Abstract
Background
Building the European Antimicrobial Resistance Surveillance network in Veterinary medicine (EARS-Vet) was proposed to strengthen the European One Health antimicrobial resistance (AMR) surveillance approach.
Objectives
To define the combinations of animal species/production types/age categories/bacterial species/specimens/antimicrobials to be monitored in EARS-Vet.
Methods
The EARS-Vet scope was defined by consensus between 26 European experts. Decisions were guided by a survey of the combinations that are relevant and feasible to monitor in diseased animals in 13 European countries (bottom-up approach). Experts also considered the One Health approach and the need for EARS-Vet to complement existing European AMR monitoring systems coordinated by the ECDC and the European Food Safety Authority (EFSA).
Results
EARS-Vet plans to monitor AMR in six animal species [cattle, swine, chickens (broilers and laying hens), turkeys, cats and dogs], for 11 bacterial species (Escherichia coli, Klebsiella pneumoniae, Mannheimia haemolytica, Pasteurella multocida, Actinobacillus pleuropneumoniae, Staphylococcus aureus, Staphylococcus pseudintermedius, Staphylococcus hyicus, Streptococcus uberis, Streptococcus dysgalactiae and Streptococcus suis). Relevant antimicrobials for their treatment were selected (e.g. tetracyclines) and complemented with antimicrobials of more specific public health interest (e.g. carbapenems). Molecular data detecting the presence of ESBLs, AmpC cephalosporinases and methicillin resistance shall be collected too.
Conclusions
A preliminary EARS-Vet scope was defined, with the potential to fill important AMR monitoring gaps in the animal sector in Europe. It should be reviewed and expanded as the epidemiology of AMR changes, more countries participate and national monitoring capacities improve.
Introduction
Antimicrobial resistance (AMR) cannot be tackled effectively without well-performing monitoring systems, covering the animal and human sectors.1,2 Currently, the ECDC coordinates the European Antimicrobial Resistance Surveillance Network (EARS-Net), which monitors AMR in invasive bacterial isolates from humans with bloodstream infections or meningitis,3 and the European Food- and Waterborne Diseases and Zoonoses Network (FWD-Net), which monitors AMR in human Salmonella and Campylobacter infections.4 In the food sector, the European Food Safety Authority (EFSA) coordinates active monitoring of AMR in indicator commensal bacteria (Escherichia coli primarily, but countries may also report for Enterococcus faecalis and Enterococcus faecium) and zoonotic bacteria (Salmonella spp., Campylobacter coli and Campylobacter jejuni), according to Directive 2003/99/CE and Commission Implementing Decision (EU) 2020/1729.5 Caecal samples from healthy food-producing animals (cattle under 1 year, chickens, turkeys and swine) are taken at slaughterhouse, as well as samples of meat thereof at retail. As no European system currently monitors AMR in clinical isolates from diseased animals, data on AMR hotspots in specific animal infections are still lacking at a European level, although necessary to tailor strategies to rationalize antimicrobial usage in the animal sector.6 However, national monitoring systems for AMR in clinical isolates from diseased animals are in place in at least 12 European countries.7 In addition, veterinary AMR monitoring programmes exist outside of Europe.8
In this context, the EU Joint Action on Antimicrobial Resistance and Healthcare-Associated Infections (EU-JAMRAI), co-funded by the Third Health Programme of the EU, proposed to establish the European Antimicrobial Resistance Surveillance network in Veterinary medicine (EARS-Vet), to strengthen a One Health AMR surveillance approach in Europe.6 During EU-JAMRAI, it was agreed that EARS-Vet should work as a European network of national monitoring systems in diseased animals (similarly to EARS-Net) and aim to complement and integrate with the AMR monitoring systems of the ECDC and EFSA. Its objectives shall be to report on the AMR situation, follow AMR trends and detect emerging AMR in bacterial pathogens of animals in Europe. This information would contribute to: advising policy makers on interventions to mitigate AMR in the animal sector; monitoring the impact of European efforts to tackle AMR in the animal sector; evaluating or revising marketing authorizations of veterinary antimicrobials; supporting antimicrobial stewardship initiatives, especially the development of veterinary antimicrobial treatment guidelines; generating missing epidemiological cut-off values (ECOFFs) and then clinical breakpoints for the interpretation of antimicrobial susceptibility testing (AST) results in veterinary medicine; assessing the risk of transmission of resistant bacteria or resistance genes between animals and humans via non-food-related routes, e.g. by direct contact between humans and companion or food-producing animals; and estimating the burden of AMR in animal health, e.g. attributable animal deaths caused by infections with antimicrobial-resistant bacteria.
Following a review and analysis of national AMR monitoring systems in clinical animal isolates,7 EARS-Vet plans to follow EUCAST standards, collect quantitative data (MICs or inhibition zone diameters) and interpret AST results using ECOFFs. However, until harmonization on the AST method is reached among participating countries, AST data produced by different standards shall be accepted by EARS-Vet, similarly to what EARS-Net did for two decades before accepting only those produced according to EUCAST standards.6
A major step in the design of EARS-Vet was the definition of its monitoring scope, i.e. the combinations of animal species/production types/age categories/bacterial species/specimens (i.e. sample types)/antimicrobials to be monitored in EARS-Vet. The present study aims to describe these combinations and the bottom-up and One Health approach that was followed to determine them.
Methods
Bottom-up approach: collection and analysis of 13 national veterinary AMR monitoring scopes
To define an EARS-Vet scope composed of combinations that are both relevant and feasible to monitor in Europe, the national veterinary AMR monitoring scopes of 13 countries were collected and analysed. Such data collection was done by completing an Excel template recording the combinations of animal species/production types/age categories/bacterial species/specimens/antimicrobials monitored by 10 countries with a national monitoring system (the Czech Republic, Denmark, Estonia, Finland, France, Germany, Ireland, the Netherlands, Norway and Sweden) or considered as future monitoring targets in three countries that were in the process of establishing a monitoring system (Spain) or planning to build their system (Belgium and Greece) at the time of study (2019–20). The national scopes of Belgium, Greece and Spain were defined via the consultation of relevant national experts, who were advised to follow the recommendations of the World Organisation for Animal Health in this regard.9 These national veterinary AMR monitoring scopes, which may already reflect a One Health perspective, were analysed to identify the most frequently included animal species, production types, age categories, bacterial species, specimens and antimicrobials.
One Health approach: exploring the feasibility for countries to monitor AMR in the bacterial species monitored by EARS-Net
Due to possible One Health implications and to explore integration opportunities between EARS-Net and EARS-Vet, the 13 countries sharing their national veterinary AMR monitoring scope were also asked about the feasibility to monitor AMR in clinical animal isolates for seven of the eight bacterial species monitored by EARS-Net (E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, Staphylococcus aureus, E. faecalis and E. faecium), among the animal species already included in their national monitoring scope. Streptococcus pneumoniae, also monitored in EARS-Net, was not included in this survey as it is primarily a human pathogen. For each combination of animal species/bacterial species, countries had the choice between three answers: (i) the monitoring is feasible and the combination is already included in the national scope (‘feasible and included’); (ii) the monitoring is feasible, but the combination is not included in the national scope (‘feasible, but not included’); or (iii) the monitoring is ‘currently not feasible’. Justifications were required in the case of answering (ii) or (iii).
Expert consensus on the EARS-Vet scope
Guided by the information generated through the bottom-up and One Health approach, a group of 26 experts (veterinary and human microbiologists, veterinary epidemiologists and Ministry representatives) from 14 European countries (the 13 countries that shared their national scope and Italy) defined the EARS-Vet scope by consensus through six teleconferences in 2020. The decision process followed this stepwise approach: (i) determination of the animal species; (ii) determination of the bacterial species within each selected animal species; (iii) determination of accepted clinical specimens for each combination of animal species/bacterial species; (iv) determination of stratification per production type and/or age category for each combination of animal species/bacterial species/specimen; and (v) determination of the antimicrobials for each combination of animal species/bacterial species/specimen/production type/age category.
Results
National veterinary AMR monitoring scopes were collected from 13 countries, with 10 of these having a monitoring system in place and 3 others in the process of establishing or planning to build their systems. These scopes are available in Table S1 (available as Supplementary data at JAC Online).
Determination of the animal species
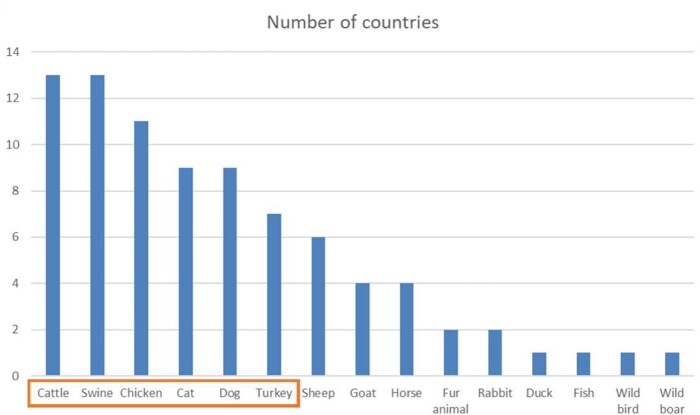
Figure 1 shows the animal species included in the 13 national scopes. All countries incorporated cattle and swine, with a majority also including chickens and turkeys among food-producing animals. Nine countries included companion animals (cats and dogs) and four included horses. The expert panel decided to monitor in EARS-Vet the six animal species most frequently included in national scopes: cattle, swine, chickens, cats, dogs and turkeys.
Figure 1.
Distribution of the animal species present in the national veterinary AMR monitoring scopes of 13 European countries (the orange box indicates the animal species selected for the EARS-Vet scope). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Determination of the bacterial species within each selected animal species
Bacterial species determination was performed in two steps, first by considering national scopes and then by exploring the feasibility to include more EARS-Net bacteria in the EARS-Vet scope.
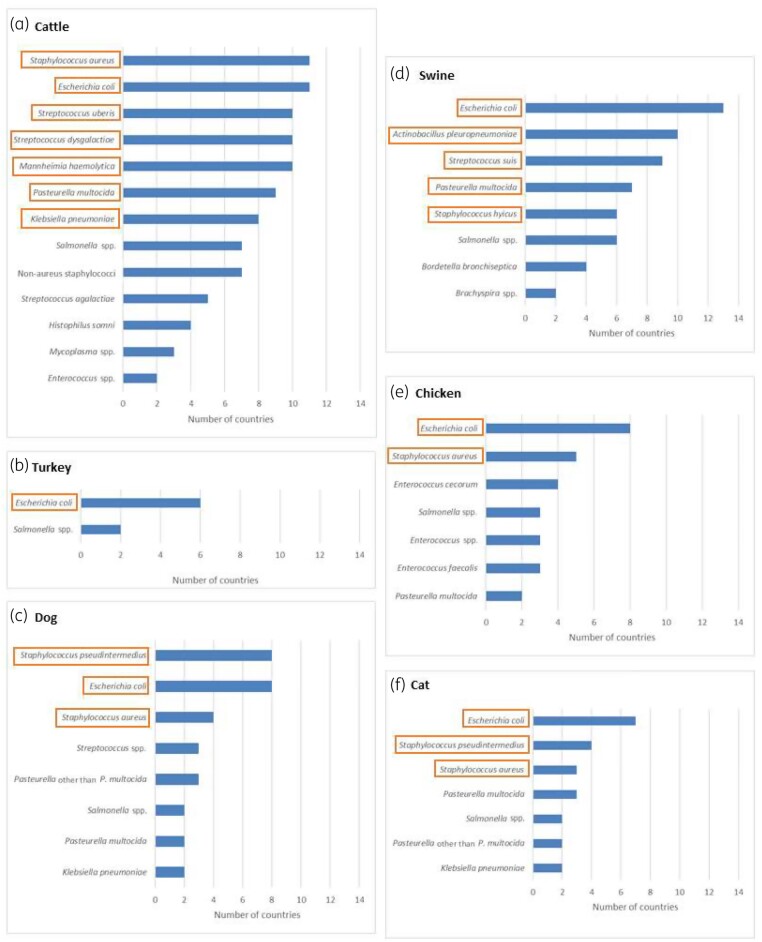
Figure 2 shows the bacterial species included in the 13 national scopes for each of the six chosen animal species. The expert group decided to include in the EARS-Vet scope the most frequently reported bacterial species within each animal species. No threshold defined the selection of bacterial species, but, in practice, none was selected if included by fewer than three countries. Salmonella was not included as it was not considered a major animal pathogen in Europe. Moreover, AMR monitoring in this bacterium has already been covered and prioritized from a public health perspective by EFSA. Although S. aureus is not considered among the most frequent causes of clinical infection in cats, dogs and chickens, the expert group decided to include it for these animal species (in addition to cattle where it is a frequent cause of mastitis) to address a possible risk for public health through direct contact.
Figure 2.
Distributions of the bacterial species present in the national veterinary AMR monitoring scopes of 13 European countries per cattle, swine, turkey, chickens, cats and dogs (orange boxes indicate the bacterial species selected for the EARS-Vet scope). Only bacterial species selected by at least two countries are displayed. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The feasibility of monitoring AMR in clinical animal isolates for seven bacterial species monitored by EARS-Net (E. coli, P. aeruginosa, K. pneumoniae, A. baumannii, S. aureus, E. faecalis and E. faecium) is described in detail in Tables S2 and S3. The combinations that most countries found feasible to monitor and already had in their national scope were E. coli in cattle and swine, and S. aureus in cattle, whereas no country included A. baumannii. Combinations of bacterial species/animal species that were categorized ‘feasible, but not included’ were assessed in order to evaluate whether countries could expand their monitoring systems as part of the EARS-Vet programme. For only 16 (out of 42) animal species/bacterial species combinations the answer ‘feasible, but not included’ was given. However, the number of countries never exceeded three, except for the combination dog/E. faecalis. The most frequent reason for such an answer was that the bacterium was not a monitoring priority. The most common reason for reporting ‘currently not feasible’ for an EARS-Net bacterium was that it was too rarely obtained from animal clinical specimens (countries used thresholds ranging from 10 to 100 isolates minimum per year). Finally, the expert group concluded that the EARS-Vet scope should not include more bacterial species than those initially selected, based on national scopes.
Determination of accepted specimens for each animal species/bacterial species combination
Table 1 summarizes expert decisions on the specimens to be accepted within EARS-Vet. The monitoring of AMR could be stratified per specimen only for E. coli in cattle, between milk samples (in the case of mastitis), inner organs or blood (in the case of septicaemia) and faeces or intestinal content (in the case of diarrhoea). In swine, it was decided to monitor E. coli in faeces or intestinal content only, as national systems rarely monitor it in blood or inner organs. When it comes to E. coli from swine faecal or intestinal samples, 8 of the 10 countries with a monitoring system in place monitor AMR only for virulent isolates or stratify their monitoring between virulent and non-virulent isolates, identified by PCR, serotyping, haemolytic profile or a combination of them. Thus, the expert group advised EARS-Vet to collect data on the haemolytic profile, serotypes and virulence factors of swine E. coli to stratify AMR monitoring based on typing information.
Table 1.
Proposed animal species, production types, specimens and bacterial species to be monitored in EARS-Vet
| Animal species | Production type | Specimens | Bacterial species |
|---|---|---|---|
| Cattle | any | faeces or intestinal content | E. coli a |
| blood and inner organs | E. coli | ||
| milk | E. coli | ||
| K. pneumoniae | |||
| S. aureus | |||
| S. uberis | |||
| S. dysgalactiae | |||
| lungs and other samples from the lower or upper respiratory tract | M. haemolytica | ||
| P. multocida | |||
| Swine | any | faeces or intestinal content | E. coli a |
| inner organs (including lungs, spleen, joints etc.) | Staphylococcus hyicus | ||
| S. suis | |||
| lungs and inner organs | P. multocida | ||
| A. pleuropneumoniae | |||
| Chicken | broilers | inner organs (including spleen, bone marrow, joints etc.) | E. coli |
| laying hen | inner organs (including spleen, bone marrow, joints etc.) | E. coli | |
| broilers | inner organs (including spleen, bone marrow, joints etc.) | S. aureus | |
| laying hen | inner organs (including spleen, bone marrow, joints etc.) | S. aureus | |
| Turkey | – | inner organs (including spleen, bone marrow, joints etc.) | E. coli |
| Dog | - | urine | E. coli |
| skin and ear | S. pseudintermedius | ||
| S. aureus | |||
| Cat | - | urine | E. coli |
| skin and ear | S. pseudintermedius | ||
| S. aureus |
Information on the virulence profile shall be collected in EARS-Vet.
Determination of stratification per production type and age category for each animal species/bacterial species/specimen combination
Some participating countries stratify their national monitoring per production type and age category. For instance, in the Czech Republic, AMR monitoring in swine pathogens is stratified between pre-weaning piglets, post-weaning piglets, fattening pigs and sows. However, this is not systematic across national monitoring systems. Experts pointed out that this information was often missing in their national databases, except for chickens, where broilers and laying hens are most often distinguished. Therefore, EARS-Vet could stratify its monitoring per production type for chickens only and not per age category (Table 1).
Determination of antimicrobials for each animal species/bacterial species/specimen/production type combination
Different countries often monitor different antimicrobial agents for a given combination of animal species/bacterial species/specimen/production type. Therefore, the expert group decided that EARS-Vet should monitor AMR for different categories of antimicrobials: antimicrobial classes (e.g. tetracyclines), when resistance to one agent (e.g. oxytetracycline) usually implies resistance to all other agents of the class; individual antimicrobial agents (e.g. neomycin), when resistance to these agents does not usually imply resistance to the other members of their antimicrobial classes, due to disparate resistance mechanisms (e.g. aminoglycosides); and antimicrobials used to detect specific resistance phenotypes, e.g. methicillin resistance in staphylococci.
When it comes to an antimicrobial class or antimicrobials used to detect a specific resistance phenotype, EARS-Vet shall accept and collate AMR data that correspond to different antimicrobial agents, as in EARS-Net.3
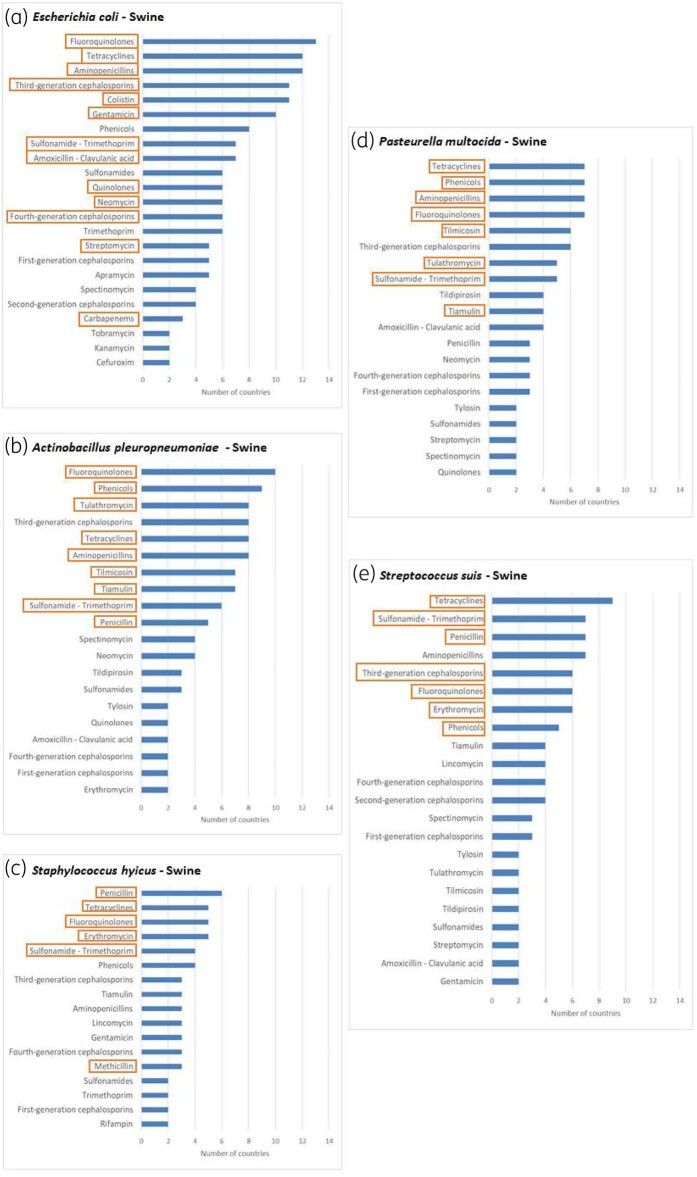
Figure 3 shows the antimicrobial categories included for each of the bacterial species selected for swine. Similar figures are available for cattle (Figure S1), chickens and turkeys (Figure S2) and cats and dogs (Figure S3). In general, the antimicrobial categories most frequently included in national scopes were selected for the EARS-Vet scope, with a few notable exceptions: cephalosporins and penicillins+β-lactamase inhibitors were not selected for Pasteurella multocida, Mannheimia haemolytica and Actinobacillus pleuropneumoniae, as resistance to β-lactams is not common in Europe and penicillins alone are already included;10 third-generation cephalosporins were not selected for staphylococci, as in vitro susceptibility testing of these agents is less reliable than other antimicrobials testing resistance to cephalosporins indirectly (i.e. those used for monitoring methicillin resistance);11 amphenicols were not selected when they were not commonly used to treat the conditions in question (e.g. to treat mastitis in cattle) according to the experts, although frequently included in national scopes for several combinations of animal species/bacterial species; sulphonamides and trimethoprim were not included as separate classes in swine/E. coli, as they are mostly used as fixed combinations in clinical practice;12 tildipirosin was not included for respiratory pathogens, as two other macrolides were already included in the EARS-Vet scope (tulathromycin and tilmicosin); and fusidic acid was not included for S. aureus and Staphylococcus pseudintermedius in cats and dogs, as this drug and its derivatives are only used for topical treatment in veterinary medicine13 (in that regard, little evidence exists between AST results and clinical efficacy when it comes to topical agents11).
Figure 3.
Distribution of antimicrobial categories present in the national veterinary AMR monitoring scopes of 13 European countries for E. coli, S. suis, S. hyicus, A. pleuropneumoniae and P. multocida isolated from swine (orange boxes indicate the antimicrobial categories selected for the EARS-Vet scope). Only antimicrobial categories selected by at least two countries are displayed. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
For bacterial species included in both EARS-Vet and EARS-Net (no common bacterial species between EARS-Vet and the AMR monitoring component of FWD-Net),4 all AST results for antimicrobial classes that are important from a public health perspective and monitored by EARS-Net shall also be collected,3 even though they were not frequently included in national scopes. Thus, for E. coli and K. pneumoniae, EARS-Vet shall collect AST results for piperacillin/tazobactam, carbapenems and tigecycline and, for S. aureus, vancomycin, rifampicin, linezolid and daptomycin.
The complete EARS-Vet scope at an antimicrobial agent level is presented in Table 2 for swine. Similar tables are available for cattle (Table S4), chickens and turkeys (Table S5) and cats and dogs (Table S6). The lists of antimicrobial agents were determined to maximize country participation and minimize the diversity of accepted antimicrobial agents by EARS-Vet. For example, in the case of isolated E. coli from cats, countries reported in their national scopes: enrofloxacin, ciprofloxacin, marbofloxacin, danofloxacin among fluoroquinolones. However, all countries reporting marbofloxacin and/or danofloxacin also reported enrofloxacin, whereas some countries reported only enrofloxacin or only ciprofloxacin. Thus, the list for E. coli from cats comprises only enrofloxacin and ciprofloxacin.
Table 2.
Proposed EARS-Vet scope in terms of bacterial species and antimicrobials for swine
| Bacterium | Antimicrobial category | Antimicrobial agents |
|---|---|---|
| E. coli | aminopenicillins | amoxicillin, ampicillin |
| amoxicillin+clavulanic acid | amoxicillin+clavulanic acid | |
| third-generation cephalosporins | cefotaxime, ceftiofur | |
| fourth-generation cephalosporins | cefquinome, cefepime | |
| quinolones | flumequine, nalidixic acid | |
| fluoroquinolones | enrofloxacin, ciprofloxacin | |
| tetracyclines | tetracycline | |
| colistin | colistin | |
| gentamicin | gentamicin | |
| neomycin | neomycin | |
| streptomycin | streptomycin | |
| sulphonamide/trimethoprim | sulphonamide/trimethoprim | |
| piperacillin/tazobactam | piperacillin/tazobactam | |
| carbapenems | imipenem, meropenem, ertapenem | |
| tigecycline | tigecycline | |
| S. hyicus | penicillin | penicillin |
| methicillin | oxacillin, cefoxitin | |
| fluoroquinolones | enrofloxacin, ciprofloxacin | |
| erythromycin | erythromycin | |
| tetracyclines | tetracycline | |
| sulphonamide/trimethoprim | sulphonamide/trimethoprim | |
| S. suis | penicillin | penicillin |
| third-generation cephalosporins | ceftiofur | |
| fluoroquinolones | enrofloxacin, ciprofloxacin | |
| erythromycin | erythromycin | |
| amphenicols | florfenicol, chloramphenicol | |
| tetracyclines | tetracycline | |
| sulphonamide/trimethoprim | sulphonamide/trimethoprim | |
| P. multocida and A. pleuropneumoniae | penicillin (only for A. pleuropneumoniae) | penicillin |
| aminopenicillins | amoxicillin, ampicillin | |
| fluoroquinolones | enrofloxacin, marbofloxacin, ciprofloxacin | |
| tulathromycin | tulathromycin | |
| tilmicosin | tilmicosin | |
| amphenicols | florfenicol, chloramphenicol | |
| tetracyclines | tetracycline, oxytetracycline, doxycycline | |
| sulphonamide/trimethoprim | sulphonamide/trimethoprim | |
| tiamulin | tiamulin |
Finally, by selecting the most frequently included antimicrobial compound for each combination, three sets of antimicrobials could be proposed and used by veterinary laboratories to cover all the combinations of veterinary relevance of the EARS-Vet scope: set A for E. coli and K. pneumoniae, ampicillin, amoxicillin+clavulanic acid, cefotaxime or ceftiofur (cefotaxime being preferred for the detection of ESBL-producing Enterobacterales), cefquinome, cefalexin, cefoxitin, nalidixic acid or flumequine, enrofloxacin, tetracycline, colistin, gentamicin, neomycin, streptomycin and sulfamethoxazole/trimethoprim; set B for P. multocida, M. haemolytica and A. pleuropneumoniae, penicillin or ampicillin, enrofloxacin, tulathromycin, tilmicosin, tiamulin, gentamicin, florfenicol, tetracycline and sulfamethoxazole/trimethoprim; set C for S. aureus, S. pseudintermedius, Streptococcus suis, Streptococcus dysgalactiae and Streptococcus uberis, penicillin, oxacillin (to detect methicillin-resistant S. pseudintermedius), cefoxitin (to detect MRSA), ceftiofur or cefotaxime, enrofloxacin, lincomycin, erythromycin, tetracycline, gentamicin, streptomycin, florfenicol and sulfamethoxazole/trimethoprim.
Countries may of course supplement these sets with antimicrobials of more local clinical relevance, as well as with antimicrobials included in the EARS-Vet scope with a public health perspective only (e.g. carbapenems for E. coli). However, field veterinary laboratories should not provide AST results for these latter antimicrobials to their customers, so as not to promote their use.
In addition to phenotypical AMR data, as a starting point for molecular AMR monitoring, the expert group also recommended EARS-Vet to collect molecular results confirming the presence of a methicillin resistance profile or differentiating isolates with ESBLs and AmpC cephalosporinases, when this information is available and by specifying the method used. However, not all countries could systematically provide this information. At the time of study, among the 10 countries with a monitoring system in place, 8 and 4 systematically tested the presence of mec genes in the case of suspected MRSA or methicillin-resistant S. pseudintermedius, respectively (9 countries monitor S. pseudintermedius); 5 and 3 systematically confirmed the presence of an ESBL or AmpC profile when suspected phenotypically. Other countries also carried out these analyses, but not systematically.
Discussion
In this work, consensus among experts was reached on the combinations of animal species/bacterial species/specimens/production types/antimicrobials to be monitored in EARS-Vet. Although with a primary focus on animals, strong efforts were made to define a scope that is relevant from a One Health perspective.
This work was based on 13 national veterinary AMR monitoring scopes. Such a bottom-up approach proved particularly helpful to guide expert discussions, by providing information about what is relevant and feasible to monitor in a large part of Europe. Most of the 10 countries with a national monitoring system in place are Northern and Central European countries, but the inclusion in this work of two Southern European countries, Spain and Greece, improved European representativeness. Collecting the national scope of countries building or planning to build their system also enabled us to anticipate a future expansion of the network. However, they might not have the experience to assess accurately their capacity to monitor some combinations of animal species/production types/age categories/bacterial species/specimens/antimicrobials. For countries with a system in place, a strength of our approach has been direct interaction with national coordinators, rather than solely relying on AMR monitoring reports as a source of information. It helped locate and share non-published data in some countries and revealed that surrogate antimicrobials are sometimes used. For example, AMR data for enrofloxacin can be published in a report, but ciprofloxacin (which is not authorized for animal use) is actually tested as a surrogate antimicrobial. It enabled us to better understand what epidemiological data are collected by monitoring systems, e.g. when AMR data are reported for poultry and not per poultry species. Finally, some countries need to collect AST results over several years to reach a sufficient sample size to calculate meaningful resistance proportions. Thus, some monitoring results are only present in some yearly reports. With our interactive approach with coordinators, we could overcome these challenges and consider more recent combinations being monitored, for example when test panels have changed since their last monitoring report.
The expert group decided to include six animal species in the EARS-Vet scope, covering both food-producing and companion animals. The three countries that were either building or planning to build their system (Spain, Belgium and Greece) did not include companion animals in their scope, as focusing on food-producing animals was the priority at their stage.
Major bacterial pathogens, considered as important drivers for veterinary antimicrobial usage in Europe, were selected for each animal species. Some of them are also directly relevant for public health, such as S. aureus, which is still an important AMR monitoring gap in the animal sector that EARS-Vet could contribute to filling.
Different specimens were selected in the EARS-Vet scope, depending on the animal species/bacterial species combination. Invasive samples, if they are not contaminated, ensure that isolated bacteria are the cause of infection. However, it can be challenging or impossible to identify the cause of infection for samples taken from sites with a commensal flora or when there is a high risk of contamination. This is the case, for example, where E. coli is isolated from faecal samples. Although laboratories usually investigate the serotypes or virulence factors of E. coli from piglets, it is not commonly performed for E. coli isolates from calves. This might introduce a source of bias in the EARS-Vet monitoring to be considered when interpreting results. By comparison with the human sector, EARS-Net includes only invasive samples (from blood and CSF), but this is not the case for the Global Antimicrobial Resistance Surveillance System, coordinated by the WHO, which also includes for instance stool samples for the monitoring of Salmonella spp. and Shigella spp.14
Information on production type and age is essential for AMR monitoring, as they can dramatically influence AMR levels within the same animal species. Although it did not seem possible for EARS-Vet to widely collect such information (except for production types in chickens), this difficulty could be overcome as production type and age category can often be inferred from the bacterial species and/or specimen. For example, pathogens isolated from cattle milk almost exclusively originate from adult dairy cows and E. coli from swine faecal samples typically originate from piglets with neonatal or post-weaning diarrhoea. In practice, countries should still be able and encouraged to report data on production type and age to EARS-Vet for any kind of isolate.
Antimicrobials of both animal and human relevance were selected in the EARS-Vet scope. They cover all categories of the Antimicrobial Advice ad hoc Expert Group of EMA, which were defined based on their potential consequences to public health (due to increased AMR) when used in animals, but also considering the need to use them in veterinary medicine.12 Three sets of antimicrobials covering all the combinations of veterinary relevance in the EARS-Vet scope were also defined and could be useful to countries or diagnostic laboratories aiming to customize their antimicrobial testing panels. In the future, EARS-Vet should recommend optimal dilution ranges for the three antimicrobial sets. However, EUCAST ECOFFs have not yet been established for all EARS-Vet combinations. Teale and Borriello15 have recently proposed dilution ranges for several bacterial species (A. pleuropneumoniae, E. coli, M. haemolytica, P. multocida, S. aureus and Streptococcus spp.) and many antimicrobials of the EARS-Vet scope, that were designed where possible to include both clinical breakpoints and ECOFFs (where available from EUCAST). In addition to phenotypical information, some molecular results shall also be collected to confirm methicillin resistance or differentiate ESBL from AmpC isolates, as a starting point towards molecular monitoring. However, most national monitoring systems are based on the passive data collection of AST results from routine veterinary diagnostic laboratories, which do not test antimicrobials reserved for humans and rarely confirm phenotypes at a molecular level. Thus, wide country participation in this area would require specific external funding. In the meantime, EARS-Vet could still act as a platform for the collection and sharing of such data, even if extensive molecular monitoring is not yet feasible.
With this scope, EARS-Vet has the potential to fill important AMR monitoring gaps in Europe, by covering companion animals, animal bacterial pathogens and antimicrobials of relevance to veterinary medicine, which are not covered in EARS-Net and the EFSA monitoring. However, integration between these three European monitoring systems shall be possible. Both EARS-Vet and the EFSA monitoring include cattle, swine, chickens and turkeys in their scopes. In terms of bacterial species, E. coli is included in both systems, which is sensible as previous studies have shown different proportions and evolutions of AMR between commensal and pathogenic E. coli in food-producing animals.16,17 In the EFSA monitoring, antimicrobials are selected from a public health perspective and are monitored as individual compounds. Still, many of the antimicrobials monitored by EFSA in commensal E. coli have also been included in the EARS-Vet scope for E. coli (ampicillin, cefotaxime, chloramphenicol, nalidixic acid, ciprofloxacin, colistin, gentamicin, tetracycline, tigecycline, meropenem, imipenem and ertapenem). In terms of molecular analyses, EARS-Vet, just as EFSA, plans to collect data on ESBL and AmpC detection for E. coli.
Integration between EARS-Net and EARS-Vet shall be possible for E. coli, S. aureus and K. pneumoniae. All antimicrobial categories monitored by EARS-Net for those three bacterial species are included in EARS-Vet, although aforementioned limitations regarding the collection of AMR data for antimicrobials that are not authorized represents a challenge for broad country participation. In addition, antimicrobial groupings may vary between EARS-Net and EARS-Vet, as tested antimicrobial compounds often differ in veterinary and human medicine. For instance, EARS-Net collates resistance data for ciprofloxacin, levofloxacin and ofloxacin to monitor fluoroquinolone resistance in E. coli,3 whereas EARS-Vet plans to accept AST results for enrofloxacin, marbofloxacin and ciprofloxacin for E. coli from cattle mastitis. In contrast to EARS-Net, it was also decided for EARS-Vet not to monitor aminoglycosides and macrolides as antimicrobial classes, but as individual compounds, since they may have different resistance profiles (e.g. neomycin versus gentamicin or erythromycin versus tulathromycin).3 In terms of molecular monitoring, commonalities between EARS-Net and EARS-Vet consist of the collection of data on ESBL detection for E. coli and K. pneumoniae, as well as mec gene detection for S. aureus.
Integrated data analyses between EARS-Net, EARS-Vet and the EFSA monitoring should be possible for E. coli, which could act as a monitoring indicator across animal species and the three European monitoring systems. In terms of antimicrobials, integrated data analyses could be carried out for aminopenicillins, aminoglycosides, third-generation cephalosporins, fluoroquinolones and carbapenems. Information on ESBL detection shall be collected in the three systems, although systematically only in the EFSA monitoring. Although molecular analyses, and especially whole genome sequencing, are expected to be conducted more and more frequently, integrated molecular monitoring of AMR is expected to remain limited in the mid-term. Comparisons of AMR levels should be done with caution in light of possible biases, which remain to be explored in EARS-Vet. Comparisons of AMR trends should be preferred to direct comparisons of AMR prevalence as sampling biases are likely to be rather constant over time. In particular, EARS-Vet could complement the pool of data being analysed in the Joint Inter-Agency Antimicrobial Consumption and Resistance Analyses,18 to better understand the complex epidemiology of AMR across sectors and contribute to devising more efficient interventions against AMR.
Finally, it is noteworthy to point out that the EARS-Vet scope should be reviewed and expanded, as the epidemiology of AMR changes, more countries participate and the feasibility to monitor specific combinations changes.
Supplementary Material
Acknowledgements
We are very grateful to all professionals involved in the definition of national veterinary AMR monitoring scopes and consulted experts from the Federation of Veterinarians in Europe, ECDC, EMA, EFSA and EUCAST, as well as to Lucie Collineau (French Agency for Food, Environmental and Occupational Health and Safety) for providing sound feedback on this work.
EU-JAMRAI partners
Evelyne JOUVIN-MARCHE, Marie-Cécile PLOY, Sadika BERNARD, Yohann LACOTTE, Céline PULCINI, Marielle BOUQUEAU, Anton HLAVA, Vera BUHMANN, Eline VANDAEL, Lieven DE RAEDT, Blazenka HUNJAK, Bojana RAICKOVIC, Barbora MACKOVÁ, Helena ŽEMLIČKOVÁ, Ute WOLFF SÖNKSEN, Sissel SKOVGAARD, Jüri RUUT, Ljudmila LINNIK, Arina ZANUZDANA, Nadiya OEZCELIK, Flora KONTOPIDOU, Mariana TSANA, Alkiviadis VATOPOULOS, Achilleas GIKAS, Aimilia MAGKANARAKI, Alessandra COZZA, Domenico MARTINELLI, Rosa PRATO, Annalisa PANTOSTI, Francesca PRESTINACI, Luca BUSANI, Roberta CRETI, Uga DUMPIS, Asta DAMBRAUSKIENE, Astra VITKAUSKIENE, Silvija KIVERYTE, Agniete MAZZELLA, Rolanda VALINTELIENE, Robertas PETRAITIS, Jasper CLAESSEN, Rosa PERÁN, Elma SMEETS, Pita SPRUIJT, Svein HØEGH HENRICHSEN, Christine ÅRDAL, Mari MOLVIK, Oliver KACELNIK, Anne Margrete URDAHL, Dorota ŻABICKA, Sérgio GOMES, Razvan CIORTEA, Maja ŠUBELJ, Nina JEMEC, António LÓPEZ NAVAS, Cristina MUÑOZ MADERO, Laura ALONSO IRUJO, María SANTACREU GARCÍA, Gloria OLIVA, Marta MASSANÉS, Elena FERRAGUT, Eusebi CASTAÑO, Casimiro JIMÉNEZ GUILLÉN, Marisol FRAGOSO, Germán PEÑALVA, José Miguel CISNEROS, Milena ESTEVEZ, Sophie MONTEAU, María José GONZÁLEZ DE SUSO, Pilar GALLEGO BERCIANO, Daniele ALIOTO, Lotta EDMAN, Axana HAGGAR, Elisabet LINDAL, Jakob OTTOSON, Anna NORDENFELT, Björn BENGTSSON, Patriq FAGERSTEDT, Birgitta LYTSY, Jean Yves MADEC, Lucie COLLINEAU, Rodolphe MADER, Anne BERGER-CARBONNE, Karl PEDERSEN, Monika LARSSON, Annicka REUSS, Anne SWALUE, Cristina PORTUGAL, Hans Fredrik WILHELMSEN, Jesús OTEO-IGLESIAS, Janicke FISCHER, Helga Katharina HAUG, Maria da GRACA FREITAS and María del Pilar LÓPEZ ACUÑA.
Funding
This work was supported by the Health Programme of the EU (2014–2020) under grant agreement no. 761296.
Transparency declarations
None to declare.
Disclaimer
The views expressed in this publication are those of the authors and do not necessarily reflect the opinion of consulted experts and organizations.
Supplementary data
Tables S1 to S6 and Figures S1 to S3 are available as Supplementary data at JAC Online.
Contributor Information
EU-JAMRAI:
Cindy Demay, Evelyne Jouvin-Marche, Marie-Cécile Ploy, Olivier Barraud, Sadika Bernard, Yohann Lacotte, Céline Pulcini, Jérôme Weinbach, Christine Berling, Marielle Bouqueau, Anton Hlava, Claudia Habl, Eva Kernstock, Reinhild Strauss, Robert Muchl, Vera Buhmann, Ann Versporten, Anne Ingenbleek, Eline Vandael, Greet Haelterman, Lieven De Raedt, Blazenka Hunjak, Bojana Raickovic, Barbora Mackova, Eva Niklova, Helena Žemličková, Lucia Hrivňáková, Vlastimil Jindrak, Brian Kristensen, Mikkel Lyndrup, Sissel Skovgaard, Ute Wolf Sönksen, Birgit Aasmäe, Jüri Ruut, Ljudmila Linnik, Olga Sadikova, Pille Märtin, Aryna Zanuzdana, Gülay Kizilkaya-Güneser, Nadiya Oezcelik, Tim Eckmanns, Ageliki Lambrou, Flora Kontopidou, Maria Papadaki, Mariana Tsana, Nikos Maroulis, Alkiviadis Vatopoulos, Emmanouel Papadogiannakis, Marietta Kontarini, Achilleas Gikas, Aimilia Magkanaraki, Alessandra Cozza, Domenico Martinelli, Francesca Fortunato, Rosa Prato, Anna Maria Marella, Annalisa Pantosti, Francesca Prestinaci, Luca Busani, Patrizio Pezzoti, Roberta Creti, Rosa Maria Martoccia, Silvio Brusaferro, Aija Vilde, Aiva Jakovela, Elina Langusa, Liga Grudule, Madara Grinsteine, Uga Dumpis, Asta Dambrauskiene, Astra Vitkauskiene, Daiva Tirvaitė, Lukas Cemnalianskis, Edita Kazenaite, Ilma Lozoraitiene, Ruta Adomaitiene, Ruta Ambrazaitiene, Silvija Kiveryte, Agniete Maciulaityte, Jolanta Kuklyte, Justė Petrene, Rolanda Valinteliene, Virginija Kanapeckiene, Asta Razmiene, Brigita Kairiene, Giedre Aleksiene, Ginreta Valinciute, Robertas Petraitis, Arjen Elsemulder, Ashna Nakched, Jasper Claessen, Lili Gui, Marcel de Kort, Rosa Perán, Alieke Van Leeuwen, Elma Smeets, Marcel Mennen, Pita Spruijt, Robbin Westerhof, Andreas Skulberg, Eirik Rødseth Bakka, Kadri Miard, Svein Høegh Henricsen, Anneli Pellerud, Cecilia Kallberg, Christine Årdal, Hanne-Merete Eriksen, Katrine Kranstad, Mari Molvik, Oliver Kacelnik, Patrycja Sollund, Roar Samuelsen, Therese Bakke, Anne Margrete Urdahl, Madelaine Norström, Anna Olczak-Pienkowska, Anna Skoczynska, Dorota Żabicka, Jarosław Bysiek, Joanna Rekawek, Ana Lebre, Eva FalcãO, Gianina Scripcaru, Isabel Neves, Sérgio Gomes, Nuno Pereira, Andrei Mihai Malutan, Cristian Iuhas, Loredana Szakacs, Mara Kissiedou-Bob, Razvan Ciortea, Eva Grilc, Irena Klavs, Katja Turk, Maja Šubelj, Mitja Vrdelja, Mojca Serdt, Nina Jemec, Uroš Glavan, Zoran Simonović, Ana Navarro Tamayo, António López Navas, Cristina Muñoz Madero, José Luis Alonso Lebrero, Laura Alonso Irujo, María Santacreu García, Paloma Crespo Robledo, Gloria Oliva, Marta Massanés, Antonio Oliver Palomo, Atanasio García Pineda, Elena Ferragut, Estrella Rojo, Eusebi Castaño, Leonor Periañez, Alberto Manuel Torres Cantero, Casimiro Jiménez Guillén, Hana Hukelova, Manuel Alcaraz, María ángeles Carlos, María del Pilar López Acuña, Alberto Gil Setas, Arantxa Ibarrola Segura, Carmen Ezpeleta, Claire Gahigiro Merino, María Eugenia Portillo Bordonabe, Marisol Fragoso, Xabier Beristáin Rementería, German Peñalva, José Miguel Cisneros, Milena Estevez, Sophie Monteau, Lucia Del Rio, María José González De Suso, Pilar Gallego Berciano, Ainhoa Aranguren Oyarzábal, Daniele Alioto, José Manuel Izquierdo Palomares, María José Calvo Alcántara, Raisa González Pérez, Teresa Havarria, Anette Hulth, Karin Carlin, Lotta Edman, Malin Grape, Olov Aspevall, Axana Haggar, Elisabet Lindal, Andrea Burgos, Jakob Ottoson, Marica Ostman, Mia Egervärn, Anna Nordenfelt, Björn Bengtsson, Ingrid Söderman, Anders Bjers, Jan-Ingvar JöNsson, Maria Starborg, Mikaela Laine, Patriq Fagerstedt, Andrew Metcalfe, Jenny Soder, Birgitta Lytsy, Jean Yves Madec, Lucie Collineau, Rodolphe Mader, Anne Berger-Carbonne, and Melanie Colomb-Cotinat
References
- 1. WHO. Global Action Plan on Antimicrobial Resistance. https://www.who.int/publications/i/item/9789241509763.
- 2. European Commission. A European One Health Action Plan Against Antimicrobial Resistance (AMR). https://ec.europa.eu/health/sites/health/files/antimicrobial_resistance/docs/amr_2017_action-plan.pdf.
- 3. ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report for 2019. https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2019.pdf.
- 4. ECDC. EU Protocol for Harmonised Monitoring of Antimicrobial Resistance in Human Salmonella and Campylobacter Isolates. https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-Salmonella-Campylobacter-harmonised-monitoring.pdf. [DOI] [PubMed]
- 5. European Food Safety Authority, ECDC. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2017/2018. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2020.6007. [DOI] [PMC free article] [PubMed]
- 6. Mader R, Damborg P, Amat J-P. et al. Building the European Antimicrobial Resistance Surveillance network in veterinary medicine (EARS-Vet). Euro Surveill 2021; 26: pii=2001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mader R, Muñoz Madero C, Aasmäe B. et al. Review and analysis of national monitoring systems for antimicrobial resistance in animal bacterial pathogens in Europe: a basis for the development of the European Antimicrobial Resistance Surveillance network in Veterinary medicine (EARS-Vet). https://zenodo.org/record/5205371. [DOI] [PMC free article] [PubMed]
- 8. FDA. NARMS 2017 Animal Pathogen AMR Data. https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/2017-animal-pathogen-amr-data.
- 9. World Organisation for Animal Health. Harmonisation of national antimicrobial resistance surveillance and monitoring programmes. In: Terrestrial Animal Health Code. 2018.
- 10. Michael GB, Bossé JT, Schwarz S.. Antimicrobial resistance in Pasteurellaceae of veterinary origin. Microbiol Spectr 2018; 6. doi: 10.1128/microbiolspec.ARBA-0022-2017. [DOI] [PubMed] [Google Scholar]
- 11. EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 11.0. 2021.
- 12. De Briyne N, Atkinson J, Borriello SP. et al. Antibiotics used most commonly to treat animals in Europe. Vet Rec 2014; 175: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. EMA. Categorisation of antibiotics in the European Union. https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific_en.pdf.
- 14. WHO. Global Antimicrobial Resistance Surveillance System: Manual for Early Implementation. https://www.who.int/publications/i/item/9789241549400.
- 15. Teale C, Borriello P.. A proposed scheme for the monitoring of antibiotic resistance in veterinary pathogens of food animals in the UK. Vet Rec 2021; 189: e201. [DOI] [PubMed] [Google Scholar]
- 16. Hendriksen RS, Mevius DJ, Schroeter A. et al. Occurrence of antimicrobial resistance among bacterial pathogens and indicator bacteria in pigs in different European countries from year 2002 – 2004: the ARBAO-II study. Acta Vet Scand 2008; 50: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bourély C, Chauvin C, Jouy É. et al. Comparative epidemiology of E. coli resistance to third-generation cephalosporins in diseased food-producing animals. Vet Microbiol 2018; 223: 72–8. [DOI] [PubMed] [Google Scholar]
- 18. ECDC, European Food Safety Authority, EMA. Third Joint Inter-Agency Report on Integrated Analysis of Antimicrobial Agent Consumption and Occurrence of Antimicrobial Resistance in Bacteria From Humans and Food-Producing Animals in the EU/EEA, JIACRA III. https://www.ecdc.europa.eu/sites/default/files/documents/JIACRA-III-Antimicrobial-Consumption-and-Resistance-in-Bacteria-from-Humans-and-Animals.pdf. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.