Abstract
CYP2D6 metabolically inactivates several neurotoxins, including beta-carbolines, which are implicated in neurodegenerative diseases. Variation in CYP2D6 within the brain may alter local inactivation of neurotoxic beta-carbolines, thereby influencing neurotoxicity. The beta-carboline harmine, which induces hypothermia and tremor, is metabolized by CYP2D6 to the non-hypothermic/non-tremorgenic harmol. Transgenic mice (TG), expressing human CYP2D6 in addition to their endogenous mouse CYP2D, experience less harmine-induced hypothermia and tremor compared with wild-type mice (WT). We first sought to elucidate the role of CYP2D in general within the brain in harmine-induced hypothermia and tremor severity. A 4-h intracerebroventricular (ICV) pretreatment with the CYP2D inhibitor propranolol increased harmine-induced hypothermia and tremor in TG and increased harmine-induced hypothermia in WT. We next sought to specifically demonstrate that human CYP2D6 expressed in TG brain altered harmine response severity. A 24-h ICV propranolol pretreatment, which selectively and irreversibly inhibits human CYP2D6 in TG brain, increased harmine-induced hypothermia. This 24-h pretreatment had no impact on harmine response in WT, as propranolol is not an irreversible inhibitor of mouse CYP2D in the brain, thus confirming no off-target effects of ICV propranolol pretreatment. Human CYP2D6 activity in TG brain was sufficient in vivo to mitigate harmine-induced neurotoxicity. These findings suggest that human CYP2D6 in the brain is protective against beta-carboline-induced neurotoxicity and that the extensive interindividual variability in CYP2D6 expression in human brain may contribute to variation in susceptibility to certain neurotoxin-associated neurodegenerative disorders.
Keywords: CYP2D6, Drug metabolism, Neurotoxicity, Harmine, Propranolol
Introduction
Members of the cytochrome P450 enzyme (CYP) family catalyze the oxidative metabolism of most drugs and toxins [1]. CYP2D is a CYP subfamily, which includes CYP2D6 in humans and various CYP2D isoforms in other species [2]. CYP2D metabolizes approximately 25% of all clinically used drugs, many of which are centrally acting, including opioids, psychostimulants, and antipsychotics [3, 4]. Additionally, CYP2D metabolizes neurotoxins, including tetrahydroisoquinolines [5], 1-methyl-4-phe-nyl-1,2,3,6-tetrahydropyridine (MPTP) [6], and beta-carbolines [7], which are causally implicated in neurodegeneration [8–10]. Human CYP2D6 is highly genetically polymorphic; variation in CYP2D6 is associated with differences in drug pharmacokinetics and resultant drug response [3, 4], as well as with differences in susceptibility to neurodegenerative diseases [11, 12]. While CYP2D is expressed at high levels in the liver, CYP2D is also expressed and enzymatically active in the brain [13, 14], where local metabolism can alter brain levels of substrates (e.g., centrally acting drugs and neurotoxins) and their metabolites [15, 16]. Hepatic CYP2D is considered uninducible, but CYP2D in the brain is readily induced by exposure to xenobiotics including chronic nicotine [17, 18]. CYP2D6 variation in the human brain, in addition to variation in the liver, may influence drug-induced neurotoxicity and neurodegeneration by changing levels of neurotoxins and their metabolites within the brain.
Exposure to beta-carbolines can occur through consumption of certain foods, alcohol, and inhalation of tobacco smoke [19, 20]. Elevated levels of certain beta-carbolines are positively correlated with measures of neurodegeneration [21]and are associated with neurodegenerative diseases including Parkinson’s disease[22] and essential tremor [23]. Harmine is a beta-carboline that induces hypothermia [24] and tremor [25], which serve as measures of neurotoxicity in rodent models of neurodegenerative disorders [26]. CYP2D6 metabolism constitutes a major inactivation pathway for many beta-carbolines including harmine, which is metabolized by CYP2D6 to the inactive metabolite harmol [7, 27]. This suggests that CYP2D6 may protect against beta-carboline-induced neurotoxicity. Humanized CYP2D6-expressing transgenic mice (TG), which express human CYP2D6 in addition to mouse CYP2D, metabolize harmine (and related compounds) more rapidly than wild-type mice (WT) [7]; they also exhibit less severe hypothermia [28] and tremor [29]re-sponses, consistent with a protective role for CYP2D6 metabolism. Harmine readily enters and distributes throughout the brain [30], suggesting a potential important contribution of CYP2D6- and CYP2D-mediated metabolism in the brain to mitigating neurotoxicity.
In cultured human neurons, inhibiting CYP2D6 increased MPTP-induced neurotoxicity in vitro [31, 32]. CYP2D6 protein was lower in human brains of those with Parkinson’s disease compared with brains from age-matched healthy controls, even after controlling for CYP2D6 genotype [33]. This is consistent with lower CYP2D6 in the human brain reducing protection against xenobiotic-induced neurotoxicity and neurodegeneration, while elevated CYP2D6 in brain may be protective. We hypothesized a role for CYP2D6 within the brain, whereby local CYP2D6-mediated beta-carboline inactivation may mitigate beta-carboline-induced neurotoxicity. The aims of the current study were (1) to assess the role of CYP2D in general within the brain in neurotoxicity-related responses following administration of the beta-carboline harmine, and (2) to demonstrate specifically that human CYP2D6 expressed within the brain was sufficient to mitigate harmine-induced neurotoxicity. Selective pharmacological manipulation of CYP2D in the brain provides an effective tool to study the impact of CYP2D metabolism in the brain [15, 16]. We recently developed novel approaches, using these TG and WT, to selectively inhibit human CYP2D6 and/or mouse CYP2D in the brain, without impacting hepatic metabolism in either mouse line [34]. Propranolol acts as an irreversible inhibitor of human CYP2D6 expressed in TG brain and a competitive inhibitor of mouse CYP2D in TG and WT brain [34]. To assess the role of CYP2D in general within the brain in harmine-induced hypothermia and tremor responses (aim 1), we used a 4-h intracerebroventricular (ICV) propranolol pretreatment, which irreversibly inhibits human CYP2D6 in the TG brain and competitively inhibits mouse CYP2D in the brain of TG and WT (Fig. 1)[34]. To study the impact of human CYP2D6 specifically in the brain (aim 2), we used a 24-h ICV propranolol pretreatment, which selectively and irreversibly inhibits human CYP2D6 in TG brain but does not inhibit mouse CYP2D in the brain of TG or WT (i.e., there is no competitive inhibition remaining 24 h after propranolol pretreatment) (Fig. 1)[34]. This novel approach was used to demonstrate both the general role for CYP2D in the brain and, for the first time, that human CYP2D6 expressed in the brain of a mammal is sufficient to alter response to a neurotoxin, specifically the beta-carboline harmine.
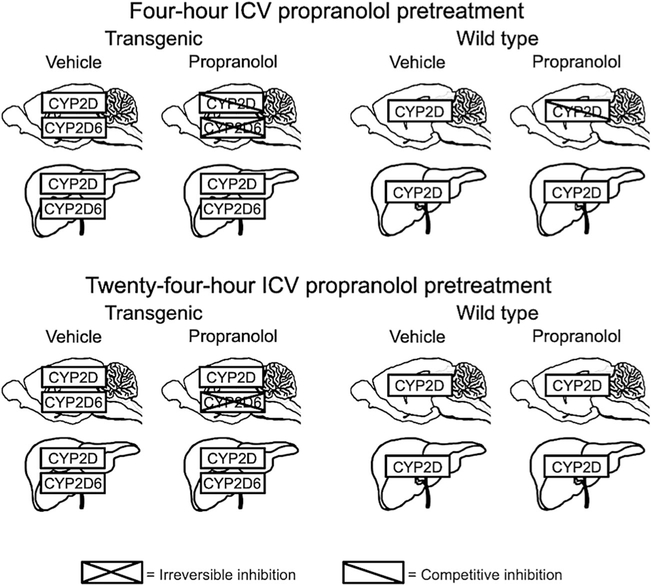
Fig. 1.
Pretreatment effects on CYP2D and CYP2D6 in brain and liver of TG and WT. Four-hour ICV propranolol pretreatment irreversibly inhibits human CYP2D6 in TG brain and competitively inhibits mouse CYP2D in TG and WT brain. Twenty-four-hour ICV propranolol pretreatment irreversibly inhibits human CYP2D6 in TG brain but no longer inhibits mouse CYP2D in TG or WT brain. Following 4-and 24-h ICV propranolol pretreatments, there is no inhibition of human CYP2D6 in TG liver, or of mouse CYP2D in TG or WT liver, indicating that propranolol given ICV does not reach the liver in sufficient amounts to alter hepatic metabolism. For more details, see text and Tolledo et al. [34]
Methods
Animals
TG were produced by microinjecting fertilized FVB/N mouse eggs with an insert that included one copy of the human CYP2D6 gene sequence (exon 1–9), the 5'-and 3'-flanking sequences, and the pseudogenes CYP2D7P1 and CYP2D8P1 (Genbank accession number BX247885, PAC clone RP4–669P10) [29]. TG founders underwent successive matings with C57BL/6J mice, and polymerase chain reaction and Southern blot analyses were used to confirm the incorporation of the full-length CYP2D6 gene [29]. For these experiments, all TG were homozygous and this was confirmed by genotyping prior to use, as previously described [35]. Age-matched adult male TG [29] and WT (Charles River, Saint-Constant, QC, Canada) mice were housed in groups of 1–4, given food and water ad libitum, and kept under a 12-h light/dark cycle with testing during the light phase. All procedures were approved by the Animal Care Committee at the University of Toronto and were conducted in accordance with the guidelines of the Canadian Council on Animal Care and the National Institutes of Health.
Intracerebroventricular Cannulation Surgery
Mice were anesthetized with 2% isofluorane and implanted with 26-gauge stainless steel guide cannulas into the right lateral ventricle (anterior-posterior - 1.0 mm, lateral - 0.5 mm, from bregma, and dorsoventral - 2.2 mm) [36]. Guide cannulas were secured using dental cement and small stabilizing screws. Dummy cannulas were inserted after surgery. Animals recovered for at least 7 days prior to experimentation.
Drugs and Drug Administration
As previously described [34], propranolol hydrochloride (Sigma, Oakville, ON, Canada) was dissolved in its vehicle, a 20% (w/v) solution of 2-hydroxypropyl-β-cyclodextrin (Sigma, Oakville, ON, Canada) in distilled water, to a final concentration of 40 μg propranolol base/μl cyclodextrin vehicle solution. A total volume of 2 μl(80 μg propranolol total or cyclodextrin vehicle) was injected ICV at a rate of 1 μl/min through the guide cannula with an automated injector system (Harvard Apparatus Pump 11 Pico Plus Elite, Holliston, MA, USA). Following the ICV injection, mice rested for 1 min before injectors were removed and cannula dummies replaced. In rodents, ICV injected compounds (e.g., dyes and CYP inhibitors) diffuse bilaterally and produce CYP inhibition across brain regions (e.g., cerebellum to frontal cortex) [16, 37, 38]. ICV pretreatment injections were given either 24 or 4 h prior to harmine treatment. Harmine hydrochloride (Sigma, Oakville, ON, Canada) was dissolved in distilled water. Harmine (or distilled water vehicle) was injected intraperitoneally (IP) at a volume of 0.1 ml/kg body weight. Harmine doses of 5.0, 7.5, and 10.0 mg base/kg were tested in the dose response experiments (n =6–8 per dose, per mouse line), and 7.5 mg/kg harmine base was used in subsequent experiments with ICV pretreatments, because this dose allowed for the detection of an increase or decrease in harmine response.
Harmine-Induced Hypothermia and Tremor Assessment
Hypothermia was calculated as the change in body temperature compared with a baseline, where baseline was measured 15 min prior to harmine injection. Body temperature was measured using a digital thermometer and lubricated thermistor probe inserted 1.0 cm rectally. Tremor was scored based on 30 s of constant observation per time point and using a modified version of a previously published scale [29]. Briefly, this included whole number scores of 0 (no tremor), 1.0 (mild infrequent tremor), 2.0 (modest intermittent tremor), 3.0 (severe frequent tremor), and 4.0 (severe constant tremor), and scoring was performed using half unit increments (e.g., 0.5, 1.0, 1.5).
Four-Hour ICV Pretreatment and Harmine-Induced Hypothermia and Tremor
A pilot study was conducted in WT (n =12),and the pretreatment effect size for hypothermia was used to derive the WT sample size (n = 8) needed; TG sample size (n = 16) was obtained by doubling that of the WT. TG and WT received ICV propranolol (or cyclodextrin vehicle) pretreatment 4 h prior to harmine (or distilled water vehicle) IP treatment. To obtain within-animal data, ICV pretreatment and IP treatment conditions were crossed over (i.e., each mouse was tested in each of the four combinations: propranolol/harmine, vehicle/harmine, propranolol/vehicle, and vehicle/vehicle). Each test session was separated by a 7-day washout; the order was randomized and counterbalanced. Hypothermia and tremor were assessed at baseline and at 15-min intervals for 90 min.
Twenty-Four-Hour ICV Pretreatment and Harmine-Induced Hypothermia and Tremor
Sample sizes were increased in this experiment to account for a smaller predicted effect size in TG, compared with the effect size observed following 4-h pretreatment. TG 0n 25) and WT (n = 24) mice received ICV propranolol (or cyclodextrin vehicle) pretreatment 24 h prior to IP harmine treatment. To obtain within-animal data, ICV pretreatment conditions were crossed over after a 7-day washout; the order was randomized and counterbalanced. Hypothermia and tremor were assessed at baseline and at 15-min intervals for 90 min.
Four- and 24-h ICV Pretreatment and In Vitro Brain and Liver CYP2D Activity
Four- or 24-h ICV propranolol (or cyclodextrin vehicle) pretreatment was administered to TG and WT (n =5–6 per pretreatment per mouse line for 4- and 24-h experiments). Mice were then euthanized, and the cerebellums and livers were collected. The cerebellum was used to assess CYP2D activity in vitro in brain, due to high CYP2D expression and activity in this region in rodents [29, 39]. Brain (i.e., cerebellum) membrane preparation and incubations were performed on the same day, while livers were stored at − 80 °C with microsome preparation and incubations performed on a subsequent day [35].
Total membranes were prepared from cerebellum, and microsomal membranes were prepared from liver as previously described [35, 40, 41]. Dextromethorphan hydrobromide (Sigma, Oakville ON, Canada) was used as a CYP2D probe substrate in both brain and liver incubations; dextromethorphan undergoes CYP2D-specific O-demethylation to dextrorphan [42], with Km values of 4.5 and 3.6 μM in TG and WT, respectively [35], similar to the ~ 5 μM Km in human liver microsomes [43]. Incubation conditions were optimized for linear dextrorphan formation by mouse brain membranes and liver microsomes [34, 35]. For brain, membranes prepared fresh from whole cerebellums (300–400 μg) were incubated with 50 μM dextromethorphan (approximate Vmax) and 1 mM NADPH in artificial cerebrospinal fluid (ACSF) (pH 7.4) for 90 min and at 37 °C under 95% O2/5% CO2 in a final volume of 1 ml [34]. For liver, microsomes (50 μg protein) were incubated with 5 μM dextromethorphan (approximate Km) and 1 mM NADPH in 100 mM potassium phosphate buffer (pH 7.4) for 10 min and at 37 °C in a final volume of 0.5 ml [35]. Incubation reactions were started by adding dextromethorphan, and reactions were stopped by adding an equal volume of a hexane-butanol (95:5 v/v) solution. Immediately before extraction, 5 ng dextrorphan base (dextrorphan tartrate, Sigma, Oakville ON, Canada) was added to each brain incubate; this was added to ensure that dextrorphan concentrations were above the limit of quantification [34]. This was subsequently subtracted from the concentration measured in each sample to calculate enzymatically formed dextrorphan. All samples were then extracted (72.8% dextrorphan recovery), the organic layer collected and dried under nitrogen, and the residue dissolved in mobile phase for analysis by high-performance liquid chromatography, as previously described [16, 34, 35].
Statistical Analysis
All analyses were performed using Prism6 (GraphPad version 6.0c, La Jolla, CA, USA) software, and all outliers were included in original analyses. Mixed-design ANOVAs were used to compare responses (i.e., either hypothermia or tremor) across time, for each IP treatment (i.e., mouse line × pretreatment, within harmine or distilled water IP treatment); subsequent analyses were only performed when there was a significant effect of group (i.e., mouse line × pretreatment). Repeated-measure (RM) ANOVAs were then used to compare responses between ICV propranolol and vehicle pretreatments within mouse line, and mixed-design ANOVAs were used to compare responses between vehicle-pretreated TG and WT. Area under the response-time curve (AUC0–90) was analyzed across all groups (mouse line × pretreatment) using two-factor mixed-design ANOVAs. AUC0–90 was compared between ICV propranolol and vehicle pretreatments (within mouse line) using Bonferroni-adjusted paired two-tailed t tests, and between vehicle-pretreated TG and WT using unpaired two-tailed t tests. Mean response was calculated by dividing AUC0–90 bytherecordedresponsedura-tion (90 min). Mean response was compared between ICV propranolol and vehicle pretreatments (within mouse line) using Bonferroni-adjusted paired two-tailed t tests. Dextrorphan formation (i.e., CYP2D enzymatic activity for brain and for liver) was compared between ICV propranolol and vehicle pretreatments (within mouse line) usingunpairedtwo-tailed t tests. For all unpaired t tests, Welch’s correction was applied when the F test comparing group variance was significant. Between-animal data is graphed showing standard deviation, and within-animal data is graphed showing standard error of the mean.
Results
Harmine Hypothermia and Tremor Dose Response
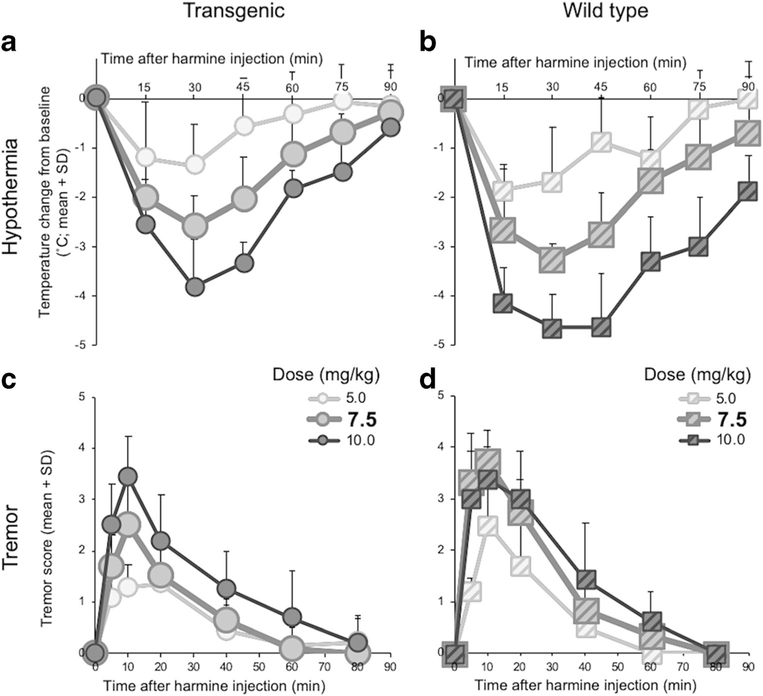
Harmine dose-dependently induced hypothermia in TG (dose, F(3, 23) = 23.0, p < 0.0001) (Fig. 2a) and in WT (dose, F(3, 20) = 48.0, p < 0.0001) (Fig. 2b). Harmine dose-dependently induced tremor in TG (dose, F(2, 20) = 7.61, p = 0.0035) (Fig. 2c) and in WT (dose, F(2, 18) = 15.7, p = 0.0001) (Fig. 2d). The 7.5 mg/kg harmine dose was selected for subsequent studies, because it allowed for the detection of an increase or decrease in response in TG and WT.
Fig. 2.
Harmine dose-dependently induced hypothermia and tremor in TG and WT. Change in body temperature from baseline after injection with harmine (5.0, 7.5, or 10.0 mg/kg IP) of a TG and b WT. Tremor score after injection with harmine (5.0, 7.5, or 10.0 mg/kg IP) in c TG and d WT. Symbols for the harmine 7.5 mg/kg dose effects are enlarged, to illustrate the effects of the dose that was used in subsequent experiments. SD standard deviation
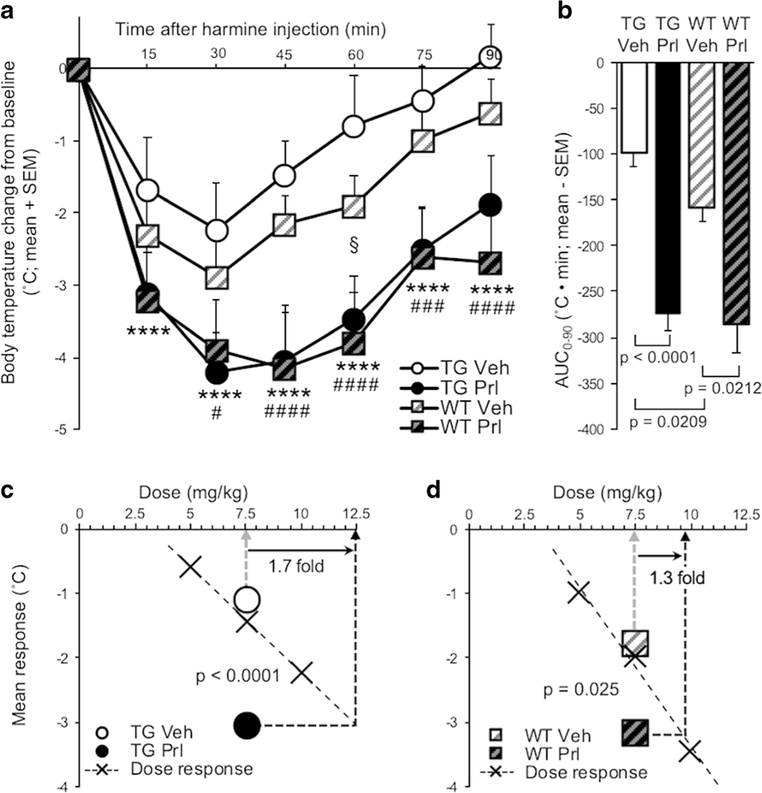
Four-Hour ICV Propranolol Pretreatment Exacerbated Harmine-Induced Hypothermia in TG and in WT
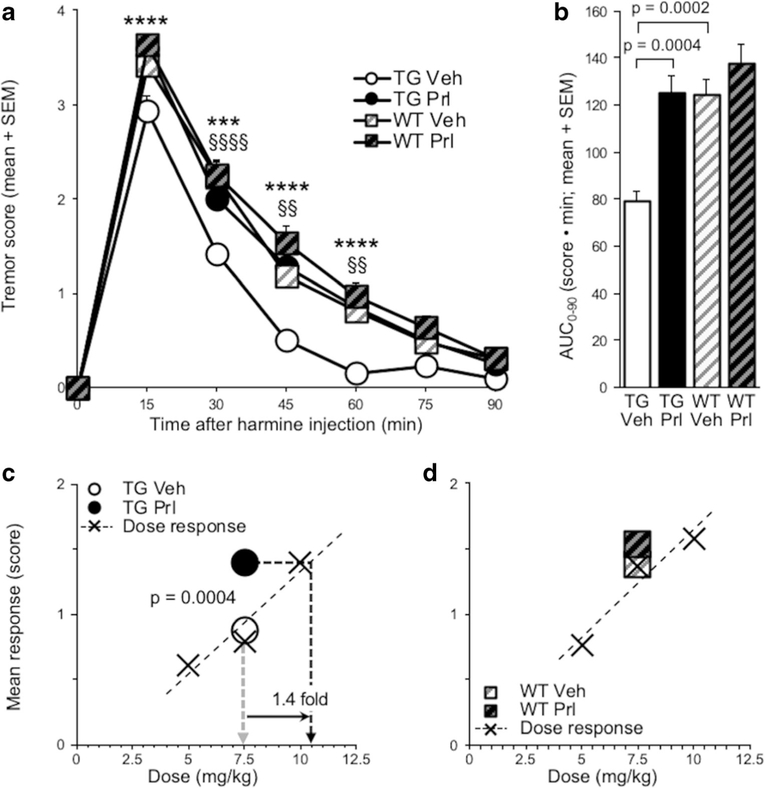
Four-hour ICV propranolol (versus vehicle) pretreatment increased harmine-induced hypothermia in TG (pretreatment, F(1, 15) = 94.4, p < 0.0001) and in WT (pretreatment, F(1, 7) = 13.4, p = 0.0081) (Fig. 3a). There was a main effect of pretreatment on hypothermia, evaluated by AUC0–90 (F(1, 22) = 67.6, p < 0.0001), due to propranolol (versus vehicle) pretreatment increasing AUC0–90 in TG(t (15) = 9.42, p <0.0001)andinWT(t(7) = 3.46, p = 0.0212) (Fig. 3b). In propranolol (versus vehicle) pretreated TG, the increase in mean response (t(15) = 9.57, p < 0.0001) corresponded to a 1.7-fold increase in apparent harmine dose (Fig. 3c). In propranolol (versus vehicle) pretreated WT, the increase in mean response (t(7) = 3.34, p = 0.025) corresponded to a 1.3-fold increase in apparent harmine dose (Fig. 3d). There was no effect of ICV propranolol (versus vehicle) pretreatment on baseline body temperature measured before IP harmine (or distilled water) treatment (p > 0.05; data not shown).
Fig. 3.
Four-hour ICV propranolol (versus vehicle) pretreatment exacerbated harmine-induced hypothermia in TG and WT. a Four hours after ICV propranolol or vehicle pretreatment, harmine treatment was administered, and change in body temperature from baseline was recorded for 90 min in TG and WT. b AUC0–90 of ICV propranolol or vehicle-pretreated TG and WT. Mean response of ICV propranolol or vehicle-pretreated c TG and d WT; values are superimposed on dose-response data from Fig. 2a, b, respectively. Propranolol versus vehicle-pretreated TG: ***p < 0.001, ****p < 0.0001; propranolol versus vehicle-pretreated WT: #p <0.05, ###p < 0.001, ####p < 0.0001; vehicle-pretreated WT versus TG: §p <0.05. Vehvehicle, Prl propranolol, SEM standard error of the mean, AUC area under the curve
Hypothermia was more severe in vehicle-pretreated WT compared with TG (mouse line, F(1, 22) = 6.69, p =0.0168) Fig. 3a), and the AUC0–90 was greater in vehicle pretreatment WT compared with TG (t(22) = 2.49, p = 0.0209) (Fig. 3b). IP distilled water treatment had no effect on body temperature, and body temperature following IP distilled water did not differ between ICV pretreatment groups or mouse lines ANOVA, all p > 0.05) (Online Resource 1).
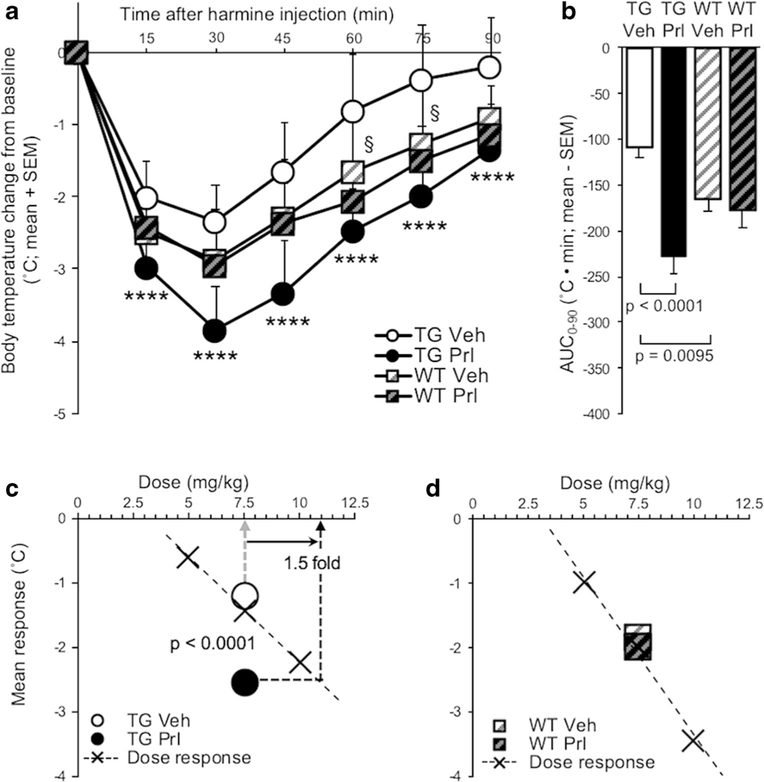
Twenty-Four-Hour ICV Propranolol Pretreatment Exacerbated Harmine-Induced Hypothermia in TG, But Not in WT
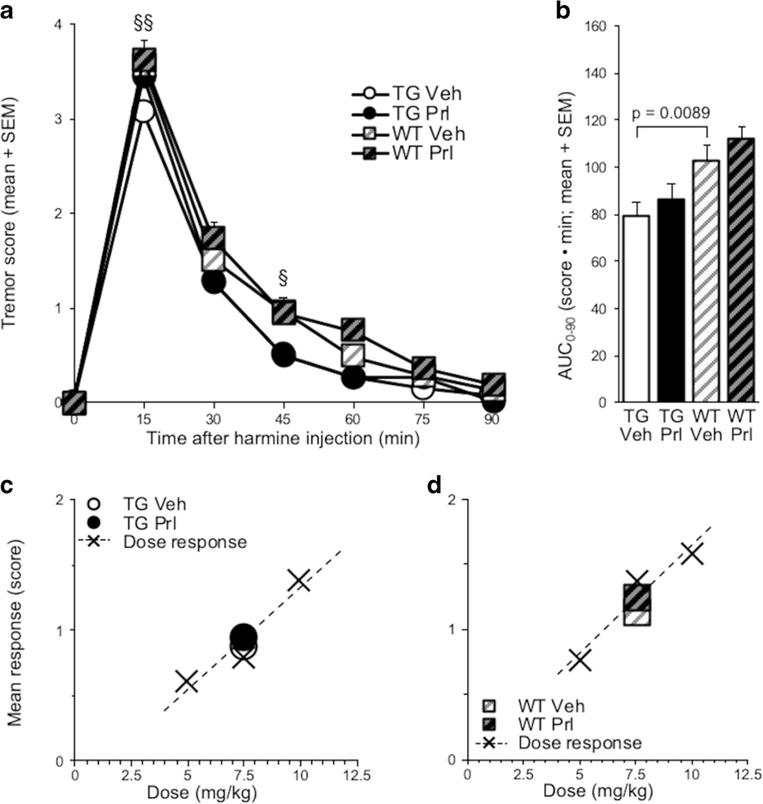
Twenty-four-hour ICV propranolol (versus vehicle) pretreatment increased harmine-induced hypothermia in TG (pretreatment, F(1, 24) = 49.7, p < 0.0001), but had no effect in WT (Fig. 4a). There was an interaction effect of pretreatment and mouse line on hypothermia, evaluated by AUC0–90 (F(1, 47) = 12.2, p = 0.001), as well as a main effect of pretreatment (F(1, 47) = 19.1, p < 0.0001), due to propranolol (versus vehicle) increasing AUC in TG (t(24) = 7.06,p < 0.0001)(Fig. 4b). In propranolol (versus vehicle) pretreated TG, the increase in mean response (t(24) = 7.06, p < 0.0001) corresponded to a 1.5-fold increase in apparent dose (Fig. 4c). In propranolol (versus vehicle) pretreated WT, there was no change in mean response (Fig. 4d). There was no effect of ICV propranolol (versus vehicle) pretreatment on baseline body temperature measured before IP harmine treatment (p > 0.05; data not shown).
Fig. 4.
Twenty-four-hour ICV propranolol (versus vehicle) pretreatment exacerbated harmine-induced hypothermia in TG. a Twenty-four hours after ICV propranolol or vehicle pretreatment, harmine treatment was administered and change in body temperature from baseline was recorded for 90 min in TG and WT. b AUC0–90 of ICV propranolol or vehicle-pretreated TG and WT. Mean response of ICV propranolol or vehicle-pretreated c TG and d WT; values are superimposed on dose response data from Fig. 2a, b, respectively. Propranolol versus vehicle-pretreated TG: ***p < 0.001, ****p < 0.0001; vehicle-pretreated WT versus TG: §p <0.05. Vehvehicle, Prl propranolol, SEM standard error of the mean, AUC area under the curve
Hypothermia was more severe in vehicle-pretreated WT compared with TG (mouse line, F(1, 47) = 8.09, p =0.0066) (Fig. 4a). The AUC was greater in vehicle-pretreated WT compared with TG (t(38.47) = 2.73, p = 0.0095) (Fig. 4b).
Four-Hour ICV Propranolol Pretreatment Exacerbated Harmine-Induced Tremor in TG, But Not in WT
Four-hour ICV propranolol (versus vehicle) pretreatment increased harmine-induced tremor in TG (pretreatment, F(1, 15) = 24.3, p = 0.0002), but not in WT (Fig. 5a). There was a main effect of pretreatment on tremor, evaluated by AUC (F(1, 22) = 9.61; p = 0.0052), due to propranolol (versus vehicle) pretreatment increasing AUC0–90 in TG(t(15) = 4.96,p= 0.0004) (Fig. 5b). In propranolol (versus vehicle) pretreated TG, the increase in mean response (t(15) = 4.82, p = 0.0004) corresponded to a 1.4-fold increase in apparent dose (Fig. 5c). In propranolol (versus vehicle) pretreated WT, there was no change in mean response (Fig. 5d). Propranolol (versus vehicle) pretreatment also increased peak tremor (measured at 15 min after harmine injection) in TG (t(15) = 4.20, p = 0.0008) (Online Resource 2a), but not in WT (Online Resource 2b).
Fig. 5.
Four-hour ICV propranolol (versus vehicle) pretreatment exacerbated harmine-induced tremor in TG. a Four hours after ICV propranolol or vehicle pretreatment, harmine treatment was administered, and tremor was scored for 90 min in TG and WT. b AUC0–90 of ICV propranolol or vehicle-pretreated TG and WT. Mean response of ICV propranolol or vehicle-pretreated c TG and d WT; values are superimposed on dose-response data from Fig. 2c, d, respectively. Propranolol versus vehicle-pretreated TG: ***p < 0.001, ****p < 0.0001; vehicle-pretreated WT versus TG: §§p < 0.01, §§§§p < 0.0001. Veh vehicle, Prl propranolol, SEM standard error of the mean, AUC area under the curve
Tremor was more severe in vehicle-pretreated WT compared with TG (mouse line, F(1, 22) = 20.4, p = 0.0002) (Fig. 5a). There was a main effect of mouse line on tremor, evaluated by AUC0–90 (F(1,22)=13.6,p=0.0013),due to AUC0–90 being greater in vehicle-pretreated WT compared with TG (t(22) = 4.38, p = 0.0002) (Fig. 5b).
Twenty-Four-Hour ICV Propranolol Pretreatment Did Not Alter Harmine-Induced Tremor in TG or WT
Twenty-four-hour ICV propranolol (versus vehicle) pretreatment had no effect on harmine-induced tremor in TG or in WT (Fig. 6a). Propranolol (versus vehicle) pretreatment had no effect on tremor, evaluated by AUC0–90 (Fig. 6b) or on mean response of either TG or WT (Fig. 6c, d). Propranolol (versus vehicle) pretreatment trended towards increasing peak tremor in TG (t(24) = 1.77, p = 0.0887) (Online Resource 2c), but not in WT (Online Resource 2d).
Fig. 6.
Twenty-four-hour ICV propranolol (versus vehicle) pretreatment did not alter harmine-induced tremor. a Twenty-four hours after ICV propranolol or vehicle pretreatment, harmine treatment was administered, and tremor was scored for 90 min in TG and WT. b AUC0–90 of propranolol or vehicle-pretreated TG and WT. Mean response of propranolol or vehicle-pretreated c TG and d WT; values are superimposed on dose-response data from Fig. 2c, d, respectively. Vehicle-pretreated WT versus TG: §p <0.05,§§p < 0.01. Veh vehicle, Prl propranolol, SEM standard error of the mean; AUC area under the curve
Tremor was more severe in vehicle-pretreated WT compared with TG (mouse line, F(1, 47) = 7.48, p = 0.0088) (Fig. 6a). There was a main effect of mouse line on tremor, measured by AUC0–90 (F(1, 47) = 15.5, p = 0.0003), due to AUC0–90 being greater in vehicle-pretreated WT compared with TG (t(47) = 2.73, p = 0.0089) (Fig. 6b).
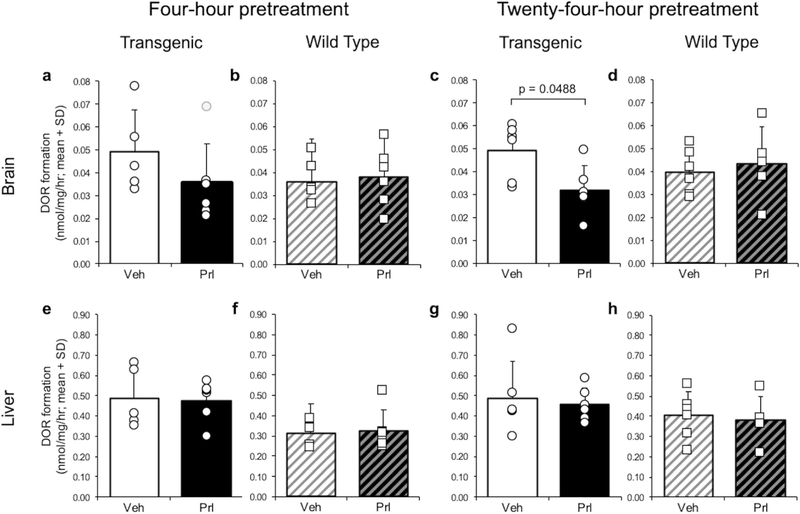
Effect of ICV Pretreatment on In Vitro CYP2D Enzymatic Activity in the Brain and in the Liver
Four-hour ICV propranolol (versus vehicle) pretreatment yielded a non-significant decrease in in vitro brain dextrorphan formation in TG (t(9) = 1.28, p = 0.2312); removal of one outlier in the propranolol group revealed a significant difference (t(8) = 2.32, p = 0.0488) (Fig. 7a). There was no effect of 4-h ICV propranolol (versus vehicle) pretreatment in WT (Fig. 7b). Twenty-four-hour ICV propranolol (versus vehicle) pretreatment reduced in vitro brain dextrorphan formation in TG (t(10) = 2.63, p = 0.0253) (Fig. 7c), but not in WT (Fig. 7d). Taken together, the effects of the 4-h and 24-h pretreatments are consistent with ICV propranolol irreversibly inhibiting human CYP2D6 expressed in TG brain, but not irreversibly inhibiting mouse CYP2D in TG or WT brain. There was no effect of 4- or 24-h ICV propranolol (versus vehicle) pretreatment on in vitro liver dextrorphan formation in TG or WT (Fig. 7e–h), consistent with ICV propranolol not crossing into the peripheral system in sufficient amounts to inhibit CYP2D6 in TG liver or mouse CYP2D in TG and WT liver.
Fig. 7.
ICV propranolol (versus vehicle) pretreatment reduced in vitro brain CYP2D activity in TG. In vitro brain dextrorphan formation after 4-h ICV propranolol or vehicle pretreatment in a TG (outlier in gray) and b WT, and after 24-h ICV pretreatment in c TG and d WT. In vitro liver dextrorphan formation after 4-h ICV propranolol or vehicle pretreatment in e TG and f WT, and after 24-h ICV pretreatment in g TG and h WT. Data were normalized to the vehicle-pretreated TG group in the 4-h ICV pretreatment experiment. DOR dextrorphan, Veh vehicle, Prl propranolol, SD standard deviation
Discussion
To study the role of CYP2D in general within the brain in harmine-induced hypothermia and tremor responses, a 4-h pretreatment with ICV propranolol was given. This 4-h ICV propranolol pretreatment, which irreversibly inhibits human CYP2D6 in TG brain and competitively inhibits mouse CYP2D in TG and WT brain, exacerbated harmine-induced hypothermia and tremor in TG and exacerbated harmine-induced hypothermia in WT. This indicates a role for CYP2D in general in the brain to alter this neurotoxicity. To then study the role of human CYP2D6 specifically within the brain in harmine-induced hypothermia and tremor responses, a 24-h pretreatment with ICV propranolol was given. This 24-h ICV propranolol pretreatment, which selectively and irreversibly inhibits human CYP2D6 in TG brain, exacerbated harmine-induced hypothermia in TG. This study is the first demonstration that human CYP2D6 specifically, expressed in mammalian brain, has sufficient activity in vivo to mitigate neurotoxin-induced response.
Beta-carbolines and their precursors are consumed in animal protein and some plant-derived foods [19, 20], as well as in alcoholic beverages and through tobacco smoke inhalation [20]; beta-carbolines can also be formed endogenously and are detected at picogram to nanogram concentrations in various human tissues [22, 44–46]. Beta-carbolines can be neurotoxic in vitro and in vivo [47, 48]. Many beta-carbolines are structurally similar to MPTP and can be bioactivated in the brain to N-methyl-beta-carbolinium cations [46], which are analogs of the Parkinsonism-inducing neurotoxic metabolite MPP+ [10, 49]. The CYP2D6-mediated detoxification of beta-carbolines could provide an alternative protective route in competition with a bioactivation pathway. Elevated exposure to beta-carbolines, either through consumption or endogenous production, may contribute to the etiology of some neurodegenerative disorders [21, 50]. Harmine is a beta-carboline and a metabolite of harmane[51], and both are present in human tissue [45]. Harmane levels are higher in those with essential tremor [23]and Parkinson’s disease [52]. In animals, harmine causes hypothermia [24] and tremor [25]. Human CYP2D6 and mouse CYP2D catalyze the O-demethylation of harmine to its non-hypothermic and non-tremorgenic metabolite harmol [7].
Harmine-induced hypothermia is centrally mediated, with some evidence for the involvement of a serotonergic pathway in the hypothalamus [24, 53]. Many neurotoxins, including MPTP and chlorpyrifos, induce hypothermia via central mechanisms [54, 55]. A 4-h ICV propranolol pretreatment exacerbated harmine-induced hypothermia in TG and WT. Of note, this pretreatment had no effect on body temperature measured after IP treatment with distilled water, suggesting a lack of off-target effects of ICV propranolol pretreatment. This 4-h pretreatment effect is consistent with irreversible inhibition of human CYP2D6 in TG brain and competitive inhibition of mouse CYP2D in TG and WT brain, whereby harmine inactivation to harmol was reduced in the brain, exacerbating harmine response in both TG and WT. Although harmine levels were not measured in brain, the findings suggest that CYP2D isoforms in the brain metabolize harmine and that more of this metabolism confers protection against beta-carboline-induced neurotoxicity. Consistent with previous reports, hypothermia was less severe in TG compared with WT [28], owing to their expression of human CYP2D6 in addition to mouse CYP2D in both liver and brain, yielding more rapid inactivation of harmine to harmol [7]. Pretreatment with ICV propranolol does not inhibit hepatic metabolism or alter peripheral drug levels [34], meaning ICV propranolol-pretreated TG (versus WT) presumably have more rapid harmine metabolism in the liver due to human CYP2D6 and mouse CYP2D activity in TG liver. Nevertheless, hypothermia response did not differ between propranolol-pretreated TG and WT, suggesting that harmine metabolism in the brain may be a more important determinant of hypothermia severity than metabolism in the liver.
A 24-h ICV propranolol pretreatment irreversibly inhibits human CYP2D6 in TG brain and has no effect on mouse CYP2D in TG or WT brain. This pretreatment increased harmine-induced hypothermia in TG and had no effect in WT, used to control for the off-target effects of 24-h ICV propranolol pretreatment. The impact of 24-h pretreatment on TG (versus WT) is consistent with reduced CYP2D6-mediated harmine inactivation to harmol in TG brain exacerbating response, providing further evidence that harmine metabolism in the brain (versus the liver) is an important determinant of hypothermia severity. This suggests that human CYP2D6, expressed in TG brain, contributes significantly to harmine inactivation and is sufficient to mitigate this measure of beta-carboline-induced neurotoxicity.
Essential tremor is the most common neurodegenerative movement disorder, affecting approximately 5% of those over the age of 65 [56]. Beta-carbolines have been causally implicated in essential tremor etiology [57], as levels in blood [58] and in the brain [23] were elevated in those with essential tremor compared with healthy controls. Furthermore, among a group of individuals with essential tremor, beta-carboline levels in blood were associated with lower metabolic function, indicating more degeneration, in the cerebellum [21]. In fact, cerebellar degeneration is a common feature of essential tremor [59] and of beta-carboline administration in animal models [60]. Beta-carbolines, including harmine and its congener harmaline, are commonly used in rodents to model essential tremor [57, 61].
The 4-h ICV propranolol pretreatment, which irreversibly inhibits human CYP2D6 and competitively inhibits mouse CYP2D in TG brain, increased harmine-induced tremor in TG. This is consistent with inhibition of CYP2D6- and CYP2D-mediated harmine inactivation in the brain exacerbating harmine response. Contrary to our hypothesis, 4-h pretreatment did not exacerbate tremor in WT. In our dose-response experiments, a harmine dose of 7.5 mg/kg IP (used in this experiment) produced a near-maximal tremor response in WT, indicating a potential ceiling effect. Additionally, the observational tremor scale may lack the sensitivity to detect subtle differences in tremor severity. Therefore, it may not have been possible to detect an increase in tremor severity following 4-h pretreatment in WT, especially if the effect was not robust.
Following a 24-h ICV propranolol pretreatment, which selectively and irreversibly inhibits human CYP2D6 in TG brain, we observed an increase in peak tremor that trended towards significance in TG, suggesting that inhibiting CYP2D6-mediated harmine inactivation to harmol in the brain may enhance tremor response. Although we did not observe as large of an effect on tremor of the 24-h pretreatment compared with the 4-h pretreatment, this is consistent with a lesser effect of inhibiting human CYP2D6 but not mouse CYP2D in TG brain, due to the uninhibited mouse CYP2D in TG brain metabolizing harmine. Nevertheless, the smaller effect of 24-h pretreatment on harmine-induced tremor response contrasts with the robust effect on harmine-induced hypothermia. The difference between hypothermia and tremor may be due to these responses being generated via discrete central mechanisms, which may be differentially impacted by local CYP2D and/or CYP2D6 metabolism in the brain. Thus, peripheral drug levels and hepatic metabolism, which are not affected by pretreatment [34], may have a greater impact on harmine-induced tremor severity, in contrast with the greater impact of brain drug levels and brain metabolism on harmine-induced hypothermia response.
Propranolol is used as a treatment for essential tremor in humans and, given as a 30-min pretreatment, it suppresses harmine-induced tremor in rodents [62]. After a 24-h ICV pretreatment, propranolol does not remain in the brain, due to its ∼ 2-h half-life in mouse brain [63], and should have no direct suppressant effect on harmine-induced tremor. Given as a 4-h pretreatment, propranolol remains in the brain and, if in sufficient amounts, would have suppressed tremor. The 4-h ICV propranolol pretreatment enhanced tremor in TG, suggesting that the tremor-suppressant effect was diminished after 4 h, or that the enhancement of tremor superseded this effect. Propranolol pretreatments could also have indirectly altered harmine responses via inhibition of CYP2D6- and/or CYP2D-mediated metabolism of endogenous compounds. For example, CYP2D isozymes may contribute to alternative pathways of serotonin and/or dopamine synthesis in the brain, which has been tested under specific conditions [64, 65]. Reduced serotonin and dopamine signaling would attenuate harmine-induced hypothermia and tremor [24, 66]; thus, inhibiting CYP2D6 and/or CYP2D in TG and WT brain, if sufficient to meaningfully reduce serotonin and dopamine synthesis, should have reduced harmine-induced hypothermia and tremor severity. However, inhibitor pretreatment exacerbated these responses, consistent with propranolol inhibiting harmine metabolism and increasing harmine in the brain. Although brain drug levels were not measured, we used several controls that, taken together, suggest there were no relevant off-target effects of propranolol on body temperature in general, or on harmine-induced hypothermia and tremor.
CYP2D6 metabolism constitutes an inactivation pathway for several neurotoxins, suggesting a protective role for CYP2D6 for these toxins [5–7]. Genetic CYP2D6 poor metabolizers are at higher risk for developing Parkinson’s disease [12], and this risk is increased further by lifetime exposure to environmental toxins, which are causally implicated in idiopathic Parkinson’s disease, as well as being CYP2D6 substrates [67, 68]. This suggests a gene-environment interaction whereby CYP2D6-mediated inactivation following xenobiotic neurotoxin exposure reduces neurotoxicity and subsequent neurodegeneration.
Human CYP2D6 expressed in the TG brain was sufficient to mitigate specific neurotoxicity-related responses to harmine, despite harmine being administered peripherally and undergoing first-pass metabolism. This suggests that CYP2D6 expressed in the human brain may play a role in protecting against beta-carboline-induced neurotoxicity, even in the case of peripheral exposure, such as that occurring through consumption of certain foods and beverages. The large interindividual variation in CYP2D6 levels in the human brain [33] may influence local metabolism of beta-carbolines and other neurotoxins, which could then impact the severity of neurotoxicity and resultant neurodegeneration. CYP2D6 is expressed in brain regions that support a role in protecting against essential tremor (cerebellum) and Parkinson’s disease (substantia nigra and caudate-putamen) [33, 69]. Despite CYP2D in the liver being uninducible, CYP2D in the brain can be induced by xenobiotic exposure. For example, repeated daily nicotine administration increases CYP2D in the brain (but not in the liver) of mice [70], rats [18], and non-human primates [17]. Consistent with this, human cigarette smokers have more CYP2D6 in the brain compared with non-smoking controls [17]. Furthermore, CYP2D6 levels were higher in the cerebellum and substantia nigra [17]; neurodegeneration in these regions is associated with essential tremor [59] and Parkinson’s disease [71]. Cigarette smoking is associated with decreased risk for essential tremor [72]and Parkinson’s disease [73], suggesting that the neuroprotective effect of smoking may be due, at least in part, to higher CYP2D6 in the brain increasing local neurotoxin inactivation. This could confer protection against neurotoxicity and the subsequent neurodegeneration characteristic of these neurodegenerative disorders.
CYP2D6 metabolism in the human brain is of increasing interest. CYP2D6 is expressed in neurons and glia within discrete brain regions [13, 33], and its substrates include centrally acting endogenous compounds. CYP2D6 was shown in vitro to catalyze the 6β-, 21-, and 16α-hydroxylation of progesterone [74], as well as the formation of dopamine from tyramine [75] and serotonin from 5-methoxytryptamine, with evidence for the latter occurring in vivo [65, 76]. Variation in CYP2D6 in the brain may impact neurological, cognitive, and psychosocial function, as evidenced by their associations with CYP2D6 genotype and central mechanisms. For example, the genotype-derived CYP2D6 activity score was found to be inversely related with cerebral blood flow in the thalamus, where CYP2D6 is expressed [13, 77]. Genetic CYP2D6 poor metabolizers scored higher on measures of impulsiveness, perfectionism, and sustained attention, and they scored lower on general psychopathology, as assessed in a series of personality and cognitive tests [78]. Genetic CYP2D6 extensive and ultra-rapid metabolizers were also more frequent among patients with eating disorders compared with healthy controls [79]. Thus, the ability to selectively manipulate human CYP2D6 in a mouse brain and use WT as controls, as applied herein, provides a novel tool to investigate these and other endogenous functions of CYP2D6 in the brain, as well as its impact on various cognitive and behavioral outcomes.
Using this novel approach, we demonstrated previously that CYP2D6 is functional in vivo and can alter brain drug and metabolite levels, as well as drug response [34]. Here, we show for the first time that human CYP2D6 in brain can also alter neurotoxin response following peripheral beta-carboline administration; specifically, CYP2D6 in brain mitigated harmine-induced neurotoxicity. In conclusion, CYP2D6 in the human brain may confer some neuroprotection against beta-carboline-induced neurotoxicity. This represents a potential novel source of variation in susceptibility to neurotoxicity and to neurodegenerative disorders that are associated with elevated neurotoxin (e.g., beta-carboline) exposure, including essential tremor and Parkinson’s disease. More broadly, this model can be used to test novel neurotoxins and centrally acting drugs for the impact of CYP2D, and human CYP2D6 specifically, in the brain on resulting behaviors in vivo.
Supplementary Material
Acknowledgments
This research was undertaken, in part, thanks to funding from the Canada Research Chairs program (Dr. Tyndale, the Canada Research Chair in Pharmacogenomics), the Canadian Institutes of Health Research (foundation grant FDN-154294 and MOP 136937), the Centre for Addiction and Mental Health and the CAMH Foundation, and the National Institutes of Health Intramural Research Program (ZIA BC005708). We also acknowledge the support of Dr. Bin Zhao for his technical assistance with the LC-MS/MS and Qian Zhou for genotyping the mice.
Footnotes
Conflict of Interest R.F.T. has consulted for Quinn Emanuel and Ethismos Research Inc. All other authors declare no conflict of interest.
Compliance with Ethical Standards
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12035-020-02050-w) contains supplementary material, which is available to authorized users.
Data and Material Availability Supporting data and material can be found in the additional files and can be requested from the corresponding author.
Ethics Approval All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Experiments were performed in accordance with the NIH guidelines for the care and use of laboratory animals, and with approval of the University of Toronto Animal Care Committee.
References
- 1.Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138(1):103–141. 10.1016/j.pharmthera.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 2.Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14(1):1–18. 10.1097/00008571-200401000-00001 [DOI] [PubMed] [Google Scholar]
- 3.Bertilsson L, Dahl ML, Dalen P, Al-Shurbaji A (2002) Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol 53(2): 111–122. 10.1046/j.0306-5251.2001.01548.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingelman-Sundberg M (2005) Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J 5(1):6–13. 10.1038/sj.tpj.6500285 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Fujita S, Narimatsu S, Masubuchi Y, Tachibana M, Ohta S, Hirobe M (1992) Cytochrome P450 isozymes catalyzing 4hydroxylation of parkinsonism-related compound 1,2,3,4tetrahydroisoquinoline in rat liver microsomes. FASEB J 6(2): 771–776. 10.1096/fasebj.6.2.1537468 [DOI] [PubMed] [Google Scholar]
- 6.Modi S, Gilham DE, Sutcliffe MJ, Lian LY, Primrose WU, Wolf CR, Roberts GC (1997) 1-methyl-4-phenyl-1,2,3,6tetrahydropyridine as a substrate of cytochrome P450 2D6: allosteric effects of NADPH-cytochrome P450 reductase. Biochemistry 36(15):4461–4470. 10.1021/bi962633p [DOI] [PubMed] [Google Scholar]
- 7.Yu AM, Idle JR, Krausz KW, Kupfer A, Gonzalez FJ (2003) Contribution of individual cytochrome P450 isozymes to the O-demethylation of the psychotropic beta-carboline alkaloids harmaline and harmine. J Pharmacol Exp Ther 305(1):315–322. 10.1124/jpet.102.047050 [DOI] [PubMed] [Google Scholar]
- 8.Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219(4587):979–980. 10.1126/science.6823561 [DOI] [PubMed] [Google Scholar]
- 9.Nagatsu T (1997) Isoquinoline neurotoxins in the brain and Parkinson’s disease. Neurosci Res 29(2):99–111. 10.1016/s0168-0102(97)00083-7 [DOI] [PubMed] [Google Scholar]
- 10.Collins MA, Neafsey EJ, Matsubara K, Cobuzzi RJ Jr, Rollema H (1992) Indole-N-methylated beta-carbolinium ions as potential brain-bioactivated neurotoxins. Brain Res 570(1–2): 154–160. https://d0i.0rg/l0.1016/0006-8993(92)90576-u [DOI] [PubMed] [Google Scholar]
- 11.Bon MA, Jansen Steur EN, de Vos RA, Vermes I (1999) Neurogenetic correlates of Parkinson’s disease: apolipoprotein-E and cytochrome P450 2D6 genetic polymorphism. Neurosci Lett 266(2): 149–151. 10.1016/s0304-3940(99)00278-5 [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Mo C, Zeng Z, Chen S, Xie Y, Peng Q, He Y, Deng Y et al. (2013) CYP2D6*4 allele polymorphism increases the risk of Parkinson’s disease: evidence from meta-analysis. PLoS One 8(12):e84413. 10.1371/joumal.pone.0084413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinta SJ, Pai HV, Upadhya SC, Boyd MR, Ravindranath V (2002) Constitutive expression and localization of the major drug metabolizing enzyme, cytochrome P4502D in human brain. Brain Res Mol Brain Res 103(1–2):49–61. 10.1016/s0169328x(02)00177-8 [DOI] [PubMed] [Google Scholar]
- 14.Voirol P, Jonzier-Perey M, Porchet F, Reymond MJ, Janzer RC, Bouras C, Strobel HW, Kosel M et al. (2000) Cytochrome P-450 activities in human and rat brain microsomes. Brain Res 855(2): 235–243. 10.1016/s0006-8993(99)02354-9 [DOI] [PubMed] [Google Scholar]
- 15.McMillan DM, Miksys S, Tyndale RF (2019) Rat brain CYP2D activity alters in vivo central oxycodone metabolism, levels and resulting analgesia. Addict Biol 24(2):228–238. 10.1111/adb.12590 [DOI] [PubMed] [Google Scholar]
- 16.Miksys S, Wadji FB, Tolledo EC, Remington G, Nobrega JN, Tyndale RF (2017) Rat brain CYP2D enzymatic metabolism alters acute and chronic haloperidol side-effects by different mechanisms. Prog Neuro-Psychopharmacol Biol Psychiatry 78:140–148. 10.1016/j.pnpbp.2017.04.030 [DOI] [PubMed] [Google Scholar]
- 17.Mann A, Miksys S, Lee A, Mash DC, Tyndale RF (2008) Induction of the drug metabolizing enzyme CYP2D in monkey brain by chronic nicotine treatment. Neuropharmacology 55(7): 1147–1155. 10.1016/j.neuropharm.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue J, Miksys S, Hoffmann E, Tyndale RF (2008) Chronic nicotine treatment induces rat CYP2D in the brain but not in the liver: an investigation of induction and time course. J Psychiatry Neurosci 33(1):54–63 [PMC free article] [PubMed] [Google Scholar]
- 19.Herraiz T (2000) Tetrahydro-beta-carboline-3-carboxylic acid compounds in fish and meat: possible precursors of co-mutagenic beta-carbolines norharman and harman in cooked foods. Food Addit Contam 17(10):859–866. 10.1080/026520300420439 [DOI] [PubMed] [Google Scholar]
- 20.Herraiz T (2004) Relative exposure to beta-carbolines norharman and harman from foods and tobacco smoke. Food Addit Contam 21(11):1041–1050. 10.1080/02652030400019844 [DOI] [PubMed] [Google Scholar]
- 21.Louis ED, Zheng W, Mao X, Shungu DC (2007) Blood harmane is correlated with cerebellar metabolism in essential tremor: a pilot study. Neurology 69(6):515–520. 10.1212/01.wnl.0000266663.27398.9f [DOI] [PubMed] [Google Scholar]
- 22.Kuhn W, Muller T, Grosse H, Rommelspacher H (1996) Elevated levels of harman and norharman in cerebrospinal fluid of parkinsonian patients. J Neural Transm (Vienna) 103(12):1435–1440. 10.1007/BF01271257 [DOI] [PubMed] [Google Scholar]
- 23.Louis ED, Factor-Litvak P, Liu X, Vonsattel JP, Galecki M, Jiang W, Zheng W (2013) Elevated brain harmane (1-methyl-9H-pyrido[3,4-b]indole) in essential tremor cases vs. controls. Neurotoxicology 38:131–135. 10.1016/j.neuro.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Fattah AF, Matsumoto K, Gammaz HA, Watanabe H (1995) Hypothermic effect of harmala alkaloid in rats: involvement of serotonergic mechanism. Pharmacol Biochem Behav 52(2):421–426. 10.1016/0091-3057(95)00131-f [DOI] [PubMed] [Google Scholar]
- 25.Kelly DM, Naylor RJ (1974) Mechanisms of tremor induction by harmine. Eur J Pharmacol 27(1):14–24. 10.1016/0014-2999(74)90197-6 [DOI] [PubMed] [Google Scholar]
- 26.Wilms H, Sievers J, Deuschl G (1999) Animal models of tremor. Mov Disord 14(4):557–571. [DOI] [PubMed] [Google Scholar]
- 27.Herraiz T, Guillen H, Aran VJ (2008) Oxidative metabolism of the bioactive and naturally occurring beta-carboline alkaloids, norharman and harman, by human cytochrome P450 enzymes. Chem Res Toxicol 21(11):2172–2180. 10.1021/tx8002565 [DOI] [PubMed] [Google Scholar]
- 28.Wu C, Jiang XL, Shen HW, Yu AM (2009) Effects of CYP2D6 status on harmaline metabolism, pharmacokinetics and pharmacodynamics, and a pharmacogenetics-based pharmacokinetic model. Biochem Pharmacol 78(6):617–624. 10.1016/j.bcp.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng J, Zhen Y, Miksys S, Beyoglu D, Krausz KW, Tyndale RF, Yu A, Idle JR et al. (2013) Potential role of CYP2D6 in the central nervous system. Xenobiotica 43(11):973–984. 10.3109/00498254.2013.791410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moncrieff J (1989) Determination of pharmacological levels of harmane, harmine and harmaline in mammalian brain tissue, cerebrospinal fluid and plasma by high-performance liquid chromatography with fluorimetric detection. J Chromatogr 496(2):269–278. 10.1016/s0378-4347(00)82576-1 [DOI] [PubMed] [Google Scholar]
- 31.Mann A, Tyndale RF (2010) Cytochrome P450 2D6 enzyme neuroprotects against 1-methyl-4-phenylpyridinium toxicity in SH-SY5Y neuronal cells. Eur J Neurosci 31(7):1185–1193. 10.1111/j.1460-9568.2010.07142.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matoh N, Tanaka S, Takehashi M, Banasik M, Stedeford T, Masliah E, Suzuki S, Nishimura Y et al. (2003) Overexpression of CYP2D6 attenuates the toxicity of MPP+ in actively dividing and differentiated PC12 cells. Gene Expr 11(3–4):117–124. 10.3727/000000003108749017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mann A, Miksys SL, Gaedigk A, Kish SJ, Mash DC, Tyndale RF (2012) The neuroprotective enzyme CYP2D6 increases in the brain with age and is lower in Parkinson’s disease patients. Neurobiol Aging 33(9):2160–2171. 10.1016/j.neurobiolaging.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 34.Tolledo C, Stocco MR, Miksys S, Gonzalez FJ, Tyndale RF (2020) Human CYP2D6 is functional in brain in vivo: evidence from humanized CYP2D6 transgenic mice. Mol Neurobiol 57:2509–2520. https://d0i.0rg/l0.1007/sl2035-020-01896-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolledo EC, Miksys S, Gonzalez FJ, Tyndale RF (2020) Propranolol is a mechanism-based inhibitor of CYP2D and CYP2D6 in humanized CYP2D6-transgenic mice: Effects on activity and drag responses. Br J Pharmacol 177(3):701–712. 10.1ll1/bph.14884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franklin KBJ, Paxinos G (2013) Paxinos and Franklin's The mouse brain in stereotaxic coordinates. Fourth edition. Academic Press, an imprint of Elsevier, Amsterdam [Google Scholar]
- 37.DeVos SL, Miller TM (2013) Direct intraventricular delivery of drugs to the rodent central nervous system. J Vis Exp 75:e50326. 10.3791/50326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khokhar JY, Tyndale RF (2011) Drug metabolism within the brain changes drug response: selective manipulation of brain CYP2B alters propofol effects. Neuropsychopharmacology 36(3):692–700. 10.1038/npp.2010.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miksys S, Rao Y, Sellers EM, Kwan M, Mendis D, Tyndale RF (2000) Regional and cellular distribution of CYP2D subfamily members in rat brain. Xenobiotica 30(6):547–564. 10.1080/004982500406390 [DOI] [PubMed] [Google Scholar]
- 40.Siu EC, Wildenauer DB, Tyndale RF (2006) Nicotine selfadministration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology 184(3–4):401–408. 10.1007/s00213-006-0306-6 [DOI] [PubMed] [Google Scholar]
- 41.Tyndale RF, Li Y, Li NY, Messina E, Miksys S, Sellers EM (1999) Characterization of cytochrome P-450 2D1 activity in rat brain: high-affinity kinetics for dextromethorphan. Drug Metab Dispos 27(8):924–930. [PubMed] [Google Scholar]
- 42.Schmid B, Bircher J, Preisig R, Kupfer A (1985) Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther 38(6):618–624. 10.1038/clpt.1985.235 [DOI] [PubMed] [Google Scholar]
- 43.Kerry NL, Somogyi AA, Bochner F, Mikus G (1994) The role of CYP2D6 in primary and secondary oxidative metabolism of dextromethorphan: in vitro studies using human liver microsomes. Br J Clin Pharmacol 38(3):243–248. 10.1111/j.13652125.1994.tb04348.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fekkes D, Tuiten A, Bom I, Pepplinkhuizen L (2001) Tryptophan: a precursor for the endogenous synthesis of norharman in man. Neurosci Lett 303(3):145–148. 10.1016/s03043940(01)01750-5 [DOI] [PubMed] [Google Scholar]
- 45.Zheng W, Wang S, Barnes LF, Guan Y, Louis ED (2000) Determination of harmane and harmine in human blood using reversed-phased high-performance liquid chromatography and fluorescence detection. Anal Biochem 279(2): 125–129. 10.1006/abio.1999.4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsubara K, Collins MA, Akane A, Ikebuchi J, Neafsey EJ, Kagawa M, Shiono FI (1993) Potential bioactivated neurotoxicants, N-methylated beta-carbolinium ions, are present in human brain. Brain Res 610(1):90–96. 10.1016/0006-8993(93)91221-d [DOI] [PubMed] [Google Scholar]
- 47.Cobuzzi RJ Jr, Neafsey EJ, Collins MA (1994) Differential cytotoxicities of N-methyl-beta-carbolinium analogues of MPP+ in PC12 cells: insights into potential neurotoxicants in Parkinson’s disease. J Neurochem 62(4): 1503–1510. 10.1046/j.1471-4159.1994.62041503.x [DOI] [PubMed] [Google Scholar]
- 48.Ostergren A, Fredriksson A. Brittebo EB (2006) Norharmaninduced motoric impairment in mice: neurodegeneration and glial activation in substantia nigra. J Neural Transm (Vienna) 113(3): 313–329. 10.1007/s00702-005-0334-0 [DOI] [PubMed] [Google Scholar]
- 49.Albores R, Neafsey EJ, Drucker G, Fields JZ, Collins MA (1990) Mitochondrial respiratory inhibition by N-methylated beta-carboline derivatives structurally resembling N-methyl-4phenylpyridine. Proc Natl Acad Sci U S A 87(23):9368–9372. 10.1073/pnas.87.23.9368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsubara K, Gonda T, Sawada H, Uezono T, Kobayashi Y, Kawamura T, Ohtaki K, Kimura K et al. (1998) Endogenously occurring beta-carboline induces parkinsonism in nonprimate animals: a possible causative protoxin in idiopathic Parkinson’sdis-ease. J Neurochem 70(2):727–735. 10.1046/j.1471-4159.1998.70020727.x [DOI] [PubMed] [Google Scholar]
- 51.Guan Y, Louis ED, Zheng W (2001) Toxicokinetics of tremorogenic natural products, harmane and harmine, in male Sprague-Dawley rats. J Toxicol Environ Health A 64(8):645–660. 10.1080/152873901753246241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Louis ED, Michalec M, Jiang W, Factor-Litvak P, Zheng W (2014) Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in Parkinson’s disease. Neurotoxicology 40:52–56. 10.1016/j.neuro.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang XL, Shen HW, Yu AM (2015) Potentiation of 5-methoxy-N, N-dimethyltryptamine-induced hyperthermia by harmaline and the involvement of activation of 5-HT1A and 5-HT2A receptors. Neuropharmacology 89:342–351. 10.1016/j.neuropharm.2014.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satoh N, Yonezawa A, Tadano T, Kisara K, Arai Y, Kinemuchi H (1987) Acute effects of a parkinsonism-inducing neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on mouse body temperature. Life Sci 41(11):1415–1424. 10.1016/0024-3205(87)90617-5 [DOI] [PubMed] [Google Scholar]
- 55.Gordon CJ, Grantham TA (1999) Effect of central and peripheral cholinergic antagonists on chlorpyrifos-induced changes in body temperature in the rat. Toxicology 142(1):15–28. 10.1016/s0300-483x(99)00121-3 [DOI] [PubMed] [Google Scholar]
- 56.Louis ED, Ferreira JJ (2010) How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 25(5):534–541. 10.1002/mds.22838 [DOI] [PubMed] [Google Scholar]
- 57.Louis ED (2008) Environmental epidemiology of essential tremor. Neuroepidemiology 31(3):139–149. 10.1159/000151523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P (2005) Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology 65(3):391–396. 10.1212/01.wnl.0000172352.88359.2d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D (2002) Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett 333(1):17–20. 10.1016/s0304-3940(02)00966-7 [DOI] [PubMed] [Google Scholar]
- 60.O'Hearn E, Molliver ME (1993) Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience 55(2):303–310 [DOI] [PubMed] [Google Scholar]
- 61.Miwa H (2007) Rodent models of tremor. Cerebellum 6(1):66–72. 10.1080/14734220601016080 [DOI] [PubMed] [Google Scholar]
- 62.Kulkarni SK, Kaul PN (1979) Modification by levo-propranolol of tremors induced by harmine in mice. Experientia 35(12):1627–1628. 10.1007/BF01953232 [DOI] [PubMed] [Google Scholar]
- 63.Levy A, Ngai SH, Finck AD, Kawashima K, Spector S (1976) Disposition of propranolol isomers in mice. Eur J Pharmacol 40(1):93–100. 10.1016/0014-2999(76)90358-7 [DOI] [PubMed] [Google Scholar]
- 64.Bromek E, Haduch A, Golembiowska K, Daniel WA (2011) Cytochrome P450 mediates dopamine formation in the brain in vivo. J Neurochem 118(5):806–815. 10.1111/j.1471-4159.2011.07339.x [DOI] [PubMed] [Google Scholar]
- 65.Haduch A, Bromek E, Kot M, Kaminska K, Golembiowska K, Daniel WA (2015) The cytochrome P450 2D-mediated formation of serotonin from 5-methoxytryptamine in the brain in vivo: a microdialysis study. J Neurochem 133(1):83–92. 10.1111/jnc.13031 [DOI] [PubMed] [Google Scholar]
- 66.Arshaduddin M, Al Kadasah S, Biary N, Al Deeb S, Al Moutaery K, Tariq M (2004) Citalopram, a selective serotonin reuptake inhibitor augments harmaline-induced tremor in rats. Behav Brain Res 153(1):15–20. 10.1016/j.bbr.2003.10.035 [DOI] [PubMed] [Google Scholar]
- 67.Anwarullah AM, Badshah M, Abbasi R, Sultan A, Khan K, Ahmad N, von Engelhardt J (2017) Further evidence for the association of CYP2D6*4 gene polymorphism with Parkinson’sdisease:acase control study. Genes Environ 39:18. 10.1186/s41021-017-0078-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elbaz A, Levecque C, Clavel J, Vidal JS, Richard F, Amouyel P, Alperovitch A, Chartier-Harlin MC et al. (2004) CYP2D6 polymorphism, pesticide exposure, and Parkinson’s disease. Ann Neurol 55(3):430–434. 10.1002/ana.20051 [DOI] [PubMed] [Google Scholar]
- 69.Miksys S, Rao Y, Hoffmann E, Mash DC, Tyndale RF (2002) Regional and cellular expression of CYP2D6 in human brain: higher levels in alcoholics. J Neurochem 82(6):1376–1387. 10.1046/j.1471-4159.2002.01069.x [DOI] [PubMed] [Google Scholar]
- 70.Singh S, Singh K, Patel DK, Singh C, Nath C, Singh VK, Singh RK, Singh MP (2009) The expression of CYP2D22, an ortholog of human CYP2D6, in mouse striatum and its modulation in 1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease phenotype and nicotine-mediated neuroprotection. Rejuvenation Res 12(3):185–197. 10.1089/rej.2009.0850 [DOI] [PubMed] [Google Scholar]
- 71.Alexander GE (2004) Biology of Parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin Neurosci 6(3):259–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benito-Leon J, Louis ED, Bermejo-Pareja F, Neurological Disorders in Central Spain Study G (2008) Population-based case-control study of cigarette smoking and essential tremor. Mov Disord 23(2):246–252. 10.1002/mds.21810 [DOI] [PubMed] [Google Scholar]
- 73.Alves G, Kurz M, Lie SA, Larsen JP (2004) Cigarette smoking in Parkinson’s disease: influence on disease progression. Mov Disord 19(9):1087–1092. 10.1002/mds.20117 [DOI] [PubMed] [Google Scholar]
- 74.Niwa T, Hiroi T, Tsuzuki D, Yamamoto S, Narimatsu S, Fukuda T, Azuma J, Funae Y (2004) Effect of genetic polymorphism on the metabolism of endogenous neuroactive substances, progesterone and p-tyramine, catalyzed by CYP2D6. Brain Res Mol Brain Res 129(1–2):117–123. 10.1016/j.molbrainres.2004.06.030 [DOI] [PubMed] [Google Scholar]
- 75.Hiroi T, Imaoka S, Funae Y (1998) Dopamine formation from tyramine by CYP2D6. Biochem Biophys Res Commun 249(3): 838–843. 10.1006/bbrc.1998.9232 [DOI] [PubMed] [Google Scholar]
- 76.Yu AM, Idle JR, Byrd LG, Krausz KW, Kupfer A, Gonzalez FJ (2003) Regeneration of serotonin from 5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics 13(3):173–181. 10.1097/01.fpc.0000054066.98065.7b [DOI] [PubMed] [Google Scholar]
- 77.Kirchheiner J, Seeringer A, Godoy AL, Ohmle B, Maier C, Beschoner P, Sim EJ, Viviani R (2011) CYP2D6 in the brain: genotype effects on resting brain perfusion. Mol Psychiatry 16(3): 237, 333–241. 10.1038/mp.2010.42 [DOI] [PubMed] [Google Scholar]
- 78.Penas LEM, Dorado P, Pacheco R, Gonzalez I, LLerena A (2009) Relation between CYP2D6 genotype, personality, neurocognition and overall psychopathology in healthy volunteers. Pharmacogenomics 10(7):1111–1120. 10.2217/pgs.09.75 [DOI] [PubMed] [Google Scholar]
- 79.Penas-Lledo EM, Dorado P, Aguera Z, Gratacos M, Estivill X, Fernandez-Aranda F, Llerena A (2012) CYP2D6 polymorphism in patients with eating disorders. Pharmacogenomics J 12(2):173–175. 10.1038/tpj.2010.78 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.