Abstract
Canine distemper morbillivirus (CDV) infection causes a frequently fatal systemic disease in a broad range of carnivore species, including domestic dogs. In CDV infection, classical serology provides data of diagnostic and prognostic values (kinetics of seroconversion) and is also used to predict the optimal vaccination age of pups. Routine CDV serology is still based on time- and cost-intensive virus neutralization assays (V-NA). Here, we describe a new capture-sandwich enzyme-linked immunosorbent assay (ELISA) that uses recombinant baculovirus-expressed nucleocapsid (N) protein of a recent CDV wild-type isolate (2544/Han95) for the detection of CDV-specific antibodies in canine sera. Recombinant antigen was produced with high efficacy in Heliothis virescens larvae. The capture-sandwich ELISA enabled a clear-cut qualitative evaluation of the CDV-specific immunoglobulin G (IgG) and IgM serostatuses of 196 and 35 dog sera, respectively. Inter-rater agreement analysis (κ = 0.988) indicated that the ELISA can be used unrestrictedly as a substitute for the V-NA for the qualitative determination of CDV-specific IgG serostatus. In an attempt to semiquantify N-specific antibodies, a one-step-dilution (alpha method) IgG-specific ELISA was implemented. Alpha values of ≥50% showed very good inter-rater agreement (κ = 0.968) with V-NA titers of ≥1/100 50% neutralizing dose (ND50) as measured against the central European CDV wild-type isolate 2544/Han95 in canine sera originating from northern Germany. An ND50 titer of 1/100 is considered a threshold, and titers of ≥1/100 indicate a resilient, protective immunity. CDV N-specific antibodies of the IgM class were detected by the newly developed ELISA in 9 of 15 sera obtained from dogs with symptoms of acute distemper. In leucocytes of 5 of the 15 dogs (all of which were also IgM positive) CDV RNA was detected by reverse transcription (RT)-PCR. The recombinant capture-sandwich ELISA detecting N-specific antibodies of the IgG class provided superior sensitivity and specificity and thus represents a rapid and cost-effective alternative to classical CDV V-NA. By detection of specific IgM antibodies, the ELISA will be complementary to RT-PCR and V-NA in the diagnosis of acute distemper infections.
Canine distemper virus (CDV), a morbillivirus in the Paramyxoviridae family, induces a highly contagious, systemic, and often fatal disease in domestic dogs as well as in a broad, and seemingly expanding, range of wild carnivore species (1, 20). Reservoirs of wild-type (wt) virulent CDV are probably maintained in local feral carnivore species, and spillovers into the canine population are likely to occur, since CDV has been shown to cross species borders almost without hindrance (1, 4, 20, 35). Modified live-attenuated CDV vaccines are available for use in dogs, and in general, they efficiently induce protective immunity (8). However, even in domestic dog populations in which a broad vaccination coverage is maintained, sporadic cases and outbreaks of canine distemper in regions of endimicity occasionally occur (6). In populations showing good herd immunity rates, young pups with waning maternal immunity are at greatest risk of wt CDV infection associated with clinically overt distemper. Dogs exhibiting titers of CDV-neutralizing antibodies of <1/100 50% neutralizing dose (ND50) are considered to be susceptible, and titers of maternal antibodies of ≥1/20 may interfere with vaccination success in pups (3, 8). The examination of the CDV-specific serostatuses of dogs, therefore, sets out to (i) determine the ideal time point for vaccination of a pup, (ii) evaluate vaccination success, and (iii) determine the diagnoses and prognoses of acute wt CDV infections.
Routine measurement of CDV-specific antibodies is based on virus neutralization assays (V-NA), which are costly as well as time-consuming (at least 4 days) and require specialized laboratories (2, 18, 35). Several approaches to develop more convenient enzyme-linked immunosorbent assay (ELISA) techniques for the detection of CDV-specific antibodies have been sought (5, 13, 29). Despite promising sensitivity and specificity results compared to those of the V-NA, these ELISA applications have obviously not received widespread acceptance. This fact is at least in part related to the assay’s source of viral antigen, which requires purification by density gradient centrifugation from supernatants of infected Vero cell cultures. CDV, however, grows poorly in cell culture and rarely exceeds infectivity titers of 106.0 50% tissue culture infective doses (TCID50) per ml. In addition, purified cell culture-derived CDV proteins are highly susceptible to proteolytic degradation. In contrast, several widely used ELISA applications have been developed for the detection of antibodies against other morbillivirus species such as the viruses that cause measles, rinderpest, or peste-des-petits-ruminants, which are antigenically related to CDV. These ELISAs use recombinant preparations of the specific viral nucleocapsid (N) protein that represents the immunodominant morbillivirus protein, although N proteins do not carry neutralization sites (10, 12, 22, 23).
The objective of this study was to improve the detection of CDV-specific antibodies in canine sera. We designed a capture-sandwich ELISA using a recombinant wt CDV N protein which was produced in the baculovirus expression system. The resulting assay proved to be superior to V-NA with respect to sensitivity and specificity. With an immunoglobulin M (IgM)-specific conjugate, the capture-sandwich ELISA also supported the diagnosis of acute CDV infections.
MATERIALS AND METHODS
Cells and viruses.
wt CDV isolates from Germany, 5804/Han89 and 2544/Han95, were grown as described previously (17) in Vero cells at 37°C in Dulbecco modified Eagle medium supplemented with 2% fetal calf serum. Spodoptera frugiperda (Sf9) cells were propagated as monolayer cultures at 27°C in TC 100 medium (Sigma, St. Louis, MO.) supplemented with 10% fetal calf serum. The production and growth properties of a recombinant Autographa californica nuclear polyhedrosis virus (AcNPV)-based baculovirus which expresses the N protein of CDV 2544/Han95 under the transcriptional control of the polyhedrin promoter (Bac-CDV-N) is reported elsewhere (19).
Canine serum samples.
A total of 196 serum specimens from dogs were sampled. Seventy-two sera were obtained from various ambulant patients of the Small Animal Clinic, Hannover Veterinary School, Hannover, Germany. Fifteen of these dogs were clinically suspected of having a distemper infection based on the presence of at least three of the following symptoms: fever, mucopurulent nasal and/or conjunctival discharge, bronchopneumonia, central nervous system disorders, and gastroenteritis. Another 121 sera were collected from different parts of Siberia, Russia, from clinically healthy dogs without a CDV vaccination record. Three sera originated from specific pathogen-free (SPF) dogs of a breeding colony at the National German Cancer Institute.
Detection of CDV RNA in clinical specimens.
An EDTA blood sample was taken from dogs clinically suspected of distemper (n = 15). Total RNA was isolated from buffy coat cells with an RNeasy kit (Qiagen). Following reverse transcription (RT), cDNA specific for the phosphoprotein (P) gene was amplified by PCR with so-called universal P gene primers, since they were shown to anneal to the P gene of all morbillivirus species tested so far. Details of this PCR have been communicated elsewhere (16). Amplified sequences of 453 bp were detected by horizontal agarose gel electrophoresis according to standard protocols.
MAbs.
For the establishment of CDV N-specific hybridomas, BALB/c mice were immunized subcutaneously three times at 14-day intervals with 100 μl of a 1/1 emulsion of ABMS-multiplier (Linaris) with lysate of Bac-CDV-N-infected Sf9 cells or with partially purified authentic CDV 5804/Han89 as described previously (14). Spleen cells of immunized mice were fused with P3X63-Ag8.653 myeloma cells. Hybridomas secreting N protein-specific antibodies were selected by an immune-peroxidase monolayer assay (IPMA) with heat-fixated CDV-infected Vero cells or Bac-CDV-N-infected Sf9 cells as described previously (18). Monoclonal antibodies (MAbs) secreted from stably producing hybridomas were characterized with regard to their Ig subclass and their binding affinity compared with those of the parent wt CDV and baculovirus-expressed CDV N antigen (calculation of the affinity index was done according to the method described in reference 28).
Neutralization assay.
Microneutralization assays were performed as previously described (36). In brief, serial twofold dilutions (starting from 1/10) of serum specimens in duplicate were prepared in Dulbecco modified Eagle medium and mixed with 50 μl of a virus suspension containing 100 TCID50 of CDV 2544/Han95. After an incubation period of 1 h at 37°C in a humidified CO2 atmosphere, 50 μl of a suspension containing 200,000 Vero cells was added to each well. The plates were incubated then at 37°C for 3 days, after which viral antigen was stained immunocytochemically (IPMA) as described below with a MAb (CDTC-16) specific for the CDV N protein (20). Neutralizing antibody titers (ND50s) were calculated as reciprocals of the highest serum dilution giving 50% protection (25).
IPMA.
Fifty microliters of a suspension containing 200,000 Vero cells was added to each well of a microtiter plate. One hundred microliters of a suspension containing 10 TCID50 of CDV 2544/Han95 was added, and the plates were incubated at 37°C for 3 days. After removal of culture medium, the cells were fixed and permeabilized in situ by incubation for 7 h at 80°C. If not used instantly, plates were sealed in airtight plastic bags and stored up to 6 months at −20°C. MAbs or canine serum samples were probed in serial twofold dilutions (100 μl) in duplicate starting from a dilution of 1/20 in phosphate-buffered saline containing 0.05% Tween 20 (PBS-T) for their binding activities against cell-associated CDV antigens. All incubation steps were carried out on a rocker plate. After incubation for 1 h at room temperature (RT), the plates were washed three times with PBS-T and incubated for 1 h at RT with a corresponding peroxidase-labelled, affinity-purified secondary antibody. Following another washing cycle with PBS-T, 100 μl of a solution containing 3-amino-9-ethyl-carbazole per well was used as a chromogene. Antibody titers were calculated as reciprocals of the highest antibody dilution which still enabled clear-cut detection of intracellular viral antigen by light microscopy.
Western blotting.
Vero cell cultures (approximately 107.0 cells) infected with CDV 2544/Han95 or mock infected were harvested at 72 h postinfection (p.i.), lysed directly in Laemmli loading buffer (27) containing 5 mM dithiothreitol, subjected to electrophoresis in sodium dodecyl sulfate (SDS)–10% polyacrylamide minigels, and transferred onto a polyvinylidene difluoride membrane as described previously (27). Nonspecific binding sites were blocked with PBS-T containing 1% (wt/vol) skim milk powder (PBS-TM) at 4°C overnight. The membrane was probed with MAbs or canine serum samples (diluted 1/20 or 1/100 in PBS-TM, respectively) for 2 h at RT. After being washed extensively in PBS-T, the membrane was probed with the corresponding peroxidase (POD)-labelled, affinity-purified secondary antibody. All incubations were carried out on a rocker plate. Teton (4-[1,4,7,10-tetraoxadecyl]-1-naphthol [Boehringer Mannheim] in substrate buffer, 50 mM MgCl2, 100 mM NaCl, 100 mM Tris-HCl [pH 8.0], 0.03% hydrogen peroxide) was used in the chromogenic reaction.
Production of recombinant CDV N antigen in vitro.
Sf9 insect cell cultures infected with Bac-CDV-N at a multiplicity of infection of 1 were harvested at 72 h p.i. Approximately 2 × 107 cells were washed two times with cold Tris-buffered saline (TBS) and pelleted at 3,000 × g at 4°C. The pellets were resuspended in 500 μl of one of the following solutions: double-distilled water, 1% (vol/vol) Triton X-100 in TBS (pH 8.7), 1% (wt/vol) SDS in TBS (pH 8.7), 4 M urea in TBS (pH 8.7), 20 mM octyl-beta-d-thioglucopyranoside (beta-OTG) in TBS (pH 8.7) or 20 mM beta-OTG in 0.1 M glycine (pH 9.6). Samples were incubated for 30 min on ice, sonificated, and clarified at 15,000 × g for 2 min. Supernatants were aliquoted and stored at −80°C until use.
Production of recombinant CDV N antigen in larvae.
Fourth-instar Heliothis virescens larvae from a breeding colony at Agrevo, Frankfurt am Main, Germany, were injected subcuticularly with approximately 5 × 104 TCID50 of Bac-CDV-N. Control larvae were given an equal dose of wt AcNPV by the same route. Infected larvae were kept a 27°C on an artificial diet (Stonefly Industries). At 3, 4, and 5 days p.i., antigen from two larvae each was prepared following shock freezing in liquid nitrogen and subsequent pulverization. Lysates were prepared from powdered larval material by resuspension in TBS (pH 8.7) containing 2% (wt/vol) SDS. The rationale to use this buffer was based on favorable results with infected Sf9 cell cultures. Insoluble remnants were pelleted, and supernatants were frozen at −80°C.
Antigen potency testing by indirect ELISA.
Microtiter plates (Maxisorb; Nunc) were directly coated with duplicate serial twofold dilutions (starting at 1/100) of antigen preparations from Sf9 cell cultures or larvae in TBS at 100 μl per well, and then the plates were incubated overnight at 4°C while being gently shaken. Nonspecific binding sites were blocked by incubation with 1% (wt/vol) ELISA blocking reagent (Boehringer Mannheim) in double-distilled water for 1 h at RT. Subsequently, incubations with MAb CDRCT-8 and POD-labelled, affinity-purified goat anti-mouse IgG were carried out at RT for 1 h each. Washing cycles between all incubation steps were performed with PBS-T. 2,2′-Azino-bis-[3-ethyl-benzthiazoline]-6-sulfonic acid (ABTS; 100 μl; Sigma) at 0.2 mM in citrate buffer (pH 4.0) containing 0.03% hydrogen peroxide was used as the substrate. Optical densities at 405 nm (OD405) were determined.
Capture-sandwich ELISA.
Microtiter plates were coated with affinity-purified N-specific MAbs diluted in TBS to 2 μg per ml for 4 h at RT. Nonspecific binding sites were blocked by incubation with 1% (wt/vol) ELISA blocking reagent in double-distilled water for 1 h at RT. Antigen preparations (100 μl) of recombinant CDV N protein appropriately diluted in TBS containing 0.05% Tween 20 were added, and the plates were incubated overnight at 4°C while being shaken. All further incubations were carried out on a rocker plate as well. The plates were then washed three times with PBS-T. Starting at a dilution of 1/20 in PBS-T containing 1% (wt/vol) ELISA blocking reagent, canine sera (100 μl) were added in duplicate and the plates were incubated at RT for 2 h. The washing cycle described above was then repeated. POD-conjugated caprine IgG, specific for canine IgG or IgM (Wherl, Wolfsburg, Germany) and diluted appropriately in PBS-T, was added to each well, and the plates were incubated at RT for 1 h. Following a further washing cycle, ABTS (100 μl) was added and the plates were incubated for 30 min, after which the optical densities at 405 nm were read. In a separate series, ELISA plates were coated with MAbs and antigen at the appropriate dilutions and then fixed with 1.5% (vol/vol) formaldehyde in double-distilled water for 15 min at RT. The formaldehyde was removed, and the wells were washed three times with PBS-T. Plates were then air dried, sealed in airtight plastic bags, and stored for at least 3 months at −20°C. When completely defrosted, these plates were further processed as described above.
Statistic analysis.
Routine statistics were performed with programs supplied with the MedCalc software package, version 4.16g (33). Performance characteristics of the capture-sandwich ELISA were calculated according to the method of Ryan (32). For comparison of different serologic assays, inter-rater agreement analysis was carried out (9).
RESULTS
MAbs.
Hybridoma clones secreting CDV-specific MAbs were identified by IPMA. MAbs produced by six established hybridomas (Table 1) reacted with a 60- to 62-kDa double band in a Western blot analysis with either authentic antigen of CDV 2544/Han95 from infected Vero cells or recombinant antigen from Bac-CDV-N-infected Sf9 cells (Fig. 1). The affinities of these MAbs for recombinant CDV N, evaluated by IPMA and indirect ELISA, however, proved to be 4 to 20 times lower than those for authentic CDV N; the highest ratios were obtained with the MAbs CDRCT-8 and CDTC-16 (Table 1).
TABLE 1.
Characterization of established CDV-N-specific murine MAbs
| MAb | Antigen for immunization | IgG subclass | Affinity indexa
|
|
|---|---|---|---|---|
| Authentic CDV | Bac-CDV-N | |||
| CDRCT-2 | Bac-CDV-N | 1 | 1,923 | 230 |
| CDRCT-5 | Bac-CDV-N | 2b | 941 | 176 |
| CDRCT-8 | Bac-CDV-N | 1 | 45,455 | 6,060 |
| CDTC-11 | CDV 5804/Han89 | 1 | 37,594 | 1,882 |
| CDTC-14 | CDV 5804/Han89 | 1 | 23,256 | 1,116 |
| CDTC-16 | CDV 5804/Han89 | 1 | 29,850 | 5,731 |
Calculations of affinity indices were made according to equations in reference 28.
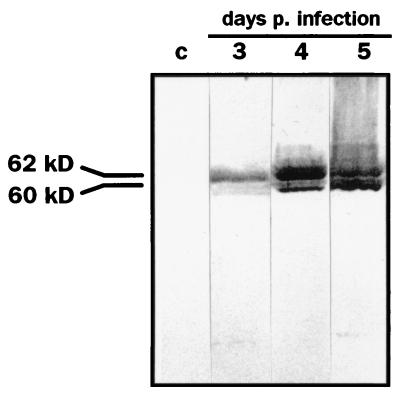
FIG. 1.
Expression of CDV 2544/Han95 N protein by recombinant baculovirus in H. virescens larvae as demonstrated by Western blot analysis. Larvae were prepared 3 to 5 days following subcuticular infection with 5 × 104 TCID50 of recombinant Bac-CDV-N or with wt AcNPV (c, control). Blots were probed with the N-specific MAb CDRCT-8 (Table 1). In each lane 10 μl of a lysate (prepared with 2% SDS in TBS) was loaded. At least 500 μl of lysate was produced from a single larva. p., post.
Production of recombinant CDV N protein.
Maximum expression of the CDV N protein with Bac-CDV-N in Sf9 cell cultures has been reported to occur at 72 h after infection at a multiplicity of infection of 1 to 2 (19). All antigen remained cell associated at this time point, as was evident from Western blot assays with antigen from infected cells and cell-free supernatant (data not shown). Yields of CDV N protein from infected larvae peaked between days 4 and 5 p.i. as shown in Fig. 1. The N protein was recovered as a 60- to 62-kDa protein from the larvae similar in size to the authentic viral protein; no evidence of proteolytic degradation was apparent until day 5 (Fig. 1).
Development of ELISA applications with recombinant CDV N protein. (i) Indirect ELISA.
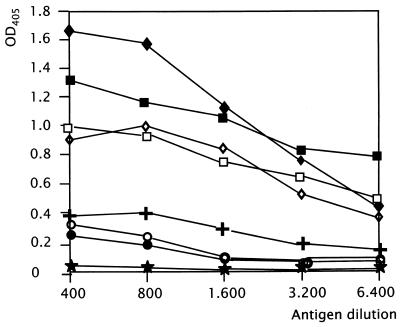
Since the vast majority of the baculovirus-expressed N protein from both Sf9 cultures and larvae was found to be cell associated, lysing conditions were expected to have a significant effect on the relative yield and quality of the recombinant antigen for use in ELISAs. Treatment of infected Sf9 cells with a 4 M urea buffer or TBS buffers containing either 1% SDS or Triton X-100 gave the best results as measured by an indirect ELISA with MAb CDRCT-8 for detection (Fig. 2). For use in an indirect ELISA the amount of antigen recovered from a single larva was sufficient to coat up to 200 microtiter plates, 10 times more that obtained from harvests of in vitro cultures of approximately 4 × 107.0 infected Sf9 cells. However, when canine sera were probed in this assay, we obtained unacceptably high background signals which were probably due to reactivity to cocoated cellular or baculovirus antigens (data not shown).
FIG. 2.
Yields and suitability of recombinant CDV N protein prepared from infected Sf9 in vitro cultures and from H. virescens larvae under different lysing conditions. Results were obtained by indirect ELISA with MAb CDRCT-8. Mock-infected Sf9 cells were used as a negative control. ⧫, 4 M urea in TBS (pH 8.7); ■, 2% SDS in TBS (pH 8.7) with lysate from larvae at a starting dilution of 1/1,000; ◊, 1% Triton X-100 in TBS (pH 8.7); □, 1% SDS in TBS (pH 8.7); +, aqua bidest; ○, beta-OTG in TBS (pH 8.7); ●, beta-OTG in 0.1 M glycine (pH 9.6); ★, negative control.
(ii) Capture-sandwich ELISA.
In order to minimize background noise and simultaneously avoid further purification steps, a capture assay was established. Four different N-specific MAbs were tested for their ability to capture and present the recombinant N protein from lysates of Bac-CDV-N-infected Sf9 cells prepared with 4 M urea, 1% SDS, or Triton X-100 buffers. The different combinations of capture antibody and antigen were then probed with a known positive canine serum (CDV specific; ND50, 1/5,000) and a certified negative canine serum (CDV specific; ND50, <1/5; serum from an SPF dog). MAb CDTC-16 in combination with antigen obtained by use of the TBS–1% SDS lysis buffer gave the highest signal-to-noise ratios (data not shown). This system was further optimized by fine-tuning MAb and antigen concentrations as well as the time, mode, and temperature of incubation. The following conditions were found to be optimal. The preferred MAb was determined to be CDTC-16 at 2 μg per ml in TBS buffer after incubation for 2 to 4 h at RT. The best antigen from Sf9 cells was the lysate that was obtained with 1% SDS in TBS, diluted 1/200 to 1/400 in TBS plus ELISA blocking reagent (1%), and incubated overnight at 4°C. The best antigen from larvae was the lysate that was obtained with 2% SDS in TBS, diluted 1/4,000, and incubated overnight at 4°C. Canine sera were best at a minimal dilution (1/20) in PBS-T.
Performance characteristics of the recombinant CDV N protein capture-sandwich ELISA.
The CDV-neutralizing antibody titers of 196 canine sera, starting from a dilution of 1/10, were determined. Lower dilutions, particularly of the Russian sera, were frequently cytotoxic. All samples were tested at least twice in independent assays. Sera showing an ND50 of <1/10 (n = 64; V-NA seronegative) were further examined by IPMA and Western blotting. Fifty-three of these sera were concordantly found to be free of CDV-specific IgG antibodies. These sera were used to adjust the ELISA cutoff level. For this purpose each serum was examined in duplicate in three independent ELISA runs at a single dilution of 1/20. The mean adsorbance at 405 nm of these samples amounted to 0.1 (standard deviation [SD] = 0.01). A value two times higher than the arithmetric mean (0.2) was defined as the cutoff value (15).
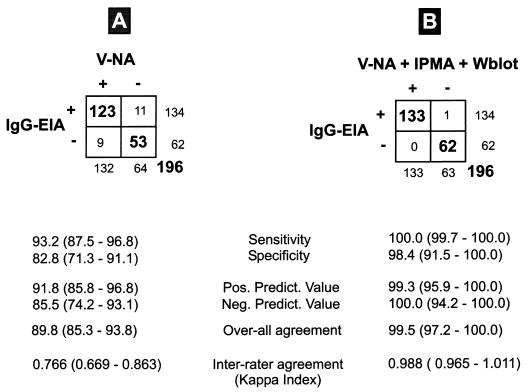
All 196 sera were then examined in duplicate serial twofold titration series by the capture-sandwich ELISA. The ELISA endpoint titer of a serum was expressed as the reciprocal of the maximum dilution showing an absorbance value of ≥0.21. Titers of ≥1/20 were considered seropositive. Results are shown in Fig. 3A. A total of 176 sera showed concordant qualitative results (correct positive versus correct negative results). Twenty sera gave discrepant results. Eleven Siberian samples, which were found to be free of CDV-neutralizing antibodies (ND50 < 1/10), gave a positive signal in the ELISA. In 10 of these, N protein-specific antibodies were also found in both the IPMA and the Western blot analysis. These sera, therefore, were considered truely positive in the ELISA. A single discrepant serum which gave a positive signal exclusively in the ELISA remained. Nine further samples obtained from dogs of northern Germany and showing ND50 between 1/10 and 1/120 in the V-NA reacted negatively in the ELISA as well as in the IPMA and Western blot analysis with an IgG-specific anticanine conjugate. However, when an anti-IgM conjugate was used, all nine sera reacted positively in these three assays. Therefore, these nine sera were considered truely negative in the IgG-specific ELISA. On the basis of the resolved data, the general performance characteristics of the IgG-specific capture-sandwich ELISA were recalculated (Fig. 3B).
FIG. 3.
Characteristics of the IgG-specific CDV N protein-based capture-sandwich ELISA compared to those of V-NA alone (A) and in combination with other nonneutralizing IgG-relevant test systems (B). Values in the boxes are numbers of samples. Performance parameters were calculated according to equations in reference 31. Values in parentheses are 95% confidence intervals. Wblot, Western blotting; EIA, enzyme immunoassay; Pos. Predict. Value, positive predictive value; Neg. Predict. Value, negative predictive value.
Correlation between ND50 and N-specific ELISA values.
We were particularly interested to learn whether the ELISA could be used for quantification of CDV N-specific antibodies and how the values related to ND50 (Fig. 4). A neutralizing titer of ≥1/100 induced by vaccination has been shown to correlate with protection (3, 25). Correlations were performed with 173 sera which gave initially concordant qualitative results when we compared their ELISA titers with their V-NA titers (this excludes three sera from SPF dogs). For ease of performance these sera were reexamined in a one-step-dilution (alpha) application of the ELISA at a single dilution of 1/100 in duplicate. Results were recorded as percentages of activity relative to those of a known positive and a known negative standard serum (15), determined with the equation percentage of activity = (ODsample − ODneg)/(ODpos − ODneg) × 100, where ODsample, ODneg, and ODpos are the OD405s of the sample, the negative standard serum, and the positive standard serum, respectively. A serum from one SPF dog which was used as standard negative serum (ND50 < 1/5) showed an OD405 of 0.09 (SD = 0.01). The standard positive serum (ND50 = 1/5,000) had been raised in a beagle dog against the CDV vaccine strain Rockborn (24). The ODpos − ODneg value was adjusted to 0.55 (SD = 0.05) by diluting the standard positive serum appropriately.
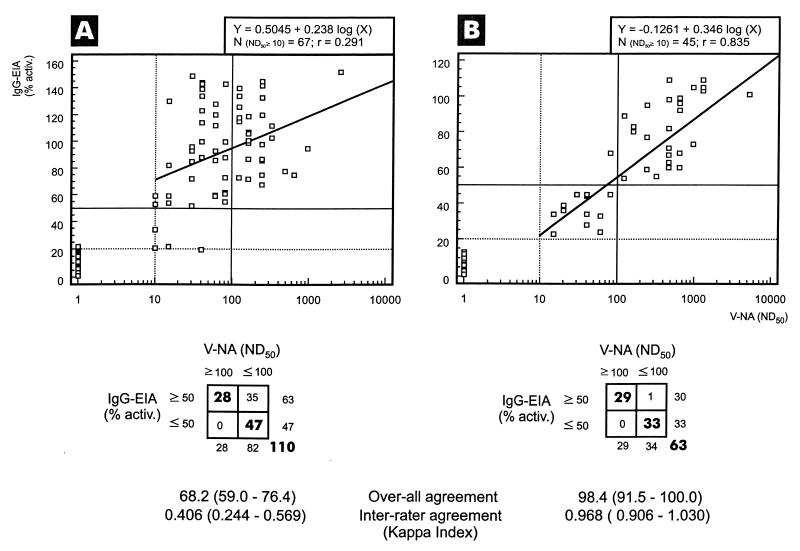
FIG. 4.
Correlation between values obtained by the one-step capture-sandwich ELISA (alpha method) and CDV-neutralizing antibody titers (ND50) in concordantly IgG-positive or -negative canine sera originating from Siberia, Russia (A), or from northern Germany (B). Sera were examined in duplicate by the recombinant CDV N protein-based ELISA at a single dilution of 1/100. Results were recorded as percentages of activity relative to those of a standard positive serum and a negative serum obtained with the following equation: percentage of activity = (ODsample − ODneg)/(ODpos − ODneg) × 100. Correlation (Spearman’s rank analysis) and regression tests were performed for sera showing ND50 titers of ≥1/10. Inter-rater agreement analysis was extended also to seronegative samples. A threshold value of ≥50% activity indicated the presence of a (protective) ND50 titer of ≥1/100 only in sera of panel B. Dotted horizontal and vertical lines indicate thresholds distinguishing seropositive from seronegative values. Solid horizontal and vertical lines indicate thresholds discriminating protective from nonprotective titers. % activ., percentage of activity; EIA, enzyme immunoassay. Values in boxes are numbers of samples with the indicated ND50 and percentages of activity. Values in parentheses are 95% confidence intervals.
Correlation between ND50 titers and percentages of activity were calculated only for sera showing an ND50 titer of ≥1/10 (n = 108). A correlation coefficient of 0.323 (Spearman’s rank analysis), however, indicated only weak correlation (data not shown). When the samples were stratified according to their origins from dogs of Siberia, Russia, or northern Germany, sera of the first cohort (Siberia) (n = 112, of which 63 had ND50 of ≥1/10; r = 0.291) (Fig. 4A) confirmed a weak correlation whereas sera from the second cohort (northern Germany), in sharp contrast, showed good correlation (n = 63, of which 45 had ND50 of ≥1/10; r = 0.835) (Fig. 4B). The difference between the correlation coefficients was highly significant (P < 0.0001).
Measurement of alpha values allowed the following conclusions. Samples displaying an activity of <20% (corresponding to the OD405 cutoff value of <0.20) proved to be CDV seronegative (ND50 <1/10) (Fig. 4). A percentage of activity of ≥50% correlated with an ND50 of ≥1/100 only in the cohort of sera from northern Germany (Fig. 4B). In fact, inter-rater agreement analysis indicated that an ELISA percentage of activity of 50% can be used without restriction as a substitute for the ND50 threshold titer of 1/100 in this cohort (κ = 0.968). In the sera sampled in Siberia, we observed a tendency towards higher N-specific antibody titers in relation to the ND50 measured against CDV 2544/Han95, which resulted in poor inter-rater agreement (Fig. 4A).
Long-term storage effects.
In order to evaluate the suitability of the CDV N protein capture-sandwich ELISA for use in routine serologic laboratories, the stability of the MAb-antigen preparation was tested. Several plates coated with MAb and antigen (ready for use) were stored for at least 3 months at −20°C. Half of the plates were pretreated with 1.5% formaldehyde before being frozen. Six known positive (ND50 ranging from 1/10 to 1/5,000) and negative (ND50 < 1/10) canine sera were tested in duplicate in parallel on freshly prepared (control) and frozen ready-to-use plates. The ELISA titers and percentages of activity (alpha method) determined did not differ between fresh and formaldehyde-treated frozen ready-to-use plates (mean difference of OD405 values = 0.1 [SD = 0.01]), whereas a significant loss of activity was evident with frozen plates not treated with formaldehyde (data not shown). Freshly prepared plates which were not treated with formaldehyde could be stored only up to 48 h at 4°C without significant loss of activity.
Detection of acute CDV infections by an IgM-specific capture-sandwich ELISA.
The cutoff value with a canine IgM-specific conjugate was adjusted with 20 sera which were shown by IPMA and Western blotting to be free of CDV-specific antibodies of the IgM class. An OD405 value of 0.2, which represents the doubled mean ODs of these sera tested in duplicate, was finally fixed. Results of the IgM-specific capture-sandwich ELISA were expressed as an index calculated according to the following formula: index = ODsample − ODnegative control/cutoff. Indices of ≥1.2 were considered positive, those of <1.0 were considered negative, and those between 1.0 and 1.2 were considered indeterminate.
Fifteen of 72 dogs presented for various clinical conditions at the Small Animal Clinic, Hannover Veterinary School, were suspected of having canine distemper based on the criteria mentioned in Materials and Methods. All 15 dogs had mounted neutralizing antibody titers between 1/10 and 1/120 ND50. Sera obtained from nine of these dogs reacted positively in the IgM ELISA, showing indices between 1.8 and 2.6, while all nine sera reacted negatively in the IgG ELISA. In buffy coat cells of five of the IgM-positive animals, CDV RNA was detected by RT-PCR, indicating a viremic phase in the course of an active infection (Table 2). In 6 of the 15 dogs, neither CDV-specific IgM nor CDV RNA was detected. Since paired serum samples were not available from these dogs, the clinical diagnosis of distemper could not be confirmed by seroconversion.
TABLE 2.
Detection of acute CDV infection by RT-PCR and IgM capture-sandwich ELISA
| No. of dogs | No. with clinical symptomsa | No. of dogs (n = 15)
|
||
|---|---|---|---|---|
| RT-PCR positiveb | IgM ELISA positivec | IgM ELISA and RT-PCR negative | ||
| 72 | 15 | 5 | 9 | 6 |
Dogs clinically suspected of canine distemper presented with at least three of the following symptoms: fever, mucopurulent nasal and/or conjunctival discharge, bronchopneumonia, central nervous system disorders, and gastroenteritis.
Number of dogs positive by RT-PCR for CDV RNA in buffy coat cells.
Number of dogs positive by the ELISA for CDV-specific IgM in serum.
DISCUSSION
In this study we report on the development of a capture-sandwich ELISA suitable for the rapid and sensitive detection of canine IgG and IgM antibodies specific for CDV. The assay is based on a recombinant wt CDV N protein produced in the baculovirus expression system.
In a previous study we showed by immunological and biochemical analyses that CDV N protein expressed by recombinant baculoviruses in Sf9 insect cell cultures appears to be indistinguishable from authentic N protein expressed by CDV-infected Vero cells (19). In the framework of the present investigation, however, MAbs which were raised against both the authentic and the recombinant CDV N protein revealed a lowered affinity for baculovirus-expressed N protein. The basis of this phenonemon was not further investigated since it apparently did not create a significant impact on the suitability of the antigen for use in an ELISA. We have further demonstrated that the recombinant CDV N protein can be produced in H. viriscens larvae at a quality similar to that of infected Sf9 cell cultures. With respect to ease, efficacy, and costs of production, the larval system was found to be clearly superior to insect cell cultures. This result is in line with studies of the recombinant expression of N proteins of other morbilliviruses (12, 22, 23).
In a first attempt to use the recombinant antigen for the detection of canine CDV-specific antibodies, direct coating of the antigen failed to give acceptable results due to high background signals. A capture-sandwich application, using a capture antibody in combination with an antigen solution prepared in the presence of SDS, provided improved conditions. The capture antibody served to enrich for the specific antigen and, thus, reduced nonspecific reactivity due to the presence of cellular or baculoviral antigens (11). The SDS was expected to break up intermolecular N protein aggregrations as well as intramolecular secondary structures. Obviously, these conditions made (nonconformational) B-cell epitopes along the N protein accessible to canine antibodies.
Among a total of 196 sera, 11 false-positive and 9 false-negative samples were initially detected with the capture-sandwich ELISA, when the V-NA was regarded as the gold standard. In 10 of the 11 false-positive samples, all of Siberian origin, however, CDV-specific antibodies were also detected by IPMA. Western blotting revealed these antibodies to be specific for CDV N as well as, in some sera, phosphoprotein. These samples, therefore, must be regarded as CDV seropositive, although antibodies with neutralizing capacities were undetectable. The N protein is the immunodominant morbillivirus protein and elicits the most vigorous antibody response in all morbillivirus infections. Therefore, in individuals with waning morbillivirus-specific humoral immunity, N-specific antibodies may still be detectable when H- and F-specific (neutralizing) antibodies are below detection levels. The use of a N protein-based assay may be more sensitive than V-NA when the qualitative CDV serostatus is evaluated. In addition, it has been shown that in certain cases of fulminant acute distemper infections, as well as in CDV-induced chronic demyelinating central nervous system disease, there is a restricted immune response to the hemagglutinin (H) and fusion (F) glycoproteins carrying neutralization sites but that the humoral response to nucleocapsid proteins appears to be comparatively unimpaired (26, 30).
All nine samples which tested negative in the IgG ELISA but proved to harbor CDV-neutralizing antibodies were consecutively shown by IPMA, Western blotting, and an IgM-specific capture-sandwich ELISA to carry CDV-specific antibodies of the IgM class exclusively and, thus, probably reflect an early stage of a CDV infection. Indeed, these sera had been obtained from dogs presenting with clinical signs consistent with acute virulent CDV infection, and in five animals a cell-associated CDV viremia was demonstrated by RT-PCR. As such, the CDV N protein-based IgM-specific ELISA may also be helpful in the serological diagnosis of acute distemper infections.
Having resolved these discrepant results, the performance characteristics of the IgG sandwich ELISA are promising and, in terms of sensitivity, even superior to those of the V-NA as far as qualitative results are concerned. The V-NA, however, provide additional information. Although T-cell-mediated immunity is essential in clearing morbillivirus infections, the ND50 antibody titer can be used as a surrogate marker of the resilience of a CDV-specific immunity. Dogs presenting with titers of ≥1/100 are considered to be protected from distemper (26). To test for a correlation between N-specific antibody titers and the ND50 of a serum, a one-step-dilution (alpha method) ELISA was implemented. When we analyzed these results statistically, a bias related to the sera’s geographic origins (Hannover, Germany, versus Siberia, Russia) was revealed. Results with sera obtained from German dogs showed a good correlation (r = 0.835, Spearman’s rank analysis) compared to results with sera obtained from Russian dogs (r = 0.291). The Russian sera displayed comparatively higher alpha values in relation to the ND50. Alpha values of ≥50%, therefore, could be taken as surrogate markers for ND50 titers of ≥1/100, which indicate the presence of a resilient CDV-specific immunity, for sera sampled in Germany but, at present, not for sera sampled in Siberia. Three reasons may account for this discrepancy: (i) sampling and storage conditions, (ii) neutralizability of wt CDV virus used for V-NA, and (iii) phylogenetic differences of viruses used for V-NA and for immunization of dogs. (i) While the sera from Germany were sampled and stored under optimal conditions, the Russian sera were obtained and processed under semisterile circumstances and had experienced several cycles of freezing and thawing before examination. This might have affected the stability of neutralizing antibodies more than of (the presumably more abundant) N-specific antibodies. (ii) The CDV wt isolate 2544/Han95 was used in all V-NA. This isolate originated from the same area from which the German canine sera were obtained. By phylogenetic analysis, this isolate has been found to belong to a currently circulating central European CDV genotype (17). wt isolates from Russia (Lake Baikal, Siberia), in contrast, were shown to belong to a different genotype (7). The genotypic differences are reflected phenotypically at the amino acid level of the hemagglutinin protein, which elicits the majority of neutralizing antibodies. The nucleocapsid protein, however, displays an overall higher degree of nucleotide sequence and antigen conservation (19). As such, the less significant correlation between N-specific alpha values and ND50 titers of the Siberian sera might have been due to the use of a heterologous CDV isolate from Germany displaying a slightly different antigenic makeup in the hemagglutinin protein. Unfortunately, no Siberian isolate was available for use in complementing V-NA. (iii) Most of the 63 German dogs had a vaccination record, but all 110 Siberian dogs did not. This suggests that all positive Siberian sera reflect wt CDV infections but that most of the positive German sera probably reflect vaccine-induced immunity. CDV vaccine strains, however, have been shown to form yet another genotype that is distinct from both central European and Siberian wt CDV (20).
On the basis of these data it cannot be determined with certainty whether the weak correlation between ND50 titers and N-specific ELISA titers observed with the Siberian sera is due to the antigenic makeup of the CDV isolate used for V-NA or the N protein of the capture-sandwich ELISA. However, minor strain-specific influences on virus-neutralizing antibody titers have been demonstrated for rinderpest (31), measles (34), and distemper (18) viruses, although commonly the morbillivirus species are considered serologically monotypic. In CDV serology, minor strain-specific antigenic variation causes us to have reservations about the validity of the ND50 threshold titer of 1/100 since it is likely to vary with the CDV strain used in V-NA and it also challenges the role of V-NA as the gold standard. In view of our data on the Siberian sera, use of the V-NA for seroprevalence studies may lead to an underestimation of prevalence rates when no local (i.e., homologous) CDV isolate is at hand. So, at least for screening purposes, the N-specific capture-sandwich IgG ELISA may prove to be a first-choice option.
In conclusion, results of the recombinant one-step-dilution capture-sandwich ELISA introduced here showed very good agreement in an inter-rater analysis with results of V-NA, and it may therefore be a practicable and cost-effective substitute for CDV V-NA in an evaluation of qualitative CDV-specific IgG serostatus. This ELISA format likewise appears to be suitable for serologic diagnosis of acute distemper infections by detection of specific IgM. Provisional results from ongoing studies in our lab indicate that the ELISA can be easily adapted for detection of CDV-specific antibodies also in sera of noncanine species, such as the exotic felids (unpublished data).
ACKNOWLEDGMENTS
We thank G. Knauf and K. Grosser, Agrevo, Frankfurt am Main, Germany, for their gift of H. virescens larvae and for indispensable advice concerning larval culture and baculovirus infection.
Parts of this study were funded by the DAAD and the Small Animal Clinic, Hannover Veterinary School.
REFERENCES
- 1.Appel M J G, Gillespie J H. Canine distemper virus. Virol Monogr. 1972;11:1–96. [Google Scholar]
- 2.Appel M J G, Robson D S. A microneutralization test for canine distemper virus. Am J Vet Res. 1973;34:1459–1463. [PubMed] [Google Scholar]
- 3.Appel M J G, Shek W R, Sheshberadaran H, Norrby E. Measles virus and inactivated canine distemper virus induce incomplete immunity to canine distemper. Arch Virol. 1984;82:73–82. doi: 10.1007/BF01309369. [DOI] [PubMed] [Google Scholar]
- 4.Appel M G J, Yates R A, Foley G L, Bernstein J J, Santinelli S, Spelman L H, Miller L D, Arp L H, Anderson M, Barr M, Pearce-Kelling S, Summers B A. Canine distemper epizootic in lions, tigers, and leopards in North America. J Vet Diagn Investig. 1994;6:277–288. doi: 10.1177/104063879400600301. [DOI] [PubMed] [Google Scholar]
- 5.Blixenkrone-Möller M, Pedersen I R, Appel M J, Griot C. Detection of IgM antibodies against canine distemper virus in dog and mink sera employing enzyme-linked immunosorbent assay (ELISA) J Vet Diagn Investig. 1991;3:3–9. doi: 10.1177/104063879100300102. [DOI] [PubMed] [Google Scholar]
- 6.Blixenkrone-Möller M, Svansson V, Have P, Örvell C, Appel M J G, Pedersen I R, Dietz H H, Henriksen P. Studies on manifestations of canine distemper in urban dog populations. Vet Microbiol. 1993;37:163–173. doi: 10.1016/0378-1135(93)90190-i. [DOI] [PubMed] [Google Scholar]
- 7.Bolt G, Jensen T D, Gottschalk E, Arctander P, Appel M J G, Buckland R, Blixenkrone-Möller M. Genetic diversity of the attachment (H) protein gene of current field isolates of canine distemper virus. J Gen Virol. 1997;78:367–372. doi: 10.1099/0022-1317-78-2-367. [DOI] [PubMed] [Google Scholar]
- 8.Chappius G. Control of canine distemper. Vet Microbiol. 1995;44:351–358. doi: 10.1016/0378-1135(95)00028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J. A coefficient of agreement for nomical scales. Ed Psychol Meas. 1960;20:37–46. [Google Scholar]
- 10.Diallo A, Libeau G, Couacy-Hymann E, Barbron M. Recent developments in the diagnosis of rinderpest and peste-des-petits-ruminants. Vet Microbiol. 1995;44:307–317. doi: 10.1016/0378-1135(95)00025-6. [DOI] [PubMed] [Google Scholar]
- 11.Erdman D E, Anderson L J, Adams D R, Stewart J A, Markowitz L E, Bellini W J. Evaluation of monoclonal antibody-based capture enzyme immunoassays for detection of specific antibodies to measles virus. J Clin Microbiol. 1991;29:1466–1471. doi: 10.1128/jcm.29.7.1466-1471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fooks A R, Stephenson J R, Warnes A, Dowsett A B, Rima B K, Wilkinson G W G. Measles virus nucleocapsid protein expressed in insect cells assembles into nucleocapsid-linked structures. J Gen Virol. 1993;74:1439–1444. doi: 10.1099/0022-1317-74-7-1439. [DOI] [PubMed] [Google Scholar]
- 13.Gemma T, Miyashita N, Shin Y-S, Okita M, Mori T, Iwatsuki K, Mikami T, Kai C. Serological survey of canine distemper virus infection using enzyme-linked immunosorbent assay. J Vet Med Sci. 1995;57:761–763. doi: 10.1292/jvms.57.761. [DOI] [PubMed] [Google Scholar]
- 14.Greiser-Wilke I, Moennig V, Coulibaly C O Z, Dahle J, Leder L, Liess B. Identification of conserved epitopes on a hog cholera protein. Arch Virol. 1990;111:213–225. doi: 10.1007/BF01311055. [DOI] [PubMed] [Google Scholar]
- 15.Gripenberg M, Linder E, Kurki P, Engvall E. A solid phase enzyme-linked immunosorbent assay (ELISA) for the demonstration of antibodies against denatured, single-stranded DNA in patient sera. Scand J Immunol. 1978;7:151–157. doi: 10.1111/j.1365-3083.1978.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 16.Haas L, Subbarao S M, Harder T C, Liess B, Barrett T. Detection of phocid distemper virus RNA in seal tissues using slot hybridization and the polymerase chain reaction: genetic evidence that the virus is distinct from canine distemper virus. J Gen Virol. 1991;72:393–398. doi: 10.1099/0022-1317-72-4-825. [DOI] [PubMed] [Google Scholar]
- 17.Haas L, Martens W, Greiser-Wilke I, Mamaev L, Butina T, Maack D, Barrett T. Analysis of the haemagglutinin gene of current wild-type canine distemper virus isolates from Germany. Virus Res. 1997;48:65–71. doi: 10.1016/s0168-1702(97)01449-4. [DOI] [PubMed] [Google Scholar]
- 18.Harder T C, Klusmeyer K, Frey H-R, Örvell C, Liess B. Intertypic differentiation and detection of intratypic variants among canine and phocid morbillivirus isolates by kinetic neutralization using a novel immunoplaque assay. J Virol Methods. 1993;41:77–92. doi: 10.1016/0166-0934(93)90164-m. [DOI] [PubMed] [Google Scholar]
- 19.Harder, T. C., V. Von Messling, C. Örvell, V. Moennig, and L. Haas. Expression of wild-type canine distemper virus nucleocapsid, fusion and hemagglutinin proteins in recombinant baculoviruses. Submitted for publication.
- 20.Harder T C, Osterhaus A D M E. Canine distemper virus—a morbillivirus in search of new hosts? Trends Microbiol. 1997;5:120–124. doi: 10.1016/S0966-842X(97)01010-X. [DOI] [PubMed] [Google Scholar]
- 21.Harder T C, Willhaus T, Frey H-R, Liess B. Morbillivirus infection of seals (Phoca vitulina) during the 1988 epizootic in the bay of Heligoland. III. Transmission studies of cell culture propagated phocine distemper virus in harbour seals (Phoca vitulina) and grey seals (Halichoerus gryphus): clinical, virological and serological results. J Vet Med Ser B. 1990;37:641–650. [PubMed] [Google Scholar]
- 22.Hummel K B, Erdman D D, Heath J, Bellini W J. Baculovirus expression of the nucleoprotein of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J Clin Microbiol. 1992;30:2874–2880. doi: 10.1128/jcm.30.11.2874-2880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismail T, Ahmad S, d’Souza-Ault M, Bassiri M, Saliki J, Mebus C, Yilma T. Cloning and expression of the nucleocapsid gene of virulent Kabete O strain of rinderpest virus in baculovirus: use in differential diagnosis between vaccinated and infected animals. Virology. 1994;198:138–147. doi: 10.1006/viro.1994.1016. [DOI] [PubMed] [Google Scholar]
- 24.Jäger M, Liess B, Harder T C, Ising S, Stoye M. Experimental inoculation of beagle dogs permits serological differentiation of phocine and canine distemper virus. Wien Tieraerztl Monschr. 1990;77:105–108. [Google Scholar]
- 25.Kaerber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihen-versuche. Arch Exp Pathol Pharmakol. 1931;162:480. [Google Scholar]
- 26.Krakowka S, Olsen R, Confer A, Koestner A, McCullough B. Serologic response to canine distemper viral antigens in gnotobiotic dogs infected with canine distemper virus. J Infect Dis. 1975;132:384–392. doi: 10.1093/infdis/132.4.384. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleaveage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lebich M, Harder T C, Frey H-R, Visser I K G, Osterhaus A D M E, Liess B. Comparative immunological characterization of type-specific and conserved B cell epitopes of pinniped, feline and canine herpesviruses. Arch Virol. 1994;136:335–347. doi: 10.1007/BF01321062. [DOI] [PubMed] [Google Scholar]
- 29.Potgieter L N D, Ajidagba P A. Quantitation of canine distemper virus and antibodies by enzyme linked immunosorbent assays using protein A and monoclonal antibody capture. J Vet Investig. 1989;1:110–115. doi: 10.1177/104063878900100203. [DOI] [PubMed] [Google Scholar]
- 30.Rima B K, Duffy N, Mitchell W J, Summers B A, Appel M J G. Correlation between humoral immune responses and presence of virus in the CNS of dogs experimentally infected with canine distemper virus. Arch Virol. 1991;121:1–8. doi: 10.1007/BF01316739. [DOI] [PubMed] [Google Scholar]
- 31.Rossiter P B, Taylor W P, Crowther J R. Antigenic variation of rinderpest virus detected by kinetic neutralization and competition ELISA using early rabbit sera. Vet Microbiol. 1988;16:195–200. doi: 10.1016/0378-1135(88)90044-2. [DOI] [PubMed] [Google Scholar]
- 32.Ryan P. Evaluating laboratory tests and determining their usefulness. Vet Med. 1991;86:874–880. [Google Scholar]
- 33.Schoonjans F, Zalata A, Depuydt C E, Comhaire F H. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed. 1995;48:257–262. doi: 10.1016/0169-2607(95)01703-8. [DOI] [PubMed] [Google Scholar]
- 34.Tamin A, Rota P A, Wang Z D, Heath J L, Anderson L J, Bellini W J. Antigenic analysis of current wild-type and vaccine strains of measles virus. J Infect Dis. 1994;170:1269–1275. doi: 10.1093/infdis/170.4.795. [DOI] [PubMed] [Google Scholar]
- 35.Visser I K G, Kumarev V P, Örvell C, de Vries P, Broeders H W J, van de Bildt M W G, Groen J, Teppema J S, Burger M C, Uytdehaag F G C M, Osterhaus A D M E. Comparison of two morbilliviruses isolated from seals during outbreaks of distemper in North West Europe and Siberia. Arch Virol. 1990;111:149–164. doi: 10.1007/BF01311050. [DOI] [PubMed] [Google Scholar]
- 36.Zaghawa A, Liess B, Frey H-R. Antiserum raised in pigs against canine distemper virus and its utility in diagnostic procedures for morbillivirus infections (canine distemper, phocine distemper, rinderpest) J Vet Med Ser B. 1990;37:353–362. doi: 10.1111/j.1439-0450.1990.tb01069.x. [DOI] [PubMed] [Google Scholar]