A prospective cohort study is underway to evaluate the difference of oblique lateral interbody fusion (OLIF) and minimally invasive transforaminal lumbar interbody fusion with respect to perioperative data, patient-reported outcomes, radiographic measure, and complications. The results demonstrate surgeons could give preference to OLIF on the basis of a rich experience in lumbar anatomy and lumbar anterior or lateral surgery.
Keywords: minimally invasive, minimally invasive transforaminal lumbar interbody fusion, oblique lateral interbody fusion, surgical procedures
Abstract
Study Design.
Prospective cohort study.
Objective.
To assess the differences in the clinical and radiological outcomes between oblique lateral interbody fusion (OLIF) and minimally invasive transforaminal lumbar interbody fusion (MI-TLIF).
Summary of Background Data.
Nowadays, there is still a controversy regarding whether OLIF is superior to MI-TLIF in the management of degenerative lumbar disease.
Methods.
Between August 3, 2019 and February 3, 2020, 137 patients were assigned to OLIF or MI-TLIF at their request and the surgeon's discretion: 71 in the OLIF group and 66 in the MI-TLIF group. The perioperative data, patient-reported outcomes, radiographic outcomes, and complications were compared between the two groups.
Results.
The OLIF group showed shorter operation time (110.5 vs.183.8 minutes, P < 0.001), lesser estimated blood loss (123.1 vs. 232.0 mL, P < 0.001), shorter length of hospital stay (5.5 vs. 6.7 days, P < 0.001), and lower serum creatine kinase (CK) (1 day postoperatively) (376.0 vs. 541.8 IU/L, P < 0.01) than that of MI-TLIF group. Both groups showed no significant differences in the visual analog scale (VAS) scores of lower back and leg pain and the Oswestry Disability Index (ODI) scores preoperatively and at 1, 3, and 12 months postoperatively, respectively (P > 0.05). Compared with the MI-TLIF group, the OLIF group showed better restoration of disc height (DH) (4.7/4.6/4.7 vs. 3.7/3.7/3.7 mm, P < 0.01) and lumbar lordosis angle (LLA) (10.5°/10.8°/11.1° vs. 5.8°/5.7°/5.3°, P < 0.001), but not the value of segmental lordosis angle (SLA) (P > 0.05) at 1 day, 1 month, and 1 year postoperatively, respectively. The complication rate of OLIF was higher than that of MI-TLIF (29.4% vs. 9.7%, P < 0.01).
Conclusion.
Compared with MI-TLIF, OLIF showed similar results in terms of patient-reported outcomes, restoration of SLA and fusion rate, and superior results with respect to restoration of DH and LLA, operation time, estimated blood loss, length of hospital stay, and serum CK levels (1 day postoperatively). Even though the complication rate of OLIF is higher than that of MI-TLIF, it does not bring persistent and substantial damage to the patients.
Level of Evidence: 3
Conventional posterior/transforaminal lumbar interbody fusion (TLIF) and anterior lumbar interbody fusion techniques have yielded satisfactory clinical outcomes for degenerative lumbar diseases.1,2 However, iatrogenic paraspinal muscle injury, posterior tension band disruption, and approach-related complications are a concern.2,3 Thus, surgical approaches, including direct lateral interbody fusion, extreme lateral interbody fusion, and oblique lateral interbody fusion (OLIF)4 have been popularized, and minimally invasive (MI) procedures are adopted,5 alongside mini-open techniques and unilateral or bilateral Wiltse procedures.
The efficacy of OLIF and MI-TLIF for the management of degenerative lumbar disease has been demonstrated in several studies.6–8 However, based on the English literature,9–13 it is difficult to draw a conclusion regarding whether OLIF is superior to MI-TLIF because of small sample sizes, heterogeneity of study objectives and evaluation indices, and a low level of evidence. Therefore, we aimed to assess the clinical and radiological outcomes of OLIF and MI-TLIF. We suppose that OLIF provides better clinical and radiological outcomes compared with MI-TLIF.
MATERIALS AND METHODS
Sample Size Estimation
Based on data described by Li et al,7 we estimated that enrollment of 132 patients (66 patients per arm) would provide the study with 80% power to detect a 20% difference in the complication rate (30% vs.10%), at an alpha significance level of 0.05 (two-tailed) and a 10% loss to follow-up. PASS, version 15 (PASS, NCSS, LLC, 2017), was used for the sample size calculation.
Patient Population and Grouping
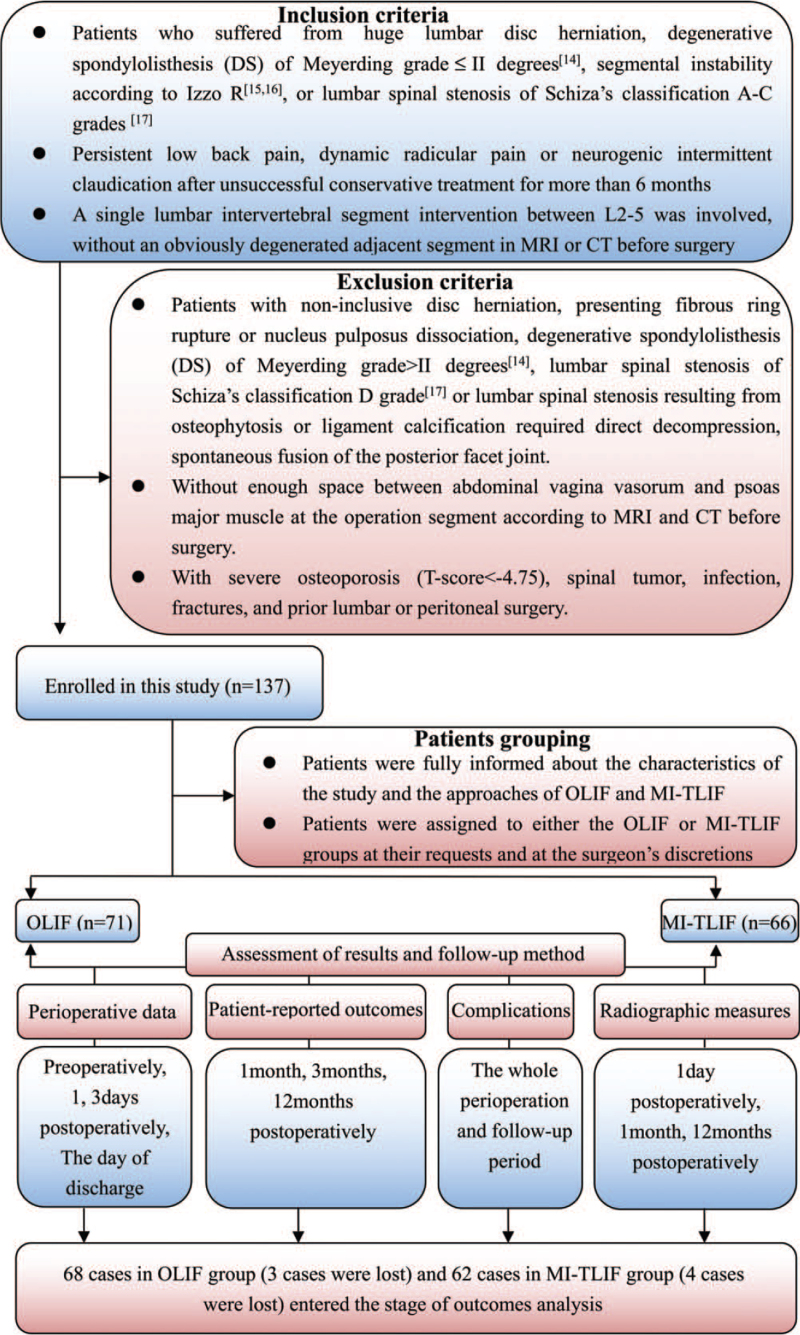
Study approval was obtained from the hospital ethics committee. From August 3, 2019 to February 3, 2020, 137 consecutive patients with huge lumbar disc herniation, degenerative spondylolisthesis (DS) of Meyerding grade14 less than or equal to II degrees, segmental instability,15,16 or lumbar spinal stenosis of Schiza classification A–C grades17 were enrolled in this study based on the inclusion and exclusion criteria (detailed in Figure 1). After rigorous screening, all the patients suitable for OLIF and MI-TLIF approaches were included. Preoperatively, demographic and clinical characteristics (Table 1) were documented and all patients were informed about the study protocols. After signing informed consent, patients were assigned to OLIF or MI-TLIF at their request and the surgeon's discretion (Figure 1). All operations were performed by a single surgical team (one surgeon, and three assistants).
Figure 1.
Flow chart for patient population, grouping, assessment of results, and follow-up method.
TABLE 1.
The Demographic and Clinical Characteristics and Perioperative Data of the Patients of OLIF and MI-TLIF Groups
| OLIF | MI-TLIF | P Value; t/Z/χ2 Value | |
| No. of patients | 68 | 62 | - |
| Sex (M/F) | 36/32 | 33/29 | P = 0.974; t = 0.001 |
| Age, yr | 60.2 ± 6.2 | 61.1 ± 5.3 | P = 0.363; t = 0.914 |
| BMI, kg/m2 | 23.2 ± 2.5 | 23.9 ± 3.6 | P = 0.168; t = 1.387 |
| Preoperative diagnosis | P = 0.962; χ2 = 0.290 | ||
| Lumbar disc herniation | 11 | 9 | - |
| DS | 28 | 25 | - |
| I degree | 16 | 13 | - |
| II degree | 12 | 12 | - |
| Segmental instability | 13 | 11 | - |
| Lumbar spinal stenosis | 16 | 17 | - |
| Operation segment | P = 0.778; χ2 = 0. 503 | ||
| L2–3 | 12 | 14 | - |
| L3–4 | 18 | 15 | - |
| L4–5 | 38 | 33 | - |
| VAS of low back | 6.7 ± 1.6 | 6.4 ± 1.3 | P = 0.317; Z = –1.001 |
| VAS of leg | 5.6 ± 2.0 | 5.5 ± 2.5 | P = 0.808; Z = –0.243 |
| ODI (%) | 61.1 ± 10.3 | 58.6 ± 11.0 | P = 0.110; Z = –1.600 |
| Sagittal alignment | |||
| DH, mm | 8.9 ± 2.2 | 8.6 ± 2.0 | P = 0.334; t = 0.969 |
| SLA, ° | 17.4 ± 5.2 | 15.8 ± 4.9 | P = 0.063; t = 1.872 |
| LLA, ° | 39.7 ± 12.0 | 41.6 ± 10.9 | P = 0.348; t = 0.943 |
| Operation time, min | 110.5 ± 37.8 | 183.8 ± 65.5 | P = 0.000; t = 7.900 |
| Estimate blood loss, mL | 123.1 ± 39.8 | 232.0 ± 83.2 | P = 0.000; t = 9.652 |
| Length of hospital stay, d | 5.5 ± 1.1 | 6.7 ± 2.0 | P = 0.000; t = 4.005 |
| Serum creatine kinase, IU/L | |||
| Preoperatively | 92.7 ± 51.4 | 88.1 ± 32.0 | P = 0.540; t = 0.615 |
| 1 day postoperatively | 376.0 ± 140.8 | 541.8 ± 400.0 | P = 0.002; t = 3.207 |
| 3 days postoperatively | 215.8 ± 124.6 | 248.6 ± 228.0 | P = 0.305; t = 1.030 |
BMI indicates body mass index; CK, creatine kinase; DH, disc height; DS, degenerative spondylolisthesis; LLA, lumbar lordosis angle; ODI, Oswestry Disability Index; SLA, segmental lordosis angle; VAS, visual analog scale.
Surgical Techniques
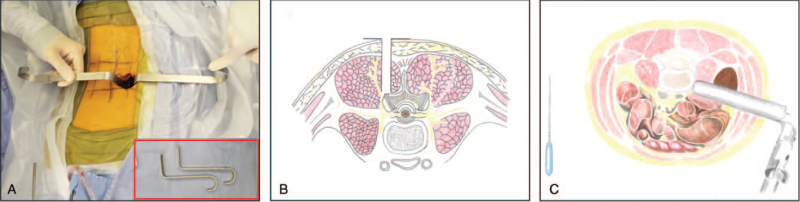
The MI-TLIF procedure was a two-step processes: first, a K-wire was used to penetrate the multifidus muscle and a trans-muscular surgical corridor was created with two micro-laminectomy retractors (Figure 2A) docking on the facet joint complex (Figure 2B). Second, TLIF was carefully performed with crescent cages (Crescent spinal system, Medtronic Sofamor Danek, Memphis, TN) filled with autograft from laminectomy under magnification. The contralateral side was handled either by the trans-muscular procedure (when decompression was needed), or an inter-muscular Wiltse procedure5 with pedicle screw insertion.
Figure 2.
Sketch map of two micro-laminectomy retractors (A), MI-TLIF procedure (B), and OLIF procedure (C). MI-TLIF indicates minimally invasive transforaminal lumbar interbody fusion; OLIF, oblique lateral interbody fusion.
The OLIF procedure was referring to our modified lateral approach described previously18–20 (Figure 2C and Video 1 [Supplemental Digital Content 1]), utilizing expandable retractors and PEEK intervertebral cages (Clydesdale Spinal System; Medtronic Sofamor Danek, Minneapolis, MN) filled with artificial bone (Wright, TN). Subsequently, patients were placed in prone position and received posterior fixation through an inter-muscular Wiltse procedure with the help of two micro-laminectomy retractors (Figure 2A).
Postoperative Management and Follow-up Method
All patients were encouraged to perform passive straight leg raising 1 day postoperatively, and moderate off-bed activity with a brace 2 to 3 days postoperatively. Return to daily life was not permitted until successful lumbar fusion was achieved.
All patients were strictly followed up through monthly outpatient visits for 6 months, and semi-annual outpatient visits subsequently, until death or loss to follow-up. The latest follow-up was carried out between August 2020 and February 2021, constituting an average follow-up period of 1 year.
Assessment of Outcomes
Clinical outcomes (primary outcomes), were independently assessed by an experienced clinical research coordinator who had not participated in the study. Radiographic outcomes (secondary outcomes), were evaluated by a blinded radiologist and a superior spine surgeon. Measurements were repeated after 3 weeks. Interobserver and intraobserver repeatability was calculated using the intraclass correlation coefficient (ICC) and formula.21
Clinical Outcomes
Perioperative Data
Operation time and estimated blood loss were recorded 1 day postoperatively. Serum CK level was measured preoperatively and 1 and 3 days postoperatively. Length of hospital stay was documented on the day of discharge.
Patient-reported Outcomes
Patient-reported outcomes included the Oswestry Disability Index (ODI) score and visual analog scale (VAS) score of low back and leg assessed at 1, 3, and 12 months postoperatively.
Complications
Complications were collected throughout the perioperative and postoperative follow-up periods and treated appropriately.
Radiographic Outcomes
Radiographic outcomes, including DH, SLA, and LLA restoration, were evaluated at1 day, 1 month, and 12 months postoperatively. Detailed measurement criteria of DH, SLA, and LLA were referenced from Zhu et al.22 The Restored value of each patient = (value after surgery − value before surgery).
The fusion rate was assessed at 12 months postoperatively. According to the improved Brantigan criteria (0–4 points)23 more than or equal to three points (Supplemental Digital Content 2) was defined as a successful fusion.
Statistical Analysis
Continuous, discrete, and rating variables are presented as mean ± standard deviation (SD), and categorical variables are expressed as frequency or percentage. Student t test was used for intergroup analysis of normal distributed continuous variables. The Mann–Whitney U test was used for intergroup analysis of discrete variables, rating variables, and continuous variables, which were not normally distributed. The chi-square test was used for categorical variables. All analyses were performed using the Statistical Package for the Social Sciences (SPSS 19.0; IBM Corp., Armonk, NY). P < 0.05 indicated statistical significance.
RESULTS
No significant differences in patients’ demographic and clinical characteristics were noted between the two groups (P > 0.05, Table 1). The loss rates for the two groups were comparable after 12-month follow-up (OLIF: 4.2% [3/71] vs. MI-TLIF: 6.1% [4/66], P = 0.626, χ2 = 0.238).
Perioperative Data
The perioperative data are shown in Table 1. Compared with MI-TLIF, OLIF demonstrated shorter operation time (110.5 ± 37.8 vs. 183.8 ± 65.5 minutes, P < 0.001), lesser estimated blood loss (123.1 ± 39.8 vs. 232.0 ± 83.2 mL, P < 0.001), and shorter length of hospital stay (5.5 ± 1.1 vs. 6.7 ± 2.0 days, P < 0.001). Serum CK level in the OLIF group was markedly lower than that in the MI-TLIF group 1 day postoperatively (376.0 ± 140.8 vs. 541.8 ± 400.0 IU/L, P < 0.01), but not preoperatively and 3 days postoperatively (P > 0.05) (Table 1).
Patient-reported Outcomes
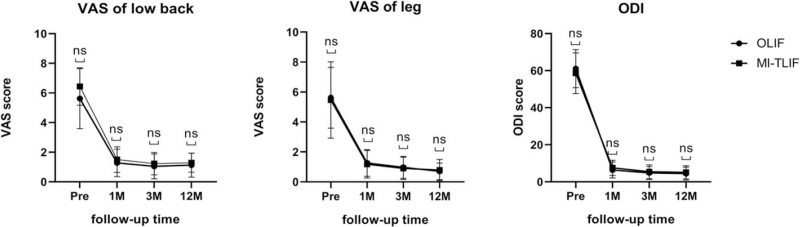
The OLIF and MI-TLIF groups showed no significant differences in the VAS scores for lower back (6.7 ± 1.6/1.3 ± 0.9/1.0 ± 0.8/1.1 ± 0.8 vs. 6.4 ± 1.3/1.5 ± 0.9/1.2 ± 0.8/1.3 ± 0.6, P > 0.05), leg pain (5.6 ± 2.0/1.3 ± 0.9/1.0 ± 0.7/0.7 ± 0.5 vs. 5.5 ± 2.5/1.2 ± 0.9/0.9 ± 0.7/0.8 ± 0.7, P > 0.05), and in the ODI scores (61.5 ± 10.3/6.4 ± 4.3/4.8 ± 3.5/4.5 ± 3.5% vs. 58.6 ± 11.0/7.5 ± 4.1/5.4 ± 3.8/5.1 ± 3.5%, P > 0.05) recorded preoperatively and 1, 3, and 12 months postoperatively, respectively (Figure 3) (Supplemental Digital Content 3).
Figure 3.
Comparison of VAS score of lower back and leg pain and ODI score between the OLIF and MI-TLIF groups preoperatively (pre), 1 month (1 M), 3 months (3 M), 12 months (12 M) postoperatively. Data represents mean ± SD. Note: ns: the difference was not significant (P > 0.05). MI-TLIF indicates minimally invasive transforaminal lumbar interbody fusion; ODI, Oswestry Disability Index; OLIF, oblique lateral interbody fusion; VAS, visual analog scale.
Complications
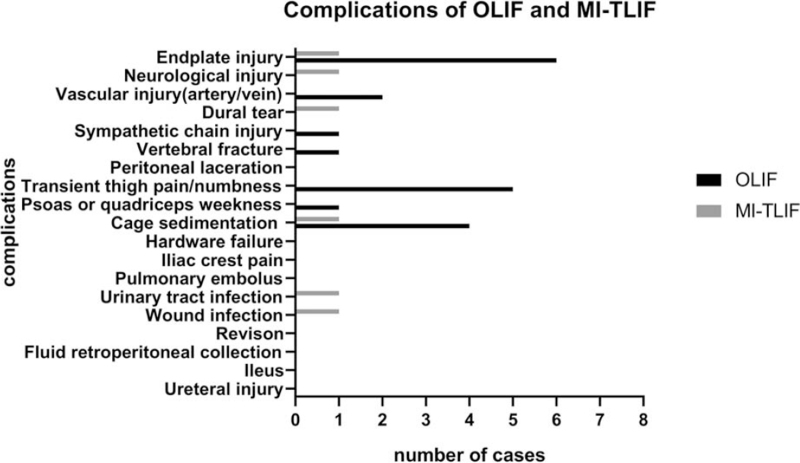
Major complications are shown in Figure 4 and all accepted immediate and effective treatments, listed in Table 2. The complication rate was significantly higher in the OLIF group than in the MI-TLIF group (29.4% [20/68] vs. 9.7% [6/62], P < 0.01, χ2 = 4.20).
Figure 4.
Comparison of perioperative and postoperative complications of OLIF and MI-TLIF. MI-TLIF indicates minimally invasive transforaminal lumbar interbody fusion; OLIF, oblique lateral interbody fusion.
TABLE 2.
Summary of Treatments of Complications of OLIF and MI-TLIF Groups
| Case | Operation | Complications | Treatment and Prognosis |
| 1–6 | OLIF | Endplate injury | Thoracic lumbar braces were used for protection and strength, and successful fusion were achieved in 10–12 months |
| 7 | OLIF | Ovarian vein injury | Hemostasis was attempted by local pressing hemostasis |
| 8 | OLIF | Segmental vessel injury | Hemostasis was attempted by vascular ligation after pressing the two ends of the damaged vessels with two periosteal detachers. |
| 9 | OLIF | Left sympathetic chain injury | Symptoms was resolved 4 days after surgery with neurotrophic treatment |
| 10 | OLIF | Vertebral fracture | The fracture in the right front of the L2 vertebral body was healed well after stay in bed for 1 month and a thoracic lumbar brace protection for 2 months |
| 11–15 | OLIF | Transient thigh pain and numbness | Symptoms was resolved 6–9 days after surgery with neurotrophic treatment |
| 16 | OLIF | Psoas weakness | Symptoms was resolved 4 days after surgery with neurotrophic treatment |
| 17–20 | OLIF | Cage sedimentation | Symptoms was controlled with the help of thoracic lumbar braces for protection |
| 21 | MI-TLIF | Endplate injury | A thoracic lumbar braces was used for protection and strength, and successful fusion were achieved in 9 months |
| 22 | MI-TLIF | Neurological injury | Symptoms was resolved 2 weeks after surgery with neurotrophic treatment |
| 23 | MI-TLIF | Dural tear | The crevasse was closed directly and the leakage of cerebrospinal fluid was not happened postoperatively |
| 24 | MI-TLIF | Cage sedimentation | Symptoms was controlled with the help of thoracic lumbar braces for protection |
| 25 | MI-TLIF | Urinary tract infection | Infection was controlled by oral levofloxacin for 2 weeks |
| 26 | MI-TLIF | Wound infection | Infection was controlled by closed continuous irrigation and suction drainage for 2 weeks |
MI-TLIF indicates minimally invasive transforaminal lumbar interbody fusion; OLIF, oblique lateral interbody fusion.
Radiographic Outcomes
The interobserver and intraobserver ICC for DH, SLA, and LLA restoration and fusion rate were between 0.85 and 0.95.
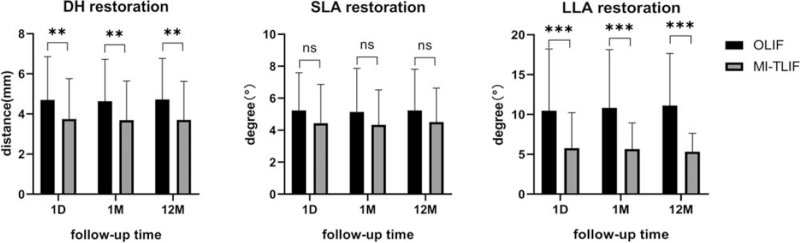
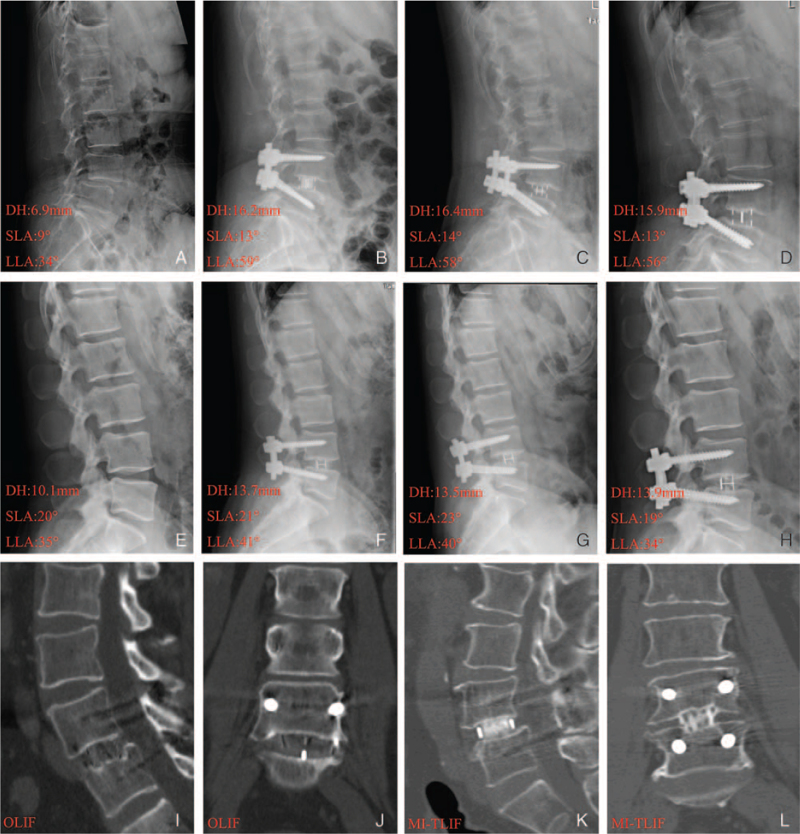
The OLIF group had significantly better DH and LLA restoration than the MI-TLIF group at 1 day, 1 month, and 12 months postoperatively (4.7 ± 2.1/4.6 ± 2.1/4.7 ± 2.0 vs. 3.7 ± 2.0/3.7 ± 2.0/3.7 ± 1.9 mm, P < 0.01; 10.5 ± 7.7°/10.8 ± 7.3°/11.1 ± 6.5° vs. 5.8 ± 4.5°/5.7 ± 3.3°/5.7 ± 3.3°, P < 0.001, respectively), but not for SLA restoration (5.2 ± 2.4°/5.1 ± 2.7°/5.2 ± 2.6° vs. 4.4 ± 2.4°/4.4 ± 2.2°/4.5 ± 2.1°, P > 0.05) (Figure 5) (Supplemental Digital Content 4). At the latest follow-up, a high fusion rate comparable to that of MI-TLIF group (98.4%, 61/62) was noted for the OLIF group (98.4% [61/62] vs. 98.5% [67/68], P = 0.614) (Supplemental Digital Content 4). Representative cases are shown in Figure 6A–L.
Figure 5.
Comparison of DH, SLA, and LLA restoration between the OLIF and MI-TLIF groups 1 day (1D), 1 month (1 M), and 12 months (12 M) postoperatively. Data represents mean ± SD. Note: ns: the difference was not significant (P > 0.05); ∗∗ and ∗∗∗: the difference was significant (P < 0.01 and P < 0.001 respectively). DH indicates disc height; LLA, lumbar lordosis angle; MI-TLIF, minimally invasive transforaminal lumbar interbody fusion; OLIF, oblique lateral interbody fusion; SLA, segmental lordosis angle.
Figure 6.
Representative cases in respect to DH, SLA, and LLA restoration in OLIF (ABCD) and MI-TLIF (EFGH) groups before operation and at 1 day, 1 month, and 12 months postoperatively, and successful lumbar fusion in OLIF(IJ) and MI-TLIF(KL) groups at 12 months postoperatively. DH indicates disc height; LLA, lumbar lordosis angle; MI-TLIF, minimally invasive transforaminal lumbar interbody fusion; OLIF, oblique lateral interbody fusion; SLA, segmental lordosis angle.
DISCUSSION
With advancements in MI techniques and concepts, OLIF and MI-TLIF have become well-established surgical techniques and are extensively used with satisfactory outcomes.6–8,24,25 Nevertheless, high quality comparative articles are urgently needed to determine the superiority of OLIF versus MI-TLIF procedures. Hence, we conducted this study and obtained preliminary research results, which were generally in agreement with other studies7,10–13 (Table 3). To the best of our knowledge, this is the first prospective cohort study to evaluate these procedures’ outcomes to date.
TABLE 3.
Literatures Review of the Clinical and Radiologic Outcomes of OLIF Compared With MI-TLIF
| Study | Properties (Mean Follow up) | Subjects (Patient No) | Results |
| Lin et al, 2018 | A matched-pair retrospective study (29 months for OLIF; 40 months for MI-TLIF) | Low-grade spondylolisthesis or lumbar spinal stenosis of L4-L5 (25 in MI-TLIF; 25 in OLIF) | Similar changes in VAS, ODI, forminal height, SLA, LLA, CSAS, and complication rate; less blood loss and shorter operative time in OLIF versus MI-TLIF; OLIF was superior to MI-TLIF in restoring DH and CASF; Fusion rate at 6 months: 80% in OLIF and 52% in MI-TLIF |
| Sheng et al, 2020 | A retrospective study (12 months for OLIF and MI-TLIF) | DS (38 in MIS-OLIF; 55 in MIS-TLIF) | Similar changes in VAS, ODI, disc angle and L1-S1 lordosis; shorter procedures and hospital stay and less blood loss in OLIF versus MI-TLIF; changes of DH and foramina dimension were greater in MIS-OLIF versus MI-TLIF. |
| Li et al, 2018 | A systematic review (10.4 months for OLIF; 25.4 months for MI-TLIF) | DS, spinal stenosis, degenerated kyphoscoliosis and discogenic low back pain (2009 in OLIF; 1488 in MI-TLIF) | Similar changes in DH, SLA, LLA, length of hospital stay, VAS, ODI, fusion rates; less operative blood loss and operative time in OLIF versus MI-TLIF; the incidence of intraoperative and postoperative complications was 9.5% and 19.9% for OLIF and 3.5% and 8.5% for MI-TLIF. |
| Koike et al, 2021 | A retrospective study (18.1 months for OLIF-LPF; 22.5 months for MI-TLIF) | Single-level DS (38 in OLIF-LPF; 48 in MIS-TLIF) | Similar changes in operation time, EBL, CRP level 5 days postoperatively, VAS and slipping length; DH changes and JOABPEQ domains improvements was greater in OLIF-LPF versus MI-TLIF; |
| Kotani et al, 2020 | A retrospective study (31 months for OLIF-LPF; 57 months for MI-TLIF) | L3 or L4 DS (92 in OLIF-LPF; 50 in MI-TLIF) | Similar changes in operation time, EBL, the percent slip reduction, fusion rate, and symptomatic ASD; JOABPEQ effectiveness rate and VAS of leg pain improvements were higher in OLIF-LPF versus MI-TLIF; the less correction loss of posterior DH in OLIF-LPF versus MI-TLIF |
LLA indicates lumbar lordosis angle; ODI, Oswestry Disability Index; MI-TLIF, minimally invasive transforaminal lumbar interbody fusion; OLIF, oblique lateral interbody fusion; SLA, segmental lordosis angle; VAS, visual analog scale.
Consistent with several previous studies,7,12,13 OLIF was associated with shorter operation times, less estimated blood loss, and muscle injury (lower serum CK level 1 day postoperatively) compared with MI-TLIF. In MI-TLIF, it is time consuming to establish the trans-multifidus decompression channel and complete laminectomy under magnification,22 damaging the posterior bony structure and paraspinal muscles to some extent and causing massive bleeding. Conversely, it is convenient and almost noninvasive for experienced operators to take the path of the retroperitoneal anatomic corridor and achieve indirect decompression through the restoration of DH26 in OLIF; the posterior bony structure and paraspinal muscles were preserved and bleeding rarely occurs. Additionally, we found that hospital stays were shorter in OLIF than in MI-TLIF, which was controversial in previous studies.7,12 In our experiences, preserving the multifidus and posterior column structures in OLIF contributed to earlier off-bed activity and hospital discharge compared with MI-TLIF.
Nevertheless, operation time, estimated blood loss, and muscle injury in OLIF may vary depending on surgical expertise, especially for beginners. Hence, we have provided two technical improvements previously (Video 1 [Supplemental Digital Content 1]). First, a minimal skin incision is recommended 2 cm back from the normal OLIF incision, facilitating oblique placement in the working channel and the orthogonal maneuver for the cage placement. Second, two special custom-made retractors are used to pull the psoas muscle to the dorsal side and pull the abdominal organs to the ventral side under direct visualization, allowing the convenient and safe exposure of the working channel without radiation.
Recent studies7,10–12 have reached a consensus that indirect decompression, performed in OLIF, can yield similar clinical outcomes compared with MI-TLIF, as denoted by the ODI score and VAS score of back or leg postoperatively. However, a matched-pair retrospective study13 demonstrated that the improvement in the VAS for back pain was significantly greater in OLIF than in MI-TLIF 6 weeks postoperatively, although no differences between the two groups were found at further follow-up, explained by avoidance of iatrogenic violation of posterior lumbar elements in OLIF. We found OLIF demonstrated similar improvement of the ODI score and VAS score of back and leg pain compared with MI-TLIF at 1, 3, and 12 months postoperatively. However, not all patients with leg pain could benefit from OLIF. In our experiences, OLIF was effective for dynamic radicular pain, mostly relieved in resting state, but was poor for static radicular pain, existing persistently in resting state. Given the expansion of spinal canal and nerve root canal from dynamic state to resting state, we suppose the degree of nerve compression of static radicular pain may be more severe than that of dynamic radicular pain, which could not be completely relieved by OLIF. Hence, patients with static radicular pain were excluded in our study. Regarding neurogenic intermittent claudication pain, the present study suggest that variation of dynamic mechanical stress on the lumbar spinal nerve roots may be the major cause, rather than static mechanical stress on the spinal nerve roots with each posture or nerve root ischemia.27 Therefore, patients with neurogenic intermittent claudication pain could benefit from OLIF due to the restoration of vertebral displacement and intervertebral stability and were included.
It is reasonable to conclude that both OLIF and MI-TLIF are associated with satisfactory complication rates, based on the findings of previous studies.18,28–31 However, only two comparative studies focus on the complication rates of both surgical approaches and draw different conclusions.7,13 Li et al7 found that a lower complication rate in MI-TLIF than in OLIF (12.1% vs.29.5%), while Lin et al13 reported similar complication rates of the two surgical approaches (32% vs. 36%). In our study, MI-TLIF showed a distinct advantage over OLIF with respect to the complication rates (9.7% vs. 29.4%, P < 0.05). Endplate injury, cage sedimentation, and transient thigh pain/numbness primarily accounted for the difference in the complication rates.
In OLIF, the reason for cage sedimentation is multifactorial and includes endplate injury, over-distraction, osteoporosis, or distraction of a severely narrowed disc, etc.32 Among these factors, endplate injury occupies an important position, mainly resulting from osteoporosis and improper practice.7 Despite the high incidence of endplate injury and cage sedimentation, the fusion rate of OLIF was similar to that of MI-TLIF in our study, comparable with Li et al's results.7 Therefore, some researchers believe that cage sedimentation may provide intervertebral stability with efficient bony fusion, enabling better contact with the bone, and facilitating sound fusion.33 We found five patients experienced transient thigh pain and/or numbness in OLIF, mainly due to the violation of the psoas major and lumbar plexus. Although the low incidence of lumbar plexus intervention in our patients is in line with reported results,7,32,34 reasonable precautions should be taken to reduce the incidence.18 Fortunately, the symptoms were transient and resolved completely with conservative treatment 1 to 2 weeks postoperatively.34
Literature revealed that the postoperative restoration of DH, SLA, and LLA between OLIF and MI-TLIF is still debatable.7,10–13,35 A recent meta-analysis7 demonstrated very similar restoration of DH, SLA, and LLA between OLIF and MI-TLIF. Conversely, several studies10–13 concluded that OLIF is superior to MI-TLIF with regard to the restoration of DH, either in the immediate postoperative period or long-term follow-up but not in the restoration of SLA and LLA. We found that OLIF had a distinct advantage over MI-TLIF in terms of the restoration of DH and LLA, both in the immediate postoperative period and long-term follow-up, but not in the case of SLA. This finding may be associated with several reasons. First, the cage of OLIF is much bigger than that of MI-TLIF, which results in bigger restoration of DH directly and can be placed on the rigid epiphysis ring around the vertebral body, in favor of distracting disc space and compressing the posterior column to restore LLA.35 Second, the cage radian from front to back is 6° or 12° in OLIF, but 0° in MI-TLIF, which is beneficial to the restoration of LLA directly. Third, compared with OLIF, the greater damage of paraspinal muscle and posterior tension band in MI-TLIF may decrease the stability of the spine and break the balance of the posterior column element of the abdominal muscles, leading to a compensatory decrease in LLA,36,37 which may be the reason for the correction loss of LLA after MI-TLIF at the long-term follow-up.
Direct evidence of the effectiveness of spinal decompression was based exclusively on radiological parameters. From above results, the indirect decompression of OLIF has certain advantages over MI-TLIF, but only if patients meet certain indications of OLIF, listed in the inclusion criteria. Unfortunately, the exact indications for indirect decompression remain inconclusive and contradictory.38 Specifically, some patients were reported unsuitable for indirect decompression, such as those with calcified discs, severe facet hypertrophy, synovial cysts, severe central canal stenosis, uncontained disc herniations, and osteophytes arising from the posterior endplates.38–40
This study has some limitations. First, in our study, patients grouping were not random, resulting in some bias, such as cofounding bias and selection bias, and compromising the outcomes. Second, the statistical results with “P > 0.05” of our study, especially for patient-reported outcomes, have a high possibility of type II error resulting from small population differences (δ), big individual differences (SD) and small sample size. Hence, multicenter, large sample prospective randomized trials with long-term follow-up periods are warranted for a more comprehensive evaluation.
In conclusion, compared with MI-TLIF, OLIF showed similar results in terms of patient-reported outcomes and fusion rate, and superior results with regard to perioperative data and radiographic outcomes. The complication rate of OLIF was higher than that of MI-TLIF, primarily owing to the endplate injury, cage sedimentation, and lumbar plexus intervention, which do not adversely affect the clinical and radiographic outcomes. Hence, with a rich experience in lumbar anterior or lateral surgery, surgeons could give preference to OLIF for the treatment of lumbar degenerative diseases.
Key Points
Compared with MI-TLIF, OLIF showed similar results in terms of the VAS score of lower back and leg pain, ODI score, SLA restoration, and fusion rate, and superior results with respect to DH restoration, LLA restoration, operation time, estimated blood loss, length of hospital stay, and serum CK levels.
Even though the complication rate of OLIF is higher than that of MI-TLIF, it does not bring persistent and substantial damage to the patients.
On the basis of a rich experience in lumbar anatomy and lumbar anterior or lateral surgery, surgeons could give preference to OLIF for the treatment of lumbar degenerative diseases.
Further multicenter, large sample prospective randomized trials with long-term follow-up periods are warranted for a more comprehensive evaluation.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication.
This work was sponsored by the National Key R&D Program of China (2020YFC1107100), National Natural Science Fund of China (81472064, 81672150), Zhejiang Medical and Health Science and Technology Project (2018KY117, 2019ZD041), New Talent in Medical Field of Zhejiang Province, and the Fundamental Research Funds for the Central Universities (2019QNA7027).
No relevant financial activities outside the submitted work.
Supplemental digital content is available for this article.
References
- 1.Lan T, Hu SY, Zhang YT, et al. Comparison between posterior lumbar interbody fusion and transforaminal lumbar interbody fusion for the treatment of lumbar degenerative diseases: a systematic review and meta-analysis. World Neurosurg 2018; 112:86–93. [DOI] [PubMed] [Google Scholar]
- 2.Richter M, Weidenfeld M, Uckmann FP. [Anterior lumbar interbody fusion. Indications, technique, advantages and disadvantages]. Orthopade 2015; 44:154–161. [DOI] [PubMed] [Google Scholar]
- 3.Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015; 1:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hah R, Kang HP. Lateral and oblique lumbar interbody fusion-current concepts and a review of recent literature. Curr Rev Musculoskelet Med 2019; 12:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MJ, Mok J, Patel P. Transforaminal lumbar interbody fusion: traditional open versus minimally invasive techniques. J Am Acad Orthop Surg 2018; 26:124–131. [DOI] [PubMed] [Google Scholar]
- 6.Garg B, Mehta N. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF): a review of indications, technique, results and complications. J Clin Orthop Trauma 2019; 10:S156–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li HM, Zhang RJ, Shen CL. Radiographic and clinical outcomes of oblique lateral interbody fusion versus minimally invasive transforaminal lumbar interbody fusion for degenerative lumbar disease. World Neurosurg 2019; 122:e627–e638. [DOI] [PubMed] [Google Scholar]
- 8.Li JX, Phan K, Mobbs R. Oblique lumbar interbody fusion: technical aspects, operative outcomes, and complications. World Neurosurg 2017; 98:113–123. [DOI] [PubMed] [Google Scholar]
- 9.Kotani Y, Ikeura A, Tokunaga H, et al. Single-level controlled comparison of OLIF51 and percutaneous screw in lateral position versus MIS-TLIF for lumbosacral degenerative disorders: clinical and radiologic study. J Orthop Sci 2020; 26:756–764. [DOI] [PubMed] [Google Scholar]
- 10.Kotani Y, Koike Y, Ikeura A, et al. Clinical and radiologic comparison of anterior-posterior single-position lateral surgery versus MIS-TLIF for degenerative lumbar spondylolisthesis. J Orthop Sci 2021; 26:992–998. [DOI] [PubMed] [Google Scholar]
- 11.Koike Y, Kotani Y, Terao H, et al. Comparison of outcomes of oblique lateral interbody fusion with percutaneous posterior fixation in lateral position and minimally invasive transforaminal lumbar interbody fusion for degenerative spondylolisthesis. Asian Spine J 2021; 15:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng SR, Geng YB, Zhou KL, et al. Minimally invasive surgery for degenerative spondylolisthesis: transforaminal or oblique lumbar interbody fusion. J Comp Effect Res 2020; 9:45–51. [DOI] [PubMed] [Google Scholar]
- 13.Lin GX, Akbary K, Kotheeranurak V, et al. Clinical and radiologic outcomes of direct versus indirect decompression with lumbar interbody fusion: a matched-pair comparison analysis. World Neurosurg 2018; 119:e898–e909. [DOI] [PubMed] [Google Scholar]
- 14.Meyerding HW. Spondylolisthesis; surgical fusion of lumbosacral portion of spinal column and interarticular facets; use of autogenous bone grafts for relief of disabling backache. J Int Coll Surg 1956; 26:566–591. [PubMed] [Google Scholar]
- 15.Izzo R, Guarnieri G, Guglielmi G, et al. Biomechanics of the spine. Part I: spinal stability. Eur J Radiol 2013; 82:118–126. [DOI] [PubMed] [Google Scholar]
- 16.Izzo R, Guarnieri G, Guglielmi G, et al. Biomechanics of the spine. Part II: spinal instability. Eur J Radiol 2013; 82:127–138. [DOI] [PubMed] [Google Scholar]
- 17.Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976) 2010; 35:1919–1924. [DOI] [PubMed] [Google Scholar]
- 18.Zeng ZY, Xu ZW, He DW, et al. Complications and prevention strategies of oblique lateral interbody fusion technique. Orthop Surg 2018; 10:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu HF, Fang XQ, Zhao FD, et al. Anteroinferior psoas technique for oblique lateral lumbar interbody fusion. Orthop Surg 2021; 13:1458–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu ZJ, Fang XQ, Zhao FD, et al. Anteroinferior psoas technique for oblique lateral lumbar interbody fusion: technical note and case series. Orthop Surg 2021; 13:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979; 86:420–428. [DOI] [PubMed] [Google Scholar]
- 22.Zhu HF, Wang GL, Zhou ZJ, et al. Prospective study of long-term effect between multifidus muscle bundle and conventional open approach in one-level posterior lumbar interbody fusion. Orthop Surg 2018; 10:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine (Phila Pa 1976) 1993; 18:2106–2107. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Feng H. Oblique lateral interbody fusion (OLIF) with supplemental anterolateral screw and rod instrumentation: a preliminary clinical study. World Neurosurg 2020; 134:e944–e950. [DOI] [PubMed] [Google Scholar]
- 25.Momin AA, Steinmetz MP. Evolution of minimally invasive lumbar spine surgery. World Neurosurg 2020; 140:622–626. [DOI] [PubMed] [Google Scholar]
- 26.Fujibayashi S, Hynes RA, Otsuki B, et al. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine (Phila Pa 1976) 2015; 40:E175–E182. [DOI] [PubMed] [Google Scholar]
- 27.Morishita Y, Hida S, Naito M, et al. Neurogenic intermittent claudication in lumbar spinal canal stenosis: the clinical relationship between the local pressure of the intervertebral foramen and the clinical findings in lumbar spinal canal stenosis. J Spinal Disord Tech 2009; 22:130–134. [DOI] [PubMed] [Google Scholar]
- 28.Wong AP, Smith ZA, Nixon AT, et al. Intraoperative and perioperative complications in minimally invasive transforaminal lumbar interbody fusion: a review of 513 patients. J Neurosurg Spine 2015; 22:487–495. [DOI] [PubMed] [Google Scholar]
- 29.Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J 2012; 6:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker CT, Farber SH, Cole TS, et al. Complications for minimally invasive lateral interbody arthrodesis: a systematic review and meta-analysis comparing prepsoas and transpsoas approaches. J Neurosurg Spine 2019; 1–15. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Woods KR, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J 2017; 17:545–553. [DOI] [PubMed] [Google Scholar]
- 32.Mehren C, Mayer HM, Zandanell C, et al. The oblique anterolateral approach to the lumbar spine provides access to the lumbar spine with few early complications. Clin Orthop Relat Res 2016; 474:2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang JD, Poffyn B, Sys G, et al. Are stand-alone cages sufficient for anterior lumbar interbody fusion. Orthop Surg 2012; 4:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abe K, Orita S, Mannoji C, et al. Perioperative complications in 155 patients who underwent oblique lateral interbody fusion surgery: perspectives and indications from a retrospective, multicenter survey. Spine (Phila Pa 1976) 2017; 42:55–62. [DOI] [PubMed] [Google Scholar]
- 35.Chen YL, Zhu ZH, Wang YK, et al. [Effects of oblique lateral interbody fusion and transforaminal lumbar interbody fusion for lordosis correction in degenerative lumbar diseases]. Zhonghua Yi Xue Za Zhi 2018; 98:1990–1995. [DOI] [PubMed] [Google Scholar]
- 36.Been E, Kalichman L. Lumbar lordosis. Spine J 2014; 14:87–97. [DOI] [PubMed] [Google Scholar]
- 37.Pope MH, Frymoyer JW, Krag MH. Diagnosing instability. Clin Orthop Relat Res 1992; 279:60–67. [PubMed] [Google Scholar]
- 38.Formica M, Quarto E, Zanirato A, et al. Lateral lumbar interbody fusion: what is the evidence of indirect neural decompression? A systematic review of the literature. HSS J 2020; 16:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010; 35:S331–S337. [DOI] [PubMed] [Google Scholar]
- 40.Wang TY, Nayar G, Brown CR, et al. Bony lateral recess stenosis and other radiographic predictors of failed indirect decompression via extreme lateral interbody fusion: multi-institutional analysis of 101 consecutive spinal levels. World Neurosurg 2017; 106:819–826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.