Analysis of 6482 eyes in the American Academy of Ophthalmology IRIS Registry found that the 12-month repositioning rate was higher after implantation of TECNIS than after AcrySof monofocal toric IOLs.
Purpose:
To determine the 12-month incidence of reoperation to realign 2 commercially available types of implanted monofocal toric acrylic intraocular lenses (IOLs).
Setting:
American Academy of Ophthalmology IRIS (Intelligent Research in Sight) Registry.
Design:
Registry retrospective study.
Methods:
Eyes that underwent cataract extraction and were implanted with a TECNIS or AcrySof monofocal toric IOL in 2016 and 2017 were identified. The rate of reoperation for IOL realignment (Current Procedural Terminology code 66825) within 365 days of implantation was determined for each IOL group. Risk factors for repositioning were evaluated using logistic regression modeling.
Results:
A total of 6482 eyes were implanted with a monofocal toric IOL, including 2013 (31.06%) with a TECNIS and 4469 (68.94%) with an AcrySof IOL. During the first postoperative year, 87 (1.3%) eyes underwent surgical IOL repositioning. The incidence of repositioning was significantly higher (P < .0001) for TECNIS-implanted (3.1%, 62/2013) than for AcrySof-implanted (0.6%, 25/4469) eyes (odds ratio [OR] 5.6; 95% CI, 3.5-8.9). Younger age (OR 0.76; 95% CI, 0.67-0.86 per 5-year increase) was associated with a higher risk for IOL repositioning.
Conclusions:
Real-world analysis of U.S. patients in the IRIS Registry revealed that the rate of surgical IOL repositioning was 5 times higher in eyes implanted with TECNIS than with AcrySof monofocal toric IOLs for astigmatic correction at the time of cataract surgery. These findings should be considered when selecting a toric IOL for correction of astigmatism in cataract patients, particularly in younger patients with a higher risk for misalignment requiring repositioning.
Corneal astigmatism in eyes undergoing cataract surgery is common. A German study of 23 239 eyes found that 73.7%, 36.0%, and 16.6% had corneal astigmatism of ≥0.5 diopter (D), ≥1.0 D, and ≥1.5 D, respectively.1 A study of 110 468 eyes from the United Kingdom found similar rates, with 78%, 42%, and 21% of eyes having corneal astigmatism of ≥0.5 D, ≥1.0 D, and ≥1.5 D, respectively.2 A systematic review of 31 studies conducted throughout the world revealed that up to 47% of eyes undergoing cataract surgery have ≥1 D of preexisting corneal astigmatism.3 The cost burden of uncorrected astigmatism after surgery—the lifelong expense of spectacles for vision correction—has been estimated at $2151 to $3440 per person in the United States and $1786 to $4629 in Europe.3
Toric intraocular lenses (IOLs) were developed to address corneal astigmatism at the time of cataract surgery. The STAAR toric IOL, which has a plate-haptic design, was the first toric IOL approved by the U.S. Food and Drug Administration (FDA) in 1998; a systematic review of visual acuity (VA) outcomes in eyes implanted with these early generation toric IOLs for corneal astigmatism demonstrated postoperative cylinder reduction and improved uncorrected distance visual acuity (UDVA) with their use.4,5 A later generation of toric IOLs had an open loop haptic design with longer overall IOL diameter, providing better rotational stability than plate-haptic IOLs.6 A systematic review and meta-analysis incorporating 13 randomized trials comparing open-loop haptic toric IOLs with nontoric IOLs concluded that toric IOLs in eyes with preoperative corneal astigmatism provided better UDVA, greater distance spectacle independence, and less residual astigmatism than nontoric IOLs even when relaxing incisions were used.7 Toric IOLs have also been shown to be cost-effective and to improve quality of life.8,9
The overall performance of toric IOLs is similar to that of monofocal IOLs.7 However, toric IOLs have a unique performance issue with regard to astigmatic axis alignment, as the IOL must be implanted and remain at the proper astigmatic meridian to optimize VA outcomes. Postoperative IOL misalignment can arise from inaccuracy in preoperative IOL calculations (eg, inaccurate corneal imaging, not accounting for posterior corneal astigmatism, or inaccurately accounting for surgically induced astigmatism), error in marking the reference axis on the eye preoperatively, intraoperative misplacement, and/or postoperative IOL rotation. Early models of toric IOLs with a plate-haptic design were associated with high rates of reoperation for realignment—on the order of 10% to 20%—compared with the <3% rate associated with open loop haptic toric IOLs.10
Most studies on the rotational stability and repositioning rates of toric IOLs have been either randomized trials or uncontrolled single-center case series. Trials are governed by protocols, often include extensive training of investigators, and may underestimate the actual performance in real-world settings. Single-center experiences reflect the skill sets of one or a small number of surgeons who may not be representative of many or most surgeons performing toric IOL implantation. Large registries include data amassed by large numbers of surgeons in a real-world setting. The American Academy of Ophthalmology (Academy) maintains the IRIS (Intelligent Research In Sight) registry, which includes real-world data from large numbers of subjects implanted with toric IOLs.11 The present study analyzed rates of reoperation for IOL alignment in patients undergoing cataract surgery with implantation of AcrySof toric IOLs (Alcon Laboratories, Inc.) or TECNIS toric IOLs (Johnson & Johnson Vision), using real-world data from the IRIS registry.
METHODS
This was a retrospective analysis of data derived from the Academy IRIS Registry, which collates data from the electronic health records of patients treated by ophthalmologists in the United States beginning in 2013.11 This analysis of deidentified data was not considered research on human subjects. The study protocol was therefore not submitted for review or approved by ethics committees.
Data were included on patients aged ≥45 years at the time of surgery who had been diagnosed with age-related cataract, as shown by an appropriate International Classification of Diseases, 10th Revision (ICD-10) diagnostic code (Table 1) 30 days prior to and including the day of the procedure, and who underwent cataract surgery, as shown by a Current Procedural Terminology (CPT) code 66984 (extracapsular cataract removal with insertion of the IOL prosthesis [1-stage procedure] and manual or mechanical technique [eg, irrigation and aspiration or phacoemulsification]) between January 1, 2016, and December 31, 2017. In addition, data were included if patients records in the IRIS Registry showed at least 2 visits within 180 days after the cataract procedure (the 2 visits were not required to be with the same care provider); if they had undergone the procedure at a practice that contributed data to the IRIS Registry for at least 365 days following the date of the procedure; and if their records included both the brand and the type (eg, monofocal vs multifocal) of implanted IOLs (Table 2). Data were excluded if patient records showed an ICD-10 code indicating specific exclusion conditions indicative of preexisting conditions that could potentially confound the outcomes (Supplemental Table 1, http://links.lww.com/JRS/A426); if records included an unspecified laterality of the diagnosis code for mature cataract or of the procedure code for cataract removal; if their records in the IRIS Registry mentioned one or fewer visits following the cataract procedure; or if their records showed a CPT code for specific procedures indicative of surgical complications (Supplemental Table 2, http://links.lww.com/JRS/A426).
Table 1.
ICD-10 Diagnoses Included in the Analysis.
| Code | Description |
| H25.011 | Cortical age-related cataract, right eye |
| H25.012 | Cortical age-related cataract, left eye |
| H25.013 | Cortical age-related cataract, bilateral |
| H25.11 | Age-related nuclear cataract, right eye |
| H25.12 | Age-related nuclear cataract, left eye |
| H25.13 | Age-related nuclear cataract, bilateral |
| H25.89 | Other age-related cataract |
| H25.21 | Age-related cataract, morgagnian type (hypermature), right eye |
| H25.22 | Age-related cataract, morgagnian type (hypermature), left eye |
| H25.23 | Age-related cataract, morgagnian type (hypermature), bilateral |
| H25.811 | Combined forms of age-related cataract, right eye |
| H25.812 | Combined forms of age-related cataract, left eye |
| H25.813 | Combined forms of age-related cataract, bilateral |
ICD-10 = International Classification of Diseases, 10th Revision
Table 2.
IOL Models Included in the Analysis.
| Type | Brand | |
| AcrySof | TECNIS | |
| Monofocal | SN60WF, SA60WF, SN60AT, and SA60AT | ZCB00 |
| Monofocal toric | SN6AT3, SN6AT4, SN6AT5, SN6AT6, SN6AT7, SN6AT8, SN6AT9, SA6AT3, SA6AT4, SA6AT5, SA6AT6, SA6AT7, SA6AT8, and SA6AT9 | ZCT150, ZCT225, ZCT300, ZCT400, ZCT425, ZCT525, and ZCT600 |
| Multifocal | SN6AD1, SV25T0, and SN6AD3 Keyword: “ReSTOR multifocal” |
ZKB00, ZLB00, and ZMB00 Keyword: “TECNIS multifocal” |
| Multifocal toric | SND1T3, SND1T4, SND1T5, SND1T6, SV25T3, SV25T4, SV25T5, and SV25T6 Keyword: “ReSTOR multifocal toric” |
NA |
| Diffractive EDoF/EDF | NA | ZXR00 Keywords: “TECNIS Symfony,” “Symfony,” “EDoF,” and “EDF” |
| Diffractive EDoF/EDF toric | NA | ZXT150, ZXT225, ZXT300, and ZXT375 Keywords: “TECNIS Symfony toric,” “Symfony toric,” “EDoF toric,” and “EDF toric” |
EDoF/EDF = extended depth of focus
Data collected included patient demographic and diagnostic information, the brand and type of IOL implanted, and the presence of CPT code 66825 (repositioning of IOL prosthesis, requiring an incision) within 365 days of the primary cataract procedure.
The primary outcome was toric IOL repositioning rate (and its 95% CI) within 365 days after implantation, stratified by lens type and brand. The chi-square test was used to compare repositioning rates between brands. Logistic regression modeling was applied to identify risk factors associated with repositioning. Formal power and sample size analysis were not performed. A convenience sample of all qualifying eyes was analyzed. All data extraction from the IRIS Registry database and statistical analyses were independently performed by Verana Health, the partner of the Academy for the curation and analysis of IRIS Registry data. Verana Health applied a set of policies and procedures to ensure data quality, integrity, security, and traceability. Data quality at Verana Health was assessed based on a matrix of 6 data quality dimensions (completeness, accuracy, traceability, consistency, generalizability, and timeliness) across 3 data quality classifications (technical, clinical, and scientific).
RESULTS
Overall, 78 796 eyes met all the eligibility criteria, with 11 012 (13.98%) of these eyes implanted with a toric IOL. Of these 11 012 eyes, 4530 (41.14%) were implanted with multifocal toric IOLs and 6482 (58.86%) with monofocal toric IOLs. Of the eyes implanted with multifocal toric IOLs, only 69 (1.52%) were implanted with AcrySof multifocal toric IOLs, whereas 4461 (98.48%) were implanted with TECNIS extended depth-of-focus (diffractive extended depth-of-focus) toric IOLs, and no TECNIS multifocal toric IOLs were FDA-approved during the study period. Because of the imbalance between the 2 types of multifocal/diffractive extended depth-of-focus toric IOLs, this analysis was limited to eyes implanted with monofocal toric IOLs. Of the 6482 eyes implanted with monofocal toric IOLs, 4469 (68.94%) were implanted with AcrySof and 2013 (31.06%) with TECNIS IOLs. The demographic characteristics of the eyes of the included subjects are shown in Table 3. Eyes were from subjects of mean age ∼70 years; of the 6482 eyes, 3909 (60.31%) were from women, and 5720 (88.24%) were from White subjects, with no significant differences between the AcrySof and TECNIS toric IOL groups.
Table 3.
Demographic Characteristics.
| Characteristic | AcrySof (N = 4469) | TECNIS (N = 2013) | P value |
| Age (y), mean ± SD | 69.9 ± 7.9 | 69.4 ± 8.3 | .05 |
| Female, n (%) | 2691 (60.2) | 1218 (60.5) | .85 |
| Race, n (%) | |||
| White | 3953 (88.5) | 1767 (87.8) | .33 |
| Black | 78 (1.7) | 46 (2.3) | |
| Other or not reported | 438 (9.8) | 200 (9.9) | |
| U.S. geographical region, n (%) | |||
| Midwest | 1442 (32.3) | 522 (25.9) | <.0001 |
| North | 456 (10.2) | 269 (13.4) | |
| South | 2030 (45.4) | 830 (41.2) | |
| West | 541 (12.1) | 392 (19.5) |
In the first postoperative year, 87 (1.3%) of the 6482 eyes with monofocal toric IOL implantation underwent surgical IOL repositioning. The incidence of repositioning was significantly higher in TECNIS-implanted (3.1%, 62/2013) than in AcrySof implanted (0.6%, 25/4469) eyes (P < .0001) (Figure 1). None of the eyes requiring repositioning were from the same patient.
Figure 1.
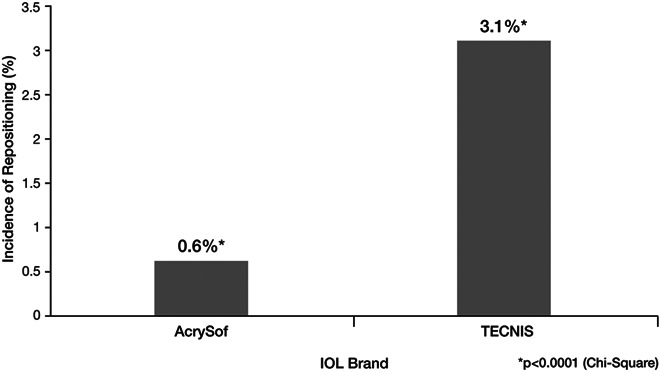
Incidence of reoperation for IOL realignment ( CPT code 66825) within 365 days of surgery by IOL brand. AcrySof toric IOLs were found to require less additional repositioning than TECNIS toric IOLS (*P < .0001; chi-square test). CPT = Current Procedural Terminology
Figure 2 shows the results of logistic regression modeling to identify risk factors for repositioning among the study cohort. Relative to eyes implanted with AcrySof monofocal toric IOLs, eyes implanted with TECNIS monofocal toric IOLs had an odds ratio (OR) of 5.57 (95% CI, 3.48-8.92) of undergoing repositioning within 365 days of the primary procedure. Younger age was also significant, with an OR of 0.76 (95% CI, 0.67-0.86) for every 5-year increase in age, indicating reduced risk with older age. Sex, race, and region of the country where the surgery took place were not significant risk factors for repositioning.
Figure 2.
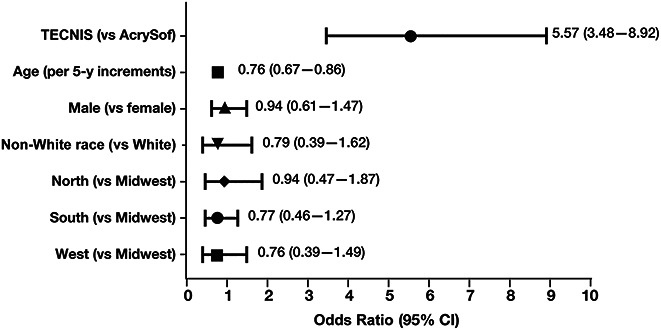
Risk factors for IOL repositioning within 365 days of primary surgery.
DISCUSSION
This analysis of real-world data drawn from the IRIS Registry found that significantly fewer eyes implanted with AcrySof monofocal toric IOLs than with TECNIS monofocal toric IOLs required secondary surgical intervention for repositioning due to IOL malalignment. Secondary surgical intervention was also more likely to occur in younger patients.
This study found that the incidence of repositioning 1 year after implantation of TECNIS monofocal toric IOLs of repositioning was 3.1%, higher than the 2.3% rate at 6 months observed in the registration trial of these lenses.12 Similarly, this study found that the incidence of repositioning 1 year after implantation of AcrySof IOLs was 0.6%, higher than the 0.4% in the registration study.13 The higher rates observed in this study were not surprising in as much as manufacturer-sponsored clinical trials are more tightly controlled than real-world clinical practice.
The sample sizes of the 2 groups were not balanced, with the number of eyes implanted with AcrySof lenses being approximately 2.2 times greater than the number of eyes implanted with TECNIS lenses. In contrast, the number of repositioning procedures was about 2.5 times greater in eyes implanted with TECNIS than with AcrySof lenses. Moreover, the 95% CI of the OR for repositioning of these lenses was higher than 1.0, indicating that balancing of the sample sizes of the 2 groups was unlikely to change the overall study results. The results of the current study are also consistent with prior reports of toric IOL misalignment or postoperative rotation, which required additional surgical procedures to address reduced visual performance.14–16 Using data drawn from a toric back-calculation dataset, developed to guide decision making regarding the value of reoperation for IOL realignment to address residual cylinder, 8229 calculations included intended lens orientation and IOL type data.14 An estimate of the denominator of overall toric IOL usage by brand found that higher proportions of implanted TECNIS lenses than AcrySof lenses had ≥5-degree misorientation from the intended axis, both in the dataset (81% vs 64%, P < .0001) and in the extrapolated analysis of all implanted IOLs (1.86% vs 0.75%, P < .0001). A single-site retrospective analysis of 1273 eyes receiving either TECNIS (n = 647) or AcrySof (n = 626) toric IOLs found that IOL rotations >5 degrees (18.2% vs 8.1%, P < .0001), >10 degrees (6.8% vs 2.2%, P = .0002), and >15 degrees (3.6% vs 1.4%, P = .02) were significantly more frequent in eyes implanted with TECNIS than with AcrySof toric IOLs.16 Reoperations were nearly twice as common in eyes implanted with TECNIS than with AryrSof IOLs (3.1% vs 1.6%), although this difference was not statistically significant (P = .10). A retrospective, multicenter analysis of 9430 eyes implanted with toric IOLs reported an overall repositioning surgery rate of 0.944%, with the rate being significantly lower for AcrySof (0.213%) than for TECNIS (1.797%) or HOYA (1.942%) (Hoya Surgical Optics, Inc.) toric IOLs (P < .0001).15
Rotation of toric IOLs reduces the effective astigmatic correction, resulting in residual postoperative astigmatism compromising VA outcomes. A 10-degree rotation results in a 33% reduction in astigmatic correction, and this may have greater visual performance impact at higher cylinder magnitude levels. The cost-effectiveness and quality-of-life improvements reported with the use of toric IOLs to improve UCDVA are likely diminished by suboptimal VA outcomes in eyes with modestly misaligned toric IOLs and the need for reoperation to realign rotated IOLs in eyes with greater misalignment.8,9 Toric IOL models with greater rotational stability and lower rates of reoperation for misalignment would thus be expected to provide greater cost-effectiveness and patient satisfaction.
Although the time to toric IOL rotation is an interesting endpoint, the primary objective of this study was to evaluate the rate of toric IOL repositioning, not time to repositioning. Most rotations occur within the first few days after implantation, whereas repositioning is frequently not performed until several weeks later. While time to toric IOL rotation is an interesting endpoint, it was not analyzed in this study as there likely was a discrepancy between IOL rotation and when the repositioning surgery actually occurred and the procedure code (CPT 66825) was entered into the IRIS registry.17
This study also found that younger age was a significant risk factor for repositioning, with an OR of 0.76 (95% CI, 0.67-0.86) for every 5-year increase in age. The reduced risk for repositioning with older age may have been due to younger patients being visually more demanding. Moreover, most lenses undergo rotation during the first hours/day after surgery.16 Thus, if younger patients are more active soon after the surgery, they may experience a higher rate of rotation. Alternatively, the biomechanics of the capsule may differ in younger and older patients. A higher prevalence of with-the-rule astigmatism in younger patients has been established, and therefore, toric location (ie, a higher percentage of torics being placed vertically) could also play a role; of note, the IRIS registry does not record toric axis, and therefore, a post hoc subanalysis was unable to be conducted. Additional clinical studies investigating the impact of age on toric IOL rotation are required.
This study's key strength is its use of real-world data from the Academy's IRIS Registry database. This database is a unique and valuable repository of real-world data extracted from the electronic health records of physicians in the United States. In 2016 alone, the IRIS Registry incorporated health data from over 36 million visits by more than 17 million unique patients to 7400 ophthalmologists at 2300 practices in the United States.10 The IRIS Registry permits analysis of large sample sizes not attainable through clinical trials, facilitating precise estimation of rates of uncommon events such as toric IOL repositioning. Also, unlike data from clinical trials—which are typically governed by strict clinical protocols that may not reflect clinical practice patterns and often include investigator training and certification for surgical studies—data from the IRIS Registry reflect real-world clinical practice and outcomes representative of surgeons from all regions, practice settings, and experience levels.
Limitations of database studies pertain primarily to the nature and quality of the data collected. This study was designed to estimate the real-world incidence of toric IOL repositioning among patients in the United States. The performance of repositioning procedure was based on the presence of CPT code 66825 in the IRIS registry. This CPT code applies to the repositioning of IOLs for purposes that include realignment for toric axis, repositioning IOL with severe tilt, decentration, or haptics out of bag. However, for toric IOLs, most repositioning procedures are performed due to misalignment either from misplacement during surgery or rotation after surgery. Thus, the incidence of toric IOL repositioning may be higher than the actual toric IOL rotation rate. Other factors such as preoperative calculation, method of intraoperative toric IOL alignment, and postoperative toric axis measurement could have impacted the results of this study; however, these details are not recorded in the IRIS database, and therefore can't be further evaluated (of note, both manufacturer calculators began to include posterior corneal astigmatism during this study: Alcon Laboratories, Inc. in August of 2016 and Johnson & Johnson Vision in October of 2016). Errors and/or omissions in documentation are not easily identified at the patient level in such large datasets and may affect analyses. Also, contribution of data to the IRIS Registry is voluntary; the surgeons and practices electing to participate may not be representative of all surgeons or practice settings. This analysis would not capture data from patients who underwent surgery with a surgeon or practice in the IRIS Registry and then were lost to follow-up because they sought postoperative care from a practice outside the IRIS Registry. In addition, it would also not capture data from patients in which the CPT code was not billed. Although these limitations affected the study cohort as a whole, they should not skew the proportions by affecting 1 IOL group in particular.
Other limitations of this study include its retrospective nature, which may have introduced a selection bias. Because this study was retrospective in nature, no repositioning criteria were predetermined in the study protocol. Moreover, there are no current standards for such secondary surgical interventions. Rather, surgery is performed if the repositioning would likely improve a patient's visual outcomes and if the surgeon prefers this approach.
Since the completion of this study, Johnson & Johnson Vision introduced the TECNIS toric II. Changes were made in the IOL design in an effort to enhance rotational stability of the TECNIS toric platform. Future studies are needed to confirm the rotational stability of these new toric IOLs.
In summary, analysis of real-world data from the Academy's IRIS Registry revealed that the rate of surgical IOL repositioning was significantly higher in eyes implanted with TECNIS monofocal toric IOLs than with AcrySof monofocal toric IOLs for astigmatic correction at the time of cataract surgery. This finding is consistent with reports from prior small studies. These findings should be considered when selecting toric IOLs for correction of astigmatism in patients with cataract, particularly in younger patients, who are at a higher risk for misalignment requiring repositioning.
WHAT WAS KNOWN
Compared with nontoric IOLs, toric IOLs provide superior visual and quality-of-life outcomes in eyes with regular corneal astigmatism.
Toric IOLs must be implanted and remain at the proper astigmatic axis alignment to optimize visual acuity outcomes.
Studies have shown differences among lenses in toric IOL rotation and the need for repositioning.
WHAT THIS PAPER ADDS
According to the Academy's IRIS Registry, the original TECNIS toric platform required more postoperative repositioning compared with the AcrySof toric platform.
Younger age is associated with higher rates of postoperative toric IOL repositioning.
Acknowledgments
The authors thank Helene Fevrier of Verana Health for extracting and statistically analyzing data from the IRIS Registry database and Aldo Martinez, PhD, of Alcon Laboratories, Inc. for reviewing the manuscript and suggesting appropriate revisions. Medical writing assistance was provided by Richard Alexander, PhD, of BelMed Inc. with funding from Alcon Laboratories, Inc.
Footnotes
Sponsored by Alcon Laboratories, Inc.
Disclosures: B.A. Kramer has received support from Alcon Vision LLC for an unrelated investigator-initiated trial. J. Berdahl has financial interests in Alcon Vision LLC, Allergan, Inc., Avedro, Inc., Aurea Medical, Bausch & Lomb, Inc., ClarVista Medical, Inc., CorneaGen, Dakota Lions Eye Bank, Envisia, Equinox, Expert Opinion, Glaukos Corp., Imprimis Pharmaceuticals, Inc., Iantech, Inc., Johnson & Johnson Vision, New World Medical, Inc., Ocular Therapeutix, Inc., Omega Ophthalmic, Ocular Surgical Data, Orasis, Rxsight, Inc., Surface, Inc., Verana Health (formerly DigiSight Technologies), Vittamed, Vance Thompson Vision, and Carl Zeiss Meditec AG. X. Gu and M. Merchea are employees of Alcon Vision LLC.

First author:
Brent A. Kramer, MD
Duke University Eye Center, Durham, North Carolina
Contributor Information
John Berdahl, Email: johnberdahl@gmail.com.
Xiaolin Gu, Email: xiaolin.gu@alcon.com.
Mohinder Merchea, Email: mo.merchea@alcon.com.
REFERENCES
- 1.Hoffmann PC, Hutz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J Cataract Refract Surg 2010;36:1479–1485 [DOI] [PubMed] [Google Scholar]
- 2.Day AC, Dhariwal M, Keith MS, Ender F, Perez Vives C, Miglio C, Zou L, Anderson DF. Distribution of preoperative and postoperative astigmatism in a large population of patients undergoing cataract surgery in the UK. Br J Ophthalmol 2019;103:993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DF, Dhariwal M, Bouchet C, Keith MS. Global prevalence and economic and humanistic burden of astigmatism in cataract patients: a systematic literature review. Clin Ophthalmol 2018;12:439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U. S. Food and Drug Administration. Premarket approval (PMA). STAAR UV-absorbing silicone posterior chamber IOLs w/toric optic. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P880091S014. Accessed June 2, 2020
- 5.Agresta B, Knorz MC, Donatti C, Jackson D. Visual acuity improvements after implantation of toric intraocular lenses in cataract patients with astigmatism: a systematic review. BMC Ophthalmol 2012;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang DF. Comparative rotational stability of single-piece open-loop acrylic and plate-haptic silicone toric intraocular lenss. J Cataract Refract Surg 2008;34:1842–1847 [DOI] [PubMed] [Google Scholar]
- 7.Kessel L, Andresen J, Tendal B, Erngaard D, Flesner P, Hjortdal J. Toric intraocular lenses in the correction of astigmatism during cataract surgery: a systematic review and meta-analysis. Ophthalmology 2016;123:275–286 [DOI] [PubMed] [Google Scholar]
- 8.Pineda R, Denevich S, Lee WC, Waycaster C, Pashos CL. Economic evaluation of toric intraocular lens: a short- and long-term decision analytic model. Arch Ophthalmol 2010;128:834–840 [DOI] [PubMed] [Google Scholar]
- 9.Mencucci R, Giordano C, Favuzza E, Gicquel JJ, Spadea L, Menchini U. Astigmatism correction with toric intraocular lenses: wavefront aberrometry and quality of life. Br J Ophthalmol 2013;97:578–582 [DOI] [PubMed] [Google Scholar]
- 10.Visser N, Bauer NJ, Nuijts RM. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg 2013;39:624–637 [DOI] [PubMed] [Google Scholar]
- 11.Chiang MF, Sommer A, Rich WL, Lum F, Parke DW, II. The 2016 American Academy of Ophthalmology IRIS Registry (Intelligent Research in Sight) database: characteristics and methods. Ophthalmology 2018;125:1143–1148 [DOI] [PubMed] [Google Scholar]
- 12.Waltz KL Featherstone K Tsai L. Trentacost D. Clinical outcomes of TECNIS toric intraocular lens implantation after cataract removal in patients with corneal astigmatism. Ophthalmology 2015;122:39–47 [DOI] [PubMed] [Google Scholar]
- 13.U. S. Food and Drug Administration. Premarket approval (PMA). Acrysof UV-absorbing intraocular lenses. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf/P930014S045b.pdf. Accessed September 23, 2020
- 14.Potvin R, Kramer BA, Hardten DR, Berdahl JP. Toric intraocular lens orientation and residual refractive astigmatism: an analysis. Clin Ophthalmol 2016;10:1829–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshika T, Fujita Y, Hirota A, Inamura M, Inoue Y, Miyata K, Miyoshi T, Nakano S, Nishimura T, Sugita T. Comparison of incidence of repositioning surgery to correct misalignment with three toric intraocular lenses. Eur J Ophthalmol 2020;30:680–684 [DOI] [PubMed] [Google Scholar]
- 16.Lee BS, Chang DF. Comparison of the rotational stability of two toric intraocular lenses in 1273 consecutive eyes. Ophthalmology 2018;125:1325–1331 [DOI] [PubMed] [Google Scholar]
- 17.Inoue Y, Takehara H, Oshika T. Axis misalignment of toric intraocular lens: placement error and postoperative rotation. Ophthalmology 2017;124:1424–1425 [DOI] [PubMed] [Google Scholar]


