Abstract
Children with severe sepsis are known to have altered zinc homeostasis and decreased circulating zinc levels, suggesting a role for zinc supplementation to improve outcomes. We tested the hypothesis that zinc supplementation would improve survival in a juvenile model of polymicrobial sepsis. Juvenile (13–14-d-old) C57BL/6 mice were treated with 10 mg/kg of zinc via i.p. injections (or vehicle) for 3 d prior to induction of polymicrobial sepsis via i.p. cecal slurry injections. Survival after sepsis was followed for 3 d, and bacterial clearance, ex vivo phagocytosis, systemic inflammatory markers and neutrophil extracellular trap (NET) formation were quantified. We found a significant survival benefit and decreased bacterial burden among zinc supplemented mice when compared with the control group. Zinc supplementation also resulted in enhanced phagocytic activity, greater neutrophil recruitment in the peritoneal cavity and NET formation, suggesting a possible mechanism for improved bacterial clearance and survival. We also noted decreased serum cytokine levels and decreased myeloperoxidase activity in lung tissue following zinc supplementation, suggesting attenuation of the systemic inflammatory response. In conclusion, zinc supplementation improves bacterial clearance, and hence survival, in juvenile mice with polymicrobial sepsis.
Keywords: Neutrophil extracellular trap, pediatric, sepsis, zinc
Introduction
Pediatric sepsis is a devastating illness that poses a significant global burden, with an estimated mortality of 25%.1 Up to 67% of children with severe sepsis may have associated multiorgan dysfunction at diagnosis, with an additional 30% of them developing new or progressive multiorgan dysfunction.1 Historically, sepsis was recognized as an exaggerated systemic immune response to an infectious etiology, resulting in a pro-inflammatory state that led to organ dysfunction. Subsequent studies have shifted the paradigm and we now understand sepsis as a maladaptive immune response that also involves activation of antiinflammatory pathways.2 This maladaptive response involves multiple concurrent processes that simultaneously involve both exaggerated inflammation and immune suppression.3
Antibiotics, source control and supportive care in intensive care units have been the mainstays of the treatment of sepsis, but a clearer understanding of the immune dysfunction suggests that modulation of the host immune response could offer potential treatment options.4 There is significant literature establishing the importance of zinc in normal immune function,5-9 suggesting that zinc supplementation might be a potential treatment option targeting the host immune response in sepsis. Data from murine polymicrobial sepsis models suggest that zinc deficiency can significantly increase systemic inflammation, organ damage and mortality, and short-term zinc repletion can ameliorate these effects.10 Consistent with these experimental observations, human studies involving critically ill adults with sepsis demonstrated an inverse correlation between plasma zinc concentrations, and inflammatory markers and markers of oxidative damage.11 A multicenter, randomized, controlled trial tested supplementation with a combination of zinc, selenium, glutamine and metoclopramide, and showed no improvement in sepsis outcomes. However, this trial used a multipronged approach making it impossible to tease out whether one of the therapies might have had a detrimental effect, potentially masking the beneficial effect of zinc.12
Genome-wide expression studies of children with sepsis demonstrated differential expression of genes that either depend on normal zinc homeostasis for normal function or directly contribute to zinc homeostasis. This observation was particularly evident in nonsurvivors of sepsis and was associated with abnormally low serum zinc concentrations.13 Subsequent studies showed improved survival and immune function after zinc supplementation in adult mice challenged with polymicrobial sepsis.14 However, the beneficial effects of zinc supplementation in adult mice cannot be assumed in juvenile mice because of well-established developmental differences in the immune response to sepsis.15 For example, inhibition of matrix metalloproteinase 8 is beneficial in adult models of sepsis but detrimental in a juvenile model of sepsis.2,12,16 To our knowledge, zinc supplementation has not been tested in a juvenile model of sepsis.
In the current study we tested the hypothesis that zinc supplementation would improve survival in a polymicrobial model of sepsis induced in juvenile mice. We further performed immunological and histochemical studies to provide a mechanistic understanding of the survival benefit conferred by zinc.
Materials and methods
Animals
All experiments were performed using juvenile (13–14-d-old) C57BL/6 mice that were bred and housed within the vivarium at Cincinnati Children’s Hospital Medical and Research Center. For the purpose of breeding, male and female adult (3-mo-old) wild type C57BL/6 mice were obtained from Charles Rivers Laboratories (Wilmington, MA, USA). All mice were housed within adequately ventilated cages, with easily accessible rodent chow and water, stable environmental and temperature conditions, and 12 h light/dark cycles. Care and handling of the animals complied with the National Institutes of Health guidelines for ethical animal treatment, and all procedures were approved by the Institutional Animal Care and Use Committee at Cincinnati Children’s Hospital Medical and Research Center.
Cecal slurry model
We prepared cecal slurry using methods previously published by Wynn et al.17 Briefly, using carbon dioxide, we euthanized three female C57BL/6 mice aged 7–10 wk, performed a midline laparotomy and excised whole cecum from each mouse. We then made an incision to the distal-most part of each cecum, and expressed and collected the contents. Cecal contents from all three donor mice were weighed together, and 5% dextrose in water (D5W) was added to make a cecal slurry at a concentration of 80 mg/ml. This slurry was vortexed and then filtered through a sterile 100-μm filter to capture any remaining large particles, producing a homogenous solution that would pass through a 27-gauge needle. For each experiment, we prepared fresh cecal slurry with three donor C57BL/6 female mice, and comparisons were made between groups of animals that received the same batch of cecal slurry.
We induced polymicrobial sepsis by removing each juvenile mouse from its mother, and administered i.p. injections of cecal slurry using a 27-gauge needle, at a dose of 0.6 mg per gram of body mass. The juvenile mouse was then returned to the cage with its mother and litter. Supportive measures such as antibiotics, analgesics or fluid resuscitation were not provided. All infected mice were followed for survival (at 6 h intervals) up to 72 h, or depending on the experiment, sacrificed at 6 or 12 h for procurement of biological specimens. In earlier experiments (unpublished data) we tested different cecal slurry doses, but found doses higher than 0.6 mg/g to be highly lethal, whereas doses <0.6 mg/g were ineffective at producing sepsis.
Zinc supplementation
Prior to induction of polymicrobial sepsis, we supplemented juvenile mice with 10 mg/kg zinc gluconate solution (Alfa Aesar; Thermo Fisher Scientific, Waltham, MA, USA), via once-daily i.p. injections using 27-gauge needles, for 3 d prior to cecal slurry administration. This dose was selected based on our prior experience and established efficacy in previously published experiments.14 Mice in the control group were administered an equal volume of PBS. We prepared and stored all solutions in a sterile manner, and warmed them to 37°C prior to injection.
Cytokine measurements
To measure cytokine levels, we collected blood samples from juvenile mice 6 h and 12 h after cecal slurry administration. Owing to significant mortality observed after the initial 12 h, we did not measure cytokine levels beyond the 12 h time point, as this would have introduced survival bias. Blood was harvested after euthanizing mice via CO2 asphyxiation, and using a 27-gauge needle to perform cardiac puncture. Blood samples were then centrifuged for 10 min at 20,817 g to separate out the components, and serum was separated and stored for testing. We then analyzed the serum samples for IL-1β, IL-2, IL-6, IL-10, macrophage inflammatory protein-1α (MIP-1α), TNF-α, and keratinocyte-derived chemokine (KC), using a Luminex Multiplex system (Millipore, Billerica, MA, USAC), as per the manufacturer’s instructions.
Myeloperoxidase activity in lung tissue
We harvested lung tissue after euthanizing juvenile mice 12 h after induction of polymicrobial sepsis, and measured myeloperoxidase (MPO) activity using a MPO Colorimetric Activity Assay Kit (Cat# MAK068; Sigma-Aldrich, St. Louis, MO, USA). Lung tissue was weighed and homogenized in the buffer solution provided with the kit, and the assay was performed per manufacturer’s recommendations. In this assay, MPO catalyzes the formation of hypochlorous acid, which reacts with taurine to form taurine chloramine. Taurine chloramine then reacts with the chromophore 5-thio-2-nitrobenzoic acid (TNB), resulting in the formation of the colorless product 5,5′-dithio-bis-2-nitrobenzoic acid. Color change was measured on a SpectraMax Plus 384 Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) using 412 nm absorbance, and the colorimetric change was used to calculate MPO concentration in each sample as per the manufacturer’s instructions.
Bacterial clearance in the peritoneum
To evaluate for bacterial clearance after induction of polymicrobial sepsis, we euthanized the juvenile mice via carbon dioxide asphyxiation, 12 h after administration of cecal slurry. We then performed a peritoneal lavage by injecting 500 μl sterile PBS into the peritoneal cavity using a 27-gauge needle, and aspirating back 150 μl after the animal was manipulated to ensure uniform distribution of the fluid within the peritoneal cavity. We made serial dilutions of the peritoneal fluid obtained, and plated it onto sheep blood agar plates for the purpose of culturing and quantification of bacterial colonies. All plates were incubated at 37°C for 48 h, and plated dilutions with 30–300 colonies were used to quantify bacterial colonies.
Phagocytosis assay for peritoneal macrophages
For these experiments, juvenile mice were not exposed to cecal slurry but did receive 3 d of zinc supplementation (or PBS for the control group). Peritoneal macrophages were harvested by performing peritoneal lavage as described above, and the peritoneal fluid from individual mice was pooled together and centrifuged for 10 min at 153 g. We re-suspended the resulting pellet in DMEM (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), counted the cells using a hemocytometer, and resuspended to a final concentration of 106 cells/ml.
We plated the cells onto a 96-well tissue culture plate at a concentration of 105 cells per well, and added DMEM to bring up the volume of each well to 150 μl. For negative controls, we added 150 μl DMEM per well. Tissue-culture plates were then covered and incubated for 2 h at 37°C and room air/5% CO2. After the 2 h incubation we emptied all wells using vacuum aspiration, and proceeded to evaluate phagocytosis of Escherichia coli or Staphylococcus aureus using the Vybrant™ Phagocytosis Assay Kit (Molecular Probes, Thermo Fisher Scientific Inc., Eugene, OR, USA) as per the manufacturer’s instructions.
Briefly, we incubated the cells with either fluorescein-labeled, non-opsonized E. coli or S. aureus for 2 h at 37°C and room air/5% CO2. The wells were then drained using vacuum aspiration, and residual extracellular fluorescein activity was inhibited using trypan blue. After 1 min incubation, the trypan blue suspension was aspirated, and we measured the intracellular fluorescein activity in a SpectraMax Plus 384 Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) using 480 nm excitation and 520 nm emission wavelength. Fluorescein readings were determined by subtracting the background fluorescence as determined by the negative-control readings, from the experimental well readings.
Flow cytometry
We performed flow cytometry to quantify the cellular composition of peritoneal cavity before cecal slurry challenge. Single-cell suspensions were prepared from peritoneal lavage samples collected following three days of zinc (or vehicle) supplementation, as described above. We suspended the cells in FACS buffer (PBS with 1% bovine albumin and 0.1% azide) and added 10% donkey serum (Invitrogen, Life Technologies, Grand Island, NY, USA) to prevent non-specific binding to cells. Cells were then stained with appropriate dilutions of the following Abs: CD11b Alex Fluor 450, Ly-6C PE Cy7, F4/80 APC, GR-1 APC Cy7 (Tonbo Biosciences, San Diego, CA, USA) and 7AAD (Life Technologies, Carlsbad, CA, USA). Non-viable cells were exclude based on 7AAD staining. CD11b+ cells were then gated for F4/80+ (macrophages) and GR-1+ (neutrophils). We analyzed all samples using FlowJo Software (FlowJo, LLC, Ashland, OR, USA).
Neutrophil extracellular trap formation and neutrophil extracellular trap efficacy
To quantify neutrophil extracellular trap (NET) formation, we euthanized juvenile mice 6 h after induction of polymicrobial sepsis, and collected peritoneal fluid as described above. One hundred μl of each peritoneal lavage sample was pipetted onto a glass slide and airdried overnight. The slides were blocked for 2 h at 20°C in PBS containing 2% BSA, followed by staining for 5 min with Sytox Green 1:10,000 and DAPI 1:50,000 (Life Technologies). Slides were then washed with PBS and mounted with ProLong Gold antifade reagent (Life Technologies). For co-staining studies, anti-neutrophil elastase Ab (1:200 dilution; Santa Cruz, Dallas, TX, USA) and anti-histone H4 Ab (1:1000 dilution; AbCam, San Francisco, CA, USA) were used with appropriate fluorophore-conjugated secondary Abs (1:200 dilution; Jackson ImmunoResearch, West Grove, PA, USA). Slides were imaged using a Nikon 90i upright wide-field microscope with LED light source, and three images per animal were captured at 20× magnification.
NET formation was quantified using Imaris (v7.7.1; Bitplane AG, Mathworks, Natick, MA, USA). The total DNA of a field was defined as signal above negative control in the green channel (Sytox Green). Nuclear surfaces were defined using a higher threshold DAPI signal which excluded non-nuclear DNA, and nuclear DNA was defined as a green signal within this surface. The fraction of DNA outside of the nucleus was then used as a measure of NET formation. To quantify the number of neutrophils and total number of cells, a surface was created using the neutrophil elastase stain, and nuclei within 1 μm of this surface were considered neutrophils and those greater than 1 μm from this surface as non-neutrophils.
To evaluate for differences in NET function, we suspended Texas Red-tagged E. coli (Life Technologies) in cecal slurry at a concentration of 1 μg/ml, and injected the mixture i.p. through a 27-gauge needle at a 0.6 mg/kg dose. Peritoneal fluid was then collected 6 h following injection of cecal slurry and tagged E. coli mixture, and slides were stained and imaged as described above. Using the Imaris software package for analysis, bacteria were defined as red signal over background and DNA was defined as green signal over negative control. We defined bacteria within 1 μm of the DNA signal as being associated with the NET, and greater than 1 μm as not associated. To normalize for differences in sample dilution, the fractional coverage of DNA (total DNA area/total image area) was used to create a ‘predicted’ bacteria count that estimated the number of bacteria that would be expected to be associated with the NET by chance. The ratio of observed-to-predicted bacteria was then calculated for each image to compare NET efficiency.
Statistical analysis
We used SigmaPlot version 13.0 (Systat Software Inc., San Jose, CA, USA) to perform statistical analyses. Continuous data with two groups were compared using Student’s t-test, and in situations where data were not normally distributed, we performed the Mann–Whitney Rank Sum test. We presented all data as medians with interquartile ranges. To compare survival between different groups, we performed a log rank survival analysis. For all analyses, P-values < 0.05 were considered significant.
Results
Zinc supplementation improves survival among juvenile mice following polymicrobial sepsis
Juvenile mice that were pretreated with zinc supplementation, demonstrated significantly improved survival at 72 h after cecal slurry administration. As shown in Figure 1, 50% of zinc-supplemented mice survived vs. 8% survival in the control group (P = 0.002). This finding supports our hypothesis that zinc supplementation confers a survival benefit in juvenile mice with sepsis secondary to polymicrobial peritonitis.
Figure 1.
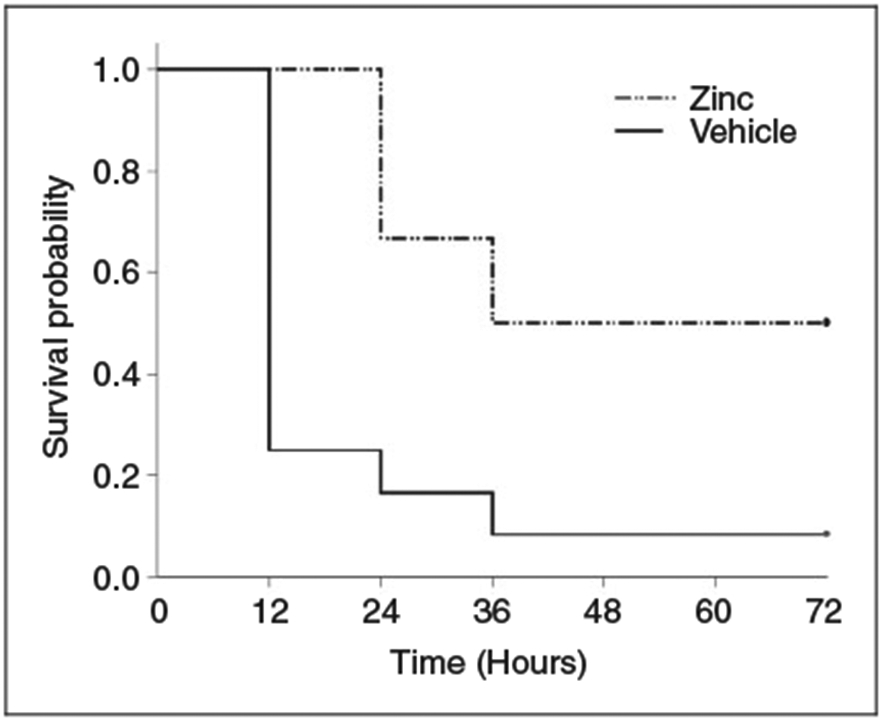
Kaplan–Meier survival curve comparing zinc-supplemented mice with control mice. Survival was monitored for 72 h, and zinc supplemented mice had significant survival advantage (n = 12 mice per group; P = 0.002).
Zinc supplementation modifies the inflammatory cytokine response following polymicrobial sepsis
To study the impact of zinc supplementation on inflammatory cytokine response, we quantified serum cytokine levels in juvenile mice at two time points: 6 h and 12 h after cecal slurry administration. As depicted in Figure 2, zinc-supplemented mice had significantly lower levels of IL-1β, IL-2 and IL-6 when compared with the control group, 6 h after induction of sepsis. At the 12-h time point, zinc-supplemented mice had significantly lower levels of IL-2, IL-6 and KC when compared with the control group. We also quantified levels of IL-10, MIP-1α and TNF-α, and these were not influenced by zinc supplementation at either time point (data not shown).
Figure 2.
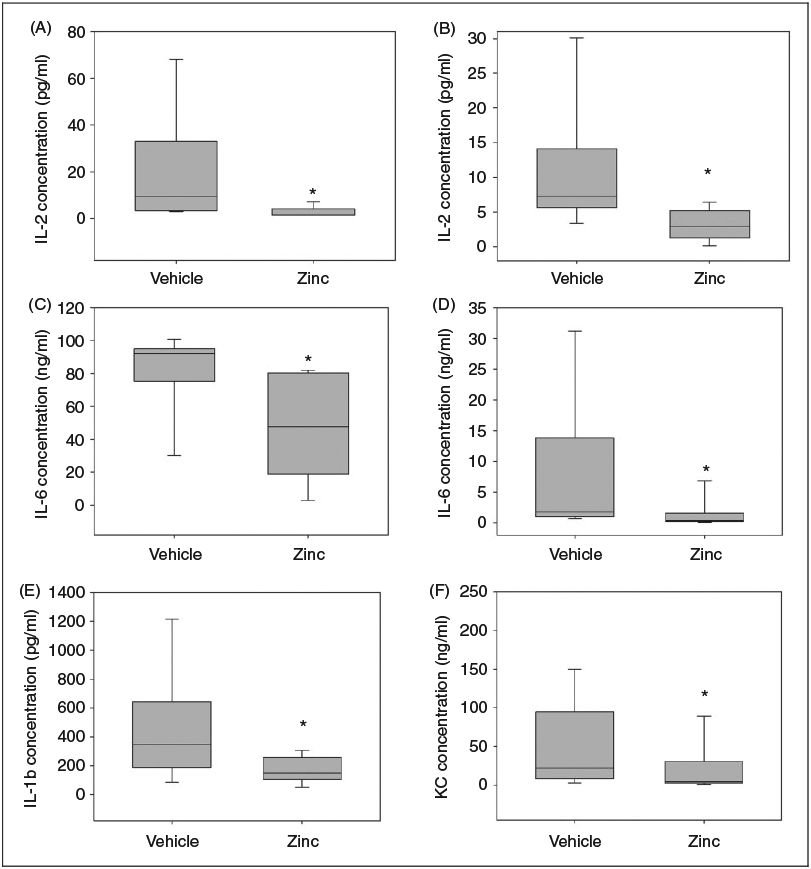
Zinc supplementation suppresses serum cytokine levels following cecal slurry administration. (A) Serum IL-2 concentration, 6 h after cecal slurry injection. (B) Serum IL-2 concentration, 12 h after cecal slurry injection. (C) Serum IL-6 concentration, 6 h after cecal slurry injection. (D) Serum IL-6 concentration, 12 h after cecal slurry injection. (E) Serum IL-1β concentration, 6 h after cecal slurry injection. (F) Serum KC concentration, 12 h after cecal slurry injection. (n = 8 mice per group for 6-h experiments, and n = 15 mice per group for 12-h experiments; *P < 0.05). Data are expressed as medians and interquartile ranges.
Zinc supplementation reduces remote inflammatory response in the lung
We assessed the impact of zinc supplementation on remote organ inflammation by measuring MPO activity in homogenized lung tissue as a surrogate marker for neutrophil infiltration. As shown in Figure 3, MPO activity in zinc-supplemented mice was significantly lower when compared with control mice 12 h after cecal slurry injection, suggesting that zinc supplementation may suppress neutrophil recruitment, inflammatory response and subsequent injury to the lung after polymicrobial sepsis.
Figure 3.
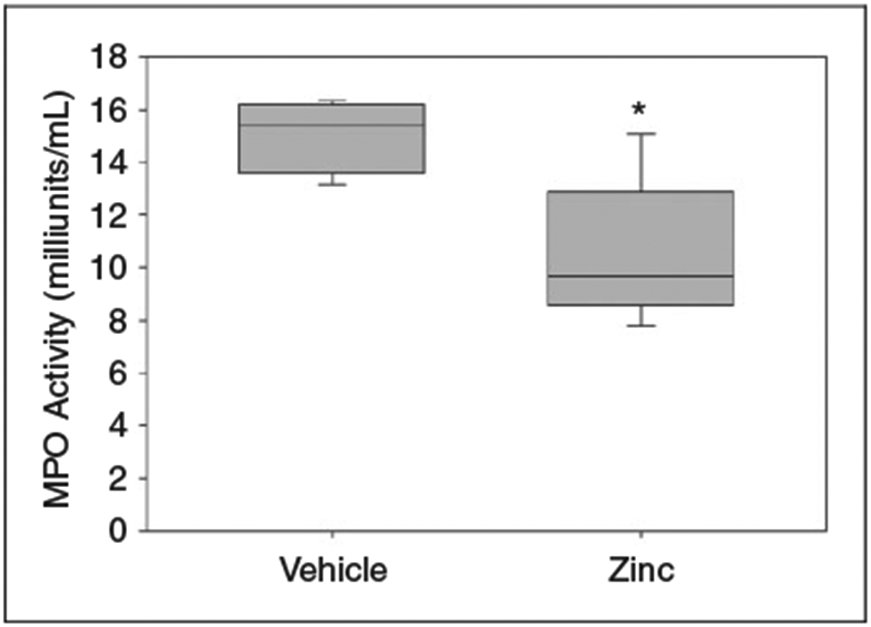
MPO activity in homogenized lung tissue, 12 h after cecal slurry injection. Zinc-supplemented mice demonstrated significantly decreased MPO activity (*P = 0.02 vs. vehicle, n = 5 mice per group). Data are expressed as medians and interquartile ranges. One unit of MPO activity (1 milliunit/ml) is defined as the amount of enzyme that hydrolyzes the substrate and generates taurine chloramine to consume 1.0 μmol TNB/min at 25°C.
Zinc supplementation augments bacterial clearance and phagocytosis in juvenile mice
To further investigate the mechanism of survival benefit with zinc supplementation, we quantified the bacterial load within peritoneal fluid, 12 h after cecal slurry administration. As shown in Figure 4, juvenile mice that received pretreatment with zinc supplementation had significantly fewer bacterial colonies when compared with the control group, indicating improved bacterial clearance.
Figure 4.
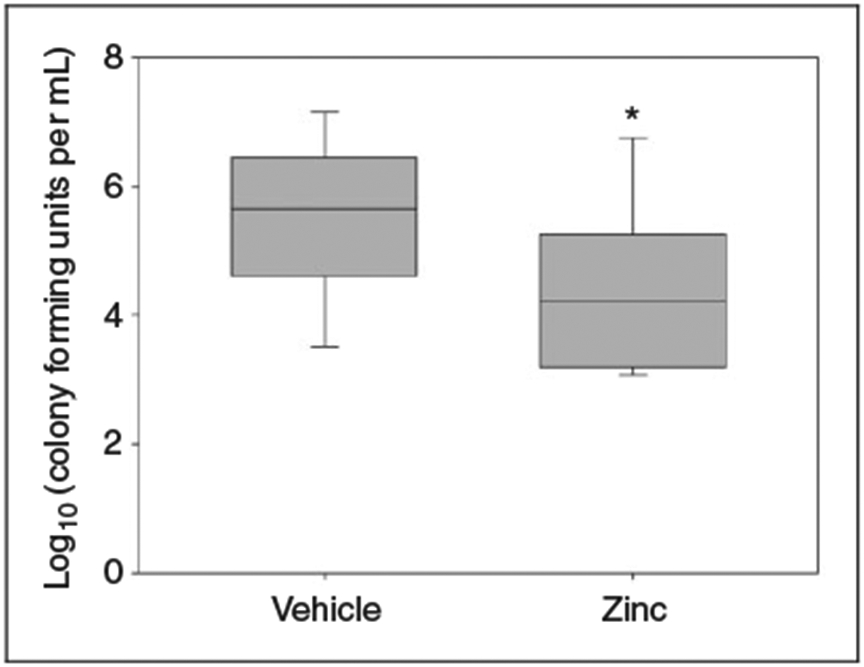
Log scale of bacterial colony counts from peritoneal lavage, 12 h after cecal slurry administration. Zinc-supplemented mice demonstrated significantly lower colony counts compared with controls (n = 18 mice per group, *P = 0.039 vs. vehicle). Data are expressed as medians and interquartile ranges.
We then performed an ex vivo phagocytosis assay, and found that peritoneal macrophages harvested from zinc-supplemented mice had significantly enhanced phagocytic activity when exposed to fluorescently tagged E. coli (Figure 5A), compared with peritoneal macrophages from control mice. When exposed to fluorescently tagged S. aureus, we found no difference between the peritoneal macrophages from zinc-supplemented mice and control mice (Figure 5B).
Figure 5.
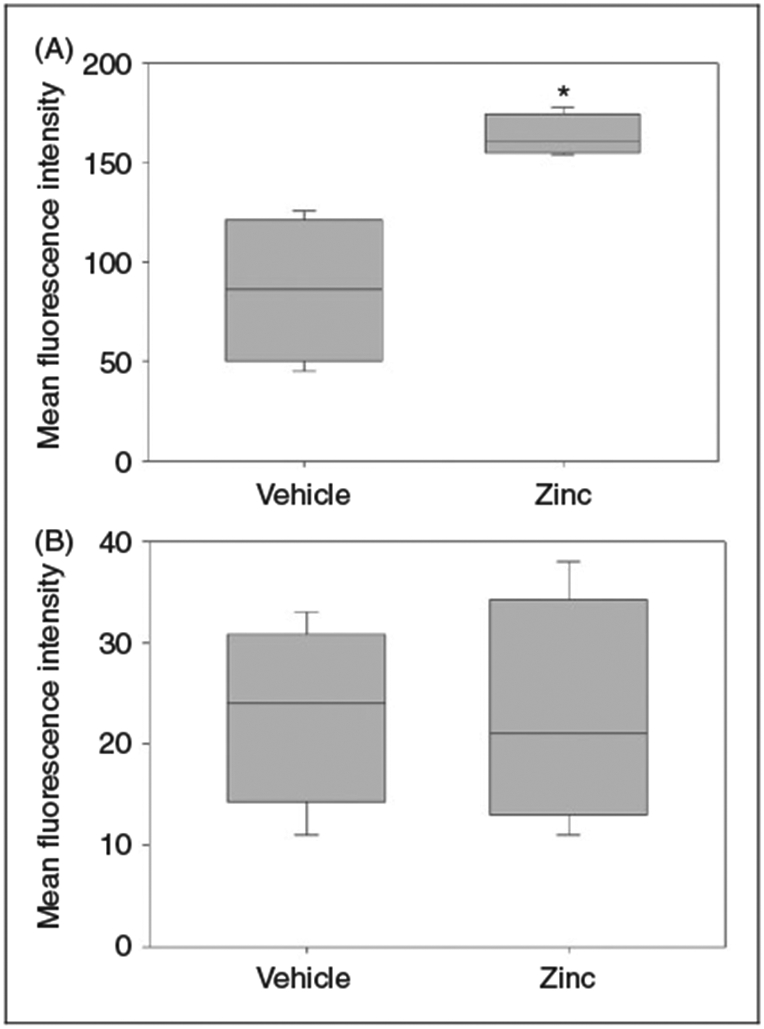
Ex vivo phagocytosis assay performed using peritoneal macrophages from zinc supplemented and control mice. (A) Peritoneal macrophages from zinc-supplemented mice demonstrated increased phagocytic activity when tested with fluorescently labeled E. coli (*P = 0.029 vs. vehicle; n = 12 mice per group). (B) No difference in phagocytic activity observed with fluorescently labeled S. aureus (P = 0.487; n = 12 mice per group). Data expressed as medians and interquartile ranges.
These findings suggest that the survival benefit with zinc pretreatment might be secondary to enhanced phagocytosis activity that leads to improved bacterial clearance of Gram-negative organisms and a lower bacterial burden.
Zinc supplementation does not influence baseline cellular composition of the peritoneal cavity
We performed flow cytometry to assess the impact of zinc supplementation on neutrophil and macrophage counts within the peritoneal cavity prior to cecal slurry challenge. Macrophages were the predominant cells in zinc-supplemented mice, as well as the control group (72% and 67%, respectively), followed by neutrophils (12% and 11%, respectively). As illustrated in Figure 6, there was no significant difference in the neutrophil or macrophage counts among the two groups.
Figure 6.
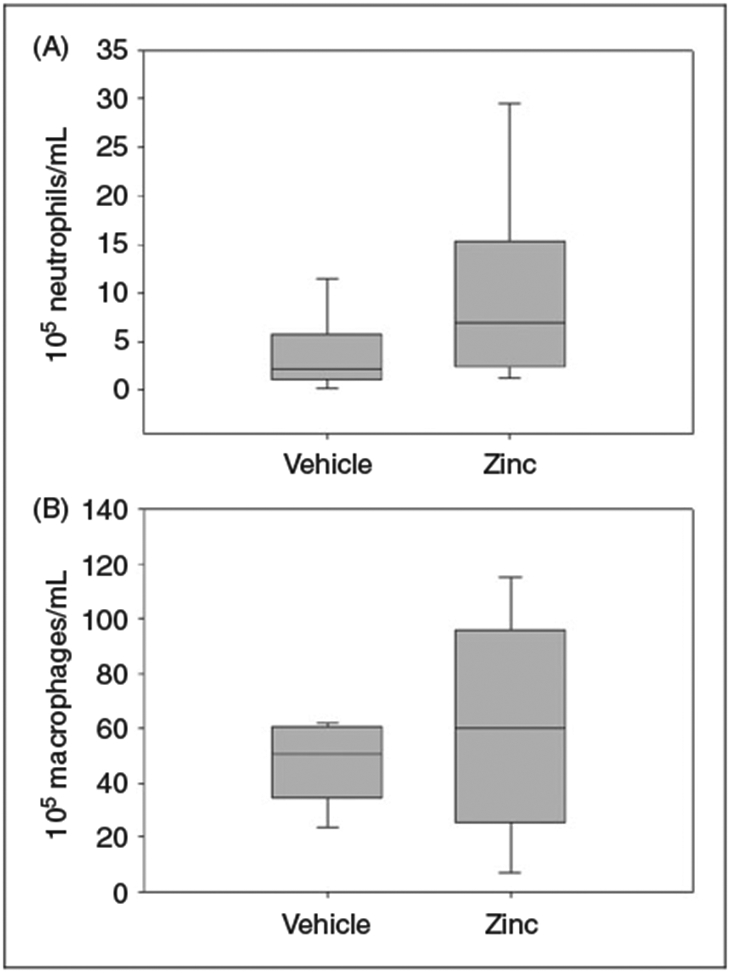
Flow cytometry performed on peritoneal fluid from zinc-supplemented and control mice. (A) No significant difference in neutrophil counts following zinc supplementation (P = 0.31 vs. vehicle; n = 6 mice per group). (B) No significant difference in macrophage counts following zinc supplementation (P = 0.99 vs. vehicle; n = 6 mice per group).
Zinc supplementation increases neutrophil recruitment and NET content in the peritoneum but does not influence NET efficacy
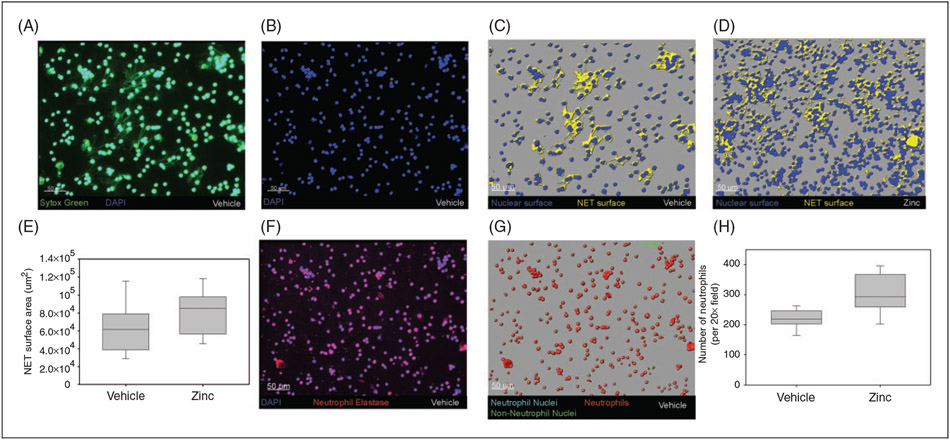
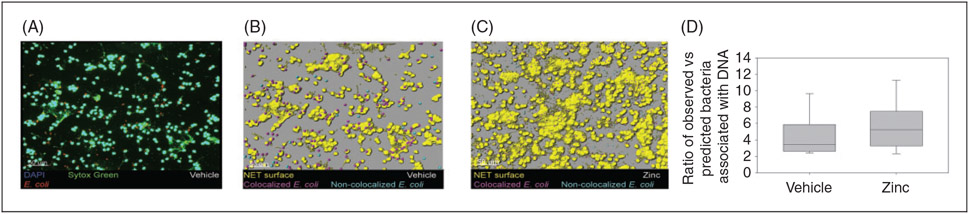
To further understand the enhanced bacterial clearance observed in our experiments, we assessed for differences in NET formation between zinc-supplemented mice and the control group, 6 h after cecal slurry administration. As illustrated in Figure 7, we found a significantly higher number of peritoneal neutrophils among zinc-supplemented mice (as visualized on stained slides), and a larger total NET surface area when compared with control mice. Figure 6 displays representative slides from vehicle-supplemented and zinc-supplemented animals, showing a greater number of neutrophils and significant NET formation following zinc administration. We then tested NET efficacy by evaluating entrapment of Texas Red-labeled E. coli bioparticles within the neutrophil traps. Pretreatment with zinc did not produce any significant change in the proportion of tagged E. coli associated with NETs, when compared with control mice (Figure 8).
Figure 7.
Neutrophilia and increased NET content in zinc-supplemented mice. (A) Representative slide from peritoneal lavage of vehicle-treated mouse, stained with Sytox Green (green) and DAPI (blue). (B) Attenuated DAPI-alone image to identify nuclei. (C) Representative nuclear (blue) and NET (yellow) surfaces. (D) Nuclear and NET surfaces on representative zinc-treated mouse. (E) Boxplot comparing total NET surface area of vehicle and zinc-supplemented mice (P = 0.03). (F) Slide from same vehicle-treated mouse, stained for neutrophil elastase (red). (G) Neutrophils (aqua spheres) differentiated from non-neutrophils (green spheres) based on association with neutrophil elastase. (H) Boxplot comparing number of neutrophils per 20× field among vehicle and zinc-treated mice (P < 0.001).
Figure 8.
Zinc supplementation does not increase bacterial adherence to NETs. (A) Representative slide from peritoneal lavage of vehicle-treated mouse, with Sytox Green (green) and DAPI (blue) staining, and Texas Red-labelled E. coli (red). (B) Representative slide from vehicle treated mouse, showing NET surface (yellow) with associated (magenta) and non-associated (aqua) E. coli. (C) Representative image from a zinc-treated mouse. (D) Boxplot comparing ratio of bacteria associated with NETs among vehicle and zinc-treated mice (P = 0.123).
These findings show that while zinc supplementation did not influence NET efficacy, it was associated with more robust recruitment of neutrophils at the site of insult and a resultant increase in NET formation, possibly further explaining the improved bacterial clearance.
Discussion
In this study we demonstrate improvement in survival among juvenile mice with polymicrobial sepsis when pretreated with zinc. Our findings are consistent with prior animal studies performed using adult mice,10,14 but this is the first time that beneficial effects of zinc have been tested in a juvenile model of murine sepsis. Zinc supplementation was associated with decreased bacterial burden in the peritoneal fluid, and increased ex vivo phagocytosis of E. coli by peritoneal macrophages. These findings suggest that the survival benefit following zinc supplementation is, in part, secondary to improved phagocytic activity of peritoneal macrophages, and a resulting enhancement of bacterial clearance. We also demonstrated that zinc supplementation led to greater recruitment of peritoneal neutrophils and a resulting increase in NET formation, which would also account for the enhanced bacterial clearance and survival advantage observed after zinc supplementation.
Generalized peritonitis produced by cecal ligation and puncture (CLP) is the more commonly used model of polymicrobial sepsis in adult mice. However, CLP is technically challenging to perform on younger mice owing to their small size, gut friability and the increased risk for maternal cannibalization after the procedure. Additionally, CLP is associated with a higher risk of inter-operator variability.17 To overcome these technical limitations, we used the cecal slurry model of polymicrobial sepsis for our experiments.
To our knowledge, this is the first study that has explored the impact of zinc on NET formation in a murine sepsis model. NETosis (formation of NETs) was first reported in 2004 by Brinkmann et al.,18 and described as a unique antimicrobial mechanism whereby activated neutrophils release a mesh-like structure capable of ensnaring and eliminating microbes. These NETs are composed primarily of nuclear DNA and histones, and contain antimicrobial peptides housed within neutrophil granules.18 The DNA fibers serve to immobilize and physically contain microbes, whereas histones and granular proteins such as neutrophil elastase, bacterial permeability increasing protein, MPO, cathepsin G and defensins confer microbicidal activity.19,20 The exact mechanism by which zinc supplementation enhances NET formation remains to be demonstrated, but appears to be related to increased neutrophil recruitment to the peritoneal compartment.
Zinc supplementation also has modulatory effects on the inflammatory cascade, as evident by the suppressed levels of circulating serum IL-2, IL-6, IL-1β and KC seen in our experiments. Theoretically, this suppression would dampen the hyper-inflammatory response to infectious agents that is typically observed in sepsis, and therefore prevent end-organ injury. This is also corroborated by our observation of decreased MPO levels in the lungs of zinc-supplemented mice, suggesting decreased neutrophil infiltration and decreased inflammation in the lung tissue. Zinc supplementation did not influence TNF-α levels in our experiments, and similar findings have also been noted in adult mice.14 We do not have a clear mechanistic understanding of this finding, but it does suggest that zinc exerts its immune-modulating effects independent of TNF-α mediated pathways. Zinc deficiency reportedly enhances the acute phase response to sepsis through up-regulation of the JAK-STAT3 pathway, thereby perpetuating increased inflammation that may lead to increased morbidity and mortality.21 More recently, a zinc importer called ZIP14 has been implicated in zinc dyshomeostasis during polymicrobial sepsis, with ablation of ZIP14 in murine models leading to increased cytokine concentrations (IL-6, IL-10 and TNF-α). This effect was overcome through dietary zinc supplementation, leading to reduced severity of sepsis, as shown by amelioration of cytokine expression.22 The existing literature, in addition to our findings, lends support to the role of zinc as an immunomodulatory agent in sepsis.
Although we report data from a pediatric model of murine polymicrobial sepsis, several clinical studies from developing countries have consistently shown the beneficial effects of zinc supplementation among children in rural settings. It has been shown to decrease incidence, severity and duration of diarrheal illnesses,23 and a trial from India established a significant reduction in acute lower respiratory tract infections and associated morbidity following zinc supplementation in preschool children.24 Similar findings were reported by a meta-analysis that showed preventive zinc supplementation led to reduction in diarrhea and pneumonia related mortality.25 It can be argued that owing to the presumably better nutritional status and lack of zinc deficiency among most children in the developed world, these clinical studies cannot be generalized. However, as we have mentioned above, sepsis can be associated with marked zinc dyshomeostasis that can be reversed with zinc supplementation.
Our previous experiments tested zinc supplementation as a therapeutic intervention after induction of sepsis in adult mice, but failed to show benefit or harm (unpublished data). Therefore, we only pursued prophylactic zinc supplementation in our experiments on juvenile mice. The fact that we administered zinc supplementation prior to induction of sepsis may be construed as a weakness of our study. However, we note that a significant number of patients develop sepsis while admitted to inpatient wards or ICUs for other indications. This was corroborated by a recent study of more than 800,000 surgical patients, which showed that patients with pneumonia were seven times as likely to develop sepsis, and patients with surgical site infections were 13 times as likely to develop sepsis.26 This provides an opportunity to administer relatively inexpensive and safe prophylactic treatments for patients at high risk of developing sepsis. Our study design suggests that zinc supplementation might be a candidate for this type of approach. Of note, however, zinc supplementation for neonatal sepsis was assessed in a randomized clinical trial in India, and showed no significant benefit.27 It is unclear whether our current dosing strategy of zinc in mice is commensurate with this previous clinical trial. Studies involving intravenous zinc supplementation in critically ill children demonstrate that relatively high doses are required to restore normal zinc levels in this population.28 Clearly, further studies need to be conducted in order to optimize zinc supplementation as a prophylactic strategy in the clinical setting.
Conclusion
This is the first study to establish the survival benefits of zinc supplementation in a juvenile murine model of polymicrobial sepsis. The mechanism of protection appears to involve enhanced bacterial clearance mediated by enhanced phagocytosis, and enhanced neutrophil recruitment leading to increased NET formation. A generalized anti-inflammatory effect of zinc supplementation might also contribute to the mechanism of protection. Additional studies are required to further elucidate the mechanisms of protection afforded by zinc supplementation.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants R01GM099773 and R01GM108025, and the Cincinnati Children’s Hospital Research Foundation.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191: 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson SJ, Nolan M, Klingbeil L, et al. Intestine-derived matrix metalloproteinase-8 is a critical mediator of polymicrobial peritonitis. Crit Care Med 2016; 44: e200–e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga WJ, Leopold SJ, Cranendonk DR and van der Poll T. Host innate immune responses to sepsis. Virulence 2014; 5: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall JC. Understanding the global burden of pediatric sepsis. Am J Respir Crit Care Med 2015; 191: 1096–1098. [DOI] [PubMed] [Google Scholar]
- 5.Haase H and Rink L. Multiple impacts of zinc on immune function. Metallomics 2014; 6: 1175–1180. [DOI] [PubMed] [Google Scholar]
- 6.Haase H and Rink L. Zinc signals and immune function. Biofactors 2014; 40: 27–40. [DOI] [PubMed] [Google Scholar]
- 7.Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med 2008; 14: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rink L and Gabriel P. Zinc and the immune system. Proc Nutr Soc 2000; 59: 541–552. [DOI] [PubMed] [Google Scholar]
- 9.Salgueiro MJ, Zubillaga M, Lysionek A, et al. Zinc status and immune system relationship: a review. Biol Trace Elem Res 2000; 76: 193–205. [DOI] [PubMed] [Google Scholar]
- 10.Knoell DL, Julian MW, Bao S, et al. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med 2009; 37: 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mertens K, Lowes DA, Webster NR, et al. Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation. Br J Anaesth 2015; 114: 990–999. [DOI] [PubMed] [Google Scholar]
- 12.Solan PD, Dunsmore KE, Denenberg AG, et al. A novel role for matrix metalloproteinase-8 in sepsis. Crit Care Med 2012; 40: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong HR, Shanley TP, Sakthivel B, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics 2007; 30: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowak JE, Harmon K, Caldwell CC and Wong HR. Prophylactic zinc supplementation reduces bacterial load and improves survival in a murine model of sepsis. Pediatr Crit Care Med 2012; 13: e323–e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn J, Cornell TT, Wong HR, et al. The host response to sepsis and developmental impact. Pediatrics 2010; 125: 1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkinson SJ, Varisco BM, Sandquist M, et al. Matrix metalloproteinase-8 augments bacterial clearance in a juvenile sepsis model. Mol Med, Epub ahead of print 8 August 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn JL, Scumpia PO, Delano MJ, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 2007; 28: 675–683. [DOI] [PubMed] [Google Scholar]
- 18.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004. 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 19.Manda A, Pruchniak MP, Arazna M and Demkow UA. Neutrophil extracellular traps in physiology and pathology. Cent Eur J Immunol 2014; 39: 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camicia G, Pozner R and de Larranaga G. Neutrophil extracellular traps in sepsis. Shock 2014; 42: 286–294. [DOI] [PubMed] [Google Scholar]
- 21.Liu MJ, Bao S, Napolitano JR, et al. Zinc regulates the acute phase response and serum amyloid A production in response to sepsis through JAK-STAT3 signaling. PLOS ONE 2014; 9: e94934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessels I and Cousins RJ. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am J Physiol Gastrointest Liver Physiol 2015; 309: G768–G778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black RE. Therapeutic and preventive effects of zinc on serious childhood infectious diseases in developing countries. Am J Clin Nutr 1998; 68: 476S–479S. [DOI] [PubMed] [Google Scholar]
- 24.Sazawal S, Black RE, Jalla S, et al. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: a double-blind, controlled trial. Pediatrics 1998; 102: 1–5. [DOI] [PubMed] [Google Scholar]
- 25.Yakoob MY, Theodoratou E, Jabeen A, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011; 11(Suppl. 3): S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakeam E, Hyder JA, Jiang W, et al. Risk and patterns of secondary complications in surgical inpatients. JAMA Surg 2015; 150: 65–73. [DOI] [PubMed] [Google Scholar]
- 27.Mehta K, Bhatta NK, Majhi S, et al. Oral zinc supplementation for reducing mortality in probable neonatal sepsis: a double blind randomized placebo controlled trial. Indian Pediatr 2013; 50: 390–393. [DOI] [PubMed] [Google Scholar]
- 28.Cvijanovich NZ, King JC, Flori HR, et al. A safety and dose escalation study of intravenous zinc supplementation in pediatric critical illness. JPEN J Parenter Enteral Nutr 2016; 40: 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]