Abstract
Background
The community pharmacy-led Sore Throat Test and Treat (STTT) service in Wales allowed pharmacists to undertake a structured clinical assessment with FeverPAIN/Centor scores and a point-of-care test (POCT) for Group A Streptococcus (GAS) infection. A new service model was temporarily agreed as a result of COVID-19, without routine use of POCT.
Objectives
To explore the impact of removing the requirement for GAS POCT from a community pharmacy STTT service on antibiotic supply.
Methods
Analysis of STTT consultation data, obtained for two periods: November 2018 (date the service went live) to September 2019 (pre-pandemic); and November 2020 (date the new service model was introduced) to May 2021.
Results
For consultations eligible for POCT, the antibiotic supply rate increased from 27% (922/3369) (95% CI: 26%–29%) with the pre-pandemic service model (FeverPAIN/Centor + POCT) to 63% (93/147) (95% CI: 55%–71%) with the new model (FeverPAIN/Centor only); the percentage of patients who were not issued an antibiotic, despite their high clinical score, decreased from 56% (646/1154) to 9.3% (8/86).
Conclusions
Preliminary data suggest that for every 100 STTT consultations with patients with a Centor score of ≥3 or a FeverPAIN score of ≥2, the use of POCT may spare up to 36 courses of antibiotics, increasing to 47 for patients with higher clinical scores, suggesting that the pre-COVID delivery model (FeverPAIN/Centor + POCT) is the optimal pathway and POCT in addition to clinical scores may result in fewer antibiotic prescriptions for sore throat symptoms. These findings have implications for STTT service delivery during and beyond the COVID-19 pandemic.
Introduction
Acute sore throat is amongst the most common reasons for consulting a GP in the UK. In recent years it has been government policy to ensure patients use the most appropriate NHS service that meets their needs. This has included a drive to shift the management of many common ailments either to self-care or to professionals other than GPs, with the intention of freeing up GP time for those with more complex and urgent medical needs. One such strategy has been to better utilize the skills of community pharmacists in managing acute minor ailments. The Sore Throat Test and Treat (STTT) service in Wales has been extensively researched. Introduced in November 2018, STTT extended Wales’ pre-existing national Common Ailment Service to allow pharmacists to undertake a structured clinical assessment with FeverPAIN/Centor scores, a point-of care test (POCT) for Group A Streptococcus (GAS) in those with FeverPAIN score ≥2 and Centor score ≥3, and to supply antibiotics in line with NICE guidance for those in whom GAS was detected.1
Previous research evaluating the first 5 months of the pilot included 1725 STTT consultations undertaken in 56 participating community pharmacies. Over the period, the availability of STTT was associated with greater reductions in prescribing of phenoxymethylpenicillin than in areas where STTT was not available (−3.8% versus −3.4%, respectively).2 Sore throat consultation rates in one GP surgery adjacent to four STTT pilot sites decreased from 0.71 per 1000 patients in March 2018 (prior to STTT) to 0.36 per 1000 patients in March 2019 (4 months after STTT was introduced).2 The service was highly acceptable to community pharmacists and patients. Pharmacists recognized the role of STTT in educating patients and contributing to antimicrobial stewardship;3 98% of patients (499/510) were satisfied with the service, with 99% stating that they would return to the pharmacy for subsequent sore throat symptoms.4
As a result, national roll-out of the pilot service was agreed by all health boards in Wales, with an estimated 50% of community pharmacies planned to provide STTT by the end of winter 2020. Key evidence gaps remained relating to the long-term impact of pharmacy STTT and the wider roll-out would provide an opportunity for further evaluation.
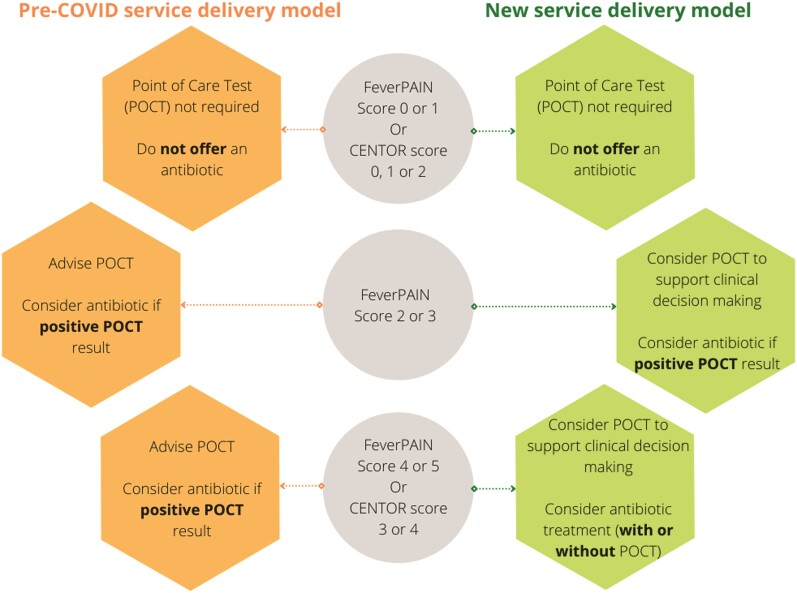
The wider roll-out has been affected by the impact of COVID-19, similarly to other healthcare services in the UK. The significant pressures experienced in community pharmacies are well documented.5 In March 2020, the STTT service was temporarily suspended as part of the wider strategy to support the delivery of critical NHS services such as dispensing, and to minimize the risk of COVID-19 transmission from avoidable presentations in pharmacies. Primary care services rapidly adapted to deliver remote consultations where possible, and the Welsh Government extended the availability of video consultations to community pharmacies. This enabled temporary changes to the delivery of STTT, in agreement with commissioners, to align with measures that safeguarded patient safety. For a time-limited period, a ‘new normal’ removed the requirement for routine POCT and allowed antibiotic supply to patients with a FeverPAIN score of ≥2 and a Centor score of ≥3 who wished to take antibiotics after discussion with the pharmacist. Community pharmacists used their professional judgement before supplying an antibiotic, and only after discussion with the patients, who may or may not have been asked to visit the pharmacy for a POCT, as per the ‘pre-COVID’ delivery model (Figure 1). The new delivery model was introduced in a staged approach in November 2020, immediately prior to the second wave of the pandemic.
Figure 1.
Comparison of the STTT service delivery models in the pre-COVID period (November 2018 to September 2019) and during the COVID period (November 2020 to May 2021). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The change provided a unique opportunity to extend the evaluation to include the new delivery model and consider how sore throat assessment in primary care could be optimized. Although stratification of patients based on clinical scoring is supported by NICE guidance, the role of an additional GAS POCT is less clear, particularly in community pharmacy.6
The primary objective of this study was to explore the impact of removing the requirement for a GAS POCT from a community pharmacy STTT service on antibiotic supply.
Materials and methods
We performed secondary data analysis of STTT consultation data, obtained from Choose Pharmacy, the national IT platform used in 98% of community pharmacies in Wales, to record service consultations. Information was collected on the number of STTT consultations, number eligible for a POCT alongside the Centor or FeverPAIN score, and whether antibiotics were supplied or not. Data were obtained for two periods: between 1 November 2018 (when the STTT service initially went live) and 30 September 2019 (the pre-pandemic period); and between 1 November 2020 (when the new delivery model was introduced) and 31 May 2021 (the COVID period). In the UK, patients do not register with a particular community pharmacy. The absence of meaningful denominators for pharmacy populations meant it was not possible to construct pharmacy consultation rates. Descriptive statistics (n and %) were used to describe the service in both periods (patients eligible for POCT test, antibiotics supplied overall and by Centor/FeverPAIN scores based on eligible population). Rates of antibiotic supplied were accompanied by 95% CIs, both overall and by Centor/FeverPAIN scores. Differences in antibiotic rates (alongside 95% CIs) between the two periods were calculated and a chi-squared test performed.7,8 Data were analysed using IBM SPSS Statistics Version 26.
Ethics
Ethical approval was granted by Cardiff School of Pharmacy and Pharmaceutical Sciences Research and Ethics Committee (reference: 2021-15).
Results
In the pre-pandemic period, a total of 4468 STTT consultations were completed between 1 November 2018 and 30 September 2019 in 56 pharmacies across two health boards in Wales. The overall antibiotic supply rate, for any FeverPAIN or Centor score, was 21% (940 patients out of 4468 consultations in total) (95% CI: 20%–22%).
Of the 3369 patients eligible for a POCT (FeverPAIN ≥ 2, Centor ≥ 3), 3175 (94%) were provided with one, with 974 (31%) testing positive. For this cohort of patients, the antibiotic supply rate was 27% (922/3369) (95% CI: 26%–29%). A further 18 patients received antibiotics based on the pharmacist’s clinical judgement or after discussions with the GP, despite not meeting the criteria for POCT; the antibiotic supply rate was higher in those with higher scores (63% for FeverPAIN = 5) (Table 1). In accordance with NICE guidance,1 around one-third (34%; 1154/3369) would have been offered an immediate or back-up antibiotic prescription on the basis of their clinical score alone (score FeverPAIN = 4/5 or Centor = 3/4); more than half of these (56%; 646/1154) were not offered antibiotics following a negative POCT result.
Table 1.
Comparison of antibiotic supply rates for STTT consultations eligible for POCT (FeverPAIN scores ≥ 2 and Centor scores ≥ 3) in pre-pandemic and COVID delivery models (excludes FeverPAIN score of 1 and 0, and Centor score of 1 and 2)
| Clinical scores | Pre-COVIDa with routine POCT | COVIDb without routine POCT | COVID period minus pre-COVID period | |||||
|---|---|---|---|---|---|---|---|---|
| STTT consultations | antibiotics suppliedc | % (95% CI) | STTT consultations | antibiotics suppliedc | % (95% CI) | Difference, % (95% CI) | Chi-squared; P value | |
| FeverPAIN | ||||||||
| 2 | 1136 | 147 | 13 (11–15) | 34 | 2 | 5.9 (1.6–19) | −7.1 (−12 to 6.3) | 1.48; 0.22 |
| 3 | 1079 | 267 | 25 (22–27) | 27 | 13 | 48 (31–66) | 23 (5.8–41) | 7.63; 0.0057 |
| 4 | 673 | 280 | 42 (38–45) | 47 | 42 | 89 (77–95) | 48 (35–55) | 42.5; 1.9 × 10−10 |
| 5 | 259 | 164 | 63 (57–69) | 16 | 15 | 94 (72–99) | 30 (7.6–38) | 6.14; 0.013 |
| Centor | ||||||||
| 3 | 166 | 40 | 24 (18–31) | 21 | 19 | 91 (71–97) | 66 (46–75) | 37.0; 7.0 × 10−10 |
| 4 | 56 | 24 | 43 (31–56) | 2 | 2 | 100 (34–100) | 57 (−44 to 70) | 0.08; 0.78 |
| Total | 3369 | 922 | 27 (26–29) | 147 | 93 | 63 (55–71) | 36 (28–43) | 88.4; 5.4 × 10−21 |
| FeverPAIN = 4/5 or Centor = 3/4 | 1154 | 508 | 44 (41–47) | 86 | 78 | 91 (83–95) | 47 (38–52) | 70.0; 6.0 × 10−17 |
November 2018 to September 2019.
November 2020 to May 2021.
Number of consultations that resulted in supply of antibiotics.
Under the new delivery model, a total of 199 consultations were completed between 1 November 2020 and 31 May 2021 in 25 of the 56 pharmacies involved in the previous study. Antibiotics were supplied in 48% of all consultations (95/199; 95% CI: 41%–55%) and in 63% of consultations eligible for a POCT (93/147; 95% CI: 56%–71%). The antibiotic rate was consistently higher for all FeverPAIN and Centor scores when compared with the pre-pandemic period, with the exception of a FeverPAIN score of 2 (Table 1). Differences were observed between the specific percentages of antibiotic supply for STTT consultations eligible for POCT in the pre-pandemic and COVID periods, with the exception of patients with a FeverPAIN score of 2 (−7.1%; 95% CI: −12% to 6.3%) and patients with a Centor score of 4 (57%; 95% CI: −44% to 70%), where fewer patients were seen.
Significant differences in the supply of antibiotics were found between the two periods, with an overall increase of 36 percentage points (95% CI: 28%–44%) from the pre-pandemic period to the COVID period, and 47 percentage points (95% CI: 38%–52%) specifically in those with severe symptoms (FeverPAIN = 4/5; Centor = 3/4). When we consider the 86 patients with Centor = 3/4 or FeverPAIN = 4/5, 78 patients (91%, 95% CI: 83%–95%) were provided with an antibiotic, meaning that only approximately 10% of the patients who would have been offered an antibiotic prescription according to NICE guidance ended up without antibiotics being supplied.
Discussion
Diagnostic scores such as FeverPAIN and Centor help clinicians to identify which patients are most likely to have GAS infection, thus improving targeted antibiotic prescribing in line with efforts to improve antibiotic stewardship.9 Adding a rapid GAS POCT could optimize this pathway further. In the UK, the use of GAS POCT is not considered cost-effective within GP consultations;6 however, internationally there are diverging opinions on this matter.10
The pre-pandemic community pharmacy STTT service involves a stepwise approach to the management of acute sore throat through structured clinical assessment, prognostic scoring and POCT. Changes to the delivery model necessitated by the COVID-19 pandemic reduced the number of steps prior to community pharmacists offering antibiotics, and consequently the number of opportunities to rule out GAS infection and target antibiotics more appropriately. Preliminary data from this study suggest that community pharmacists were significantly more likely to offer antibiotics when they relied on clinical scoring without a POCT to confirm diagnosis of GAS. When not using a POCT, pharmacist antibiotic supply rates were higher and similar to those reported for GPs.11
In the absence of longer-term follow-up data, our analysis suggests that for every 100 STTT consultations with patients with a Centor score of ≥3 or a FeverPAIN score of ≥2, the use of POCT may spare up to 36 courses of antibiotics. This increases to up to 47 courses spared when only those with higher clinical scores are included. This has significant implications for antimicrobial stewardship.
The study relates to two different periods of time; whilst this may limit the generalizability of our findings, we suggest that the pre-pandemic delivery model has advantages over pathways in which POCT is not routinely used. POCT in addition to clinical scoring tools may play a role in antimicrobial stewardship, by reducing the number of antibiotics prescribed for sore throat symptoms. These findings have implications for STTT service delivery during and beyond the COVID-19 pandemic. Given the importance placed on reducing unnecessary antibiotic use as part of a multifaceted approach to combatting antimicrobial resistance, we recommend that policymakers ensure pharmacists providing STTT should do so only when diagnosis is confirmed by the use of a validated POCT.
Acknowledgements
We would like to thank Ryan Southcott, Senior Support & Business Analyst in Digital Health and Care Wales, for his support with obtaining monthly data.
Funding
This study was carried out as part of our routine work.
Transparency declarations
None declared.
References
- 1. NICE . Sore throat (acute): antimicrobial prescribing. NICE guideline [NG84]. 2018. https://www.nice.org.uk/guidance/ng84.
- 2. Mantzourani E, Evans A, Cannings-John Ret al. . Impact of a pilot NHS-funded sore throat test and treat service in community pharmacies on provision and quality of patient care. BMJ Open Quality 2020; 9: e000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mantzourani E, Hicks R, Evans Aet al. . Community pharmacist views on the early stages of implementation of a pathfinder sore throat test and treat service in Wales: an exploratory study. Integr Pharm Res Pract 2019; 8: 105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mantzourani E, Cannings-John R, Evans Aet al. . Understanding the impact of a new pharmacy sore throat test and treat service on patient experience: a survey study. Res Soc Admin Pharm 2021; 17: 969–77. [DOI] [PubMed] [Google Scholar]
- 5. Baker M, Lindsey L, Rathbone AP. Community pharmacists’ experience of workplace pressure: the impact of COVID-19. Pharm J 2021; 306. https://pharmaceutical-journal.com/article/letters/community-pharmacists-experiences-of-workplace-pressure-the-impact-of-covid-19. [Google Scholar]
- 6. NICE . Rapid tests for group A streptococcal infections in people with a sore throat. Diagnostics guidance [DG38]. 2019. https://www.nice.org.uk/guidance/dg38.
- 7. Wilson, EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927; 22: 209–12. [Google Scholar]
- 8. Newcombe, RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 1998; 17: 873–90. [DOI] [PubMed] [Google Scholar]
- 9. Little P, Hobbs FD, Moore Met al. . PRImary care Streptococcal Management (PRISM) study: in vitro study, diagnostic cohorts and a pragmatic adaptive randomised controlled trial with nested qualitative study and cost-effectiveness study. Health Technol Assess 2014; 18: vii–xxv, 1–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gunnarsson R, Ebell MH, Wächtler Het al. . Association between guidelines and medical practitioners’ perception of best management for patients attending with an apparently uncomplicated acute sore throat: a cross-sectional survey in five countries. BMJ Open 2020; 10: e037884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gulliford MC, Dregan A, Moore MVet al. . Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014; 4: e006245. [DOI] [PMC free article] [PubMed] [Google Scholar]