Abstract
Severe coronavirus disease 2019 (COVID-19) is characterized by an increased risk of thromboembolic events, a leading cause for adverse outcomes in patients afflicted by the more serious manifestation of the disease. These thromboembolic complications expressed as sepsis-induced coagulopathy, disseminated intravascular coagulation, venous and arterial thromboembolism, pulmonary embolism, microthrombosis, and thrombotic microangiopathy have been observed to affect different organs such as the lungs, heart, kidneys, and brain. Endothelial injury and dysfunction have been identified as the critical pathway towards thrombogenesis, and contributions of other mechanisms such as hypercoagulability, cytokine storm, neutrophils have been studied. However, the contribution of hemodynamic pathways towards thrombosis in severe COVID-19 cases has not been investigated. From the classical theory of Virchow's triad to the contemporary studies on the effect of shear enhanced platelet activation, it is well established that hemodynamics plays a role in the initiation and growth of thrombosis. This article reviews recent studies on COVID-19 related thrombotic events and offers hypotheses on how hemodynamics may be responsible for some of the adverse outcomes observed in severe COVID-19 cases. While thrombogenesis through endothelial injury and the effects of hypercoagulability on thrombosis are briefly addressed, the crux of the discussion is focused on hemodynamic factors such as stasis, turbulent flow, and non-physiological shear stress and their effects on thrombosis. In addition, hemodynamics-dependent venous, arterial, and microvascular thrombosis in COVID-19 cases is discussed. We also propose further investigation of diagnostic and therapeutic options that address the hemodynamics aspects of COVID-19 thrombus formation to assess their potential in patient care.
Keywords: COVID-19, Thrombosis, Hemodynamics, Endothelial injury, Hypercoagulability, Shear stress
1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the ongoing global COVID-19 pandemic, enters and infects the human body through the lungs. SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE2) to facilitate entry into cells, and ACE2 is present widely on lung type II alveolar cells and endothelial cells throughout the body [1], [2]. Most patients experience mild or no symptoms; however, thromboembolic complications and respiratory failure are associated with severe disease manifestations [3]. Both arterial and venous thromboembolism is common in patients with severe COVID-19, and thrombosis has been observed in the lungs, heart, kidneys, and brain [4], [5]. Thrombosis in the vasculature arises from endothelial injury leading to excessive inflammation, platelet activation, and stasis [6]. These manifestations can be classified as an affliction of the endothelium, which serves as one unifying hypothesis in understanding COVID-19 [7], [8].
In patients with severe manifestations of COVID-19, a hypercoagulable state with widespread thrombosis and fibrinolysis has been observed, along with high levels of D-dimer, von Willebrand factor (vWF), and factor VIII [9], [10]. Other biological markers associated with thrombosis in COVID-19 cases include prothrombin time, platelet count, ADAMTS13, Interleukin 6 (IL-6), fibrinogen, and antiphospholipid antibodies [9], [11]. These markers have been monitored to help understand the severity of the associated thrombosis [11]. Increased coagulopathy appears unrelated to intrinsic viral activity but a combination of underlying comorbidities, hospitalization, and resultant thrombo-inflammation [11]. The hyperinflammatory state has been associated with elevated levels of markers such as interleukins, interferons, tumor necrosis factors, chemokines, and several other mediators – termed the “cytokine storm,” which have been linked to the severity of the disease [12]. While the cytokine storm is a concerning issue in the development of the severe COVID-19 disease, it is not the sole cause of thromboembolic events as indicated by relatively normal levels of IL-6 in some severe COVID-19 cases [13], and by the outcome of clinical trials involving therapies targeting IL-6 in COVID-19 patients [14]. Case studies show severe COVID-19 is also associated with a significant increase in neutrophils and neutrophil extracellular traps (NETs) embedded within the microthrombi. Even though elevated levels of neutrophils and NETs have been associated with a higher risk of thrombotic events, their overall role in COVID-19-associated thrombosis remains unclear [15], [16].
Understanding the thrombotic manifestations of COVID-19 is critical not only to grasp the underlying pathophysiology of the disease but also for improved patient treatment outcomes. We propose that SARS-CoV-2, upon triggering an endothelial response, simultaneously activates multiple parallel reactive pathways, many of which have a positive feedback loop on each other, disrupting hemostasis, ultimately leading to the runaway thrombotic events characteristic of severe COVID-19. While significant attention focused on endothelial injury and its associated biological pathways leading to thrombosis in severe COVID-19; comparatively little attention has been given to all the direct and indirect hemodynamic factors and their contributions [17], [18], [19]. In this article, we take a closer look at the contribution of hemodynamics to understand the underpinnings of the factors leading to a varied thrombosis response in severe COVID-19 cases.
2. Overview of the pathophysiology of thrombosis
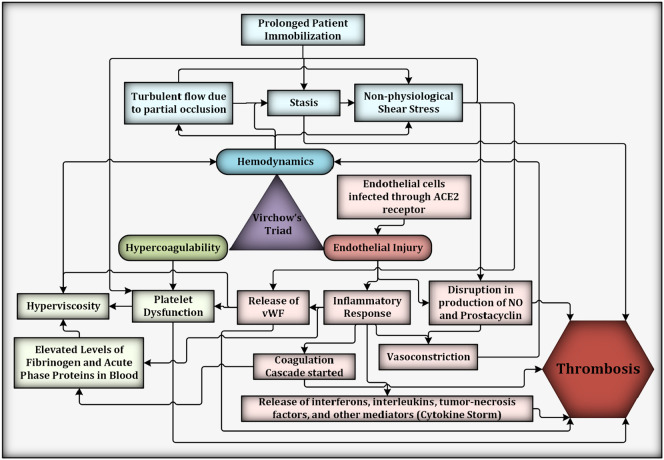
Initiation and growth of thrombus is a complex phenomenon depending on an evolving host of biological and mechanical factors that heavily influence its biochemical makeup and the thrombotic reaction pathways. The classic interpretation of Virchow's triad purports that the pathophysiologic mechanisms leading to thrombosis are dependent on the interplay between hypercoagulability, endothelial injury and dysfunction, and abnormal blood flow or non-physiological hemodynamics [20]. These interconnected pathways are complex, involve many multiscale interactions – from the molecular to macroscopic scales and have a significant effect on each other in tilting the balance of hemostasis beyond recovery (Fig. 1 ). Hemodynamics, or blood flow in the vasculature, is one of the factors that dictates the rate of thrombosis by delivering and disseminating reactive components. Flow-dependent factors such as increased shear conditions activate platelets and increase their propensity to bind to vWF. Alternatively, red blood cells and platelets can aggregate with each other or with endothelial cells under low shear conditions, causing blockages and increasing the local blood viscosity [21]. While other effects such as endothelial injury and inflammation, cytokine storm, NETs, platelet dysfunction, and disruption of prostacyclin and nitric oxide (NO) have a direct influence on thrombosis, these pathways also have an indirect effect on the hemodynamics which ultimately increases the propensity for thrombosis. Fig. 1 illustrates the complex interactions between the different factors. These direct and indirect parameters affecting hemodynamics related thrombosis are further described in the succeeding sections.
Fig. 1.
Virchow's triad and a few interconnected pathways contributing to thrombosis in severe COVID-19 cases. Per Virchow's triad, hypercoagulability, endothelial injury, and hemodynamics are the factors that contribute to thrombosis. While many biological pathways result in thrombosis, shown here are subfactors that either directly (e.g., prolonged patient immobilization, which leads to stasis induced thrombosis) or indirectly influence the hemodynamics (e.g., increased fibrinogen levels contribute to greater resistance to flow by increasing blood viscosity). The production of antithrombotic elements (e.g., NO and prostacyclin) initiated by endothelial injury is also disrupted through non-physiological shear stress. Some factors have a cascading effect, such as the release of vWF post endothelial injury leading platelet dysfunction, which could cause hyperviscosity leading to stasis and non-physiological shear stress.
2.1. Endothelial injury
Healthy vascular endothelium exposed to normal physiological shear stresses serves as a non-thrombogenic surface with properties that maintain hemostasis [21]. Vascular endothelium, while providing an essential interface between blood and tissue, furnishes a surface that, in physiological conditions, maintains blood in a liquid state under prolonged contact and controls vasomotion, inflammation, oxidative stress, vascular permeability, and structure [7]. SARS-CoV-2 enters host cells by binding to ACE2 receptors expressed on endothelial cells, found across multiple organs [22]. Infection of endothelial cells by SARS-CoV-2 leads to accumulation of inflammatory cells and eventually to endothelial and inflammatory cell death [22]. SARS-CoV-2 caused endotheliitis also leads to the release of large quantities of vWF from endothelial cells [23], [24]. vWF, a glycoprotein found in blood plasma, megakaryocytes, and subendothelial connective tissue, performs two essential functions in hemostasis; it mediates the adhesion of platelets to subendothelial connective tissue, and it binds blood clotting factor VIII [25], [26]. High vWF levels reflect endothelial damage or dysfunction, enhance the aggregation of platelets at the injury site, and over-activate the coagulation cascade to initiate thrombosis [27]. High vWF levels have also been a predictor in patients with ischemic heart disease, peripheral vascular disease, and inflammatory vascular disease [28], [29], [30]. Patients with such comorbidities are at high risk of contracting the severe form of COVID-19. The effect of endothelial injury on hemodynamics is multi-fold. Vasoconstriction and inflammation lead to reduction in vessel lumen size and cause increased resistance to flow and causes higher velocities through the blood vessel. In alveolar capillaries, vasoconstriction may have a significant impact, leading to massive capillary congestion, diffuse microthrombi, and organ damage (Fig. 2 ). In smaller arteries, there is a reduction of the vessel lumen due to blood clot formation and vasoconstriction, leading to higher velocity and higher shear rate. The effect of increased shear rate on thrombosis is described in subsequent sections. Endothelial injury can also lead to creation of recirculation/stagnation zones which alter normal hemodynamics and are associated with platelet deposition. In a study of 10 COVID-19 patients using functional respiratory imaging, impaired gas exchange in the lungs was observed. This phenomenon was attributed to redistribution of blood away from the small pulmonary vessels due to increased vascular resistance due to either vasoconstriction of distal pulmonary arteries or presence of microthrombi or both [31], [32].
Fig. 2.
Schematics of hemodynamics parameters in a blood vessel for possible thrombus formation in COVID-19 patients. A) SARS-CoV-2 binds to ACE2 receptors causing endothelial cell injury which initiates various thrombotic pathways to form a blood clot under different environments. Endothelial cell injury exposes tissue factor, vWF, collagen, and other thrombotic factors into the bloodstream which initiate both the intrinsic and extrinsic pathways of the coagulation cascade. Furthermore, the vessel lumen reduces due to injury-induced vasoconstriction. In small vessels and capillaries, such as alveolar capillaries, vasoconstriction may have a significant impact, leading to massive capillary congestion, diffuse microthrombi, and organ damage. B) In smaller arteries, there is a reduction of the vessel lumen due to blood clot formation and vasoconstriction, leading to higher velocity and higher shear rate. Increased shear rate induces morphological changes of vWF, activating platelets and increasing thrombus formation. Downstream the thrombus, recirculation/stagnation zones are likely to present themselves, and these areas are associated with platelets deposition. C) Increase in plasma viscosity is observed in COVID-19 patients, which in smaller veins and capillaries leads to lower velocities and lower shear rates. Non-physiological low values of shear influence platelets agglutination.
2.2. Hypercoagulability
In severe COVID-19, a hypercoagulable state arises due to systemic extrapulmonary hyper inflammation and hypercytokinemia. Specifically, cytokine storm has been linked to greater thrombin generation, platelet activation, and platelet aggregation which leads to disruption in hemostasis or altered hemodynamics [33]. Cytokines also promote NET formation, which triggers the extrinsic and intrinsic coagulation pathways, resulting in thrombin generation. Conversely, NETs promote the release of inflammatory cytokines [34]. In these cases, increases in cellular or plasma components, such as fibrinogen, vWF, cytokines, and immunoglobulin have a twofold effect on hemodynamics related thrombosis. It leads to increased plasma viscosity [10], [35] and an increased propensity for thrombosis due to higher levels of prothrombotic factors in the bloodstream. In smaller veins and capillaries, higher viscosity leads to lower velocities and lower shear rates. Non-physiological low values of shear influence platelets agglutination. Physiologically in smaller blood vessels, especially capillaries and microcapillaries, red blood cells drift towards the center of the vessel, while along the wall, the plasma layer is enriched with platelets [36]. This decrease in hematocrit level (Fåhræus effect) and decrease in apparent blood viscosity (Fåhræus-Lindqvist effect) as blood flows from a larger vessel to a smaller vessel is critical for blood flow in the microcirculation [37]. Hyperviscosity disrupts the Fåhræus-Lindqvist effect and could result in the formation of in-situ capillary thrombosis. Hyperviscosity in microcirculation can also impair the delivery of oxygen and nutrients to the tissues [38]. In a study of 15 severe COVID-19 patients, it is observed that all patients had high values for plasma viscosity ranging from 1.9–4.2 cP (normal range 1.4–1.8 cP) [39].
2.3. Hemodynamics
Post endothelial injury, multiple thrombotic pathways are activated. These pathways depend on hemodynamics and create positive feedback loops that perpetuate thrombosis formation (Fig. 1). Hemodynamic factors affecting thrombosis can be broadly classified into three interdependent categories: stasis, turbulent flow, and non-physiological shear stress. In the vasculature, shear stress – the frictional force generated by blood flow, is proportional to the velocity gradient perpendicular to the flow direction and viscosity of blood and is given by:
| (1) |
where τ is the shear stress, μ is viscosity, and γ is the velocity gradient or shear rate. Blood viscosity, i.e., its resistance to flow, depends on hematocrit, plasma viscosity as a function of its components, red cell deformation under high shear rates, and red cell aggregation under low shear rates [40]. Shear rate, and consequently shear stress, is maximum at the vessel wall and minimum at the center of the vessel.
2.3.1. Stasis
Blood stasis, commonly understood as a reduction in linear velocity of blood, arises from primarily three factors: physical activity or lack thereof, changes in intrinsic blood properties (viscosity), and obstruction of the flow path. Of these, the widely attributed hemodynamic factor leading to thrombosis in severe COVID-19 cases is blood stasis due to prolonged patient immobilization [41]. While instances of deep vein thrombosis (DVT) can be linked to extended periods of bed rest, this by itself does not explain the totality of hemodynamic contribution to other types of thrombosis seen in COVID-19 patients.
With hyperviscosity, the resistance to blood flow increases sharply, compromising flow through the microcirculatory system. Hyperviscosity slows the blood transit time through the microcirculation, with possible adhesion of red blood cells and ultra-large vWF multimers to the endothelium, which can initiate stasis [42].
Obstruction of the flow path may occur through a couple of different mechanisms. SARS-CoV-2 initiated endothelial injury leads to inflammation and may result in vasoconstriction. Vasoconstriction reduces the blood flow at the injury site and is more pronounced in small vessels and capillaries, such as alveolar capillaries; the site of diffuse alveolar damage and massive capillary congestion accompanied by microthrombi in severe COVID-19 cases [43]. Another mechanism of flow path obstruction is due to thrombus build-up in the vasculature (Fig. 2), causing flow stasis downstream of the thrombus. Flow path obstruction also increases resistance to blood flow.
2.3.2. Turbulent flow
Vessel injury and activation of blood constituents due to turbulence can contribute to thrombosis. Turbulence-induced thrombosis is primarily initiated due to shear effects on blood and vasculature. The blood flow path can be significantly altered due to endothelial injury-triggered vasoconstriction or due to the build-up of localized thrombus (Fig. 2). Turbulence can lead to the formation of local recirculation or stagnation regions, which have been associated with platelet deposition. In one study with several COVID-19 patients, emergency ultrasound found chaotic or turbulent flow in the deep venous system [44]. The ultrasound findings indicate the possibility of the vessel's partial occlusion, leading to turbulence and recirculation zones downstream of the blockage.
In severe COVID-19 patients, the probability that turbulent blood flow occurs in the microcirculation due to partial occlusion caused by microthrombi is low, given the small length scales in capillaries. In capillaries, it is more likely that any reduction in flow path leads to rapid occlusion due to the failure of red blood cells to pass through. Turbulence is far more likely in larger vessels where partial occlusions cause increases in blood velocities and shear rates (Fig. 2B).
2.3.3. Shear stress
Non-physiological low wall shear stress and oscillatory wall shear stress found in localized regions of stasis or turbulence promote endothelial proliferation, morphological variations, vasoconstriction, coagulation, and platelet agglutination [45], [46]. Adversely, non-physiological high wall shear stress damages the endothelium [47], causing exocytosis of vWF, which anchors to the vessel wall binding and retaining platelets [48]. Platelets then quickly activate, causing platelet aggregation, and the activated platelets can also promote activation of the clotting factors. The aggregates grow to a size that can either no longer be tethered to the endothelial cells by vWF against the force of blood flow and are released into the circulation or, if the blood flow force is insufficient to dislodge them, their growth leads to eventual blockage of the vessel [42]. Physiological wall shear stress influences vascular endothelial cells through activation of cell signaling cascades. This subsequently results in the acute release of vasodilators, key regulators of homeostasis, and antithrombotic factors, such as nitric oxide and prostacyclin [49], [50]. Nitric oxide is also an important inhibitor of platelet aggregation and leukocyte migration. Endothelial dysfunction suppresses endothelial nitric oxide synthase with an accompanying decrease in nitric oxide production in severe COVID-19 patients [51]. Deficiency in nitric oxide is thought to contribute to hypertension and thrombus formation [52].
In addition to shear stress acting on the endothelium, it also acts on blood and its constituents. Non-physiological shear stress levels induce vWF structural transition from a globular state to an extended chain conformation, initiating platelet adhesion [53], [54]. Plasma vWF adheres to the exposed collagen ensnaring circulating platelets. The captured platelets are exposed to longer durations of high shear rates, causing activation, promoting further release of vWF, and increased platelet capture. As the thrombus grows, it increasingly occludes the vessel and further increases shear rate and shear stress [55]. It is well established that higher shear stresses lead to greater platelet aggregation under the presence of vWF [56], [57]. In patients with COVID-19, drastically increased levels of vWF have been observed [58], [59], leading to the hypothesis that vWF along with high shear stress are significant contributors to disease pathogenesis and clinical prothrombotic manifestations of COVID-19-associated coagulopathy.
2.4. Venous and microvascular thrombosis
Severe COVID-19 is associated with venous thromboembolic events (VTE), including pulmonary embolism (PE), DVT, and cerebral venous sinus thrombosis (cVST), but the actual prevalence and characteristics of VTE are still unclear. There is significant variability in the occurrence rate of VTE reported in different studies (from 0.7 to 57%) due to differences in disease severity and frequency of diagnostic imaging studies performed [60], [61], [62], [63]. The rate of VTE in COVID-19 patients is high besides anticoagulant prophylaxis, much higher than the occurrence of VTE in non-COVID patients receiving thromboprophylaxis [62]. DVT is not present in all patients showing PE, leading to the hypothesis that the high rate of PE in severe COVID-19 patients is due to in situ thrombosis. Autopsy studies show that thrombotic lesions were more prevalent in the peripheral pulmonary arteries [63], and nine times more alveolar-capillary microthrombi were found in COVID-19 than influenza patients [64], It is increasingly reported that COVID-19 may be a risk factor for cVST – an uncommon form of stroke that happens when a blood clot forms in the brain's venous sinuses. cVST is reported in COVID-19 patients who fall outside the typical demographic of cVST patients and show signs of a generalized vaso-occlusive crisis. cVST is generally associated with both infection and pro-coagulopathy state [65]. It seems that patients without severe respiratory or systemic symptoms may still suffer from comorbidities associated with viral hyperviscosity and capillary occlusion [66], Microvascular thrombosis is also observed in autopsy results of microvessels of organs such as lung, heart, kidney, and liver leading to multi-organ failure [4].
We propose that the flow-related mechanisms involved in VTE are different at different sites of the venous vasculature. Doppler sonographic studies have shown that quantitatively slow venous flow in the lower extremities is associated with a mildly increased rate of subsequent DVT development [67]. We hypothesize that in larger peripheral veins, hyperviscosity induced by SARS-CoV-2 leads to lower flow velocity-induced stasis and abnormal lower values of viscous shear stress, leading to coagulation with the subsequent formation of DVT.
A different mechanism leads to thrombosis and occlusion in the pulmonary capillaries, the cerebral venous sinus, and microvascular thrombosis observed in the vasculature of multiple organs. COVID-19 patients with acute respiratory distress syndrome exhibit modified microcirculation characterized by an increased number of small vessels with slower or stopped blood flow [68]. Here, endothelial injury activates the immune response and coagulation cascade leading to increased blood viscosity due to increased cellular or plasma components, such as fibrinogen, vWF, cytokines, and immunoglobulin. Increased viscosity disrupts the Fåhræus-Lindqvist effect, which is crucial in maintaining perfusion through the microvessels. Impeded flow throughout the microvasculature leads to greater interaction between the released prothrombotic factors resulting in in-situ thrombosis in the capillaries/microvessels.
2.5. Arterial thrombosis
Pathogenesis of arterial thrombosis (AT) usually involves completely occlusive build-up or rupture of an atherosclerotic plaque in areas of disturbed flow. They can be fatal due to sudden obstruction of microcirculation, leading to stroke or cardiac death. However, AT is associated in severe COVID-19 cases even in the absence of atherosclerosis or plaque rapture [69], [70], [71]; COVID-19 instigated excessive endothelial activation has been linked to AT in regions such as the aorta, renal, and peripheral arteries [72]. Literature review suggests AT occurs in about 2.2% to 8.8% of seriously affected COVID-19 patients [35], with thrombosis observed in the superior mesenteric artery (8%), coronary arteries (5% to 9%), great vessels (aorta, common iliac, common carotids, and brachiocephalic artery; 14% to 19%), cerebral arteries (18% to 24%) and limb arteries (24% to 39%) [35], [73]. Despite the relatively low incidence of AT in COVID-19 patients, the mortality rate in these patients is as high as 20%, mainly due to end-organ injury [35].
We hypothesize that the hemodynamic factor associated with AT in COVID-19 patients is the non-physiological values of shear stress. In the arteries, including the aorta, supra-aortic trunks, coronary, and cerebral arteries, hyperviscosity induced by COVID-19 leads to non-physiological high wall shear stresses (Eq. (1)), disrupting the endothelial homeostasis, leading to greater platelet activation and subsequent aggregation to vWF. In addition, localized currents produced by the impact of built-up coagulation components can make the flow turbulent (Fig. 2B). This altered hemodynamics can speedup thrombosis, collectively with a hypercoagulable blood state and other factors already present due to COVID-19, such as high inflammatory response, increased blood viscosity, and large presence of vWF due to endothelial damage [35], [74], [75].
2.6. Diagnosis and treatment options
Diagnosis of abnormal levels of hemodynamic factors in COVID-19 can be difficult. Specifically, measurement of high shear areas or flow disruptions can be challenging as the thrombosis is disseminated throughout the vasculature. Imaging techniques such as compression ultrasound, echocardiography, Computed Tomography (CT), and Magnetic Resonance Imaging are used if manifestation or suspicions of thrombus are present [76], [77] and can provide information on localized hemodynamic disruptions. Studies that included pulmonary CT angiography for all the patients showed a higher rate of PE presence [63] indicating that the actual incidence of PE is underestimated. Indirectly, measurement of parameters such as blood viscosity and vWF have been used to understand whether there is an impact on hemodynamics [24], [78], [79]. It has also been shown that low brachial artery flow-mediated dilation, a marker of endothelial dysfunction, predicts an unfavorable in-hospital prognosis in COVID-19 patients [80], [81].
Directly targeting hemodynamics pathways that influence COVID-19 thrombosis is extremely challenging, but some efforts have been made in this direction for potential treatment. Due to the varied thrombotic events caused by COVID-19, anticoagulation and antiplatelet drugs have been widely explored to treat COVID-19 patients for maintaining blood viscosity within safer levels. Different pharmacological approaches that dysregulate the coagulation cascade at different stages have been investigated, such as heparin, aspirin, clopidogrel, and tissue plasminogen activator [15]. Thromboembolic complications widespread in COVID-19 patients are often present despite anticoagulant and antiplatelet therapies, and the appropriate dosage of these therapies during the different stages of the disease is still under study [9]. COVID-19 triggers multiple pathways that lead to thrombus formation in different parts of the vasculature. For this reason, other therapeutic options have been proposed in conjunction with anticoagulation, such as targeting vWF/ADAMTS13 homeostasis by using recombinant ADAMTS13 [82]. Blood hyperviscosity is detrimental for thrombus formation in the microcirculation. Therapeutic plasma exchange (TPE) is a promising treatment tested to combat this mechanism and decrease blood viscosity; large trials are needed to establish whether TPE is a viable treatment option for COVID-19 patients [78]. As discussed previously, physiological values of wall shear stress are fundamental to activate NO production by endothelial cells. NO has been previously shown to inhibit SARS-CoV-1 replication [83], [84], and may contribute to vasodilation and mitigate thrombogenesis [51]. Case studies suggest possible benefits in COVID-19, and phase 2 clinical trials with inhaled NO in COVID-19 are ongoing [1]. We believe that further exploration and research of alternative therapies that target hemodynamics related to thrombus formation in COVID-19 patients is needed.
3. Conclusion
Complications arising from severe COVID-19, particularly thromboembolic incidents, lead to fatal consequences in patients. There is a significant interplay between endothelial injury, hypercoagulability, and hemodynamic pathways leading to thromboembolic events. Based on the available literature, we hypothesize how different hemodynamic parameters could compound the observed complications in severe COVID-19 patients through various mechanisms such as hypercoagulability state, stasis, turbulent flow, and shear mediated platelet activation and aggregation. While some diagnostic and treatment options are available, further research is necessary to understand the underlying pathophysiology of COVID-19 and its ramifications in disease progression, including its impact on systemic hemodynamics.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kim B., Arany Z. Could shear stress mimetics delay complications in COVID-19? Trends Cardiovasc. Med. 2021 doi: 10.1016/j.tcm.2021.01.004. Epub 2021/01/31. PubMed PMID: 33515686; PubMed Central PMCID: PMC838584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein R.A., Young L.M. From ACE2 to COVID-19: a multiorgan endothelial disease. Int. J. Infect. Dis. 2020;100:425–430. doi: 10.1016/j.ijid.2020.08.083. Epub 2020/09/09. PubMed PMID: 32896660; PubMed Central PMCID: PMC7832810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atri D., Siddiqi H.K., Lang J., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl. Sci. 2020;5(5):518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinayagam S., Sattu K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118431. Epub 2020/09/19. PubMed PMID: 32946915; PubMed Central PMCID: PMC7490584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F., Kruip M., Van der Meer N., Arbous M., Gommers D., Kant K., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mui L.W., Lau J.F., Lee H.K. Thromboembolic complications of COVID-19. Emerg. Radiol. 2020 doi: 10.1007/s10140-020-01868-0. Epub 2020/11/08. PubMed PMID: 33159219; PubMed Central PMCID: PMC7647885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. Epub 2020/09/04. PubMed PMID: 32882706; PubMed Central PMCID: PMC7470753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panfoli I. Potential role of endothelial cell surface ectopic redox complexes in COVID-19 disease pathogenesis. Clin. Med. (Lond.) 2020;20(5) doi: 10.7861/clinmed.2020-0252. e146-e7. Epub 2020/07/01. PubMed PMID: 32601125; PubMed Central PMCID: PMC7539732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Ani F., Chehade S., Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb. Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. Epub 2020/06/03. PubMed PMID: 32485418; PubMed Central PMCID: PMC7255332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. Epub 2020/04/18. PubMed PMID: 32302448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. Epub 2020/04/28. PubMed PMID: 32339221; PubMed Central PMCID: PMC7273827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowenstein C.J., Solomon S.D. Severe COVID-19 is a microvascular disease. Circulation. 2020;142(17):1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinha P., Matthay M.A., Calfee C.S. Is a “cytokine storm” relevant to COVID-19? JAMA Intern. Med. 2020;180(9):1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 14.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonaventura A., Vecchié A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuo Y., Zuo M., Yalavarthi S., Gockman K., Madison J.A., Shi H. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis. 2021;51(2):446–453. doi: 10.1007/s11239-020-02324-z. Epub 2020/11/06. PubMed PMID: 33151461; PubMed Central PMCID: PMC7642240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed S., Zimba O., Gasparyan A.Y. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clin. Rheumatol. 2020;39(9):2529–2543. doi: 10.1007/s10067-020-05275-1. Epub 2020/07/13. PubMed PMID: 32654082; PubMed Central PMCID: PMC7353835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favaloro E.J., Lippi G. Thieme Medical Publishers, Inc; 2021. Maintaining Hemostasis and Preventing Thrombosis in Coronavirus Disease 2019 (COVID-19): Part II. Seminars in Thrombosis and Hemostasis. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Gonzalez F.J., Ziccardi M.R., McCauley M.D. Virchow’s triad and the role of thrombosis in COVID-related stroke. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.769254. Epub 2021/01/31. PubMed PMID: 33515686; PubMed Central PMCID: PMC7838584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung I., Lip G.Y. Virchow’s triad revisited: blood constituents. Pathophysiol. Haemost. Thromb. 2003;33(5–6):449–454. doi: 10.1159/000083844. [DOI] [PubMed] [Google Scholar]
- 21.Hathcock J.J. Flow effects on coagulation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2006;26(8):1729–1737. doi: 10.1161/01.ATV.0000229658.76797.30. Epub 2006/06/03. PubMed PMID: 16741150. [DOI] [PubMed] [Google Scholar]
- 22.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. Epub 2020/04/24. PubMed PMID: 32325026; PubMed Central PMCID: PMC7172722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward S.E., Curley G.F., Lavin M., Fogarty H., Karampini E., McEvoy N.L. Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID-19): evidence of acute and sustained endothelial cell activation. Br. J. Haematol. 2021;192(4):714–719. doi: 10.1111/bjh.17273. Epub 2020/12/17. PubMed PMID: 33326604. [DOI] [PubMed] [Google Scholar]
- 25.Sadler J.E. Biochemistry and genetics of von willebrand factor. Annu. Rev. Biochem. 1998;67(1):395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z., Mondal N.K., Ding J., Koenig S.C., Slaughter M.S., Wu Z.J. Paradoxical effect of nonphysiological shear stress on platelets and von Willebrand factor. Artif. Organs. 2016;40(7):659–668. doi: 10.1111/aor.12606. Epub 2015/11/20. PubMed PMID: 26582038; PubMed Central PMCID: PMC4871771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas S., Thakur V., Kaur P., Khan A., Kulshrestha S., Kumar P. Blood clots in COVID-19 patients: simplifying the curious mystery. Med. Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110371. Epub 2020/11/24. PubMed PMID: 33223324; PubMed Central PMCID: PMC7644431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lip G.Y., Blann A. von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc. Res. 1997;34(2):255–265. doi: 10.1016/s0008-6363(97)00039-4. Epub 2020/11/24. PubMed PMID: 33223324; PubMed Central PMCID: PMC7644431. [DOI] [PubMed] [Google Scholar]
- 29.Mannucci P.M. von Willebrand factor: a marker of endothelial damage? Arterioscler. Thromb. Vasc. Biol. 1998;18(9):1359–1362. doi: 10.1161/01.atv.18.9.1359. Epub 1998/09/22. PubMed PMID: 9743222. [DOI] [PubMed] [Google Scholar]
- 30.Makin A.J., Blann A.D., Chung N.A., Silverman S.H., Lip G.Y. Assessment of endothelial damage in atherosclerotic vascular disease by quantification of circulating endothelial cells. Relationship with von Willebrand factor and tissue factor. Eur. Heart J. 2004;25(5):371–376. doi: 10.1016/j.ehj.2003.04.001. Epub 2004/03/23. PubMed PMID: 15033248. [DOI] [PubMed] [Google Scholar]
- 31.Thillai M., Patvardhan C., Swietlik E.M., McLellan T., De Backer J., Lanclus M., et al. Functional respiratory imaging identifies redistribution of pulmonary blood flow in patients with COVID-19. Thorax. 2021;76(2):182–184. doi: 10.1136/thoraxjnl-2020-215395. [DOI] [PubMed] [Google Scholar]
- 32.Poor H.D. Pulmonary thrombosis and thromboembolism in COVID-19. Chest. 2021;160(4):1471–1480. doi: 10.1016/j.chest.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulanowska M., Olas B. Modulation of hemostasis in COVID-19; blood platelets may be important pieces in the COVID-19 puzzle. Pathogens. 2021;10(3):370. doi: 10.3390/pathogens10030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S., Zhang J., Wang C., Chen X., Zhao X., Jing H., et al. COVID-19 and ischemic stroke: mechanisms of hypercoagulability. Int. J. Mol. Med. 2021;47(3) doi: 10.3892/ijmm.2021.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheruiyot I., Kipkorir V., Ngure B., Misiani M., Munguti J., Ogeng’o J. Arterial thrombosis in coronavirus disease 2019 patients: a rapid systematic review. Ann. Vasc. Surg. 2021;70:273–281. doi: 10.1016/j.avsg.2020.08.087. Epub 2020/09/01. PubMed PMID: 32866574; PubMed Central PMCID: PMC7453204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flamm M.H., Diamond S.L. Multiscale systems biology and physics of thrombosis under flow. Ann. Biomed. Eng. 2012;40(11):2355–2364. doi: 10.1007/s10439-012-0557-9. Epub 2012/03/31. PubMed PMID: 22460075; PubMed Central PMCID: PMC3422600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chebbi R. Dynamics of blood flow: modeling of the Fahraeus-Lindqvist effect. J. Biol. Phys. 2015;41(3):313–326. doi: 10.1007/s10867-015-9376-1. Epub 2015/02/24. PubMed PMID: 25702195; PubMed Central PMCID: PMC4456490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farber P.L. Can erythrocytes behavior in microcirculation help the understanding the physiopathology and improve prevention and treatment for covid-19? Clin. Hemorheol. Microcirc. 2021 doi: 10.3233/CH-201082. PubMed PMID: 33523046. [DOI] [PubMed] [Google Scholar]
- 39.Maier C.L., Truong A.D., Auld S.C., Polly D.M., Tanksley C.L., Duncan A. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395(10239):1758–1759. doi: 10.1016/S0140-6736(20)31209-5. Epub 2020/05/29. PubMed PMID: 32464112; PubMed Central PMCID: PMC7247793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowe G., Lee A., Rumley A., Price J., Fowkes F. Blood viscosity and risk of cardiovascular events: the Edinburgh artery study. Br. J. Haematol. 1997;96(1):168–173. doi: 10.1046/j.1365-2141.1997.8532481.x. [DOI] [PubMed] [Google Scholar]
- 41.Mehta J.L., Calcaterra G., Bassareo P.P. COVID-19, thromboembolic risk, and Virchow's triad: lesson from the past. Clin. Cardiol. 2020;43(12):1362–1367. doi: 10.1002/clc.23460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varatharajah N., Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand Factor (eULVWF) decorated-platelet strings. Fed. Pract. 2020;37(6):258–259. Epub 2020/07/17 PubMed PMID: 32669777; PubMed Central PMCID: PMC7357889. [PMC free article] [PubMed] [Google Scholar]
- 43.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. Epub 2020/05/05. PubMed PMID: 32364264; PubMed Central PMCID: PMC7496150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson M., Shi D., Gordon M., Chavda Y., Grimaldi C., Bajaj T. Where there’s smoke, there’s fire: a case report of turbulent blood flow in lower extremity point-of-care ultrasound in COVID-19. Clin. Pract. Cases Emerg. Med. 2021;5(1):30–34. doi: 10.5811/cpcem.2020.10.48809. Epub 2021/02/10. PubMed PMID: 33560947; PubMed Central PMCID: PMC7872592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paszkowiak J.J., Dardik A. Arterial wall shear stress: observations from the bench to the bedside. Vasc. Endovasc. Surg. 2003;37(1):47–57. doi: 10.1177/153857440303700107. [DOI] [PubMed] [Google Scholar]
- 46.Papaioannou T.G., Stefanadis C. Vascular wall shear stress: basic principles and methods. Hell. J. Cardiol. 2005;46(1):9–15. [PubMed] [Google Scholar]
- 47.Blann A.D. Is raised von Willebrand factor a marker of endothelial cell damage? Med. Hypotheses. 1993;41(5):419–424. doi: 10.1016/0306-9877(93)90118-a. Epub 1993/11/01. PubMed PMID: 8145653. [DOI] [PubMed] [Google Scholar]
- 48.Bernardo A., Ball C., Nolasco L., Choi H., Moake J.L., Dong J.F. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J. Thromb. Haemost. 2005;3(3):562–570. doi: 10.1111/j.1538-7836.2005.01122.x. Epub 2005/03/08. PubMed PMID: 15748247. [DOI] [PubMed] [Google Scholar]
- 49.Ballermann B.J., Dardik A., Eng E., Liu A. Shear stress and the endothelium. Kidney Int. Suppl. 1998;67 doi: 10.1046/j.1523-1755.1998.06720.x. Epub 2020/12/17. PubMed PMID: 33326604. [DOI] [PubMed] [Google Scholar]
- 50.Wang W., Diamond S.L. Does elevated nitric oxide production enhance the release of prostacyclin from shear stressed aortic endothelial cells? Biochem. Biophys. Res. Commun. 1997;233(3):748–751. doi: 10.1006/bbrc.1997.6548. [DOI] [PubMed] [Google Scholar]
- 51.Green S.J. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect. 2020;22(4):149–150. doi: 10.1016/j.micinf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tousoulis D., Kampoli A.-M., Tentolouris Nikolaos Papageorgiou C., Stefanadis C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012;10(1):4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 53.Siedlecki C.A., Lestini B.J., Kottke-Marchant K.K., Eppell S.J., Wilson D.L., Marchant R.E. Shear-dependent changes in the three-dimensional structure of human von willebrand factor. Blood. 1996;88(8):2939–2950. Epub 1996/10/15 PubMed PMID: 8874190. [PubMed] [Google Scholar]
- 54.Galbusera M., Zoja C., Donadelli R., Paris S., Morigi M., Benigni A., et al. Fluid shear stress modulates von willebrand factor release from human vascular endothelium. Blood. 1997;90(4):1558–1564. Epub 1997/08/15 PubMed PMID: 9269774. [PubMed] [Google Scholar]
- 55.Casa L.D., Deaton D.H., Ku D.N. Role of high shear rate in thrombosis. J. Vasc. Surg. 2015;61(4):1068–1080. doi: 10.1016/j.jvs.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 56.Moake J.L., Turner N.A., Stathopoulos N.A., Nolasco L.H., Hellums J.D. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J. Clin. Invest. 1986;78(6):1456–1461. doi: 10.1172/JCI112736. Epub 1986/12/01 PubMed PMID: 3491092; PubMed Central PMCID: PMC423893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikeda Y., Handa M., Kawano K., Kamata T., Murata M., Araki Y. The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress. J. Clin. Invest. 1991;87(4):1234–1240. doi: 10.1172/JCI115124. PubMed PMID: 2010539; PubMed Central PMCID: PMC295144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goshua G., Pine A.B., Meizlish M.L., Chang C.-H., Zhang H., Bahel P., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ladikou E.E., Sivaloganathan H., Milne K.M., Arter W.E., Ramasamy R., Saad R. Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin. Med. (Lond.) 2020;20(5) doi: 10.7861/clinmed.2020-0346. e178-e82. Epub 2020/07/23. PubMed PMID: 32694169; PubMed Central PMCID: PMC7539718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. Epub 2020/04/10 PubMed PMID: 32271988; PubMed Central PMCID: PMC7262324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klok F.A., Kruip M., Arbous M.S., Gommers D., Kant K.M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. Epub 2020/04/16 PubMed PMID: 32291094; PubMed Central PMCID: PMC7146714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. Epub 2021/01/31 PubMed PMID: 33515686; PubMed Central PMCID: PMC7838584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suh Y.J., Hong H., Ohana M., Bompard F., Revel M.P., Valle C. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298(2) doi: 10.1148/radiol.2020203557. Epub 2020/04/18. PubMed PMID: 32302448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. Epub 2020/04/18. PubMed PMID: 32302448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cavalcanti D.D., Raz E., Shapiro M., Dehkharghani S., Yaghi S., Lillemoe K. Cerebral venous thrombosis associated with COVID-19. AJNR Am. J. Neuroradiol. 2020;41(8):1370–1376. doi: 10.3174/ajnr.A6644. Epub 2020/06/20. PubMed PMID: 32554424; PubMed Central PMCID: PMC7658892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dakay K., Cooper J., Bloomfield J., Overby P., Mayer S.A., Nuoman R. Cerebral venous sinus thrombosis in COVID-19 infection: a case series and review of the literature. J. Stroke Cerebrovasc. Dis. 2021;30(1) doi: 10.1016/j.jstrokecerebrovasdis.2020.105434. Epub 2020/11/16. PubMed PMID: 33190109; PubMed Central PMCID: PMC7833244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jensen C.T., Chahin A., Amin V.D., Khalaf A.M., Elsayes K.M., Wagner-Bartak N. Qualitative slow blood flow in lower extremity deep veins on doppler sonography: quantitative assessment and preliminary evaluation of correlation with subsequent deep venous thrombosis development in a tertiary care oncology center. J. Ultrasound Med. 2017;36(9):1867–1874. doi: 10.1002/jum.14220. Epub 2017/05/05. PubMed PMID: 28470976; PubMed Central PMCID: PMC5568938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanoore Edul V.S., Caminos Eguillor J.F., Ferrara G., Estenssoro E., Siles D.S.P., Cesio C.E. Microcirculation alterations in severe COVID-19 pneumonia. J. Crit. Care. 2021;61:73–75. doi: 10.1016/j.jcrc.2020.10.002. Epub 2020/09/01. PubMed PMID: 32866574; PubMed Central PMCID: PMC7453204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bussmann B.M., Shabbir A., Warwick A., Orr W. Acute coronary artery thrombosis presenting as asymptomatic ST-elevation myocardial infarction in a patient with COVID-19 pneumonia. BMJ Case Rep. 2021;14(2) doi: 10.1136/bcr-2021-241856. Epub 2015/02/24. PubMed PMID: 25702195; PubMed Central PMCID: PMC4456490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perini P., Nabulsi B., Massoni C.B., Azzarone M., Freyrie A. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395(10236):1546. doi: 10.1016/S0140-6736(20)31051-5. Epub 2020/05/20. PubMed PMID: 32423583; PubMed Central PMCID: PMC7200129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schweblin C., Hachulla A.L., Roffi M., Glauser F. Delayed manifestation of COVID-19 presenting as lower extremity multilevel arterial thrombosis: a case report. Eur. Heart J. Case Rep. 2020;4(6):1–4. doi: 10.1093/ehjcr/ytaa371. Epub 2021/01/14. PubMed PMID: 33437919; PubMed Central PMCID: PMC7717202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kenizou D., Perrin C., Harzallah I., Bresson D., Allimant P., Calcaianu M. Multiple arterial thrombosis in a 78-year-old patient: catastrophic thrombotic syndrome in COVID-19. CJC Open. 2021;3(2):198–200. doi: 10.1016/j.cjco.2020.09.020. Epub 2020/11/24. PubMed PMID: 33223324; PubMed Central PMCID: PMC7644431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Indes J.E., Koleilat I., Hatch A.N., Choinski K., Jones D.B., Aldailami H., et al. Early experience with arterial thromboembolic complications in patients with COVID-19. J. Vasc. Surg. 2021;73(2):381–389. doi: 10.1016/j.jvs.2020.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al Raizah A., Al Askar A., Shaheen N., Aldosari K., Alnahdi M., Luhanga M. High rate of bleeding and arterial thrombosis in COVID-19: Saudi multicenter study. Thromb. J. 2021;19(1):13. doi: 10.1186/s12959-021-00265-y. Epub 2021/03/05. PubMed PMID: 33658062; PubMed Central PMCID: PMC7928187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pellegrini D., Kawakami R., Guagliumi G., Sakamoto A., Kawai K., Gianatti A. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143(10):1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. Epub 2021/01/23. PubMed PMID: 33480806. [DOI] [PubMed] [Google Scholar]
- 76.Poggiali E., Bastoni D., Ioannilli E., Vercelli A., Magnacavallo A. Deep vein thrombosis and pulmonary embolism: two complications of COVID-19 pneumonia? Eur. J. Case Rep. Intern. Med. 2020;7(5) doi: 10.12890/2020_001646. Epub 2015/02/24. PubMed PMID: 25702195; PubMed Central PMCID: PMC4456490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ropper A.H., Klein J.P. Cerebral venous thrombosis. N. Engl. J. Med. 2021;385(1):59–64. doi: 10.1056/NEJMra2106545. Epub 2021/07/01. PubMed PMID: 34192432. [DOI] [PubMed] [Google Scholar]
- 78.Truong A.D., Auld S.C., Barker N.A., Friend S., Wynn A.T., Cobb J. Therapeutic plasma exchange for COVID-19-associated hyperviscosity. Transfusion. 2021;61(4):1029–1034. doi: 10.1111/trf.16218. Epub 2017/05/05. PubMed PMID: 28470976; PubMed Central PMCID: PMC5568938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez Rodriguez M., Castro Quismondo N., Zafra Torres D., Gil Alos D., Ayala R., Martinez-Lopez J. Increased von willebrand factor antigen and low ADAMTS13 activity are related to poor prognosis in covid-19 patients. Int. J. Lab. Hematol. 2021 doi: 10.1111/ijlh.13476. PubMed PMID: 33502080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ergül E., Yılmaz A.S., Öğütveren M.M., Emlek N., Kostakoğlu U., Çetin M. COVID 19 disease independently predicted endothelial dysfunction measured by flow-mediated dilatation. Int. J. Cardiovasc. Imaging. 2021;1–8 doi: 10.1007/s10554-021-02356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bianconi V., Mannarino M.R., Figorilli F., Schiaroli E., Cosentini E., Batori G., et al. Low brachial artery flow-mediated dilation predicts worse prognosis in hospitalized patients with COVID-19. J. Clin. Med. 2021;10(22):5456. doi: 10.3390/jcm10225456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turecek P.L., Peck R.C., Rangarajan S., Reilly-Stitt C., Laffan M.A., Kazmi R. Recombinant ADAMTS13 reduces abnormally up-regulated von Willebrand factor in plasma from patients with severe COVID-19. Thromb. Res. 2021;201:100–112. doi: 10.1016/j.thromres.2021.02.012. Epub 2021/03/05. PubMed PMID: 33662796; PubMed Central PMCID: PMC7890348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abdul-Cader M.S., Amarasinghe A., Abdul-Careem M.F. Activation of toll-like receptor signaling pathways leading to nitric oxide-mediated antiviral responses. Arch. Virol. 2016;161(8):2075–2086. doi: 10.1007/s00705-016-2904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Åkerström S., Mousavi-Jazi M., Klingström J., Leijon M., Ak Lundkvist, Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79(3):1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]