Abstract
Lessons drawn from successes and failures with coronavirus disease 2019 (COVID-19) and Ebola virus disease (EVD) should help shaping a robust health innovation system for infectious disease epidemics. Epidemic response research and development (R&D) can be mobilized quickly for public health priorities and yield medicinal products within months. However, to resolve epidemics, technological advances must be equitably accessible and deployed, and these examples expose the limitations of a supply-driven, fragmented R&D ecosystem relying primarily on the private sector to deliver health products. Efficient epidemic response requires a coordinated public health-focused, end-to-end R&D ecosystem for the development, registration, availability, and use of pharmaceutical products. Because pivotal clinical trials can only be conducted during outbreaks, significant preparation must be done beforehand: strengthening clinical research capacity and developing pre-positioned trial protocols and clinical characterization protocols, as well as conducting discovery and pre-clinical research, manufacturing, and early clinical testing of candidate products. This will allow for speedy execution of clinical research early into an outbreak and delivering products within a short time. Effective interventions should be adopted and deployed ensuring equitable access during the ongoing outbreak. Measures to make products available where and when needed must be integrated throughout the R&D value chain.
Key Words: infectious disease epidemic, preparedness, response, medicinal products, health technology, innovation, COVID-19, Ebola virus disease
Graphical abstract
Response to the COVID-19 pandemic shows that collective resources and intelligence can be swiftly mobilized to generate technological developments. However, our ability to effectively prevent and curb epidemics depends on fixing the lack of a globally coordinated end-to-end research and development ecosystem for the development, registration, availability, and use of pharmaceutical products.
The context
An epidemic is “an increase, often sudden, in the number of cases of a disease above what is normally expected in that population in that area.”1 Many conditions would therefore qualify as an epidemic, including both communicable and non-communicable diseases—e.g., the ongoing “obesity epidemic”2 and the “opioid overdose epidemic.”3
Even when focusing on infectious disease epidemics, we still have very different scenarios: seasonal or occasional outbreaks of endemic infections (e.g., dengue and other arboviruses), seasonal spill-overs from animal reservoirs (e.g., Ebola virus disease [EVD], Lassa fever, and other viral hemorrhagic diseases, plague), new diseases (be it a known pathogen introduced in a new geographical area, or a previously unknown pathogen for humans, like sever acute respiratory disease coronavirus 2). Most of these epidemics are geographically and time limited. The spread of an epidemic over several countries and continents may prompt the World Health Organization (WHO) to declare a pandemic.
Between the great influenza pandemic of 1918 and the COVID-19 pandemic, many epidemics have occurred, mostly under the radar screen of media and public attention, but also neglected by science and investments in preparedness or response capacity, including tools development. Occasionally, some epidemics overcome this high tolerance threshold and trigger attention and reactions, essentially when they are perceived as a threat to “global health security,” i.e., potentially affecting high-income countries.4
Therefore, there is no one-size-fit-all approach to responding to infectious disease epidemics. However, there certainly are cross-cutting elements underpinning a successful prevention, preparedness, and response system. They are the sum of reliable disease surveillance, robust health systems, responsive medical innovation, equitable access to medicine and health care—which means essentially reaching the sustainable development goals 3 (good health and well-being) and 10 (reduced inequalities).5
Epidemic preparedness and response research and development
Diagnostics, treatments, and vaccines are critical health technologies for public health emergency preparedness and response. Ensuring their timely development and availability is, therefore, a collective public health responsibility. However, even for well-known microbial threats, such technologies are often not (sufficiently) available when needed, or come late during an outbreak, are ill-adapted to the health systems, or are only accessible for few.
It is important to understand why and how these health technologies are developed, how they are deployed, and what makes them fail or succeed.
At a different scale, both the West African EVD epidemic and the COVID-19 pandemic have shown how dependent the success of public health interventions is on accurate education and science communication and how misinformation can undermine epidemic response. The pandemic has also revealed how collectively unprepared we are to exposing unfiltered scientific debate—which feeds on diversity of opinions—to public scrutiny, often resulting in either naive or malevolent misinterpretations. Scientific journals have also been overwhelmed with submissions, also revealing the limits of the peer review system and its overall capacity to critically absorb a large influx of papers, with sometimes negative consequences on decision-making.6
The dearth of available health technologies for epidemic preparedness and response is commonly ascribed to “market failure”—an expression of the current health innovation ecosystem relying largely on the private sector for pharmaceutical product research and development (R&D) and supply. The general lack of commercial attractiveness of infectious diseases (often requiring short-term therapies, as opposed to more lucrative chronic treatments)7 is compounded with a weak “business case,” given the unpredictable size and timing of “demand” in the case of epidemic outbreaks, and a reluctancy of public health systems and the international community to invest in structural interventions and preventive measures, including stockpiles that might remain unused, expire, and needing replacement.
While the COVID-19 pandemic may have temporarily transformed epidemic preparedness and response R&D from an area of market failure to a highly profitable market opportunity, it has still relied on massive public investments and other policy interventions to mobilize and reward the private sector. Despite the successful development of multiple effective vaccines at unprecedented speed, access to these vaccine remains profoundly unequal, which is a likely engine of persistent transmission and mutations in viral genome.8 This lack of a globally coordinated end-to-end R&D ecosystem and a public health-oriented governance mechanism when it comes to the availability and use of interventions is hindering our capacity to effectively prevent and curb epidemics—now and in the future—if radical changes are not set in place.9,10
We need collective intelligence and coordination, not fragmentation and competition
In responding to global health threats, governments and their health authorities are naturally in charge of defining the response strategies and outbreak control measures, from non-pharmaceutical interventions like physical distancing, protective barriers, travel restrictions, and lockdowns, to testing, treatment, and vaccination strategies. Public health authorities would also be expected to (1) define which type of pharmaceutical interventions they need—i.e. determining the target product profile; (2) coordinate a portfolio of R&D projects that covers priority health needs in order to identify and facilitate the development of the most suited products; (3) oversee the research methodology and study designs to ensure they address the most important public health questions in a timely way; and (4) secure the availability, accessibility, and optimal deployment of the health interventions for epidemic preparedness and response.
However, this is hardly the case. When infectious disease epidemics arise, and especially when they are not just a public health concern but are also considered a global health security threat, there is a flurry of activities by the global health community to start R&D or revive dormant workstreams, in what is often a very reactive and mostly competitive—uncoordinated, uncollaborative—mode. The R&D ecosystems functions like a conductor-less orchestra, with research groups, public health institutions, funders, and pharmaceutical companies each pursuing their objectives, but not necessarily in harmony toward shared public health goals. For instance, the responsibility to bring investigational products into clinical development and subsequent registration is largely left to commercial product developers that will select which proprietary technologies will be progressed—whether it corresponds or not with the most desirable public health interventions. At the same time, academic and other public researchers—often competing for funding through research financing mechanisms that are ill-adapted to respond to health emergencies—will launch a variety of investigator-driven studies using available products or interventions (e.g., by repurposing or combining drugs).
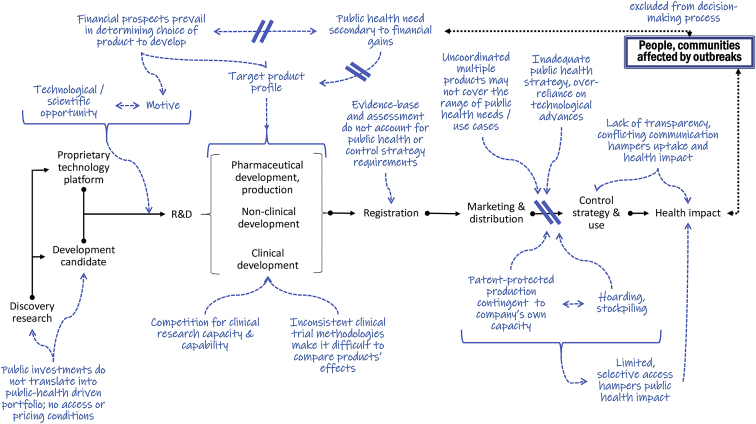
Figure 1 summarizes the critical disruptions at the main steps in product development and use conspiring against mounting an effective epidemic response system.
Figure 1.
Medicinal product value chain from discovery to availability and use for health impact, providing examples of inefficiencies of the current competitive, supply-driven, fragmented R&D ecosystem specifically for epidemic preparedness and response
For instance, as of November 2021, 4,299 treatment trials (1,172 recruiting, 1,044 completed, and 760 inactive, suspended, terminated, or withdrawn) and 1,104 vaccine trials (1,104 recruiting, 513 completed, and 242 inactive, suspended, terminated, or withdrawn) are registered just in clinicaltrials.gov.11 It is difficult to know which of these trial results are published where, or when or if they will be published at all.12 Without a mechanism to channel resources to the most promising interventions and ensure global collaboration and coordination rather than fragmented competition, there will be too many ill-designed, underpowered, or otherwise inconclusive or non-comparable trials.13,14 Companies also have no obligation to ensure that products are registered, available, and accessible in countries where outbreaks occur, while research funders typically only finance certain types of activities—for instance, clinical trials—but not necessarily manufacturing, registration, availability, or access. There is no clear division of labor and responsibility across the R&D value chain, nor any agreed plans around ownership and sharing of scientific data, intellectual property (IP) management, strategic use of manufacturing capacity, or allocation of final products in the interest of global public health. Finally, there is currently no collective mechanism to ensure end-to-end financing of epidemic preparedness and response R&D, including equitable global access. Given that the availability of financing is a critical driver of many activities along the value chain, there is an important role for research funders in directing the right kind of studies and coordination, and put conditionalities as needed to the financing to ensure that research efforts are pursued in ways that maximize chances to result in equitable access, including research data sharing.
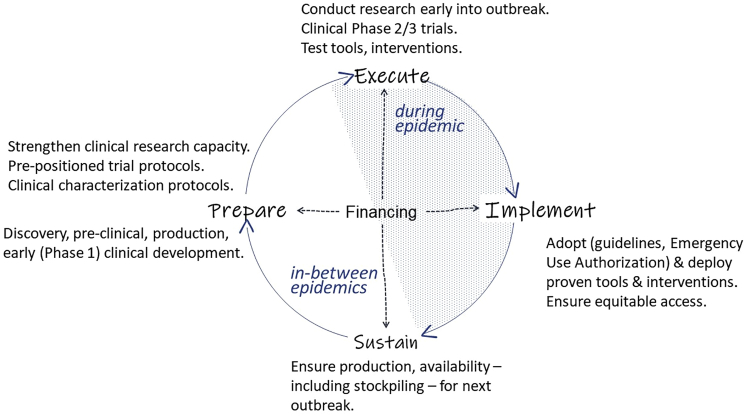
Figure 2 depicts the proposed essential elements of a global research and response architecture for infectious disease epidemics.
Figure 2.
Building a global research and response architecture for infectious disease epidemics
Strengthening the clinical research architecture for outbreak diseases preparedness and response
A specific challenge with medical innovation for epidemic diseases is that interventions can only be tested clinically during an outbreak, often as integral part of response. Since when and where an outbreak will occur is often unpredictable, most of the preparatory work must be done outside (ahead of) epidemics to enable a prompt and coordinated response when an outbreak does occur. This requires strengthening clinical research capacity in “peacetime,” as during epidemics health care systems are often stretched dealing with providing medical treatment to patients, which makes it difficult for them to absorb and implement trials, especially in low-resource settings. It also requires a public health-driven priority setting to ensure that the most promising interventions are tested in priority, not just the ones that were able to amass sufficient resources quickly.
We have seen various cases where trials did only start past the peak of transmission or where research groups competed for access to patients, thereby fragmenting the effort, with the result that the projected sample size could not be enrolled due to declining number of cases. A case in point are multiple treatment trials set up during the 2016–2018 West Africa EVD epidemic.15,16
Yet, neither the West Africa EVD epidemic nor the COVID-19 pandemic are the archetypal outbreak given their size and duration; most epidemics are short lived, have low caseload, and may be geographically dispersed. For instance, between 2003 and 2021, a total of 862 laboratory-confirmed human cases of avian influenza A (H5N1) have been reported from 16 countries.17 Similarly, 1,516 human cases of avian influenza A (H7N9) have been reported between 2013 and 2017, with the largest epidemic being 776 cases occurring in China over 12 months in 2016 and 2017. Under these circumstances, it is particularly challenging to conduct proper clinical trials to test new interventions, despite their high case fatality rates (about one-half and one-third, respectively) and evident epidemic potential.18 This warrants piloting innovative trial designs that can span multiple outbreaks, in multiple countries, as was intended with the original design of the Ebola PALM trial.19
The longer-lasting 2009–2010 influenza A (H1N1)pdm09 pandemic represents instead a failed, belated response, and a hugely missed opportunity to gather timely information essential to setting up an effective response. The US Centers for Disease Control and Prevention estimate that between 151,700 and 575,400 deaths,20 which, contrary to seasonal flu, occurred mostly in a younger population. By the time a specific vaccine became available in large enough quantities, it was already late in the pandemic. Clinical research was of insufficient quality and the results became available mostly well after the pandemic was over.21
Clinical research also requires observational studies to understand clinical presentation and risks of unfavorable outcomes to inform case management practices. This entails the development of pre-positioned and approved clinical record forms for rapid uptake to generate harmonized datasets. These adaptable research tools exist. The International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) is a global federation of clinical research networks that work collaboratively to prevent illness and deaths from infectious disease outbreaks. The ISARIC adheres to principles of open-source research22 and collaboration instead of competition.23 The ISARIC-WHO clinical record form was quickly adopted at the onset of the COVID-19 pandemic,24 leading to a dataset of more than 700,000 COVID-19 cases at the time of this writing25 that can be accessed for analyses. This shows that a global collaborative approach, based on research readiness in a peer-to-peer network, is achievable and effective, and can be replicated for future epidemics.26
Last but not least: Ensuring availability and access
Too often, the battle about access is unsuitably centered on the final product, when in reality it starts much upstream.
A key characteristic of market failure is that market demand-and-supply dynamics do not leverage R&D, availability, and access when the purchasing power of those affected is too small to attract commercial interest. Fixing the market failure for R&D—for instance, through public funding or other market incentives—does not guarantee that the resulting interventions will be registered, available, and accessible in countries where outbreaks occur. Outside an ongoing outbreak, there may be little urge for companies to seek product registration, or for countries or public health authorities to purchase (for stockpiling) the new product if registered—unless motivated by reasons like health security. As a result, critical health technologies that have been developed are not necessarily available where and when an outbreak occurs.
Since licensure is granted to pharmaceutical companies, even if the research was done by broad consortia of public and private partners, the control over product availability—how much is being manufactured and sold to whom and at what price—is essentially in the hands of the private sector. A company typically holds both IP rights via patents on products and technologies and retains exclusivity on the use of data contained in the regulatory dossier, precluding others to produce and sell the products. Such monopolies compound the fragility of an already precarious supply chain. Reliance on a single source for the supply of critical health technologies is a recipe for scarcity, high prices, or both, with major impact on equitable access. The inequalities in COVID-19 vaccine access in Africa for instance are explained in part to the lack of vaccine manufacturing capacity on the continent (currently importing 99% of all vaccines used).27
Too often, companies will prioritize licensure in markets of interest, primarily the United States, Europe, and a handful of other countries that may be interested in establishing strategic stockpiles for certain diseases of epidemic potential. For products that are eligible to receive a US Food and Drug Administration (FDA) priority review voucher, an incentive to stimulate the development of drugs or vaccines against neglected tropical diseases, filoviruses (including Ebola), rare pediatric indications, and medical countermeasures for certain global health security threats,28 the financial reward is obtained upon registration at the US FDA. Unfortunately, obtention of the reward is not linked to any obligation for availability or access where needed.29
Lessons learnt from EVD and COVID-19
As illustrated below, EVD and COVID-19 are two examples of exceptional epidemic R&D efforts where, despite much heralded successes in product development, inadequate public health-focused leadership and governance has resulted in unfinished research agendas, critical availability gaps, and major access inequities. While different in size and health and economic impact, both benefited from massive public investments to support and accelerate the development and availability of medical technologies to help curb the epidemics. Yet, this was not accompanied by commensurate public oversight and end-to-end steering to ensure maximal impact of investments, with major public health, access, and ethics implications.
Ebola virus disease
While known since the 1970s, EVD is a typical case of market failure: no treatment, diagnostic, or vaccine was available when a long-expected, major outbreak devastated Guinea, Liberia, and Sierra Leone in 2014–2016, killing more than 11,000 people and creating a global panic.30 Yet, thanks to an impressive mobilization of public health institutions, governments, researchers, philanthropic organizations, pharmaceutical companies, and humanitarian actors coming together on an ad hoc basis, a burst of R&D activities ensued, which continued throughout the 2018–2019 outbreaks in the Democratic Republic of Congo (DRC).
By early 2021, there were two vaccines and two treatments registered by FDA or European Medicines Agency (EMA), an impressive achievement, yet their availability, affordability, and effective use for current and future EVD outbreaks remain wrought with questions and uncertainties.
Of the four new Ebola products that received FDA or EMA marketing authorization, only the rVSV-ZEBOV vaccine (Ervebo) produced by Merck is registered in DRC and in a few other Ebola-prone African countries. It is also WHO prequalified. In January 2021, a GAVI-funded global emergency stockpile of 500,000 doses was created under the auspices of the International Coordinating Group on Vaccine Provision, accessible to all countries.31 While lower income countries can obtain vaccines for free, it is not known how much other countries will be charged, nor the price Merck has charged GAVI. It is also not clear how the supply will be sustained in the future.
Johnson & Johnson's (JNJ) prime-boost vaccine (Zabdeno [Ad26.ZEBOV] and Mvabea [MVA-BN-Filo]) was WHO pre-qualified, is being used in further studies but is not yet registered in African countries. The two treatments, Regeneron's Inmazeb (the association of three monoclonal antibodies: atoltivimab, maftivimab, and odesivimab-ebgn) and Ridgeback Biotherapeutics' Ebanga (the monoclonal antibody ansuvimab), are also not registered in any African country. It is thought that the US government has established stockpiles of at least one, if not both, Ebola treatments, but no details of size and price are publicly available.
Critically, none of these registered products seems readily available for use in disease-endemic countries, including the DRC, which has been experiencing a series of EVD outbreaks. During the 2018–2020 Kivu EVD outbreak—the second largest recorded outbreak—control efforts made use of remaining clinical supplies of the still-investigational products with little transparency on how to access these stocks, and with often restricted conditions for use because still under restrictive “study conditions.”
In addition, many outstanding research questions remain around both vaccines and treatments, and there is no pathways or clear responsibilities as to how to address them. The fact that none of these products can be bought through regular procurement channels (there are also no official prices, and secrecy over prices paid for the stockpiles) has hampered operational research to better understand how to best deploy these different tools for epidemic preparedness and response.
The pivotal efficacy trial for Merck's rVSV-ZEBOV vaccine in Guinea was designed as a reactive cluster ring vaccination study—whereby contacts and contacts of contacts of cases are randomized to receive vaccine or placebo. The subsequent use of this vaccine in the DRC followed the same approach, and the WHO Strategic Advisory Group of Experts on Immunization (SAGE) continues to recommend it as primary strategy in case of an EVD outbreak, partly because of limited supplies.32 Other questions still unresolved include duration of protection and effectiveness when used as a preventive vaccine—i.e., given at population level, whether individuals had been already exposed or not to a source of infection. The difference is important. The ring vaccination approach is triggered by an ongoing outbreak, as opposed to vaccinating a population to prevent outbreaks altogether.
The deployment of rVSV-ZEBOV as a primary outbreak response has effectively rendered demonstrating clinical efficacy of the JNJ's Ad26.ZEBOV/MVA-BN-Filo prime-boost vaccine practically unworkable. This vaccine was granted marketing authorization by the EMA in 2020 based on correlates of protection—animal studies and levels of immune response in humans—and was pre-qualified by the WHO in 2021. This approach, whereby the prime and boost are administered eight weeks apart, makes this vaccine “not suitable for an outbreak response where immediate protection is necessary.”33 Meanwhile, since antibodies were detectable up to 2 years after immunization, and a booster shot with Ad26.ZEBOV resulted in a rapid increase of specific antibodies,34 the WHO SAGE now suggests that countries to consider pre-emptive vaccination of EVD response teams with the prime-boost vaccine. However, wider use a as preventive vaccine remains questionable, primarily for the lack of clinical efficacy data.
The case of these two vaccines shows a failure to look collectively beyond single trial designs and to understand the consequences of the fact that there typically is only a one-off chance to gather pivotal safety and efficacy evidence in clinical trials, and thus ensure that the most important public health questions are answered. Here, early trial design choices and unattended evidence gaps now stand in the way of a regional and public health-focused disease control strategy, including targeted preventive vaccination.
As for treatments, research questions include their potential as post-exposure prophylaxis, possible dose optimization (related also to the cost of goods and therefore potentially price), or their use in combination with other antivirals. The lack of straightforward availability for use and follow-on research, including the reportedly prohibitive pricing (lack of transparency precludes knowing how much), reflects a failure to apply public health-focused conditionalities to the agreements between public and private partners involved in the development. Such governance challenges illustrate the mainly voluntary “coalition of the willing” nature of the research partnerships, with gaps and lack of transparency around decision-making and accountability, against the background of complex power dynamics.
The case of both EVD vaccines and therapies also shows an inadequate distribution of ownership and control between the many partners involved in clinical research and implementation. For instance, trial data ownership and decision-making over industrial and regulatory strategy lies solely with the commercial partner, which also controls availability (manufacturing decisions) and access (local registration, availability, and price). Yet, as is typically the case for epidemic R&D, most of the Ebola research was financed through public and philanthropic resources and was conducted by public and non-profit institutions. Still, the financial rewards such as the proceeds of the FDA's priority review voucher go solely to the company, without any obligation to commit to invest in further research or even to make the product equitably available at an affordable price. In fact, it will be the same public resources (US government and other multilateral health donors) that finance the creation of stockpiles (often at undisclosed prices) of products for which they had already largely financed the development. Meanwhile, a lack of coordination and transparent decision-making between local, national, and international health authorities results in sub-optimal response strategies.
COVID-19
The COVID-19 epidemic response R&D has differed substantially from EVD’s, especially for vaccines. The exceptional size and scale of the pandemic made it the primary health and economic concern of wealthy countries, generating huge commercial and political interest. Early on in the pandemic, pharmaceutical companies quickly took up vaccine R&D, building on existing proprietary technology platforms, already developed or under research for other indications (e.g., messenger RNA [mRNA],35 adenoviral vector vaccines36); some also partnered with academic groups with promising technologies that they acquired under exclusive licence.37 At the same time, unprecedented amounts of government funding are mobilized to support and de-risk the companies, by both directly financing the R&D and making advanced market commitments.38 This meant that these companies were confident to start industrial development and scaling up vaccine manufacturing ahead of safety and efficacy demonstration in clinical trials, thus accelerating considerably the process toward marketing. In addition, key regulatory agencies like the US FDA and EMA signaled willingness to consider accelerated review and approval pathways based on preliminary data, such as the Emergency Use Authorization, to speed up the availability and deployment of the vaccines.
As a result, vaccine R&D was set in motion and progressed at an unprecedented pace. The first vaccine to receive regulatory authorization for use in August 2020 was the adenoviral vector-based vaccine Sputnik from Gamalea (authorized in Russia), followed by the first-ever mRNA vaccines by Moderna and Pfizer (US FDA emergency use authorization in December 2020, followed by regulators in other countries), and Astra Zeneca (UK MHRA emergency use authorization in December 2020, followed by regulators in other countries). As of November 2021, 23 vaccines have received emergency use or full regulatory authorizations, 107 vaccines are reportedly in clinical trials, and more than 75 are in preclinical testing.39
The concomitant development of several effective COVID-19 vaccines in just a year is a testimony of our global scientific and technological capabilities and shows what is possible when political will exists and (public) resources are made available. However, the stark inequities in access to these innovations, fueled by a combination of private sector control over the IP rights and manufacturing capabilities, together with vaccine nationalism by wealthy country governments (hoarding the majority of dosages), are leaving large parts of the world exposed to the risk of COVID-19 and its health, economic, and societal consequences.40,41 As of November 19, 2021, high-income countries have administered on average more than 145 doses per 100 persons and are rolling out booster shots, while many people in low-income countries are yet to receive their first dose (the average number of doses per 100 people is 7).42 This collective failure to translate technological progress into an effective global health response to the pandemic and protect the most vulnerable everywhere is not only a moral failure, as WHO Director General pointed out,43 but also a colossal failure in R&D governance to respond to the most important challenge of our time.44 It is also a hugely miscalculated strategy as letting the virus free to replicate unchecked exposes the same high-income countries to the risk of more transmittable and less controllable virus variants.45
As global demand for equitable access to COVID-19 vaccines intensifies, health and socio-economic justice advocates supported by global leaders like the UN Secretary General and WHO Director-General are calling for vaccine manufactures to share IP, know-how, and technologies to enable the scale up of vaccine production in low- and middle-income countries.46 However, it is clear that companies dominating the market are unwilling to give up control over their vaccines, in particular IP and production technology and know-how. This, with full endorsement of high-income countries unwilling to use the leverage of their massive public investment to force companies to share critical technologies for pandemic preparedness and response47 means that global access will, for now, continue to rely on the goodwill of vaccine manufacturers to produce sufficient quantities and sell vaccines at prices they deem the buyer is willing to pay according to ruthless demand-supply market dynamics.
Another damaging characteristic of market-based R&D is its reliance on competition rather than collaboration and collective intelligence to bring health interventions forward, even during epidemics.48 Instead of taking a portfolio approach to enable complementary technologies (from an epidemic control perspective) to advance in parallel, it incentivizes a race for the first to obtain regulatory approval—often emergency use authorization based on preliminary evidence.49
After a critical review of the global response to COVID-19, the Independent Panel for Pandemic Preparedness and Response has started to lay out a framework for future “pre-negotiated systems for a global health commons approach to mobilize pandemic tools,” including an end-to-end R&D platform, predictable financing, adequate industrial policies, inclusive governance, and regional platforms.50 Similarly, global health leaders have begun to articulate what biomedical R&D toward the global public interest must look like,51 which is also echoed the WHO Council on the Economics of Health for All.52
Transparency—and a lack thereof
For the public to accept measures such as lockdowns, vaccination, and other interventions against epidemics, trust is essential, and trust requires transparency both about research results and decision-making. Sadly, such has not been the case for COVID-19,53,54 but that is not new. An example in the recent past is oseltamivir (Tamiflu) and the controversies over the lack of public availability of clinical trial data and the interpretation of the results of company-sponsored trials that informed the decision by WHO to include the drug on the essential medicines list and by various governments to stockpiling this medication.55 Many have challenged the fact that pharmaceutical companies retain full control over clinical trial data, including what and when to publish, which is the norm in the sector, and a growing number of policies are being adopted to increase disclosure and transparency requirements,56 but progress has been slow.57
In contrast with company-sponsored trials, the publicly funded RECOVERY trial has made available the results of putative COVID-19 treatments tested in more than 44,000 hospitalized patients, all being immediately actionable, including the use of inexpensive, available medicines.58 They have shown that the immune-modulators dexamethasone59 (tested in 6,429 patients) and tocilizumab60 (n = 4,169), and the casirivimab-imdevimab antibody cocktail61 (n = 9,785) work, and also shown that many other do not (hydroxychloroquine,62 lopinavir-ritonavir,63 convalescent plasma,64 aspirin,65 azithromycin,66 and colchicine67).
Meanwhile, results from pharmaceutical company-sponsored COVID-19 treatment and vaccine trials, and the ensuing policy decisions including major procurement contracts, do not seem to deviate from the secrecy patterns.68 Examples abound in the past year of announcements made through press releases, that, among other things, have huge effects on stock markets, as was the case again after the recent announcements by Merck of positive trial results from its drug candidate.69 The transparency adopted by the US FDA when they reviewed applications for COVID-19 vaccines, including the online publication of the company's data as well as an independent analysis performed by the US FDA scientists, and a public hearing with experts, during which these are discussed, stands as a lone and positive example of the type of data transparency that can be achieved to build trust. However, the same authorities were criticized for a lack of transparency and non-evidence-based decision making around other applications.70
Finally, governments must strengthen surveillance systems and ensure timely sharing of information on circulating pathogens, which is essential for directing research efforts to rapidly deliver appropriate health technologies for epidemic response.
Conclusions
Infectious disease epidemics occur all the time, but only those perceived as global health threats attract attention and investments toward the development of countermeasures like vaccines, diagnostics, and treatments. To mount an effective preparedness and response strategy for epidemics, one-off opportunistic partnerships of the willing are not always adequate to overcome the many challenges and gaps of an R&D ecosystem that is geared to seize market opportunities rather than addressing public health needs, especially for populations in low-resource countries.
As shown during both the COVID-19 and EVD epidemics, with sufficient public investments it is possible to overcome market failures and harness our collective knowledge and technological capacity to speedily develop and test new medical interventions. However, we fell short of scaling up vaccine manufacturing for the speedy deployment and failed on vaccine equity. The lack of a coordinated end-to-end R&D architecture to steer the development, registration, availability and use of pharmaceutical products hampers our collective capacity to effectively prevent and curb epidemics and exposes the limitations of a supply-driven and fragmentary R&D pipeline that relies primarily on the private sector to deliver these products.
What we need instead is to accept that epidemic preparedness and response is a public responsibility requiring global health focused leadership and adequate financing to build and manage a portfolio of R&D projects that can adapt to the changing needs for epidemic control, and has equitable access built-in component from the start. This requires strong public health direction to build symbiotic partnerships between public and private sectors toward the shared goal of delivering appropriate health interventions as common goods, not private commodities.
Acknowledgments
E.T.’s contribution is partly based on an Epidemic Ethics/WHO initiative, which was supported by FCDO/Wellcome Grant 214711/Z/18/Z. P.O. receives salary support from the UK Foreign, Commonwealth, and Development Office; and the Wellcome Trust [215091/Z/18/Z].
Author contributions
Both authors contributed equally to the conceiving, writing, and editing this paper.
Declaration of interest
Both authors declare no conflict of interest.
References
- 1.US Department of Health and Human Services . 3rd edition. 2006. Principles of Epidemiology in Public Health Practice.https://www.cdc.gov/csels/dsepd/ss1978/SS1978.pdf [Google Scholar]
- 2.González-Muniesa P., Mártinez-González M.A., Hu F., Deprés J.P., Matsuzawa Y., Loos R.J.F., Moreno L.A., Bray G.A., Martinez J.A. Obesity. Nat. Rev. Dis. Primers. 2017;3:17034. doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 3.Skolnick P. The opioid epidemic: crisis and solutions. Annu. Rev. Pharmacol. Toxicol. 2018;58:143–159. doi: 10.1146/annurev-pharmtox-010617-052534. [DOI] [PubMed] [Google Scholar]
- 4.Heymann D.L., Chen L., Takemi K., Fidler D.P., Tappero J.W., Thomas M.J., Kenyon T.A., Frieden T.R., Yach D., Nishtar S., et al. Global health security: the wider lessons from the west African Ebola virus disease epidemic. Lancet. 2015;385:1884–1901. doi: 10.1016/S0140-6736(15)60858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United Nations . 2015. 17 Sustainable Development Goals.https://sdgs.un.org/goals [Google Scholar]
- 6.Davey M., Kirchgaesser S., Boseley S. Surgisphere: Governments and WHO changed Covid-19 policy based on suspect data from tiny US Company (2020) The Guardian. 2020 https://www.theguardian.com/world/2020/jun/03/covid-19-surgisphere-who-world-health-organization-hydroxychloroquine 3 June. [Google Scholar]
- 7.Kim T. 2018. Goldman Sachs Asks in Biotech Research Report: ‘Is Curing Patients a Sustainable Business Model?’ CNBC 18 April.https://www.cnbc.com/2018/04/11/goldman-asks-is-curing-patients-a-sustainable-business-model.html [Google Scholar]
- 8.World Health Organization Vaccine inequity undermining global economic recovery. News Release. 2021;22 https://www.who.int/news/item/22-07-2021-vaccine-inequity-undermining-global-economic-recovery [Google Scholar]
- 9.Lurie N., Keusch G.T., Dzau V.J. Urgent lessons from COVID 19: why the world needs a standing, coordinated system and sustainable financing for global research and development. Lancet. 2021;27:1229–1236. doi: 10.1016/S0140-6736(21)00503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torreele E., Kazatchkine M., Mazzucato M. Preparing for the next pandemic requires public health-focused industrial policy. BMJ. 2021 https://blogs.bmj.com/bmj/2021/04/01/preparing-for-the-next-pandemic-requires-public-health-focused-industrial-policy/ 1 April. [Google Scholar]
- 11.https://clinicaltrials.gov
- 12.Jones C.W., Adams A.C., Murphy E., King R.P., Saracco B., Stesis K.R., Cavanaugh S., Roberts B.W., Platts-Mills T.F. Delays in reporting and publishing trial results during pandemics: cross sectional analysis of 2009 H1N1, 2014 Ebola, and 2016 Zika clinical trials. BMC Med. Res. Methodol. 2021;21:120. doi: 10.1186/s12874-021-01324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe D. The problem with COVID-19 clinical trials. 2021. https://www.science.org/content/blog-post/problem-covid-19-clinical-trials Science Blog 17 March.
- 14.Manoharan L., Olliaro P., Horby P.W., Watson C.H. Chemoprophylaxis trial designs in epidemics: insights from a systematic review of COVID-19 study registrations. Trials. 2021;22:370. doi: 10.1186/s13063-021-05323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojek A., Horby P., Dunning J. Insights from clinical research completed during the west Africa Ebola virus disease epidemic. Lancet Infect. Dis. 2017;17:e280–e292. doi: 10.1016/S1473-3099(17)30234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olliaro P.L. Initiation and publication time-lags of treatment trials for Ebola virus disease. Lancet Infect. Dis. 2018;18:28–29. doi: 10.1016/S1473-3099(17)30698-9. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Influenza at the human-animal interface. Summary and assessment, from 23 June to 8 August 2021. 2021. https://cdn.who.int/media/docs/default-source/influenza/human-animal-interface-risk-assessments/influenza_summary_ira_ha_interface_08_08_2021.pdf
- 18.Centers for Disease Control and Prevention. Asian Lineage Avian Influenza A/H7N)) Virus. https://www.cdc.gov/flu/avianflu/h7n9-virus.htm
- 19.Mulangu S. The PALM Consortium: a multicenter, multi-outbreak randomized controlled trial of Ebola virus disease therapeutics. Open Forum Infect. Dis. 2019;6:S12–S13. [Google Scholar]
- 20.Centers for Disease Control and Prevention H1N1 Pandemic (H1N1pdm09 Virus) 2009. https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html
- 21.Rojek A.M., Martin G.E., Horby P.W. Compassionate drug (mis)use during pandemics: lessons for COVID-19 from 2009. BMC Med. 2020;18:265. doi: 10.1186/s12916-020-01732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunning J.W., Merson L., Rohde G.G.U., Gao Z., Semple M.G., Tran D., Gordon A., Olliaro P.L., Khoo S.H., Bruzzone R., et al. Open source clinical science for emerging infections. Lancet Infect. Dis. 2014;14:8–9. doi: 10.1016/S1473-3099(13)70327-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ISARIC Clinical Characterisation Group Global outbreak research: harmony not hegemony. Lancet Infect. Dis. 2020;20:770–772. doi: 10.1016/S1473-3099(20)30440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Severe Acute Respiratory and emerging Infection Consortium. ISARIC Covid-19 Timeline 2019-2021. https://isaric.org/covid-19-pandemic-response-timeline/.
- 25.ISARIC Clinical Characterisation Group. Baillie J.K., Baruch J., Beane A., Blumberg L., Bozza F.A., Broadley T., Burrell A., Carson G., Citarella B.W., Dunning J., et al. ISARIC COVID-19 clinical data report Issued: 15 December 2021. medRxiv. 2021 doi: 10.1101/2020.07.17.20155218. Preprint at. [DOI] [Google Scholar]
- 26.ISARIC Clinical Characterisation Group The value of open-source clinical science in pandemic response: lessons from ISARIC. Lancet Infect. Dis. 2021;21:1623–1624. doi: 10.1016/S1473-3099(21)00565-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin A. How COVID spurred Africa to plot a vaccines revolution. 2021. https://www.nature.com/articles/d41586-021-01048-1 Nature 21 April. [DOI] [PubMed]
- 28.US Food and Drug Administration Tropical Disease Priority Review Voucher Program. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/tropical-disease-priority-review-voucher-program
- 29.Sunyoto T., Potet J., Boelaert M. Why miltefosine, a life-saving drug for leishmaniasis, is unavailable to people who need it the most. BMJ Glob. Health. 2018;3:e000709. doi: 10.1136/bmjgh-2018-000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torreele E., Olliaro P. Ebola in west africa is a wake-up call. What the Ebola crisis tells us about our failing drug development system. 2014. http://www.aljazeera.com/indepth/opinion/2014/11/ebola-west-africa-wake-up-call-2014112125429230915.html Al Jazeera 6 Nov.
- 31.Gavi The Vaccine Alliance Ebola vaccine to be made available to countries for outbreak response. News Release. 2021;12 https://www.gavi.org/news/media-room/500000-doses-ebola-vaccine-be-made-available-countries-outbreak-response [Google Scholar]
- 32.World Health Organization Meeting of the strategic advisory group of experts on immunization, 22–24 March 2021: conclusions and recommendations. 2021. https://apps.who.int/iris/bitstream/handle/10665/341623/WER9622-eng-fre.pdf
- 33.European Medicines Agency New vaccine for the prevention of ebola virus disease recommended for approval in the European Union. 2020. https://www.ema.europa.eu/en/news/new-vaccine-prevention-ebola-virus-disease-recommended-approval-european-union Press Release 29 May.
- 34.Ishola D., Manno D., Afolabi M.O., Keshinro B., Bockstal V., Rogers B., Owusu-Kyei K., Serry-Bangura A., Swaray I., Lowe B., et al. Safety and long-term immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Sierra Leone: a combined open-label, non-randomised stage 1, and a randomised, double-blind, controlled stage 2 trial. Lancet Infect. Dis. 2021;22:97–109. doi: 10.1016/S1473-3099(21)00125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May M. After COVID-19 successes, researchers push to develop mRNA vaccines for other diseases. Nat. Med. 2021;27:930–932. doi: 10.1038/s41591-021-01393-8. https://www.nature.com/articles/s41591-021-01393-8 [DOI] [PubMed] [Google Scholar]
- 36.Mendonça S.A., Lorincz R., Boucher P., Curiel D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines. 2021;6:97. doi: 10.1038/s41541-021-00356-x. https://www.nature.com/articles/s41541-021-00356-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.AstraZeneca AstraZeneca and Oxford University announce landmark agreement for COVID-19 vaccine. 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/astrazeneca-and-oxford-university-announce-landmark-agreement-for-covid-19-vaccine.html Press Release 30 April.
- 38.Hoecklin M. €93 Billion Spent by Public Sector on COVID Vaccines and Therapeutics in 11 Months, Research Finds. 2021. https://healthpolicy-watch.news/81038-2/ Health Policy Watch 12 Jan.
- 39.The New York Times Corona Virus Tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- 40.Amnesty International . 2021. G20’s Bitter Divide on Global Vaccine Inequality Could Condemn World to an “Endless Pandemic”, Charities Warn.https://www.amnesty.org/en/latest/news/2021/10/g20s-bitter-divide-on-global-vaccine-inequality-could-condemn-world-to-an-endless-pandemic-charities-warn/ News Release 30 October. [Google Scholar]
- 41.United Nations Development Program. Global Dashboard for Vaccine Equity, https://data.undp.org/vaccine-equity/.
- 42.Our World in Data Coronavirus (COVID-19) Vaccinations, https://ourworldindata.org/covid-vaccinations.
- 43.United Nations . UN News; 2021. WHO chief warns against ‘catastrophic moral failure’ in COVID-19 vaccine access.https://news.un.org/en/story/2021/01/1082362 [Google Scholar]
- 44.Mazzucato M., Li S.L., Torreele E. Project Syndicate; 2020. Designing vaccines for people, not profits.https://www.project-syndicate.org/commentary/covid-vaccines-for-profit-not-for-people-by-mariana-mazzucato-et-al-2020-12 [Google Scholar]
- 45.Elliott L. 2021. The Omicron Variant Reveals the True Global Danger of ‘vaccine Apartheid’.https://www.theguardian.com/business/2021/nov/28/the-omicron-variant-reveals-the-true-global-danger-of-vaccine-apartheid The Guardian 28 November. [Google Scholar]
- 46.World Health Organization . 2020. Solidarity Call to Action. Making the Response to COVID-19 a Public Common Good.https://www.who.int/initiatives/covid-19-technology-access-pool/solidarity-call-to-action [Google Scholar]
- 47.Médecins Sans Frontières . 2021. Countries Obstructing COVID-19 Patent Waiver Must Allow Negotiations to Start.https://www.msf.org/countries-obstructing-covid-19-patent-waiver-must-allow-negotiations Press release 9 March. [Google Scholar]
- 48.Torreele E. Collective intelligence, not market competition, will deliver the best COVID-19 vaccine. 2020. https://www.statnews.com/2020/06/10/collective-intelligence-not-market-competition-deliver-best-covid-19-vaccine/ STAT First Opinion, June.
- 49.Torreele E. The rush to create a COVID-19 vaccine can do more harm than good. BMJ. 2020;370:m3209. doi: 10.1136/bmj.m3209. [DOI] [PubMed] [Google Scholar]
- 50.Ramchandani R., Kazatchkine M., Liu J., Sudan P., Dybul M., Matsoso P., Nördstrom A., Phelan A., Legido-Quigley A., Singh S., Mabuchi S. Vaccines, therapeutics, and diagnostics for covid-19: redesigning systems to improve pandemic response. BMJ. 2021;375:e067488. doi: 10.1136/bmj-2021-067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swaminathan S., Pécoul B., Abdullah H., Christou C., Gray G., IJsselmuiden C., et al. Re-orient biomedical R&D towards the global public interest. Nature. 2022;602:207–210. doi: 10.1038/d41586-022-00324-y. [DOI] [PubMed] [Google Scholar]
- 52.The WHO Council on the Economics of Health for All . 2021. Governing Health Innovation for the Common Good.https://www.who.int/publications/m/item/governing-health-innovation-for-the-common-good Council Brief No. 1. [Google Scholar]
- 53.Haseltine W. 2020. Moderna’s Claim of Favorable Results in its Vaccine Trial Is an Example of ‘publication by Press Release’.https://www.washingtonpost.com/opinions/2020/05/19/rush-share-good-news-covid-19-drugs-is-undermining-science/ Washington Post Opinion May 19. [Google Scholar]
- 54.Tanveer S., Rowhani-Farid A., Hong K., Jefferson T., Doshi P. Transparency of COVID-19 vaccine trials: decisions without data. BMJ Evid. Based Med. 2021 doi: 10.1136/bmjebm-2021-111735. [DOI] [PubMed] [Google Scholar]
- 55.Goldacre B. What the Tamiflu Saga tells us about drug trials and big pharma. The Guardian. 2014 https://www.theguardian.com/business/2014/apr/10/tamiflu-saga-drug-trials-big-pharma [Google Scholar]
- 56.Zaninelli M., Paarlberg R. Clinical trial disclosure and data transparency: obligation or opportunity? Applied Clinical Trials. 2021 https://www.appliedclinicaltrialsonline.com/view/clinical-trial-disclosure-and-data-transparency-obligation-or-opportunity [Google Scholar]
- 57.Mitra-Majumdar M., Kesselheim A.S. Reporting bias in clinical trials: progress toward transparency and next steps. PLoS Med. 2022;19:e1003894. doi: 10.1371/journal.pmed.1003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Águas R., Mahdi A., Shretta R., Horby P., Landray M., White L., CoMo Consortium Potential health and economic impacts of dexamethasone treatment for patients with COVID-19. Nat. Commun. 2021;12:915. doi: 10.1038/s41467-021-21134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., et al. Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.RECOVERY Collaborative group Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.06.15.21258542. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.RECOVERY Collaborative Group. Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., et al. Effect of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.RECOVERY Collaborative Group Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.RECOVERY Collaborative Group Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.RECOVERY Collaborative Group Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.06.08.21258132. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.RECOVERY Collaborative Group Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:605–612. doi: 10.1016/S0140-6736(21)00149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.RECOVERY Collaborative Group Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.05.18.21257267. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Transparency International Lack of transparency over vaccine trials, secretive contracts and “science by press release” risk success of global Covid-19 response. 2021. https://www.transparency.org/en/press/covid-19-vaccines-lack-of-transparency-trials-secretive-contracts-science-by-press-release-risk-success-of-global-response Press release 25 May.
- 69.Krauskopf L., Maddipatla M. Merck COVID-19 pill success slams moderna shares, shakes up healthcare sector. 2021. https://www.reuters.com/business/healthcare-pharmaceuticals/merck-covid-19-pill-success-slams-moderna-shares-shakes-up-healthcare-sector-2021-10-01/
- 70.Bendicksen L., Sharfstein J.M., Kesselheim A.S. Increase transparency at the FDA: we need sunlight to fight the pandemic. STAT. First Opin. 2020;29 https://www.statnews.com/2020/09/29/increase-transparency-at-the-fda-we-need-sunlight-to-fight-the-pandemic/ [Google Scholar]