Abstract
Nanozyme is a series of nanomaterials with enzyme-mimetic activities that can proceed with the catalytic reactions of natural enzymes. In the field of biomedicine, nanozymes are capturing tremendous attention due to their high stability and low cost. Enzyme-mimetic activities of nanozymes can be regulated by multiple factors, such as the chemical state of metal ion, pH, hydrogen peroxide (H2O2), and glutathione (GSH) level, presenting great promise for biomedical applications. Over the past decade, multi-functional nanozymes have been developed for various biomedical applications. To promote the understandings of nanozymes and the development of novel and multifunctional nanozymes, we herein provide a comprehensive review of the nanozymes and their applications in the biomedical field. Nanozymes with versatile enzyme-like properties are briefly overviewed, and their mechanism and application are discussed to provide understandings for future research. Finally, underlying challenges and prospects of nanozymes in the biomedical frontier are discussed in this review.
Graphical Abstract
Keywords: Nanozyme, Oxidative stress, Reactive oxygen species, Disease therapy
Background
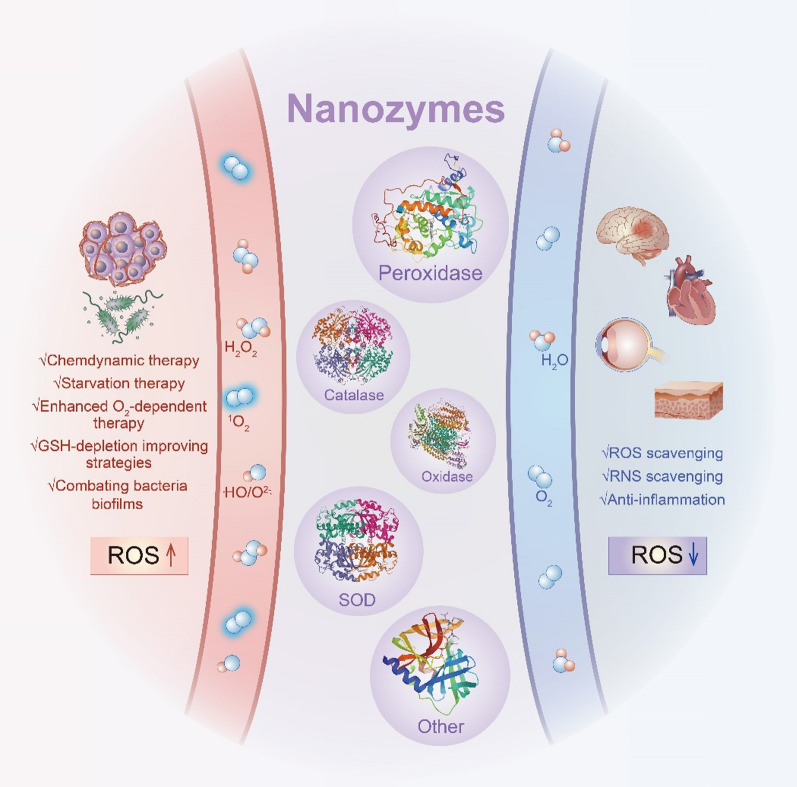
Nanozymes, as artificial enzymes, are nanomaterials with enzyme mimetic activities, which have attracted considerable interest due to their relatively higher physiochemical stability against harsh environments, higher durability, and lower costs than natural enzymes [1]. In the past decades, numerous nanomaterials have been revealed to elucidate the oxidase (OXD), glucose oxidase (GOD), peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) mimicking activities with extensive biomedical applications [2, 3]. At present, nanozymes are mainly composed of metal and metal oxides, since the metallic active center can effectively mimic the catalytic electronic redox process enabled by natural enzymes. Specifically, the enzyme-mimicking activities are affected by various factors, such as the oxidation states of the metallic center, reduction agent, temperature, and pH in the surrounding environment [4, 5]. Interestingly, the disease features differ from normal tissues provide typical therapeutic options for rational design and application of nanozymes in biomedicine. It is well known that the tumor microenvironment (TME) exhibits higher redox potential levels than the normal tissues. Such characteristics in the tumor can catalyze enzyme-like activities of the nanozymes [6–8]. For instance, metallic ions (such as Fe3+, Cu2+, and Mn4+, etc.) can be reduced to lower-valent metallic ions (Fe2+, Cu+, and Mn3+) by intracellular GSH [9–11]. Hence, the POD activities and catalytic efficiency of the nanozymes could be altered remarkably in the specific pathological microenvironment.
Although numerous nanozymes having been made in the biomedicine field, it is still challenging to obtain a fundamental insight into the key factors that affect the catalytic performance, enzymatic-likes properties, as well as the substrate selectivity of nanozymes, on the basis of the interplay between intrinsic structure and extrinsic environment [12, 13]. Moreover, the catalytic mechanisms of metal oxide nanozymes are pivotal to rationally design novel nanozymes with inherent catalytic capacities and this approach has been widely applied in biomedicine as a controllable multifunctional platform [14, 15]. Recently, many types of nanoparticles with inherent catalytic properties have been reported to achieve various biomedical applications, including oxygen-dependent tumor therapy, radiotherapy, chemodynamic therapy, bacterial infection diseases, and reactive oxygen species (ROS)-related diseases, etc. [16–20]. Therefore, recent advances in the field of nanozyme’s biomedical application may bring new insights into the popularization of nanoparticles in the treatment of the biomedical field.
In this review, Different metal- or metal-based nanozymes have been overviewed and described as classified according to their catalytic active center, which significantly impacts the functionalities and activities of the nanozymes during certain catalytic reactions. The versatile enzymes-like properties, mechanism of nanozymes, and the factors that affect the catalytic performance are initially summarized. Then, recently administrated strategies of nanozymes in the therapeutic frontier have been introduced (Scheme 1). Finally, the current challenges of the development of nanozymes and prospects are discussed. We hope that the present review will be of significant benefit for different biomedical fields and provide insightful ideas for the design and development of nanozymes.
Scheme 1.
Schematic illustration of metal-based nanozymes for biomedical application
Cerium-based nanozymes
Cerium (Ce)-based nanoparticles have been exploited for biomedical applications since they exhibit multiple enzyme-like activities such as catalase- (CAT), peroxidase- (POD), cytochrome c oxidase-, and superoxide dismutase-mimetic (SOD) functions [21–23]. The underlying mechanism of nanoceria-mediated enzymatic reactions was associated with the chemical state of the cerium element. The reduction state (Ce3+) and oxidation state (Ce4+) affect the enzyme-like performance of CeO2 [24, 25]. Singh et al. reported that CeVO4 nanoparticles exhibited cytochrome c oxidase (CcO) activity, which can dismutase oxygen into water at physiological pH conditions due to the electron transfer between Ce3+, Ce4+, and V5+ [26]. Interestingly, the ratio of Ce3+/Ce4+ of CeVO4 has been shown to affect the CcO-like activity of CeVO4. The authors suggested that the lower ratio Ce3+/Ce4+ had higher CcO-like activity in CeVO4 while the higher ratio of lowered valence states (Ce3+) corresponds to the higher SOD-like activity. The present study demonstrated the enzyme-mimetic activities of Ce-based nanozymes associated with available oxidation states. Importantly, recent studies manifested that the surface defect characteristics of CeO2 could partly affect the enzyme-like capability. Recently, Wang et al. revealed the SOD- and CAT mimicking mechanisms of CeO2 by first-principles calculations [27]. Their results suggested that oxygen vacancies played critical roles to scavenge superoxide anion (O2•−) and hydrogen peroxide (H2O2). The oxygen vacancies impacting enzyme-mimic activities were mainly ascribed to the reduced activation energy and the formation of the intermediate species. This research suggests that the reduction of activation energy by CeO2 is critically important in exploring the catalytic processes. Their catalytic activity is substantially affected by the intrinsic properties (e.g., dimensions, oxygen vacancy) and physiological factors such as pH, GSH, and temperature. Meanwhile, the oxygen vacancy concentrations were highly dependent on the particle size of CeO2 [28].
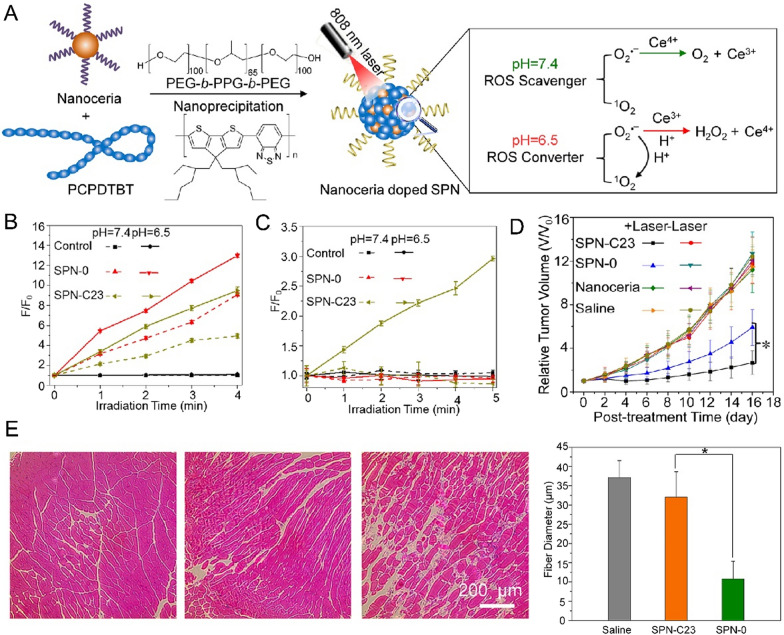
Despite the advancements made in biomedicine, nanoceria has key limitations that need to be overcome. For instance, the precise regulation of the enzyme activities remains challenging for nanoceria to meet the biomedical application. Moreover, the toxicity of nanoceria against normal tissues still presents great challenges in achieving clinical application. Previous studies have explored the toxicity effect of CeO2 of varied shapes in RAW264.7 cell line. Compared with the cubic/octahedral morphological nanoparticles, the rod-like CeO2 shows increasing serum concentration of tumor necrosis factor alpha (TNF-α) and lactate dehydrogenase (LDH) release, demonstrating the morphological-dependent cytotoxicities [29]. The biocompatibility of CeO2 nanoparticle was demonstrated in further in vivo studies in rats when administrating CeO2 of as high as 20 mg/kg [30]. These observations provide an important inspiration for other nanozyme to achieve higher therapeutic effect with minimal toxicity. To solve the low selectively and poor therapeutic effects, it is highly appealing to design controllable nanoceria-based therapeutic systems. Noteworthily, the catalytic behavior of nanoceria is also determined by physiologically catalytic environments. By controlling enzyme activities in a TME or light stimuli, a desired therapeutic effect with low tissue damage could be achieved. Zhu et al. reported that the self-regulated nanoceria-doped poly-(cyclopentadithiophene-alt-benzothiadiazole) (SPN-C23) as smart nanoplatforms for tumor photodynamic therapy (PDT) [31]. When near-infrared (NIR) laser was irradiated against the tumor tissue with acidic microenvironment (pH = 6.5), SPN-C23, acted as a ROS converter, was able to amplify PDT damage against tumor tissues through catalyzing O2•− to produce H2O2 (Fig. 1A). When exposed to the normal microenvironment (pH = 7.4), Ce4+ of SPN-C23 could transform O2•− to O2 with generated Ce3+ due to oxygen vacancies in the surface of nanoceria. Consequently, SPN-C23 exhibited high singlet oxygen sensor green (SOSG) fluorescence enhancement at pH = 6.5 and possessed relatively low fluorescence intensity at pH = 7.4 (Fig. 1B), demonstrating that pH-dependent single oxygen species (1O2) production of SPN-C23. SPN-C23 also possesses higher fluorescence at pH = 6.5 with NIR laser irradiation, indicating that the generation of H2O2 (Fig. 1C). PDT efficiency of SPN-C23 was evaluated on in vivo 4-T1 xenograft tumor model. Under NIR laser irradiation, the growth of the tumor in SPN-C23 treated group was significantly suppressed after intravenous injection of SPN-C23 for 16 days (Fig. 1D). Tissue damage by SPN-0 and SPN-C23 were examined by histological (H&E) staining respectively. The damaged area of the healthy muscle tissues in the SPN-C23 group was significantly decreased as compared to the SPN-0 treatment group (Fig. 1E). Their study provides an effective approach by utilizing ceria-based nanozyme to regulate PDT against cancer with high biocompatibility.
Fig. 1.
A Scheme for nanoceria of self-regulated photodynamic properties at various tissue physiological conditions. B SOSG detects 1O2 production of control, SPN-0, and SPN-C23 under 808 nm laser irradiation. C HRP/Amplex Red detects H2O2 generated at pH = 6.5 or pH = 7.4 under NIR laser irradiation. D Relative tumor growth curves after intravenous injection of saline and varied treatment groups. E H&E staining for evaluated tissues damaged after PDT [31].
Copyright 2017 American Chemical Society
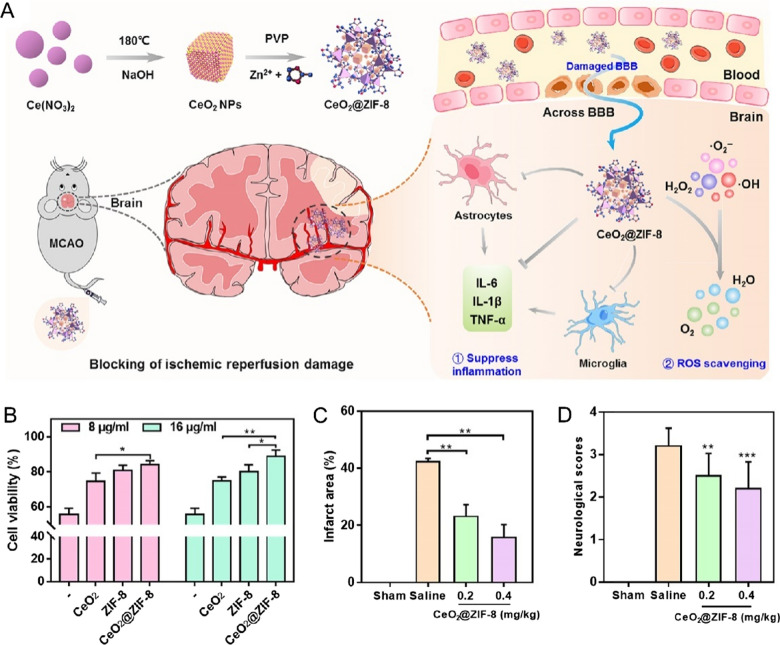
ROS as the signaling messengers play an essential function in the physiological signal transduction pathway [32]. Endogenous and low concentrations of ROS can be produced from normal metabolic processes in the living cells [33, 34]. However, ROS are highly toxic at higher concentrations and can damage protein, RNA, and DNA, leading to cell death [32, 35]. Therefore, ameliorating the oxidative stress induced by ROS has been proved to be beneficial in pathological therapy, such as age-related macular degeneration (AMD), traumatic brain injury, and ischemic disease [23, 36–38]. Nanoceria is often employed as an antioxidant agent to treat ROS-relevant diseases. For instance, AMD is associated with irreversible ROS damage against the macular that may lead to blindness [39, 40]. Generally, evidence showed that AMD pathology can be treated by counteracting the overproduction of ROS. Mitra et al. reported a nanoceria with dominated Ce3+ that can scavenge the ROS, such as H2O2 and ·OH, and inhibit neovascularization formation [41]. Moreover, Yan et al. developed a single-atom Pt/CeO2 for traumatic brain injury treatment [36]. Compared with CeO2, Pt/CeO2 exhibits highly enzymatic activity that can scavenge O2•−, ·NO, and ·OH. Furthermore, in vivo administration of Pt/CeO2 to C57BL/6 mice can significantly improve the wound recovery by up to 100%, higher than the mice in the untreated group with 50% of the wound closure. Similarly, ischemic stroke is one of the inflammations associated with excessive ROS generation. With excellent antioxidation activity, ceria nanozymes have been applied for efficient treatment of reperfusion-induced injury in ischemic stroke [42]. Although nanoceria with ROS eliminating ability can protect the cells from ROS damage, physiological stability and biocompatibility remained a challenge towards further clinical prospects. Regarding the present issue, He et al. designed a multifunctional nano-system for treating ischemic stroke with prolonged blood circulation time and higher biosafety [43]. CeO2@ZIF-8 were prepared by in-situ capping of CeO2 with zeolitic imidazolate framework-8 (ZIF-8) (Fig. 2A). In their design, ZIF-8 was served as peroxidase to maintain the activity of CeO2, as well as to enhance the penetration and accumulation of CeO2 to brain tissue. In PC12 neuronal cells, CeO2@ZIF-8 can effectively protect cells from tert-butyl hydroperoxide (t-BOOH) induced cell apoptosis (Fig. 2B). In vivo administration of CeO2@ZIF-8 could significantly reduce the infarcted area and increased the neurological scores of mice, confirming that CeO2@ZIF-8 can effectively treat mice with ischemic stroke (Fig. 2C, D).
Fig. 2.
A Fabrication methodologies and therapeutic mechanism of CeO2@ZIF-8 nanozymes. B The viability of PC12 cells co-treated with t-BOOH and nanozymes for two days. C Infarct areas of various groups after treatment (n = 4). D Neurological scores of different groups with CeO2@ZIF-8 therapy for three days (n = 10) [43].
Copyright the Authors 2020
Another interesting application of nanoceria was the elimination of extracellular DNA (eDNA) for anti-biofilms. Biofilms, a community of bacteria cells, prevents a majority of approaches to treat bacterial infectious diseases in humans. eDNA is a crosslinking component of bacteria biofilm, which provides a potential survival benefit for bacterial infection. It has been established that eDNA has a profound impact on the process of biofilm formation. Therefore, eradicating eDNA is an effective strategy to treat biofilm infection. Functionalization of CeO2 offers a versatile approach in combating biofilm formation and bacterial infection. Liu et al. designed metal–organic framework (MOF)/Ce-based nanozymes to combat biofilms [44]. The MOF/Ce nanozymes with DNase mimic activities could not only inhibit the biofilm formation but also eradicate established biofilm matrix components by hydrolyzing eDNA. As compared to primitive MOF, MOF/Ce nanozymes possessed higher bactericidal activity via co-incubation. The mechanism of the bactericidal activity of MOF/Ce nanozymes is that two adjacent Ce4+ could bind to the oxygen atom of the phosphate group by withdrawing the electrons, resulting in phosphodiester linkage cleaving [45].
Ferrum-based nanozymes
Ferrum (Fe)-based nanoparticles have gained extensive attention for various biomedical applications due to their magnetic resonance imaging (MRI) performance, POD-mimetic activity, and CAT mimetic properties [46, 47]. Huo et al. reported that Fe3O4 nanoparticles and GOD co-encapsulated into the mesoporous silica nanoparticles could be effective for tumor catalytic therapy [48]. The intrinsic POD-mimetic activity of Fe3O4 could generate a considerable amount of ·OH from H2O2 produced by the GOD catalysis from glucose. Importantly, the efficiency of ·OH production efficiency involving the amount of Fe2+. Compared with the reaction kinetics of Fe2+ with H2O2 (40–80 L mol−1 s−1), the reaction rate of Fe3+ (9.1 × 10–7 L mol−1 s−1) with H2O2 is relatively low [49, 50]. In addition, several reports have established links between POD-mimetic activity and CAT mimicking property of Fe3O4 under various conditions. Gao et al. investigated the catalytic mechanism of Fe2+/Fe3+ proportion in Fe3O4 with the use of either a reducing agent (NaBH4) or an oxidizing agent (NaIO4) [51]. They found that increased Fe2+/Fe3+ proportion of Fe3O4 could be achieved by NaBH4 treatment, with the correspondingly enhanced peroxidase-like activity of Fe3O4. On the contrary, decreased proportion of Fe2+/Fe3+ treated by NaIO4 reduces the POD-like activity of Fe3O4. In addition, the authors showed that the pH, temperature, and dimension of nanoparticles could effectively influence the enzyme-like activity of the Fe3O4. These pieces of evidence provide insights into the metallic species and their impacts on the enzyme activities of Fe3O4. Another study by Wang et al. showed that reducing agents in the physiological environment (l-cysteine/NADPH) can restore Fe3+ to Fe2+ on the surface of Fe2O3, enhancing the abilities of ·OH generation by Fe2O3 [52]. To further understand the impact of the physiological environment, Chen et al. investigated the enzymes-like properties of Fe-oxide (Fe3O4 and γ-Fe2O3) nanoparticles at the cellular level by electron spin resonance (ESR) and multi-parameter water quality meter [53]. They demonstrated that Fe3O4 and γ-Fe2O3 nanoparticles exhibit higher POD-like activities at pH = 4.8, while CAT-like activities were observed at pH = 7.4. The controllable enzymatic activities in targeted microenvironments provide flexibility and high sensitivity for diverse biomedical applications. Owing to the role of reducing agents in enzyme-like activity, it is feasible that the Fe3+ is reduced to Fe2+ by the overexpressed GSH in tumor tissues, contributing to the elevation of the ROS generation and resultant tumor destruction.
Fe-based nanozyme with CAT-like activity have been reported to broaden the therapy for ROS-involved cerebral malaria. Zhao et al. designed and synthesized recombinant human ferritin (HFn) modified Fe3O4 (Fenozyme) with blood–brain barrier crossing and ROS-scavenging activity to treat cerebral malaria [54]. From the in vivo murine cerebral malaria experiment, it has been revealed that fenozyme possessed significant ROS scavenging abilities of Fe3O4 and prominent blood–brain barrier crossing performance of HFn. Administration of the fenozyme can significantly ameliorate the lesion of cerebral malaria and enhance the survival rate of infected mice induced by the parasite.
Copper-based nanozymes
Copper (Cu) oxide nanomaterials have received significant attention due to their enzyme-mimetic activity [55–57]. The POD-mimetic activity of Cu oxide nanoparticles has focused on ROS production activity by Fenton-like catalysis by Cu+ and/or Cu0 [58]. Besides, the reaction rate of Cu+ with H2O2 was high than Fe2+ because the redox potential of Cu2+/Cu+ is lower, indicating that Cu+ exhibited relatively higher POD-like activities than Fe2+ [59, 60]. Similar to Fe2+, the Fenton-like activity of Cu-based nanoparticles is the potent antitumor agent. In tumor microenvironment mediated therapy, intratumoral reductive agents (such as GSH) can reduce Cu2+ to Cu+ species, leading to high selectivity and efficiency. For instance, Ma et al. reported copper-amino acid mercaptide nanomaterials (Cu-Cys) with GSH depletion and Cu+ production within the tumor microenvironment [60]. After their accumulation at the tumor sites, Cu+ species reacted with H2O2 and produced sufficient ROS, initiating the tumor apoptosis via a Fenton-like reaction. Besides tumor treatment, the POD-like activity of Cu oxide nanoparticles has been employed as antibacterial treatment. Xi et al. designed Cu/carbon nanozymes that can effectively kill Gram-positive and Gram-negative bacteria [61]. Especially, they confirmed that the enzyme-like properties were dependent on the chemical state of Cu. Cu0 exhibits high POD-like activities than Cu2+ and kill bacteria by Fenton-like reaction under H2O2-rich environment. The research progress of Cu-based nanozymes shows their promising biomedicine applications in the targeted disease microenvironment.
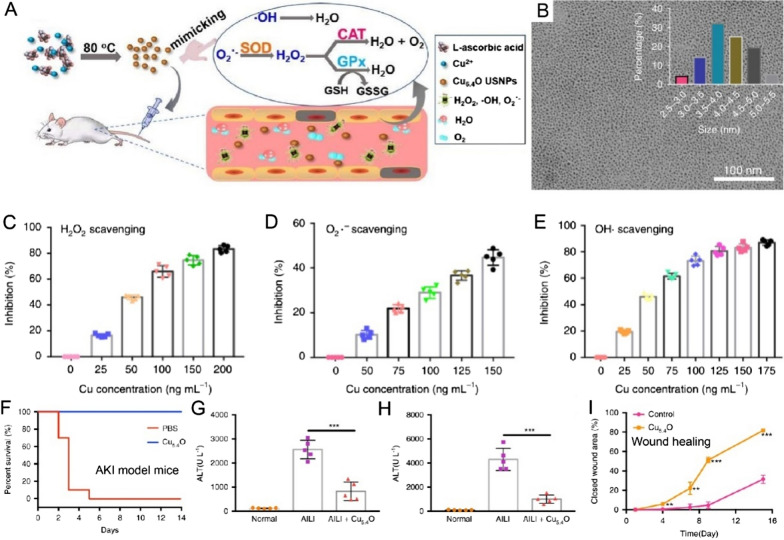
Regulating the activity of nanozymes by utilizing an external stimulus may be highly desirable. It has been demonstrated that visible light could modulate the antibacterial activity of CuO. Nurul Karim et al. fabricated a CuO-nanorod that exhibits POD-mimic activities, and the enzymes-like activities were controlled by visible light [62]. Due to the favorable band structure (1.44 eV), CuO exhibit relatively high POD-like efficiency in the presence of light to kill Gram-negative bacteria efficiently. Besides, the Cu2O nanoparticles can mimic the cytochrome c oxidase activity [63]. It is worth noting that CuxO nanoparticles synthesized in the presence of the structure-directing agent phenylalanine (Phe) can mimic multienzyme activities, such as GPx, POD, superoxide dismutase (SOD), and catalase, enabling the ROS scavenging performance for Parkinson’s disease amelioration [64]. The potential mechanism is that CuxO could reduce the intracellular ROS levels and alleviating oxidative damages. On the other hand, Korschelt et al. reported a copper hydroxide (Cu(OH)2) nanoparticle with glycine functionalized (Gly-Cu(OH)2) that served as SOD mimics, eliminating O2•− radicals generated while smoking [65]. The mechanism of the SOD-like activity by Gly-Cu(OH)2 was further investigated. They found that the reduction and re-oxidized by Cu2+ of Gly-Cu(OH)2 play a prominent role to eliminate O2•−. Importantly, Lin et al. reported an interesting Cu2+-tannic acid (TA) complex nanozyme (Cu-TA) that exhibits SOD-like activity and catalase-like activity for ROS scavenging [66]. The high SOD-like activity of Cu-TA was dependent on the coordination of Cu2+ and TA, which enhance the redox potential of Cu2+. Additionally, the Cu-TA nanozyme can eliminate ·OH and decompose H2O2 to H2O. The ROS scavenging efficiency of nanozyme was further investigated after Cu-TA being loaded into the cigarette filter, and the scavenging efficiency was calculated to be 87.0%, 68.9%, and 34.6% of O2•−, H2O2, and ·OH, respectively. To increase therapeutic benefit and reduce systemic toxicity, it is highly desirable to develop the Cu-based nanoparticles with higher antioxidant activity. Other groups also reported multienzymes-like activities of Cu-based nanoparticles, Liu et al. fabricated ultrasmall Cu5.4O nanoparticles with extensive ROS scavenging efficiency and abilities to treat ROS-related disease (Fig. 3) [67]. They demonstrated that Cu5.4O could exhibit CAT-, SOD-, and GPx-mimicking for enhanced treatment effect against various ROS-mediated diseases at extremely magnitude such as acute kidney injury (AKI) (2 µg/mg for treatment in vivo), liver damage (6 µg /mg for treatment in vivo), as well as wound healing. Moreover, pharmacokinetics and biodistribution experiments revealed that Cu5.4O possesses highly renal clearance advances and outstanding biocompatibility.
Fig. 3.
A Schematic illustration of the synthesis of ultrasmall Cu5.4O nanoparticles with multi enzyme-like properties. B TEM image of Cu5.4O nanoparticles; C H2O2-, D O2•−-, and E ·OH-scavenging ability of Cu5.4O nanoparticles. F Survival rate of the AKI model mice after Cu5.4O treatment. Detection of G serum AST and H ALT levels of acetaminophen (APAP)-induced acute liver injury. I Wound area of diabetic mice after Cu5.4O treatment [67].
Copyright the Authors 2020
Manganese-based nanozymes
Manganese (Mn)-oxide nanoparticles have been demonstrated with intrinsic activities of POD-, GPx-, CAT-, and SOD-like activities due to the variable Mn valent states [68, 69]. In the presence of Cl–/HCO3– environment, the Mn2+ exhibit POD-like property that can decompose H2O2 into ·OH for tumor therapy [9, 70]. Similar to Fe3+ and Cu2+, the GSH could reduce Mn3+/Mn4+ into Mn2+ in the tumor microenvironment, the depletion of GSH could sensitize the ROS-based therapeutic strategies such as chemodynamic therapy and photodynamic therapy [71–74]. Recently, Fu et al. constructed Mn-doped calcium phosphate nanoparticles with loaded GOD (GOD-MnCaP) for tumor therapy [70]. Under the tumor microenvironment, GOD could catalyze the intracellular glucose into H2O2 for Mn2+-mediated ·OH generation and gluconic acid for enhanced Mn2+-mediated reaction (Mn2+ + H2O2 → Mn3+ + ·OH + OH−) [75]. Mn-containing nanomaterials have also been served as CAT-like nanozymes for O2 generation, capturing widespread attention in O2 mediated therapeutic strategy [72, 76–78]. The Mn-oxide nanoparticles have been established as ROS scavenging agents for the treatment of oxidation-stress mediated diseases [69, 79, 80]. Decreasing ROS levels is one of the important therapeutic mechanisms for Mn-oxidated nanoparticles. However, the targeted delivery strategies remain a great challenge for current Mn-based nanozymes to meet different disease criteria. Based on these challenges, a material design approach may provide an appropriate option to satisfy such demand. Shi et al. reported the Mn3O4 encapsulated erythrocyte with T7 peptides functionalization system (Mn3O4@nanoerythrocyte-T7) for ischemic stroke protection [81]. After being accumulated into the infarcted sites via T7 peptides targeting, the Mn3O4@nanoerythrocyte-T7 can efficiently scavenge the ROS and supply oxygen before thrombolysis stroke, with O2 supply to the hemoglobin in the erythrocyte after thrombolysis. Selective targeting to ischemic stroke provides an attractive strategy to achieve a strong Mn-based nanozymes therapeutic effect.
Interestingly, the GOD enzyme-like activity of MnO2 nanosheets was reported by Tang et al. [82]. They synthesized MnO2 nanosheets (M-NSs) by a one-step wet-chemical method that had high glucose affinity and thermal stability as compared to the natural GOD. Under NIR laser irradiation, M-NSs could achieve the photothermal conversion while the glucose was gradually transformed to gluconic acid and H2O2, resulting in glucose deprivation enhanced photothermal therapy. Such inorganic nanozyme with GOD-like activity provides a new strategy for the evolution of glucose deprivation and ROS-mediated cancer therapy.
Molybdenum-based nanozymes
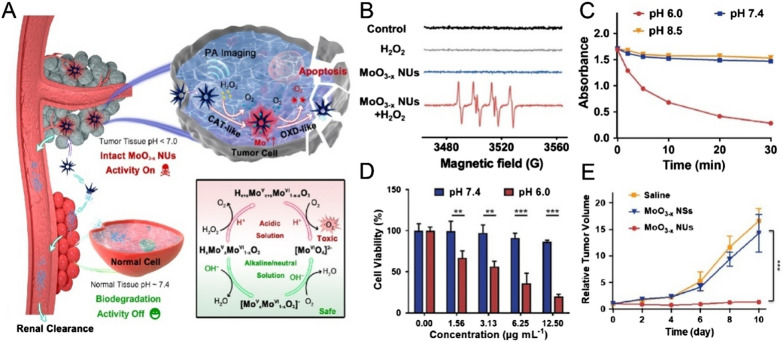
Molybdenum (Mo) nanoparticles have attracted considerable attention as nanozymes [83–85]. There have been many reports regarding the catalytic activity of SOD, CAT, OXD, and sulfite oxidase, etc. [86, 87]. Although Mo-based nanozymes display outstanding enzyme-mimicking performance, it is still difficult to further expand their application to biomedicine. One of the main constraints for the application of Mo-based nanozymes is that these nanozymes carry both antioxidative and oxidative activities simultaneously, and may fail in their application to inhibit oxidative-mediated injury with satisfied outcome. Han et al. synthesized MoO3-x nanodots with CAT- and SOD- mimic activities for Alzheimer's disease treatment [88]. However, the OXD-activities should be considered and may affect the therapeutic benefits of MoO3-x. Considering these issues, new strategies should be developed to design intelligent nanozymes with controlled enzyme activity at specific microenvironments with acidity. Hu et al. constructed MoO3-x nanourchins (MoO3-x NUs) with pH-dependent multi-enzymatic activity for tumor-specific therapy (Fig. 4) [89]. Under normal physiological pH environment, MoO3-x possessed high biocompatibility due to their stimuli-responsive biodegradation behavior. MoO3-x exhibits excellent catalase enzyme activity under acidic and high H2O2 conditions such as reduce to the high proportion of Mo5+ atoms. Furthermore, MoO3-x exhibits OXD-like activity that could convert O2 by disintegrated endogenous H2O2 to O2•− for tumor-specific catalytic therapy. This research provides a new potential therapeutic strategy to reduce the toxicity of nanozymes by controlling their acidic-responsive behavior.
Fig. 4.
A Schematic illustration of the MoO3-x NUs with multi-enzyme mimicking activities for pH-responsive tumor therapy. B Electron paramagnetic resonance spectra of MoO3-x NUs in the presence of H2O2 (1 mM). C DPBF degradation curves at different pH conditions (pH 6.0, pH 7.4, and pH 8.5). D Cytotoxicity of MoO3-x NUs against B16 melanoma cells at pH 7.4 and pH 6.0; E B16 xenograft tumor growth curves after different treatments (n = 5) [89].
Copyright 2019 American Chemical Society
Cobalt-based nanozymes
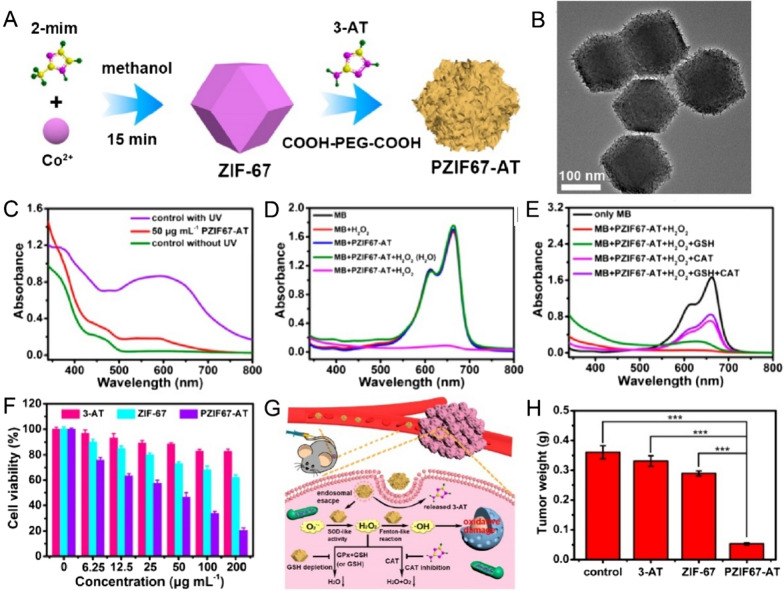
Intrinsic multienzyme-like activities of cobalt (Co)-based nanoparticles have been reported [90–92]. Dong et al. reported the Co3O4 possesses the pH-dependent enzyme-like property and the reaction basis is similar to Fe3O4 [93]. The Co3O4 showed optimal CAT-like reactivity and SOD-like activity at higher pH conditions (pH ≥ 7.4), and the CAT-like reactivity of Co3O4 was significantly high than Fe3O4 at the same conditions. Additionally, Co3O4 exhibits higher POD-like activity in an acidic medium (pH = 3.6). The catalyzing efficacy of Co-based nanozyme limits their biomedical application. Thus, improving the enzyme activity of nanozymes may be therapeutically attractive for better antitumor efficacy. Recently, Sang et al. developed a polyethylene glycol decorated PZIF67-AT nanoparticles by combining the multienzyme-like activities of Co-based zeolitic imidazole framework-67 and 3-amino-1,2,4-triazole (3-AT) [94]. In their design, the SOD-mimetic activity of PZIF67-AT initially converts O2•− to H2O2 (Fig. 5), subsequently, the production of H2O2 was converted to ·OH by PZIF67-AT for cancer therapy. The CAT-like activity of PZIF67-AT was inhibited by 3-AT through binding of the Co-active center. In addition, the overexpressed GSH in TME could also be depleted by PZIF67-AT. This study offers an insight into nanozymes in the applications of tumor therapy.
Fig. 5.
A Illustration depicting the preparation of PZIF67-AT nanoparticles. B Representative TEM image of PZIF67-AT nanoparticles. C UV–Vis absorbance detection of the O2•−-eliminated activity of PZIF67-AT. D Methylene blue (MB) degradation demonstrated the Fenton-like ability of PZIF67-AT nanoparticles. E MB degradation of PZIF67-AT in the presence of CAT or GSH solutions. F HeLa cell viability with various treatments. G The therapeutic process and mechanism of PZIF67-AT nanoparticles under in vivo conditions [94].
Copyright 2020, American Chemical Society
Platinum-based nanozymes
Tumor hypoxia and overproduced H2O2 is the unique feature of solid tumor, which is critical to tumor proliferation and metastasis [95, 96]. Importantly, the therapeutic efficiency of current methods was limited by tumor hypoxia microenvironments, such as photodynamic therapy (PDT) and radiotherapy [97–99]. Unfortunately, oxygen-dependent PDT was severely discouraged due to the low intratumoral oxygen level [100–102]. Fortunately, nanozymes with catalase-like activities could provide a feasible method to improve tumor oxygen-involved therapeutic methods [103, 104]. Based on the catalase-like activity, platinum (Pt) based nanomaterials have been widely applied to decompose the endogenous H2O2 to O2, thus relieving tumor hypoxia for tumor therapy, including PDT and radiotherapy [105, 106]. Zhang et al. report a Pt-PCN-224 nano-platform by decorating Pt on PCN-224 [106, 107]. In the overexpressed H2O2 microenvironment, Pt is capable of producing O2 for the photosensitizer (PCN-224) during the photosensitization process to form 1O2, which could remarkably enhance the outcome of tumor PDT. Besides, Li et al. synthesized porous Pt nanoparticles that can absorb X-ray and convert H2O2 to O2, improving the radiotherapy efficiency against the malignant tumor [108].
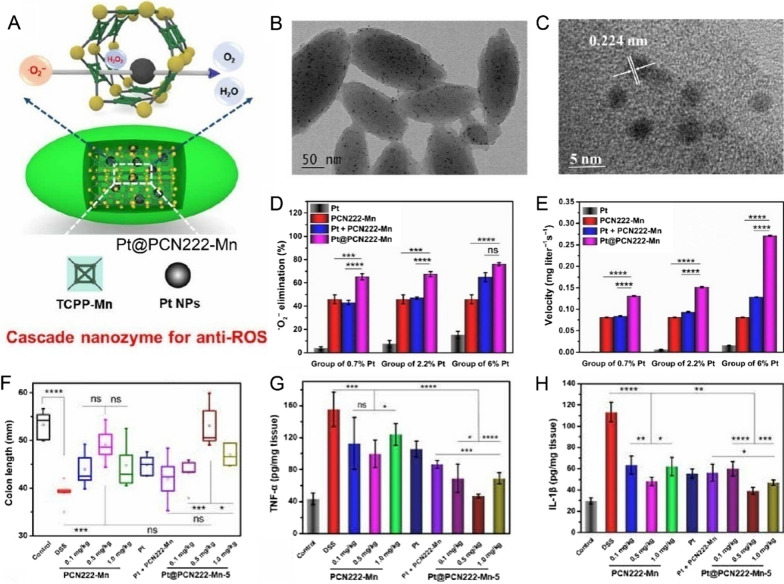
In addition, Pt-based nanozymes could also be used for ROS and inflammation associated with diseases. In another work, Lin et al. synthesized a cascade nanozyme of Pt@PCN222-Mn to realize anti-inflammatory therapy (Fig. 6) [109]. The PCN222-Mn with SOD-like activity can react with endogenous O2•−, resulting in superoxide depletion and subsequent H2O2 generation. Then the Pt nanoparticles exhibited strong CAT-like performance to catalyze H2O2 for O2 generation, thereby benefiting the inflammatory bowel diseases. This research provides the paradigm that the rationally designed nanozymes could have better cascade enzymatic performance against pathologies in a variety.
Fig. 6.
A Schematic illustration of the design of Pt@PCN222-Mn. B TEM and C high-resolution TEM of Pt@PCN222-Mn. D O2– elimination ability and E oxygen-generated velocities of Pt@PCN222-Mn. F the corresponding colon lengths after treatment with various concentrations of Pt@PCN222-Mn. Levels of G Inflammatory cytokine IL-1 and H TNF-α in colon homogenates after treatment [109].
Copyright the Authors 2020
Gold-based nanozymes
Enzymatic properties of gold nanoparticles (Au NPs) have widespread uses in biomedical applications [110]. Many studies have reported that Au NPs exhibited multiple-enzymes mimicking abilities such as peroxidase-mimetic activity and glucose oxidase (GOD) activity [111–113]. The GOD-like activity of Au nanoparticles can deplete the glucose and generate H2O2, which could effectively consume glucose nutrients and inducing cell starving in tumor tissues. For example, Gao et al. synthesized the Au-containing inorganic nanozyme platform (DMSN-Au-Fe3O4-PEG) [114]. Firstly, Au specifically catalyzes glucose to H2O2, which was reacted with Fenton agent (Fe3O4) to produce highly toxic hydroxyl radicals (·OH) by typical Fenton reaction for tumor suppression. Additionally, the GOD-mimetic activity of Au nanoparticles has been reported to be synergized with CAT-mimetic nanomaterial enhanced tumor therapy efficiency. Liu et al. loaded Pt and Au into the porphyrin metal–organic frameworks (PCN) with folic acid decoration (P@Pt@P-Au-FA) [115]. The authors showed that endogenous H2O2 could be catalyzed by Pt to O2 for enhanced PDT. The oxygen molecules act as the substrate for gold nanoparticles to convert glucose into H2O2, supplying the reactant of Pt repeatedly. Within the oxygen cycle, remarkably consumption of glucose and production of gluconic acid could accelerate the catalytic efficiency and the antitumor efficiency of Au. However, the total O2 level was not increased during this reaction cycle, low intracellular O2 levels still constrain tumor therapies that are oxygen-dependent.
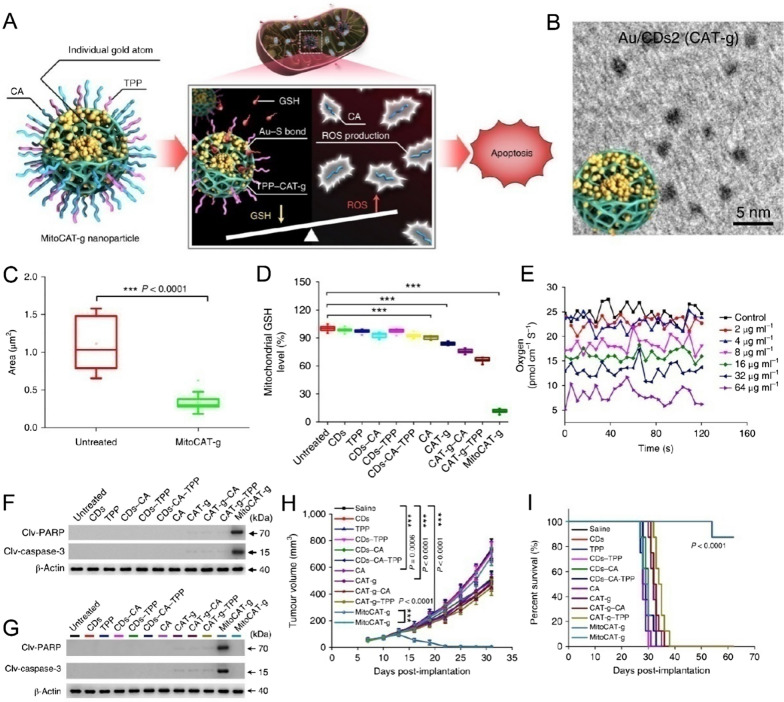
The elevated GSH level in cancer cells enables tumor cells to maintain redox homeostasis and resistance to overexpression of ROS [116]. Depletion of GSH has been developed as a smart strategy for enhanced chemodynamic therapy, chemotherapy, photodynamic therapy, and radiotherapy [117]. However, redox homeostasis destruction has been rarely reported for cancer treatment. Based on the biochemical reactions between Au and thiol of GSH, Gong et al. synthesized single-atom Au nanoagents with GPx-like activity to amplify mitochondrial ROS for tumor therapy (Fig. 7) [118]. First, single-atom Au was incorporated into carbon dot (CAT-g) as metal centers catalysis of GSH. Then, triphenylphosphine and cinnamaldehyde were further employed to modify CAT-g (MitoCAT-g) to enable the mitochondria targeting ability and ROS generating ability. As a result, MitoCAT-g effectively strengthened the oxidative stress in the mitochondrial of tumor cells and trigger apoptosis for cell death.
Fig. 7.
A Schematic illustration of the synthesis and therapeutic mechanism of CAT-g. B TEM image of MitoCAT-g. Statistics of C intracellular mitochondrion area and D perimeter of untreated cells and MitoCAT-g-treated cells. E The oxygen flux of single HepG-2 cells after treatment with various doses of MitoCAT-g. G Western blotting determination of pro-apoptotic proteins (F) and (H) apoptotic proteins in tumor tissues after different treatments. Relative tumor volumes and I survival rate of mice after different treatments [118].
Copyright the Authors 2019
Iridium-based nanozymes
Other CAT-like nanozymes containing metal oxide-based nanomaterials, such as iridium (Ir) based nanoparticles, have been developed for biomedical applications [119, 120]. The mechanism of these metal oxide-based nanozymes was associated with the oxidation valent of metal species. For example, Su et al. investigated the connections between the CAT-like property of PVP-Ir(0) NPs and chemical state, demonstrating that the formation of IrO2 upon exposure to H2O2 enables the PVP-IrNPs to exhibit CAT-like activity [121]. The POD activities of PVP-IrNPs were originated from electron transfer mediators. Zhang et al. demonstrated that PVP-IrNPs can scavenge ROS and reactive nitrogen species (RNS) to alleviate AKI [122]. In their work, ultrasmall PVP-IrNPs (1.5 nm) could rapidly accumulate to the kidney after intravenous administration, protecting ROS- or RNS-mediated cellular damage. Furthermore, PVP-IrNPs could be easily excreted to urine by the kidney and exhibit lower systemic toxicity. Besides, Ir-oxide (IrOx) has been reported that acid-activated OXD-like and pH-dependent CAT-like functions for targeted tumor therapies [123]. At neutral normal tissues, the IrOx presented dominantly CAT-like activities. While the POD-like and OXD-like activities were greatly improved along with the gluconic acid generation by GOD catalysis. Importantly, the glutathione (GSH) can be consumed by Ir4+, dramatically reduced antioxidative species and enhanced lethality could be ultimately achieved.
Ruthenium-based nanozymes
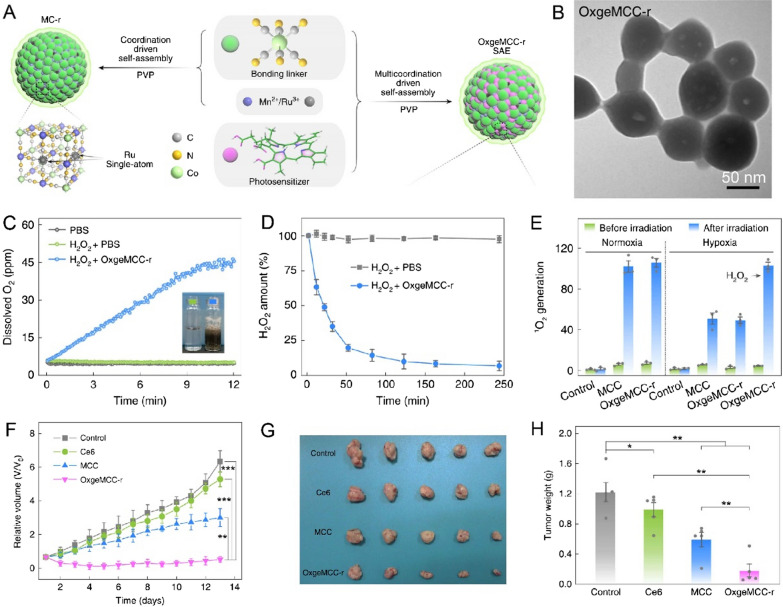
Recently, Xu et al. also discovered that ruthenium (Ru)-based nanoparticles with catalase-like activity could be constructed for highly efficient phototherapy against 4-T1 tumors [124]. In their work, RuO2@BSA was first prepared by alkaline precipitation methods, and photosensitizer (IR-808-Br2) was subsequently decorated into the protein shell to form RuO2@BSA@IR-808-Br2. First, the RuO2 possesses high CAT-like activity, endowing IR-808-Br2 with highly efficient PDT activity. Second, the RuO2 has photothermal conversion efficiency for PTT. As a result, RuO2@BSA@IR-808-Br2 achieves sufficient tumor inhibition by synergistically enhanced efficacy of PDT and PTT. The catalase-mimetic activity of RuO2 was activated after being exposed to the tumor microenvironment, and immediately convert H2O2 to oxygenate the IR-808-Br2 for the photodynamic process under near-infrared irradiation. Wei et al. reported a multi-functional IrRu-GOD@PEG NPs that could realize tumor starvation therapy and oxidative therapy by chemical catalysis from H2O2 to 1O2 [125]. Such an oxidative therapeutic strategy through IrRu alloy nanoparticles provides a new insight for tumor therapy. Recently, the emerging single atom Ru has drawn extensive attention for endogenous O2 generation. Wang et al. reported an O2 generation single-atom Ru nano-platform (OxgeMCC-r) to enhance the therapeutic efficacy of PDT by self-assembly in the presence of PVP, Mn/Ru, and Ce6 (Fig. 8) [126]. Single-atom Ru allows effective O2 generation at a low concentration of the Ru to overcome tumor hypoxia for 1O2-mediated tumor killing.
Fig. 8.
A Schematic illustration of the OxgeMCC-r formation process. B TEM images of OxgeMCC-r. C Time-dependent O2 production by OxgeMCC-r with H2O2. D H2O2 Degradation curve of H2O2 by OxgeMCC-r. E DPBF determines the 1O2 generation ability of different groups with or without laser irradiation. F Tumor volume curves of mice after different treatments. G Photo images of tumors collected from different groups after the end of the treatment periods. H Tumor weights from different groups of mice after the end of the treatment [126].
Copyright the Authors 2020
Conclusion and outlook
The fast development and revolution of nanoscience and nanotechnology has broadened extensive research interests for their application in biomedicine. Nanozymes is one of the emerging research frontiers that exhibit great prospects for disease therapy. Herein, we have summarized and discussed the most recent development of nanozymes with their intrinsic therapeutic features for versatile biomedical applications. Despite the tremendous advantages of nanozymes in biomaterial applications, some critical issues and challenges are still needed to be considered. (1) The catalytic efficiency of most nanozymes should be further improved, with controllable enzyme-like activity. It is expected that the enzymatic reactions could be highly lesion site-specific, guaranteeing the biocompatibility and therapeutic specificity. To achieve high enzyme-like activity, introduction of the single-atom nanozymes is the most attractive strategy to achieve such issue, owing to their highly dispersion of the catalytic active sites and atomic utilization efficiency. Yet the loading efficiency of the single metal atoms is limited, challenges for the performance advancement of single-atom nanozymes are remained. In addition, rational design of cascade nanozymes may represent a promising strategy to improve the catalytic efficiency. (2) The molecular mechanism of nanozymes with multienzyme activities are still not clear. For instance, nanozymes mimicking dual-enzymes of CAT and POD, are supposed to exhibit self-competition performance during biomedical applications (as these enzymes both consume H2O2). The exact molecular mechanism of the electron movements within the metallic species should be further investigated under different conditions. In this regard, disease microenvironment holds great potential to regulate the desired enzymatic performance of specific nanozyme. Numerous studies have demonstrated the feasibility and responsiveness of nanozymes in broad biomedicine applications under characteristic stimuli, such as pH condition, GSH, and light, etc. Another strategy to regulate the specific enzymatic performance of the nanozyme lies in the application of specific inhibitors. (3) The selectivity and specificity of nanozymes should be further improved and optimized. During tumor therapy, although pH- or GSH-responsive nanozymes have exhibited “smart” enzyme-like activities to kill tumor cells, low selectivity and specificity have limited their further applications. To address this dilemma, rationally designed controllable nanozymes are significant to achieve high specificity of its enzyme-mimetic activity against various diseases. Recently, many studies have demonstrated that exogenous stimuli, such as light and ultrasonic, could serve as the trigger to control nanozymes activation. These modalities may provide feasible options to achieve the desired prominent site-specificity. (4) Biocompatibility and biodegradability should be considered. Overcoming the in vivo toxicity of nanozymes during therapeutics is still a barrier toward clinical application. Currently, systemic injection of nanozymes will inevitably cause adverse effect against normal tissues. For metal-based nanozymes, the toxicity is largely associated to the metallic species of the constructed metal-based nanozymes. Although numerous studies have demonstrated the cytoprotective role and biocompatible character of nanozymes, metal ion release is still considered as the possible factor to cause the side impact against normal tissues due to the metal overload. For example, copper or iron overloaded in normal tissue/cells may trigger Fenton or Fenton-like reaction that could severely damage the biomacromolecules as well as the nucleic acids. Therefore, pharmacokinetics of the nanozymes are of critically important during the biocompatibility and biosafety evaluation. The surface tunable properties of nanozymes provide an opportunity to design biosafety agents. Taken into considerations, surface modification is one of the alternative strategies to overcome the limitation of nanozymes. Moreover, considering the ligands of nanozymes could influence therapeutic outcomes, bioavailability, clearance dynamics, and systemic toxicity. From this perspective, it is necessary to carefully choose a suitable ligand and endow nanozymes with higher biosafety.
Abbreviations
- GSH
Glutathione
- OXD
Oxidase
- GOD
Glucose oxidase
- POD
Peroxidase
- CAT
Catalase
- SOD
Superoxide dismutase
- GPx
Glutathione peroxidase
- TME
Tumor microenvironment
- O2•−
Superoxide anion
- H2O2
Hydrogen peroxide
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- CcO
Cytochrome c oxidase
- TNF-α
Tumor necrosis factor alpha
- LDH
Lactate dehydrogenase
- SPN-C23
Self-regulated nanoceria-doped poly-(cyclopentadithiophene-alt-benzothiadiazole)
- PDT
Photodynamic therapy
- NIR
Near-infrared
- SOSG
Singlet oxygen sensor green
- AMD
Age-related macular degeneration
- 1O2
Single oxygen species
- AMD
Age-related macular degeneration
- ZIF-8
Zeolitic imidazolate framework-8
- t-BOOH
Tert-butyl hydroperoxide
- eDNA
Extracellular DNA
- MOF
Metal–organic framework
- MRI
Magnetic resonance imaging
- ESR
Electron spin resonance
- HFn
Human ferritin
- Cu-Cys
Copper-amino acid mercaptide nanomaterials
- Phe
Phenylalanine
- TA
Tannic acid
- M-NSs
MnO2 nanosheets
- MoO3-x Nus
MoO3-x nanourchins
- 3-AT
3-Amino-1,2,4-triazole
- PCN
Porphyrin metal–organic frameworks
Authors' contributions
XR and DC wrote the manuscript, YW, HL, and YZ checked different sections of the manuscript. HC , XL and MH edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 22005327), China Postdoctoral Science Foundation Funded Project (Grant Nos. 2020M671243, BX20200345), Post-Doctor Research project, West China Hospital, Sichuan University (No. 20HXBH141). X. Ren and D. Chen contributed equally to this work.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiangyi Ren and Dongxu Chen contributed equally to this study
Contributor Information
Hongying Chen, Email: chenhongying@wchscu.cn.
Minfeng Huo, Email: mfhuo@mail.sic.ac.cn.
References
- 1.Wu J, Wang X, Wang Q, et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II) Chem Soc Rev. 2019;48:1004–1076. doi: 10.1039/c8cs00457a. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Jana D, Zhao Y. Metal-organic framework derived nanozymes in biomedicine. Acc Chem Res. 2020;53:1389–1400. doi: 10.1021/acs.accounts.0c00268. [DOI] [PubMed] [Google Scholar]
- 3.Fan Y, Liu S, Yi Y, et al. Catalytic nanomaterials toward atomic levels for biomedical applications: from metal clusters to single-atom catalysts. ACS Nano. 2021;15:2005–2037. doi: 10.1021/acsnano.0c06962. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Zhang L, Deng H, et al. In vivo activation of pH-responsive oxidase-like graphitic nanozymes for selective killing of Helicobacter pylori. Nat Commun. 2021;12:2002. doi: 10.1038/s41467-021-22286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao L, Yan X. Nanozymes: biomedical applications of enzymatic Fe3O4 nanoparticles from in vitro to in vivo. Adv Exp Med Biol. 2019;1174:291–312. doi: 10.1007/978-981-13-9791-2_9. [DOI] [PubMed] [Google Scholar]
- 6.Yang M, Li J, Gu P, et al. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact Mater. 2021;6:1973–1987. doi: 10.1016/j.bioactmat.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F, Lin L, Zhang Y, et al. A tumor-microenvironment-activated nanozyme-mediated theranostic nanoreactor for imaging-guided combined tumor therapy. Adv Mater. 2019;31:e1902885. doi: 10.1002/adma.201902885. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Li Z, Sun Z, et al. Visualization nanozyme based on tumor microenvironment "unlocking" for intensive combination therapy of breast cancer. Sci Adv. 2020;6:eabc8733. doi: 10.1126/sciadv.abc8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin LS, Song J, Song L, et al. Simultaneous fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew Chem Int Ed. 2018;57:4902–4906. doi: 10.1002/anie.201712027. [DOI] [PubMed] [Google Scholar]
- 10.Cao S, Fan J, Sun W, et al. A novel Mn-Cu bimetallic complex for enhanced chemodynamic therapy with simultaneous glutathione depletion. Chem Commun. 2019;55:12956–12959. doi: 10.1039/c9cc06040e. [DOI] [PubMed] [Google Scholar]
- 11.Fu LH, Wan Y, Qi C, et al. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv Mater. 2021;33:e2006892. doi: 10.1002/adma.202006892. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Wang H, Xu B, et al. Photo-responsive nanozymes: mechanism, activity regulation, and biomedical applications. View. 2020;2:20200045. [Google Scholar]
- 13.Dong H, Fan Y, Zhang W, et al. Catalytic mechanisms of nanozymes and their applications in biomedicine. Bioconjugate Chem. 2019;30:1273–1296. doi: 10.1021/acs.bioconjchem.9b00171. [DOI] [PubMed] [Google Scholar]
- 14.Ji S, Jiang B, Hao H, et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat Catal. 2021;4:407–417. [Google Scholar]
- 15.Huang Y, Ren J, Qu X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev. 2019;119:4357–4412. doi: 10.1021/acs.chemrev.8b00672. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Wan K, Shi X. Recent advances in nanozyme research. Adv Mater. 2019;31:e1805368. doi: 10.1002/adma.201805368. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Gao Y, Chandrawati R, et al. Therapeutic applications of multifunctional nanozymes. Nanoscale. 2019;11:21046–21060. doi: 10.1039/c9nr06596b. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Jiang C, Li B, Liu Z, et al. A core-shell Au@Cu2-xSe heterogeneous metal nanocomposite for photoacoustic and computed tomography dual-imaging-guided photothermal boosted chemodynamic therapy. J Nanobiotechnol. 2021;19:1–18. doi: 10.1186/s12951-021-01159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng X, Li D, Chen L, et al. High-performance self-cascade pyrite nanozymes for apoptosis-ferroptosis synergistic tumor therapy. ACS Nano. 2021;15:5735–5751. doi: 10.1021/acsnano.1c01248. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Wang A, Liu S, et al. A titanium nitride nanozyme for pH-responsive and irradiation-enhanced cascade-catalytic tumor therapy. Angew Chem Int Ed. 2021;60:25328–25338. doi: 10.1002/anie.202106750. [DOI] [PubMed] [Google Scholar]
- 21.Ghorbani M, Derakhshankhah H, Jafari S, et al. Nanozyme antioxidants as emerging alternatives for natural antioxidants: achievements and challenges in perspective. Nano Today. 2019;29:100775. [Google Scholar]
- 22.Liu C, Yao J, Hu J, et al. Navigating nMOF-mediated enzymatic reactions for catalytic tumor-specific therapy. Mater Horiz. 2020;7:3176–3186. [Google Scholar]
- 23.Li F, Qiu Y, Xia F, et al. Dual detoxification and inflammatory regulation by ceria nanozymes for drug-induced liver injury therapy. Nano Today. 2020;35:100925. [Google Scholar]
- 24.Fan Y, Li P, Hu B, et al. A smart photosensitizer-cerium oxide nanoprobe for highly selective and efficient photodynamic therapy. Inorg Chem. 2019;58:7295–7302. doi: 10.1021/acs.inorgchem.9b00363. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Liu Y, Sun S, et al. Catalytic patch with redox Cr/CeO2 nanozyme of noninvasive intervention for brain trauma. Theranostics. 2021;11:2806–2821. doi: 10.7150/thno.51912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh N, Mugesh G. CeVO4 nanozymes catalyze the reduction of dioxygen to water without releasing partially reduced oxygen species. Angew Chem Int Ed. 2019;58:7797–7801. doi: 10.1002/anie.201903427. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Shen X, Gao X, et al. Simultaneous enzyme mimicking and chemical reduction mechanisms for nanoceria as a bio-antioxidant: a catalytic model bridging computations and experiments for nanozymes. Nanoscale. 2019;11:13289–13299. doi: 10.1039/c9nr03473k. [DOI] [PubMed] [Google Scholar]
- 28.Esch F, Fabris S, Zhou L, et al. Electron localization determines defect formation on ceria substrates. Science. 2005;309:752–755. doi: 10.1126/science.1111568. [DOI] [PubMed] [Google Scholar]
- 29.Forest V, Leclerc L, Hochepied J, et al. Impact of cerium oxide nanoparticles shape on their in vitro cellular toxicity. Toxicol In Vitro. 2017;38:136–141. doi: 10.1016/j.tiv.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Qian Q, Zhang Y, Chen Y, et al. Assessment of pulmonary toxicity of potential antioxidant drug PEGylated nanoceria after intratracheal instillation in rats. J Appl Toxicol. 2021;41:941–952. doi: 10.1002/jat.4079. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, Fang Y, Miao Q, et al. Regulating near-infrared photodynamic properties of semiconducting polymer nanotheranostics for optimized cancer therapy. ACS Nano. 2017;11:8998–9009. doi: 10.1021/acsnano.7b03507. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Wang X, Du J, et al. Reactive oxygen species-regulating strategies based on nanomaterials for disease treatment. Adv Sci. 2021;8:2002797. doi: 10.1002/advs.202002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian M, Xue Z, Qiao X, et al. Movable hollow nanoparticles as reactive oxygen scavengers. Chem. 2019;5:2378–2387. [Google Scholar]
- 34.Stavros P, Sikha S. Therapeutic benefits of nanoparticles in stroke. Front Neurosci. 2015;9:182. doi: 10.3389/fnins.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nash K, Ahmed S. Nanomedicine in the ROS-mediated pathophysiology: applications and clinical advances. Nanomedicine. 2015;11:2033–2040. doi: 10.1016/j.nano.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan R, Sun S, Yang J, et al. Nanozyme-based bandage with single-atom catalysis for brain trauma. ACS Nano. 2019;13:11552–11560. doi: 10.1021/acsnano.9b05075. [DOI] [PubMed] [Google Scholar]
- 37.Yildirim Z, Ucgun N, Yildirim F. The role of oxidative stress and antioxidants in the pathogenesis of age-related macular degeneration. Clinics. 2011;66:743–746. doi: 10.1590/S1807-59322011000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang K, Tu M, Gao W, et al. Hollow Prussian blue nanozymes drive neuroprotection against ischemic stroke via attenuating oxidative stress, counteracting inflammation, and suppressing cell apoptosis. Nano Lett. 2019;19:2812–2823. doi: 10.1021/acs.nanolett.8b04729. [DOI] [PubMed] [Google Scholar]
- 39.Abokyi S, To C, Lam T, et al. Central role of oxidative stress in age-related macular degeneration: evidence from a review of the molecular mechanisms and animal models. Oxid Med Cell Longevity. 2020;2020:7901270. doi: 10.1155/2020/7901270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kauppinen A, Paterno JJ, Blasiak J, et al. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73:1765–1786. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitra RN, Gao R, Zheng M, et al. Glycol chitosan engineered autoregenerative antioxidant significantly attenuates pathological damages in models of age-related macular degeneration. ACS Nano. 2017;11:4669–4685. doi: 10.1021/acsnano.7b00429. [DOI] [PubMed] [Google Scholar]
- 42.Ni D, Wei H, Chen W, et al. Ceria nanoparticles meet hepatic ischemia-reperfusion injury: the perfect imperfection. Adv Mater. 2019;31:e1902956. doi: 10.1002/adma.201902956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He L, Huang G, Liu H, et al. Highly bioactive zeolitic imidazolate framework-8-capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Sci Adv. 2020;6:eaay9751. doi: 10.1126/sciadv.aay9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Wang F, Ren J, et al. A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. Biomaterials. 2019;208:21–31. doi: 10.1016/j.biomaterials.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Xu F, Lu Q, Huang PJ, et al. Nanoceria as a DNase I mimicking nanozyme. Chem Commun. 2019;55:13215–13218. doi: 10.1039/c9cc06782e. [DOI] [PubMed] [Google Scholar]
- 46.Huo M, Wang L, Wang Y, et al. Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano. 2019;13:2643–2653. doi: 10.1021/acsnano.9b00457. [DOI] [PubMed] [Google Scholar]
- 47.Xu B, Cui Y, Wang W, et al. Immunomodulation-enhanced nanozyme-based tumor catalytic therapy. Adv Mater. 2020;32:e2003563. doi: 10.1002/adma.202003563. [DOI] [PubMed] [Google Scholar]
- 48.Huo M, Wang L, Chen Y, et al. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun. 2017;8:357. doi: 10.1038/s41467-017-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou W, Gao J, Zhao H, et al. The role of quinone cycle in Fe2+-H2O2 system in the regeneration of Fe2+ Environ Technol. 2017;38:1887–1896. doi: 10.1080/09593330.2016.1240241. [DOI] [PubMed] [Google Scholar]
- 50.Pham AL, Doyle FM, Sedlak DL. Kinetics and efficiency of H2O2 activation by iron-containing minerals and aquifer materials. Water Res. 2012;46:6454–6462. doi: 10.1016/j.watres.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao L, Zhuang J, Nie L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 52.Wang B, Yin JJ, Zhou X, et al. Physicochemical origin for free radical generation of iron oxide nanoparticles in biomicroenvironment: catalytic activities mediated by surface chemical states. J Phys Chem C. 2012;117:383–392. [Google Scholar]
- 53.Chen Z, Yin J, Zhou Y, et al. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano. 2012;6:4001–4012. doi: 10.1021/nn300291r. [DOI] [PubMed] [Google Scholar]
- 54.Zhao S, Duan H, Yang Y, et al. Fenozyme protects the integrity of the blood-brain barrier against experimental cerebral malaria. Nano Lett. 2019;19:8887–8895. doi: 10.1021/acs.nanolett.9b03774. [DOI] [PubMed] [Google Scholar]
- 55.Shan J, Li X, Yang K, et al. Efficient bacteria killing by Cu2WS4 nanocrystals with enzyme-like properties and bacteria-binding ability. ACS Nano. 2019;13:13797–13808. doi: 10.1021/acsnano.9b03868. [DOI] [PubMed] [Google Scholar]
- 56.Sun S, Chen Q, Tang Z, et al. Tumor microenvironment stimuli-responsive fluorescence imaging and synergistic cancer therapy by carbon-dot-Cu2+ nanoassemblies. Angew Chem Int Ed. 2020;59:21041–21048. doi: 10.1002/anie.202007786. [DOI] [PubMed] [Google Scholar]
- 57.Khoris IM, Ganganboina AB, Suzuki T, et al. Self-assembled chromogen-loaded polymeric cocoon for respiratory virus detection. Nanoscale. 2021;13:388–396. doi: 10.1039/d0nr06893d. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Li Z, Hu Y, et al. Photothermal conversion-coordinated Fenton-like and photocatalytic reactions of Cu2-xSe-Au Janus nanoparticles for tri-combination antitumor therapy. Biomaterials. 2020;255:120167. doi: 10.1016/j.biomaterials.2020.120167. [DOI] [PubMed] [Google Scholar]
- 59.Soltani T, Lee BK. Enhanced formation of sulfate radicals by metal-doped BiFeO3 under visible light for improving photo-Fenton catalytic degradation of 2-chlorophenol. Chem Eng J. 2017;313:1258–1268. [Google Scholar]
- 60.Ma B, Wang S, Liu F, et al. Self-assembled copper-amino acid nanoparticles for in situ glutathione and H2O2 sequentially triggered chemodynamic therapy. J Am Chem Soc. 2019;141:849–857. doi: 10.1021/jacs.8b08714. [DOI] [PubMed] [Google Scholar]
- 61.Xi J, Wei G, An L, et al. Copper/carbon hybrid nanozyme: tuning catalytic activity by the copper state for antibacterial therapy. Nano Lett. 2019;19:7645–7654. doi: 10.1021/acs.nanolett.9b02242. [DOI] [PubMed] [Google Scholar]
- 62.Karim MN, Singh M, Weerathunge P, et al. Visible-light-triggered reactive-oxygen-species-mediated antibacterial activity of peroxidase-mimic CuO nanorods. ACS Appl Mater Interfaces. 2018;1:1694–1704. [Google Scholar]
- 63.Chen M, Wang Z, Shu J, et al. Mimicking a natural enzyme system: cytochrome c oxidase-like activity of Cu2O nanoparticles by receiving electrons from cytochrome c. Inorg Chem. 2017;56:9400–9403. doi: 10.1021/acs.inorgchem.7b01393. [DOI] [PubMed] [Google Scholar]
- 64.Hao C, Qu A, Xu L, et al. Chiral molecule-mediated porous CuxO nanoparticle clusters with antioxidation activity for ameliorating Parkinson's disease. J Am Chem Soc. 2019;141:1091–1099. doi: 10.1021/jacs.8b11856. [DOI] [PubMed] [Google Scholar]
- 65.Korschelt K, Ragg R, Metzger CS, et al. Glycine-functionalized copper(II) hydroxide nanoparticles with high intrinsic superoxide dismutase activity. Nanoscale. 2017;9:3952–3960. doi: 10.1039/c6nr09810j. [DOI] [PubMed] [Google Scholar]
- 66.Lin S, Cheng Y, Zhang H, et al. Copper tannic acid coordination nanosheet: a potent nanozyme for scavenging ROS from cigarette smoke. Small. 2019;27:e1902123. doi: 10.1002/smll.201902123. [DOI] [PubMed] [Google Scholar]
- 67.Liu T, Xiao B, Xiang F, et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun. 2020;11:2788. doi: 10.1038/s41467-020-16544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuthati Y, Busa P, Goutham Davuluri VN, et al. Manganese oxide nanozymes ameliorate mechanical allodynia in a rat model of partial sciatic nerve-transection induced neuropathic pain. Int J Nanomed. 2019;14:10105–10117. doi: 10.2147/IJN.S225594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh N, Savanur MA, Srivastava S, et al. A manganese oxide nanozyme prevents the oxidative damage of biomolecules without affecting the endogenous antioxidant system. Nanoscale. 2019;11:3855–3863. doi: 10.1039/c8nr09397k. [DOI] [PubMed] [Google Scholar]
- 70.Fu L, Hu Y, Qi C, et al. Biodegradable manganese-doped calcium phosphate nanotheranostics for traceable cascade reaction-enhanced anti-tumor therapy. ACS Nano. 2019;13:13985–13994. doi: 10.1021/acsnano.9b05836. [DOI] [PubMed] [Google Scholar]
- 71.He M, Chen Y, Tao C, et al. Mn-porphyrin-based metal-organic framework with high longitudinal relaxivity for magnetic resonance imaging guidance and oxygen self-supplementing photodynamic therapy. ACS Appl Mater Interfaces. 2019;11:41946–41956. doi: 10.1021/acsami.9b15083. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Pan Y, Wei C, et al. A tumor microenvironment responsive biodegradable CaCO3/MnO2- based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics. 2019;23:6867–6884. doi: 10.7150/thno.37586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jia Q, Ge J, Liu W, et al. A magnetofluorescent carbon dot assembly as an acidic H2O2-driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv Mater. 2018;30:e1706090. doi: 10.1002/adma.201706090. [DOI] [PubMed] [Google Scholar]
- 74.Fan H, Yan G, Zhao Z, et al. A smart photosensitizer-manganese dioxide nanosystem for enhanced photodynamic therapy by reducing glutathione levels in cancer cells. Angew Chem Int Ed. 2016;55:5477–5482. doi: 10.1002/anie.201510748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ding B, Zheng P, Ma P, et al. Manganese oxide nanomaterials: synthesis, properties, and theranostic applications. Adv Mater. 2020;32:e1905823. doi: 10.1002/adma.201905823. [DOI] [PubMed] [Google Scholar]
- 76.Kim J, Cho HR, Jeon H, et al. Continuous O2-evolving MnFe2O4 nanoparticle-anchored mesoporous silica nanoparticles for efficient photodynamic therapy in hypoxic cancer. J Am Chem Soc. 2017;139:10992–10995. doi: 10.1021/jacs.7b05559. [DOI] [PubMed] [Google Scholar]
- 77.Yin SY, Song G, Yang Y, et al. Persistent regulation of tumor microenvironment via circulating catalysis of MnFe2O4@Metal-organic frameworks for enhanced photodynamic therapy. Adv Funct Mater. 2019;29:1901417. [Google Scholar]
- 78.Meng L, Cheng Y, Tong X, et al. Tumor oxygenation and hypoxia inducible factor-1 functional inhibition via a reactive oxygen species responsive nanoplatform for enhancing radiation therapy and abscopal effects. ACS Nano. 2018;12:8308–8322. doi: 10.1021/acsnano.8b03590. [DOI] [PubMed] [Google Scholar]
- 79.Singh N, Savanur MA, Srivastava S, et al. A redox modulatory Mn3O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a Parkinson's disease model. Angew Chem Int Ed. 2017;56:14267–14271. doi: 10.1002/anie.201708573. [DOI] [PubMed] [Google Scholar]
- 80.Yao J, Cheng Y, Zhou M, et al. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem Sci. 2018;9:2927–2933. doi: 10.1039/c7sc05476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi J, Yu W, Xu L, et al. Bioinspired nanosponge for salvaging ischemic stroke via free radical scavenging and self-adapted oxygen regulating. Nano Lett. 2020;20:780–789. doi: 10.1021/acs.nanolett.9b04974. [DOI] [PubMed] [Google Scholar]
- 82.Tang W, Fan W, Zhang W, et al. Wet/Sono-chemical synthesis of enzymatic two-dimensional MnO2 nanosheets for synergistic catalysis-enhanced phototheranostics. Adv Mater. 2019;31:e1900401. doi: 10.1002/adma.201900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, Chen T, Wu X, et al. Oxygen vacancy-engineered PEGylated MoO3-x nanoparticles with superior sulfite oxidase mimetic activity for vitamin B1 detection. Small. 2019;15:e1903153. doi: 10.1002/smll.201903153. [DOI] [PubMed] [Google Scholar]
- 84.Ni D, Jiang D, Kutyreff C, et al. Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat Commun. 2018;9:5421. doi: 10.1038/s41467-018-07890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li S, Jiang D, Ehlerding EB, et al. Intrathecal administration of nanoclusters for protecting neurons against oxidative stress in cerebral ischemia/reperfusion injury. ACS Nano. 2019;13:13382–13389. doi: 10.1021/acsnano.9b06780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y, Li D, Tan J, et al. Near-infrared regulated nanozymatic/photothermal/ photodynamic triple-therapy for combating multidrug-resistant bacterial infections via oxygen-vacancy molybdenum trioxide nanodots. Small. 2021;17:e2005739. doi: 10.1002/smll.202005739. [DOI] [PubMed] [Google Scholar]
- 87.Ren C, Li D, Zhou Q, et al. Mitochondria-targeted TPP-MoS2 with dual enzyme activity provides efficient neuroprotection through M1/M2 microglial polarization in an Alzheimer's disease model. Biomaterials. 2020;232:119752. doi: 10.1016/j.biomaterials.2019.119752. [DOI] [PubMed] [Google Scholar]
- 88.Han Q, Wang X, Liu X, et al. MoO3-x nanodots with dual enzyme mimic activities as multifunctional modulators for amyloid assembly and neurotoxicity. J Colloid Interface Sci. 2019;539:575–584. doi: 10.1016/j.jcis.2018.12.093. [DOI] [PubMed] [Google Scholar]
- 89.Hu X, Li F, Xia F, et al. Biodegradation-mediated enzymatic activity-tunable molybdenum oxide nanourchins for tumor-specific cascade catalytic therapy. J Am Chem Soc. 2019;142:1636–1644. doi: 10.1021/jacs.9b13586. [DOI] [PubMed] [Google Scholar]
- 90.Mu J, Wang Y, Zhao M, et al. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem Comm. 2012;48:2540–2542. doi: 10.1039/c2cc17013b. [DOI] [PubMed] [Google Scholar]
- 91.Wang J, Wang Y, Zhang D, et al. Intrinsic oxidase-like nanoenzyme Co4S3/Co(OH)2 hybrid nanotubes with broad-spectrum antibacterial activity. ACS Appl Mater Interfaces. 2020;12:29614–29624. doi: 10.1021/acsami.0c05141. [DOI] [PubMed] [Google Scholar]
- 92.Li S, Sun W, Luo Y, et al. Hollow PtCo alloy nanospheres as a high-Z and oxygen generating nanozyme for radiotherapy enhancement in non-small cell lung cancer. J Mater Chem B. 2021;23:4643–4653. doi: 10.1039/d1tb00486g. [DOI] [PubMed] [Google Scholar]
- 93.Dong J, Song L, Yin JJ, et al. Co3O4 nanoparticles with multi-enzyme activities and their application in immunohistochemical assay. ACS Appl Mater Interfaces. 2014;6:1959–1970. doi: 10.1021/am405009f. [DOI] [PubMed] [Google Scholar]
- 94.Sang Y, Cao F, Li W, et al. Bioinspired construction of a nanozyme-based H2O2 homeostasis disruptor for intensive chemodynamic therapy. J Am Chem Soc. 2020;142:5177–5183. doi: 10.1021/jacs.9b12873. [DOI] [PubMed] [Google Scholar]
- 95.Wang L, Huo M, Chen Y, et al. Tumor microenvironment-enabled nanotherapy. Adv Healthcare Mater. 2018;7:e1701156. doi: 10.1002/adhm.201701156. [DOI] [PubMed] [Google Scholar]
- 96.Cao Z, Zhang L, Liang K, et al. Biodegradable 2D Fe-Al hydroxide for nanocatalytic tumor-dynamic therapy with tumor specificity. Adv Sci. 2018;5:1801155. doi: 10.1002/advs.201801155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jarosz-Biej M, Smolarczyk R, Cichoń T, et al. Tumor microenvironment as a "Game Changer" in cancer radiotherapy. Int J Mol Sci. 2019;20:3212. doi: 10.3390/ijms20133212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X, Kwon N, Guo T, et al. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew Chem Int Ed. 2018;57:11522–11531. doi: 10.1002/anie.201805138. [DOI] [PubMed] [Google Scholar]
- 99.Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomed. 2018;13:6049–6058. doi: 10.2147/IJN.S140462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwiatkowski S, Knap B, Przystupski D, et al. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 101.Zhang C, Chen W, Zhang T, et al. Hybrid nanoparticle composites applied to photodynamic therapy: strategies and applications. J Mater Chem B. 2020;8:4726–4737. doi: 10.1039/d0tb00093k. [DOI] [PubMed] [Google Scholar]
- 102.Chen J, Fan T, Xie Z, et al. Advances in nanomaterials for photodynamic therapy applications: status and challenges. Biomaterials. 2020;237:119827. doi: 10.1016/j.biomaterials.2020.119827. [DOI] [PubMed] [Google Scholar]
- 103.Gao Z, Li Y, Zhang Y, et al. Biomimetic platinum nanozyme immobilized on 2D Metal-organic frameworks for mitochondrion-targeting and oxygen self-supply photodynamic therapy. ACS Appl Mater Interfaces. 2020;12:1963–1972. doi: 10.1021/acsami.9b14958. [DOI] [PubMed] [Google Scholar]
- 104.Wang D, Zhang N, Jing X, et al. A tumor-microenvironment fully responsive nano-platform for MRI-guided photodynamic and photothermal synergistic therapy. J Mater Chem B. 2020;8:8271–8281. doi: 10.1039/d0tb01373k. [DOI] [PubMed] [Google Scholar]
- 105.Cao H, Yang Y, Liang M, et al. Pt@polydopamine nanoparticles as nanozymes for enhanced photodynamic and photothermal therapy. Chem Commun. 2021;57:255–258. doi: 10.1039/d0cc07355e. [DOI] [PubMed] [Google Scholar]
- 106.Yang Y, Zhu D, Liu Y, et al. Platinum-carbon-integrated nanozymes for enhanced tumor photodynamic and photothermal therapy. Nanoscale. 2020;12:13548–13557. doi: 10.1039/d0nr02800b. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Wang F, Liu C, et al. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano. 2018;12:651–661. doi: 10.1021/acsnano.7b07746. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Yun KH, Lee H, et al. Porous platinum nanoparticles as a high-Z and oxygen generating nanozyme for enhanced radiotherapy in vivo. Biomaterials. 2019;197:12–19. doi: 10.1016/j.biomaterials.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 109.Liu Y, Cheng Y, Zhang H, et al. Integrated cascade nanozyme catalyzes in vivo ROS scavenging for anti-inflammatory therapy. Sci Adv. 2020;6:eabb2695. doi: 10.1126/sciadv.abb2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petree JR, Yehl K, Galior K, et al. Site-selective RNA splicing nanozyme: DNAzyme and RtcB conjugates on a gold nanoparticle. ACS Chem Biol. 2018;13:215–224. doi: 10.1021/acschembio.7b00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen J, Ma Q, Li M, et al. Glucose-oxidase like catalytic mechanism of noble metal nanozymes. Nat Commun. 2021;12:3375. doi: 10.1038/s41467-021-23737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fan L, Xu X, Zhu C, et al. Tumor catalytic-photothermal therapy with yolk-shell Gold@Carbon nanozymes. ACS Appl Mater Interfaces. 2018;10:4502–4511. doi: 10.1021/acsami.7b17916. [DOI] [PubMed] [Google Scholar]
- 113.Dan Q, Hu D, Ge Y, et al. Ultrasmall theranostic nanozymes to modulate tumor hypoxia for augmenting photodynamic therapy and radiotherapy. Biomater Sci. 2020;8:973–987. doi: 10.1039/c9bm01742a. [DOI] [PubMed] [Google Scholar]
- 114.Gao S, Lin H, Zhang H, et al. Nanocatalytic tumor therapy by biomimetic dual inorganic nanozyme-catalyzed cascade reaction. Adv Sci. 2019;6:1801733. doi: 10.1002/advs.201801733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu C, Xing J, Akakuru OU, et al. Nanozymes-engineered metal-organic frameworks for catalytic cascades-enhanced synergistic cancer therapy. Nano Lett. 2019;19:5674–5682. doi: 10.1021/acs.nanolett.9b02253. [DOI] [PubMed] [Google Scholar]
- 116.Guo X, Wang L, Duval K, et al. Dimeric drug polymeric micelles with acid-active tumor targeting and FRET-traceable drug release. Adv Mater. 2018;30:1705436. doi: 10.1002/adma.201705436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang TT, Xu CH, Zhao W, et al. A redox-activated theranostic nanoagent: toward multi-mode imaging guided chemo-photothermal therapy. Chem Sci. 2018;9:6749–6757. doi: 10.1039/c8sc02446d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gong N, Ma X, Ye X, et al. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat Nanotechnol. 2019;14:379–387. doi: 10.1038/s41565-019-0373-6. [DOI] [PubMed] [Google Scholar]
- 119.Feng L, Dong Z, Liang C, et al. Iridium nanocrystals encapsulated liposomes as near-infrared light controllable nanozymes for enhanced cancer radiotherapy. Biomaterials. 2018;181:81–91. doi: 10.1016/j.biomaterials.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 120.Zhen W, Liu Y, Lin L, et al. BSA-IrO2: catalase-like nanoparticles with high photothermal conversion efficiency and a high X-ray absorption coefficient for anti-inflammation and antitumor theranostics. Angew Chem Int Ed. 2018;57:10309–10313. doi: 10.1002/anie.201804466. [DOI] [PubMed] [Google Scholar]
- 121.Xu P, Wang X, Li T, et al. Biomineralization-inspired nanozyme for single-wavelength laser activated photothermal-photodynamic synergistic treatment against hypoxic tumors. Nanoscale. 2020;12:4051–4060. doi: 10.1039/c9nr08930f. [DOI] [PubMed] [Google Scholar]
- 122.Su H, Liu DD, Zhao M, et al. Dual-enzyme characteristics of polyvinylpyrrolidone-capped iridium nanoparticles and their cellular protective effect against H2O2-induced oxidative damage. ACS Appl Mater Interfaces. 2015;7:8233–8242. doi: 10.1021/acsami.5b01271. [DOI] [PubMed] [Google Scholar]
- 123.Zhang DY, Younis MR, Liu H, et al. Multi-enzyme mimetic ultrasmall iridium nanozymes as reactive oxygen/nitrogen species scavengers for acute kidney injury management. Biomaterials. 2021;271:120706. doi: 10.1016/j.biomaterials.2021.120706. [DOI] [PubMed] [Google Scholar]
- 124.Zhen W, Liu Y, Wang W, et al. Specific "Unlocking" of a nanozyme-based butterfly effect to break the evolutionary fitness of chaotic tumors. Angew Chem Int Ed. 2020;59:9491–9497. doi: 10.1002/anie.201916142. [DOI] [PubMed] [Google Scholar]
- 125.Wei C, Liu Y, Zhu X, et al. Iridium/ruthenium nanozyme reactors with cascade catalytic ability for synergistic oxidation therapy and starvation therapy in the treatment of breast cancer. Biomaterials. 2020;238:119848. doi: 10.1016/j.biomaterials.2020.119848. [DOI] [PubMed] [Google Scholar]
- 126.Wang D, Wu H, Phua S, et al. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat Commun. 2020;11:357. doi: 10.1038/s41467-019-14199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.