Abstract
Background
Scleral cross-linking can enhance the biomechanical strength of the sclera and is expected to be a new operative method for the prevention of myopia. However, studies investigating the changes in intraocular pressure (IOP) and ocular pulse amplitude (OPA) after blue light-riboflavin induced scleral collagen cross-linking (SXL) in rhesus monkeys are limited. This study aimed to investigate the changes in IOP and OPA in three-year-old rhesus macaques 1 week, 1 month, and 3 months after blue light-riboflavin SXL.
Methods
Seven three-year-old rhesus macaques (14 eyes) were randomly divided into two groups, with 4 monkeys in group A (8 eyes) and 3 monkeys in group B (6 eyes). The right eye of each rhesus macaque was used as the experimental eye, whereas the left eye was used as the control. In group A, one quadrant of each right eye was irradiated. In group B, two quadrants of each right eye and one quadrant of each left eye were irradiated. The IOP and OPA of both eyes were measured in all seven rhesus macaques before SXL and 1 week, 1 month, and 3 months postoperatively, and differences in the IOP and OPA between the experimental and control eyes were evaluated via the paired t test.
Results
In groups A and B, there were no significant differences between the experimental and control eyes in the IOP or OPA before SXL or 1 week, 1 month, or 3 months postoperatively (P > 0.05).
Conclusions
The IOP and OPA are not significantly affected in 1 vs 0 or in 1 vs 2 quadrants of blue light SXL.
Keywords: Blue-light, Cross-linking, Rhesus macaques, Intraocular pressure, Ocular pulse amplitude
Background
Myopia is an ocular disease affecting public health globally. It is estimated that by 2050, almost half the world’s population will have myopia, and one-tenth of the world’s population will develop high myopia [1]. Myopia, especially high myopia, primarily manifests as scleral thinning and progressive growth of the ocular axis; hence, methods that enhance the biomechanical strength of the sclera with a resultant delay in scleral thinning and axial growth can effectively prevent and treat myopia and its complications. Scleral cross-linking (SXL) with riboflavin and blue light has a stiffening effect on the sclera in rabbits [2]. The biomechanical strength of the human sclera may be enhanced by collagen cross-linking with riboflavin/460-nm blue-light irradiation [3]. Wang M et al. confirmed that the scleral biomechanics after equatorial SXL are stronger than those after posterior SXL and that the equatorial region of the sclera is the preferred position for SXL [4]. Anatomically, the vortex veins pass through the equatorial sclera, and any abnormality in the vortex veins can induce a pathological change in the intraocular pressure (IOP) and ocular blood flow. The ocular pulse amplitude (OPA) refers to the difference in the IOP between the systolic and diastolic periods of the cardiac cycle and is a parameter reflecting the blood flow in the eyes. IOP fluctuations are indicative of the rhythmic haemodynamic status of the eye corresponding to each heartbeat. There have been many reports confirming that patients with diseases associated with changes in the blood flow in the eye have relatively low OPA values. Abeg. O Pinto L et al. found that the OPA value in patients with primary open-angle glaucoma and normal-IOP glaucoma was lower than that in healthy people of the same age [5]. Wang et al. proved that there was no significant difference in the OPA between the two eyes in patients with bilateral disease but that there was a significant difference in patients with unilateral disease [6]. Ebru Nevin Çetin et al. confirmed that the OPA in patients with Behcet's disease with ocular lesions was lower than that in patients with Behcet's disease without ocular lesions and that in the normal controls [7]. Ebru n. Cetin et al. also confirmed that the OPA in patients with multiple sclerosis was lower than that in the controls [8]. Based on the experiences of our research group from a previous investigation, this study aimed to observe the changes in the IOP and OPA after blue light-riboflavin induced SXL in the eyes of rhesus monkeys and provide some experimental basis for the clinical application of the blue light SXL technique.
Methods
Study design
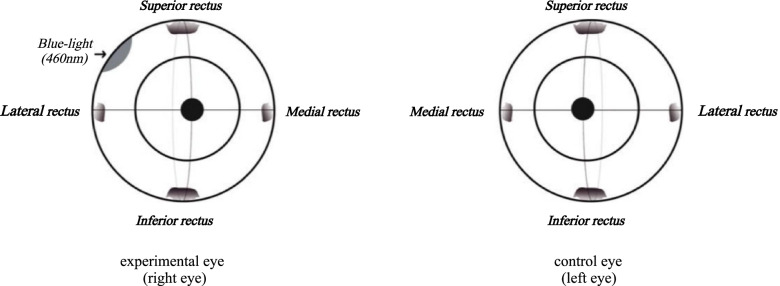
Seven three-year-old rhesus macaques (14 eyes) were divided into two groups using a completely randomized design, with 4 monkeys in group A (8 eyes) and 3 monkeys in group B (6 eyes). The right eye of each rhesus macaque was used for the experiment, and the left eye was used as a control. In group A, the superior temporal quadrant of the right eye was irradiated with blue light (Fig. 1). In group B, the superior and inferior temporal quadrants of the right eye and the superior temporal quadrant of the left eye were irradiated with blue light (Fig. 2). The estimation of the sample size in each group for this study was performed based on other reports in the literature [9–11]. Both eyes of all the rhesus macaques were examined clinically before SXL and 1 week, 1 month, and 3 months postoperatively, and the examiners were blinded to the group allocation during the experiment.
Fig. 1.
Front views of rhesus macaque eyeballs showing the scleral collagen cross-linking locations in group A
Fig. 2.
Front views of rhesus macaque eyeballs showing the scleral collagen cross-linking locations in group B
Subjects
Seven healthy rhesus macaques (age, 3.0–3.5 years; weight, 4.3–6.0 kg) were obtained from the Experimental Animal Centre of the People’s Liberation Army Academy of Military Medical Sciences in China. They were raised in the laboratory animal breeding room of Capital Medical University. They were housed individually and provided free access to food and water throughout the study. The room temperature was maintained between 23 °C and 27 °C, and the relative humidity was maintained between 45 and 55%. The general condition of all macaques met the national health monitoring standards for primate laboratory animals. They underwent anterior-segment and fundus examinations before initiation of the experimental procedure to exclude potential health factors that might affect the IOP measurements. They showed no abnormities, and baseline measurements of the IOP showed no pathological findings. The use of experimental animals in this study was approved by the Institutional Animal Care and Use Committee of Capital Medical University (AEEI-2014–127), and all macaques included in the study were acquired and used in compliance with the Association for Research in Vision and Ophthalmology’s Statement for the Use of Animals in Ophthalmic and Vision Research. After the experiment, the rhesus macaques continued to receive good care and were used in subsequent studies.
Surgery and SXL
All macaques were anaesthetized with a mixture of ketamine hydrochloride (10%; 20 mg/kg) and xylazine hydrochloride (5 mg/kg) injected intramuscularly into the buttocks. All surgical procedures were performed by one surgeon (Mengmeng Wang). All eyes underwent a 360-degree conjunctival peritomy, and Tenon’s capsule was dissected to expose the equatorial sclera in the corresponding quadrant. Two scleral traction wires were placed in front of the planned cross-linking site; these wires were pulled to rotate the eyeball and expose the irradiation zone. A 0.5% solution of riboflavin was instilled on the exposed scleral surface 20 min before irradiation. The diameter of the irradiation zone in the equatorial sclera in all animals was 10 mm. A collimated beam of blue light (460 nm) was generated with a beam illumination system (Beijing Obodi Optoelectronic Technology Co., Ltd., Beijing, China), which conformed to the national standards. The exposed sclera was irradiated from a distance of 5 cm, with a resulting surface area of irradiation of 22.5 mW/cm2; riboflavin solution was dripped onto the scleral surface every 20 min during irradiation. Blank irradiation zones were not irradiated, but the 0.5% riboflavin solution was administered in the same manner. Postoperatively, the preset sutures were removed, and the conjunctiva was sutured with absorbable surgical sutures. A 0.3% gatifloxacin eye gel was applied to all eyes to avoid infection, 4 times a day for 1 week.
IOP Measurements
The IOP measurements were performed before SXL and 1 week, 1 month, and 3 months after the SXL operation. After the induction of general anaesthesia, each macaque was secured in a seated position. An appropriately experienced examiner then measured the IOP of the experimental and control eyes in groups A and B using a Model 30 pneumatonometer (Reichert, America) and TonoLab rebound tonometer (iCare Finland Oy, Helsinki, Finland). When performing measurements using the Model 30 pneumatonometer, the operator held the probe and moved the sensor towards the eye gently to touch the corneal apex. As the membrane touched the cornea, the sensor handle was continuously moved towards the eye until an audible tone was generated, which indicated that the most accurate reading was obtained. The tone changed to a noticeably low pitch when the standard deviation among IOP readings was maintained below 1.0 mmHg for three seconds. Additionally, the instrument displayed the average IOP and standard deviation. When performing measurements using the TonoLab rebound tonometer, the IOP was assessed 5 times, and for each of these assessments, it was measured 6 times, and the mean IOP values were calculated.
Measurement of OPA
The OPA measurements were performed before SXL and 1 week, 1 month, and 3 months after the SXL operation. After the induction of general anaesthesia, the rhesus monkeys were placed in a sitting position. An appropriately experienced examiner then measured the OPA of the experimental and control eyes in groups A and B using the Model 30 pneumatonometer. When measuring the OPA in pulse tonometry mode, the operator held the probe in contact with the cornea. A changed tone was generated when the pneumatonometer sensed five ocular pulses, indicating that the instrument had enough samples to compute an average OPA; the test ended when the instrument sensed ten ocular pulses. The reading displayed was the average of all the detected pulses. The measurement was repeated 3 times, and the mean value of the 3 measurements was obtained.
Statistical Analysis
SPSS version 20.0 (SPSS, Chicago, IL, USA) was used to analyse the results. The IOP and OPA data are recorded as the mean ± standard deviation. The Shapiro–Wilk test was used to test normality. We evaluated differences in the IOP and OPA between the experimental and control eyes via the paired t test at the scheduled follow-up time points within both groups. P < 0.05 was considered statistically significant.
Results
Seven three-year-old rhesus macaques (14 eyes) were included and randomly divided into two groups: group A (8 eyes) and group B (6 eyes). Complete 3-month follow-up data were obtained for all eyes. Postoperatively, all macaques had normal corneas, anterior chambers, and clear lenses, and none exhibited any vitreous or retinal lesions. Moreover, there were no clinical signs of inflammation after blue light SXL.
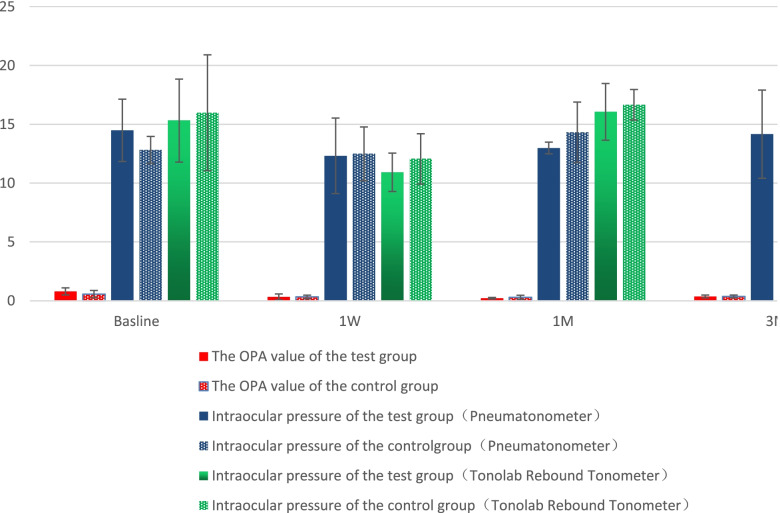
The baseline OPA in the seven rhesus monkeys (14 eyes) was 0.65 ± 0.06 mmHg. The IOP and OPA measured by the TonoLab rebound tonometer/pneumatonometer and the pneumatonometer before SXL and 1 week, 1 month, and 3 months postoperatively showed no significant differences between the experimental and control eyes in group A (P > 0.05; Fig. 3, Table 1).
Fig. 3.
IOP and OPA measurements in the experimental and control eyes in group A. IOP: Intraocular pressure; OPA: Ocular pulse amplitude
Table 1.
Demographics and characteristics of the study animals at baseline
| Animal# | Sex | Weight(kg) | IOP(mmHg) | OPA(mmHg) | ||
|---|---|---|---|---|---|---|
| R | L | R | L | |||
| 1 | M | 5.6 | 15.2 | 14.4 | 0.65 | 0.5 |
| 2 | M | 4.9 | 19.2 | 20.8 | 0.5 | 0.5 |
| 3 | F | 6.0 | 21.4 | 21.2 | 0.6 | 0.6 |
| 4 | F | 5.4 | 17.4 | 17.0 | 0.9 | 0.8 |
| 5 | M | 4.3 | 19.4 | 21.6 | 0.8 | 0.3 |
| 6 | F | 5.2 | 13.6 | 12.4 | 1.1 | 0.9 |
| 7 | F | 4.8 | 13.0 | 14.0 | 0.5 | 0.5 |
Additionally, the IOP and OPA measured by the TonoLab rebound tonometer/pneumatonometer and the Model 30 pneumatonometer, respectively, before SXL, and 1 week, 1 month, and 3 months postoperatively showed no significant differences between the experimental and control eyes in group B (P > 0.05; Fig. 4, Table 2). However, there was a significant difference in the IOP measured by the pneumatonometer and TonoLab rebound tonometer. Moreover, the values obtained by the pneumatonometer were significantly lower than those obtained by the TonoLab tonometer (t = -2.671, P = 0.010).
Fig. 4.
IOP and OPA measurements in the experimental and control eyes in group B. IOP: Intraocular pressure; OPA: Ocular pulse amplitude
Table 2.
IOP and OPA measurements in the experimental and control eyes in two groups
| IOPa(mmHg) | IOPb(mmHg) | OPA(mmHg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E eye | C eye | P value | E eye | C eye | P value | E eye | C eye | P value | ||
| Group A | Base | 18.30 ± 2.64 | 18.35 ± 3.24 | 0.931 | 13.33 ± 2.82 | 13.25 ± 1.55 | 0.919 | 0.66 ± 0.17 | 0.60 ± 0.14 | 0.194 |
| 1w | 12.70 ± 6.25 | 11.40 ± 3.01 | 0.556 | 11.00 ± 3.24 | 11.63 ± 1.75 | 0.600 | 0.58 ± 0.30 | 0.53 ± 0.15 | 0.718 | |
| 1 m | 12.10 ± 5.00 | 12.85 ± 2.92 | 0.582 | 10.50 ± 1.91 | 12.63 ± 1.65 | 0.110 | 0.50 ± 0.12 | 0.65 ± 0.44 | 0.519 | |
| 3 m | 11.40 ± 3.67 | 12.40 ± 3.13 | 0.239 | 11.88 ± 2.63 | 12.13 ± 2.10 | 0.495 | 0.55 ± 0.19 | 0.63 ± 0.17 | 0.391 | |
| Group B | Base | 15.33 ± 3.53 | 16.00 ± 4.92 | 0.572 | 14.50 ± 2.65 | 12.83 ± 1.15 | 0.199 | 0.80 ± 0.30 | 0.57 ± 0.31 | 0.250 |
| 1w | 10.93 ± 1.63 | 12.07 ± 2.14 | 0.360 | 12.33 ± 3.21 | 12.50 ± 2.29 | 0.995 | 0.33 ± 0.25 | 0.33 ± 0.15 | 1.000 | |
| 1 m | 16.07 ± 2.41 | 16.67 ± 1.30 | 0.449 | 13.00 ± 0.50 | 14.33 ± 2.57 | 0.448 | 0.23 ± 0.06 | 0.30 ± 0.17 | 0.423 | |
| 3 m | 13.80 ± 2.03 | 13.27 ± 2.10 | 0.094 | 14.17 ± 3.75 | 14.33 ± 2.75 | 0.874 | 0.37 ± 0.12 | 0.37 ± 0.12 | 0.423 | |
IOPa Intraocular pressure (Tonolab Rebound Tonometer), IOPb Intraocular pressure (Pneumatonometer), OPA Ocular pulse amplitude, E eye experimental eye, C eye control eye, Base baseline, w week, m month
Discussion
The progression of myopia is primarily manifests as scleral thinning and progressive growth of the ocular axis. The use of SXL to increase scleral rigidity and reduce axial eye elongation has been suggested, and most of the current related research has been based on collagen cross-linking (CXL) by riboflavin/ultraviolet –A light. Compared to ultraviolet light, blue light is more controllable because of its longer wavelength. Blue light-riboflavin induced SXL was found to increase the biomechanical strength of the sclera in rabbits and donated human eyes [2, 3]. Wang M et al. confirmed that the scleral biomechanics were stronger after equatorial ultraviolet- and riboflavin-induced SXL than after posterior ultraviolet riboflavin SXL [4]. Moreover, equatorial SXL prevents damage to the optic nerve; hence, the equatorial region of the sclera is the preferred location for SXL [4]. Our research group conducted a series of studies on equatorial SXL, Yu Li et al. [12] irradiated one quadrant in their study on the safety and long-term scleral biomechanical stability of rhesus eyes after scleral cross-linking with blue light irradiation; Yu Li et al. [13] irradiated one or two quadrants with blue light for riboflavin scleral cross-linking in an ocular safety evaluation. Anatomically, the vortex veins pass through the equatorial sclera. They transport uveal blood outside the eyeball and maintain the outflow of partial aqueous fluid or humour; therefore, any abnormality in the vortex veins can induce a pathological change in the IOP and ocular blood flow. At present, the OPA is mostly analyzed in the studies of glaucoma and other ocular ischemic diseases, but it has not been used to study SXL. In addition, compared with other experimental animals, monkeys have eyes that are more structurally similar to human eyes physiologically. This study is the first to investigate the OPA in rhesus macaques and has more clinical significance than studies performed in other experimental animals.
The OPA refers to the difference in IOP between the systolic and diastolic periods of the cardiac cycle and is indicative of the rhythmic haemodynamic status of the eye corresponding to each heartbeat. The mechanism of change in the OPA is as follows: during the systolic period of the cardiac cycle, blood pumped from the heart enters the blood vessels of the eye, of which 90–95% enters the choroidal blood vessels. OPA values in normal individuals fluctuate between 2 and 3 mmHg but are usually maintained at less than 5 mmHg; moreover, binocular differences are often less than 0.5 mmHg [14–17]. In this study, the baseline OPA in the rhesus macaques was 0.65 ± 0.06 mmHg, which is lower than previously reported values between 2 and 3 mmHg. This difference can be attributed to the influence of general anaesthesia on OPA measurements. Pianka et al. reported that both sub-Tenon's and peribulbar anaesthesia can significantly reduce the OPA [18]. Zuche et al. reported significantly reduced IOP and OPA values under general anaesthesia [19]. However, this difference could also be due to the species-specific differences between rhesus monkeys and humans.
The Model 30 pneumatonometer is a novel, noninvasive pneumatic tonometer; its probe contains a gentle, floating pneumatic sensor that touches the surface of the cornea with the exact amount of applanating force required to take a tonometry measurement. In this experiment, there were significant differences in the IOP measured by the Model 30 pneumatonometer and the TonoLab rebound tonometer; the values obtained by the Model 30 pneumatonometer were significantly lower than those obtained by the TonoLab tonometer (P < 0.05). Barkana et al. found that the IOP values measured by the Model 30 pneumatonometer in the seated and supine positions (both measured when the individual was awake) were higher than those measured by the iCare rebound tonometer [20]. Therefore, the differences in this study might be related to general anaesthesia. The difference between the two tonometers may be associated with the rapid fluctuations in IOP after the induction of general anaesthesia. Van der Walt et al. found that the IOP decreased 8 min after the administration of an intramuscular ketamine injection [21]. Ding et al. found that in animal experiments, the IOP could increase rapidly and subsequently decrease quickly to a lower level within 3 min after the induction of general anaesthesia by ketamine [22]. The relatively small sample size may also be one of the reasons for this difference.
Gool et al. measured the OPA in normal individuals and glaucoma patients and reported that the OPA and IOP values were positively correlated; for every 10 mmHg increase in the IOP, the OPA increased by 1.6 mmHg [23]. Hence, OPA values should be compared on the basis of similar IOP values. In this study, the IOP values in both groups A and B showed no significant difference between the experimental and control eyes. Therefore, the comparison of the OPA values in this experiment is of clinical significance. In this study, there were no significant differences between the experimental and the control eyes in the IOP or OPA before SXL or 1 week, 1 month, or 3 months postoperatively in group A or group B (P > 0.05). This result indicats that the IOP and OPA in rhesus macaques were not significantly affected by blue light SXL in one or two quadrants. However, the results could also indicate that SXL has a limited therapeutic scope and may not adequately affect the blood flow in the entire eyeball. Scleral crosslinking may increase ocular rigidity (OR). OR is an index to quantify the compliance of the globe and has been used to characterize the biomechanical properties of the ocular wall. Friedenwald [24] uses the OR coefficient (E) in a logarithmic equation to express the pressure–volume relationship: E = (log IOP 1 − log IOP 2)/(V1 − V2). In the formula, the slope E is the OR coefficient. Wang proposed that the OPA and pulsatile ocular blood flow could be used to approximately replace the (log IOP 1 − log IOP 2) and (V1 − V2) values in the above formula to obtain an estimated value E (Er) [25]. A higher OPA may be found in eyes with increased rigidity, due to either a higher rigidity coefficient or a higher IOP [26]. Girard MJ et al. considered that changes in the scleral properties detected in eyes of monkeys subjected to elevated IOP include stiffening, and these changes are believed to reflect remodelling of the scleral extracellular matrix [27]. Therefore, OPA may not be directly related to simple ocular rigidity, but ocular rigidity and OPA value change owing to the increase in intraocular pressure. Kimball and Elizabeth considered scleral crosslinking capable of increasing the risk of glaucoma; however, we found that the increase in IOP was induced in a separate step. It was not a result of the stiffening. Local change in ocular rigidity is unlikely to affect global ocular rigidity. This is also documented in the following publication, where a substantial part of the corneal stromal thickness was excised and the overall rigidity did not change significantly [28]. Moreover, the postoperative OPA values in both the experimental and control eyes were lower than the baseline values in group B, which may be because the surgical intervention results in a decrease in the OPA. Therefore, we can include other detection indices of ocular blood flow in future experiments to further understand the changes in blood flow. However, the lack of a significant difference between the two eyes in the two groups provides a basis for the safety of the surgery. In addition, some limitations of the study should be noted. First, the results are credible based on the sample size reported in other studies in the literature, but the sample size in this investigation was still small, and we plan to expand the sample size in further research. Second, the OPA measurements indicate the blood flow in the entire eyeball and not the blood flow only in the irradiated area. However, other equipment that can measure local blood flow cannot obtain IOP values in a short time. Third, although the IOP and OPA were measured according to the manufacturers' instructions and we took the average values of multiple measurements, both the IOP and OPA are variable values, and the data may be affected by fluctuation.
Conclusion
In conclusion, the IOP and OPA are not significantly affected in 1 vs 0 or in 1 vs 2 quadrants of blue light SXL. This study provides an experimental basis for the clinical application of the blue light SXL technique. Further studies incorporating scleral biomechanical sclera stability will be performed to obtain a relatively optimized schema of SXL.
Acknowledgements
Not applicable.
Abbreviations
- IOP
Intraocular pressure
- OPA
Ocular pulse amplitude
- CXL
Collagen crosslinking
- SXL
Scleral collagen crosslinking
- OR
Ocular rigidity
Authors’ contributions
FJZ made contribution to supervision and final approval, made critical revision of the manuscript and fund application. CL collected, analyzed and interpreted data, wrote the manuscript. YL collected data and made critical revision of the manuscript. MMW implement surgical intervention of experimental animals. JL provided expert technical assistance with the measurement and made contribution to the design of the experiment. NLW provided the instrument used in the experiment and made contribution to the design of the work. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (numbers 81570877 and 81873682) and the 215 High-Level Talent Fund of Beijing Health Government (number 2013–2-023). The funders helped us to purchase the animals, reagents and consumables needed for the experiment, such as eye drops.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Animal Care and Use Committee of Capital Medical University. All macaques included in the study were acquired and used in compliance with the Association for Research in Vision and Ophthalmology’s Statement for the Use of Animals in Ophthalmic and Vision Research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chong Liu, Email: 13581796267@163.com.
Yu Li, Email: lipuyu1991@126.com.
Mengmeng Wang, Email: wangmengmg@163.com.
Jing Li, Email: lijinghappy2012@126.com.
Ningli Wang, Email: wningli@vip.163.com.
Fengju Zhang, Email: zhangfj126@126.com.
References
- 1.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Iseli HP, Spoerl E, Wiedemann P, et al. Efficacy and safety of blue-light scleral cross-linking[J] J Refract Surg. 2008;24(7):S752. doi: 10.3928/1081597X-20080901-21. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, Zou Y, Zhang F, et al. Efficacy of blue-light cross-linking on human scleral reinforcement. Optom Vis Sci. 2015;92:873–878. doi: 10.1097/OPX.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Zhang F, Qian X, et al. Regional biomechanical properties of human sclera after cross-linking by riboflavin/ultraviolet A[J] J Refract Surg. 2012;28(10):723. doi: 10.3928/1081597X-20120921-08. [DOI] [PubMed] [Google Scholar]
- 5.Abeg.o Pinto L, Vandewalle E, WillekensK, Marques-Neves C, Stalmans I. Ocular pulse amplitude and Doppler waveform analysis in glaucoma patients. Acta Ophthalmol 2014;92:e280–e285 [DOI] [PubMed]
- 6.WANG Ren-Yan, ZHONG Yong, DONG Fang-Tian, SHI Wei. Dynamic contour tonometer for patients with nonarteritic anterior ischemic optic neuropathy.Recent Advances In Ophthalmology, 2010, 30(1):81–84.(In Chinese)
- 7.Cetin EN, Bulgu Y, Taslı L, Cobankara V, Yildirım C. Ocular pulse amplitude in Behçet disease. Ocul Immunol Inflamm. 2011;19(5):376–378. doi: 10.3109/09273948.2011.601844. [DOI] [PubMed] [Google Scholar]
- 8.Cetin EN, Erdogan C, Acer S, et al. Decreased ocular pulse amplitude and retinal nerve fibre layer in multiple sclerosis[J] Neuro-Ophthalmology. 2013;37(3):5. doi: 10.3109/01658107.2013.785001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin H, Liu L, Ding H, et al. Comparison of femtosecond laser-assisted corneal intrastromal xenotransplantation and the allotransplantation in rhesus monkeys[J] BMC Ophthalmol. 2017;17(1):202. doi: 10.1186/s12886-017-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downs JC, Burgoyne CF, Seigfreid WP, et al. 24-Hour IOP Telemetry in the Nonhuman Primate: implant system performance and initial characterization of IOP at Multiple Timescales[J] Invest Ophthalmol Vis Sci. 2011;52(10):7365–7375. doi: 10.1167/iovs.11-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smit-Mcbride Z, Nguyen J, Elliott GW, et al. Effects of aging and environmental tobacco smoke exposure on ocular and plasma circulatory microRNAs in the Rhesus macaque[J] Mol Vis. 2018;24:633–646. [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Zhang F, Sun M, Lai L, Lv X, Liu C, Wang M, Wang N. Safety and long-term scleral biomechanical stability of rhesus eyes after scleral cross-linking by Blue Light. Curr Eye Res. 2021;46(7):1061–1070. doi: 10.1080/02713683.2020.1853781. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Liu C, Sun M, Lv X, Wang M, Jiao X, Zhang L, Wang N, Zhang F. Ocular safety evaluation of blue light scleral cross-linking in vivo in rhesus macaques. Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1435–1442. doi: 10.1007/s00417-019-04346-7. [DOI] [PubMed] [Google Scholar]
- 14.Perkins ES. The ocular pulse. Curr Eye Res. 1981;1:19–23. doi: 10.3109/02713688109019968. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann C, Bachmann LM, Robert YC, Thiel MA. Ocular pulse amplitude in healthy subjects as measured by dynamic contour tonometry. J Glaucoma. 2005;14:344–350. doi: 10.1097/01.ijg.0000176936.16015.4e. [DOI] [PubMed] [Google Scholar]
- 16.Knecht PB, Bosch MM, Michels S, et al. The ocular pulse amplitude at different intra ocular pressure:a prospective study. Acta Ophthalmol. 2011;89:e466–e471. doi: 10.1111/j.1755-3768.2011.02141.x. [DOI] [PubMed] [Google Scholar]
- 17.Pourjavan S, Boelle PY, Detry-marel M, De Potter P. Physiological diurnal variability and characteristrics of the ocular pulse amplitude( OPA ) with the dynamic contour tonometer (DCT-Pascal) Int Ophthalmol. 2007;27:357–360. doi: 10.1007/s10792-007-9161-7. [DOI] [PubMed] [Google Scholar]
- 18.Pianka P, Weintraubpadova H, Lazar M, et al. Effect of sub-Tenon’s and peribulbar anesthesia on intraocular pressure and ocular pulse amplitude.[J]. J Cataract Refract Surg. 2001;27(8):1221–6. [DOI] [PubMed]
- 19.Zuche H, Morinello E, Viestenz A, et al. Reduction of intraocular pressure and ocular pulse amplitude during general anesthesia. Ophthalmologe. 2015;112(9):764–769. [DOI] [PubMed]
- 20.Barkana Y, Gutfreund S. Measurement of the difference in intraocular pressure between the sitting and lying body positions in healthy subjects: direct comparison of the Icare Pro with the Goldmann applanation tonometer, Pneumatonometer and Tonopen XL[J] Clin Experiment Ophthalmol. 2014;42(7):608–614. doi: 10.1111/ceo.12272. [DOI] [PubMed] [Google Scholar]
- 21.van der Walt JG, Roodt F, Tinley C. How does sevoflurane induction, followed by a ketamine maintenance infusion, affect intraocular pressure? Establishment of an anaesthetic protocol for paediatric glaucoma examinations under anaesthesia. Br J Ophthalmol. 2018;102(7):902–905. [DOI] [PubMed]
- 22.Ding C, Wang P, Tian N. Effect of general anesthetics on IOP in elevated IOP mouse model[J] Exp Eye Res. 2011;92(6):0–520. doi: 10.1016/j.exer.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gool SV, Pauwels F, Solie L, et al. Ocular pulse amplitude measurement using pascal dynamic contour tonometer in glaucoma, patients[J] Klin Monbl Augenheilkd. 2014;231(04):363–367. doi: 10.1055/s-0034-1368220. [DOI] [PubMed] [Google Scholar]
- 24.Friedenwald JS. Contribution to the theory and practice of tonometry. Am J Ophthalmol. 1937;20:985–1024. doi: 10.1016/S0002-9394(37)90425-2. [DOI] [Google Scholar]
- 25.Wang J, Freeman EE, Descovich D, Harasymowycz PJ, Kamdeu Fansi A, Li G, Lesk MR. Estimation of ocular rigidity in glaucoma using ocular pulse amplitude and pulsatile choroidal blood flow. Invest Ophthalmol Vis Sci. 2013;54:1706–1711. doi: 10.1167/iovs.12-9841. [DOI] [PubMed] [Google Scholar]
- 26.Dastiridou AI, et al. “Ocular rigidity, ocular pulse amplitude, and pulsatile ocular blood flow: the effect of intraocular pressure.” Invest Ophthalmol Vis Sci. 2009;50(12):5718–22. [DOI] [PubMed]
- 27.Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011;52:5656–5669. doi: 10.1167/iovs.10-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kymionis GD, et al. “Ocular rigidity evaluation after photorefractive keratectomy: an experimental study.” J Refract Surg. 2008;24(2):173–7. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.