Abstract
The pathophysiology of SARS-CoV-2 is unique in the different pathways the virus utilizes to induce severe systemic inflammation, leading to a higher potential for critical care requirements and a poorer prognosis. Although there are several risk factors for the development of severe infection, one associated with poorer outcome is obesity. In this report, we present a case of bowel perforation in an obese patient with severe COVID-19 infection.
Keywords: Bowel perforation, coronavirus, COVID-19, critical care, obesity, SARS-CoV-2
When SARS-CoV-2 was first reported in December 2019, no one could have fathomed the scale in which this virus would spread across the world.1 In vivo studies of respiratory cell lines and in vitro studies of ferrets revealed that SARS-CoV-2 reduced the antiviral responses of type I and III interferons and significantly upregulated proinflammatory chemokines.2 Thus, the SARS-CoV-2 virus can blunt both the innate and adaptive immune responses of the human body. Many of the cases that resulted in unfavorable outcomes (prolonged hospital stay or death) had exacerbated inflammatory responses.3 Some notable risk factors for the development of severe infection include diabetes, cardiovascular disease, and advanced age.4 One specific risk factor, obesity, is associated with a higher potential for critical care requirements and possible invasive mechanical ventilation in the setting of COVID-19 infection. This is due to the increased basal inflammatory state of patients with obesity. In a study examining COVID-19 hospitalizations in New York, patients with body mass index > 30 kg/m2 were between 1.8 and 3.6 times more likely to require critical care than patients with a body mass index < 30 kg/m2.5
CASE DESCRIPTION
A 33-year-old man with known hypertension, obesity (body mass index 72 kg/m2), and obstructive sleep apnea presented to the emergency department with dyspnea. He tested positive for the COVID-19 virus 5 days prior to presentation. His initial imaging confirmed findings of extensive bilateral pulmonary opacities, concerning for COVID-19 pneumonia. He was noted to have severe hypoxia and placed on high-flow nasal cannula oxygen. His initial blood pressure was 209/135 mm Hg and temperature, 102.5°F. The B-type natriuretic peptide was 337 pg/mL (normal <100 pg/mL), procalcitonin was 0.35 ng/mL (normal <0.15 ng/mL), white blood cell count was 5700/μL, and troponin was 0.2 ng/mL (normal <0.03 ng/mL). His initial inflammatory laboratory tests were notable for a ferritin of 693 µg/L (normal 24–336 µg/L), lactate dehydrogenase of 610 U/L (normal 140–280 U/L), erythrocyte sedimentation rate of 25 mm/h (normal 0–22 mm/h), and C-reactive protein of 75.5 μg/mL (normal <10 μg/mL). The patient was started on remdesivir and dexamethasone and was transferred to the intensive care unit due to increased oxygen requirements. He was then transferred to the internal medicine wards approximately 8 days after presentation.
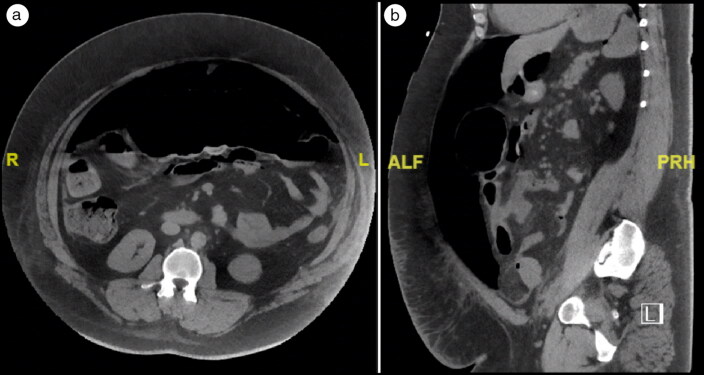
Three days after his transfer to the medicine wards (and 11 days after presentation), he began to have severe, unprovoked abdominal pain. He also had findings of acute abdomen with rebound tenderness. Imaging of the abdomen/pelvis revealed a large amount of free intraperitoneal air, compatible with viscus perforation (Figure 1). The C-reactive protein was 128.6 μg/mL, erythrocyte sedimentation rate was 94 mm/h, ferritin was 942 μg/L, and lactate dehydrogenase was 450 U/L.
Figure 1.
High-resolution CT of the abdomen, (a) axial view and (b) sagittal view, showing free air in the abdominal cavity.
The patient was immediately taken to the operating room. Approximately 4.5 cm of his ascending colon and 37.0 cm of his transverse colon were removed, with colostomy creation 2 days later to allow for bowel rest. Gross pathologic exam of the resected colon showed transmural defects and associated serositis/abscess, but no thrombosis was identified. Imaging of the brain revealed a left anterior cerebral artery infarct. Two days after that event, tracheostomy was performed due to failure to wean from mechanical ventilation. Serial chest x-rays revealed worsening respiratory status with findings of interval increase in multifocal pneumonia and development of acute respiratory distress syndrome. After ongoing conversations about goals of care, the patient’s family elected to transition to comfort care. At 45 days postpresentation, the patient passed away.
DISCUSSION
The pathway that links this patient with severe COVID-19–related illness is related to the association between obesity, the up-regulation of the immune system, and the dysregulation of the adaptive immune response. Obese patients are already in a heightened basal inflammatory state, so contracting this virus creates the perfect storm for a severe systemic inflammatory response. In terms of the objective data, the severity of this patient’s hospital course was evidenced by his rising inflammatory markers (C-reactive protein, erythrocyte sedimentation rate, ferritin, and lactate dehydrogenase) all the way up to the discovery of his acute bowel perforation.
The virus utilizes the angiotensin-converting enzyme 2 (ACE-2) receptor for entry.6 ACE-2 receptors are expressed on the surface of lung alveolar epithelial cells and on the surface of enterocytes in the small intestine and colon—thus explaining the respiratory and gastrointestinal symptoms of a COVID-19 infection, respectively.7 Knowing this information, it is reasonable to deduce that the SARS-CoV-2 virus could potentially directly invade colonic tissue by utilizing the ACE-2 receptor. In this patient, there was direct transmural colonic injury with the formation of an abscess that was the precursor to subsequent perforation. This presentation of potential direct injury provides more evidence in support of the theory that the virus can directly enter and injure the colonic mucosa. However, most of the current research on the association between severe SARS-CoV-2 viral infection and gastrointestinal perforation is in the form of case reports and is therefore more anecdotal than correlational. More research is needed to identify additional cases and create an aggregated set of data.
References
- 1.Carvalho T, Krammer F, Iwasaki A.. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol. 2021;21(4):245–256. doi: 10.1038/s41577-021-00522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Zhang Z, Tian J, Xiong S.. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Ann Palliat Med. 2020;9(2):428–436. doi: 10.21037/apm.2020.03.26. [DOI] [PubMed] [Google Scholar]
- 5.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H.. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]