Abstract
Background
Literature regarding exosomes as mediators in intercellular communication to promote progression in mycosis fungoides (MF) is lacking.
Objectives
To characterize MF-derived exosomes and their involvement in the disease.
Methods
Exosomes were isolated by ultracentrifugation from cutaneous T-cell lymphoma (CTCL) cell lines, and from plasma of patients with MF and controls (healthy individuals). Exosomes were confirmed by electron microscopy, Nano-Sight and CD81 staining. Cell-line exosomes were profiled for microRNA array. Exosomal microRNA (exomiRNA) expression and uptake, and plasma-cell-free microRNA (cfmiRNA) were analysed by reverse-transcriptase quantitative polymerase chain reaction. Exosome uptake was monitored by fluorescent labelling and CD81 immunostaining. Migration was analysed by transwell migration assay. Results MyLa- and MJ-derived exosomes had a distinctive microRNA signature with abundant microRNA (miR)-155 and miR-1246. Both microRNAs were delivered into target cells, but only exomiR-155 was tested, demonstrating a migratory effect on target cells. Plasma levels of cfmiR-1246 were significantly highest in combined plaque/tumour MF, followed by patch MF, and were lowest in controls (plaque/tumour > patch > healthy), while cfmiR-155 was upregulated only in plaque/tumour MF vs. controls. Specifically, exomiR-1246 (and not exomiR-155) was higher in plasma of plaque/tumour MF than in healthy controls. Plasma exosomes from MF but not from controls increased cell migration.
Conclusions
Our findings show that MF-derived exosomes promote cell motility and are enriched with miR-155, a well-known microRNA in MF, and miR-1246, not previously reported in MF. Based on their plasma expression we suggest that they may serve as potential biomarkers for tumour burden.
Mycosis fungoides (MF), the most common type of primary cutaneous T-cell lymphoma (CTCL), is typified by a protracted cutaneous clinical course with slow progression from slightly scaly flat skin lesions (patches) to elevated or infiltrated lesions (plaques) and eventually to tumours with the potential to spread systemically. Although both patches and plaques represent lesions of early-stage MF, plaques confer a higher risk of disease progression and are associated with worse disease-specific survival.1 Sézary syndrome (SS) is an aggressive, erythrodermic and leukaemic variant of CTCL with poor prognosis.2,3
Exosomes are a subtype of extracellular vesicles (EV) and are composed of a lipid bilayer with a diameter of 20–150 nm.4 The role of exosomes is to carry and transmit biological information from donor cells to proximal and distant sites, by releasing their lipid, protein and nucleic acid cargo into the cytoplasm of recipient cells.5–7
Cancer cells release a significantly higher total number of EVs, particularly exosomes, than normal cells.8 They use exosomal microRNAs (exomiRNAs) to shape local and distant regions to promote tumour progression, by regulating vascularization, stroma cells and matrix, immune response, immune escape, chemoresistance and metastasis.8–10 Both disease presence and progression have been associated with an increase in exosome release and their molecular content.8 In haematological neoplasms, such as acute and chronic myeloid leukaemia, chronic lymphocytic leukaemia and multiple myeloma,11,12 malignant cells secrete exosomes for tumorigenesis, maintain tumour persistence by shaping the tumour niche, modify anti-cancer immunity, and evade destruction by chemotherapy.
Cell-free microRNAs (cfmiRNAs), including circulating free microRNAs (miRNAs) and miRNAs incorporated into EVs such as exosomes, are epigenetic modifiers, and their detection can serve as potential diagnostic, predictive and prognostic biomarkers in cancer.13–18 While the miRNA profile of tissue samples of CTCL has been widely investigated,19 the literature regarding cfmiRNAs in CTCL is strikingly sparse.20
The aims of the present study were as follows. Firstly, to detect the presence of exosomes in MF and CTCL and to characterize their miRNA content, and their biological significance and cancerous roles in vitro and ex vivo. Secondly, to investigate whether selected exomiRNAs isolated from MF and CTCL cell lines can be detected in plasma of patients with MF as exosomes and cfmiRNAs, and whether they reflect tumour burden.
Materials and methods
Cell culture
Three CTCL cell lines were used: HH (aggressive CTCL); Hut78 (SS) and MyLa (MF). In addition, MJ was also used. This is listed by the American Tissue Culture Collection as an MF cell line that harbours human T-lymphotropic virus (HTLV) (Appendix S1; see Supporting Information). MJ is considered by some authors as an MF-like adult T-cell leukaemia/lymphoma cell line, based on a recent study that demonstrated genetic differences between HTLV-1+ and HTLV-1− CTCL cell lines, although many similarities were found with respect to gene expression profiling and ability to produce tumours in mice.21 MJ previously transduced with a lentiviral plasmid expressing anti-miR-155 was also used.22
Isolation of normal peripheral blood mononuclear cells
This process is detailed in Appendix S2 (see Supporting Information).
Plasma samples
Blood samples of patients with pretreated MF and controls were collected in K3EDTA tubes under the local Helsinki protocol of the Rabin Medical Center, Israel, and City of Hope, USA. All patients were staged according to Olsen et al.23 (Table S1; see Supporting Information). Samples were centrifuged at 1000 g for 10 min at 4 °C and then at 2000 g for 10 min.
Exosome isolation
Exosomes were isolated by differential centrifugation and ultracentrifugation at 4 °C. In brief, 300 mL of cell-line supernatant and 3 mL of human plasma were centrifuged with serial centrifugation. A process of 1300 g for 5 min followed by 2000 g for 10 min was done only for cell-line supernatant. Cell-line supernatant and plasma were then centrifuged as follows: 10 000 g for 30 min, filtration through a 0·22-μm filter, then 110 000 g for 90 min. The exosome pellet was then suspended and centrifuged again at 110 000 g for 90 min. The supernatant was discarded from the exosome pellet, and the pellet was resuspended with the remaining volume of left-over phosphate-buffered saline (PBS).
Transmission electron microscopy
Exosome samples were adsorbed on Formvar carbon-coated grids and stained with 2% aqueous uranyl acetate, and then analysed with a JEM-1400Plus transmission electron microscope (JEOL Ltd, Tokyo, Japan).
Nanoparticle tracking analysis
Exosome samples were diluted in PBS and analysed by a NanoSight LM10 HS system with a tuned 405-nm laser (NanoSight Ltd, Amesbury, UK).
Exosome uptake for tracking exosome internalization
Samples of 20 μL of exosomes were mixed with 1·5 mL of Diluent C and 4 μL of the red membrane dye PKH26 (Sigma-Aldrich, St Louis, MO, USA) and incubated for 10 min. The labelled exosomes were washed twice with PBS at 100 000 g; PBS from the top of the washed labelled exosome tube was used as the control. Thereafter, 5 × 105 normal peripheral blood mononuclear cells (NPBMCs) or MyLa cells were incubated with labelled exosomes (15 μL) for 24 h. Target cells that internalized the exosomes were analysed by two methods. The first method was fluorescence microscopy. Cells were centrifuged with the Shandon Cytospin® cytocentrifuge (Thermo Fisher Scientific Inc., Waltham, MA, USA), fixed with 4% paraformaldehyde, washed with PBS, and covered with a mounting medium containing 4′,6-diamidino-2-phenylindole (BioLegend, San Diego, CA, USA). Images were taken with an Axio Imager Z2 microscope (magnitude × 40; Zeiss, Jena, Germany). The second method was fluorescence-activated cell sorting (FACS). Cells were washed with PBS and analysed for PKH26-positive cells by FACS (Gallios™; Beckman Coulter, Brea, CA, USA).
RNA purification
RNA was isolated from cell lines with TRIzol (Thermo Fisher), from cell-line-derived exosomes with the Total Exosome RNA and Protein Isolation Kit (Thermo Fisher), and from plasma samples or plasma exosomes with the miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany). During RNA purification from plasma samples, 3·5 μL of Spike-In lyophilized Caenorhabditis elegans miR-39 (Qiagen) was added.
MicroRNA array
All experiments were performed using the Affymetrix miRNA 4·0 array (Thermo Fisher).24
Reverse-transcriptase quantitative polymerase chain reaction
A 5-ng sample of total RNA was used for reverse cDNA reaction with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). For reverse-transcriptase quantitative polymerase chain reaction (RT-qPCR), we used the TaqMan Fast Advanced Master Mix on a StepOnePlus™ Real-Time PCR System (both Applied Biosystems). Primers and reference miRNAs for normalization are detailed in Appendix S3 (see Supporting Information).
Migration assay
Cell migration was evaluated using a transwell chamber assay (Thermo Fisher). A sample of 100 μL of serum-free medium containing 2 × 104 cells with or without exosomes (20 μL) was plated for 24 h in the top chamber of a transwell with 8-μm-diameter pores; 600 μL of medium was placed in the lower chamber + 10% serum. Insert staining and migration analysis were done as previously described.25 The percentage of migrated cells was calculated as:
Flow cytometry analysis for CD81
Latex FACS beads (20 μL) were incubated with exosomes (50 μL) for 15 min at room temperature and then rotated with PBS overnight at 4 °C. Next, the exosome–beads complex was centrifuged for 5 min at 4500 g at 4 °C, the supernatant was discarded, and 200 μL of 100-mmol L−1 glycine was added for 15 min at 4 °C followed by vortexing five or six times and incubation for another 15 min at room temperature. The beads were centrifuged and the supernatant was discarded. CD81-allophycocyanin (APC) (1 : 50; Milteny Biotec, Bergisch, Germany) or APC IGg-1 isotype (1 : 100; Milteny Biotec) was added and incubated for 12 min at 4 °C. The beads were washed with washing buffer, resuspended in sample buffer and analysed by FACS (Gallios; Beckman Coulter).
Data analysis
All experiments were performed in triplicate, and data were analysed with GraphPad Prism 8 (GraphPad Inc., La Jolla, CA, USA). The significance of the differential effects among the groups was determined with two-tailed Student’s t-test. The results of the microscopy-based migration assay were analysed using ImageJ (National Institutes of Health, Bethesda, MD, USA). The variance was similar in the compared groups. The fold change in expression level of specific miRNAs in the study samples compared with the reference group was calculated as described previously.24 The nonparametric unpaired Mann–Whitney test was used for the comparisons. Significant differences were recorded as *0·01 < P < 0·05, **0·005 < P < 0·01, ***P < 0·005. Linear correlation was based on the calculated Pearson correlation coefficient.
Results
Cutaneous T-cell lymphoma cell lines secrete exosomes
Four CTCL cell lines were cultured for 5 days with serum-free medium, and exosomes were isolated from the conditioned medium by ultracentrifugation. Transmission electron microscopy verified the typical cup shape and small size (50–150 nm) of exosomes (Figure 1a), and nanoparticle tracking analysis showed a peak diameter of 106–143 nm (Figure 1b). FACS analysis of CTCL cell-line exosomes revealed high positivity for the exosomal marker CD81 (Figure 1c), indicating that the isolated EVs were exosome enriched.
Figure 1.


Isolation and characterization of exosomes released by cutaneous T-cell lymphoma (CTCL) cell lines. Exosomes were isolated from conditioned media of four CTCL cell lines (total ~1·3 × 108 cells) by differential centrifugation including ultracentrifugation and were analysed as follows. (a) Electron microscopy study showing typical cup-shape morphology and small size of exosomes. (b) Nanoparticle tracking analysis showing size distribution and concentration of exosomes for samples diluted 1 : 100, except for MyLa exosomes at 1 : 50, as follows: HH, 7 × 106; MJ, 5·2 × 107; Hut78, 7·2 × 106; MyLa, 4·7 × 106 particles isolated from ~320 mL cell supernatant. (c) Conjugation of exosomes to latex beads followed by fluorescence-activated cell sorting analysis for CD81 (an exosome marker), showing the rate of CD81-positive particles higher than 80%. APC, allophycocyanin.
Mycosis fungoides cell-line exosomes carry high levels of miR-155 and miR-1246 and deliver them to recipient cells
Affymetrix microRNA array demonstrated that the MyLa and MJ exosomes contained abundant miRNA expression with an overlapping signature, whereas exosomes derived from the SS cell line (Hut 78) and the aggressive CTCL cell line (HH) contain low miRNA content (Figure 2a). In total 56 miRNAs had signal intensity ≥ 15 in at least one of the exosome cell groups (Table S2; see Supporting Information). Comparing the expression of the 56 miRNAs among exosomes from CTCL cell lines revealed an expanded miRNA repertoire in the MF exosomes from MyLa (45) and MJ (48), and a low miRNA repertoire in non-MF exosomes (25 in Hut78 and 27 in HH) (Figure S1; see Supporting Information).
Figure 2.

MicroRNA profile of cutaneous T-cell lymphoma (CTCL) cell-line-derived exosomes and delivery of highly expressed exosomal microRNAs. (a) Hierarchical clustering analysis of differentially expressing microRNAs among CTCL cell-line-derived exosomes using Affymetrix microRNA microarray. (b) Reverse-transcriptase quantitative polymerase chain reaction (RT-qPCR) analysis of differential expression of miR-155, miR-1246 and nonrelevant miR-199 normalized to U6 in exosomes of CTCL cell lines, presented as relative quantification (RQ), where MJ exosomal microRNA has RQ = 1. (c–e) RT-qPCR analysis of miR-155 and miR-1246 expression in MyLa, Hut78 and HH cell lines with vs. without the addition of MJ exosomes (24 h) (c); and in normal peripheral blood mononuclear cells (NPBMCs) with and without addition of MyLa and MJ exosomes (24 h) for miR-155 (d) and for miR-1246 (e) normalized to U6, presented as the RQ, where cells without exosomes have RQ = 1.
Exosomes from MyLa and MJ had the highest number of shared miRNAs (n = 39), indicating their molecular similarities. The highest expression was found for miR-1246 and miR-155 in all four cell-line exosomes, with both known as exosomal oncomiRs in haematological and solid neoplasms.9,26 RT-qPCR analysis yielded the highest expression in MJ exosomes compared with MyLa and other CTCL cell-line exosomes (Figure 2b). A nonrelevant miRNA, miR-199, used as the negative control, showed a different expression pattern from the relevant miRNAs (Figure 2b).
To determine whether exomiR-155 and exomiR-1246 are taken up by various types of cells, we incubated exosomes isolated from MJ cells with other CTCL cell lines for 24 h. RT-qPCR revealed a significant increase in intracellular miR-155 and miR-1246 in all CTCL cell lines compared with cells without exosomes (Figure 2c).
As MyLa and MJ cells were derived from patients sharing similar clinical features of MF, and given the overlapping molecular landscape of both cell lines,21 we further concentrated on these two cell lines. NPBMCs were incubated with MyLa or MJ exosomes for 24 h and then analysed for exomiR-155 and exomiR-1246 uptake by RT-qPCR. The NPBMCs displayed upregulation of intracellular miR-155 (Figure 2d) and miR-1246 (Figure 2e), indicating that MF cell-line exosomes carry miR-155 and miR-1246 and deliver them to recipient cells.
Exosomes of mycosis fungoides cell lines incorporate into recipient cells and increase their migration through exomiR-155
As cancer-derived exosomes and specifically exomiR-15527,28 and exomiR-124629,30 promote the migration capacity of target cells as oncogenic mediators, we sought to check whether MF cell-line exosomes also increase the motility of recipient cells. Firstly, we tracked the uptake of MF exosomes into target cells by labelling MyLa and MJ exosomes with red fluorescent PKH26, and added them to NPBMCs for 24 h. PKH26 was detected by FACS in NPBMCs with MyLa and MJ exosomes (Figure 3a). Red fluorescent exosomes were seen as foci within the cytoplasm of the target cells incubated with MyLa or MJ exosomes, but not in NPBMCs without exosomes (Figure 3b). Secondly, we analysed the exosome migration effect by transwell migration assay. MyLa and MJ exosomes were incubated with NPBMCs for 24 h, and the number of migrated cells was compared with the results from cells without exosomes. An increase of 32% was seen following incubation with MJ exosomes (P = 0·0057) (Figure 4a), and an increase of 59% following incubation with MyLa exosomes (P < 0·001) (Figure 4b).
Figure 3.
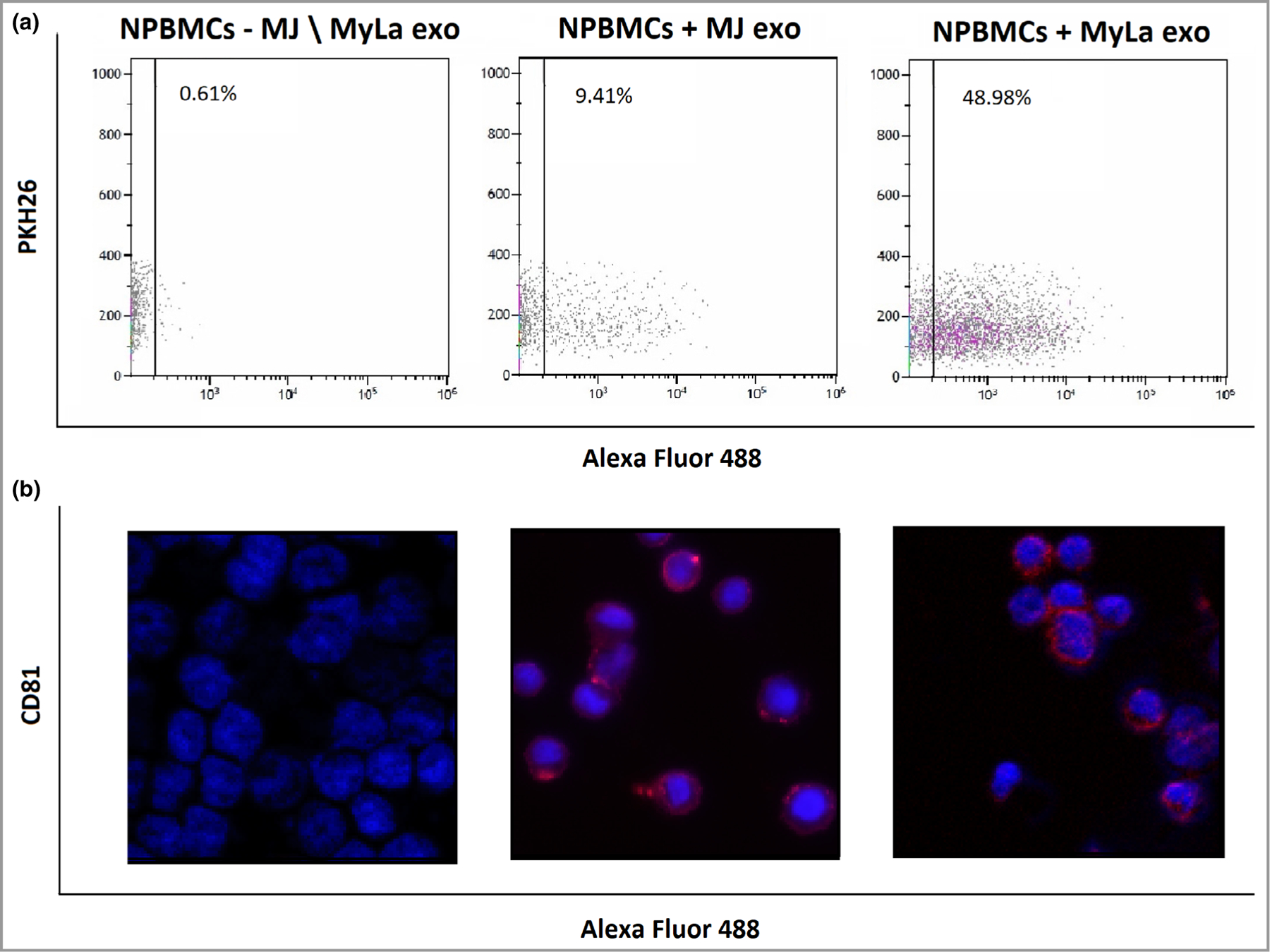
Uptake of mycosis fungoides cell line exosomes into NPBMCs. Fluorescence-activated cell sorting for PKH26 in normal peripheral blood mononuclear cells (NPBMCs) with PKH26-labelled MJ or MyLa exosomes (a) and immunostaining of CD81 in NPBMCs with unlabelled exosomes (b).
Figure 4.

The effect of mycosis fungoides exosomes on cell motility. Transwell migration assay showing migration enhancement of normal peripheral blood mononuclear cells (NPBMCs) with MJ (a) or MyLa exosomes (b) vs. without. **P < 0·01, ***P < 0·005.
To understand whether MF exosomes mediate the migration of other MF cells as part of intercellular communication in tumour heterogeneity, we analysed the migration effect of MJ exosomes on MyLa cells. The internalization of MJ exosomes into MyLa cells was detected by CD81 immunostaining and PKH26 (Figure 5). Both CD81 and PKH26 staining in MyLa cells incubated with MJ exosomes showed a cytoplasmic dot staining pattern typical in intracellular vesicle organization. The number of migrated MyLa cells increased by 49% after incubation with MJ exosomes (P < 0·001) (Figure 6a).
Figure 5.

Uptake of MJ exosomes into MyLa cells. Immunostaining of CD81 in MyLa cells with MJ exosomes. Fluorescence microscopy and fluorescence-activated cell sorting for PKH26 in MyLa cells with or without MJ-labelled exosomes.
Figure 6.

The effect of MJ exosomes on the motility of MyLa cells through exosomal miR-155. Transwell migration assay showing migration enhancement of MyLa cells with vs. without MJ exosomes (a) and reduction in migration of MyLa cells with exosomes from MJ cells transduced with anti-miR-155 vs. without exosomes (b). (c) miR-155 expression in the exosomes used in (b). RQ, relative quantification. *P < 0·05, ***P < 0·005.
Next we evaluated the effect of specifically miR-155-containing exosomes on cell migration. On incubation of MyLa cells with exosomes from MJ cells previously transduced with anti-miR-155,22 there was no increase but actually a reduction in MyLa cell migration, by 27·7% (P = 0·015) (Figure 6b). RT-qPCR analysis confirmed the low level of miR-155 in exosomes of the transduced MJ cells compared with exosomes of original MJ cells, with a fold change of −3 (Figure 6c). Taken together, these data suggest that miR-155-containing exosomes may play a tumour-supporting role by enhancing cell migration of malignant cells in MF.
Exosomes from plasma of patients with mycosis fungoides increased the migration of normal peripheral blood mononuclear cells
To determine whether plasma exosomes of patients with MF regulate the migration of target cells, similarly to exosomes of MF cell lines, we isolated exosomes from plasma samples of patients with active patch MF (n = 4) and controls (n = 4). Based on nanoparticle tracking analysis, their particle diameter was about 90 nm (Figure S2; see Supporting Information). FACS analysis of plasma exosomes showed that they were highly positive for CD81 (97–100%), indicating that the isolated plasma EVs are exosome enriched (Figure S3; see Supporting Information).
Exosome uptake was tracked by incubating NPBMCs with plasma exosomes from patients with patch MF and controls for 24 h. Immunostaining showed cytoplasmic foci of CD81, indicating uptake of exosomes from both sources (Figure 7a). NPBMCs incubated with plasma exosomes from patients with patch MF and controls were evaluated. The percentage of migrated NPBMCs with plasma MF exosomes was significantly higher (by 22·8%) than the percentage of migrated NPBMCs with control exosomes (P = 0·0074) (Figure 7b). No difference was detected in exosome uptake between healthy controls and patients with MF (Figure 7a). Thus, the difference in the migration effect was not due to differences in exosome uptake, but rather to biological differences in exosome content between patients with patch MF and controls.
Figure 7.

Exosomes from plasma of patients with mycosis fungoides (MF) and healthy controls: exosome internalization and exosome-mediated migration. Exosomes from plasma of patients with early patch MF (n = 4) and healthy controls (n = 4) were added to normal peripheral blood mononuclear cells (NPBMCs) for 24 h. (a) CD81 immunostaining showing internalization of exosomes into NPBMCs. (b) Transwell migration assay showing the effect of exosomes on cell migration. **P < 0·01.
cfmiR-1246 was upregulated in plasma of patients with mycosis fungoides (MF) in a stage-dependent manner, compared with controls, while cfmiR-155 was upregulated in plaque/tumour MF vs. patch MF or healthy controls
To determine whether expression of plasma cfmiR-155 and cfmiR-1246 is stage dependent in MF, we extracted RNA from plasma samples of patch MF (n = 10), plaque-stage MF (n = 9), tumour-stage MF (n = 8) and controls (n = 11) (clinical data are delineated in Table S1). The expression levels of cfmiR-155 and cfmiR-1246 were analysed by RT-qPCR and normalized to hsa-miR-16, a stably expressed endogenous reference miRNA, previously used for normalization of circulating cfmiR-155 and cfmiR-1246 in cancer31,32 (Appendix S3). The level of plasma cfmiR-1246 seemed to be highest in tumour-stage MF and lowest in controls in a decreasing fashion; however, only combining tumour and plaque type into one group in comparison with patch MF yielded statistically significant results: plaque/tumour > patch > healthy (Figure 8a, Table 1; and Figure S4a; see Supporting Information). cfmiR-155 was also upregulated in plaque/tumour MF vs. healthy controls or patch MF (Figure 8b, Table 1; and Figure S4b), with no difference between patch MF and healthy controls. Of the 17 patients with plaque or tumour MF, low blood involvement (B1) was detected in three patients based on FACS. Of the 10 patients with patch MF, B1 was detected in two patients (Table S1). No correlation was found between plasma cfmiR-1246 and cfmiR-155 levels and blood FACS results (R = −0·25 and R = −0·24, respectively). A relevant blood clone was detected in two of the seven patients with plaque or tumour MF who were tested. Blood genotyping analysis was not done in any of the patients with patch MF.
Figure 8.

The expression of cell free and exosomal miR-155 and miR-1246 in plasma of MF patients and healthy controls. Plasma samples of patch mycosis fungoides (MF) (n = 10), combined plaque/tumour MF (n = 17) and healthy controls (n = 11) were used for reverse-transcriptase quantitative polymerase chain reaction (RT-qPCR) to analyse the expression of (a) plasma cell-free microRNA (cfmiR)-155 and (b) cfmiR-1246 in RNA extracted directly from plasma; and (c) exosomal microRNA (exomiR)-1246 and (d) exomiR-155 in RNA extracted from plasma exosomes. RT-qPCR data were normalized to miR-16, presented as the relative quantification (RQ) where the value for healthy controls was set at RQ = 1. #No significant difference from healthy controls. *P < 0·05, **P < 0·01, ***P < 0·005 vs. healthy controls.
Table 1.
The relative quantification (RQ) of cell-free microRNA (cfmiR)-1246 and cfmiR-155 expression among plasma of patients with mycosis fungoides and controls
| microRNA | Comparison | RQ | P-value |
|---|---|---|---|
| cfmiR-1246 | Patch vs. healthy | 2·48 | 0·019 |
| cfmiR-1246 | Plaque/tumour vs. patch | 11·2 | 0·043 |
| cfmiR-1246 | Plaque/tumour vs. healthy | 27·7 | < 0·001 |
| cfmiR-155 | Plaque/tumour vs. healthy | 4·70 | 0·020 |
| cfmiR-155 | Plaque/tumour vs. patch | 5·02 | 0·018 |
The RT-qPCR data of plasma cfmiR-155 and cfmiR-1246 were normalized to cel-miR-39 (a commonly used spike-in miRNA for normalization of extracellular miRNAs),33,34 showing a similar trend of significant increase to the normalization to miR-16 (Figure S5a, b; see Supporting Information).
exomiR-1246 was upregulated in plasma of patients with plaque/tumour mycosis fungoides compared with controls, with no difference in exomiR-155
The expression level of exomiR-1246 was higher in patients with plaque/tumour MF than in healthy controls, with a fold change of 7·57 (P = 0·0018) (Figure 8c; and Figure S4c). There was no difference in exomiR-1246 between plasma of patch MF vs. healthy, or patch MF vs. plaque/tumour MF (Figure 8c; and Figure S4c). exomiR-155 was not differentially expressed among all the compared groups (Figure 8d). Normalization to cel-miR-39 showed a similar trend of increase in exomiR-1246 in plasma of plaque/tumour MF vs. healthy controls, but with borderline significance (P = 0·054) (Figure S5c, d).
Discussion
For the first time, our study has identified exosomes in MF and documented their relevance in this disease. We isolated exosomes and demonstrated that the most abundant miRNAs in MF cell-line exosomes are miR-155, a well-known miRNA in CTCL, and miR-1246, a new miRNA not previously reported in CTCL. Both miRNAs were delivered into target cells, but only exomiR-155 was tested, and demonstrated a migratory effect.
Thus, our finding of exosomes enriched in miR-155 and its significance in cell motility are in line with many other reports on the oncogenic role of cellular miR-155 in MF.22,35–43 Therefore, we may expect that MF cells expressing high levels of miR-155 secrete miR-155 in order to spread their cancerous phenotype by entering into recipient cells, and reducing the expression of miR-155 target tumour-suppressor genes.
miR-155 in cancer exosomes was found to promote migration, proliferation and chemoresistance in solid cancer27,44,45 and haematological cancer.28,46 Here, for the first time, miR-1246 was reported in MF, as it is a relatively newly discovered miRNA.47 Its cellular form plays an oncogenic role in T-cell acute lymphoblastic leukaemia48 and several solid tumours,49–53 and its extracellular form is tightly linked to cancer progression of acute myeloid leukaemia54 and some solid cancers.29,30,55
To investigate the in vivo relevance of exosomes in MF, we isolated exosomes from plasma of patients with MF and healthy controls. Similarly to the in vitro results, the level of miR-1246 in exosomes from plasma of patients with plaque/tumour MF was higher than in controls, but levels in patch MF were not higher, probably due to the overall low tumour burden. Exosomes of metastatic cancer cells enriched with miR-1246 were found to be delivered to nonmetastatic cancer cells or nonmalignant cells and to enhance their viability, migration and therapy resistance.29,30,55 Therefore, we assume that circulating exosomes and specifically exomiR-1246 in plasma of patients with MF might be involved in MF progression. Unlike in our in vitro findings, exomiR-155 was not upregulated in plasma of patients with MF vs. controls. A possible explanation for this contradiction is that exosomes of MF cell lines expressing high exomiR-155 resemble the skin tissue exosomes, which are different from the circulating plasma exosomes. Therefore there was no contribution of exomiR-155 to the migratory effect of exosomes from plasma of patients with patch MF vs. healthy controls demonstrated in our study. This suggests that other exosomal miRNAs or proteins mediate the migration effect.
Plasma samples of patients with plaque/tumour MF had high levels of cfmiR-155 and cfmiR-1246 relative to controls. None of the patients had leukaemic involvement (B2), with no correlation of our plasma findings to lower blood involvement (B0, B1). Therefore, we assume that the increased levels of these cfmiRNAs reflect the increased expression of those miRNAs in the cutaneous rather than circulating lymphoma cells. Thus, cfmiR-155 and cfmiR-1246 might be indicative markers for higher cutaneous tumour burden, with a further application as liquid biopsy. The aggressive course of tumour MF is well documented, and plaques have been found to be associated with decreased survival compared with patch MF.3,56 A previous study has already demonstrated that plasma cfmiR-155 was upregulated in MF and SS compared with patients with benign lesions, and shown reduction in its expression within response to treatment.20 A high level of miR-155 was also detected in plasma exosomes of patients with chronic lymphocytic leukaemia,46 and a high level of cfmiR-155 in plasma of patients with breast cancer vs. controls.57 Nevertheless, herein, cfmiR-1246 was upregulated in plasma of patients with MF in line with their lesion stage, suggesting its involvement and indication for tumour progression. High levels of cfmiR-1246 have also been reported in serum or plasma from patients with different types of solid tumours.33,34,57–59
Our study was limited by the small number of patients, which precluded comparison of the relevant miRNA levels between stage IA MF (body surface area < 10%) and stage IB (≥ 10%), and by the lack of blood genotyping analysis in the majority of our patients.
In summary, our in vitro study demonstrates the secretion and role of MF exosomes and specifically exomiR-155 and exomiR-1246. Our in vivo study provided evidence that high plasma levels of exomiR-1246, cfmiR-155 and cfmiR-1246 are all associated with more advanced lesions of MF and can serve as targets for novel treatments. They are also promising noninvasive biomarkers for MF.
Supplementary Material
Appendix S1 Cutaneous T-cell lymphoma cell lines.
Appendix S2 Peripheral blood mononuclear cells.
Appendix S3 Primers for microRNAs.
Figure S1 Venn diagram of shared microRNAs between exosomes derived from various cutaneous T-cell lymphoma cell lines.
Figure S2 Size distribution of exosomes isolated from plasma of patients with mycosis fungoides and healthy donors.
Figure S3 Expression of exosome marker CD81 on exosomes isolated from plasma of patients with mycosis fungoides and healthy donors.
Figure S4 Expression of cell-free and exosomal miR-155 and miR-1246 in plasma of patients with mycosis fungoides and healthy donors.
Figure S5 Expression of cell-free and exosomal miR-155 and miR-1246 in plasma of patients with mycosis fungoides and healthy donors.
Table S1 Characteristics of patients with mycosis fungoides.
Table S2 The miRNA content of cutaneous T-cell lymphoma cell-line-derived exosomes based on Affymetrix micro-RNA array 4.
What is already known about this topic?
Cancer-derived exosomes mediate tumour progression by delivering microRNAs such as miR-155 and miR-1246 into recipient cells, and their cell-free (cf) components are upregulated in plasma of patients with cancer.
What does this study add?
Mycosis fungoides (MF) cell-line exosomes carry miR-155 and miR-1246, deliver them to recipient cells (malignant and benign), and increase their migration through miR-155.
cfmiR-1246, cfmiR-155 and exosomal miR-1246 are upregulated in plasma of patients with MF, mainly in tumour/plaque-stage disease.
What is the translational message?
Extracellular miR-155 and miR-1246 may serve as targets for novel treatments and as promising noninvasive biomarkers for tumour burden in MF.
Funding sources
This study was supported by an International Collaboration Grant from the Jacki and Bruce Barron Cancer Research Scholars’ Program, a partnership of the ICRF and City of Hope, as supported by the Harvey L. Miller Family Foundation. C.Q. is supported by an NIH/NCI grant (R01 CA229510–01) and the Leukemia Lymphoma Society Clinical Scholar Award.
Footnotes
Conflicts of interest
C.Q. has been an advisory board and/or steering committee member for Miragen, Bioniz, Trillium, Kyowa Kirin and Helsinn, and has received a research grant from Celgene.
Data availability:
The authors encourage sharing of the data from this study.
References
- 1.Agar NS, Wedgeworth E, Crichton S et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol 2010; 28:4730–9. [DOI] [PubMed] [Google Scholar]
- 2.Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 2017; 92:1085–102. [DOI] [PubMed] [Google Scholar]
- 3.Trautinger F, Eder J, Assaf C et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Update 2017. Eur J Cancer 2017; 77:57–74. [DOI] [PubMed] [Google Scholar]
- 4.Dörsam B, Reiners KS, von Strandmann EP. Cancer-derived extracellular vesicles: friend and foe of tumour immunosurveillance. Biol Sci 2018; 373:20160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longjohn MN, Hudson BJ, Smith NC et al. Deciphering the messages carried by extracellular vesicles in hematological malignancies. Blood Rev 2021; 46:100734. [DOI] [PubMed] [Google Scholar]
- 6.Jeppesen DK, Fenix AM, Franklin JL et al. Reassessment of exosome composition. Cell 2019; 177:428–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valadi H, Ekström K, Bossios A et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654–9. [DOI] [PubMed] [Google Scholar]
- 8.Dilsiz N. Role of exosomes and exosomal microRNAs in cancer. Future Sci OA 2020; 6:FSO465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pontecorvi G, Bellenghi M, Puglisi R et al. Tumor-derived extracellular vesicles and microRNAs: functional roles, diagnostic, prognostic and therapeutic options. Cytokine Growth Factor Rev 2020; 51:75–83. [DOI] [PubMed] [Google Scholar]
- 10.Mills J, Capece M, Cocucci E et al. Cancer-derived extracellular vesicle-associated microRNAs in intercellular communication: one cell’s trash is another cell’s treasure. Int J Mol Sci 2019; 20:6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kok VC, Yu CC. Cancer-derived exosomes: their role in cancer biology and biomarker development. Int J Nanomedicine 2020; 15:8019–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharifi H, Shafiee A, Molavi G et al. Bringing oncogenic signals to blood cells. J Cell Biochem 2019; 120:16307–15. [DOI] [PubMed] [Google Scholar]
- 13.Goossens N, Nakagawa S, Sun X, Hoshida Y. Cancer biomarker discovery and validation. Transl Cancer Res 2015; 4:256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell PS, Parkin RK, Kroh EM et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105:10513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koga Y, Yasunaga M, Moriya Y et al. Exosome can prevent RNase from degrading microRNA in feces. J Gastrointest Oncol 2011; 2:215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neill CP, Gilligan KE, Dwyer RM. Role of extracellular vesicles (EVs) in cell stress response and resistance to cancer therapy. Cancers (Basel) 2019; 11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Z, Zhang D, Lee H et al. Macrophage-derived apoptotic bodies promote the proliferation of the recipient cells via shuttling microRNA-221/222. J Leukoc Biol 2017; 101:1349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Zhang D, Zhu Z et al. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep 2016; 12:35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gluud M, Willerslev-Olsen A, Gjerdrum LMR et al. MicroRNAs in the pathogenesis, diagnosis, prognosis and targeted treatment of cutaneous T-cell lymphomas. Cancers (Basel) 2020; 12:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dusílková N, Bašová P, Polívka J et al. Plasma miR-155, miR-203, and miR-205 are biomarkers for monitoring of primary cutaneous T-cell lymphomas. Int J Mol Sci 2017; 18:2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Netchiporouk E, Gantchev J, Tsang M et al. Analysis of CTCL cell lines reveals important differences between mycosis fungoides/Sézary syndrome vs. HTLV-1+ leukemic cell lines. Oncotarget Actions 2017; 8:95981–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyal L, Yehezkel S, Gorovitz B et al. Oncogenic role of microRNA-155 in mycosis fungoides: an in vitro and xenograft mouse model study. Br J Dermatol 2017; 177:791–800. [DOI] [PubMed] [Google Scholar]
- 23.Olsen E, Vonderheid E, Pimpinelli N et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007; 110:1713–22. [DOI] [PubMed] [Google Scholar]
- 24.Moyal L, Yehezkel S, Gorovitz B et al. Unilesional mycosis fungoides is associated with increased expression of miR-17~92, and Th1 skewing. Br J Dermatol 2019; 180:1123–34. [DOI] [PubMed] [Google Scholar]
- 25.Aronovich A, Moyal L, Gorovitz B et al. Cancer-associated fibroblasts in mycosis fungoides promote tumor cell migration and drug resistance via CXCL12/CXCR4. J Invest Dermatol 2021; 141:619–27. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z, Shi K, Yang S et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer 2018; 17:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos JC, Lima NDS, Sarian LO et al. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci Rep 2018; 8:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Saghir J, Nassar F, Tawil N, El-Sabban M. ATL-derived exosomes modulate mesenchymal stem cells: potential role in leukemia progression. Retrovirology 2016; 13:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li XJ, Ren ZJ, Tang JH, Yu Q. Exosomal microRNA miR-1246 promotes cell proliferation, invasion and drug resistance by targeting CCNG2 in breast cancer. Cell Physiol Biochem 2017; 44:1741–8. [DOI] [PubMed] [Google Scholar]
- 30.Sakha S, Muramatsu T, Ueda K, Inazawa J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci Rep 2016; 6:38750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anwar SL, Tanjung DS, Fitria MS et al. Dynamic changes of circulating miR-155 expression and the potential application as a noninvasive biomarker in breast cancer. Asian Pac J Cancer Prev 2020; 21:491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeshita N, Hoshino I, Mori M et al. Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br J Cancer 2013; 108:644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones K, Nourse JP, Keane C et al. Plasma microRNA are disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res 2014; 20:253–64. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Zhang C, Zhang P et al. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med 2018; 7:1670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ralfkiaer U, Hagedorn PH, Bangsgaard N et al. Diagnostic micro-RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood 2011; 118:5891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moyal L, Barzilai A, Gorovitz B et al. miR-155 is involved in tumor progression of mycosis fungoides. Exp Dermatol 2013; 22:431–3. [DOI] [PubMed] [Google Scholar]
- 37.Sandoval J, Díaz-Lagares A, Salgado R et al. MicroRNA expression profiling and DNA methylation signature for deregulated micro-RNA in cutaneous T-cell lymphoma. J Invest Dermatol 2015; 135:1128–37. [DOI] [PubMed] [Google Scholar]
- 38.Van Kester MS, Ballabio E, Benner MF et al. miRNA expression profiling of mycosis fungoides. Mol Oncol 2011; 5:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garaicoa FH, Roisman A, Arias M et al. Genomic imbalances and microRNA transcriptional profiles in patients with mycosis fungoides. Tumour Biol 2016; 37:13637–47. [DOI] [PubMed] [Google Scholar]
- 40.Marosvári D, Téglási V, Csala I et al. Altered microRNA expression in folliculotropic and transformed mycosis fungoides. Pathol Oncol Res 2015; 21:821–5. [DOI] [PubMed] [Google Scholar]
- 41.Maj J, Jankowska-Konsur A, Sadakierska-Chudy A et al. Altered microRNA expression in mycosis fungoides. Br J Dermatol 2012; 166:331–6. [DOI] [PubMed] [Google Scholar]
- 42.Kopp KL, Ralfkiaer U, Gjerdrum LM et al. STAT5-mediated expression of oncogenic miR-155 in cutaneous T-cell lymphoma. Cell Cycle 2013; 12:1939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Querfeld C, Foss FM, Kim YH et al. Phase 1 trial of cobomarsen, an inhibitor of Mir-155, in cutaneous T cell lymphoma. Blood 2018; 132 (Suppl. 1):2903. [Google Scholar]
- 44.Kirave P, Gondaliya P, Kulkarni B et al. Exosome mediated miR-155 delivery confers cisplatin chemoresistance in oral cancer cells via epithelial–mesenchymal transition. Oncotarget 2020; 11:1157–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun JF, Zhang D, Gao CJ et al. Exosome-mediated miR-155 transfer contributes to hepatocellular carcinoma cell proliferation by targeting PTEN. Med Sci Monit Basic Res 2019; 25:218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeh YY, Ozer HG, Lehman AM et al. Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling. Blood 2015; 125:3297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morin RD, O’Connor MD, Griffith M et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res 2008; 18:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo M, Zhang Q, Xia M et al. Differential co-expression and regulatory network analysis uncover the relapse factor and mechanism of T cell acute leukemia. Mol Ther Nucleic Acids 2018; 12:184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S, Zeng Y, Zhou J-M et al. MicroRNA-1246 promotes growth and metastasis of colorectal cancer cells involving CCNG2 reduction. Mol Med Rep 2016; 13:273–80. [DOI] [PubMed] [Google Scholar]
- 50.Sun Z, Meng C, Wang S et al. MicroRNA-1246 enhances migration and invasion through CADM1 in hepatocellular carcinoma. BMC Cancer 2014; 14:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chai S, Ng K-Y, Tong M et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology 2016; 64:2062–76. [DOI] [PubMed] [Google Scholar]
- 52.Kim G, An H-J, Lee M-J et al. Hsa-miR-1246 and hsa-miR-1290 are associated with stemness and invasiveness of non-small cell lung cancer. Lung Cancer 2016; 91:15–22. [DOI] [PubMed] [Google Scholar]
- 53.Zhang WC, Chin TM, Yang H et al. Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat Commun 2016; 21:11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdelhamed S, Butler JT, Doron B et al. Extracellular vesicles impose quiescence on residual hematopoietic stem cells in the leukemic niche. EMBO Rep 2019; 20:e47546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan D, Xu J, Wang J et al. Extracellular miR-1246 promotes lung cancer cell proliferation and enhances radioresistance by directly targeting DR5. Oncotarget 2016; 7:32707–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zackheim HS. Cutaneous T cell lymphoma: update of treatment. Dermatology 1999; 199:102–5. [DOI] [PubMed] [Google Scholar]
- 57.Moshiri F, Salvi A, Gramantieri L et al. Circulating miR-106b-3p, miR-101–3p and miR-1246 as diagnostic biomarkers of hepatocellular carcinoma. Oncotarget 2018; 9:15350–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhagirath D, Yang T, Bucay N et al. microRNA-1246 is an exosomal biomarker for aggressive prostate cancer. Cancer Res 2018; 78:1833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Y, Hannafon B, Zhao Y et al. Plasma exosome miR-196a and miR-1246 are potential indicators of localized pancreatic cancer. Oncotarget 2017; 8:77028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Cutaneous T-cell lymphoma cell lines.
Appendix S2 Peripheral blood mononuclear cells.
Appendix S3 Primers for microRNAs.
Figure S1 Venn diagram of shared microRNAs between exosomes derived from various cutaneous T-cell lymphoma cell lines.
Figure S2 Size distribution of exosomes isolated from plasma of patients with mycosis fungoides and healthy donors.
Figure S3 Expression of exosome marker CD81 on exosomes isolated from plasma of patients with mycosis fungoides and healthy donors.
Figure S4 Expression of cell-free and exosomal miR-155 and miR-1246 in plasma of patients with mycosis fungoides and healthy donors.
Figure S5 Expression of cell-free and exosomal miR-155 and miR-1246 in plasma of patients with mycosis fungoides and healthy donors.
Table S1 Characteristics of patients with mycosis fungoides.
Table S2 The miRNA content of cutaneous T-cell lymphoma cell-line-derived exosomes based on Affymetrix micro-RNA array 4.
Data Availability Statement
The authors encourage sharing of the data from this study.


