Abstract
Hepatocyte nuclear factor 1α (HNF1α) is a transcription factor required for normal insulin secretion and maintenance of β-cell number in the pancreas. HNF1α is also expressed in pancreatic α-cells, but its role in these cells is unknown. The aim of this study was to clarify the role of HNF1α in α-cells. Male Hnf1a+/− mice with a mixed background were backcrossed to outbred ICR mice. Glucose tolerance, glucagon and insulin secretion, islet histology, and gene expression were investigated in ICR Hnf1a−/− and Hnf1a+/+ mice. Regulation of Slc5a1 (encoding sodium glucose cotransporter 1 [SGLT1]) expression by HNF1α and the effect of SGLT1 inhibition on glucagon secretion were also explored. ICR Hnf1a−/− mice were glucose intolerant and exhibited impaired glucose-stimulated insulin secretion. The β-cell area of ICR mice was decreased in Hnf1a−/− mice, but the α-cell area in the pancreas was similar between Hnf1a−/− and Hnf1a+/+ mice. Hnf1a−/− mice showed higher fasting glucagon levels and exhibited inadequate suppression of glucagon after glucose load. In addition, glucagon release in response to hypoglycemia was impaired in Hnf1a−/− mice, and glucagon secretion after 1.1 mM glucose administration, was also decreased in Hnf1a−/− islets. Slc5a1 expression was decreased in Hnf1a−/− islets, while HNF1α activated the Slc5a1 promoter in αTC1–6 cells. Inhibition of SGLT1 suppressed 1.1 mM glucose-stimulated glucagon secretion in islets and αTC1–6 cells, but SGLT1 inhibition had no additional inhibitor effect in HNF1α-deficient cells. Our findings indicate that HNF1α modulates glucagon secretion α-cells through the regulation of Slc5a1.
Keywords: Hepatocyte nuclear factor (HNF) 1α, Maturity-onset diabetes of the young (MODY), Pancreatic α-cell, Glucagon, Sodium-glucose cotransporter (SGLT)
1. Introduction
Hepatocyte nuclear factor 1α (HNF1α), a transcription factor belonging to the homeodomain superfamily, is expressed in the liver, kidney, intestine, and pancreas (including β-cells) [1]. We identified that heterogenous mutations in the gene encoding HNF1α as a cause of a form of maturity-onset diabetes of the young (MODY3) [2], which is characterized by the progressive impairment of insulin secretion [3,4]. Hnf1α-null (−/−) mice and transgenic mice expressing a naturally occurring human dominant-negative P291fsinsC HNF1A mutation in pancreatic β-cells develop early-onset diabetes with impaired insulin secretion and a reduction of β-cell number [5,6]. These results indicate that HNF1α is required for normal insulin secretion and the maintenance of β-cell number.
Glucagon, which is secreted from pancreatic α-cells, counters the actions of insulin and corrects hypoglycemia by enhancing hepatic glucose output [7,8]. Glucagon secretion is stimulated by hypoglycemia and is suppressed by hyperglycemia and intra-islet paracrine factors, including insulin. We previously demonstrated that HNF1α is also expressed in glucagon-producing pancreatic α-cells [9], and Haliyur et al. [10], recently reported that glucagon release after stimulation with low glucose and epinephrine was abrogated in human islets expressing an HNF1A variant. To clarify the role of HNF1α in pancreatic α-cells, we investigated the number of pancreatic α-cells and glucagon secretion in Hnf1α−/− mice on an outbred ICR background because Hnf1α−/− mice on a C57BL/6J background showed early postnatal lethality. In contrast to the decreased β-cell area in Hnf1a−/− mice, the total area of glucagon-positive α-cells was similar between control and Hnf1a−/− mice. However, Hnf1a−/− mice exhibited defective glucagon secretion at low glucose and impaired suppression of glucagon secretion at high glucose. Sodium glucose cotransporter 1 (SGLT1), a glucose transporter expressed in pancreatic α-cells, controls glucagon secretion [11]. We found that HNF1α regulated the transcription of Slc5a1 (encoding SGLT1) in α-cells, and that SGLT1 expression was decreased in Hnf1a−/− islets. Furthermore, inhibition of SGLT1 suppressed glucagon secretion at low glucose, but SGLT1 inhibition had no additional inhibitory effect in HNF1α-deficient cells. Our findings indicate that HNF1α plays an important role in glucagon secretion in α-cells, and the decreased expression of SGLT1 might be involved, at least in part, in the dysregulated secretion of glucagon.
2. Materials and methods
2.1. Cell culture
Mouse αTC1 clone 6 (αTC1–6, CRL-2934) cells were obtained from the American Type Culture Collection (Manassas, VA). The cells were maintained at 37 °C in a 5% (v/v) CO2 incubator in Dulbecco's modified Eagle's medium containing 25 mM glucose, 44 mM sodium bicarbonate, 10% (v/v) fetal bovine serum, 15 mM HEPES, 0.1 mM nonessential amino acids, and antibiotics (50 U/mL penicillin and 50 U/mL streptomycin). Islets isolated from mice were cultured in RPMI-1640 medium (Gibco, Grand Island, NY) including 10% (v/v) fetal bovine serum, HEPES, sodium pyruvate, and antibiotics.
2.2. Mouse models
Hnf1a+/− mice (MGI:2384429) [12] with a mixed background (129Svj × C57BL/6J × FVB/N) were backcrossed to C57BL/6J or ICR mice (KBT Oriental Co., Ltd., Saga, Japan) for at least six generations before their use in this study. Another line of Hnf1a+/− mice on a C57BL/6J background was produced as previously described [13,14]. A targeting vector was constructed containing 6.25 kb of the 5' homologous region and 3.24 kb of the 3' homologous region flanking the initiation ATG codon in exon 1 of Hnf1a. We used a RENKA embryonic stem (ES) cell line derived from C57BL/6 mice [15]. Heterozygous Hnf1a+/− mice were crossed to generate Hnf1a−/− mice. The percentage of Hnf1a+/+, Hnf1a+/−, and Hnf1a−/− mice that survived for more than 10 days after birth was calculated. The survival rates between genotypes were analyzed by the Kaplan-Meier method. The mice were maintained under specific pathogen-free conditions in a 12-h light (07:00–19:00)/12-h dark (19:00–07:00) cycle with free access to water and normal mouse chow (CE-2; CLEA, Tokyo, Japan). To maintain the survival of newborn Hnf1a−/− pups, pregnant female mice were fed a pelleted commercial diet (CMF; Oriental Yeast Co., Ltd., Tokyo, Japan) until they gave birth and their pups were weaned. Room temperature was maintained at 22 ± 1–2 °C. The handling and killing of the mice were performed in compliance with the animal care guidelines of Kumamoto University. This research was approved by the animal research committee of Kumamoto University, and all animal experimental procedures were approved by the Kumamoto University Ethics Review Committee for Animal Experimentation.
2.3. Metabolic parameters and metabolic studies
Body weights and plasma glucose levels were measured in mice aged from 3 to 20 weeks. After a 6-h (for an intraperitoneal glucose tolerance test [IPGTT] and glucose-stimulated insulin secretion [GSIS] test) and 4-h (for an insulin tolerance test [ITT]) fasting period, 3- to 8-week-old mice were intraperitoneally administered either 2 g/kg glucose (for IPGTT), 3 g/kg glucose (for GSIS), or 2.0 U/kg human insulin (Humulin-R; Eli Lilly and Co., Indianapolis, IN) (for ITT). Blood glucose was measured by a glucose sensor (Glutest Neo Super; Sanwa Kagaku, Nagoya, Japan). Tail blood was collected at the indicated times, and aprotinin (250 KIU/mL) and EDTA (0.75 mg/mL) were added to blood samples for plasma glucagon measurements. Plasma insulin and glucagon levels were determined using a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (type S) (Shibayagi Co., Gunma, Japan) and a mouse glucagon ELISA kit (Mercodia AB, Uppsala, Sweden).
2.4. Histology
Pancreas tissues from ad libitum-fed mice were fixed with 10% (v/v) neutral buffered formalin (Wako Pure Chemical Industries, Ltd.) for 18–24 h at 4 °C. Fixed samples were embedded in paraffin, cut into 10-μm cross-sections, mounted on MAS-coated slides (Matsunami Glass, Osaka, Japan), and deparaffinized for standard histological staining with hematoxylin and eosin (HE). Six paraffin-embedded pancreas sections cut at 100-μm intervals were stained with HE, and the pancreatic islet area of each section was circled and calculated by using ImageJ software (US National Institutes of Health, Bethesda, MD). The distribution of islet size was categorized based on islet area (μm2).
2.5. Immunofluorescence and cell distribution analysis
To determine the α- or β-cell composition of each islet, 10 μm-thick paraffin-embedded pancreas sections were subjected to antigen retrieval using HistoVT One (L6F9587; Nacalai Tesque, Kyoto, Japan), and double-stained with an anti-insulin antibody (A0564, 1:400; Dako, Santa Clara, CA) and anti-glucagon antibody (#ab92517, 1:400; Abcam, Cambridge, MA). After reaction with fluorescent dye-conjugated secondary antibodies, fluorescent signals were captured using an all-in-one fluorescent microscope (BZ-X700; Keyence, Tokyo, Japan). The total area (μm2), glucagon-positive area (μm2), and insulin-positive area (μm2) of each islet was measured by Keyence software. Small islet-like cell clusters (ICCs) were defined as a cell population that is made up of only insulin-positive cells or glucagon-positive cells.
2.6. Islet isolation, glucagon content, and glucagon secretion
Pancreatic islets were isolated from Hnf1a+/+ and Hnf1a−/− mice as previously described [16]. Briefly, after bile duct cannulation and digestion of the pancreas using a mixture of 1.5 mg/mL collagenase L (Nitta Gelatin, Osaka, Japan), 1.5 mg/mL hyaluronidase (H3506; Sigma-Aldrich, St. Louis, MO), and 0.1% (v/v) protease inhibitor cocktail (Nacalai Tesque, Inc., Kyoto, Japan), isolated islets were manually collected. Islet glucagon was extracted by an acid–ethanol extraction method as previously described [17]. To assess glucagon secretion from isolated islets in vitro, size-matched islets were pre-incubated in pH 7.4 HEPES-buffered Krebs-Ringer bicarbonate solution (KRBH) buffer (120 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 2.4 mM CaCl2, 1.2 mM MgCl2, 20 mM NaHCO3, and 10 mM HEPES) containing 11 mM glucose and then stimulated with KRBH buffer containing 1.1 or 5.6 mM glucose, or with 20 mM KCl for the indicated time. To examine the effect of SGLT inhibition on glucagon secretion, isolated islets were pre-incubated with 20 μM sotagliflozin for 2 h, after which a glucagon secretion assay was performed in the presence of 20 μM sotagliflozin. Glucagon concentration was determined by a mouse glucagon enzyme-linked immunosorbent assay (ELISA) kit (Mercodia AB, Uppsala, Sweden) and glucagon levels were normalized by islet number.
2.7. Western blotting
Western blotting analysis was performed as previously described [18]. Twenty μg of total protein lysates from isolated islets and αTC1–6 cells were subjected to gel electrophoresis and protein transfer onto PVDF membrane. The following primary antibodies were used: anti-HNF1α (610,902; BD Biosciences, San Jose, CA), anti-β-actin (clone AC15; Sigma-Aldrich), and anti-SGLT1 (#ab14685; Abcam). After reaction with secondary antibodies, the signals were detected by using Chemi-Lumi One Super type (Nacalai Tesque) and a ChemiDoc imaging system (BR170–8265; Bio-Rad Laboratories, Hercules, CA).
2.8. Quantitative real-time RT-PCR
Liver tissues and αTC1–6 cells were homogenized in Sepasol-RNA I Super G solution (Nacalai Tesque), and total RNA was isolated using a conventional phenol-chloroform-based RNA extraction method. Pancreatic islet RNA was purified with an RNeasy Micro Kit (Qiagen, Valencia, CA). cDNA was prepared using a PrimeScript RT reagent kit and gDNA Eraser (RR047A; TaKaRa Bio, Inc., Shiga, Japan). Quantitative PCR (qPCR) was performed using SYBR Premix Ex Taq II (RR820A; TaKaRa Bio, Inc.) and an ABI 7300 thermal cycler (Applied Biosystems, Foster City, CA). For each gene, mRNA levels were determined by the comparative Cycle threshold (Ct) method (ΔΔCt) and levels of each mRNA were normalized to 18S rRNA or TATA-binding protein (Tbp) mRNA. Primer sequences for Cltrn, Slc2a2, Hnf4a, Hgfac, Agxt, G6pc, Got1, Sglt1, Sglt2, and Slc2a1 mRNAs are listed in Supplementary Table 1.
2.9. Chromatin immunoprecipitation quantitative real-time PCR (ChIP qPCR)
αTC1–6 cells were fixed in 1% formaldehyde at room temperature and the reaction was quenched by 150 mM glycine. The fixed cells were incubated in 0.5% Nonidet P-40 buffer on ice for 15 min and lysed in SDS lysis buffer (50 mM Tris-HCl, pH 8.0, 1% SDS, and 10 mM EDTA). DNA was sheared using a Bioruptor Sonicator (Diagenode, Denville, NJ) by 7 cycles of sonication (30 s on, 30 s off, high output). The sheared chromatin was diluted 5-fold in ChIP dilution buffer (50 mM Tris-HCl, pH 8.0, 167 mM NaCl, 1.1% Triton X-100, and 0.11% sodium deoxycholate). The chromatin was incubated with 2 μg anti-HNF1 antibody (sc-8986; Santa Cruz Biotechnology, Dallas, TX) or control IgG (2729; Cell Signaling Technology, Danvers, MA) overnight at 4 °C. The chromatin was incubated with magnetic beads (Dynabeads protein A and protein G; Invitrogen, Carlsbad, CA) for 6 h at 4 °C, followed by sequential washing with low-salt RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, and 0.1% sodium deoxycholate), high-salt RIPA buffer (50 mM Tris-HCl, pH 8.0, 500 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, and 0.1% sodium deoxy-cholate), LiCl wash buffer (10 mM Tris-HCL, pH 8.0, 250 mM LiCl, 1 mM EDTA, 0.5% Nonidet P-40, and 0.5% sodium deoxycholate), and Tris-EDTA buffer. The chromatin was eluted and reverse cross-linked in ChIP direct elution buffer (50 mM Tris-HCl, pH 8.0, 5 mM EDTA, and 0.5% SDS) overnight at 65 °C. DNA fragments, which were manually extracted and collected by using phenol-chloroform extraction and ethanol precipitation, were evaluated by qPCR with primers for the Tbp gene body (P1) (5'-CCCCTTGTACCCTTCACC AAT-3' and 5'-GAAGCTGCGGTACAATTCCAG-3'), primers for the Slc5a1 promoter region without the HNF1 binding motif (P2) (5'-TTG TCTCTTGCTCTTTGAGG-3' and 5'-GGTGATGTTTCATAGCTTGC-3'), and primers for the Slc5a1 promoter region containing the HNF1 binding motif (P3) (5'-ACACCTAGGAGCTGCTTCC-3' and 5'-TCAAGG TGCTACTGTCCATG-3').
2.10. Retrovirus-mediated transduction of short hairpin RNA (shRNA) and small interfering RNA (siRNA) transfection
For knocking down Hnf1a expression, a specific shRNA target sequence (5'-GCCTAATGGCCTTGGAGAAAC-3') was designed for mouse Hnf1a using the BLOCK-iT™ RNAi Designer (Thermo Fisher Scientific, Waltham, MA). Oligonucleotides encoding shRNA were cloned into a pSIREN-RetroQ shRNA expression vector (Clontech Laboratories, Inc., Mountain View, CA). Then, the pSIREN-RetroQ control vector or pSIREN-RetroQ-Hnf1a vector was transfected into Plat-E cells, a retroviral packaging cell line, using the jetPRIME reagent (Polyplus, New York, NY). αTC1–6 cells were infected with retrovirus-containing medium and selected by incubation with 5 μg/mL puromycin to generate αTC1–6 cells stably expressing HNF1α shRNA (HNF1α KD αTC1–6 cells) or negative control shRNA (control αTC1–6 cells). For Slc5a1 knockdown, αTC1–6 cells (3.0 × 105 cells, 24-well plate) were transfected with control scramble siRNA or mouse Slc5a1 Silencer Select siRNA (s73953, Ambion; Thermo Fisher Scientific) at a final concentration of 10 nM using the Lipofectamine RNAiMAX reagent (Thermo Fisher Scientific), according to the manufacturer's instructions.
2.11. Transient transfection and luciferase reporter assay
The mouse Sglt1 promoter containing a putative HNF1α binding site was amplified by PCR using a pair of primers (5'-AACTCGAGGCAGAC TCTCTTGAG-3' and 5'-AAGCTTGGGATGGGTGCATCGGTGGCAGT AAC-3'), and it was sub-cloned into the pGL4.10 basic reporter (Promega, Madison, WI). The transcription factor binding site was altered by PCR-based mutagenesis using a pair of primers (5'-GCTGTTA ACTCAAAAGCAGTATAAG-3' and 5'-CAGCCCCGAGGGAG-3') to produce an HNF1α binding site mutant (CTGGCTGTTAAC). The pcDNA3.1-wild-type (WT)-HNF1α and pcDNA3.1-P291fsinsC mutant-HNF1α expression plasmids were previously described [19]. αTC1–6 cells (2.0 × 105 cells) were seeded into each well of a 48-well plate at 24 h before transfection. Transient transfection was performed using the jetPRIME reagent (Polyplus) according to the manufacturer's instructions. At 24 or 48 h after transfection, luciferase activity was measured by using a Dual-Luciferase reporter assay system (Promega).
2.12. Glucose uptake assay
Size-matched islets isolated from Hnf1a+/+ and Hnf1a−/− mice were pre-cultured in RPMI-1640 medium with DMSO or 20 μM sotagliflozin (LX4211; Cayman Chemical, Ann Arbor, MI) for 2 h. After washing once with KRBH buffer containing 1.1 mM glucose, they were incubated in KRBH buffer containing 1.1 mM glucose, 200 μM of 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose (2-NBDG, Peptide Institute, Osaka, Japan), and DMSO or 20 μM sotagliflozin for 1 h. After washing with KRBH buffer, images were captured by an all-in-one fluorescence microscope (BZ-X700; Keyence, Osaka, Japan) and fluorescence intensity in the peripheral area (approximately 50% outside of the radius) of the islets was measured.
2.13. Statistical analysis
Data are presented as mean values ± standard error (S.E.) of the mean of the indicated number of experiments (n). The significance of differences was assessed with an unpaired t-test and a log-rank (Mantel-Cox) test, and a value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. Generation of Hnf1a−/− mice with an ICR background
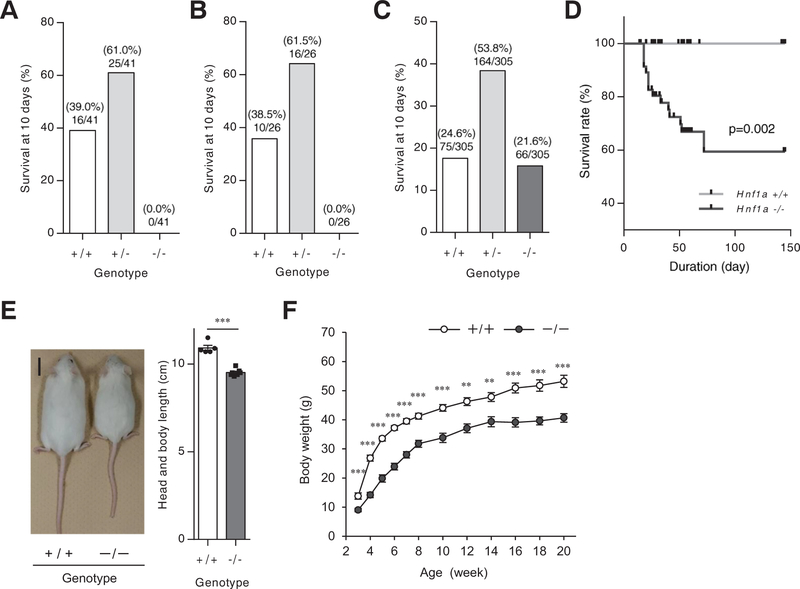
To clarify the role of HNF1α in pancreatic α-cells, heterozygous Hnf1a+/− mice (mixed background of 129Svj × C57BL/6J × FVB/N) [12] were crossed over six generations to generate a pure C57BL/6J background. C57BL/6J Hnf1a−/− mice were not embryonic lethal, but none of the Hnf1a−/− newborns lived for longer than 10 days (Fig. 1A). The early postnatal lethality of Hnf1a−/− mice with the C57BL/6J background was also detected in another line of Hnf1a−/− mice [13] (Fig. 1B). Since postnatal lethality in an inbred strain can often be rescued in an outbred line [20,21], Hnf1a+/− mice with a mixed background [12] were backcrossed to outbred ICR mice for 6 generations. On the ICR background, most of the Hnf1a−/− mice lived more than 10 days (observed ratio at 10 days: Hnf1a+/+ 24.6%, Hnf1a+/− 53.8%, Hnf1a−/− 21.6%) (Fig. 1C). The survival rate of Hnf1a null mice on the ICR background (ICR Hnf1a−/− mice) gradually decreased, but these mice still lived longer than previously reported Hnf1a−/− mice (Fig. 1D) [22]. ICR Hnf1a−/− mice were smaller than Hnf1a+/+ mice, and body weight was significantly lower in Hnf1a−/− mice relative to control mice (Fig. 1E, F), as previously described [12,22]. The liver of ICR Hnf1a−/− mice showed increased triglyceride and glycogen content compared with Hnf1a+/+ mice (Supplementary Fig. 1), consistent with previous reports [12,22–24].
Fig. 1.
Phenotypic analyses of Hnf1a−/−mice on the ICR background. (A-C) Genotypic distribution at 10 days after birth. The number of Hnf1a wild-type (+/+), Hnf1a heterozygous (+/−), and Hnf1a null (−/−) mice born after being backcrossed 6 times with C57BL/6J mice (A), or with a pure C57BL/6J background (B), or born after being backcrossed 6 times with ICR mice (C) was counted (3–5 generations). The ratio on the bars indicates genotype-matched mouse number/total mouse number from multiple litters. (D) Kaplan-Meier survival curves for Hnf1a+/+ (n = 39; gray) and Hnf1a−/− (n = 66; black) littermates. p values were calculated using a log-rank (Mantel-Cox) test. (E) Gross appearance of 10-week-old Hnf1a+/+ (left) and Hnf1a−/− (right) mice. Scale bar, 2 cm. Body length (from the tip of the nose to the base of the tail) (n = 5–7) was measured. (F) Body weight chart for male Hnf1a+/+ (n = 63) and Hnf1a−/− (n = 65) mice from 3 to 20 weeks of age. All data are presented as mean ± S.E. (S.E.; error bars). **p < 0.01; ***p < 0.001.
3.2. Defective insulin secretion in ICR Hnf1a−/− mice
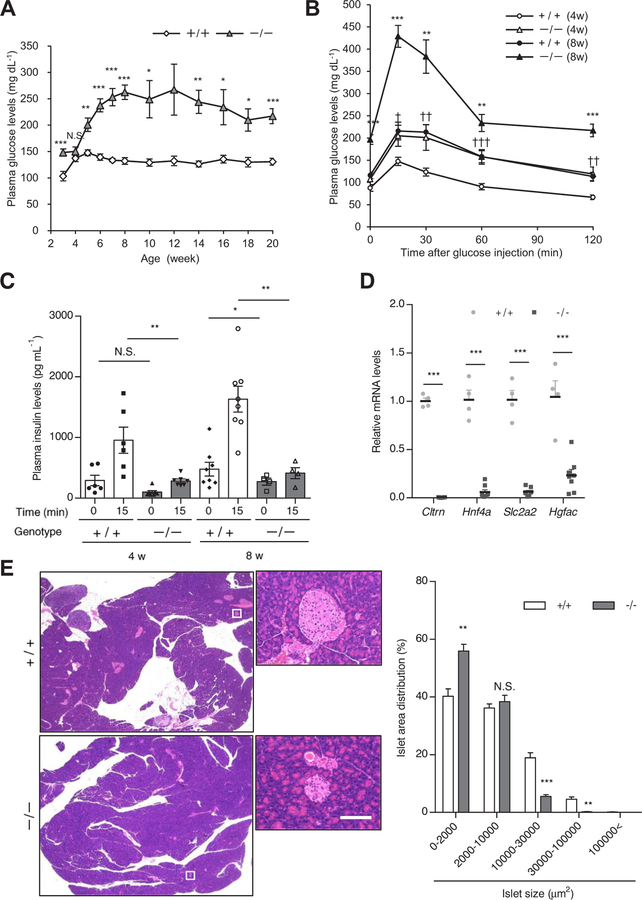
Diabetic phenotype, pancreatic islet size, and β-cell mass are reported to be profoundly affected by genetic background in Hnf1a−/− mice [25]. The non-fasting blood glucose levels of ICR Hnf1a−/− mice were significantly higher than those of ICR Hnf1a+/+ mice after 5 weeks of age (Fig. 2A). Although blood glucose levels were similar between ICR Hnf1a+/+ and Hnf1a−/− mice at 4 weeks of age, an intraperitoneal glucose tolerance test (IPGTT) demonstrated that ICR Hnf1a−/− mice were glucose intolerant at this age (Fig. 2B). As previously reported [5], the insulin secretory response to glucose was significantly impaired in Hnf1a−/− mice (Fig. 2C). The expression of Cltrn, Hnf4a, Slc2a2, and Hgfac mRNAs, which are direct target genes of HNF1α [1,26], was significantly decreased in the islets of Hnf1a−/− mice (Fig. 2D). The islets of ICR Hnf1a−/− mice were smaller than those of Hnf1a+/+ mice (Fig. 2E). These results are consistent with the phenotypes of previously reported Hnf1a−/− mice [5].
Fig. 2.
β-Cell dysfunction in Hnf1a−/− mice. (A) Plasma glucose chart for male Hnf1a+/+ (n = 63) and Hnf1a−/− (n = 65) mice from 3 to 20 weeks. (B, C) IPGTT (B) and GSIS (C) of 4- and 8-week-old Hnf1a+/+ (n = 6–8) and Hnf1a−/− (n = 4–7) mice. (D) Islet gene expression of 8-week-old mice was quantified by qPCR (n = 4). The expression levels of Cltrn, Hnf4a, Slc2a2, and Hgfac were normalized to that of Tbp. (E) H&E staining of pancreas sections from 8-week-old mice (left). Scale bar, 100 μm. Profiling of islet size distribution in Hnf1a+/+ and Hnf1a−/− mice (right) (n = 4). +/+ and −/− indicate Hnf1a+/+ and Hnf1a−/− mice, respectively. All data are presented as mean ± S.E. (S.E.; error bars). N.S., not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
3.3. Pancreatic β-cells and α-cells in ICR Hnf1a−/− mice
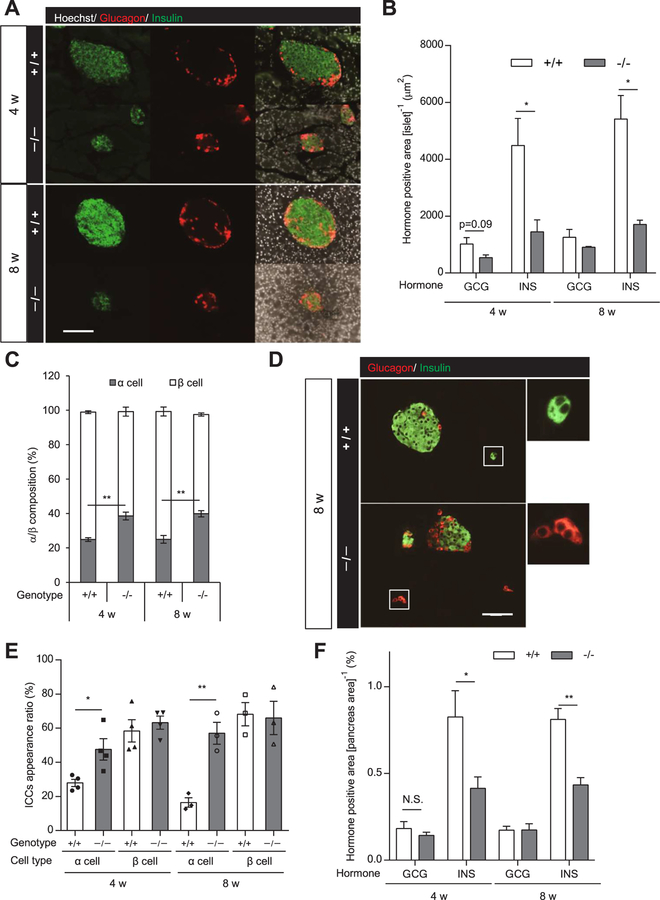
Immunohistochemical analysis revealed that the proportion of insulin-positive cells in ICR Hnf1a−/− islets was markedly decreased compared with that of ICR Hnf1a+/+ islets (Fig. 3A). As previously reported [5], the β-cell area in the islets of ICR Hnf1a−/− mice was significantly decreased compared with Hnf1a+/+ mice (Fig. 3A, B). There was also a tendency for a decreased α-cell area in the islets of ICR Hnf1a−/− mice, but the difference did not reach statistical significance (Fig. 3B). Due to the decrease of β-cell number, the ratio of α-cells in Hnf1a−/− islets was significantly increased compared with that in Hnf1a+/+ islets (Fig. 3C). A comparison of similarly sized islets (total islet area, 500–2000 μm2) also revealed that the ratio of α-cells was significantly increased in Hnf1a−/− islets compared with Hnf1a +/+ islets (Supplementary Fig. 2). Small islet-like cell clusters (ICCs), which consist exclusively of insulin-positive cells or glucagon-positive cells, were occasionally detected in the pancreas (Fig. 3D). The appearance rate of ICCs, consisting of only insulin-positive cells, was similar between Hnf1a−/− and Hnf1a+/+ mice, but the appearance rate of ICCs consisting exclusively of glucagon-positive cells was significantly increased in Hnf1a−/− mice (Fig. 3E). Collectively, the α-cell area in the pancreas was similar between Hnf1a−/− and Hnf1a +/+ mice, even though the islets were smaller in Hnf1a−/− mice (Fig. 3F).
Fig. 3.
Islet cell composition and α-cell area in the pancreas of Hnf1a−/− mice. (A) α-cell and β-cell composition in each islet of 4- and 8-week-old mice. Immunofluorescence for insulin (green), glucagon (red), and Hoechst (white). Scale bars, 100 μm. (B) Glucagon (GCG)-positive and insulin (INS)-positive areas (μm2) in the islet area on the section were measured (n = 3–4). (C) Percentage of α-cell area and β-cell area to islet total area is shown (n = 3–4). (D) Representative image of ICCs in 8-week-old mouse pancreas. Immunofluorescence for insulin (green) and glucagon (red). Scale bars, 100 μm. (E) Appearance ratio of ICCs in 4- and 8-week-old mouse pancreas (n = 3–4). (F) Glucagon (GCG)-positive and insulin (INS)-positive areas in the whole pancreas area on the section were calculated (n = 3–6). +/+ and −/− indicate Hnf1a+/+ and Hnf1a−/− mice, respectively. All data are presented as mean ± S.E. (S.E.; error bars). N.S., not significant; *p < 0.05; **p < 0.01.
3.4. Dysregulation of glucagon secretion in ICR Hnf1a−/− mice
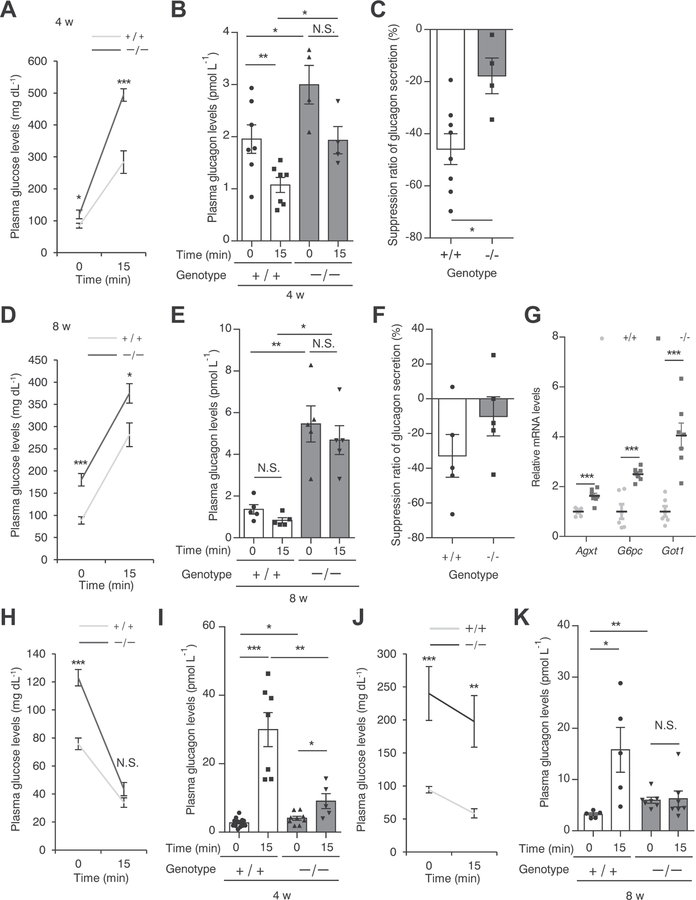
To address the functional impact of the loss of HNF1α in pancreatic α-cells, we examined glucagon secretion in response to the intraperitoneal administration of glucose in 4- and 8-week-old ICR Hnf1a−/− mice. ICR Hnf1a−/− mice showed higher fasting blood glucose levels (Fig. 4A, D) and plasma glucagon levels (Fig. 4B, E) compared with Hnf1a+/+ mice at both ages. Plasma glucagon levels were significantly decreased after intraperitoneal glucose load in 4-week-old Hnf1a+/+ mice (Fig. 4B). The suppression of glucagon secretion after glucose load was inhibited in Hnf1a−/− mice (Hnf1a +/+ 46.0%, Hnf1a−/− 17.8%, p < 0.05) (Fig. 4C). Hyperglucagonemia in Hnf1a−/− mice persisted at 8 weeks of age (Fig. 4E), and there was no significant reduction of plasma glucagon levels between before and after glucose load (Fig. 4E, F). The liver is a major target organ for glucagon action, and glucagon regulates the hepatic expression of Agxt, G6pc, and Got1 [27]. Consistently, the expression of these genes was significantly increased in the liver of hyperglucagonemic Hnf1a−/− mice (Fig. 4G).
Fig. 4.
Dysregulated glucagon secretion in Hnf1a−/− mice (A–F). Plasma glucose levels (A, D) and plasma glucagon levels (B, E) before and at 15 min after glucose injection (3 g/kg) of 4- and 8-week-old Hnf1a+/+ (n = 5–7) and Hnf1a−/− (n = 4–5) mice. Suppression ratio of glucagon secretion (15 min after glucose injection vs. 0 min) in 4- and 8-week-old Hnf1a+/+ (n = 5–8) and Hnf1a−/− (n = 4–5) mice (C, F). (G) Hepatic gene expression of glucagon target genes in 8-week-old ad libitum-fed mice. The expression levels of Agxt, G6pc, and Got1 were normalized to that of 18S rRNA (n = 6–7). (H–K) Plasma glucose levels (H, J) and plasma glucagon levels (I, K) before and at 15 min after human insulin injection (2.0 U/kg) of 4-week-old mice (H, I) and 8-week-old mice (J, K) of both genotypes (n = 5–15). +/+ and −/− indicate Hnf1a+/+ and Hnf1a−/− mice, respectively. All data are presented as mean ± S.E. (S.E.; error bars). N.S., not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
We next examined glucagon secretion in response to intraperitoneal insulin injection. Plasma glucagon levels were significantly increased (11.1-fold) by injection of 4-week-old Hnf1a+/+ mice with insulin (Fig. 4H, I). Notably, this increase of glucagon secretion was significantly impaired in Hnf1a−/− mice. Similarly, 8-week-old Hnf1a−/− mice also exhibited a lack of glucagon secretion after insulin injection (Fig. 4J, K).
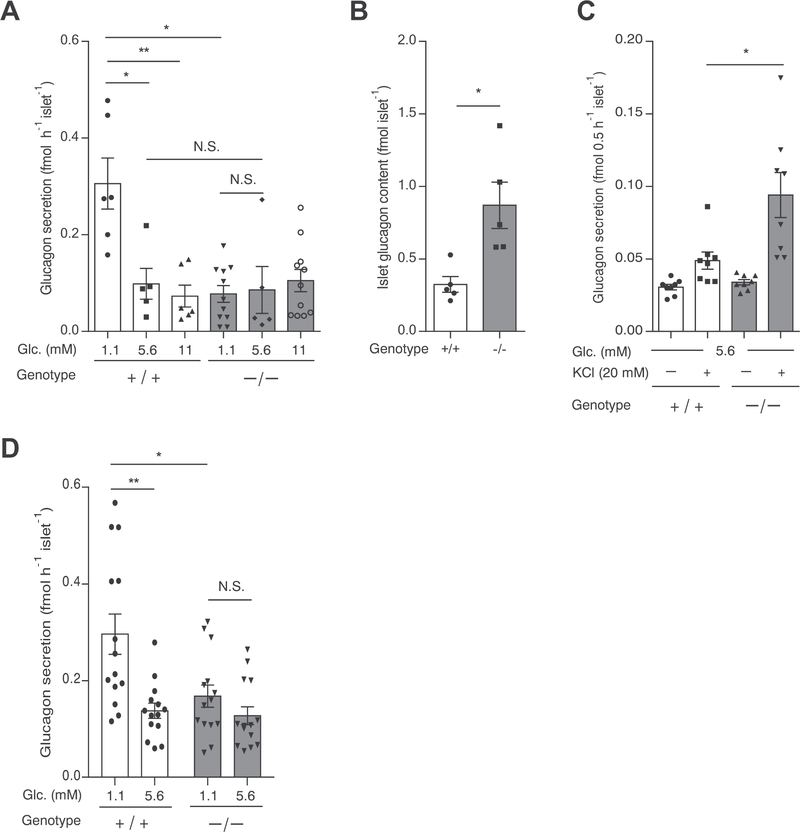
We then investigated glucagon secretion in islets isolated from 4-week-old Hnf1a+/+ and Hnf1a−/− mice. In Hnf1a+/+ islets, glucagon secretion after 1.1 mM glucose treatment was significantly elevated (2.3-fold) compared with that after exposure to 5.6 mM glucose (Fig. 5A). In contrast, glucagon secretion at 1.1 mM glucose was reduced by 74.6% (Hnf1a+/+ 0.31 fmol h−1 islet−1, Hnf1a−/− 0.078 fmol h−1 islet−1, p < 0.05) in Hnf1a−/− islets compared with Hnf1a +/+ islets (Fig. 5A). The elevation of glucose concentration (5.6 mM and 11 mM) exerted no additional inhibitory effect on glucagon secretion in Hnf1a−/− islets (Fig. 5A). Glucagon content was significantly increased in Hnf1a−/− islets compared with Hnf1a+/+ islets (Fig. 5B), and stimulation with 20 mM KCl significantly increased glucagon secretion in Hnf1a−/− islets compared with Hnf1a+/+ islets (Fig. 5C). Therefore, the reduction of glucagon secretion at 1.1 mM glucose is unlikely due to decreased glucagon content in α-cells. Consistent with the results from 4-week-old mice, glucagon secretion at 1.1 mM glucose was significantly decreased in Hnf1a−/− islets compared with that in Hnf1a+/+ islets (Fig. 5D). Taken together, these results indicate that HNF1α deficiency leads to dysregulated glucagon secretion in response to hypoglycemia.
Fig. 5.
Glucagon secretion defects in the islets of Hnf1a−/− mice. (A) Glucagon secretion in isolated islets from 4-week-old Hnf1a+/+ and Hnf1a−/− mice at 1.1, 5.6, and 11 mM glucose (n = 5–11 experiments using islets from 4 to 5 mice per genotype). (B) Islet glucagon content was normalized to islet number. (C) Glucagon secretion in the isolated islets from 4-week-old Hnf1a+/+ and Hnf1a−/− mice at 5.6 mM glucose with or without 20 mM KCl (n = 8 experiments using islets from 4 to 5 mice per genotype). (D) Glucagon secretion in isolated islets from 8-week-old Hnf1a+/+ and Hnf1a−/− mice at 1.1, and 5.6 mM glucose (n = 14 experiments using islets from 3 to 4 mice per genotype). +/+ and −/− indicate Hnf1a+/+ and Hnf1a−/− mice, respectively. All data are presented as mean ± S.E. (S.E.; error bars). N.S., not significant; *p < 0.05; **p < 0.01.
3.5. HNF1α regulates SGLT1 expression in pancreatic α-cells
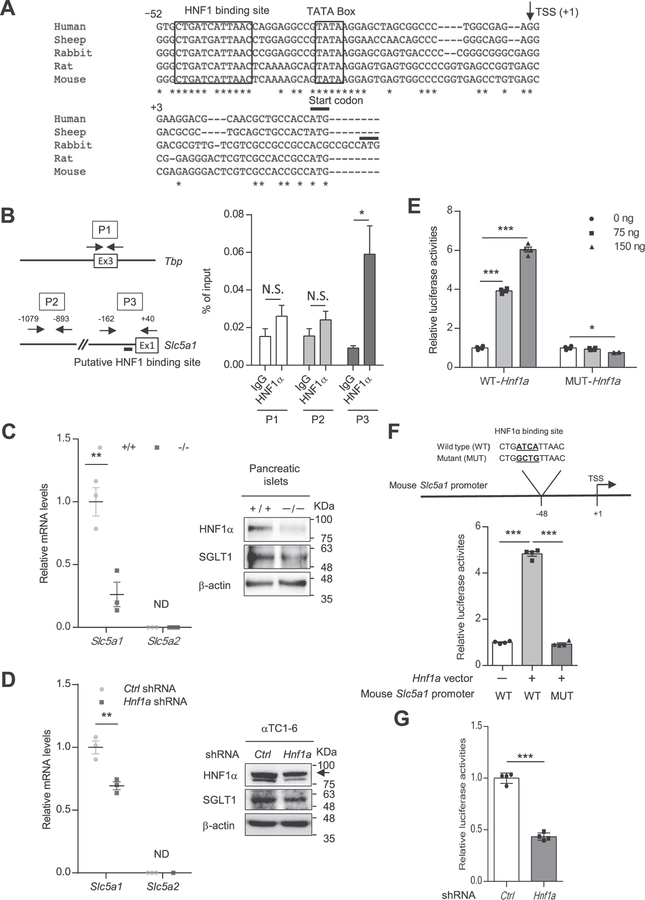
SGLT1 is selectively expressed in small intestinal villus cells, renal tubule cells, heart, and pancreatic α-cells [11,28–30]. Suga et al. reported that SGLT1 inhibition significantly suppressed glucagon secretion from islets at 1 mM glucose [11]. There is a conserved HNF1 binding site in the promoter region of Slc5a1 (encoding SGLT1) (Fig. 6A). HNF1α binds to the promoter region and activates transcription of Slc5a1 in intestinal cells [31]. Accordingly, we investigated whether HNF1α controls glucagon secretion by regulating SGLT1 expression. First, we examined the recruitment of HNF1α to the HNF1 binding site in the Slc5a1 promoter region in αTC1–6 cells, a pancreatic α-cell line, by a ChIP assay. Specific binding of HNF1α to the promoter region of Slc5a1 was identified in αTC1–6 cells (Fig. 6B). We then investigated whether Slc5a1 transcription is dependent on HNF1α in pancreatic α-cells. The expression of Slc5a1 mRNA and SGLT1 protein was significantly decreased in the islets of Hnf1a−/− mice compared with those of Hnf1a+/+ mice (Fig. 6C). It was reported that SGLT2 is expressed in pancreatic α-cells and controls glucagon secretion [29]. However, Slc5a2 (encoding SGLT2) mRNA expression was not detected in islets (Fig. 6C) as reported in recent studies [11,32]. Knocking down Hnf1a expression led to the downregulation of SGLT1 expression in αTC1–6 cells without affecting Slc5a2, Slc2a1 (encoding solute carrier family 2, member 1, GLUT1), and Slc2a2 (encoding GLUT2) expression in αTC1–6 cells (Fig. 6D, Supplementary Fig. 3). The promoter region of the mouse Slc5a1 gene was cloned into the pGL4-luciferase vector and co-transfected into αTC1–6 cells with WT-HNF1α or P291fsinsC mutant-HNF1α expression vectors. WT-HNF1α activated the reporter gene, while P291fsinsC mutant-HNF1α lacked transactivation activity (Fig. 6E). Mutation of the HNF1α binding site in the reporter gene reduced (80.8% decrease, p < 0.001) the transactivation activity of HNF1α (Fig. 6F). Furthermore, knocking down Hnf1a expression reduced reporter gene activity in αTC1–6 cells (Fig. 6G). These results indicate that HNF1α regulates SGLT1 expression in pancreatic α-ells.
Fig. 6.
SGLT1 is a direct target of HNF1α in α-cells. (A) Multiple sequence alignment of mammalian Slc5a1 promoters, depicting the high degree of conservation of the HNF1 binding motif. Alignments were generated by using CLUSTALW (https://www.genome.jp/tools-bin/clustalw). The arrow denotes the transcription start site. Highly conserved nucleotide sequences among species are indicated by an asterisk (*). (B) Binding of HNF1α to the mouse Slc5a1 gene promoter. ChIP-qPCR analysis was performed using 3 different primers: primer 1 (P1) targeted exon 3 of mouse Tbp, P2 targeted the mouse Slc5a1 promoter without the HNF1 binding site, and P3 targeted the mouse Slc5a1 promoter with the putative HNF1 binding site. Enrichment values at the indicated sites (P1–P3) were normalized to input DNA (n = 5). (C, D) qPCR and western blot analyses of SGLT expression in Hnf1a+/+ and Hnf1a−/− mouse islets (C). qPCR and western blot analyses of SGLT expression in control shRNA and Hnf1a shRNA-overexpressing αTC1–6 cells (D). The relative expression levels of Slc5a1 and Slc5a2 were normalized to that of Tbp (n = 3). β-Actin was used as a loading control. (E) Dose-dependent effect of HNF1α WT or HNF1α dominant negative mutant P291fsinsC-Hnf1a on Slc5a1 promotor activity. αTC1–6 cells were co-transfected with either 75 ng (square) or 150 ng (triangle) pcDNA3.1-HNF1α WT or pcDNA3.1-P291fsinsC-Hnf1a expression vector, as well as 50 ng pGL4.10-Slc5a1 promoter WT and 25 ng pRL-TK (n = 4). (F) Schematic diagram of a putative HNF1 binding site on the mouse Slc5a1 promoter. The sequence of the binding mutant for HNF1α is shown. αTC1–6 cells were co-transfected with 75 ng pcDNA3.1-Hnf1a WT, 50 ng pGL4.10 basic control or pGL4.10-Slc5a1 promoter WT or pGL4.10-Slc5a1 promoter mutant and 25 ng pRL-TK (n = 4). (G) Slc5a1 promotor activity in control shRNA vs. Hnf1a KD αTC1–6 cells. The cells were co-transfected with 50 ng pGL4.10-Slc5a1 promoter and 25 ng pRL-TK (n = 4). All data are presented as mean ± S.E. (S.E.; error bars) *p < 0.05; **p < 0.01; ***p < 0.001.
3.6. SGLT1 controls low glucose-stimulated glucagon secretion
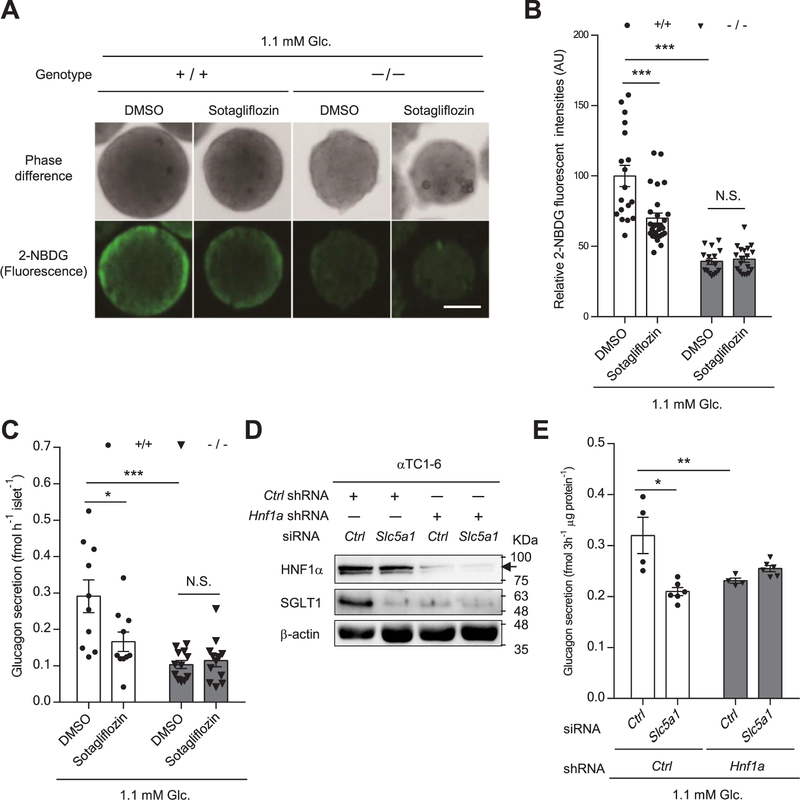
We next investigated whether inhibition of SGLT1 affects glucagon secretion in α-cells. A fluorescent derivative of glucose, 2-NBDG, can be transported into living cells through SGLTs and GLUTs [33]. First, we examined 2-NBDG uptake in mouse islets at 1.1 mM glucose in the presence or absence of sotagliflozin, which is a non-selective SGLT inhibitor [34]. Pancreatic islets in mice are composed of an internal core of β-cells surrounded by a mantle of α-cells, which express GLUT1 and SGLT1 [11,35,36]. Fluorescence intensity was weak in the core of Hnf1a+/+ islets, whereas strong intensity was detected in the periphery (rich in α-cells) (Fig. 7A). Interestingly, the fluorescence intensity in peripheral Hnf1a+/+ cells was significantly reduced by 30.0% in the presence of sotagliflozin (Fig. 7A, B). In addition, peripheral fluorescence intensity was significantly decreased in Hnf1a−/− islets compared with that in Hnf1a+/+ islets, and sotagliflozin treatment exerted no additional inhibitory effect (Fig. 7A, B). These results suggest that SGLT1 activates glucose uptake in α-cells under low glucose conditions. We then examined the effect of SGLT1 on glucagon secretion. Inhibition of SGLT1 in mouse islets by sotagliflozin and in αTC1–6 cells by siRNA significantly decreased glucagon secretion at 1.1 mM glucose (Fig. 7C–E). Glucagon secretion in the presence of low glucose was significantly reduced in Hnf1a−/− islets, coincident with decreased SGLT1expression (Fig. 6C) compared with Hnf1a+/+ islets (Fig. 7C). Notably, sotagliflozin did not exert an additional inhibitory effect in Hnf1a−/− islets (Fig. 7C). Similarly, Hnf1a knockdown in αTC1–6 cells led to a reduction of glucagon secretion at 1.1 mM glucose, and there was no additional decrease of glucagon secretion by concomitant knockdown of Slc5a1 (Fig. 7D, E). Collectively, these results indicate that SGLT1 inhibition suppresses glucagon secretion at low glucose conditions.
Fig. 7.
Role of SGLT1 in the dysfunction of glucagon secretion in Hnf1a−/− mouse islets and Hnf1a KD αTC1–6 cells. (A, B) Effect of sotagliflozin on 2-NBDG uptake in the islets of Hnf1a+/+ and Hnf1a−/− mice at 1.1 mM glucose was evaluated. Representative light (top panel), and fluorescence (bottom panel) images of 2-NBDG-accumulated islets are presented (A), and quantification of relative 2-NBDG uptake at the periphery of the islets is shown (n = 16–18 islets from 2 to 3 mice per genotype). Scale bars, 50 μm (B). (C) Glucagon secretion at 1.1 mM glucose in isolated islets from Hnf1a+/+ and Hnf1a−/− mice in the presence of DMSO or sotagliflozin was examined by ELISA (n = 10–12). (D) Western blot analyses of HNF1α and SGLT1 expression in control or Hnf1a KD αTC1–6 cells transfected with control siRNA or Slc5a1 siRNA. β-Actin was used as a loading control. (E) The effect of Slc5a1 knockdown on glucagon secretion at 1.1 mM glucose in control and Hnf1a KD αTC1–6 cells was examined (n = 4–6). Glucagon secretion for 3 h was normalized to total protein content. All data are presented as mean ± S.E. (S.E.; error bars) *p < 0.05; **p < 0.01; ***p < 0.001.
4. Discussion
Mutations in the gene encoding HNF1α cause MODY3, which is associated with impaired insulin secretion. HNF1α is expressed in pancreatic α-cells, but its role in these cells is unknown. In the present study, we revealed that the fasting glucagon levels of Hnf1a−/− mice were higher than those of control mice, and Hnf1a−/− mice exhibited inadequate suppression of glucagon after glucose load. Glucagon secretion is under paracrine control by insulin [8], and insulin secretion is severely impaired in Hnf1a−/− mice. The deficiency in insulin secretion in Hnf1a−/− β-cells might be responsible for the hypersecretion of glucagon observed in these mice. In agreement with these findings, a clinical study showed inappropriate glucagon suppression after glucose administration in patients with MODY3 [37]. The hypersecretion of glucagon might exacerbate postprandial hyperglycemia in Hnf1a−/− mice and in patients with MODY3.
Subjecting the mice to hyperinsulinemic hypoglycemia revealed that glucagon release was strikingly impaired in Hnf1a−/− mice compared with Hnf1a+/+ mice. In addition, glucagon secretion at 1.1 mM glucose was also reduced in Hnf1a−/− islets. Consistent with our findings, Haliyur et al. reported that glucagon release after stimulation with low glucose and epinephrine was abrogated in the islets of a patient with MODY3 [10]. Further studies will be necessary, but these findings strongly suggest that HNF1α deficiency causes impaired glucagon secretion as well as impaired insulin secretion. Patients with MODY3 often exhibit hypersensitivity to sulfonylureas compared with type 2 diabetic patients, and even low doses frequently result in hypoglycemia [38–40]. A dysfunction in glucagon secretion might exacerbate the risk of hypoglycemia in patients with MODY3. From a mechanistic point of view, we found that SGLT1 expression was decreased in Hnf1a−/− islets and in Hnf1a knockdown αTC1–6 cells. SGLT1 inhibition was reported to suppress glucagon release from isolated islets at 1 mM glucose [11]. Consistent with this report, we also found that 1.1 mM glucose-stimulated glucagon secretion was notably suppressed by SGLT1 inhibition. Low glucose activates voltage-dependent Na+ channels and stimulates the amplitude of action potentials in pancreatic α-cells. This stimulates P/Q-type Ca2+-current activation and glucagon exocytosis [41,42]. At this point, we do not know the exact mechanism underlying the regulation of glucagon secretion by SGLT1, but the SGLT1 current carried by Na+ co-transport is a depolarizing current. Inhibition of SGLT1 blocks the entry of positive Na+ ions, and thus has a hyperpolarizing effect. Hyperpolarization could compromise glucagon secretion because the threshold for action potential generation will be not reached. In agreement with this idea, it was reported that the SGLT1 current is responsible for initiating electrical activity and glucagon-like peptide 1 secretion in intestinal L cells [43–45]. Meanwhile, ATP-sensitive potassium (KATP) channel activity also plays an important role in glucagon secretion. Increased KATP channel activity as a result of metabolic disturbance (e.g., oligomycin treatment) leads to the loss of glucagon secretion at low glucose [41]. Indeed, KATP channel activity is increased in Hnf1a-deficient β-cells due to a defect in ATP production [46]. Thus, increased KATP channel activity might also be involved in the dysregulation of glucagon secretion in Hnf1a−/− α-cells. Experiments with α-cell conditional Hnf1a−/− mice and Slc5a1−/− mice could provide additional useful information about the roles of HNF1α in α-cells.
Hnf1a−/− mice exhibit a marked reduction of β-cell number with a reduced β-cell proliferation rate [5,6]. In contrast, we did not detect a significant decrease in α-cell area between Hnf1a+/+ and Hnf1a−/− mice, suggesting that the contribution of HNF1α to cell growth is different between β- and α-cells. Furthermore, we found that ICCs, which consist exclusively of α-cells, were increased in the pancreas of Hnf1a−/− mice. It has been reported that hepatic glucagon signaling regulates α-cell proliferation [47,48]; however, the expression of Gcgr mRNA, which encodes the glucagon receptor, was unchanged in the liver of Hnf1a−/− mice (data not shown). At present, the mechanism underlying this effect is unknown, but it might be worth noting that the ablation of HNF1α in human embryonic stem cells increases the development of α-cells in vitro [49].
In conclusion, we found that ICR Hnf1a−/− mice are a novel model of human MODY3, and that HNF1α plays a critical role in proper glucagon secretion in mice. Further studies are necessary to determine whether the same abnormalities are characteristic of human MODY3. Detailed studies of ICR Hnf1a−/− mice may lead to a better understanding of the molecular basis of glucagon secretion in patients with MODY3.
Supplementary Material
Acknowledgements
The authors thank all the technical staff of laboratory for their assistance.
Funding
This study was supported by a Grant-in-Aid for Scientific Research (B) (19H03711), a grant from the Japan Agency for Medical Research and Development (JP19gm5010002), a grant from Japan Diabetes Foundation, and a grant from the Takeda Science Foundation.
Abbreviations:
- HNF
hepatocyte nuclear factor
- MODY
maturity-onset diabetes of the young
- ICCs
islet-like cell clusters
- SGLT
sodium-glucose cotransporter
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbadis.2020.165898.
References
- [1].Yamagata K, Roles of HNF1α and HNF4α in pancreatic β-cells: lessons from a monogenic form of diabetes (MODY), in: Litwack G (Ed.), Vitamins & Hormones, vol. 95, Academic Press, Cambridge, USA, 2014, pp. 407–423. [DOI] [PubMed] [Google Scholar]
- [2].Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta F, Vaxillaire M, Southam L, Cox RD, Lathrop GM, Boriraj VV, et al. , Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3), Nature 384 (1996) 455–458. [DOI] [PubMed] [Google Scholar]
- [3].Byrne MM, Sturis J, Menzel S, Yamagata K, Fajans SS, Dronsfield MJ, Bain SC, Hattersley AT, Velho G, Froguel P, et al. , Altered insulin secretory responses to glucose in diabetic and nondiabetic subjects with mutations in the diabetes susceptibility gene MODY3 on chromosome 12, Diabetes. 45 (11) (1996) 1503–1510. [DOI] [PubMed] [Google Scholar]
- [4].Lehto M, Tuomi T, Mahtani M, Widén E, Forsblom C, Sarelin L, Gullström M, Isomaa B, Lehtovirta M, Hyrkkö A, et al. , Characterization of the MODY3 phenotype. Early-onset diabetes caused by an insulin secretion defect, J. Clin. Invest. 99 (4) (1997) 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pontoglio M, Sreenan S, Roe M, Pugh W, Ostrega D, Doyen A, Pick AJ, Baldwin A, Velho G, Froguel P, et al. , Defective insulin secretion in hepatocyte nuclear factor-1 α-deficient mice, J. Clin. Invest. 101 (10) (1998) 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yamagata K, Nammo T, Moriwaki M, Ihara A, Iizuka K, Yang Q, Satoh T, Li M, Uenaka R, Okita K, et al. , Overexpression of dominant-negative mutant hepatocyte nuclear factor-1α in pancreatic β-cells causes abnormal islet architecture with decreased expression of e-cadherin, reduced β-cell proliferation, and diabetes, Diabetes. 51 (1) (2002) 114–123. [DOI] [PubMed] [Google Scholar]
- [7].Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, Rorsman P, Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes. Metab. 13 (2011) 95–105. [DOI] [PubMed] [Google Scholar]
- [8].Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford E, Herrera PL, Polonsky KS, McGuinness OP, Kulkarni R, et al. , Insulin signaling in alpha cells modulates glucagon secretion in vivo, Cell Metab 9 (4) (2009) 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nammo T, Yamagata K, Hamaoka R, Zhu Q, Akiyama TE, Gonzalez FJ, Miyagawa J, Matsuzawa Y, et al. , Expression profile of MODY3/HNF-1α protein in the developing mouse pancreas, Diabetologia. 45 (2002) 1142–1153. [DOI] [PubMed] [Google Scholar]
- [10].Haliyur R, Tong X, Sanyoura M, Shrestha S, Lindner J, Saunders DC, Aramandla R, Poffenberger G, Redick SD, Bottino R, et al. , Human islets expressing HNF1A variant have defective β cell transcriptional regulatory networks, J. Clin. Invest. 129 (1) (2019) 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Suga T, Kikuchi O, Kobayashi M, Matsui S, Yokota-Hashimoto H, Wada E, Kohno D, Sasaki T, Takeuchi K, Kakizaki S, et al. , SGLT1 in pancreatic α cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels, Mol Metab. 19 (2019) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee YH, Sauer B, Gonzalez FJ, Laron dwarfism and non-insulin dependent diabetes mellitus in the Hnf-1alpha knockout mouse, Mol. Cell. Biol. 18 (5) (1998) 3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ohki T, Sato Y, Yoshizawa T, Yamamura K, Yamada K, Yamagata K, Identification of hepatocyte growth factor activator (Hgfac) gene as a target of HNF1α in mouse β-cells, Biochem. Biophys. Res. Commun. 425 (3) (2012) 619–624. [DOI] [PubMed] [Google Scholar]
- [14].Araki K, Imaizumi T, Sekimoto T, Yoshinobu K, Yoshimuta J, Akizuki M, Miura K, Araki M, Yamamura K, Exchangeable gene trap using the Cre/mutated lox system, Cell. Mol. Biol. 45 (1999) 737–750. [PubMed] [Google Scholar]
- [15].Mishina M, Sakimura K, Conditional gene targeting on the pure C57BL/6 genetic background, Nuerosci. Res 58 (2007) 105–112. [DOI] [PubMed] [Google Scholar]
- [16].Sato Y, Tsuyama T, Sato C, Karim MF, Yoshizawa T, Inoue M, Yamagata K, Hypoxia reduces HNF4α/MODY1 protein expression in pancreatic β-cells by activating AMP-activated protein kinase, J. Biol. Chem. 292 (21) (2017) 8716–8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sato Y, Inoue M, Yoshizawa T, Yamagata K, Moderate hypoxia induces β-cell dysfunction with HIF-1-independent gene expression changes, PLoS One 9 (12) (2014) e114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yoshizawa T, Karim MF, Sato Y, Senokuchi T, Miyata K, Fukuda T, Go C, Tasaki M, Uchimura K, Kadomatsu T, et al. , Sirt7 controls hepatic lipid metabolism by regulating the ubiquitin-proteasome pathway, Cell Metab 19 (4) (2014) 712–721. [DOI] [PubMed] [Google Scholar]
- [19].Yamagata K, Yang Q, Yamamoto K, Iwahashi H, Miyagawa J, Okita K, Yoshiuchi I, Miyazaki J, Noguchi T, Nakajima H, et al. , Mutation P291fsinsC in the transcription factor hepatocyte nuclear factor-1α is dominant negative, Diabetes. 47 (8) (1998) 1231–1235. [DOI] [PubMed] [Google Scholar]
- [20].Nishimoto M, Katano M, Yamagishi T, Hishida T, Kamon M, Suzuki A, Hirasaki M, Nabeshima Y, Nabeshima Y, Katsura Y, et al. , In vivo function and evolution of the eutherian-specific pluripotency marker UTF1, PLoS One 8 (7) (2013) e68119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Festing MFW, Evidence should trump intuition by preferring inbred strains to outbred stocks in preclinical research, ILAR J 55 (3) (2014) 399–404. [DOI] [PubMed] [Google Scholar]
- [22].Pontoglio M, Barra M, Hadchouel A, Doyen C, Kress JP, Bach C, Babinet M Yaniv, Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal fanconi syndrome, Cell. 84 (4) (1996) 575–585. [DOI] [PubMed] [Google Scholar]
- [23].Akiyama TE, Ward JM, Gonzalez FJ, Regulation of the liver fatty acid-binding protein gene by hepatocyte nuclear factor 1 α (HNF1 α). Alterations in fatty acid homeostasis in HNF1 α -deficient mice, J. Biol. Chem. 275 (35) (2000) 27117–27122. [DOI] [PubMed] [Google Scholar]
- [24].Hiraiwa H, Pan CJ, Lin B, Akiyama TE, Gonzalez FJ, Chou JY, A molecular link between the common phenotypes of type 1 glycogen storage disease and HNF1alpha-null mice, J. Biol. Chem. 276 (11) (2001) 7963–7967. [DOI] [PubMed] [Google Scholar]
- [25].Garcia-Gonzalez MA, Carette C, Bagattin A, Chiral M, Makinistoglu MP, Garbay S, Prévost G, Madaras C, Hérault Y, Leibovici M, Pontoglio M, A suppressor locus for MODY3-diabetes, Sci. Rep. 6 (2016) 33087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fukui K, Yang Q, Cao Y, Takahashi N, Hatakeyama H, Wang H, Wada J, Zhang Y, Marselli L, Nammo T, et al. , The HNF-1 target collectrin controls insulin exocytosis by SNARE complex formation, Cell Metab. 2 (6) (2005) 373–384. [DOI] [PubMed] [Google Scholar]
- [27].Watanabe C, Seino Y, Miyahira H, Yamamoto M, Fukami A, Ozaki N, Takagishi Y, Sato J, Fukuwatari T, Shibata K, et al. , Remodeling of hepatic metabolism and hyperaminoacidemia in mice deficient in proglucagon-derived peptides, Diabetes. 61 (1) (2012) 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tsimihodimos V, Elisaf M, Effects of incretin-based therapies on renal function, Eur. J. Pharmacol. 818 (2018) 103–109. [DOI] [PubMed] [Google Scholar]
- [29].Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, et al. , Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion, Nat. Med. 21 (2015) 512–517. [DOI] [PubMed] [Google Scholar]
- [30].Zhou L, Cryan EV, D’Andrea MR, Belkowski S, Conway BR, Demarest KT, Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1), J. Cell. Biochem. 90 (2003) 339–346. [DOI] [PubMed] [Google Scholar]
- [31].Kekuda R, Saha P, Sundaram S, Role of Sp1 and HNF1 transcription factors in SGLT1 regulation during chronic intestinal inflammation, Am. J. Physiol. Gastrointest. Liver Physiol 294 (6) (2008) G1354–G1361. [DOI] [PubMed] [Google Scholar]
- [32].Solini A, Sebastiani G, Nigi L, Santini E, Rossi C, Dotta F, Dapagliflozin modulates glucagon secretion in an SGLT2-independent manner in murine alpha cells, Diabetes Metab. 43 (6) (2017) 512–520. [DOI] [PubMed] [Google Scholar]
- [33].Blodgett AB, Kothinti RK, Kamyshko I, Petering DH, Kumar S, Tabatabai NM, A fluorescence method for measurement of glucose transport in kidney cells, Diabetes Technol. Ther. 13 (7) (2011) 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cefalo CMA, Cinti F, Moffa S, Impronta F, Sorice GP, Mezza T, Pontecorvi A, Giaccari A, Sotagliflozin, the first dual SGLT inhibitor: current outlook and perspectives, Cardiovasc. Diabetol. 18 (1) (2019) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Heimberg H, De Vos A, Pipeleers D, Thorens B, Schuit F, Differences in glucose transporter gene expression between rat pancreatic alpha- and beta-cells are correlated to differences in glucose transport but not in glucose utilization, J. Biol. Chem. 270 (15) (1995) 8971–8975. [DOI] [PubMed] [Google Scholar]
- [36].Kuhre RE, Ghiasi SM, Adriaenssens AE, Wewer Albrechtsen NJ, Andersen DB, Aivazidis A, Chen L, Mandrup-Poulsen T, Ørskov C, Gribble FM, Reimann F, Wierup N, Tyrberg B, Holst JJ, No direct effect of SGLT2 activity on glucagon secretion, Diabetologia. 62 (6) (2019) 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Østoft SH, Bagger JI, Hansen T, Pedersen O, Holst JJ, Knop FK, Vilsbøll T, Incretin effect and glucagon responses to oral and intravenous glucose in patients with maturity-onset diabetes of the young-type 2 and type 3, Diabetes. 63 (8) (2014) 2838–2844. [DOI] [PubMed] [Google Scholar]
- [38].Hansen T, Eiberg M, Rouard M, Vaxillaire AM, Møller SK, Rasmussen M, Fridberg SA, Urhammer JJ, Holst K, Almind SM, Hansen Echwald L., Bell GI, Pedersen O, Novel MODY3 mutations in the hepatocyte nuclear factor-1alpha gene: evidence for a hyperexcitability of pancreatic beta-cells to intravenous secretagogues in a glucose-tolerant carrier of a P447L mutation, Diabetes. 46 (4) (1997) 726–730. [DOI] [PubMed] [Google Scholar]
- [39].Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT, Genetic cause of hyperglycaemia and response to treatment in diabetes, Lancet. 362 (9392) (2003) 1275–1281. [DOI] [PubMed] [Google Scholar]
- [40].Tuomi T, Honkanen EH, Isomaa B, Sarelin L, Groop LC, Improved prandial glucose control with lower risk of hypoglycemia with nateglinide than with glibenclamide in patients with maturity-onset diabetes of the young type 3, Diabetes Care 29 (2) (2006) 189–194. [DOI] [PubMed] [Google Scholar]
- [41].Zhang Q, Ramracheya R, Lahmann C, Tarasov A, Bengtsson M, Braha O, Braun M, Brereton M, Collins S, Galvanovskis J, et al. , Role of K-ATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes, Cell Metab. 18 (6) (2013) 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pedersen MG, Ahlstedt I, Hachmane MFEL, Göpel SO, Dapagliflozin stimulates glucagon secretion at high glucose: experiments and mathematical simulations of human A-cells, Sci. Rep. 6 (2016) 31214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gribble FM, Williams L, Simpson AK, Reimann F, A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line, Diabetes. 52 (5) (2003) 1147–1154. [DOI] [PubMed] [Google Scholar]
- [44].Parker HE, Adriaenssens A, Rogers G, Richards P, Koepsell H, Reimann F, Gribble FM, Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion, Diabetologia. 55 (9) (2012) 2445–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kuhre RE, Frost CR, Svendsen B, Holst JJ, Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine, Diabetes. 64 (2) (2015) 370–382. [DOI] [PubMed] [Google Scholar]
- [46].Dukes ID, Sreenan S, Roe M, Levisetti M, Zhou YP, Ostrega D, Bell GI, Pontoglio M, Yaniv M, Philipson L, Polonsky KS, Defective pancreatic beta-cell glycolytic signaling in hepatocyte nuclear factor-1alpha-deficient mice, J. Biol. Chem. 273 (38) (1998) 24457–24464. [DOI] [PubMed] [Google Scholar]
- [47].Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, et al. , Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice, Proc. Natl. Acad. Sci. U. S. A. 100 (3) (2003) 1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Longuet C, Robledo AM, Dean ED, Dai C, Ali S, McGuinness I, de Chavez V, Vuguin PM, Charron MJ, Powers AC, Drucker DJ, Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: evidence for a circulating α-cell growth factor, Diabetes. 62 (4) (2013) 1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cardenas-Diaz FL, Osorio-Quintero C, Diaz-Mirand MA, Kishore S, Leavens K, Jobaliya C, Stanescu D, Ortiz-Gonzalez X, Yoon C, Chen S, et al. , Modeling monogenic diabetes using human ESCs reveals developmental and metabolic deficiencies caused by mutations in HNF1A, Cell Stem Cell 25 (2) (2019) 273–289.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.