ABSTRACT
TEX264 (testes expressed gene 264) is a single-pass transmembrane protein, consisting of an N-terminal hydrophobic region, a gyrase inhibitory (GyrI)-like domain, and a loosely structured C terminus. TEX264 was first identified as an endoplasmic reticulum (ER)-resident Atg8-family-binding protein that mediates the degradation of portions of the ER during starvation (i.e., reticulophagy). More recently, TEX264 was identified as a cofactor of VCP/p97 ATPase that promotes the repair of covalently trapped TOP1 (DNA topoisomerase 1)-DNA crosslinks. This review summarizes the current knowledge of TEX264 as a protein with roles in both autophagy and DNA repair and provides an evolutionary and structural analysis of GyrI proteins. Based on our phylogenetic analysis, we provide evidence that TEX264 is a member of a large superfamily of GyrI-like proteins that evolved in bacteria and are present in metazoans, including invertebrates and chordates.
Abbreviations: Atg8: autophagy related 8; Atg39: autophagy related 39; Cdc48: cell division cycle 48; CGAS: cyclic GMP-AMP synthase; DPC: DNA-protein crosslinks; DSB: DNA double-strand break; ER: endoplasmic reticulum; GyrI: gyrase inhibitory domain; LRR: leucine-rich repeat; MAFFT: multiple alignment using fast Fourier transform; MAP1LC3/LC3: microtubule-associated protein 1 light chain 3; MTOR: mechanistic target of rapamycin kinase; STUBL: SUMO targeted ubiquitin ligase; SUMO: small ubiquitin-like modifier; TEX264: testis expressed gene 264; TOP1cc: topoisomerase 1-cleavage complex; UBZ: ubiquitin binding Zn finger domain; VCP: valosin containing protein
KEYWORDS: Autophagy, DNA repair, gyrase inhibitory-like proteins, nucleophagy, reticulophagy, TEX264
Introduction
Protein homeostasis is essential for cellular viability. The two major branches of protein homeostasis are autophagy and ubiquitin-dependent proteasomal degradation, which share notable similarities. Both processes enable cells to dispose of excess, aggregated, and damaged organelles or proteins. Dedicated receptor proteins target specific cargo/substrates to facilitate their trafficking into autophagosomes or their presentation to proteasome, and often recognize ubiquitin chains on cargo/substrates [1]. Moreover, certain chaperones, such as the VCP/Cdc48/p97 ATPase, have critical roles in both degradative processes [2].
The autophagic degradation of portions of the ER, known as reticulophagy, has recently been recognized as an important response to nutrient deprivation and the accumulation of misfolded ER lumenal proteins [3]. Reticulophagy is mediated by receptor proteins that are tethered to the ER membrane and bind LC3/GABARAP proteins on phagophore membranes. The Gyrase inhibitory (GyrI)-like domain-containing protein TEX264 was recently shown to be a major reticulophagy receptor, which is sequestered by phagophores via its interaction with LC3-family members and mediates the autophagic degradation of many ER membrane and lumenal proteins upon starvation [4,5]. More recently, we identified an important role for TEX264 as a cofactor of the VCP ATPase at the inner nuclear membrane, where it helps to preserve genome stability [6]. As a cofactor of VCP, TEX264 promotes the degradation of DNA lesions known as TOP1 cleavage complexes (TOP1ccs), which are composed of TOP1 covalently bound to a single-stranded DNA break, and its evolutionarily conserved GyrI-like domain is critical for this function.
Overall, three recent papers have reported distinct functions of the TEX264 protein with a common theme, whereby TEX264 acts as a membrane-anchored receptor to promote the degradation of ER proteins during reticulophagy or of nuclear substrates during DNA repair. This raises fascinating questions regarding potential overlap between these roles. Here, we review the recent reports on TEX264, discuss its potential role in bridging DNA repair and autophagy, and provide a phylogenetic and structural analysis of the GyrI superfamily of proteins.
TEX264 in reticulophagy
The first reports on TEX264 revealed its critical role as a receptor for reticulophagy (also known as ER-phagy), a process by which portions of the ER are sequestered into autophagosomes during nutrient deprivation. Chino et al. identified TEX264 in a mass spectrometry analysis of proteins that interact preferentially with wild-type LC3B versus an LC3-interacting region (LIR) binding-defective variant [4]. An et al. meanwhile, identified TEX264 in a global quantitative proteome analysis as a protein whose abundance is decreased upon either MTOR inhibition or amino acid deprivation in an ATG7- and RB1CC1 (RB1 inducible coiled-coil 1)-dependent manner [5]. TEX264 was shown to undergo trafficking from the ER to lysosomes upon nutrient deprivation, which was dependent both on canonical autophagy pathway components and an LIR motif in TEX264’s C terminus. The long, intrinsically disordered nature of TEX264’s C terminus is also crucial for its reticulophagy function. Due to their large size, ribosomes on the ER membrane may prevent the direct association of the ER and phagophore membranes; however, TEX264’s long C terminus bridges this spatial gap by extending into the cytosol and binding LC3 on phagophores [4].
Of the seven known mammalian reticulophagy receptors, TEX264 appears to play a major role in regulating reticulophagy flux [7–13]. By comparing the effects of individually depleting TEX264 and other known reticulophagy receptors, it was observed that TEX264 knockdown most dramatically suppressed reticulophagy in HeLa cells [4]. Similarly, based on global quantitative proteome mass spectrometry, it was estimated that approximately 50% of all reticulophagy flux upon starvation is driven by TEX264 in HEK293T cells [5]. A more recent genome-wide CRISPR interference screen of reticulophagy regulators found only a modest reduction in reticulophagy activity upon TEX264 knockdown, which is consistent with there being, at least partial, functional redundancy between different reticulophagy receptors [14]. The extent of reticulophagy flux and the impact of the different receptors may vary between tissues and cell types and could be influenced by the differential expression of reticulophagy receptors, with TEX264 appearing to be the most broadly expressed [4].
During nutrient deprivation, TEX264 loss stabilizes many ER membrane and lumenal proteins but does not affect others [5]. This raises important questions as to how TEX264 achieves cargo specificity. One possibility is that the sub-regional differences in TEX264 expression or activation on the ER membrane regulate the differential turnover of cargo. Another is that specific interactions between TEX264 and proteins on the lumenal side of the inner ER membrane enable selective protein degradation [15]. The UFL1 (ubiquitin-fold modifier 1) E3 ligase was recently shown to be required for the autophagic degradation of ER sheets [14]. UFL1 is recruited to the ER membrane by DDRGK1 (DDRGK containing protein 1), where it UFMylates the oligosaccharyltransferase (OST) complex subunit, RPN1, and the ribosomal protein, RPL26. Depletion of DDRGK1 specifically impairs reticulophagy mediated by receptors on ER sheets, such as TEX264, but not by those on ER tubules. Thus, it will be very important to understand how UFMylation on the ER surface is recognized prior to reticulophagy and how this impacts TEX264’s function.
TEX264 in DNA repair
We identified TEX264 in a mass spectrometry analysis of proteins that interact with VCP inside the nucleus [6]. VCP is an ATPase, which mediates protein unfolding, typically to present them to the proteasome for degradation [16]. An intriguing aspect of TEX264 was that it possessed a putative VCP interaction motif, known as a SHP box, in its loosely structured C-terminus, which we found mediates its direct interaction with VCP in vitro.
As discussed below, the GyrI-like domain of TEX264 suggested it may play a role in regulating topoisomerases, possibly in collaboration with VCP. The yeast homolog of VCP, Cdc48, was previously implicated in repairing a DNA lesion composed of TOP1 (DNA topoisomerase 1) covalently bound to the 3ʹ end of a single-stranded DNA break, known as a TOP1cc [17]. TOP1ccs impede DNA replication and transcription, and defects in their repair contribute to various neurological disorders [18–21]. Abrogating VCP activity in human cells significantly impaired TOP1cc repair [6]. As VCP/Cdc48 requires cofactors to be recruited to its substrates, we speculated that TEX264 might fulfill the role of targeting VCP to TOP1ccs. Accordingly, we found that TEX264 is needed to bridge VCP and TOP1 both in vitro and in vivo (Figure 1) [6].
Figure 1.
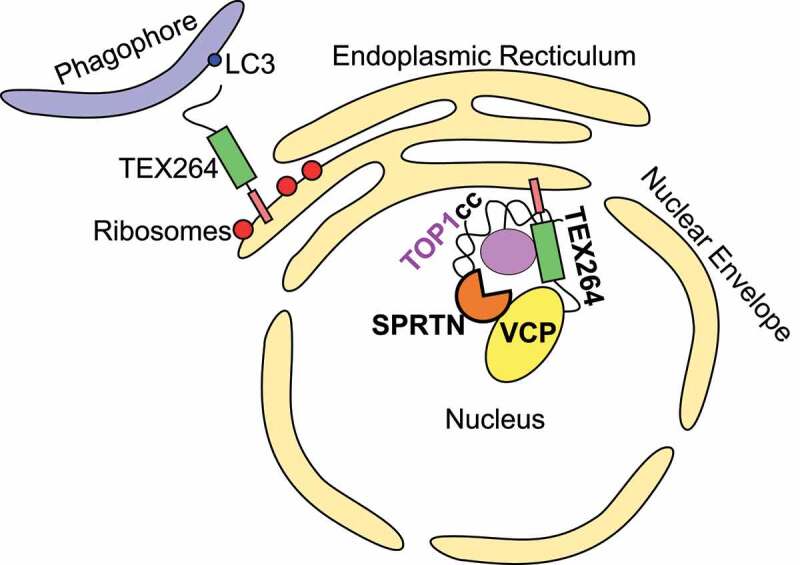
A model for TEX264 function in the ER and nucleus. TEX264 is anchored at both the ER and inner nuclear membrane via its N-terminal single-pass transmembrane domain. TEX264 promotes degradation of portions of the ER during starvation by binding LC3-coated phagophores via its C-terminal LIR. At the inner nuclear membrane, TEX264 associates with VCP-SPRTN subcomplexes via its C-terminal SHP box and promotes TOP1cc repair.
TEX264-deficient cells accumulate endogenous TOP1ccs, exhibit basal replication stress and DNA damage, and are sensitive to low doses of TOP1cc-stabilizing drugs. TEX264’s ability to promote TOP1cc repair relies on motifs in/neighboring its GyrI-like domain as well as a SUMO-interacting motif contained within this domain [6]. As recombinant TEX264 and unmodified TOP1 directly interact in vitro, it seems most plausible that SUMO represents an additional signal to enable TEX264 to distinguish transient TOP1ccs from trapped TOP1ccs, which are extensively modified with SUMO [22]. Indeed, in yeast, Cdc48 promotes the repair of SUMOylated TOP1ccs via its SUMO-binding cofactors, Ufd1 and the metalloprotease Wss1 [17,23,24]. In addition, TOP1cc SUMOylation may enhance the binding affinity between TEX264 and TOP1.
In metazoans, the metalloprotease, and VCP cofactor, SPRTN/DVC1 also proteolytically digests TOP1ccs, as well as other DNA-protein crosslinks (DPCs) [25–27]. TEX264 is necessary to bridge the interaction between TOP1 and SPRTN but is dispensable for general DPC repair [6]. Overall, we propose that VCP unfolds TOP1 such that it can be proteolytically digested by SPRTN. The resulting DNA-bound peptide remnant can only then be excised by the phosphodiesterase TDP1, thus completing TOP1cc repair [20,28].
SPRTN bares motifs which enable it to interact with the DNA replication clamp loader, PCNA, via a proliferating cell nuclear antigen (PCNA)-interacting peptide (PIP) box and ubiquitinated proteins, via its ubiquitin-binding Zn finger (UBZ) domain, and its role in DPC repair is coupled to DNA replication [26,29–32]. This begs the question of why an additional cofactor is needed for its recruitment to substrates. There is some evidence that SPRTN’s PIP box and UBZ domain are not required for its recruitment to chromatin upon DPC formation and its role in DPC repair [25,33]. This indicates that there must be other modes of recruiting SPRTN to specific DPC substrates. Indeed, the requirement for an additional recruitment factor, such as TEX264, is particularly important in the case of TOP1ccs, which are linked to the 3ʹ end of single-stranded DNA breaks, and therefore would not be directly encountered by the elongating DNA polymerase. Moreover, owing to SPRTN’s small active site, which can only be accessed by flexible peptide substrates, there must be additional factors, such as VCP, that enable the processing/unfolding of bulky DPCs [34]. The involvement of additional substrate-recognition factors for specific DPCs could also be a mechanism of restraining SPRTN’s potentially deleterious protein sequence-unspecific cleavage activity by uncoupling DPC recognition from DPC proteolysis.
TEX264 is localized predominantly at the ER and the nuclear periphery, where it is tethered by its N-terminal transmembrane leucine-rich repeat (LRR) [5]. A variant of TEX264 that lacks this LRR redistributes into the cytosol as well as the inner nuclear space [6]. This is consistent with a sub-population of TEX264 being localized to the inner nuclear membrane, facing inwards, as well as the ER membrane, facing the cytosol. Interestingly, we detected TEX264 at DNA replication forks by isolation of proteins on nascent DNA (iPOND) and by immunofluorescent co-localization with nascent DNA [6]. As there is no experimental evidence of alternatively spliced TEX264 isoforms that lack the LRR nor for a cleavage-mediated mechanism for releasing TEX264 from the inner nuclear membrane, TEX264 is likely to be acting at DNA replication forks in the vicinity of the nuclear envelope. This is interesting in the context of recent work, which demonstrated that TOP1 acts on R-loops at nuclear lamina-associated heterochromatic regions [35]. Indeed, these chromatin regions are highly prone to topological stress and could suggest that TOP1ccs frequently arise in the vicinity of the inner nuclear membrane. Further supporting this possibility, it was found that, upon TOP1cc-induced DNA replication fork stalling, the SLFN11 (schlafen family member 11) protein is recruited by RPA1 to DNA replication sites at the nuclear periphery where SLFN11’s ATPase activity blocks DNA replication by changing chromatin structure across replication sites [36].
The SUMO modification machinery is also active at the inner nuclear membrane. For example, modification of LMNA (lamin A/C) by SUMO1 in response to DNA damage is proposed to stimulate its interaction with LC3B and promote its clearance by nucleophagy [37]. A role in relocalizing SUMOylated proteins at DNA lesions to the nuclear periphery has been widely described in yeast. For example, in the S/G2 phases of the cell cycle, mono-SUMOylation of unidentified factors triggers the relocalization of a persistent DNA double-strand break (DSB) to the inner nuclear membrane [38]. Moreover, mono-SUMOylation of various repair proteins promotes the recruitment of collapsed DNA replication forks to the nuclear pore complex [39]. Similarly, in Drosophila, DSBs in heterochromatic DNA regions move to the inner nuclear membrane in a SUMO-dependent manner [40]. Whether DNA lesions are relocalized to the nuclear periphery in human cells is less well explored, although the association between the nuclear lamina and various human DNA repair and replication factors is important for maintaining genome stability [41,42].
Because a SUMO-targeted ubiquitin ligase (STUbL) is required for these relocalized lesions to be repaired, it has been speculated that SUMOylated proteins at collapsed replication forks or resected DSBs need to be degraded by the proteasome to ensure appropriate repair [43]. Some of the targets of this STUbL activity are likely to also be Cdc48 substrates, given the cooperative activity of Cdc48 and STUbL in maintaining genome stability, including in the repair of TOP1ccs [17]. Whether TEX264 also acts with VCP to present TOP1ccs to the proteasome is unknown; however, proteasomal proteolysis is thought to largely occur at the nuclear envelope (and rough ER), where VCP/Cdc48 also has diverse roles [44,45]. This possibility is further supported by the findings that the human STUbL RNF4 (ring finger protein 4) is required for proteasomal TOP1cc degradation, and is known to mark DNA repair factors for extraction by VCP and SPRTN [46,47].
Intersection of DNA repair and autophagy?
As a transmembrane protein, TEX264 acts as a receptor, either at the ER membrane facing the cytosol (for reticulophagy) or the inner nuclear membrane facing the nucleus (for DNA repair). Whether there is any further overlap between its distinct reported roles remains unknown (Figure 1).
Numerous lines of evidence indicate that autophagy contributes to the maintenance of genome stability through the degradation of nuclear proteins, micronuclei, and cytosolic chromatin fragments. In yeast, the DNA repair protein Uba2-Sae2 (UBA2-RBBP8/CtIP in humans) is degraded by autophagy when histone deacetylases are inhibited, resulting in impaired DNA end resection and increased cellular sensitivity to DNA damaging agents [48]. In human cells, the levels of the autophagy cargo receptor SQSTM1/p62 (sequestosome 1) influence DNA repair. For example, nuclear SQSTM1 interacts with and inhibits the DNA repair E3 ligase RNF168 (ring finger protein 168), resulting in defective homology-dependent DNA repair [49]. Nuclear SQSTM1 also promotes the degradation of the DNA repair protein, RAD51, by the proteasome [50]. Thus, the autophagic degradation of nuclear SQSTM1 facilitates homologous recombination repair.
Besides these direct roles in DNA repair, autophagy also mediates the degradation of nuclear components in mammalian cells during DNA damage- or oncogene-induced senescence [51,52]. Nuclear autophagy (i.e., nucleophagy) was first described in yeast, where Atg39 mediates the autophagic degradation of the nuclear envelope and inner nuclear membrane proteins in response to starvation [53]. While no human homolog of Atg39 has been identified, recent work has shown that numerous autophagy proteins are present in the nuclei of mammalian cells, including LC3, ATG5 and ATG7 [52,54]. Indeed, nuclear proteins, such as LMNB1 (lamin B1) and SIRT1 (sirtuin 1), undergo stress-induced degradation in a manner that requires their direct interaction with nuclear LC3B and is mediated by the canonical cytosolic autophagy machinery [51,52]. Importantly, mammalian nucleophagy appears to be distinct from yeast nucleophagy in that it is not induced by conventional stresses, such as starvation or MTOR inhibition [52]. Rather, mammalian nucleophagy is triggered during DNA damage- and oncogene-induced replicative senescence and cells that fail to induce nucleophagy escape senescence [52]. A detailed understanding of how nuclear proteins are targeted for autophagic degradation is lacking and, to date, no mammalian counterpart of Atg39 has been identified. It is plausible that such a receptor protein(s) exists to facilitate the shuttling of nuclear components to cytosolic autophagosomes by directly interacting with either nuclear LC3B or the substrates themselves. Some of the known reticulophagy receptors could possibly also regulate nucleophagy, given that the ER and inner nuclear membranes are contiguous. TEX264 potentially fulfills the criteria of a nucleophagy receptor since it localizes to the perinuclear ER membrane and nuclear envelope and interacts with LC3 family members [5,6]. Accordingly, it will be interesting to determine if TEX264 and LC3 interact inside the nucleus. Additionally, the reticulophagy receptors, CCPG1 (cell cycle progression 1), which localizes to the perinuclear ER, and CDK5RAP3/C53/LZAP (CDK5 regulatory subunit associated protein 3), which is also present within the nucleus, may be candidate nucleophagy receptors [9,55].
Recent work has shown that cytosolic DNA triggers autophagy, which, in turn, drives the clearance of DNA from the cytosol. After replicative stress, damaged chromatin fragments bud from the nucleus into the cytosol and are targeted to the lysosome by SQSTM1 [56]. Similarly, micronuclei harboring damaged chromatin are coated with SQSTM1 and subjected to autophagic degradation [57]. Cytosolic DNA species generated by telomeric DNA damage activates autophagy via the CGAS-STING1 (stimulator of interferon response cGAMP interactor 1) pathway, triggering autophagic cell death, presumably through the degradation of vital cellular components [58]. Intriguingly, TOP1ccs on cytosolic chromatin fragments were recently proposed to be crucial for CGAS activation during senescence because they can be directly bound by CGAS, enhancing its binding to DNA [59].
Autophagy has previously been implicated in the SUMO- and Cdc48-dependent repair of TOP1ccs. In yeast, the DPC protease Wss1 forms a complex with Cdc48 and Doa1, another Cdc48 cofactor implicated in selective autophagy [24]. In response to replication stress, Wss1 relocalizes to vacuoles, suggesting a link between DPC repair and autophagy. It is unclear if DPCs could be degraded by autophagy. Given the high endogenous cellular concentrations of formaldehyde and its propensity to induce protein-protein as well as DNA-protein crosslinks, it is possible that autophagy helps to evict aggregated protein-protein and DNA-protein crosslinks from the nucleus. This could possibly involve the recycling of the liberated protein fragments generated by SPRTN- or Wss1-mediated DPC proteolysis. Interestingly, tandem-affinity mass spectrometry data revealed that the interaction between TEX264 and TOP1 is significantly increased during starvation, however the functional relevance of this is unclear [5].
Proximity biotinylation mass spectrometry analysis of TEX264 detected many VCP-derived peptides, the number of which was not altered during starvation or by mutating TEX264’s LIR, indicating that the association between TEX264 and VCP is unaffected by TEX264’s ability to traffic into autophagosomes [5]. VCP/Cdc48 is required for autophagic degradation, including ribophagy and mitophagy, because it promotes autophagosome–lysosome fusion, yet the precise mechanisms underlying its role are unclear [2,60]. In cooperation with TEX264, VCP might be required to enable engulfment of the ER membrane by phagophores. Another possibility is that direct VCP-dependent extraction of modified ribosomes from the ER membrane is necessary to enable its engulfment. Arguing against this is the observation that VCP depletion by CRISPR interference enhances reticulophagy [14]. This could result from diminished VCP-dependent ER-associated degradation (ERAD), leading to an increased reliance on reticulophagy to clear misfolded proteins from the ER, which could mask any negative impact VCP depletion has on reticulophagy. Nevertheless, VCP could facilitate reticulophagy by, for example, removing ER membrane proteins to expose other substrates for ubiquitination or UFMylation, via a mechanism that would be analogous to its proposed role in mitophagy and the Endo‐Lysosomal Damage Response [61–63].
Evolutionary and structural analysis of GyrI proteins
A particularly noteworthy feature of TEX264 is its GyrI-like domain, which makes it a member of an evolutionarily ancient superfamily of proteins with diverse functions that include inhibitors of the type II topoisomerase gyrase and transcriptional regulators [64–68]. TEX264’s GyrI-like domain is required for TEX264 to bind TOP1, but its relevance, if any, for reticulophagy is unknown.
Most GyrI proteins have acquired domains that confer a diverse array of additional functions, some of which are illustrated in Figure 2A. For example, the GyrI domain of the transcription factors, Rob and BmrR, is fused to an N-terminal helix-turn-helix (HTH) motif that binds DNA. The prototypical member of the GyrI superfamily is Escherichia coli SbmC (renamed GyrI), which was shown to protect cells from microcin b17, a peptide that traps covalent DNA-gyrase intermediates [69]. Interestingly, the expression of SbmC/GyrI is induced in response to both DNA damage and nutrient starvation, potentially providing a distant evolutionary basis for the roles of human TEX264 in autophagy and DNA repair. Subsequent work found that GyrI co-purified with gyrase and suppressed its supercoiling activity, most likely by either sequestering gyrase or inhibiting its binding to DNA [65,67,70]. GyrI also counteract the cytotoxic effects of quinolones, a non-proteinaceous class of antibiotics that stabilize DNA-gyrase complexes, and other DNA-damaging agents, such as mitomycin C [71]. It will be interesting to know if TEX264 adopts a similar mechanism of action to GyrI to suppress TOP1ccs, specifically, by addressing whether the direct binding of TEX264 to topoisomerases inhibits their decatenation activity on DNA templates in vitro. They appear to be distinct mechanisms as TEX264 recruits TOP1cc repair factors, requires VCP activity, and is epistatic with TDP1 in the repair of endogenous TOP1ccs. Moreover, a GyrI-derived 8-amino-acid-long peptide that inhibits gyrase does not inhibit TOP1 activity [67]. Interestingly, a subgroup of prokaryotic GyrI-like proteins (but not GyrI itself) was recently shown to possess hydrolase activity [72]. This activity catalyzes the hydrolysis of DNA-alkylating agents and thereby confers cellular resistance to cytotoxic xenobiotics. The catalytic activity of these proteins depends on pairs of aromatic and acidic residues, however, TEX264 does not contain corresponding residues required for catalysis.
Figure 2.
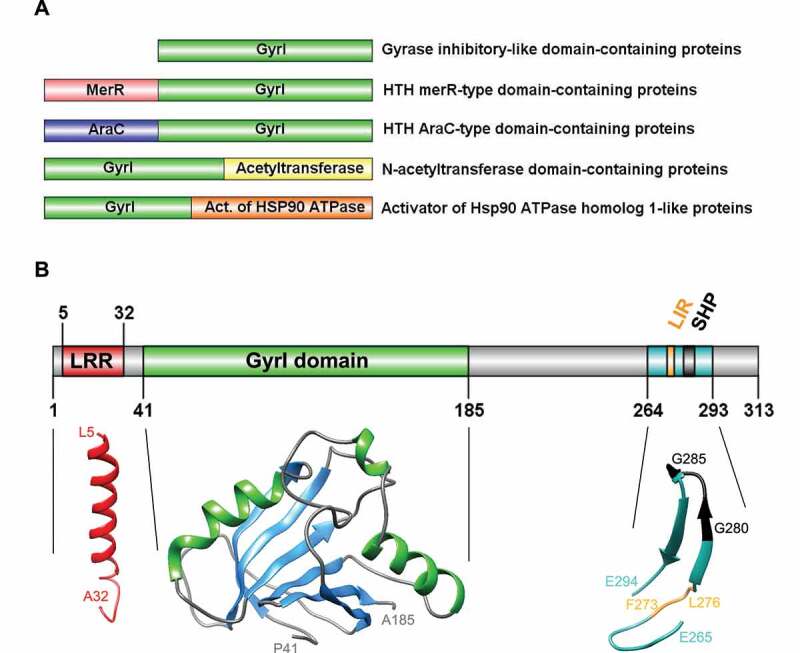
Diversity, topology, and structural models of GyrI proteins. (A) Representative schematics of a subset of GyrI superfamily members, from a total of 73 distinct domain organizations. (B) Topology and structural models of human TEX264 protein motifs and domains. All models were created using the SWISS-MODEL workspace and/or Phyre2 server. GyrI domain was modeled with high to very high confidence based on three templates: SbmC, E. coli Rob transcription factor 2 (1D5Y), and an uncharacterized protein from Chlorobium tepidum (2KCU). The N-terminus of TEX264 bears a leucine-rich repeat (LRR) structural motif that forms an α/β horseshoe fold. The LRR motif was modeled with good to high confidence based on the photosystem II reaction center protein J (6J3Y). The C-terminal part containing the LC3-interacting region (LIR) and VCP-interacting motif (SHP) were modeled with lower confidence based on a Thermotoga maritima mannanase (Man5) carbohydrate-binding module (CBM) (1OF3) and show at least 2 beta-sheets with good model confidence.
Intrigued by the fact that homologs of TEX264 are present in vertebrates but absent in established model organisms such as yeast (Saccharomyces cerevisiae and Schizosaccharomyces pombe), we decided to investigate the evolutionary history of GyrI domain-containing proteins. Blastp searches using bacterial and human GyrI domain sequences through bacteria, yeast, plants, fungi, invertebrate and chordate species was followed by multiple sequence alignment using the MAFFT algorithm (Multiple Alignments using Fast Fourier Transform), while alignment quality was assessed using Guidance software (Figure 3) [73,74]. Phylogenetic trees were constructed with maximum likelihood analysis in PhyML (Figure 4) [75,76]. Ftsa (cell division ATPase) proteins were used as an outgroup (Fig. S1) because they are functionally different from GyrI domain-containing proteins, yet they contain an SHS2 module like GyrI proteins, making it possible to reach sufficiently good multiple protein alignment for subsequent tree building. The GyrI domain of SbmC/GyrI contains two tandem SHS modules, the second of which encompasses its interaction site with Gyrase, suggesting this domain mediates protein-protein interactions [77]. The SHS2 module of TEX264 is highly conserved across TEX264 orthologs, as well as in E. coli SbmC/GyrI, indicating its functional and/or structural importance prior to the evolution of autophagy (Fig. S1).
Figure 3.
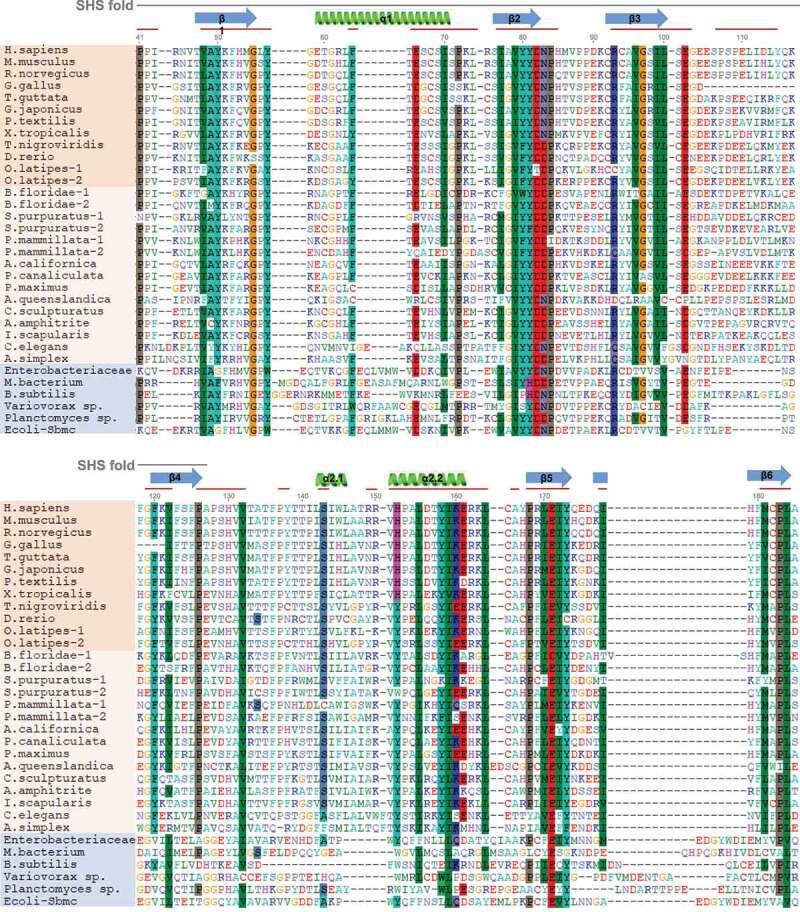
GyrI domain sequence alignments of TEX264 orthologs. The GyrI-like domain of human TEX264 corresponds to amino acids 41–185 of the full-length protein. Shown above the alignment (gray line), the SHS2 fold in human TEX264 corresponds to amino acids 21–127. The structure of human TEX264 according to 3D modeling is labeled for the corresponding protein sequence, where α-helices are shown in green and beta-sheets in blue, as in the structural model of human TEX264 in Figure 2B. Red lines designate conserved motifs and domains. TEX264 orthologs are shown in orange (dark: vertebrates; bright: invertebrates), while bacterial GyrI-domain containing proteins are shown in blue. Protein sequences were aligned using the MAFFT alignment algorithm. Alignment quality score was assessed using the Guidance2 server and was 0.752, where 1 is maximum, indicating high alignment quality.
Figure 4.
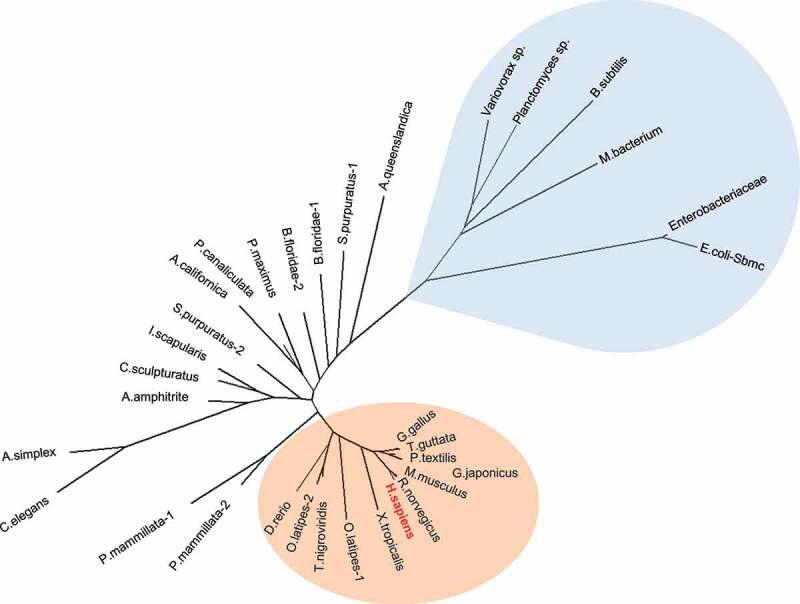
Phylogenetic analysis of TEX264 proteins. TEX264 orthologs in vertebrates are highlighted in orange (invertebrates are colorless) and bacterial GyrI-domain containing proteins are highlighted in blue. Ftsa proteins were used as an outgroup (Fig. S2). Full-length protein sequences were aligned with the MAFFT alignment algorithm. The phylogenetic tree was constructed using the maximum likelihood method. The expanded phylogenetic tree with detailed methodology is shown in Fig. S2.
We have found conserved TEX264 orthologs in invertebrate and chordate species (Figure 4 and S1), while they were absent in fungi and plants. Despite being present in bacteria and metazoans, GyrI domain-containing proteins are notably absent in yeast, similar to PARPs (poly(ADP-ribose) polymerases) [78]. Since yeast diverged prior to the evolution of metazoans, this indicates that they either independently lost GyrI domain-containing proteins or that these domains were regained via convergent evolution in the first common ancestor of all metazoans.
Phylogenetic analysis of full-length proteins (Figure 4 and S1) and of the GyrI domain (amino acids 41–185 of human TEX264) showed similar clustering (Fig. S2). Bacterial GyrI-domain containing proteins expectedly cluster closer to the TEX264 group than to the Ftsa outgroup but are quite distant to the TEX264 group and bare substantial differences in protein sequence outside of the GyrI domain. On the other hand, the GyrI domain of bacterial proteins is similar to TEX264 orthologs with several conserved regions (Figure 3), and most importantly, structural models of human TEX264 GyrI domain can be constructed with high model confidence (Figures 2B and 3). Given the phylogenetic proximity and similarity in 3D structures of the GyrI domain, bacterial GyrI domain-containing proteins might be regarded as distant ancestors of the GyrI domain in TEX264 orthologs.
Unlike its GyrI-like domain, the C terminus of TEX264 – corresponding to amino acids 186–313 of the full-length protein sequence – substantially diverges in invertebrates and is absent in bacterial GyrI domain-containing proteins (Fig. S3). In vertebrates, TEX264’s C terminus is conserved, including both the LIR and SHP motifs (Fig. S3). However, the C-terminal part diverges considerably in the invertebrate lineage (Fig. S3). Specifically, the LIR motif is only partly conserved in tunicates, mollusks, and crustaceans, while it is absent in nematodes, sponges, and a chordate lancelet (Branchiostoma floridae; Fig. S3). The SHP motif is conserved in higher vertebrate species, from reptiles to mammals, but is divergent in lower vertebrates (fish and amphibians), and completely absent in invertebrate TEX264 orthologs (Fig. S3). Bacterial GyrI domain-containing proteins are shorter than TEX264 orthologs and lack a C terminus that would resemble that of TEX264. Likewise, bacterial GyrI domain-containing proteins lack the N-terminal LRR domain, which is otherwise highly conserved throughout TEX264 orthologs within the animal kingdom (Fig. S4).
The model of TEX264’s GyrI-like domain shows two antiparallel sheets and two alpha-helices following the β1-α1-β2-β3-β4-α2-β5-β6 linear arrangement (Figures 2B and 4). Similar to bacterial GyrI proteins, E. coli SbmC and Rob2, two similar halves of GyrI domain show pseudo-two-fold symmetry (Figure 2B). The N-terminus of TEX264 bears a leucine-rich repeat (LRR) structural motif that forms an α/β horseshoe fold. The LRR motif was modeled with good-to-high confidence based on the photosystem II reaction center protein J (6J3Y; Figure 2B). The C-terminal part containing the LC3-interacting region (LIR) and VCP/Cdc48 interacting motif (SHP) were modeled with lower confidence based on a Thermotoga maritima mannanase (Man5) carbohydrate-binding module (CBM) (1OF3) and show at least two beta-sheets with good model confidence (Figure 2B).
Future perspectives
Three recent studies have reported distinct roles for the TEX264 protein as a membrane-anchored receptor either for reticulophagy or for nuclear substrates during DNA repair. TEX264 evolved from an ancient superfamily of proteins, orthologs of which are present in bacteria and metazoans. GyrI domain-containing proteins have acquired additional and diverse domains and functions throughout evolution, including transcription regulation, chromatin-remodeling, and protein homeostasis. The fact that GyrI proteins and TEX264 orthologs pre-date the evolution of autophagy hints at a distinct primordial function of these proteins, as illustrated by the role of the TEX264 relative, SbmC, in regulating bacterial gyrase. It will be interesting to understand whether the GyrI-like domain of TEX264 and its interaction with VCP is also important for reticulophagy. It will also be fascinating to explore the contribution of TEX264 to processes such as nuclear degradation in cell types that undergo extensive organelle loss during differentiation, such as erythroblasts and epidermal keratinocytes [79]. Future studies should also aim to address whether TEX264’s role in the nucleus extends beyond TOP1cc repair and whether these roles rely on its ability to promote autophagy and associate with the inner nuclear membrane.
Supplementary Material
Funding Statement
The K.R. laboratory is funded by Medical Council Research Programme grant [MC_PC_12001/1 and MC_UU_00001/1]. M.P. research group is supported by Croatian Science Foundation Installation Grant [UIP-2017-05-5258] and Project grant [IPS-2020-01-4225], Ruder Boskovic Institute (Zagreb, Croatia) and European Structural and Investment Funds STIM – REI project [KK.01.1.1.01.0003].
Disclosure statement
The authors declare no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Khaminets A, Behl C, Dikic I.. Ubiquitin-dependent and independent signals in selective autophagy. Trends Cell Biol. 2016;26:6–16. [DOI] [PubMed] [Google Scholar]
- [2].Dargemont C, Ossareh-Nazari B.. Cdc48/p97, a key actor in the interplay between autophagy and ubiquitin/proteasome catabolic pathways. Biochim Biophys Acta Mol Cell Res. 2012;1823:138–144. [DOI] [PubMed] [Google Scholar]
- [3].Dikic I. Open questions: why should we care about reticulophagy and ER remodelling? BMC Biol. 2018;16:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chino H, Hatta T, Natsume T, et al. Intrinsically disordered protein TEX264 mediates reticulophagy. Mol Cell. 2019;74(909–921):e6. [DOI] [PubMed] [Google Scholar]
- [5].An H, Ordureau A, Paulo JA, et al. TEX264 is an endoplasmic reticulum-resident ATG8-interacting protein critical for ER remodeling during nutrient stress. Mol Cell. 2019;74(891–908):e10. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fielden J, Wiseman K, Torrecilla I, et al. TEX264 coordinates p97- and SPRTN-mediated resolution of topoisomerase 1-DNA adducts. Nat Commun. 2020;11:1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Khaminets A, Heinrich T, Mari M, et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522:354–358. [DOI] [PubMed] [Google Scholar]
- [8].Stephani M, Picchianti L, Gajic A, et al. A cross-kingdom conserved reticulophagy receptor maintains endoplasmic reticulum homeostasis during stress. eLife. 2020;9:e58396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Smith MD, Harley ME, Kemp AJ, et al. CCPG1 is a non-canonical autophagy cargo receptor essential for reticulophagy and pancreatic ER proteostasis. Dev Cell. 2018;44(217–232):e11. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen Q, Xiao Y, Chai P, et al. ATL3 is a tubular Reticulophagy receptor for GABARAP-mediated selective autophagy. Curr Biol. 2019;29:846–855.e6. [DOI] [PubMed] [Google Scholar]
- [11].Liang JR, Lingeman E, Ahmed S, et al. Atlastins remodel the endoplasmic reticulum for selective autophagy. J Cell Biol. 2018;217:3354–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Delorme-Axford E, Popelka H, Klionsky DJ. TEX264 is a major receptor for mammalian reticulophagy. Autophagy. 2019;15:1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fumagalli F, Noack J, Bergmann T, et al. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat Cell Biol. 2016;18:1173–1184. [DOI] [PubMed] [Google Scholar]
- [14].Liang JR, Lingeman E, Luong T, et al. A genome-wide reticulophagy screen highlights key roles of mitochondrial metabolism and ER-resident UFMylation. Cell. 2020;180:1160–1177.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wilkinson S. Picky eating at the reticulophagy buffet. Trends Biochem Sci. 2019;44:731–733. [DOI] [PubMed] [Google Scholar]
- [16].Bodnar NO, Rapoport TA. Molecular mechanism of substrate processing by the Cdc48 ATPase complex. Cell. 2017;169:722–735.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nie M, Aslanian A, Prudden J, et al. Dual recruitment of Cdc48 (p97)-Ufd1-Npl4 ubiquitin-selective segregase by small ubiquitin-like modifier protein (SUMO) and ubiquitin in SUMO-targeted ubiquitin ligase-mediated genome stability functions. J Biol Chem. 2012;287:29610–29619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walker C, Herranz-Martin S, Karyka E, et al. C9orf72 expansion disrupts ATM-mediated chromosomal break repair. Nat Neurosci. 2017;20:1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Alagoz M, Chiang SC, Sharma A, et al. ATM deficiency results in accumulation of DNA-topoisomerase I covalent intermediates in neural cells. PLoS ONE. 2013;8:e58239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].El-Khamisy SF, Saifi GM, Weinfeld M, et al. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature. 2005;434:108–113. [DOI] [PubMed] [Google Scholar]
- [21].Katyal S, Lee Y, Nitiss KC, et al. Aberrant topoisomerase-1 DNA lesions are pathogenic in neurodegenerative genome instability syndromes. Nat Neurosci. 2014;17:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mao Y, Desai SD, Liu LF. SUMO-1 conjugation to human DNA topoisomerase II isozymes. J Biol Chem. 2000;275:26066–26073. [DOI] [PubMed] [Google Scholar]
- [23].Stingele J, Schwarz MS, Bloemeke N, et al. A DNA-dependent protease involved in DNA-protein crosslink repair. Cell. 2014;158:327–338. [DOI] [PubMed] [Google Scholar]
- [24].Balakirev MY, Mullally JE, Favier A, et al. Wss1 metalloprotease partners with Cdc48/Doa1 in processing genotoxic SUMO conjugates. eLife. 2015;4:e06763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maskey RS, Flatten KS, Sieben CJ, et al. Spartan deficiency causes accumulation of Topoisomerase 1 cleavage complexes and tumorigenesis. Nucleic Acids Res. 2017;45:4564–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vaz B, Popovic M, Newman JA, et al. Metalloprotease SPRTN/DVC1 orchestrates replication-coupled DNA-protein crosslink REPAIR. Mol Cell. 2016;64:704–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fielden J, Ruggiano A, Popović M, et al. DNA protein crosslink proteolysis repair: from yeast to premature ageing and cancer in humans. DNA Repair (Amst). 2018;71:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Interthal H, Champoux JJ. Effects of DNA and protein size on substrate cleavage by human tyrosyl-DNA phosphodiesterase 1. Biochem J. 2011;436:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mosbech A, Gibbs-Seymour I, Kagias K, et al. DVC1 (C1orf124) is a DNA damage-targeting p97 cofactor that promotes ubiquitin-dependent responses to replication blocks. Nat Struct Mol Biol. 2012;19:1084–1092. [DOI] [PubMed] [Google Scholar]
- [30].Larsen NB, Gao AO, Sparks JL, et al. Replication-coupled DNA-protein crosslink repair by SPRTN and the proteasome in xenopus egg extracts. Mol Cell. 2019;73(574–588):e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Halder S, Torrecilla I, Burkhalter MD, et al. SPRTN protease and checkpoint kinase 1 cross-activation loop safeguards DNA replication. Nat Commun. 2019;10:3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mórocz M, Zsigmond E, Tóth R, et al. DNA-dependent protease activity of human Spartan facilitates replication of DNA-protein crosslink-containing DNA. Nucleic Acids Res. 2017;45:3172–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stingele J, Bellelli R, Alte F, et al. Mechanism and regulation of DNA-protein crosslink repair by the DNA-dependent metalloprotease SPRTN. Mol Cell. 2016;64:688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li F, Raczynska JE, Chen Z, et al. Structural insight into DNA-dependent activation of human metalloprotease spartan. Cell Rep. 2019;26(3336–3346):e4. [DOI] [PubMed] [Google Scholar]
- [35].Manzo SG, Hartono SR, Sanz LA, et al. DNA Topoisomerase I differentially modulates R-loops across the human genome. Genome Biol. 2018;19:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Murai J, Tang S-W, Leo E, et al. SLFN11 blocks stressed replication forks independently of ATR. Mol Cell. 2018;69:371–384.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li Y, Jiang X, Zhang Y, et al. Nuclear accumulation of UBC9 contributes to SUMOylation of lamin A/C and nucleophagy in response to DNA damage. J Exp Clin Cancer Res. 2019;38:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Horigome C, Bustard DE, Marcomini I, et al. PolySUMOylation by Siz2 and Mms21 triggers relocation of DNA breaks to nuclear pores through the Slx5/Slx8 STUbL. Genes Dev. 2016;30:931–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Whalen JM, Dhingra N, Wei L, et al. Relocation of collapsed forks to the nuclear pore complex depends on sumoylation of DNA repair proteins and permits Rad51 association. Cell Rep. 2020;31:107635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ryu T, Bonner MR, Chiolo I. Cervantes and Quijote protect heterochromatin from aberrant recombination and lead the way to the nuclear periphery. Nucleus. 2016;7:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li W, Bai X, Li J, et al. The nucleoskeleton protein IFFO1 immobilizes broken DNA and suppresses chromosome translocation during tumorigenesis. Nat Cell Biol. 2019;21:1273–1285. [DOI] [PubMed] [Google Scholar]
- [42].Cobb AM, Murray TV, Warren DT, et al. Disruption of PCNA-lamins A/C interactions by prelamin A induces DNA replication fork stalling. Nucleus. 2016;7:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Horigome C, Gasser SM. SUMO wrestles breaks to the nuclear ring’s edge. Cell Cycle. 2016;15:3011–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Olmos Y, Hodgson L, Mantell J, et al. ESCRT-III controls nuclear envelope reformation. Nature. 2015;522:236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Enenkel C, Lehmann A, Kloetzel PM. GFP-labelling of 26S proteasomes in living yeast: insight into proteasomal functions at the nuclear envelope/rough ER. Mol Biol Rep. 1999;26:131–135. [DOI] [PubMed] [Google Scholar]
- [46].Sun Y, Miller Jenkins LM, Su YP et al. A conserved SUMO-Ubiquitin pathway directed by RNF4/SLX5-SLX8 and PIAS4/SIZ1 drives proteasomal degradation of topoisomerase DNA-protein crosslinks. BioRxiv. 2019;707661. DOI: 10.1101/707661. [DOI] [Google Scholar]
- [47].Gibbs-Seymour I, Oka Y, Rajendra E, et al. Ubiquitin-SUMO circuitry controls activated fanconi anemia ID complex dosage in response to DNA damage. Mol Cell. 2015;57:150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Robert T, Vanoli F, Chiolo I, et al. HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Nature. 2011;471:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang Y, Zhang N, Zhang L, et al. Autophagy regulates chromatin ubiquitination in DNA damage response through elimination of SQSTM1/p62. Mol Cell. 2016;63:34–48. [DOI] [PubMed] [Google Scholar]
- [50].Hewitt G, Carroll B, Sarallah R, et al. SQSTM1/p62 mediates crosstalk between autophagy and the UPS in DNA repair. Autophagy. 2016;12:1917–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Xu C, Wang L, Fozouni P, et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol. 2020;22:1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dou Z, Xu C, Donahue G, et al. Autophagy mediates degradation of nuclear lamina. Nature. 2015;527:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mochida K, Oikawa Y, Kimura Y, et al. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature. 2015;522:359–362. [DOI] [PubMed] [Google Scholar]
- [54].Lee IH, Kawai Y, Fergusson MM, et al. Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science. 2012;336:225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang J, He X, Luo Y, et al. A novel ARF-binding protein (LZAP) alters ARF regulation of HDM2. Biochem J. 2006;393:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ivanov A, Pawlikowski J, Manoharan I, et al. Lysosome-mediated processing of chromatin in senescence. J Cell Biol. 2013;202:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rello-Varona S, Lissa D, Shen S, et al. Autophagic removal of micronuclei. Cell Cycle. 2012;11:170–176. [DOI] [PubMed] [Google Scholar]
- [58].Nassour J, Radford R, Correia A, et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019;565:659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhao B, Liu P, Fukumoto T, et al. Topoisomerase 1 cleavage complex enables pattern recognition and inflammation during senescence. Nat Commun. 2020;11:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ju JS, Fuentealba RA, Miller SE, et al. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J Cell Biol. 2009;187:875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].McLelland GL, Goiran T, Yi W, et al. Mfn2 ubiquitination by PINK1/parkin gates the p97-dependent release of ER from mitochondria to drive mitophagy. eLife. 2018;7:e32866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tanaka A, Cleland MM, Xu S, et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Papadopoulos C, Kirchner P, Bug M, et al. VCP/p97 cooperates with YOD1, UBXD1 and PLAA to drive clearance of ruptured lysosomes by autophagy. Embo J. 2017;36:135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zheleznova Heldwein EE, Brennan RG. Crystal structure of the transcription activator BmrR bound to DNA and a drug. Nature. 2001;409:378–382. [DOI] [PubMed] [Google Scholar]
- [65].Nakanishi A, Oshida T, Matsushita T, et al. Identification of DNA gyrase inhibitor (GyrI) in Escherichia coli. J Biol Chem. 1998;273:1933–1938. [DOI] [PubMed] [Google Scholar]
- [66].Romanowski MJ, Gibney SA, Burley SK. Crystal structure of the Escherichia coli SbmC protein that protects cells from the DNA replication inhibitor microcin B17. Proteins. 2002;47:403–407. [DOI] [PubMed] [Google Scholar]
- [67].Nakanishi A, Imajoh-Ohmi S, Hanaoka F. Characterization of the interaction between DNA gyrase inhibitor and DNA gyrase of Escherichia coli. J Biol Chem. 2002;277:8949–8954. [DOI] [PubMed] [Google Scholar]
- [68].Kwon HJ, Bennik MHJ, Demple B, et al. Crystal structure of the Escherichia coli Rob transcription factor in complex with DNA. Nat Struct Biol. 2000;7:424–430. [DOI] [PubMed] [Google Scholar]
- [69].Baquero MR, Bouzon M, Varea J, et al. sbmC, a stationary‐phase induced SOS Escherichia coli gene, whose product protects cells from the DNA replication inhibitor microcin B17. Mol Microbiol. 1995;18:301–311. [DOI] [PubMed] [Google Scholar]
- [70].Chatterji M, Nagaraja V. GyrI: a counter-defensive strategy against proteinaceous inhibitors of DNA gyrase. EMBO Rep. 2002;3:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chatterji M, Sengupta S, Nagaraja V. Chromosomally encoded gyrase inhibitor GyrI protects Escherichia coli against DNA-damaging agents. Arch Microbiol. 2003;180:339–346. [DOI] [PubMed] [Google Scholar]
- [72].Yuan H, Zhang J, Cai Y, et al. GyrI-like proteins catalyze cyclopropanoid hydrolysis to confer cellular protection. Nat Commun. 2017;8:1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Katoh K, Misawa K, Kuma KI, et al. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Penn O, Privman E, Ashkenazy H, et al. GUIDANCE: a web server for assessing alignment confidence scores. Nucleic Acids Res. 2010;38:W23–W28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. [DOI] [PubMed] [Google Scholar]
- [76].Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol. 2006;55:539–552. [DOI] [PubMed] [Google Scholar]
- [77].Anantharaman V, Aravind L. The SHS2 module is a common structural theme in functionally diverse protein groups, like Rpb7p, FtsA, GyrI, and MTH1598/Tm1083 superfamilies. Proteins. 2004;56:795–807. [DOI] [PubMed] [Google Scholar]
- [78].Citarelli M, Teotia S, Lamb RS. Evolutionary history of the poly(ADP-ribose) polymerase gene family in eukaryotes. BMC Evol Biol. 2010;10:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rogerson C, Bergamaschi D, O’Shaughnessy RFL. Uncovering mechanisms of nuclear degradation in keratinocytes: a paradigm for nuclear degradation in other tissues. Nucleus. 2018;9:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


