Abstract
Introduction
Angelman syndrome (AS) is a rare neurodevelopmental disorder caused by mutation or loss of UBE3A and marked by intellectual disability, ataxia, autism‐like symptoms, and other atypical behaviors. One route to treatment may lie in the role that environment plays early in postnatal life. Environmental enrichment (EE) is one manipulation that has shown therapeutic potential in preclinical models of many brain disorders, including neurodevelopmental disorders. Here, we examined whether postweaning EE can rescue behavioral phenotypes in Ube3a maternal deletion mice (AS mice), and whether any improvements are sex‐dependent.
Methods
Male and female mice (C57BL/6J Ube3atm1Alb mice and wild‐type (WT) littermates; ≥10 mice/group) were randomly assigned to standard housing (SH) or EE at weaning. EE had a larger footprint, a running wheel, and a variety of toys that promoted foraging, burrowing, and climbing. Following 6 weeks of EE, animals were submitted to a battery of tests that reliably elicit behavioral deficits in AS mice, including rotarod, open field, marble burying, and forced swim; weights were also monitored.
Results
In male AS‐EE mice, we found complete restoration of motor coordination, marble burying, and forced swim behavior to the level of WT‐SH mice. We also observed a complete normalization of exploratory distance traveled in the open field, but we found no rescue of vertical behavior or center time. AS‐EE mice also had weights comparable to WT‐SH mice. Intriguingly, in the female AS‐EE mice, we found a failure of EE to rescue the same behavioral deficits relative to female WT‐SH mice.
Conclusions
Environmental enrichment is an effective route to correcting the most penetrant phenotypes in male AS mice but not female AS mice. This finding has important implications for the translatability of early behavioral intervention for AS patients, most importantly the potential dependency of treatment response on sex.
Keywords: behavior therapy, mouse, neurodevelopmental disorders, sex differences, transgenic
Environmental enrichment (EE) is a powerful behavioral manipulation that may provide a route to ameliorating the symptoms of some brain disorders. In this application of EE in mice modeling Angelman syndrome (AS), a rare neurodevelopmental disorder, we found that this intervention can correct a wide range of robust behavioral deficits commonly seen in male AS mice. Notably, we also found that female AS mice do respond to the same intervention, which constitutes a key sex difference that will need to be considered by researchers and therapists alike in the future.

1. INTRODUCTION
Angelman syndrome (AS) is a neurodevelopmental disorder that affects at least one in 20,000 individuals worldwide (Buiting et al., 2016; Dan, 2009; Mabb et al., 2011; Margolis et al., 2015), with equal numbers of males and females affected by the disorder (Buiting et al., 2016). AS is marked by developmental delay, absent or impaired speech, severe intellectual disability, sleep disruption, and a series of other behavioral abnormalities including motor dysfunction, hyperactivity, and anxiety (Margolis et al., 2015; Williams et al., 2006). Neurologically, AS patients have microcephaly, abnormal brain rhythmicity, and high seizure susceptibility (Dan, 2009; Sidorov et al., 2017). A large fraction of AS patients also has a comorbid diagnosis of autism spectrum disorder (Veltman et al., 2005). Moreover, like other neurodevelopmental disorders, AS constitutes a tremendous lifelong hardship for patients, their families, and the healthcare system (Wheeler et al., 2017). For this reason, it is imperative to develop therapeutic approaches that effectively treat the core features of AS.
Preclinical AS research has made extensive use of transgenic mouse models with disruptions of the gene Ube3a, which encodes an E3 ubiquitin ligase that is critical for brain maturation and synaptic communication (Mabb et al., 2011). The most frequently used mouse model, originally generated by Jiang and colleagues in 1998, has a behavioral profile that echoes the clinical presentation in AS patients (Jiang et al., 1998; Sonzogni et al., 2018). These mice show robust and highly reproducible deficits in a battery of behavioral tasks that includes the accelerating rotarod, which tests motor coordination and motor learning; the open‐field task, which tests activity and anxiety; and other tasks including the marble‐burying task, the nest building task, and the forced swim task (Allensworth et al., 2011; Born et al., 2017; Huang et al., 2013; Jiang et al., 1998; Sonzogni et al., 2018). Some studies have also demonstrated high seizure susceptibility, disrupted brain rhythmicity, and mild cognitive impairments (Born et al., 2017; Dan, 2009; Huang et al., 2013; Jiang et al., 1998; Sidorov et al., 2017; Sonzogni et al., 2018), as well as sex‐dependent sensory defects (Koyavski et al., 2019) in these animals. Work with AS mouse models has revealed a series of promising pharmacological (Baudry et al., 2012; Ciarlone et al., 2017; Cruz et al., 2021; Gu et al., 2019; Guzzetti et al., 2018; Hethorn et al., 2015; Huang et al., 2013; Liu et al., 2019; van Woerden et al., 2007), dietary (Ciarlone et al., 2017), and gene‐therapy (Meng et al., 2013; Schmid et al., 2021; Silva‐Santos et al., 2015; Sonzogni et al., 2019; Wolter et al., 2020) approaches that counteract the disruption of Ube3a and correct these behavioral phenotypes. Despite these efforts, the only approved clinical therapies to date are those that treat symptoms such as seizure onset and sleep loss (Bi et al., 2016; Markati et al., 2021; Rotaru et al., 2020).
Recently, there has been interest in understanding the role that early‐life experience plays in neurodevelopmental disorders and whether modification of the early‐life environment would constitute an effective route to treatment (Consorti et al., 2019; Hannan, 2014; Kelly & Hannan, 2019; Kondo & Hannan, 2019; Sale et al., 2014). Using environmental enrichment (EE)—an experimental paradigm in which researchers provide additional sensory, cognitive, social, or physical stimulation to their subjects (Nithianantharajah & Hannan, 2006; Sztainberg & Chen, 2010)—researchers have been able to correct phenotypes in mouse models of a variety of neuropsychiatric disorders. For instance, in juvenile mice modeling Rett syndrome, a disorder that shares several features with AS, cognitive and physical enrichment was able to restore motor function on the rotarod (Kondo et al., 2008; Nag et al., 2009), and earlier intervention was able to restore spatial learning (Lonetti et al., 2010). This approach has successfully rescued alterations in activity, motor function, repetitive behaviors, and other autism‐related behaviors in other mouse models of neurodevelopmental disorders (Lacaria et al., 2012; Martínez‐Cué et al., 2002; Queen et al., 2020; Restivo et al., 2005; Reynolds et al., 2013; Schneider et al., 2006; Yamaguchi et al., 2017). Promisingly, experts in autism therapy have been able to translate some aspects of EE into clinical therapies and have found some success with their patients (Woo & Leon, 2013; Woo et al., 2015). In AS mice, there is evidence that altering the sensory environment in adult animals is sufficient to rescue synaptic plasticity in the visual cortex, suggesting that other disease‐related phenotypes may also be susceptible to change by environmental enrichment (Yashiro et al., 2009). Adding to the potential of EE for AS mice, one study that applied long‐term EE to male mice showed improvement in a small number of behaviors (Jamal et al., 2017). However, it remains unclear how EE affects the most robust and highly reproducible behavior phenotypes of AS in mice, and it is important to explore how sex, enrichment time, and other parameters determine the effectiveness of EE as an intervention.
In the present study, we investigated the degree to which 6–12 weeks postweaning EE rescues behavioral phenotypes in AS model mice. We examined a battery of behaviors that has proven to be highly reproducible across investigators and AS mouse models (Sonzogni et al., 2018) and is broader in scope than previous studies. At the same time, we asked whether the rescue of any phenotype due to EE was dependent on sex, as sex differences are important to document when evaluating the effectiveness of any potential treatment (Bale & Epperson, 2017; Shansky & Woolley, 2016). We found that while this form of EE is capable of completely or partially rescuing many highly penetrant behaviors in male mice, it is considerably less effective in female mice. The substantial sex difference demonstrated here has important implications for behavioral therapeutic approaches to AS.
2. MATERIALS AND METHODS
2.1. Animals
Male and female heterozygous mice carrying a maternal deletion of Ube3a (Ube3am+/p– , denoted as AS) and wild‐type (WT) littermates were bred at Augustana University. Female heterozygous mice on the C57 background carrying a paternal deletion of Ube3a (Ube3atm1Alb ; stock No. 016590; RRID:JAX_IMSR:016590; Jiang et al., 1998) and male C57BL/6J mice (stock No. 000664; RRID:IMSR_JAX:000664) were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and paired for breeding in standard housing (SH). Genotype was confirmed by PCR testing according to previously published protocols (Jiang et al., 1998; Judson et al., 2016). At postnatal day 21, pups were separated by sex and weaned randomly into SH or environmentally enriched housing; litters were not separated by genotype (Figure 1a). In total, 110 mice from 15 litters from four breeding pairs were used (WT‐SH, 17 males and 11 females; WT‐EE, 19 males and 16 females; AS‐SH, 13 males and 11 females; and AS‐EE, 13 males and 10 females). These numbers do not deviate significantly from the expected values of 25% male WT mice, 25% male AS mice, 25% female WT mice, and 25% female AS mice from this breeding scheme (chi‐squared test, χ 2(3) = 4.255, p = .23).
FIGURE 1.

Experimental setup. (a) Angelman syndrome (AS, Ube3am‐/p+) mice on the C57BL/6J (C57) background and their wild‐type (WT) littermates arising from mothers carrying the paternal deletion (Ube3am+/p–) and WT males (left) were randomly assigned to standard housing (SH) or larger enriched housing (EE) (right). Dashed line indicates the relative size of EE housing. (b) Timeline for behavioral testing. Following the beginning of enrichment at weaning at postnatal day 21, marble burying (MB), open field (OF), accelerating rotarod (RR), and forced swim (FS) tasks were administered
All mice were housed on a 12‐h light‐dark cycle (7 a.m.–7 p.m.) in open‐top filtered mouse cages (Ancare, Bellmore, NY, USA) in groups of 2–5 littermates per cage (median = 3 littermates, interquartile range = 1 littermate). Animals had ad libitum access to food and water during this period. Cages were changed on a biweekly basis. All experimental procedures complied with NIH guidelines and were approved by the Augustana University Institutional Animal Care and Use Committee.
2.2. Housing conditions
At weaning, mice were assigned randomly and blind to genotype to one of two housing conditions (Figure 1a) and housed exclusively with their littermates. Animals from no more than one litter were assigned to the same cage. SH consisted of a 19 cm × 29.2 cm × 12.7 cm conventional open‐top filtered mouse cage (Ancare) that contained aspen bedding (Envigo, Indianapolis, IN, USA), a shelter (Fisher Scientific, Waltham, MA, USA) and compressed cotton nesting material (nestlet, Ancare) along with a food hopper and a water bottle. Environmentally enrichment housing was modeled after prior literature on environmental enrichment in mice (Nithianantharajah & Hannan, 2006; Sztainberg & Chen, 2010; Tomas et al., 2015) and consisted of opportunities for physical exercise, such as running and climbing; for exploration of a variety of textures, materials, and sensory modalities; and for digging and burrowing. The cage consisted of a 46 cm × 31.1 cm × 17.8 cm arena made of clear plastic (Sterilite, Townsend, MA, USA) custom‐adapted to fit a filtered cage top, food hopper, and water bottle (Ancare). The enclosure contained aspen bedding (Envigo); a variety of shelter options, including red and yellow translucent houses and huts (Fisher Scientific), “walk‐up” houses (Petmate, Arlington, TX, USA), and upcycled plastic piping (purchased from the Scrap Exchange, Durham, NC, USA); one of two types of exercise wheel (Fisher Scientific, and Ware Pet Products, Phoenix, AZ, USA); compressed cotton nestlets; (nestlet, Fisher Scientific) crinkle paper (Crink‐l'Nest, Lab Supply, Dallas, TX, USA); treats (Hartz) scattered throughout the bedding; and an assortment of toys, including bells, Nylabones (Neptune City, NJ, USA), wooden blocks and sticks, chains, ropes, activity rings (Bio‐Serv, Flemington, NJ, USA) Duplo blocks (LEGO, Enfield, CT, USA), and balls of a variety of sizes and textures. Enrichment devices were changed out once a week, and the locations of enrichment devices and the orientation of the enrichment cage in the housing room were rotated once a week.
2.3. Behavioral testing battery
After 6 weeks in the assigned housing, mice underwent a series of behavioral assays over the next 5 weeks (Figure 1b). These behaviors were selected from among the “gold standard” of highly reproducible phenotypes in AS mice on the C57 background across laboratories (Rotaru et al., 2020). All mice underwent marble burying, rotarod, and open‐field testing. Forced swim testing, which began after 18 mice had already completed the behavioral battery, was administered to 92 mice. Testing occurred during light hours. Prior to each testing session, the mice were acclimated to the testing room for a minimum of 30 min. Following each behavioral assay, mice were returned to their home cages. Mice were weighed weekly during the behavioral battery. All data were collected by experimenters blind to genotype.
2.3.1. Marble burying
Marble‐burying measures ethologically relevant repetitive behavior in mice (Angoa‐Pérez et al., 2013; Deacon, 2006). Mice were placed in a Plexiglas cage (Ancare) containing 5 cm of ⅛′′ corncob bedding (The Andersons Inc., Maumee, OH, USA) and 15 ¼′′ black glass marbles arranged in a 3‐by‐5 grid; testing took place in the presence of 60 dB white noise under dim light (30–40 lux). The mice were allowed to interact with the marbles for 30 min, after which the animals were removed from the cage and the number of unburied marbles was counted. A marble was counted as unburied if less than 50% of its surface was covered. The average number of unburied marbles was calculated for each cage from the independent observations of three researchers. The inside of the cage was cleaned with 70% ethanol between trials and bedding was replaced.
2.3.2. Rotarod
Testing on the accelerating rotarod, which measures motor function and motor coordination (Deacon, 2013), was carried out as previously described (Gu et al., 2019; Huang et al., 2013; Thaxton et al., 2018). Briefly, mice were tested on 2 separate days, with three trials delivered on the first day and two trials delivered 48 h later. Each day began with 30 min of habituation in a brightly lit room. During each trial, mice were placed using a wooden dowel into one of four lanes of a rod rotating at a constant speed of 4 rpm. Once the trial began, the rotarod accelerated to a speed of 40 rpm over 5 min. The trial for each mouse ended when the mouse fell off the rotarod, completed two complete somersaults around the rotarod, or reached the end of the 5 min trial; end‐of‐trial times were recorded. Subsequent trials on the same day started 10 min later. The rotarod was cleaned with 70% ethanol between trials.
2.3.3. Open field
The open‐field task measures anxiety and exploratory activity in mice (Sonzogni et al., 2018; Tanaka et al., 2012). Mice were placed in a 41 cm × 41 cm × 41 cm white Plexiglas testing chamber; testing took place under bright lights and in the presence of 60 dB white noise. The mice were allowed to freely roam the testing chamber for 30 min while being recorded by a PSEye camera (Sony Interactive Entertainment, San Mateo, CA, USA) mounted on the ceiling (Badura et al., 2018; Kloth et al., 2015), with images collected at a rate of 75 fps. After testing, the mice were removed from the testing chamber and returned to their home cages, and the inside of the testing chamber was cleaned with 70% ethanol. Video was recorded using a custom Python (RRID:SCR_008394) program using the CL‐Eye Platform (Code Laboratories, Henderson, NV, USA) (Badura et al., 2018; Kloth et al., 2015) and was analyzed using custom MATLAB (RRID:SCR_001622) code similar to previously published methods (Zhang et al., 2020). The MATLAB analysis extracted distance traveled and entries into the center (defined as at least 10.25 cm from the walls of the arena). Videos were also scored manually by experimenters blind to genotype and housing condition in order to quantify the number of rearing episodes.
2.3.4. Forced swim
The forced swim task tests depressive‐like behavior in mice (Can et al., 2012). Following 30 min habituation under dim lights (30–40 lux), mice were placed in a clear, plastic 3 L beaker (Fisher Scientific) filled with water (approximately 25°C) and were allowed to acclimate for 2 min. After acclimation, the mice were allowed to swim for another 4 min while being recorded. After testing, the video was scored manually by experimenters blind to genotype and housing condition in order to determine the amount of time each mouse spent immobile and the amount of time each mouse spent producing rhythmic, propulsive movements of the hindlimbs and forelimbs.
2.4. Statistics
Data were analyzed using two‐way analyses of variance (ANOVAs) unless otherwise noted. Homoscedasticity of residuals was confirmed using Spearman's rank correlation test, and normality of residuals was confirmed using Shapiro–Wilk's test. In some cases, data were transformed to satisfy the assumptions of the two‐way ANOVA: marble‐burying data were transformed as , while other data were transformed as . These cases are clearly indicated in Supporting Information. Significant genotype or housing effects were followed up with Bonferroni‐corrected planned comparisons between the following pairs of groups: WT‐SH versus WT‐EE, AS‐SH versus AS‐EE, WT‐SH versus AS‐SH, and AS‐EE versus WT‐SH. The data were analyzed using Graphpad Prism 9 (GraphPad Software, San Diego, CA; RRID:SCR_002798). The significance level was α = .05. All results depicted in figures are the mean ± SEM of the untransformed data. Complete statistical information can be found in Supporting Information.
3. RESULTS
Following 6 weeks of postweaning experimental housing, mice underwent behavioral testing (Figure 1). Complete results from statistical analyses can be found in Supporting Information.
3.1. Weights
First, we tested the effect of housing at 6 weeks postweaning on the excess weight phenotype documented in AS mice (Huang et al., 2013). Two‐way ANOVA analysis of weights from male mice revealed significant main effects of both genotype (p = .0068) and housing (p = .0003) (Figure 2a). Increased weight seen in AS‐SH mice (vs. WT‐SH, p = .0121) was normalized by EE: AS‐EE weights were significantly different from AS‐SH weights (p = 0.0021) and could not be distinguished from WT‐SH animals (p > .9999). Likewise, two‐way ANOVA analysis of weights from female mice revealed significant main effects of both genotype (p = .0001) and housing (p = .0003) (Figure 2b). Increased weight seen in AS‐SH mice (vs. WT‐SH, p = .0297) was also completely normalized by EE: AS‐EE weights were significantly different from AS‐SH weights (p = .0034) and could not be distinguished from WT‐SH animals (p > .9999).
FIGURE 2.

Environmental enrichment normalizes weight deficit at week 6. Improvements in excessive weights were observed in male (a) and female (b) AS mice in the enriched environment. Abbreviations: AS, Angelman syndrome mice; EE, enriched environment; SH, standard housing; WT, wild‐type littermates. Weights, in grams, displayed as mean ± SEM. Two‐way analysis of variance (ANOVA) followed by Bonferroni‐corrected planned comparisons: *p < .05, **p < .01
3.2. Marble burying
At 6 weeks postweaning, we also examined whether EE ameliorates the highly penetrant marble‐burying deficit seen in AS mice on the C57 background (Born et al., 2017; Huang et al., 2013; Sonzogni et al., 2019). Two‐way ANOVA analysis of data from male mice at 6 weeks postweaning revealed significant main effects of genotype (p < .0001) and housing (p = .0265) (Figure 3a). However, deficient marble burying in AS‐SH mice (vs. WT‐SH, p = .0005) was not significantly improved by EE (AS‐EE vs. AS‐SH, p = .1173). A follow‐up test 2 weeks later showed significant main effects of genotype (p < .0001) and housing (p = .0002) (Figure 3b). Deficient marble burying in AS‐SH mice (vs. WT‐SH, p < .0001) was completely rescued in AS‐EE mice (vs. AS‐SH, p = .0009; vs. WT‐SH, p = .2559).
FIGURE 3.
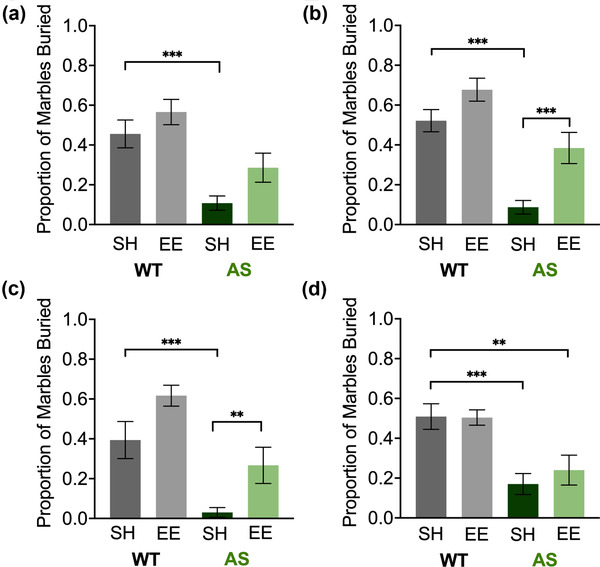
Environmental enrichment normalizes marble burying in male AS mice after 8 weeks. Improvement in the deficit in the number of marbles buried was observed at week 6 in male (a) and female (b) AS mice in the enriched environment, with this improvement strengthened in male AS mice (c) but not female AS mice (d) at a week 8 retest. Abbreviations: AS, Angelman syndrome mice; EE, enriched environment; SH, standard housing; WT, wild‐type littermates. Number of marbles ≥50% buried displayed as mean ± SEM. Two‐way analysis of variance (ANOVA) followed by Bonferroni‐corrected planned comparisons: *p < .05, **p < .01, ***p < .001
Two‐way ANOVA analysis of data from female mice at 6 weeks postweaning revealed significant main effects of genotype (p < .0001) and housing (p = .0003) (Figure 3c). Deficient marble burying in AS‐SH mice (vs. WT‐SH, p < .0001) did improve with EE (AS‐EE vs. AS‐SH, p = .0024, AS‐EE vs. WT‐SH, p = .2430). However, a follow‐up test 2 weeks later showed that the improvement at 6 weeks had disappeared, revealing a significant main effect of genotype only (p < .0001) (Figure 3d). Indeed, we observed deficient marble burying mice in the AS‐SH mice (planned comparison vs. WT‐SH, p = .0003) that did not seem to be affected by EE (AS‐EE vs. AS‐SH, p = .8139; AS‐EE vs. WT‐SH, p = .0076). These differences in marble‐burying behavior between WT‐SH and both AS‐SH and AS‐EE mice seem to have been driven by an increase in marble‐burying behavior in the AS‐SH mice, so that it more closely resembles marble‐burying behavior in AS‐SH male mice than it did at the 6‐week timepoint, as well as increased marble burying in WT‐SH mice.
3.3. Open field
Next, we examined whether 7 weeks of EE can improve behavioral deficits in the open field commonly seen in AS mice on the C57 background (Berg et al., 2021; Dutta & Crawley, 2020; Koyavski et al., 2019). Two‐way ANOVA analysis of distance traveled by male mice in the open field revealed a genotype x housing interaction (p = .0335) (Figure 4a). We observed a deficit in AS‐SH mice (vs. WT‐SH, p = .0001) that was improved in AS‐EE mice (vs. AS‐SH mice, p = .0340; vs. WT‐SH mice, p = .3132). There was no corresponding improvement in distance traveled due to housing in WT mice (p > .9999). When we examined the number of rearing movements in the same experiments, two‐way ANOVA analysis detected a significant main effect of genotype (p < .0001) with no significant main effect of housing (p = .0545) (Figure 4b). Indeed, we observed significant differences in AS rearing regardless of housing condition (AS‐SH vs. WT‐SH, p < .0001; AS‐EE vs. WT‐SH, p < .0001). When we examined the number of entries into the center zone, two‐way ANOVA analysis of data from male mice revealed a significant genotype x housing interaction (p = .0236) (Figure 4c). We observed that AS mice indeed made fewer center entries (vs. WT‐SH, p = .0001), with no significant improvement with EE (AS‐EE vs. AS‐SH, p = .1786, vs. WT‐SH, p = .0418). No improvements were seen in WT mice in EE (p = .2494).
FIGURE 4.

Environmental enrichment improves some aspects of exploratory activity in the open field in male AS mice after 7 weeks. Male AS mice in the enriched environment showed improvements in distance traveled (a), with no statistically significant improvement in deficient rearing behaviors (b) or thigmotaxis (c). Female AS mice in the enriched environment showed no statistically significant improvements in distance traveled (d), rearing behaviors (e), or thigmotaxis (f) relative to standard housing. Abbreviations: AS, Angelman syndrome mice; EE, enriched environment; SH, standard housing; WT, wild‐type littermates. All quantities displayed as mean ± SEM. Two‐way analysis of variance (ANOVA) followed by Bonferroni‐corrected planned comparisons: *p < .05, **p < .01, ***p < .001
In female mice, two‐way ANOVA analysis of distance traveled revealed a main effect of genotype (p = .0020) (Figure 4d). Planned comparisons revealed no significant group differences (p > .05), indicating mild deficits in distance traveled in AS mice. When we examined the number of rearing movements in the same experiments, two‐way ANOVA analysis of rearing events revealed a main effect of genotype (p < .0001) (Figure 4e). We confirmed a deficit in AS‐SH mice (vs. WT‐SH, p = .0037) that was not ameliorated in the presence of EE (vs. WT‐SH, p = .0057). When we examined the number of entries into the center zone, two‐way ANOVA analysis revealed a significant main effect of genotype (p = .0002) with no significant effect of housing (p = .0672) (Figure 4f). We observed that female AS mice were indeed deficient in center entries (vs. WT‐SH, p = .0024). Interestingly, center zone entries in AS‐EE mice were not significantly different from WT‐SH mice (p = .4590), suggesting improvement due to housing. However, because there were no corresponding significant differences between AS‐SH and AS‐EE mice (p = .1071), we could not conclude that this aspect of behavior had been rescued.
3.4. Rotarod
Then, we examined whether 9 weeks of EE rescues motor dysfunction on the accelerating rotarod seen in AS mice on the C57 background (Born et al., 2017; Huang et al., 2013; Jiang et al., 1998; Sonzogni et al., 2018). Two‐way ANOVA analysis of data from male mice revealed significant main effects of genotype (p = .0483) and housing (p < .0001) (Figure 5a). At this phase of the task, EE enhanced rotarod performance in both WT (p < .0001) and AS (p = .0467) mice, but notably there was no difference between WT‐SH and AS‐SH mice (p > .9999). During a retest of motor learning 48 h later, two‐way ANOVA analysis revealed significant main effects of genotype (p < .0001) and housing (p < .0001) (Figure 5b). At this phase of the task, we were able to replicate the rotarod deficit in AS‐SH mice (vs. WT‐SH, p = .0001), and we found that EE significantly normalized rotarod performance in AS‐EE mice (vs. AS‐SH, p = .0003; vs. WT‐SH, p > .9999) and improved rotarod performance in WT‐EE mice (vs. WT‐SH, p = .0029).
FIGURE 5.

Environmental enrichment normalizes rotarod performance in male mice after 9 weeks. Improvement in the motor coordination deficit on the accelerating rotarod was observed in male AS mice (a and b) but not female mice (c and d) in environmental enrichment. Data show the training period (a and c) and a retest of motor learning 48 h later (b and d). Abbreviations: AS, Angelman syndrome mice; EE, enriched environment; SH, standard housing; WT, wild‐type littermates. Latency to fall, in seconds (maximum, 300 s), displayed as mean ± SEM. Two‐way analysis of variance (ANOVA) followed by Bonferroni‐corrected planned comparisons: *p < .05, **p < .01, ***p < .001
The same experiments in female mice revealed no rescue of the rotarod phenotype. Two‐way ANOVA analysis revealed a significant main effect of genotype (p = .0008) and housing (p = .0374) (Figure 5c). However, we observed a significant difference in rotarod performance only in WT (planned comparison, WT‐SH vs. WT‐EE, p = .0186). During a retest of motor learning 48 h later, two‐way ANOVA analysis revealed a significant main effect of genotype (p < .0001) (Figure 5d). We confirmed the documented deficits in AS‐SH mice (vs. WT‐SH, p = .0161), but found no improvements in performance due to EE (vs. AS‐SH, p > .9999).
3.5. Forced swim
Finally, we tested whether EE rescues forced swim deficits that have been documented in AS mice (Silva‐Santos et al., 2015; Sonzogni et al., 2018). Two‐way ANOVA analysis of the data from the male mice revealed a significant genotype x housing interaction (p = .0198) (Figure 6a). We found a difference between mice in SH (WT‐SH vs. AS‐SH, p = .0015) that was ameliorated by EE (AS‐SH vs. AS‐EE, p = .0076; WT‐SH vs. AS‐EE, p > .9999). There was no impact of EE on WT mice (WT‐SH vs. WT‐EE, p > .9999). Two‐way ANOVA analysis of the data from female mice revealed neither significant main effects nor a significant interaction (p > .05) (Figure 6b).
FIGURE 6.

Environmental enrichment corrects forced swim performance in male mice after 11 weeks. The forced swim deficit in male AS mice improved with environmental enrichment (a), but similar changes were not seen in female mice (b). Abbreviations: AS, Angelman syndrome mice; EE, enriched environment; SH, standard housing; WT, wild‐type littermates. Floating time, as a percentage of 4 min, displayed as mean ± SEM. Two‐way analysis of variance (ANOVA) followed by Bonferroni‐corrected planned comparisons: *p < .05, **p < .01, ***p < .001
4. DISCUSSION
In the present study, we set out to determine the degree to which enrichment of the environment rescued behavioral phenotypes associated with AS in mice. We tested the effects of 6–12 weeks of postweaning sensory, cognitive, and physical enrichment on a battery of behavioral tasks that have been shown to be highly reproducible across laboratories and have face validity with AS in patients (Rotaru et al., 2020; Sonzogni et al., 2018). We recapitulated the behavioral deficits in both male and female AS mice but found that our EE protocol was far more successful at normalizing these phenotypes in male mice than in female mice. These findings suggest that EE may have therapeutic value in AS, but sex differences and other aspects of enrichment merit further consideration.
Our EE protocol was remarkably effective in male AS mice. First, we observed a complete rescue of rotarod performance at the 48‐h retest after 9 weeks. Second, we observed a complete rescue of horizontal exploratory behavior in the open field to WT levels after 7 weeks. Third, we observed a complete normalization of marble burying to WT levels after 6 weeks. Fourth, we observed a rescue of immobility in the forced swim task. Finally, we observed a correction of weight. These findings are consistent with a body of evidence showing that EE is effective at correcting some phenotypes in mouse models of neurodevelopmental disorders. In mouse models of autism—which is comorbid in most AS patients (Mertz et al., 2014)—EE has been demonstrated to correct hypoactivity in the open field (Queen et al., 2020; Suemaru et al., 2018; Yamaguchi et al., 2017) or normalize ethologically relevant repetitive behaviors like marble burying (Mansouri et al., 2021; Reynolds et al., 2013). Interestingly, these studies showed a decrease in marble burying to WT levels following EE, rather than the increase we observed here. In mouse models of Rett syndrome—a disorder that resembles AS in its developmental delay, absent speech, ataxia, and seizure susceptibility but is most distinct in its regressive phenotype (Jedele, 2007; Tan et al., 2014)—EE has been demonstrated to restore motor coordination deficits on the rotarod (Kondo et al., 2008; Lonetti et al., 2010) and correct hypoactivity in the open field (Nag et al., 2010). In mouse models of Fragile X syndrome—which shares autism incidence, sensory sensitivity, and intellectual disability with AS (Heald et al., 2020)—EE has been shown to correct hyperactivity in the open field (Restivo et al., 2005). However, none of these studies demonstrated the same high degree of correction across the same suite of behaviors examined in the current study. Thus, this combination of EE parameters seems to be potent in male AS mice.
Interestingly, 7 weeks of EE in male AS mice did not rescue rearing behavior and center zone entries in the open field, behaviors that have been used to probe anxiety in mice (Kulesskaya & Voikar, 2014; Sturman et al., 2018), including in AS mouse models (Born et al., 2017; Godavarthi et al., 2012; Guzzetti et al., 2018; Koyavski et al., 2019; Sonzogni et al., 2018). These results contrast with some prior studies described above that see improvements in anxiety‐related behavior with EE (Lacaria et al., 2012; Lonetti et al., 2010; Queen et al., 2020; Restivo et al., 2005). However, the finding of an uncorrected rearing deficit may not be explained by unmitigated anxiety; rather, it is possible that AS mice have muscle weakness that that is not ameliorated with 7 weeks of EE, despite restored horizontal movement in the open field and improvement on the rotarod after 9 weeks of EE. Indeed, recent studies have started to document subtle, clinically relevant motor deficits in AS rodent models (Berg et al., 2021, 2020; Petkova et al., 2021). Nevertheless, anxiety is a key part of the clinical presentation of AS (Wheeler et al., 2019). Therefore, future work should examine the effect of short‐term EE on highly reproducible tasks that more clearly dissociate anxiety‐related behaviors from confounding motor deficits in AS mice, including the elevated‐plus maze (Born et al., 2017; Ciarlone et al., 2016, 2017; Dutta & Crawley, 2020), the light‐dark task (Dutta & Crawley, 2020; Godavarthi et al., 2012; Jiang et al., 2010), or other tasks (Crawley, 2007). One prior study indicates that long‐term EE rescues behavior in the light‐dark task in male AS mice (Jamal et al., 2017), so it will be fruitful to examine the role of EE on this behavior on a shorter timescale in both sexes. It would also be beneficial for future work to examine more closely the effect of EE on motor behavior in AS, including clinically relevant phenotypes like gait (Berg et al., 2021).
It should be noted that we did not necessarily expect our protocol to rescue as many of the behaviors as we tested. For example, in mouse models of autism, the behaviors for which EE is documented as either being highly effective or largely ineffective can vary from model to model, as in the studies described above. There is even one example in which EE failed to rescue any of the disease‐related phenotypes examined in a mouse model of autism (Hulbert et al., 2018). The effectiveness of EE for the suite of behaviors here—or the ineffectiveness of EE on behavior elsewhere—may be due to the interaction between the environment and disease pathophysiology at a particular point in time and may depend on the type and duration of enrichment (Kondo & Hannan, 2019; Nithianantharajah & Hannan, 2006). Moreover, among studies that have tested novel therapeutic approaches in AS model mice, some of the highly reproducible behavioral phenotypes are more frequently rescued than others. Across 16 recent studies that were able to rescue at least one of the behaviors tested here, motor coordination deficits on the rotarod were most commonly corrected, with the phenotype completely or partially rescued in over half of the studies (nine of 16: Adhikari et al., 2021; Cruz et al., 2021; Guzzetti et al., 2018; Judson et al., 2021; Kumar et al., 2019; Schmid et al., 2021; Silva‐Santos et al., 2015; Van Woerden et al., 2007; Wolter et al., 2020) and no rescue in the rest of the studies (Berg et al., 2020; Gu et al., 2019; Hethorn et al., 2015; Milazzo et al., 2021; Schultz & Crawley, 2020; Sonzogni et al., 2018; Wang et al., 2019). Partial or complete rescue occurred least frequently in open field thigmotaxis (one of four studies in which it was examined: Guzzetti et al., 2018) and marble burying (four of 10 studies in which it was examined: Cruz et al., 2021; Judson et al., 2021; Schmid et al., 2021; Silva‐Santos et al., 2015). Together, this body of evidence suggests that some disease‐related phenotypes may be more easily rescued than others (though it should be noted that factors including genetic background and age of intervention may have influenced the effectiveness of any given approach). Further research should probe the mechanistic basis that underlies the ability of the combination of EE parameters in this study to shape a wide range of AS‐relevant behaviors.
In stark contrast to the success of EE in male AS mice, our EE protocol was substantially less effective in female AS mice. Here, only the previously documented weight phenotype (Ciarlone et al., 2016; Huang et al., 2013) showed improvement. Although previous work showed that there are no sex differences in the behavioral tasks we selected in AS‐SH mice (Born et al., 2017; Koyavski et al., 2019; Sonzogni et al., 2018), one recent study (Koyavski et al., 2019) showed that there were sex differences in odor perception and object exploration and hinted that other sex differences may be uncovered as the literature on female AS mice expands. Indeed, sex differences in the effectiveness of EE are not unheard of. In some studies of EE in rodents, EE was beneficial for males but not females (Doreste‐Mendez et al., 2019; Kentner et al., 2018; Queen et al., 2020; Stam et al., 2008; Wood et al., 2010), while in other studies, EE was beneficial in females but not males (Lin et al., 2011; Martínez‐Cué et al., 2002; Stam et al., 2008). How might these differences arise? Previous studies on EE in mice of both sexes have documented differences in how EE interacts with hormonal state and stress response to anxiogenic tasks (Girbovan & Plamondon, 2013; Kentner et al., 2018). Other studies suggest that EE in female mice may not engage the same molecular mechanisms to the same degree as in male mice (Chourbaji et al., 2012; Kentner et al., 2018; Lin et al., 2011; Queen et al., 2020). If the effect of EE on behavioral phenotypes in AS mice depends on brain‐deried neurotrophic factor upregulation and glucocorticoid receptor downregulation, as has been reported previously (Jamal et al., 2017), it is possible that female mice do not respond in the same way because of the failure of EE to engage these molecular mechanisms. Furthermore, the most effective EE parameters may not be identical for male and female AS mice. In other studies of EE, female mice tend to benefit more from some forms of enrichment, like social enrichment, than others (Chourbaji et al., 2008; Kondo et al., 2016; Lambert et al., 2005; Pietropaolo et al., 2004). It is also possible that other behavioral differences in AS mice, including recently detected sex differences in odor perception and object exploration (Koyavski et al., 2019), may account for fundamental differences in how male and female mice engage with the enriched environment. In the same vein, it is possible that female AS mice require longer bouts of EE or need to begin EE at a different developmental time point. It will be interesting to examine how altering enrichment parameters might make this intervention more effective for female AS mice.
Our findings are distinct from the results of the only other existing study of EE in AS mice (Jamal et al., 2017) in a few important ways. First, although both studies show that postweaning enrichment is sufficient to rescue deficits on the rotarod, we were able to observe statistically significant differences in AS mouse behavior after just 9 weeks rather than 16–32 weeks. The researchers reported a lack of a significant difference after 8 weeks, but this finding may be an indication that the experiment was underpowered or that there were important differences in EE protocol. Second, we examined behaviors that cover a larger swath of disease‐related phenotypes and are robust and highly reproduced (Sonzogni et al., 2018). Our study adds a robust battery of exploratory, anxiety‐related, and species‐typical repetitive behaviors to the previous findings. These tasks provide relatively quick endpoint assays for the effectiveness of EE, or drugs that engage the same mechanisms as EE (Kondo & Hannan, 2019; McOmish & Hannan, 2007; Solinas et al., 2021). Third, our study used both male and female mice, rather than just male mice, to document whether sex is a variable in the effectiveness of EE. In total, our findings step forward in understanding enrichment as an intervention for AS.
Our study also has important limitations. The current study was limited to Ube3atm1Alb mice (Jiang et al., 1998) on the C57BL6/J background, and EE was administered for 6–12 weeks immediately upon weaning. Differences in behavior due to background strain are well‐documented in the literature (Born et al., 2017; Huang et al., 2013; Sonzogni et al., 2018), and it is possible that our EE protocol will not be as effective on other background strains, despite our focus on highly reproducible behaviors across backgrounds. It is also possible that mouse models carrying mutations relevant to AS in human patients (Jiang et al., 1998; Rotaru et al., 2020) will respond differently to this EE protocol. In addition, it will also be useful to understand how the type and duration of EE, the time of onset of EE, and the removal of long‐term EE affect the effectiveness of the intervention. Future work addressing these areas will help us determine the broad applicability of EE to AS.
Future work should also examine other highly penetrant phenotypes associated with AS and examine the role EE has on the pathophysiology of the disease in rodents. Seizure susceptibility is one of the commonly documented features of AS in both human patients and model mice. Previous research in other mouse models indicates that EE is effective at reducing epilepsy (Morelli et al., 2014; Young et al., 1999), and it is reasonable to hypothesize that EE is also effective at reducing seizures in AS mice. It would also be useful to know how effective EE is at correcting phenotypes that echo the cognitive symptoms of AS in patients. Although deficits in cognitive tasks like the novel object recognition task, fear conditioning, and the Morris water maze have been reported, they are relatively modest (Sonzogni et al., 2018). Nevertheless, work on EE in mouse models of autism, Fragile X syndrome, and Rett syndrome shows substantial rescue of cognitive behavior (Jamal et al., 2017; Lacaria et al., 2012; Lonetti et al., 2010; Restivo et al., 2005). Finally, future research should attempt to document other forms of EE‐mediated recovery in AS mice at the cellular and systems levels. Understanding these mechanisms and the conditions under which they are engaged will increase the importance of our findings.
The translatability of these results to AS patients remains to be seen. A few studies with autism patients suggest that long‐term sensorimotor stimulation can lead to significant correction of cognitive disruption and behavioral dysfunction (Woo & Leon, 2013; Woo et al., 2015). These clinical findings show that some form of enrichment—or therapies that engage the same mechanisms as enrichment (Hill‐Yardin & Hannan, 2013)—may be effective in AS patients, given the results of the current study. To our knowledge, no such study currently exists. In addition, the finding of sex differences in response to environmental enrichment suggests that slightly different therapeutic approaches may need to be taken with males and females undergoing treatment for AS. Any future study of enrichment in patients will need to carefully account for sex, type of stimulation, and desired outcome.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2468
Supporting information
Supporting Information. Spreadsheet containing results from statistical tests presented in this study. Each set of results is displayed on separate tabs by corresponding figure number. Transformed data used where reported. Statistical significance for main effects and interactions in the two‐way ANOVA (columns F‐G) and Bonferroni‐corrected planned comparisons (columns I‐J): *p < .05, **p < .01, ***p < .001. XLS format.
ACKNOWLEDGMENTS
This work is supported by funding from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences, National Institutes of Health (P20GM103443). This South Dakota Biomedical Research Infrastructure Network (SD BRIN) funding supported summer research and a Faculty Fellowship Award (A.D.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Benjamin Philpot for supplies for generating the pilot dataset for this study, and we acknowledge Megan Hutzenbuhler, Kayla Kopczynski, and Allison Stitt for their work generating the pilot dataset for this study. We thank Brenda Rieger and Brian Vander Aarde for technical assistance, and Jill Weimer and Patrick Ronan for helpful discussion of this manuscript.
Cosgrove, J. A. , Kelly, L. K. , Kiffmeyer, E. A , & Kloth, A. D. (2022). Sex‐dependent influence of postweaning environmental enrichment in Angelman syndrome model mice. Brain and Behavior, 12, e2468. 10.1002/brb3.2468
Jameson A. Cosgrove, Lauren K. Kelly, and Elizabeth A. Kiffmeyer contributed equally to this study.
DATA AVAILABILITY STATEMENT
Data that support the findings of this study and custom code used to analyze data are available from the corresponding author upon reasonable request.
REFERENCES
- Adhikari, A. , Copping, N. A. , Beegle, J. , Cameron, D. L. , Deng, P. , O'Geen, H. , Segal, D. J. , Fink, K. D. , Silverman, J. L. , & Anderson, J. S. (2021). Functional rescue in an Angelman syndrome model following treatment with lentivector transduced hematopoietic stem cells. Human Molecular Genetics, 30(12), 1067–1083. 10.1093/hmg/ddab104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allensworth, M. , Saha, A. , Reiter, L. T. , & Heck, D. H. (2011). Normal social seeking behavior, hypoactivity and reduced exploratory range in a mouse model of Angelman syndrome. BMC Genetics, 12, 7. 10.1186/1471-2156-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa‐Pérez, M. , Kane, M. J. , Briggs, D. I. , Francescutti, D. M. , & Kuhn, D. M. (2013). Marble burying and nestlet shredding as tests of repetitive, compulsive‐like behaviors in mice. Journal of Visualized Experiments, 82, e50978. 10.3791/50978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura, A. , Verpeut, J. L. , Metzger, J. W. , Pereira, T. D. , Pisano, T. J. , Deverett, B. , Bakshinskaya, D. E. , & Wang, S. S.‐H. (2018). Normal cognitive and social development require posterior cerebellar activity. eLife, 7, e36401. 10.7554/eLife.36401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale, T. L. , & Epperson, C. N. (2017). Sex as a biological variable: Who, what, when, why, and how. Neuropsychopharmacology, 42(2), 386–396. 10.1038/npp.2016.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry, M. , Kramar, E. , Xu, X. , Zadran, H. , Moreno, S. , Lynch, G. , Gall, C. , & Bi, X. (2012). Ampakines promote spine actin polymerization, long‐term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiology of Disease, 47(2), 210–215. 10.1016/j.nbd.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, E. L. , Petkova, S. P. , Born, H. A. , Adhikari, A. , Anderson, A. E. , & Silverman, J. L. (2021). Insulin‐like growth factor‐2 does not improve behavioral deficits in mouse and rat models of Angelman syndrome. Molecular Autism, 12(1), 59. 10.1186/s13229-021-00467-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, E. L. , Pride, M. C. , Petkova, S. P. , Lee, R. D. , Copping, N. A. , Shen, Y. , Adhikari, A. , Fenton, T. A. , Pedersen, L. R. , Noakes, L. S. , Nieman, B. J. , Lerch, J. P. , Harris, S. , Born, H. A. , Peters, M. M. , Deng, P. , Cameron, D. L. , Fink, K. D. , Beitnere, U. , … Silverman, J. L. (2020). Translational outcomes in a full gene deletion of ubiquitin protein ligase E3A rat model of Angelman syndrome. Translational Psychiatry, 10(1), 39. 10.1038/s41398-020-0720-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, X. , Sun, J. , Ji, A. X. , & Baudry, M. (2016). Potential therapeutic approaches for Angelman syndrome. Expert Opinion on Therapeutic Targets, 20(5), 601–613. 10.1517/14728222.2016.1115837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born, H. A. , Dao, A. T. , Levine, A. T. , Lee, W. L. , Mehta, N. M. , Mehra, S. , Weeber, E. J. , & Anderson, A. E. (2017). Strain‐dependence of the Angelman syndrome phenotypes in Ube3a maternal deficiency mice. Scientific Reports, 7(1), 8451. 10.1038/s41598-017-08825-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting, K. , Williams, C. , & Horsthemke, B. (2016). Angelman syndrome—Insights into a rare neurogenetic disorder. Nature Reviews Neurology, 12(10), 584–593. 10.1038/nrneurol.2016.133 [DOI] [PubMed] [Google Scholar]
- Can, A. , Dao, D. T. , Arad, M. , Terrillion, C. E. , Piantadosi, S. C. , & Gould, T. D. (2012). The mouse forced swim test. Journal of Visualized Experiments, 59, e3638. 10.3791/3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji, S. , Brandwein, C. , Vogt, M. A. , Dormann, C. , Hellweg, R. , & Gass, P. (2008). Nature vs. nurture: Can enrichment rescue the behavioural phenotype of BDNF heterozygous mice? Behavioural Brain Research, 192(2), 254–258. 10.1016/j.bbr.2008.04.015 [DOI] [PubMed] [Google Scholar]
- Chourbaji, S. , Hörtnagl, H. , Molteni, R. , Riva, M. A. , Gass, P. , & Hellweg, R. (2012). The impact of environmental enrichment on sex‐specific neurochemical circuitries—Effects on brain‐derived neurotrophic factor and the serotonergic system. Neuroscience, 220, 267–276. 10.1016/j.neuroscience.2012.06.016 [DOI] [PubMed] [Google Scholar]
- Ciarlone, S. L. , Grieco, J. C. , D'Agostino, D. P. , & Weeber, E. J. (2016). Ketone ester supplementation attenuates seizure activity, and improves behavior and hippocampal synaptic plasticity in an Angelman syndrome mouse model. Neurobiology of Disease, 96, 38–46. 10.1016/j.nbd.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Ciarlone, S. L. , Wang, X. , Rogawski, M. A. , & Weeber, E. J. (2017). Effects of the synthetic neurosteroid ganaxolone on seizure activity and behavioral deficits in an Angelman syndrome mouse model. Neuropharmacology, 116, 142–150. 10.1016/j.neuropharm.2016.12.009 [DOI] [PubMed] [Google Scholar]
- Consorti, A. , Sansevero, G. , Torelli, C. , Berardi, N. , & Sale, A. (2019). From basic visual science to neurodevelopmental disorders: The voyage of environmental enrichment‐like stimulation. Neural Plasticity, 2019, 5653180. 10.1155/2019/5653180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley, J. N. (2007). Mouse behavioral assays relevant to the symptoms of autism. Brain Pathology, 17(4), 448–459. 10.1111/j.1750-3639.2007.00096.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, E. , Descalzi, G. , Steinmetz, A. , Scharfman, H. E. , Katzman, A. , & Alberini, C. M. (2021). CIM6P/IGF‐2 receptor ligands reverse deficits in Angelman syndrome model mice. Autism Research, 14(1), 29–45. 10.1002/aur.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan, B. (2009). Angelman syndrome: Current understanding and research prospects. Epilepsia, 50(11), 2331–2339. 10.1111/j.1528-1167.2009.02311.x [DOI] [PubMed] [Google Scholar]
- Deacon, R. M. J. (2006). Digging and marble burying in mice: Simple methods for in vivo identification of biological impacts. Nature Protocols, 1(1), 122–124. 10.1038/nprot.2006.20 [DOI] [PubMed] [Google Scholar]
- Deacon, R. M. J. (2013). Measuring motor coordination in mice. Journal of Visualized Experiments, 75, e2609. 10.3791/2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doreste‐Mendez, R. , Ríos‐Ruiz, E. J. , Rivera‐López, L. L. , Gutierrez, A. , & Torres‐Reveron, A. (2019). Effects of environmental enrichment in maternally separated rats: Age and sex‐specific outcomes. Frontiers in Behavioral Neuroscience, 13, 198. 10.3389/fnbeh.2019.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, R. , & Crawley, J. (2020). Behavioral evaluation of Angelman syndrome mice at older ages. Neuroscience, 445, 163. 10.1016/j.neuroscience.2019.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girbovan, C. , & Plamondon, H. (2013). Environmental enrichment in female rodents: Considerations in the effects on behavior and biochemical markers. Behavioural Brain Research, 253, 178–190. 10.1016/j.bbr.2013.07.018 [DOI] [PubMed] [Google Scholar]
- Godavarthi, S. K. , Dey, P. , Maheshwari, M. , & Jana, N. R. (2012). Defective glucocorticoid hormone receptor signaling leads to increased stress and anxiety in a mouse model of Angelman syndrome. Human Molecular Genetics, 21(8), 1824–1834. 10.1093/hmg/ddr614 [DOI] [PubMed] [Google Scholar]
- Gu, B. , Zhu, M. , Glass, M. R. , Rougié, M. , Nikolova, V. D. , Moy, S. S. , Carney, P. R. , & Philpot, B. D. (2019). Cannabidiol attenuates seizures and EEG abnormalities in Angelman syndrome model mice. Journal of Clinical Investigation, 129(12), 5462–5467. 10.1172/JCI130419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzetti, S. , Calzari, L. , Buccarello, L. , Cesari, V. , Toschi, I. , Cattaldo, S. , Mauro, A. , Pregnolato, F. , Mazzola, S. M. , & Russo, S. (2018). Taurine administration recovers motor and learning deficits in an Angelman syndrome mouse model. International Journal of Molecular Sciences, 19(4), E1088. 10.3390/ijms19041088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan, A. J. (2014). Environmental enrichment and brain repair: Harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience‐dependent plasticity. Neuropathology and Applied Neurobiology, 40(1), 13–25. 10.1111/nan.12102 [DOI] [PubMed] [Google Scholar]
- Heald, M. , Adams, D. , & Oliver, C. (2020). Profiles of atypical sensory processing in Angelman, Cornelia de Lange and Fragile X syndromes. Journal of Intellectual Disability Research, 64(2), 117–130. 10.1111/jir.12702 [DOI] [PubMed] [Google Scholar]
- Hethorn, W. R. , Ciarlone, S. L. , Filonova, I. , Rogers, J. T. , Aguirre, D. , Ramirez, R. A. , Grieco, J. C. , Peters, M. M. , Gulick, D. , Anderson, A. E. , Banko, J. L. , Lussier, A. L. , & Weeber, E. J. (2015). Reelin supplementation recovers synaptic plasticity and cognitive deficits in a mouse model for Angelman syndrome. European Journal of Neuroscience, 41(10), 1372–1380. 10.1111/ejn.12893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill‐Yardin, E. L. , & Hannan, A. J. (2013). Translating preclinical environmental enrichment studies for the treatment of autism and other brain disorders: Comment on Woo and Leon (2013). Behavioral Neuroscience, 127(4), 606–609. 10.1037/a0033319 [DOI] [PubMed] [Google Scholar]
- Huang, H.‐S. , Burns, A. J. , Nonneman, R. J. , Baker, L. K. , Riddick, N. V. , Nikolova, V. D. , Riday, T. T. , Yashiro, K. , Philpot, B. D. , & Moy, S. S. (2013). Behavioral deficits in an Angelman syndrome model: Effects of genetic background and age. Behavioural Brain Research, 243, 79–90. 10.1016/j.bbr.2012.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert, S. W. , Bey, A. L. , & Jiang, Y.‐H. (2018). Environmental enrichment has minimal effects on behavior in the Shank3 complete knockout model of autism spectrum disorder. Brain and Behavior, 8(11), e01107. 10.1002/brb3.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal, I. , Kumar, V. , Vatsa, N. , Singh, B. K. , Shekhar, S. , Sharma, A. , & Jana, N. R. (2017). Environmental enrichment improves behavioral abnormalities in a mouse model of Angelman syndrome. Molecular Neurobiology, 54(7), 5319–5326. 10.1007/s12035-016-0080-3 [DOI] [PubMed] [Google Scholar]
- Jedele, K. B. (2007). The overlapping spectrum of Rett and Angelman syndromes: A clinical review. Seminars in Pediatric Neurology, 14(3), 108–117. 10.1016/j.spen.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Jiang, Y. H. , Armstrong, D. , Albrecht, U. , Atkins, C. M. , Noebels, J. L. , Eichele, G. , Sweatt, J. D. , & Beaudet, A. L. (1998). Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long‐term potentiation. Neuron, 21(4), 799–811. 10.1016/s0896-6273(00)80596-6 [DOI] [PubMed] [Google Scholar]
- Jiang, Y. H. , Pan, Y. , Zhu, L. , Landa, L. , Yoo, J. , Spencer, C. , Lorenzo, I. , Brilliant, M. , Noebels, J. , & Beaudet, A. L. (2010). Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. Plos One, 5(8), e12278. 10.1371/journal.pone.0012278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson, M. C. , Wallace, M. L. , Sidorov, M. S. , Burette, A. C. , Gu, B. , van Woerden, G. M. , King, I. F. , Han, J. E. , Zylka, M. J. , Elgersma, Y. , Weinberg, R. J. , & Philpot, B. D. (2016). GABAergic neuron‐specific loss of Ube3a causes Angelman syndrome‐like EEG abnormalities and enhances seizure susceptibility. Neuron, 90(1), 56–69. 10.1016/j.neuron.2016.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson, M. C. , Shyng, C. , Simon, J. M. , Davis, C. R. , Punt, A. M. , Salmon, M. T. , Miller, N. W. , Ritola, K. D. , Elgersma, Y. , Amaral, D. G. , Gray, S. J. , & Philpot, B. D. (2021). Dual‐isoform hUBE3A gene transfer improves behavioral and seizure outcomes in Angelman syndrome model mice. JCI insight, 6(20), e144712. 10.1172/jci.insight.144712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, A. , & Hannan, A. J. (2019). Therapeutic impacts of environmental enrichment: Neurobiological mechanisms informing molecular targets for enviromimetics. Neuropharmacology, 145(Pt A), 1–2. 10.1016/j.neuropharm.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Kentner, A. C. , Lima, E. , Migliore, M. M. , Shin, J. , & Scalia, S. (2018). Complex environmental rearing enhances social salience and affects hippocampal corticotropin releasing hormone receptor expression in a sex‐specific manner. Neuroscience, 369, 399–411. 10.1016/j.neuroscience.2017.11.035 [DOI] [PubMed] [Google Scholar]
- Kloth, A. D. , Badura, A. , Li, A. , Cherskov, A. , Connolly, S. G. , Giovannucci, A. , Bangash, M. A. , Grasselli, G. , Peñagarikano, O. , Piochon, C. , Tsai, P. T. , Geschwind, D. H. , Hansel, C. , Sahin, M. , Takumi, T. , Worley, P. F. , & Wang, S. S.‐H. (2015). Cerebellar associative sensory learning defects in five mouse autism models. eLife, 4, e06085. 10.7554/eLife.06085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, M. A. , Gray, L. J. , Pelka, G. J. , Christodoulou, J. , Tam, P. P. L. , & Hannan, A. J. (2008). Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome—Mecp2 gene dosage effects and BDNF expression. European Journal of Neuroscience, 27(12), 3342–3350. 10.1111/j.1460-9568.2008.06305.x [DOI] [PubMed] [Google Scholar]
- Kondo, M. A. , Gray, L. J. , Pelka, G. J. , Leang, S.‐K. , Christodoulou, J. , Tam, P. P. L. , & Hannan, A. J. (2016). Affective dysfunction in a mouse model of Rett syndrome: Therapeutic effects of environmental stimulation and physical activity. Developmental Neurobiology, 76(2), 209–224. 10.1002/dneu.22308 [DOI] [PubMed] [Google Scholar]
- Kondo, M. A. , & Hannan, A. J. (2019). Environmental stimulation modulating the pathophysiology of neurodevelopmental disorders. In Oberman L. M. & Enticott P. G. (Eds.), Neurotechnology and brain stimulation in pediatric psychiatric and neurodevelopmental disorders (pp. 31–54). Academic Press. 10.1016/B978-0-12-812777-3.00003-9 [DOI] [Google Scholar]
- Koyavski, L. , Panov, J. , Simchi, L. , Rayi, P. R. , Sharvit, L. , Feuermann, Y. , & Kaphzan, H. (2019). Sex‐dependent sensory phenotypes and related transcriptomic expression profiles are differentially affected by Angelman syndrome. Molecular Neurobiology, 56(9), 5998–6016. 10.1007/s12035-019-1503-8 [DOI] [PubMed] [Google Scholar]
- Kulesskaya, N. , & Voikar, V. (2014). Assessment of mouse anxiety‐like behavior in the light‐dark box and open‐field arena: Role of equipment and procedure. Physiology & Behavior, 133, 30–38. 10.1016/j.physbeh.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Kumar, V. , Joshi, T. , Vatsa, N. , Singh, B. K. , & Jana, N. R. (2019). Simvastatin restores HDAC1/2 activity and improves behavioral deficits in angelman syndrome model mouse. Frontiers in Molecular Neuroscience, 12, 289. 10.3389/fnmol.2019.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaria, M. , Spencer, C. , Gu, W. , Paylor, R. , & Lupski, J. R. (2012). Enriched rearing improves behavioral responses of an animal model for CNV‐based autistic‐like traits. Human Molecular Genetics, 21(14), 3083–3096. 10.1093/hmg/dds124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, T. J. , Fernandez, S. M. , & Frick, K. M. (2005). Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiology of Learning and Memory, 83(3), 206–216. 10.1016/j.nlm.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Lin, E.‐J. D. , Choi, E. , Liu, X. , Martin, A. , & During, M. J. (2011). Environmental enrichment exerts sex‐specific effects on emotionality in C57BL/6J mice. Behavioural Brain Research, 216(1), 349–357. 10.1016/j.bbr.2010.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Johe, K. , Sun, J. , Hao, X. , Wang, Y. , Bi, X. , & Baudry, M. (2019). Enhancement of synaptic plasticity and reversal of impairments in motor and cognitive functions in a mouse model of Angelman Syndrome by a small neurogenic molecule, NSI‐189. Neuropharmacology, 144, 337–344. 10.1016/j.neuropharm.2018.10.038 [DOI] [PubMed] [Google Scholar]
- Lonetti, G. , Angelucci, A. , Morando, L. , Boggio, E. M. , Giustetto, M. , & Pizzorusso, T. (2010). Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biological Psychiatry, 67(7), 657–665. 10.1016/j.biopsych.2009.12.022 [DOI] [PubMed] [Google Scholar]
- Mabb, A. M. , Judson, M. C. , Zylka, M. J. , & Philpot, B. D. (2011). Angelman syndrome: Insights into genomic imprinting and neurodevelopmental phenotypes. Trends in Neurosciences, 34(6), 293–303. 10.1016/j.tins.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri, M. , Pouretemad, H. , Wegener, G. , Roghani, M. , Afshari, M. , Mallard, C. , & Ardalan, M. (2021). Dual profile of environmental enrichment and autistic‐like behaviors in the maternal separated model in rats. International Journal of Molecular Sciences, 22(3), 1173. 10.3390/ijms22031173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis, S. S. , Sell, G. L. , Zbinden, M. A. , & Bird, L. M. (2015). Angelman Syndrome. Neurotherapeutics, 12(3), 641–650. 10.1007/s13311-015-0361-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markati, T. , Duis, J. , & Servais, L. (2021). Therapies in preclinical and clinical development for Angelman syndrome. Expert Opinion on Investigational Drugs, 30, 1–12. 10.1080/13543784.2021.1939674 [DOI] [PubMed] [Google Scholar]
- Martínez‐Cué, C. , Baamonde, C. , Lumbreras, M. , Paz, J. , Davisson, M. T. , Schmidt, C. , Dierssen, M. , & Flórez, J. (2002). Differential effects of environmental enrichment on behavior and learning of male and female Ts65Dn mice, a model for Down syndrome. Behavioural Brain Research, 134(1–2), 185–200. 10.1016/s0166-4328(02)00026-8 [DOI] [PubMed] [Google Scholar]
- McOmish, C. E. , & Hannan, A. J. (2007). Enviromimetics: Exploring gene environment interactions to identify therapeutic targets for brain disorders. Expert Opinion on Therapeutic Targets, 11(7), 899–913. 10.1517/14728222.11.7.899 [DOI] [PubMed] [Google Scholar]
- Meng, L. , Person, R. E. , Huang, W. , Zhu, P. J. , Costa‐Mattioli, M. , & Beaudet, A. L. (2013). Truncation of Ube3a‐ATS unsilences paternal Ube3a and ameliorates behavioral defects in the Angelman syndrome mouse model. Plos Genetics, 9(12), e1004039. 10.1371/journal.pgen.1004039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz, L. G. , Thaulov, P. , Trillingsgaard, A. , Christensen, R. , Vogel, I. , Hertz, J. M. , & Ostergaard, J. R. (2014). Neurodevelopmental outcome in Angelman syndrome: Genotype‐phenotype correlations. Research in Developmental Disabilities, 35(7), 1742–1747. 10.1016/j.ridd.2014.02.018 [DOI] [PubMed] [Google Scholar]
- Milazzo, C. , Mientjes, E. J. , Wallaard, I. , Rasmussen, S. V. , Erichsen, K. D. , Kakunuri, T. , van der Sman, A. , Kremer, T. , Miller, M. T. , Hoener, M. C. , & Elgersma, Y. (2021). Antisense oligonucleotide treatment rescues UBE3A expression and multiple phenotypes of an Angelman syndrome mouse model. JCI insight, 6(15), e145991. 10.1172/jci.insight.145991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli, E. , Ghiglieri, V. , Pendolino, V. , Bagetta, V. , Pignataro, A. , Fejtova, A. , Costa, C. , Ammassari‐Teule, M. , Gundelfinger, E. D. , Picconi, B. , & Calabresi, P. (2014). Environmental enrichment restores CA1 hippocampal LTP and reduces severity of seizures in epileptic mice. Experimental Neurology, 261, 320–327. 10.1016/j.expneurol.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Nag, N. , Moriuchi, J. M. , Peitzman, C. G. K. , Ward, B. C. , Kolodny, N. H. , & Berger‐Sweeney, J. E. (2009). Environmental enrichment alters locomotor behaviour and ventricular volume in Mecp2 1lox mice. Behavioural Brain Research, 196(1), 44–48. 10.1016/j.bbr.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Nithianantharajah, J. , & Hannan, A. J. (2006). Enriched environments, experience‐dependent plasticity and disorders of the nervous system. Nature Reviews Neuroscience, 7(9), 697–709. 10.1038/nrn1970 [DOI] [PubMed] [Google Scholar]
- Petkova, S. P. , Duis, J. D. , & Silverman, J. L. (2021) Gait as a quantitative translational outcome measure in Angelman syndrome. bioRxiv. 10.1101/2021.08.13.456146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaolo, S. , Branchi, I. , Cirulli, F. , Chiarotti, F. , Aloe, L. , & Alleva, E. (2004). Long‐term effects of the periadolescent environment on exploratory activity and aggressive behaviour in mice: Social versus physical enrichment. Physiology & Behavior, 81(3), 443–453. 10.1016/j.physbeh.2004.02.022 [DOI] [PubMed] [Google Scholar]
- Queen, N. J. , Boardman, A. A. , Patel, R. S. , Siu, J. J. , Mo, X. , & Cao, L. (2020). Environmental enrichment improves metabolic and behavioral health in the BTBR mouse model of autism. Psychoneuroendocrinology, 111, 104476. 10.1016/j.psyneuen.2019.104476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo, L. , Ferrari, F. , Passino, E. , Sgobio, C. , Bock, J. , Oostra, B. A. , Bagni, C. , & Ammassari‐Teule, M. (2005). Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proceedings of the National Academy of Sciences, 102(32), 11557–11562. 10.1073/pnas.0504984102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, S. , Urruela, M. , & Devine, D. P. (2013). Effects of environmental enrichment on repetitive behaviors in the BTBR T+tf/J mouse model of autism. Autism Research, 6(5), 337–343. 10.1002/aur.1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru, D. C. , Mientjes, E. J. , & Elgersma, Y. (2020). Angelman syndrome: From mouse models to therapy. Neuroscience, 445, 172–189. 10.1016/j.neuroscience.2020.02.017 [DOI] [PubMed] [Google Scholar]
- Sale, A. , Berardi, N. , & Maffei, L. (2014). Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiological Reviews, 94(1), 189–234. 10.1152/physrev.00036.2012 [DOI] [PubMed] [Google Scholar]
- Schmid, R. S. , Deng, X. , Panikker, P. , Msackyi, M. , Breton, C. , & Wilson, J. M. (2021). CRISPR/Cas9 directed to the Ube3a antisense transcript improves Angelman syndrome phenotype in mice. Journal of Clinical Investigation, 131(5), 142574. 10.1172/JCI142574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, T. , Turczak, J. , & Przewłocki, R. (2006). Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: Issues for a therapeutic approach in autism. Neuropsychopharmacology, 31(1), 36–46. 10.1038/sj.npp.1300767 [DOI] [PubMed] [Google Scholar]
- Schultz, M. N. , & Crawley, J. N. (2020). Evaluation of a TrkB agonist on spatial and motor learning in the Ube3a mouse model of Angelman syndrome. Learning and Memory, 27(9), 346–354. 10.1101/lm.051201.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky, R. M. , & Woolley, C. S. (2016). Considering sex as a biological variable will be valuable for neuroscience research. Journal of Neuroscience, 36(47), 11817–11822. 10.1523/JNEUROSCI.1390-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorov, M. S. , Deck, G. M. , Dolatshahi, M. , Thibert, R. L. , Bird, L. M. , Chu, C. J. , & Philpot, B. D. (2017). Delta rhythmicity is a reliable EEG biomarker in Angelman syndrome: A parallel mouse and human analysis. Journal of Neurodevelopmental Disorders, 9, 17. 10.1186/s11689-017-9195-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva‐Santos, S. , van Woerden, G. M. , Bruinsma, C. F. , Mientjes, E. , Jolfaei, M. A. , Distel, B. , Kushner, S. A. , & Elgersma, Y. (2015). Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model. Journal of Clinical Investigation, 125(5), 2069–2076. 10.1172/JCI80554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas, M. , Chauvet, C. , Lafay‐Chebassier, C. , Jaafari, N. , & Thiriet, N. (2021). Environmental enrichment‐inspired pharmacological tools for the treatment of addiction. Current Opinion in Pharmacology, 56, 22–28. 10.1016/j.coph.2020.09.001 [DOI] [PubMed] [Google Scholar]
- Sonzogni, M. , Hakonen, J. , Bernabé Kleijn, M. , Silva‐Santos, S. , Judson, M. C. , Philpot, B. D. , van Woerden, G. M. , & Elgersma, Y. (2019). Delayed loss of UBE3A reduces the expression of Angelman syndrome‐associated phenotypes. Molecular Autism, 10, 23. 10.1186/s13229-019-0277-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonzogni, M. , Wallaard, I. , Santos, S. S. , Kingma, J. , du Mee, D. , van Woerden, G. M. , & Elgersma, Y. (2018). A behavioral test battery for mouse models of Angelman syndrome: A powerful tool for testing drugs and novel Ube3a mutants. Molecular Autism, 9, 47. 10.1186/s13229-018-0231-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam, N. C. , Nithianantharajah, J. , Howard, M. L. , Atkin, J. D. , Cheema, S. S. , & Hannan, A. J. (2008). Sex‐specific behavioural effects of environmental enrichment in a transgenic mouse model of amyotrophic lateral sclerosis. European Journal of Neuroscience, 28(4), 717–723. 10.1111/j.1460-9568.2008.06374.x [DOI] [PubMed] [Google Scholar]
- Sturman, O. , Germain, P.‐L. , & Bohacek, J. (2018). Exploratory rearing: A context‐ and stress‐sensitive behavior recorded in the open‐field test. Stress, 21(5), 443–452. 10.1080/10253890.2018.1438405 [DOI] [PubMed] [Google Scholar]
- Suemaru, K. , Yoshikawa, M. , Aso, H. , & Watanabe, M. (2018). Environmental enrichment alleviates cognitive and behavioral impairments in EL mice. Epilepsy & Behavior, 85, 227–233. 10.1016/j.yebeh.2018.06.016 [DOI] [PubMed] [Google Scholar]
- Sztainberg, Y. , & Chen, A. (2010). An environmental enrichment model for mice. Nature Protocols, 5(9), 1535–1539. 10.1038/nprot.2010.114 [DOI] [PubMed] [Google Scholar]
- Tan, W. H. , Bird, L. M. , Thibert, R. L. , & Williams, C. A. (2014). If not Angelman, what is it? A review of Angelman‐like syndromes. American Journal of Medical Genetics Part A, 164A(4), 975–992. 10.1002/ajmg.a.36416 [DOI] [PubMed] [Google Scholar]
- Tanaka, S. , Young, J. W. , Halberstadt, A. L. , Masten, V. L. , & Geyer, M. A. (2012). Four factors underlying mouse behavior in an open field. Behavioural Brain Research, 233(1), 55–61. 10.1016/j.bbr.2012.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton, C. , Kloth, A. D. , Clark, E. P. , Moy, S. S. , Chitwood, R. A. , & Philpot, B. D. (2018). Common pathophysiology in multiple mouse models of Pitt‐Hopkins syndrome. Journal of Neuroscience, 38(4), 918–936. 10.1523/JNEUROSCI.1305-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas, D. , Prijanto, A. H. , Burrows, E. L. , Hannan, A. J. , Horne, M. K. , & Aumann, T. D. (2015). Environmental modulations of the number of midbrain dopamine neurons in adult mice. Journal of Visualized Experiments, 95, 52329. 10.3791/52329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden, G. M. , Harris, K. D. , Hojjati, M. R. , Gustin, R. M. , Qiu, S. , de Avila Freire, R. , Jiang, Y. , Elgersma, Y. , & Weeber, E. J. (2007). Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nature Neuroscience, 10(3), 280–282. 10.1038/nn1845 [DOI] [PubMed] [Google Scholar]
- Veltman, M. W. M. , Craig, E. E. , & Bolton, P. F. (2005). Autism spectrum disorders in Prader‐Willi and Angelman syndromes: A systematic review. Psychiatric Genetics, 15(4), 243–254. 10.1097/00041444-200512000-00006 [DOI] [PubMed] [Google Scholar]
- Wang, T. , Wang, J. , Wang, J. , Mao, L. , Tang, B. , Vanderklish, P. W. , Liao, X. , Xiong, Z. Q. , & Liao, L. (2019). HAP1 is an in vivo UBE3A target that augments autophagy in a mouse model of Angelman syndrome. Neurobiology of Disease, 132, 104585. 10.1016/j.nbd.2019.104585 [DOI] [PubMed] [Google Scholar]
- Wheeler, A. C. , Sacco, P. , & Cabo, R. (2017). Unmet clinical needs and burden in Angelman syndrome: A review of the literature. Orphanet Journal of Rare Diseases, 12(1), 164. 10.1186/s13023-017-0716-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, A. C. , Okoniewski, K. C. , Wylie, A. , DeRamus, M. , Hiruma, L. S. , Toth, D. , & Christian, R. B. (2019). Anxiety‐associated and separation distress‐associated behaviours in Angelman syndrome. Journal of Intellectual Disability Research, 63(10), 1234–1247. 10.1111/jir.12635 [DOI] [PubMed] [Google Scholar]
- Williams, C. A. , Beaudet, A. L. , Clayton‐Smith, J. , Knoll, J. H. , Kyllerman, M. , Laan, L. A. , Magenis, R. E. , Moncla, A. , Schinzel, A. A. , Summers, J. A. , & Wagstaff, J. (2006). Angelman syndrome 2005: Updated consensus for diagnostic criteria. American Journal of Medical Genetics. Part A, 140(5), 413–418. 10.1002/ajmg.a.31074 [DOI] [PubMed] [Google Scholar]
- Wolter, J. M. , Mao, H. , Fragola, G. , Simon, J. M. , Krantz, J. L. , Bazick, H. O. , Oztemiz, B. , Stein, J. L. , & Zylka, M. J. (2020). Cas9 gene therapy for Angelman syndrome traps Ube3a‐ATS long non‐coding RNA. Nature, 587(7833), 281–284. 10.1038/s41586-020-2835-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, C. C. , Donnelly, J. H. , Steinberg‐Epstein, R. , & Leon, M. (2015). Environmental enrichment as a therapy for autism: A clinical trial replication and extension. Behavioral Neuroscience, 129(4), 412–422. 10.1037/bne0000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, C. C. , & Leon, M. (2013). Environmental enrichment as an effective treatment for autism: A randomized controlled trial. Behavioral Neuroscience, 127(4), 487–497. 10.1037/a0033010 [DOI] [PubMed] [Google Scholar]
- Wood, N. I. , Carta, V. , Milde, S. , Skillings, E. A. , McAllister, C. J. , Ang, Y. L. M. , Duguid, A. , Wijesuriya, N. , Afzal, S. M. , Fernandes, J. X. , Leong, T. W. , Morton, A. J. , & Morton, J. (2010). Responses to environmental enrichment differ with sex and genotype in a transgenic mouse model of Huntington's disease. Plos One, 5(2), e9077. 10.1371/journal.pone.0009077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, H. , Hara, Y. , Ago, Y. , Takano, E. , Hasebe, S. , Nakazawa, T. , Hashimoto, H. , Matsuda, T. , & Takuma, K. (2017). Environmental enrichment attenuates behavioral abnormalities in valproic acid‐exposed autism model mice. Behavioural Brain Research, 333, 67–73. 10.1016/j.bbr.2017.06.035 [DOI] [PubMed] [Google Scholar]
- Yashiro, K. , Riday, T. T. , Condon, K. H. , Roberts, A. C. , Bernardo, D. R. , Prakash, R. , Weinberg, R. J. , Ehlers, M. D. , & Philpot, B. D. (2009). Ube3a is required for experience‐dependent maturation of the neocortex. Nature Neuroscience, 12(6), 777–783. 10.1038/nn.2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, D. , Lawlor, P. A. , Leone, P. , Dragunow, M. , & During, M. J. (1999). Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nature Medicine, 5(4), 448–453. 10.1038/7449 [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Li, H. , & Han, R. (2020). An open‐source video tracking system for mouse locomotor activity analysis. BMC Research Notes, 13(1), 48. 10.1186/s13104-020-4916-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information. Spreadsheet containing results from statistical tests presented in this study. Each set of results is displayed on separate tabs by corresponding figure number. Transformed data used where reported. Statistical significance for main effects and interactions in the two‐way ANOVA (columns F‐G) and Bonferroni‐corrected planned comparisons (columns I‐J): *p < .05, **p < .01, ***p < .001. XLS format.
Data Availability Statement
Data that support the findings of this study and custom code used to analyze data are available from the corresponding author upon reasonable request.


