at follow-up but not at baseline were also eligible in line with the Cochrane handbook.18 No restrictions were made on the method used to assess MVPA, with data from self-report and device measures included, or on publication type, year, or language.
Search strategy
The search strategy was devised and tested in Medline, combining intervention and setting terms with established randomised controlled trial filters. We adapted the search for the following datasets: Embase, PsycINFO, CINAHL, SPORTDiscus, Sports Medicine and Education Index, ASSIA, PEDro, Bibliomap, Science Citation Index (SCI-E), Conference Proceedings Citation Index (CPCI-S), and OpenGrey. We searched the following trial registers: Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, and TRoPHI. The supplementary material provides the full search strategy. No date limitations were applied except for SCI-E, where dates were restricted to the past 10 years for manageability. AC performed the searches between 9 and 21 September 2020. Subsequently, a brief search of PubMed covering the six months after these search dates was performed before the final analyses (1 April 2021).
Study selection and data extraction
Duplicates were removed automatically in EndNote version X9 (Clarivate, Philadelphia, Pennsylvania) and the remaining results were uploaded to Covidence systematic review software,19 where additional duplicates were removed. Two independent reviewers from VEK, AJD, CDM, HG, and JJCT screened study titles and abstracts, applying the eligibility criteria except for SCI-E, CPCI-S, TRoPHI, Bibliomap, ClinicalTrials.gov, and Open Grey results which were single screened (VEK). The full texts of potentially eligible studies were retrieved and assessed independently by VEK and AJD or AEC. All decisions of inclusion or exclusion were automatically recorded in Covidence, and reviewers were blinded to each other’s decisions. Any disagreements were discussed between the two reviewers and resolved by consensus. All included full texts were examined and multiple reports from the same trial were merged in Covidence before data extraction.
Data were extracted about the characteristics of included studies and summarised (table 1, table 2). All outcome data used for the meta-analysis were independently extracted by VEK and HG or AEC. Disagreements were discussed, and the original paper was consulted to reach consensus. Corresponding authors were contacted by email if data were unreported or additional details were required.
Table 1.
Characteristics of included studies, according to study author surname (beginning with A-H)
| Study (year), country | RCT type | Participants | Intervention | Comparisons* | Follow-up (months) | MVPA measure |
|---|---|---|---|---|---|---|
| Aittasalo (2006),20 Finland | Cluster | n=265, 24% male, 20-65 years; general population, inactive | PA, brief, GP | Usual care | 6 | Self-report: IPAQ |
| Alonso-Dominguez (2019),21 Spain | Individual | n=204, 54% male, 25-70 years; type 2 diabetes mellitus, PA not part of eligibility | PA and diet, intensive, nurse | Usual care | 12 | Self-report: IPAQ |
| Apinaniz (2019),22 Spain | Individual | n=110, 28% male, 18-45 years; body mass index ≥25, inactive | PA and diet, brief, nurse or GP | Usual care | 6 | Self-report: degree of adherence to recommendations |
| Arija (2018),23 Spain | Individual | n=207, 23% male, ≥18 years; people with hypertension, PA not part of eligibility | PA, intensive, nurse | Usual care | 9 | Self-report: IPAQ |
| Carroll (2010),24 US | Cluster | n=394, 31% male, adults, mean age 46.4 years†; general population, inactive | PA, brief, GP | General preventive screening report | 6 | Self-report: 7d-PAR |
| Cheng (2018),25 US | Individual | n=404, 60% male, ≥40 years; survivors of ischaemic stroke or transient ischaemic attack, PA not part of eligibility | Stroke prevention including PA, intensive, nurse or physician assistant | Usual care | 12 | Self-report: MVPA ≥3 days/week |
| Clapperton (2020),26 Trinidad | Individual | n=130, 14% male, ≥18 years; general population, inactive | PA, multiple brief, GP | Usual care | 10 | Self-report: Brief assessment tool |
| Driehuis (2012),27 Netherlands | Individual | n=457, 48% male, 40-70 years; body mass index 25-40 and hypertension or dyslipidemia, PA not part of eligibility | PA and diet, intensive, nurse | Usual care | 36 | Self-report: SQUASH |
| Dubbert (2008),28 US | Individual | n=224, 100% male, 60-85 years; veterans with physical function limitations, inactive | PA, multiple brief, nurse | Nurse health discussion | 10 | Device measured: RT3 triaxial accelerometer |
| Duijzer (2017),29 Netherlands | Individual | n=316, 52% male, 40-70 years; increased risk of type 2 diabetes, PA not part of eligibility | PA and diet, intensive, physiotherapist | Usual care | 18 | Self-report: SQUASH |
| Dutton (2006),30 US | Cluster | n=139, 0% male, 18-65 years; low-income African American women BMI ≥25, PA not part of eligibility. | PA, multiple brief, GP | Usual care | 6 | Self-report: 7d-PAR |
| Elley (2003),31 New Zealand | Cluster | n=878, 34% male, 40-79 years; general population, inactive | PA, multiple brief, primary care health professional and exercise specialist | Usual care | 12 | Self-report: Auckland heart study questionnaire |
| Fortier (2011),32 Canada | Individual | n=120, 31% male, 18-69 years; general population, inactive | PA, intensive, GP and PA counsellor | Brief GP counselling | 6 | Device measure: Actical |
| Garcia-Ortiz (2018),33 Spain | Individual | n=833, 38% male, <70 years; general population, PA not part of eligibility | PA and diet, multiple brief, nurse | Brief nurse counselling | 12 | Device measure: ActiGraph |
| Goldstein (1999),34 US | Cluster | n=355, 76% male, ≥50 years; general population, inactive | PA, multiple brief, GP and researcher | Usual care | 8 | Self-report: PASE |
| Gomez-Huelgas (2015),35 Spain | Individual | n=601, 55% male, 18-80 years; metabolic syndrome patients, PA not part of eligibility | PA and diet, multiple brief, nurse | Usual care | 36 | Self-report: Minnesota Leisure-Time PA Questionnaire |
| Grandes (2011),36 Spain | Cluster | n=4317, 35% male, 20-80 years; general population, inactive | PA, brief, GP | Usual care | 24 | Self-report: 7d-PAR |
| Hall (2011),37
Morey (2009),38 US |
Individual | n=234, 100% male, ≥70 years; older adults with multiple morbidities, inactive | PA, multiple brief, GP and lifestyle counsellor | Usual care | 24‡ | Self-report: CHAMPS |
| Harari (2008),39 UK | Cluster | n=2503, 46% male, >65 years; general population, PA not part of eligibility | Lifestyle including PA, brief, GP | Usual care | 12 | Self-report: PASE |
| Hardeman (2020),40 UK | Individual | n=1007, 38% male, 40-74 years; general population, PA not part of eligibility | PA, brief, primary care practitioner | Usual care | 3 | Device measure: ActiGraph |
| Harris (2012),41 Australia | Cluster | n=699, 43% male, 40-64 years; hypertension, hyperlipidaemia or aged 56-64 years, PA not part of eligibility | PA, diet and lifestyle, intensive, GP or nurse and dietician or exercise specialist | Usual care | 12 | Self-report: Brief assessment tool |
| Harris (2018 PACE-Lift),42 UK | Cluster | n=298, 46% male, 60-75 years; general population, PA not part of eligibility | PA, intensive, nurse | Usual care | 48 | Device measure: ActiGraph |
| Harris (2018 PACE-UP),42 UK | Cluster | n=1023, 36% male, 45-75 years; general population, inactive | PA, multiple brief, nurse | Usual care | 36 | Device measure: ActiGraph |
| Hellgren (2020),43 Sweden | Individual | n=123, 42% male, 35-75 years; individuals with prediabetes, PA not part of eligibility | PA and lifestyle, intensive, nurse | Usual care | 60 | Self-report: leisure time PA per week |
| Hesselink (2013),44 Netherlands | Cluster | n=366, 53% male, ≥45 years; individuals with impaired fasting glucose, PA not part of eligibility | PA and diet, multiple brief, nurse | Usual care | 24 | Self-report: SQUASH |
| Huebschmann (2018),45 US | Individual | n=50, 50% male, 50-85 years; patients with type 2 diabetes, inactive | PA, multiple brief, GP and clinic staff coach | Enhanced usual care: printed materials and mailings | 3 | Device measure: ActiGraph |
The term GP includes physician, clinician, doctor, general practitioner.
7d-PAR=7-day Physical Activity Recall; CHAMPS=Community Healthy Activities Model Program for Seniors; IPAQ=International Physical Activity Questionnaire (short form); MVPA=moderate to vigorous intensity physical activity; PA=physical activity; PASE=Physical activity Scale for the Elderly; RCT=randomised controlled trial; SQUASH=Short Questionnaire to assess health-enhancing physical activity.
Usual care as stated in paper.
Mean age stated when age range was not reported in study.
Adherence to physical activity guidelines assessed at 12 months only.
Table 2.
Characteristics of included studies, according to study author surname (beginning with J-Y)
| Study (year), country | RCT type | Participants | Intervention | Comparisons* | Follow-up (months) | MVPA measure |
|---|---|---|---|---|---|---|
| Jimmy (2005),46 Switzerland | Individual | n=161, 42% male, >15 years; general population, inactive | PA, intensive, GP and PA specialist | GP feedback on current PA levels | 14 | Self-report: 7-day recall questionnaire |
| Jolly (2017),47 UK | Individual | n=577, 63% male, >18 years; patients with mild COPD, PA not part of eligibility | COPD self-management including PA, intensive, nurse | Usual care | 12 | Device measure: GENEActive |
| Kloek (2018),48 Netherlands | Cluster | n=204, 32% male, 40-80 years; people with hip or knee osteoarthritis, inactive | PA, multiple brief, physiotherapist | Usual care | 12 | Device measure: ActiGraph |
| Lawton (2008),49 New Zealand | Individual | n=1089, 0% male, 40-74 years; general population, inactive | PA, multiple brief, nurse and exercise facilitator | Usual care | 24 | Self-report: NZPAQ-LF |
| Migneault (2012),50 US | Individual | n=337, 30% male, ≥35 years; African Americans with hypertension, PA not part of eligibility | PA, diet and drugs adherence, multiple brief, GP and researcher | Educational session | 8 | Self-report: 7d-PAR |
| Mitchell (2013),51 UK | Individual | n=184, 55% male, mean age 69 years†; patients with COPD, PA not part of eligibility | COPD self-management including PA, intensive, physiotherapist | Usual care | 6 | Device measure: Sensewear |
| Moreno (2019),52 Spain | Individual | n=594, 60% male, ≥18 years; patients with type 2 diabetes, PA not part of eligibility | Diabetes self-management including PA, intensive, healthcare professional | Usual care | 24 | Self-report: 7d-PAR |
| Morey (2012),53 US | Individual | n=302, 97% male, 60-89 years; older adults with prediabetes mellitus, inactive | PA, multiple brief, GP and lifestyle counsellor | Usual care | 12 | Self-report: CHAMPS |
| Pears (2016),54 UK | Individual | n=394, 41% male, 40-74 years; general population, inactive | PA, brief, nurse or healthcare assistant | Usual care | 1 | Device measure: ActiGraph |
| Pinto (2002),55 US | Individual | n=298, 28% male, ≥25 years; general population, inactive | PA, brief, GP and researcher | Healthy eating intervention | 6 | Self-report: 7d-PAR |
| Pinto (2005),56 US | Individual | n=100, 37% male, ≥60 years; older adults, inactive | PA, intensive, GP and health educator | Brief advice only | 6 | Self-report: 7d-PAR |
| Reed (2008),57 US | Individual | n=237, 27% male, adults; general population, PA not part of eligibility | PA, brief, GP or nurse | Usual care | 2 | Self-report: IPAQ |
| Richardson (2007),58 US | Individual | n=20, 25% male, ≥60 years; geriatric population, inactive | PA, brief, GP | Usual care | 1 | Self-report: MVPA hours during past 7 days |
| Schillinger (2009),59 US | Individual | n=339, 41% male, >17 years; patients with type 2 diabetes, PA not part of eligibility | Diabetes self-management including PA, intensive, GP and health educator | Usual care | 12 | Self-report: mins of MVPA on each of the past 7 days |
| Steptoe (1999),60 UK | Cluster | n=883, 46% male, 18-69 years; adults at increased risk of coronary heart disease, inactivity was one of the possible inclusion criteria | PA, smoking and diet, multiple brief, nurse | Usual care | 12 | Self-report: Allied Dunbar National Fitness Survey |
| Taheri (2020),61 Qatar | Individual | n=158, 73% male, 18-50 years; patients with early type 2 diabetes, PA not part of eligibility | PA and diet, intensive, GP and dietician or personal trainer | Usual care | 12 | Self-report: IPAQ |
| Tiessen (2013),62 Netherlands | Individual | n=201, 69% male, 50-75 years; patients with increased cardiovascular risk, inactivity was one of the possible inclusion criteria | CVD risk including PA, multiple brief, nurse | Usual care | 12 | Self-report: SQUASH |
| Valve (2013),63 Finland | Cluster | n=3059, 0% male, 17-21 years; young women, PA not part of eligibility | PA, diet and sleep, multiple brief, nurse | Sexual health and standard lifestyle counselling | 30 | Self-report: based on previous Finnish health behaviour questionnaires |
| Van der Weegan (2015),64 Netherlands | Cluster | n=199, 49% male, 40-70 years; patients with COPD or type 2 diabetes, inactive | PA, multiple brief, nurse | Usual care | 9 | Device measure: Personal activity monitor |
| Van Sluijs (2005),65 Netherlands | Cluster | n=771, 51% male, 18-70 years; patients with hypertension, hypercholesterolemia or non-insulin dependent diabetes, inactive | PA, multiple brief, GP or nurse and PA counsellor | Usual care | 12 | Self-report: SQUASH |
| Vermunt (2012),66 Netherlands | Individual | n=925, 46% male, 40-70 years; patients at high risk of type 2 diabetes, PA not part of eligibility | PA and diet, intensive, GP or nurse and dietician or physiotherapist. | Usual care | 30 | Self-report: SQUASH |
| Volger (2013),67 US | Individual | n=390, 20% male, ≥21 years; obese (body mass index 30-50), PA not part of eligibility | PA and diet, multiple brief, GP and medical assistant | Usual care | 24 | Self-report: Paffenbarger PA survey |
| Westland (2020),68 Netherlands | Cluster | n=195, 61% male, 40-75 years; patients at risk of CVD, inactive | PA, multiple brief, nurse | Usual care | 6 | Device measure: Personal activity monitor |
| Writing Group for the Activity Counselling Trial Research Group (2001),69 US | Individual | n=874, 55% male, 35-75 years; general population, inactive | PA, intensive, GP and health educator (two groups) | Usual care | 24 | Self-report: 7d-PAR |
| Yates (2017),70 UK | Cluster | n=808, 64% male, 18-74 years; adults with a high risk of type 2 diabetes, PA not part of eligibility | PA, intensive, GP or health educator | Advice leaflet | 36 | Device measure: ActiGraph |
The term GP includes physician, clinician, doctor, general practitioner.
7d-PAR=7-day Physical Activity Recall; CHAMPS=Community Healthy Activities Model Program for Seniors; COPD=chronic obstructive pulmonary disease; CVD=cardiovascular disease; IPAQ=International Physical Activity Questionnaire (short form); MVPA=moderate-to-vigorous intensity physical activity; NZPAQ-LF=Long form of the New Zealand PA questionnaire; PA=physical activity; RCT=randomised controlled trial; SQUASH=Short Questionnaire to assess health-enhancing physical activity.
Usual care as stated in paper.
Mean age stated when age range was not reported in study.
Risk of bias and quality of evidence assessment
Two independent reviewers (VEK, CDM) assessed the risk of bias using the Cochrane risk of bias tool (version 2, ROB2).71 Any disagreements between the reviewers were discussed and resolved through consensus by referring to the full text. Figures for risk of bias were produced using ROB2 and funnel plots were created using RevMan 5.4.172 to assess risk of publication bias.
Outcomes and data synthesis
The primary outcome was minutes of MVPA each week. The proportion of participants meeting guidelines for MVPA was also included as an important secondary outcome. Other secondary outcomes were total physical activity and sedentary time. We selected these secondary outcomes because strong evidence has shown that any increase in physical activity, regardless of intensity, is also important for health,73 with similar outcomes for reducing sedentary behaviours.74 Data for weight and body mass index were also synthesised when reported in the included studies because evidence reports that physical activity can be important for weight management.13 75
Inverse variance meta-analyses using random effects models were conducted in RevMan using weighted mean differences and 95% confidence intervals to describe between group differences for change in MVPA (min/week). We used random effects models because of the variety of physical activity interventions tested and the likelihood of different intervention effects. For trials with a high loss to follow-up (>20%), change in MVPA was calculated using baseline MVPA observed carried forward76 (n=10 trials). We excluded two trials from this analysis because MVPA was measured only at follow-up in one trial54 and the other reported medians.51
The likelihood of meeting MVPA guidelines (according to the guidance used in the individual randomised controlled trials) was explored using odds ratios and 95% confidence intervals. We calculated the standardised mean difference for total physical activity because of the variety of outcomes reported (eg, minutes, accelerometer counts, steps) and the effect size was interpreted as small (0.2), moderate (0.5), or large (0.8).77 See supplementary material for additional information on data synthesis methods.
Prespecified subgroup analyses were conducted that compared device and self-report measures of MVPA. We investigated intervention intensity according to the number of contacts with an interventionalist (at least one of which must have been with a health professional in primary care) to compare the effects of brief (one session ≤30 min),8 multiple brief (more than one session ≤30 min), and intensive interventions (more than one session >30 min) on outcomes. A sensitivity analysis was conducted excluding the studies considered at high risk of bias.
Post hoc subgroup analyses were conducted that compared interventions with a high number of intervention contacts (at least five contacts) versus a low number (less than five contacts). We investigated the merits of interventions delivered solely by primary care health professionals versus those involving primary care health professionals plus other interventionalists. Because primary healthcare systems differ by country, the impact of country was examined. We also explored the effect of difference in follow-up length (0-6, 7-12, >12 months).
Patient and public involvement
No patients or the public were involved in this systematic review due to funding restrictions.
Results
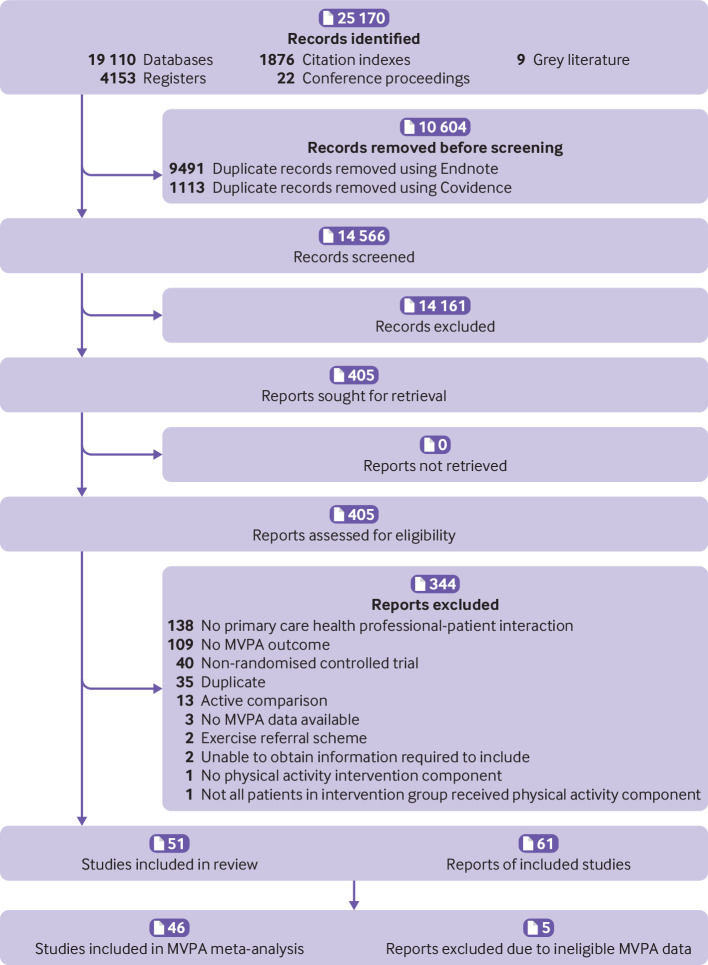
A total of 25 170 reports were identified from searches; 14 566 titles and abstracts were screened after removing duplicates. Of these, 405 full texts were assessed with 61 reports of 51 studies included in the review; 46 of these were included in the meta-analysis (fig 1). Five studies could not be meta-analysed because MVPA was not reported in a unit that would allow the data to be aggregated (kcal/week,67 episodes/week,60 MVPA score,58 unclear units,57 or ineligible for baseline observation carried forward analysis54). Of 33 study authors contacted, 10 provided further information23 25 78 79 or data.26 35 41 43 45 65
Fig 1.
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow diagram
Most studies were conducted in the United States (n=16), the UK (n=9), the Netherlands (n=9), and Spain (n=7), with the remainder in Finland, New Zealand, Trinidad, Canada, Australia, Sweden, Switzerland, and Qatar (table 1, table 2). Thirty three studies were individual randomised controlled trials and 18 were cluster randomised controlled trials. Most trials recruited participants at increased risk of disease or diseased populations (n=30) and/or inactive participants (n=24). Physical activity was the primary focus in most interventions (n=33) followed by physical activity and dietary behaviours (n=10), with others focusing on multiple health behaviours that included physical activity (n=8). GPs, nurses, and physiotherapists delivered the interventions in most trials (n=31), with others involving additional interventionalists including health educators or counsellors, exercise specialists, dieticians, and researchers. About half of the interventions were delivered in multiple brief sessions (n=23), 18 were intensive, and 10 were brief. The number of contacts with an interventionalist ranged from 1 to 72; 23 trials involved fewer than five contacts and the remainder five or more contacts (n=28). The control group was generally usual care (n=40). The length of follow-up ranged from one month to five years (see supplementary table 1 for intervention details). MVPA was measured using self-report in most trials (n=37) and using a device in 14 trials.
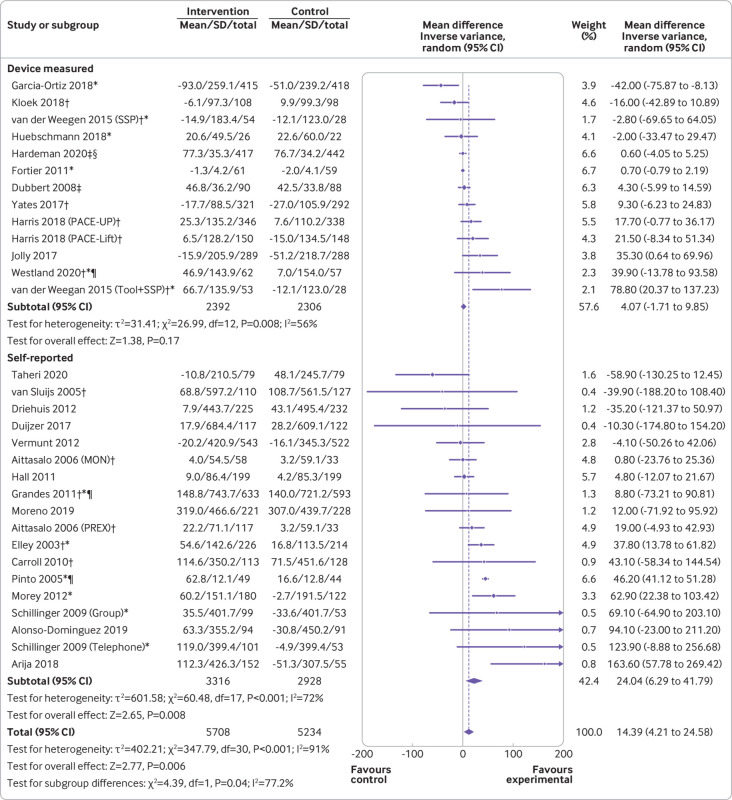
Physical activity interventions delivered by health professionals in primary care significantly increased MVPA versus control groups (mean difference 14.4 min/week, 95% confidence interval 4.2 to 24.6, P=0.006; fig 2). Heterogeneity was substantial (I2=91%, P<0.001). Limiting analyses to only trials that used a device to measure physical activity showed no significant group difference in MVPA (4.1 min/week, −1.7 to 9.9, P=0.17; I2=56%, P=0.008). Trials that used self-report measures showed that intervention participants reported achieving 24 min/week more MVPA than controls (95% confidence interval 6.3 to 41.8, P=0.008; I2=72%, P<0.001). No difference was found in minutes per week of MVPA between the groups based on the intensity of the intervention, but interventions with at least five contacts had a larger effect compared with those with less than five contacts for self-reported minutes per week of MVPA (table 3). Furthermore, interventions delivered by primary care health professionals in combination with other interventionalists significantly increased self-reported MVPA, whereas interventions delivered by primary care health professionals alone did not. No subgroup differences were observed for device measured minutes per week of MVPA.
Fig 2.
Moderate to vigorous intensity physical activity (MVPA min/week) with measurement type subgroups (device measured or self-reported). *Intention to treat analysis; †cluster randomised controlled trial; ‡follow-up values; §geometric means; ¶adjusted means. SD=standard deviation
Table 3.
Results of subgroup analyses stratified by self-reported and device measured moderate to vigorous intensity physical activity (MVPA, min/week)
| Analysis and subgroups | No of participants | Mean difference (95% CI) | P value | Heterogeneity, I2 (%) (P value) |
|---|---|---|---|---|
| Self-reported MVPA min/week | ||||
| Intervention intensity* | ||||
| Brief | 1708 | 11.0 (−5.6 to 27.5) | 0.19 | 0 (0.69) |
| Multiple brief | 1377 | 28.4 (−2.3 to 59.2) | 0.07 | 70 (0.02) |
| Intensive | 3159 | 29.6 (−7.0 to 66.2) | 0.11 | 63 (0.004) |
| Interventionalist | ||||
| PCHP only | 3245 | 19.7 (−6.1 to 45.5) | 0.13 | 36 (0.13) |
| PCHP and other | 2999 | 25.9 (2.8 to 49.1) | 0.03 | 78 (<0.001) |
| No of interventionalist contacts | ||||
| <5 | 2385 | 19.0 (4.5 to 33.6) | 0.01 | 6 (0.38) |
| ≥5 | 3859 | 28.7 (2.6 to 54.7) | 0.03 | 76 (<0.001) |
| Device measured MVPA min/week | ||||
| Intervention intensity | ||||
| Brief | 859 | 0.6 (−4.1 to 5.3) | 0.80 | NA† |
| Multiple brief | 2231 | 4.1 (−13.0 to 21.1) | 0.64 | 64 (0.007) |
| Intensive | 1608 | 9.3 (−3.1 to 21.8) | 0.14 | 56 (0.08) |
| Interventionalist | ||||
| PCHP only | 3917 | 7.3 (−4.2 to 18.7) | 0.21 | 64 (0.003) |
| PCHP and other | 781 | 0.8 (−0.7 to 2.3) | 0.31 | 0 (0.55) |
| No of interventionalist contacts | ||||
| <5 | 3533 | 13.5 (−4.2 to 31.2) | 0.13 | 70 (0.002) |
| ≥5 | 1165 | 0.8 (−0.7 to 2.3) | 0.29 | 0 (0.53) |
PCHP=primary care health professional.
Brief (one session ≤30 min), multiple brief (more than one session ≤30 min), intensive (more than one session >30 min).
Results based on one study.
When MVPA data (self-report and device measured combined) were stratified by country, larger intervention effectiveness was seen in trials conducted in the US and the UK compared with Spain, the Netherlands, and other countries (supplementary fig 1). Follow-up lengths of seven months or longer were effective at increasing minutes per week of MVPA, with the largest effect seen in follow-up lengths of 7-12 months (supplementary fig 2).
Five studies could not be included in the meta-analysis of minutes per week of MVPA; significant increases in MVPA (self-report) were reported by Volger and colleagues67 for the brief and enhanced brief lifestyle counselling groups (+593.4±175.9 and +415.4±179.6 kcal/week, respectively) compared with usual care (70.4 ±185.5 kcal/week) at 24 month follow-up. Steptoe and colleagues60 found that the intervention group increased the number of episodes of MVPA in the past four weeks (self-report) compared with the control group (+3.9 sessions, 95% confidence interval 1.0 to 6.8) after one year. Conversely, Pears and colleagues54 found no difference in device measured MVPA after one month with any of the three brief interventions tested compared with the usual care group. Richardson58 showed no difference between the intervention and control groups for MVPA (self-report). The study by Reed and colleagues57 was excluded from the MVPA meta-analysis because the units used were unclear and no response was received from the author.
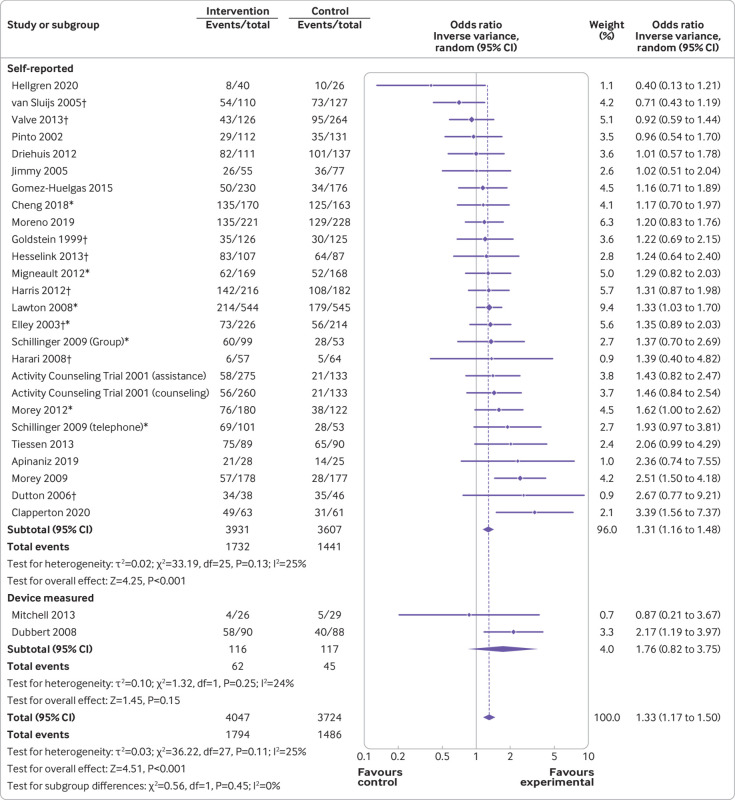
The proportion of participants meeting guidelines for MVPA was significantly higher in the intervention group versus the control group (odds ratio 1.33, 95% confidence interval 1.17 to 1.50, P<0.001), with low heterogeneity (I2=25%, P=0.11). In trials that assessed MVPA using self-report measures, this effect remained (1.31, 1.16 to 1.48, P<0.001; I2=25%, P=0.13), but not when the analysis was restricted to trials that had used device measures (1.76, 0.82 to 3.75, P=0.15, two trials; fig 3).
Fig 3.
Proportion of participants meeting moderate to vigorous intensity physical activity guidelines with measurement type subgroups. *Intention to treat analysis; †cluster randomised controlled trial
Interventions involving multiple brief contacts (odds ratio 1.43, 95% confidence interval 1.18 to 1.73, P<0.001) and intensive contact support (1.24, 1.04 to 1.47, P=0.01) significantly increased the odds of participants being sufficiently physically active in line with guidelines versus controls, with moderate (I2=49%, P=0.02) and low (I2=0%, P=0.68) heterogeneity. Brief interventions (1.18, 0.73 to 1.89, P=0.51) showed no effect from the three studies included (supplementary fig 3).
Intervention group participants significantly increased their total physical activity (all intensities of physical activity combined) with a small to moderate effect found (standardised mean difference 0.32, 95% confidence interval 0.15 to 0.49, P<0.001). Substantial heterogeneity was present (I2=91%, P<0.001; supplementary fig 4). When stratified by self-report and device measured, a larger effect was seen for total physical activity when measured using a device (0.53, 0.14 to 0.92) compared with self-reported (0.17, 0.11 to 0.24). No significant effect was observed for time spent sedentary (mean difference −3.1 min/day, 95% confidence interval −11.8 to 5.6, P=0.48; supplementary fig 5).
Eighteen trials reported weight (all objectively measured) and there was a significant reduction of 1 kg favouring the physical activity intervention groups versus controls (mean difference −1.0 kg, 95% confidence interval −1.6 to −0.5, P<0.001) with substantial heterogeneity (I2=72%, P<0.001; supplementary fig 6). A sensitivity analysis was performed with Taheri and colleagues61 removed because this intervention included an intensive diet replacement phase and therefore had a substantially larger effect on weight than other included studies. This analysis showed that the significant effect remained (−0.7 kg, −1.1 to −0.3, P<0.001) with moderate heterogeneity (I2=48%, P=0.01). No intervention effect was observed for body mass index (−0.04, −0.15 to 0.07, P=0.50; supplementary fig 7).
Of the 46 studies included in the meta-analyses of MVPA (minutes per week and the proportion of participants meeting guidelines), six were considered low risk of bias, 26 had some concerns, and 14 were high risk of bias (supplementary fig 8). For measurement of the outcome, 30 studies had some concerns because they assessed MVPA using self-report measures. Most studies did not provide a prespecified analysis plan, and so they had some concerns about the selection of the reported result (n=24). High risk of bias was typically due to incomplete MVPA data at final follow-up (n=8). A sensitivity analysis comparing the studies with low risk of bias or some concerns with high risk of bias trials did not change the results for minutes per week of MVPA (mean difference 16.5 min/week, 95% confidence interval 4.2 to 28.9, P=0.009; I2=94%, P<0.001), or the proportion who were sufficiently physically active in line with guidance (odds ratio 1.36, 95% confidence interval 1.17 to 1.57, P<0.001; I2=37%, P=0.05). No evidence was found of publication bias after examining the funnel plots (supplementary figs 9-10).
Discussion
Principal findings
Estimates from this systematic review, which included 51 randomised controlled trials, found that physical activity interventions delivered by health professionals in primary care increased participation in MVPA in patients by an average of 14 min/week versus controls. While this size of effect might seem modest, it should be interpreted within the context that MVPA has an inverse dose-response relation with all cause mortality, therefore even small increases in physical activity are clinically important.80 Other systematic reviews have reported that an increase in MVPA of 2 min/day (14 min/week) is associated with an 11% reduction in all cause mortality.81 Intervention group participants were 33% more likely to meet guidelines for MVPA and achieved significantly more overall physical activity (total activity with all intensities combined) than controls. Multiple contacts with an intervenor, including one with a primary care health professional, are needed to increase participation in MVPA. Interventions with at least five contacts had a larger effect on self-reported minutes of MVPA than those with fewer contacts.
Strengths and limitations of this review
This review has several strengths. It is a large, comprehensive systematic review that examined the effectiveness of physical activity interventions delivered by health professionals in primary care settings. Systematic reviews of randomised controlled trials investigating the effectiveness of such interventions on sedentary time or body weight are lacking. Our primary conclusions are based on a large sample of approximately 16 000 randomised participants worldwide, which increases generalisability. Comprehensive searches of published studies and grey literature were conducted with no restrictions on language or publication date, increasing the likelihood that all eligible trials were identified. Only five trials could not be included in the meta-analyses. Additionally, no evidence of publication bias was found. The focus of this review on MVPA allowed the findings to be put in a public health context and enables direct comparisons with the WHO physical activity guidelines to inform health policy decisions across the world.
This review also has some limitations. Stratified analyses by measurement type showed a significant increase in self-report measures but not in device based measures of MVPA. Self-report measures have been reported to have lower validity compared with device based measures and might overestimate physical activity82; however, this is likely to be true for the intervention and control groups, and the results do not appear to be implausibly inflated (+24 min/week, 95% confidence interval 6.3 to 42.8). While, device based measures have lower variability for validity and reliability of physical activity measurement, they also have issues with potential biases, including reactivity, incomplete data, and varying cut-off points to classify MVPA.83 84 Additionally, fewer trials have used devices to measure MVPA, and this review included all data regardless of the method used to measure MVPA. This approach allowed all the relevant data to be processed and recommendations made based on all the available evidence.
In some trials, usual care included brief physical activity advice from a primary care health professional, which could have led to an increase in physical activity in control groups (contamination). Therefore, our findings might be an underestimation of the true effects, although we know advice alone has limited effectiveness for increasing or maintaining physical activity.85 Fourteen trials were at high risk of bias, but the results remained unchanged when these trials were removed from the analyses, indicating that findings are not subject to, or dependent on, trial quality. Substantial heterogeneity was found for the outcome of minutes per week of MVPA, which appears to be related to the method used to assess physical activity. Heterogeneity was substantially reduced in a subgroup analysis when data were categorised as self-report or device measured. Although this was a large review, the data from trials using a device were limited. Only 14 trials followed participants for at least two years and one trial for five years.
Comparison with other studies
Because reviews on the effectiveness of physical activity interventions delivered by health professionals in primary care that report data on MVPA are lacking, direct comparisons with other reviews are limited. However, reviews on similar questions have reported mixed findings, with some reporting these types of interventions can be effective at increasing physical activity outcomes,8 9 10 13 15 while others have found limited evidence.11 14 Our review is most closely aligned to the review by Oloo and colleagues,15 which reported that physical activity interventions delivered in primary care increased physical activity participation (standardised mean difference 0.11), but the Oloo review only included trials that had measured overall (total) physical activity outcomes using self-report measures, and their findings were based on data from only 14 randomised controlled trials. Of note here, Goryakin and colleagues13 examined the impact of primary care initiated physical activity interventions and found that increased contacts between health professionals and patients produced a larger effect than those restricted to initial referral only. However, Goryakin and colleagues included exercise referral schemes whereas the current review did not. Nevertheless, collectively these findings highlight the importance of involving health professionals in primary care settings in providing physical activity interventions to patients.
Implications and future research
The interventions assessed in this review increased participants’ overall (total) physical activity (standardised mean difference 0.32) relative to controls at follow-up. While WHO guidelines focus on the importance of achieving 150 min/week of MVPA, they also state that all movement counts for health, regardless of intensity.2 Several studies have shown that light intensity physical activity can also improve health outcomes and this is particularly important in primary care for several reasons.86 Many patients who present to primary care health professionals might not have the means or motivation to achieve MVPA, and might be afraid to physically exert themselves to this intensity because of concerns about potential adverse outcomes (eg, older or frail patients, pregnant women, those with cardiovascular diseases or a disability). Additionally, many health professionals are reluctant to promote MVPA because they feel they lack the specialised knowledge or skills to do so, or consider it inappropriate to promote more vigorous intensity physical activity with some patient groups because of concerns about causing harm.87 The Global Action Plan for Physical Activity5 highlights the need to strengthen the training of health professionals so that competent assessments and provision of physical activity advice or counselling can be given in routine practice. Our findings can be used to reassure health professionals that interventions delivered by them in primary care can be effective in encouraging patients to be more physically active, even if this does not meet the MVPA intensity threshold recommended by WHO.
Physical activity is known to improve a wide range of health outcomes, further highlighting the importance of finding effective population based strategies to increase participation rates. This review found that patients randomised to a physical activity intervention delivered by health professionals in primary care weighed 1 kg less than control groups at follow-up. While a difference of 1 kg might appear small, this finding should be considered in the context that adults typically gain around 0.5-1 kg/year, which can contribute to the development of obesity.88 A small amount of weight loss is also important because the association between weight and all cause mortality is linear.89 Our data provide evidence that the population impacts from physical activity are likely to reduce other key health outcomes, such as weight, reducing the risk of diseases and death.
Primary care is a health context where millions of interactions between patients and health professionals take place every month. This review has highlighted the critical role that health professionals in primary care can have in supporting the public to increase their physical activity. Future research is now needed to establish the optimum number and length of contacts required to successfully initiate, and then maintain, patients’ participation in physical activity. Additionally, the effectiveness of different types of physical activity interventions and their content need to be explored in more depth. In future trials, MVPA should be measured using devices.
Conclusions
Physical activity interventions delivered by health professionals in primary care settings appear effective in increasing participation in physical activity as measured by self-report and reducing weight in adults. Health commissioners and policy makers should consider physical activity interventions that include at least one contact with a health professional in primary care to help meet the World Health Assembly target of achieving a 15% reduction in physical inactivity by 2030.5
What is already known on this topic
Increasing population levels of physical activity is a public health priority and the World Health Assembly aims to reduce physical inactivity by 15% by 2030
Most adults visit their general practice once a year, therefore health professionals in primary care have the opportunity to routinely provide physical activity interventions to patients
Previous reviews of physical activity interventions delivered in primary care have reported mixed findings and those investigating the effectiveness of such interventions for increasing moderate to vigorous intensity physical activity (MVPA) are lacking
What this study adds
Physical activity interventions delivered to patients by health professionals in primary care significantly increased MVPA compared with control groups
The results are based on data from 46 randomised controlled trials involving approximately 16 000 participants worldwide
These data could help health professionals, policy makers, and healthcare commissioners make evidence based decisions about implementing physical activity interventions during consultations delivered in primary care
Acknowledgments
Additional information and data to facilitate the meta-analysis were provided by study authors23 25 26 35 41 43 45 65 78 79 and we thank these authors for their assistance.
Acknowledgments
Funding: AJD is supported by a National Institute for Health Research (NIHR) Research Professorship award. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication. This report was supported by the NIHR Leicester Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: VEK and AJD designed the study, generated hypotheses and interpreted the data. VEK analysed the data with input from AJD and CDM. All authors critically reviewed the report. AC designed the searches with input from VEK and AJD. AC performed the searches and removed duplicates. VEK, AJD, CDM, HG, and JJCT screened the titles and abstracts. VEK, AJD, and AEC screened the full texts. VEK, HG, and AEC performed the data extraction. VEK and CDM completed the risk of bias assessment. VEK and AJD directly accessed and verified the underlying data reported in the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. VEK and AJD are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: AJD is supported by a National Institute for Health Research (NIHR) Research Professorship award; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Dissemination to participants and related patient and public communities: We will ask our media office to issue a results press release. We plan to disseminate our findings widely through social media and with a plain language summary on the CLiMB website (https://www.lboro.ac.uk/research/climb/).
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Katzmarzyk PT, Friedenreich C, Shiroma EJ, Lee I-M. Physical inactivity and non-communicable disease burden in low-income, middle-income and high-income countries. Br J Sports Med 2022;56:101-6. 10.1136/bjsports-2020-103640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451-62. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klepac Pogrmilovic B, Ramirez Varela A, Pratt M, et al. National physical activity and sedentary behaviour policies in 76 countries: availability, comprehensiveness, implementation, and effectiveness. Int J Behav Nutr Phys Act 2020;17:116. 10.1186/s12966-020-01022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health 2018;6:e1077-86. 10.1016/S2214-109X(18)30357-7 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Global action plan on physical activity 2018-2030: more active people for a healthier world. Geneva, 2018 https://apps.who.int/iris/bitstream/handle/10665/272722/9789241514187-eng.pdf?sequence=1&isAllowed=y.
- 6. van Doorslaer E, Masseria C, Koolman X, OECD Health Equity Research Group . Inequalities in access to medical care by income in developed countries. CMAJ 2006;174:177-83. 10.1503/cmaj.050584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanchez A, Bully P, Martinez C, Grandes G. Effectiveness of physical activity promotion interventions in primary care: A review of reviews. Prev Med 2015;76(Suppl):S56-67. 10.1016/j.ypmed.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 8.Campbell F, Blank L, Messina J, et al. Physical Activity: Brief Advice for Adults in Primary Care (National Institute for Health and Clinical Excellence Public Health Intervention Guidance). 2012 https://www.nice.org.uk/guidance/ph44/evidence/review-of-effectiveness-and-barriers-and-facilitators-69102685.
- 9. Orrow G, Kinmonth A-L, Sanderson S, Sutton S. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ 2012;344:e1389. 10.1136/bmj.e1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pavey TG, Taylor AH, Fox KR, et al. Effect of exercise referral schemes in primary care on physical activity and improving health outcomes: systematic review and meta-analysis. BMJ 2011;343:d6462. 10.1136/bmj.d6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stevens Z, Barlow C, Kendrick D, et al. Effectiveness of general practice-based physical activity promotion for older adults: systematic review. Prim Health Care Res Dev 2014;15:190-201. 10.1017/S1463423613000017 [DOI] [PubMed] [Google Scholar]
- 12. Richards EA, Cai Y. Integrative review of nurse-delivered physical activity interventions in primary care. West J Nurs Res 2016;38:484-507. 10.1177/0193945915581861 [DOI] [PubMed] [Google Scholar]
- 13. Goryakin Y, Suhlrie L, Cecchini M. Impact of primary care-initiated interventions promoting physical activity on body mass index: systematic review and meta-analysis. Obes Rev 2018;19:518-28. 10.1111/obr.12654 [DOI] [PubMed] [Google Scholar]
- 14. van der Wardt V, di Lorito C, Viniol A. Promoting physical activity in primary care: a systematic review and meta-analysis. Br J Gen Pract 2021;71:e399-405. 10.3399/BJGP.2020.0817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oloo MO, Wamukoya EK, Wanzala M. Efficacy of physical activity counselling interventions delivered in primary care: a systematic review and meta-analysis. Eur J Phys Educ Sport Sci 2020;6:65-141. [Google Scholar]
- 16. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Primary health care: main terminology. https://www.euro.who.int/en/health-topics/Health-systems/primary-health-care/main-terminology.
- 18. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2. 2021. [Google Scholar]
- 19.Veritas Health Innovation Melbourne Australia. Covidence systematic review software. 2021. www.covidence.org.
- 20. Aittasalo M, Miilunpalo S, Kukkonen-Harjula K, Pasanen M. A randomized intervention of physical activity promotion and patient self-monitoring in primary health care. Prev Med 2006;42:40-6. 10.1016/j.ypmed.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 21. Alonso-Domínguez R, Patino-Alonso MC, Sánchez-Aguadero N, García-Ortiz L, Recio-Rodríguez JI, Gómez-Marcos MA. Effect of a multifactorial intervention on the increase in physical activity in subjects with type 2 diabetes mellitus: a randomized clinical trial (EMID Study). Eur J Cardiovasc Nurs 2019;18:399-409. 10.1177/1474515119835048 [DOI] [PubMed] [Google Scholar]
- 22. Apiñaniz A, Cobos-Campos R, Sáez de Lafuente-Moríñigo A, et al. Effectiveness of randomized controlled trial of a mobile app to promote healthy lifestyle in obese and overweight patients. Fam Pract 2019;36:699-705. 10.1093/fampra/cmz020 [DOI] [PubMed] [Google Scholar]
- 23. Arija V, Villalobos F, Pedret R, et al. Physical activity, cardiovascular health, quality of life and blood pressure control in hypertensive subjects: randomized clinical trial. Health Qual Life Outcomes 2018;16:184. 10.1186/s12955-018-1008-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carroll JK, Lewis BA, Marcus BH, Lehman EB, Shaffer ML, Sciamanna CN. Computerized tailored physical activity reports. A randomized controlled trial. Am J Prev Med 2010;39:148-56. 10.1016/j.amepre.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng EM, Cunningham WE, Towfighi A, et al. Efficacy of a chronic care-based intervention on secondary stroke prevention among vulnerable stroke survivors: a randomized controlled trial. Circ Cardiovasc Qual Outcomes 2018;11:e003228. 10.1161/CIRCOUTCOMES.116.003228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clapperton M, Maharaj RG, Motilal S, Babwah T. The effects of structured physician delivered counselling on physical activity levels in sedentary primary care clinic attendees: A single blinded randomized controlled trial. West Indian Med J 2020;68:44. [Google Scholar]
- 27. Driehuis F, Barte JC, Ter Bogt NC, et al. Maintenance of lifestyle changes: 3-year results of the Groningen Overweight and Lifestyle study. Patient Educ Couns 2012;88:249-55. 10.1016/j.pec.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 28. Dubbert PM, Morey MC, Kirchner KA, Meydrech EF, Grothe K. Counseling for home-based walking and strength exercise in older primary care patients. Arch Intern Med 2008;168:979-86. 10.1001/archinte.168.9.979 [DOI] [PubMed] [Google Scholar]
- 29. Duijzer G, Haveman-Nies A, Jansen SC, et al. Effect and maintenance of the SLIMMER diabetes prevention lifestyle intervention in Dutch primary healthcare: a randomised controlled trial. Nutr Diabetes 2017;7:e268. 10.1038/nutd.2017.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dutton GR. Effects of a primary care weight management intervention on physical activity in low-income African American women. Diss Abstr Int Sect B Sci Eng 2006;66:3946. [Google Scholar]
- 31. Elley CR, Kerse N, Arroll B, Robinson E. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ 2003;326:793-8. 10.1136/bmj.326.7393.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fortier MS, Hogg W, O’Sullivan TL, et al. Impact of integrating a physical activity counsellor into the primary health care team: physical activity and health outcomes of the Physical Activity Counselling randomized controlled trial. Appl Physiol Nutr Metab 2011;36:503-14. 10.1139/h11-040 [DOI] [PubMed] [Google Scholar]
- 33. Garcia-Ortiz L, Recio-Rodriguez JI, Agudo-Conde C, et al. EVIDENT Investigators Group. Mobilizing Minds Research Group . Long-term effectiveness of a smartphone app for improving healthy lifestyles in general population in primary care: Randomized controlled trial (Evident II study). JMIR Mhealth Uhealth 2018;6:e107. 10.2196/mhealth.9218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldstein MG, Pinto BM, Marcus BH, et al. Physician-based physical activity counseling for middle-aged and older adults: a randomized trial. Ann Behav Med 1999;21:40-7. 10.1007/BF02895032 [DOI] [PubMed] [Google Scholar]
- 35. Gomez-Huelgas R, Jansen-Chaparro S, Baca-Osorio AJ, Mancera-Romero J, Tinahones FJ, Bernal-López MR. Effects of a long-term lifestyle intervention program with Mediterranean diet and exercise for the management of patients with metabolic syndrome in a primary care setting. Eur J Intern Med 2015;26:317-23. 10.1016/j.ejim.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 36. Grandes G, Sanchez A, Montoya I, Ortega Sanchez-Pinilla R, Torcal J, PEPAF Group . Two-year longitudinal analysis of a cluster randomized trial of physical activity promotion by general practitioners. PLoS One 2011;6:e18363. 10.1371/journal.pone.0018363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hall KS, Sloane R, Pieper CF, et al. Long-term changes in physical activity following a one-year home-based physical activity counseling program in older adults with multiple morbidities. J Aging Res 2010;2011:308407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morey MC, Peterson MJ, Pieper CF, et al. The Veterans Learning to Improve Fitness and Function in Elders Study: a randomized trial of primary care-based physical activity counseling for older men. J Am Geriatr Soc 2009;57:1166-74. 10.1111/j.1532-5415.2009.02301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harari D, Iliffe S, Kharicha K, et al. Promotion of health in older people: a randomised controlled trial of health risk appraisal in British general practice. Age Ageing 2008;37:565-71. 10.1093/ageing/afn150 [DOI] [PubMed] [Google Scholar]
- 40. Hardeman W, Mitchell J, Pears S, et al. VBI Research Team . Evaluation of a very brief pedometer-based physical activity intervention delivered in NHS Health Checks in England: The VBI randomised controlled trial. PLoS Med 2020;17:e1003046. 10.1371/journal.pmed.1003046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harris MF, Fanaian M, Jayasinghe UW, et al. A cluster randomised controlled trial of vascular risk factor management in general practice. Med J Aust 2012;197:387-93. 10.5694/mja12.10313 [DOI] [PubMed] [Google Scholar]
- 42. Harris T, Kerry SM, Limb ES, et al. Physical activity levels in adults and older adults 3-4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in UK primary care. PLoS Med 2018;15:e1002526. 10.1371/journal.pmed.1002526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hellgren MI, Jansson P-A, Lindblad U. Report from an effort to prevent type 2 diabetes development in primary care. Prim Care Diabetes 2021;15:240-4. 10.1016/j.pcd.2020.08.019 [DOI] [PubMed] [Google Scholar]
- 44. Hesselink AE, Bilo HJG, Rutten GEH, et al. Effectiveness of an intervention in primary care to prevent type 2 diabetes in people with impaired fasting glucose, a cluster-randomised controlled trial. Diabetologia 2013;56:S165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huebschmann AG, Leavitt IM, Glasgow R, Regensteiner JG, Dunn AL. A reimbursable counseling intervention improves physical activity and function for primary care patients with type 2 diabetes. Diabetes 2018;67:879P 10.2337/db18-879-P. [DOI] [Google Scholar]
- 46. Jimmy G, Martin BW. Implementation and effectiveness of a primary care based physical activity counselling scheme. Patient Educ Couns 2005;56:323-31. 10.1016/j.pec.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 47. Jolly K, Sidhu M, Hewitt C, et al. Telephone health coaching in primary care patients with MRC I/II COPD: randomised controlled trial. Eur Respir Journal Conf Eur Respir Soc Int Congr ERS 2017;50:OA2914. 15721975 [Google Scholar]
- 48. Kloek CJJ, Bossen D, Spreeuwenberg PM, Dekker J, de Bakker DH, Veenhof C. Effectiveness of a blended physical therapist intervention in people with hip osteoarthritis, knee osteoarthritis, or both: a cluster-randomized controlled trial. Phys Ther 2018;98:560-70. 10.1093/ptj/pzy045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lawton BA, Rose SB, Elley CR, Dowell AC, Fenton A, Moyes SA. Exercise on prescription for women aged 40-74 recruited through primary care: two year randomised controlled trial. BMJ 2008;337:a2509. 10.1136/bmj.a2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Migneault JP, Dedier JJ, Wright JA, et al. A culturally adapted telecommunication system to improve physical activity, diet quality, and medication adherence among hypertensive African-Americans: a randomized controlled trial. Ann Behav Med 2012;43:62-73. 10.1007/s12160-011-9319-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mitchell KE, Warrington V, Sewell L, et al. A randomised controlled trial of a self-management programme of activity coping and education-space for COPD: impact on physical activity at 6 weeks. Am J Respir Crit Care Med 2013;187:A5952. [Google Scholar]
- 52. Gamboa Moreno E, Mateo-Abad M, Ochoa de Retana García L, et al. Osakidetza Active Patient Research Group . Efficacy of a self-management education programme on patients with type 2 diabetes in primary care: a randomised controlled trial. Prim Care Diabetes 2019;13:122-33. 10.1016/j.pcd.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 53. Morey MC, Pieper CF, Edelman DE, et al. Enhanced fitness: a randomized controlled trial of the effects of home-based physical activity counseling on glycemic control in older adults with prediabetes mellitus. J Am Geriatr Soc 2012;60:1655-62. 10.1111/j.1532-5415.2012.04119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pears S, Bijker M, Morton K, et al. VBI Programme Team . A randomised controlled trial of three very brief interventions for physical activity in primary care. BMC Public Health 2016;16:1033. 10.1186/s12889-016-3684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pinto BM, Friedman R, Marcus BH, Kelley H, Tennstedt S, Gillman MW. Effects of a computer-based, telephone-counseling system on physical activity. Am J Prev Med 2002;23:113-20. 10.1016/S0749-3797(02)00441-5 [DOI] [PubMed] [Google Scholar]
- 56. Pinto BM, Goldstein MG, Ashba J, Sciamanna CN, Jette A. Randomized controlled trial of physical activity counseling for older primary care patients. Am J Prev Med 2005;29:247-55. 10.1016/j.amepre.2005.06.016 [DOI] [PubMed] [Google Scholar]
- 57. Reed J, Malvern L, Muthukrishnan S, Hardy R, King L. An ecological approach with primary-care counseling to promote physical activity. J Phys Act Health 2008;5:169-83. 10.1123/jpah.5.1.169 [DOI] [PubMed] [Google Scholar]
- 58.Richardson AH. A comparison of physician based exercise counseling protocols: a pilot study. Thesis, University of South Carolina. 2007. https://dissexpress.proquest.com/dxweb/results.html?QryTxt=&By=&Title=&pubnum=3280353
- 59. Schillinger D, Handley M, Wang F, Hammer H. Effects of self-management support on structure, process, and outcomes among vulnerable patients with diabetes: a three-arm practical clinical trial. Diabetes Care 2009;32:559-66. 10.2337/dc08-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Steptoe A, Doherty S, Rink E, Kerry S, Kendrick T, Hilton S. Behavioural counselling in general practice for the promotion of healthy behaviour among adults at increased risk of coronary heart disease: randomised trial. BMJ 1999;319:943-8. 10.1136/bmj.319.7215.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taheri S, Zaghloul H, Chagoury O, et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): an open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2020;8:477-89. 10.1016/S2213-8587(20)30117-0 [DOI] [PubMed] [Google Scholar]
- 62. Tiessen A, Smit A, Broer J, Groenier K, van der Meer K. Randomized controlled trial on cardiovascular risk management by practice nurses supported by self-monitoring in primary care. Agora Enferm 2013;17:82-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Valve P, Lehtinen-Jacks S, Eriksson T, et al. LINDA - a solution-focused low-intensity intervention aimed at improving health behaviors of young females: a cluster-randomized controlled trial. BMC Public Health 2013;13:1044. 10.1186/1471-2458-13-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van der Weegen S, Verwey R, Spreeuwenberg M, Tange H, van der Weijden T, de Witte L. It’s LiFe! Mobile and web-based monitoring and feedback tool embedded in primary care increases physical activity: a cluster randomized controlled trial. J Med Internet Res 2015;17:e184. 10.2196/jmir.4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van Sluijs EMF, van Poppel MNM, Twisk JWR, Chin A Paw MJ, Calfas KJ, van Mechelen W. Effect of a tailored physical activity intervention delivered in general practice settings: results of a randomized controlled trial. Am J Public Health 2005;95:1825-31. 10.2105/AJPH.2004.044537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vermunt PWA, Milder IEJ, Wielaard F, et al. A lifestyle intervention to reduce type 2 diabetes risk in Dutch primary care: 2.5-year results of a randomized controlled trial. Diabet Med 2012;29:e223-31. 10.1111/j.1464-5491.2012.03648.x [DOI] [PubMed] [Google Scholar]
- 67. Volger S, Wadden TA, Sarwer DB, et al. POWER-UP Research Group . Changes in eating, physical activity and related behaviors in a primary care-based weight loss intervention. Int J Obes (Lond) 2013;37(Suppl 1):S12-8. 10.1038/ijo.2013.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Westland H, Schuurmans MJ, Bos-Touwen ID, et al. Effectiveness of the nurse-led Activate intervention in patients at risk of cardiovascular disease in primary care: a cluster-randomised controlled trial. Eur J Cardiovasc Nurs 2020;19:721-31. 10.1177/1474515120919547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Blair S, Dunn A, Gibbons L, et al. Writing Group for the Activity Counseling Trial Research Group . Effects of physical activity counseling in primary care: the Activity Counseling Trial: a randomized controlled trial. JAMA 2001;286:677-87. 10.1001/jama.286.6.677 [DOI] [PubMed] [Google Scholar]
- 70. Yates T, Edwardson CL, Henson J, et al. Walking away from type 2 diabetes: a cluster randomized controlled trial. Diabet Med 2017;34:698-707. 10.1111/dme.13254 [DOI] [PubMed] [Google Scholar]
- 71.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. bmj 2019; 366: l4898. [DOI] [PubMed]
- 72.The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.4. 2020.
- 73. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol 2017;32:541-56. 10.1097/HCO.0000000000000437 [DOI] [PubMed] [Google Scholar]
- 74. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol 2018;33:811-29. 10.1007/s10654-018-0380-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jakicic JM, Powell KE, Campbell WW, et al. 2018 Physical Activity Guidelines Advisory Committee . Physical activity and the prevention of weight gain in adults: a systematic review. Med Sci Sports Exerc 2019;51:1262-9. 10.1249/MSS.0000000000001938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kaiser KA, Affuso O, Beasley TM, Allison DB. Getting carried away: a note showing baseline observation carried forward (BOCF) results can be calculated from published complete-cases results. Int J Obes (Lond) 2012;36:886-9. 10.1038/ijo.2011.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 78. Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract 2008;80:371-9. 10.1016/j.diabres.2008.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Selvaraj FJ, Mohamed M, Omar K, et al. DISSEMINATE study group . The impact of a disease management program (COACH) on the attainment of better cardiovascular risk control in dyslipidaemic patients at primary care centres (The DISSEMINATE Study): a randomised controlled trial. BMC Fam Pract 2012;13:97. 10.1186/1471-2296-13-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015;175:959-67. 10.1001/jamainternmed.2015.0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 2019; 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 2011;8:115. 10.1186/1479-5868-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dowd KP, Szeklicki R, Minetto MA, et al. A systematic literature review of reviews on techniques for physical activity measurement in adults: a DEDIPAC study. Int J Behav Nutr Phys Act 2018;15:15. 10.1186/s12966-017-0636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med 2017;47:1821-45. 10.1007/s40279-017-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lamming L, Pears S, Mason D, et al. VBI Programme Team . What do we know about brief interventions for physical activity that could be delivered in primary care consultations? A systematic review of reviews. Prev Med 2017;99:152-63. 10.1016/j.ypmed.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 86.2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee scientific report. US Department of Health and Human Services. 2018. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
- 87. Chatterjee R, Chapman T, Brannan MG, Varney J. GPs’ knowledge, use, and confidence in national physical activity and health guidelines and tools: a questionnaire-based survey of general practice in England. Br J Gen Pract 2017;67:e668-75. 10.3399/bjgp17X692513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hutfless S, Maruthur NM, Wilson RF, et al. Strategies to prevent weight gain among adults comparative effectiveness review. Rockville, MD., 2013 https://www.ncbi.nlm.nih.gov/books/NBK133218/. [DOI] [PMC free article] [PubMed]
- 89. Whitlock G, Lewington S, Sherliker P, et al. Prospective Studies Collaboration . Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083-96. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.