Abstract
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are influenced by the bacterial and fungal organisms found within the intestine. However, the intestine is also home to a vast number of viral particles, with most of them being viruses that infect prokaryotes, called bacteriophages. While use of bacteriophages to specifically target pathogenic bacterial species involved in IBD is currently under investigation, recent studies have also highlighted that these viral particles can impact the mammalian immune system. IBD is a chronic multi-factorial inflammatory condition with unknown etiology. This review will highlight the current investigations that have revealed that bacteriophage-mammalian immune cell interactions can influence disease processes beyond their known role for infecting bacteria, which might identify novel ways to treat or diagnose IBD.
Introduction
The virobiota could arguably be considered the most numerically prominent entity of the microbiota, yet is the least well understood. Only recently have compositional changes in the virome been studied using current sequencing methods, and this analysis is still being optimized [1–5]. While the intestinal virobiota contains eukaryotic viruses that are capable of infecting mammalian cells, the vast majority is composed of bacteriophages (phages). Bacteriophages can have different lifestyles based on whether they can undergo productive infection [6]. Obligately lytic (or virulent) bacteriophages take over the host cell machinery to replicate new virions which ultimately kills the host cells. Lysogenic phages (or temperate) integrate and replicate with the host DNA and upon certain signals exit the host cell with lysis. Several studies have delineated the compositional changes to the virome in IBD patients, which we will not cover in detail here [1,2]. However using whole virome sequencing, the most prominent feature of a healthy gut virome is the presence of a stable core of lytic phages, while increased temperate phage abundance is associated with individuals that have IBD [1,4]. As the inflamed intestine presents a stressful environment to resident bacteria, the observation of increased temperate phages would be consistent with phage excision and massive release of virions during disease [7].
With the growing emergence of antibiotic resistant pathogens and a growing appreciation for the importance of the beneficial microbes that reside on mammalian bodies, targeting bacterial pathogens using bacteriophages has regained interest [8]. The relative specificity of bacteriophages for their hosts, provides an opportunity to target particular pathogens without collateral damage to the other beneficial members of the microbiota and is currently under investigation for IBD therapy. In addition, the field has become aware that bacteriophages make up a vast portion of the intestinal resident viruses, that have the potential to influence the disease process. While phages do not infect mammalian cells, several recent studies have demonstrated that these prokaryotic viruses can influence, either directly or indirectly, the mammalian immune system. Here we highlight these recent studies and discuss the mechanisms by which bacteriophages can influence IBD with a focus on interactions with the mammalian immune response.
Synergy with the immune system
Antibiotics have long been used to treat most pathogenic infections with great success. However, unintended consequences of antibiotic overuse have led to the emergence of multi-drug resistance bacteria. Additionally, antibiotics are not selective and also kill beneficial commensal bacteria that prevent pathogen infection through colonization resistance or promotion of immune pathways to fend off pathogenic bacteria. Thus, bacteriophage therapy has re-emerged as a mechanism to target and kill specific infectious bacteria and allowing for commensal populations to remain in-tact. While bacteriophage therapy has classically thought to largely rely on killing of permissive bacteria by obligately lytic phages, there is a growing appreciation that bacteriophage therapy can synergize with the host immune system in what has been termed “immunophage therapy” [9].
While there are many examples of bacteriophage therapy successes, there are an equal number of failures. This is largely thought to be due to the ability of bacteria to evolve resistance to phage lysis. This highlights the need to understand the mechanisms by which bacteriophage therapy functions and move beyond in vitro models of bacteria-phage interactions and test in animal model systems. Roach et. al. set out to understand mechanisms of phage-mediated bacterial clearance using a Pseudomonas aeruginosa animal model [9]. Animals infected with P. aeruginosa followed by bacteriophage treatment two hours later survived lethal infection and were quickly able to clear the bacteria from their lungs. Interestingly, animals that lacked the signaling molecule, MyD88, the adaptor molecule for most Toll-like receptors (TLRs) that sense microbial ligands, during phage therapy, still suffered from increased mortality. While the bacteriophage therapy significantly reduced the amounts of bacteria within the lungs initially, there was a resurgence of phage-resistant bacteria after 24 hours. Neutrophils, but not T or B cells, were required for the synergistic effect. Intranasal administration of a high dose of the bacteriophage did not stimulate cytokine production within the lung on its own, leading the authors to conclude that the immune system was not necessarily being directly activated by the phage itself, rather that the neutrophils were required to kill phage-resistant bacteria.
Currently, bacteriophage therapy targeting two commonly identified organisms in individuals with IBD, Klebsiella pneumoniae and adherent invasive E. coli (AIEC), is being trialed in humans. The findings by Roach et. al. suggest that for bacteriophage therapy to be efficacious, the host must have a competent immune system that also helps eradicate phage resistant strains. As individuals with IBD have chronic inflammation within the intestine, it is unclear how this might influence this process. Recent studies have tested phage therapy against AIEC in acute mouse models of IBD [10]. Animals were colonized with AIEC followed by induction of IBD and a single dose of an AIEC targeting bacteriophage cocktail. The three phages were sufficient to reduce colonization of AIEC up to 15 days after phage treatment. Moreover, phage treated animals had reduced blood in the stool, diarrhea, and weight loss, suggesting that this therapy could work in humans. However, IBD is a multi-factorial, chronic, disease, thus, future studies will need to investigate the requirement for immune cells during bacteriophage therapy and determine whether it is effective over longer time courses.
Direct immune stimulation
Lysing of bacteria by phages release bacterial surface molecules and intercellular contents that could potentially stimulate the immune system through TLR signaling. However, bacteriophages are known to reside within the mucus layer of many animals, where they have been proposed to act as a firewall against mucosal invasion of intestinal bacteria [11]. Studies demonstrating that bacteriophage viral particles can be transcytosed from the apical to basolateral surface of intestinal epithelial cells makes it possible for these viral particles to come in direct contact with underlying immune cells of the intestine and stimulate various immune responses [12,13]. Direct immune stimulation by bacteriophages was originally explored in vitro and these experiments have been reviewed in [14,15]. While these studies suggested that bacteriophages could directly stimulate eukaryotic immune processes, many of these did not use highly purified bacteriophage preparations for their assays. As bacteriophages must be amplified for experiments by replicating within bacteria, care must be taken to purify out any contaminating LPS or bacterial molecules that could also stimulate the immune response. Moreover, in vivo animal studies conducted on this topic have analyzed mice whereby the host bacteria for the bacteriophage was present, and thereby bacterial lysis and subsequent immune stimulation by bacterial molecules could not be ruled out. Thus, whether phages could directly stimulate immune responses in the gut remained unclear.
To address this, our group orally fed a highly purified bacteriophage cocktail to germfree (GF) mice that are completely devoid of colonization by any commensal organisms [16]. Since the bacterial hosts were not present within the animals to aid replication of the phage particles, phages were continuously provided. Interestingly, bacteriophage fed, but not heat-killed phage fed, animals had significant induction of total CD8+ and CD4+ T cells as well as IFN-γ producing Th1 cells. The levels of T cells elicited by bacteriophages were similar to those found in normal colonized animals. Phage particles were visualized inside of host dendritic of cells and IFN-γ production induced by bacteriophages was dependent on endosomal TLR9. These sets of experiments demonstrate that bacteriophage particles can directly stimulate intestinal inflammatory responses even in the absence of bacterial organisms. This could suggest that bacteriophages are part of priming immunity within the gut during development.
Another investigation studying a filamentous temperate bacteriophage within the P. aeruginosa genome, called Pf phage, demonstrated the ability of this phage to suppress inflammatory responses [17]. The Pf phage has a temperate lifecycle that releases the virions through extrusion without bacterial lysis. Released filamentous virions became structural elements in P. aeruginosa biofilms promoting the formation of a liquid viscous matrix which provides the protection of biofilm embedded bacteria from desiccation and enhance their virulence [18,19]. P. aeruginosa mutants lacking the phage are phagocytosed at much higher rates than wild-type strains, suggesting the phage can influence host immune responses. Treatment of bone marrow derived macrophages with the purified phage reduced TNF-α production in response to LPS stimulation [17]. Blockage of TNF-α production by the Pf phage was dependent on its ability to stimulate Type I interferon production through TLR3. Thus, the expression of the filamentous phage by P. aeruginosa enhanced virulence through direct down-regulation of immunity. Collectively, these experiments demonstrate that bacteriophages can have distinct effects on the immune response which could have important implications for intestinal development, tolerance and disease.
An expansion in Caudovirales bacteriophages has been reported in patients with IBD and confirmed in animal models [2,20]. To test whether direct activation of the immune system by bacteriophages could influence IBD severity, we took advantage of animals that were not colonized by E. coli yet possessed a diverse microbiota [16]. These animals were orally gavaged with a cocktail of three E. coli bacteriophages, followed by induction of colitis. Animals treated with bacteriophages developed significantly worsened disease characterized by increased weight loss and intestinal damage, that was dependent on IFN-γ and TLR9. Supporting these findings, the abundance of mucosal IFN-γ production in humans is significantly correlated with the abundance of Caudovirales bacteriophages in IBD patients that failed to respond to fecal microbiota transplant (FMT). These data suggest that as bacteriophages within the bacterial microbiota are excised from their hosts during inflammation, they have the potential to further stimulate immunity and exacerbate disease. While it has yet to be investigated, as other bacteriophages are able to dampen immunity [17], it is possible that some bacteriophages might actually help to temper immunity and prevent disease.
Microbial community changes
One of the most appreciated roles of bacteriophages is lysis of their target bacteria. Therefore, it is no surprise that some studies have suggested and demonstrated that bacteriophages can naturally influence the composition of the bacterial microbiota [21]. The composition of bacteriophages in stunted children is distinct from non-stunted children, where non-stunted children possessed a greater abundance of temperate bacteriophages [22]. When bacteria from non-stunted children were exposed to phages from stunted children, Proteobacteria significantly increased, demonstrating that bacteriophages present in stunted children select for a Proteobacteria rich microbiota. Another study utilized a defined consortia of bacteria, assembled in germfree mice, of 10 distinct organisms [23]. Obligately lytic bacteriophages were selected against four of these bacterial species and provided individually or in combination to GF animals with the defined consortia and carefully tracked using sequencing and q-PCR methods. Tracking of these populations demonstrated that phage predation on their host bacteria can lead to cascading effects on the composition of the microbiota which in turn influenced changes in metabolites.
Clostridium difficile infection is a debilitating intestinal infection, that primarily results from predominance of this pathogen after an insult on microbiota composition, such as depletion through antibiotics. Restoration of the intestinal microbiota using a crude fecal preparation, termed fecal microbiota transplant (FMT) from a healthy a donor has been shown to be highly curative in patients with C. difficile [24]. One study successfully utilized fecal homogenate that was cleared of live bacterial organisms, leaving behind bacterial metabolites, DNA/RNA molecules and bacteriophages [25]. While the specific contribution of bacteriophages to clearance of C. difficle was not explicitly defined in this this study, it opens up the possibility that bacteriophage may contribute to the success of an FMT [26]. Supporting a contribution of bacteriophages during FMT in IBD, we have demonstrated persistence of a specific subset of donor derived bacteriophages in individuals who failed to respond to FMT, suggesting that certain bacteriophages might prevent successful FMT [16]. This could be due to a direct influence of these bacteriophages on beneficial microbiota composition. Given the large number of studies that delineate specific mechanisms by which bacterial organisms can influence immune system development [27], future studies should be aimed at uncovering how phage predation can impact the mammalian immune response
Temperate phage gene expression
Temperate phages can encode a number of genes that are not necessarily involved in phage assembly or replication but can benefit their prokaryotic host in various ways. The expression of these phage encoded genes can in turn influence mammalian biology. Perhaps one of the most well-studied examples of this is the cholera toxin, (CTX), that is expressed on a temperate bacteriophage in Vibrio cholera [28,29]. The CTX toxin is released from bacterial cells harboring the temperate phage within the intestine and binds to a receptor expressed on intestinal epithelial cells leading to severe fluid loss. While this phenomenon has not been extensively studied in commensal gut microbes, a recent study has identified a phage encoded molecule expressed by a common intestinal bacteria, Enterococcus hirae, can cross-react with T cells that recognize tumor antigens [30].
Zitvogel and colleagues had previously shown that E. hirae would become translocated during chemotherapeutic treatment in individuals with cancer [31]. Translocation of E. hirae stimulated immune responses that helped to fight cancer. They identified that a specific protein, called tailed length tape measure protein (TMP1), expressed by a prophage within E. hirae, was presented on class I MHC and cross-reacted with T cells that were specific for a proteosome subunit protein expressed by the mammalian host [30]. Enhancement of these cross-reactive T cell responses synergized with chemotherapeutic treatment to eradicate cancer in mice. Thus, prophages harbored by commensal bacteriophages might harbor unique and interesting genes that influence various aspects of mammalian immunity and IBD that have yet to be explored.
Conclusion
Commensal microbial organisms are clearly important in many physiological processes, and the mechanisms by which this occur are only being uncovered. As bacteriophages appear to be a large component of the commensal virome, it is likely that these organisms will also play a role in mammalian biology and diseases such as IBD. As bacteriophage-immune interactions are studied more in depth, novel therapeutic interventions or diagnostics might emerge. Studies of the Pf phage, mentioned above, have demonstrated that immunization of a mammalian host against the Pf coat protein can protect from P. aeruginosa wound infections [17]. Thus, immune responses against phages could be harnessed for protective responses. Moreover, antibody responses against bacteriophages have been identified in animal models and humans, highlighting the potential for these to be used as biomarkers [32,33]. Based on these findings, this emerging area of bacteriophage-immune dynamics has many novel pathways for discovery.
Fig 1. Mechanisms of bacteria-immune interactions that could impact IBD.
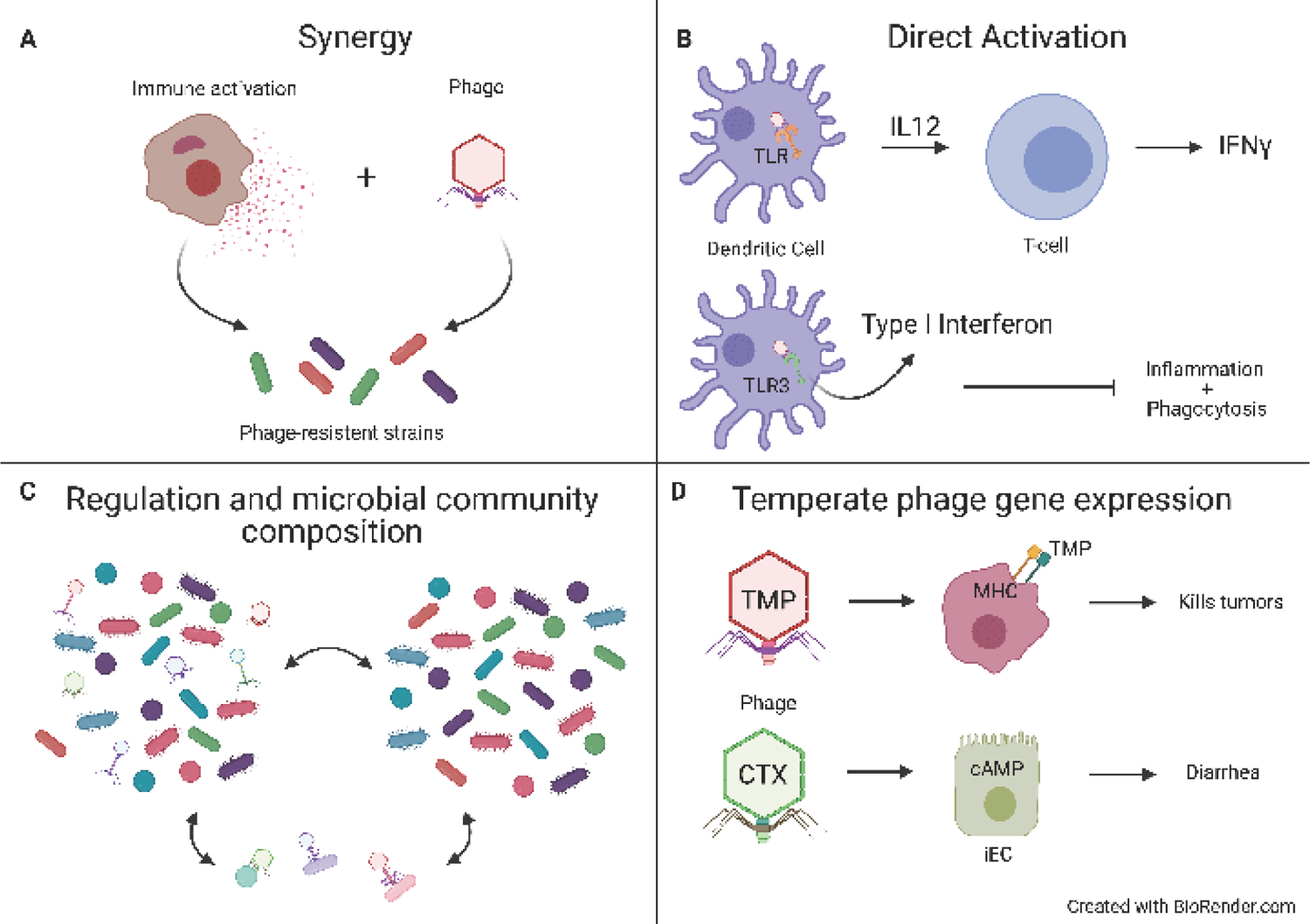
(A) Synergy with the immune system: Phage resistant strains often arise during bacteriophage therapy. Recent studies have identified that synergistic interactions with neutrophils helps to clear pathogenic strains of bacteria that acquire resistance to phages. (B) Direct activation: Bacteriophage virions have now been shown to be taken up by dendritic cells and macrophages and stimulate TLR9 and TLR3 to amplify or dampen immune responses respectively. (C) Bacteria are well document to influence immune system development. Bacteriophages can indirectly influence the immune response by regulating bacterial communities. (D) Temperate phage gene expression: Some prophages can express genes that influence the host response without necessarily expressing the entire virion. The most well documented of these is the cholera toxin that causes severe diarrhea and fluid loss. More recent studies have shown that bacteriophages can encode molecules that can act as “molecular mimics” to stimulate T cells that normally recognize mammalian host proteins.
Acknowledgments
Work in the laboratory of J.L.R is supported by the Crohn’s and Colitis Foundation, The Helmsley Foundation, the W.M. Keck Foundation, the Burrough’s Wellcome Foundation and the NIH (R01DK124336 and R01DK124317).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Nothing declared.
References
- 1. Clooney AG, Sutton TDS, Shkoporov AN, Holohan RK, Daly KM, O’Regan O, Ryan FJ, Draper LA, Plevy SE, Ross RP, et al. : Whole-Virome Analysis Sheds Light on Viral Dark Matter in Inflammatory Bowel Disease. Cell Host Microbe 2019, 26:764–778 e765. • Used a unbiased database to annotate bacteriophages in the intestine.
- 2.Reyes A, Haynes M, Hanson N, Angly FE, Heath AC, Rohwer F, Gordon JI: Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature 2010, 466:334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuo T, Lu XJ, Zhang Y, Cheung CP, Lam S, Zhang F, Tang W, Ching JYL, Zhao R, Chan PKS, et al. : Gut mucosal virome alterations in ulcerative colitis. Gut 2019, 68:1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shkoporov AN, Clooney AG, Sutton TDS, Ryan FJ, Daly KM, Nolan JA, McDonnell SA,Khokhlova EV, Draper LA, Forde A, et al. : The Human Gut Virome Is Highly Diverse, Stable, and Individual Specific. Cell Host Microbe 2019, 26:527–541 e525. [DOI] [PubMed] [Google Scholar]
- 5.Camarillo-Guerrero LF, Almeida A, Rangel-Pineros G, Finn RD, Lawley TD: Massive expansion of human gut bacteriophage diversity. Cell 2021, 184:1098–1109 e1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobbs Z, Abedon ST: Diversity of phage infection types and associated terminology: the problem with ‘Lytic or lysogenic’. FEMS Microbiol Lett 2016, 363. [DOI] [PubMed] [Google Scholar]
- 7.Diard M, Bakkeren E, Cornuault JK, Moor K, Hausmann A, Sellin ME, Loverdo C, Aertsen A,Ackermann M, De Paepe M, et al. : Inflammation boosts bacteriophage transfer between Salmonella spp. Science 2017, 355:1211–1215. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez B, Domingo-Calap P: Phage Therapy in Gastrointestinal Diseases. Microorganisms 2020, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roach DR, Leung CY, Henry M, Morello E, Singh D, Di Santo JP, Weitz JS, Debarbieux L: Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen. Cell Host Microbe 2017, 22:38–47 e34. • Demonstrated that successful bacteriophage therapy requires an intact immune system
- 10.Galtier M, De Sordi L, Sivignon A, de Vallee A, Maura D, Neut C, Rahmouni O, Wannerberger K, Darfeuille-Michaud A, Desreumaux P, et al. : Bacteriophages Targeting Adherent Invasive Escherichia coli Strains as a Promising New Treatment for Crohn’s Disease. J Crohns Colitis 2017, 11:840–847. [DOI] [PubMed] [Google Scholar]
- 11.Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J, Stotland A, Wolkowicz R, Cutting AS, Doran KS, et al. : Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci U S A 2013, 110:10771–10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen S, Baker K, Padman BS, Patwa R, Dunstan RA, Weston TA, Schlosser K, Bailey B,Lithgow T, Lazarou M, et al. : Bacteriophage Transcytosis Provides a Mechanism To Cross Epithelial Cell Layers. mBio 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorski A, Wazna E, Dabrowska BW, Dabrowska K, Switala-Jelen K, Miedzybrodzki R: Bacteriophage translocation. FEMS Immunol Med Microbiol 2006, 46:313–319. [DOI] [PubMed] [Google Scholar]
- 14.Gorski A, Nowaczyk M, Weber-Dabrowska B, Kniotek M, Boratynski J, Ahmed A, Dabrowska K, Wierzbicki P, Switala-Jelen K, Opolski A: New insights into the possible role of bacteriophages in transplantation. Transplant Proc 2003, 35:2372–2373. [DOI] [PubMed] [Google Scholar]
- 15.Gorski A, Dabrowska K, Miedzybrodzki R, Weber-Dabrowska B, Lusiak-Szelachowska M,Jonczyk-Matysiak E, Borysowski J: Phages and immunomodulation. Future Microbiol 2017, 12:905–914. [DOI] [PubMed] [Google Scholar]
- 16. Gogokhia L, Buhrke K, Bell R, Hoffman B, Brown DG, Hanke-Gogokhia C, Ajami NJ, Wong MC,Ghazaryan A, Valentine JF, et al. : Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe 2019, 25:285–299 e288. • First to show that bacteriophages can induce immune system reactions in the intestine in a germfree mouse setting.
- 17. Sweere JM, Van Belleghem JD, Ishak H, Bach MS, Popescu M, Sunkari V, Kaber G,Manasherob R, Suh GA, Cao X, et al. : Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 2019, 363. • Demonstrated that vaccination against a prophage coat protein could protect from wound infection
- 18.Secor PR, Michaels LA, Smigiel KS, Rohani MG, Jennings LK, Hisert KB, Arrigoni A, Braun KR,Birkland TP, Lai Y, et al. : Filamentous Bacteriophage Produced by Pseudomonas aeruginosa Alters the Inflammatory Response and Promotes Noninvasive Infection In Vivo. Infect Immun 2017, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Secor PR, Sweere JM, Michaels LA, Malkovskiy AV, Lazzareschi D, Katznelson E, Rajadas J,Birnbaum ME, Arrigoni A, Braun KR, et al. : Filamentous Bacteriophage Promote Biofilm Assembly and Function. Cell Host Microbe 2015, 18:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duerkop BA, Kleiner M, Paez-Espino D, Zhu W, Bushnell B, Hassell B, Winter SE, Kyrpides NC,Hooper LV: Murine colitis reveals a disease-associated bacteriophage community. Nat Microbiol 2018, 3:1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyes A, Wu M, McNulty NP, Rohwer FL, Gordon JI: Gnotobiotic mouse model of phage-bacterial host dynamics in the human gut. Proc Natl Acad Sci U S A 2013, 110:20236–20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan Mirzaei M, Khan MAA, Ghosh P, Taranu ZE, Taguer M, Ru J, Chowdhury R, Kabir MM,Deng L, Mondal D, et al. : Bacteriophages Isolated from Stunted Children Can Regulate Gut Bacterial Communities in an Age-Specific Manner. Cell Host Microbe 2020, 27:199–212 e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu BB, Gibson TE, Yeliseyev V, Liu Q, Lyon L, Bry L, Silver PA, Gerber GK: Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe 2019, 25:803–814 e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoruts A, Staley C, Sadowsky MJ: Faecal microbiota transplantation for Clostridioides difficile: mechanisms and pharmacology. Nat Rev Gastroenterol Hepatol 2021, 18:67–80. [DOI] [PubMed] [Google Scholar]
- 25.Ott SJ, Waetzig GH, Rehman A, Moltzau-Anderson J, Bharti R, Grasis JA, Cassidy L, Tholey A,Fickenscher H, Seegert D, et al. : Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients With Clostridium difficile Infection. Gastroenterology 2017, 152:799–811 e797. [DOI] [PubMed] [Google Scholar]
- 26.Nale JY, Redgwell TA, Millard A, Clokie MRJ: Efficacy of an Optimised Bacteriophage Cocktail to Clear Clostridium difficile in a Batch Fermentation Model. Antibiotics (Basel) 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ost KS, Round JL: Communication Between the Microbiota and Mammalian Immunity. Annu Rev Microbiol 2018, 72:399–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLeod SM, Kimsey HH, Davis BM, Waldor MK: CTXphi and Vibrio cholerae: exploring a newly recognized type of phage-host cell relationship. Mol Microbiol 2005, 57:347–356. [DOI] [PubMed] [Google Scholar]
- 29.Novick RP: Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus. Plasmid 2003, 49:93–105. [DOI] [PubMed] [Google Scholar]
- 30. Fluckiger A, Daillere R, Sassi M, Sixt BS, Liu P, Loos F, Richard C, Rabu C, Alou MT, Goubet AG, et al. : Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 2020, 369:936–942. • Identified a molecular mimic within a commensal bacteria that was encoded on a prophage element that induced cross reactive T cells that aided in tumor clearance
- 31.Daillere R, Vetizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, Duong CPM,Flament C, Lepage P, Roberti MP, et al. : Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45:931–943. [DOI] [PubMed] [Google Scholar]
- 32.Smith LL, Buckley R, Lugar P: Diagnostic Immunization with Bacteriophage PhiX 174 in Patients with Common Variable Immunodeficiency/Hypogammaglobulinemia. Front Immunol 2014, 5:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubinstein A, Mizrachi Y, Bernstein L, Shliozberg J, Golodner M, Liu GQ, Ochs HD: Progressive specific immune attrition after primary, secondary and tertiary immunizations with bacteriophage phi X174 in asymptomatic HIV-1 infected patients. AIDS 2000, 14:F55–62. [DOI] [PubMed] [Google Scholar]


