Abstract
Acute kidney injury is common among hospitalized COVID-19 patients, with the incidence ranging from 0.5% to 80%, due to diverse pathologies including acute tubular injury, collapsing glomerulopathy, and thrombotic microangiopathy (TMA). While macrovascular thrombosis is common in these individuals, the frequent finding of extensive microvascular thromboses in several series and case reports raises the possibility of thrombotic microangiopathy (TMA) being a contributing factor in the thrombotic and multiorgan complications of the disease. TMA has been described as either the primary finding or in concert with other pathologic findings in COVID-19 patients and carries a poor prognosis, with all patients requiring dialysis. We present a case of TMA with retinal injury and bowel perforation in addition to pulmonary and renal manifestations.
Keywords: COVID-19, dialysis, eculizumab, thrombotic microangiopathy
Thrombotic microangiopathies (TMAs) are a group of disorders characterized by the presence of microangiopathic hemolytic anemia, thrombocytopenia, and end-organ capillary thromboses causing ischemic organ damage.1 The mechanisms that have been proposed in TMAs associated with viral infections include (a) direct endothelial injury; (b) acquired inhibitors to a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) protein; (c) the presence of lupus anticoagulants; and (d) uncontrolled activation of the complement system.1 The alternative complement pathway has been shown to be contributory in the predisposition to COVID-19–associated TMAs.2
CASE DESCRIPTION
A 27-year-old black man was admitted with a 4-day history of effort intolerance, nasal congestion, cough, and lethargy. He had hypertension since his teenage years but was inconsistently treated. Four months earlier, his creatinine was 3.0 mg/dL with normal-sized kidneys. He was afebrile, with a blood pressure of 214/136 mm Hg, a respiratory rate of 24 breaths per minute, and oxygen saturation of 98% on room air. Lungs revealed bibasilar crackles. Initial laboratory results are shown in Table 1. A nasopharyngeal swab was positive for SARS-CoV-2 infection. Chest radiograph revealed bilateral pleural effusions with ground-glass opacities. Dialysis was initiated with a plan for continuing outpatient treatments, and the patient was discharged home to complete a 10-day course of dexamethasone for COVID pneumonia.
Table 1.
Initial laboratory results
| Test | Result | Test | Result |
|---|---|---|---|
| Blood urea nitrogen (mg/dL) | 112 | Haptoglobin (mg/dL) | 10 |
| Creatinine (mg/dL) | 22.2 | Hemoglobin (g/dL) | 9.3 |
| Sodium (mEq/L) | 131 | Hematocrit (%) | 25.7 |
| Potassium (mEq/L) | 3.1 | Platelets (10*9/L) | 136 |
| Chloride (mEq/L) | 89 | White blood cells (109/L) | 13.5 |
| Carbon dioxide (mEq/L) | 20 | ADAMTS13 activity (%) | 59 |
| Anion gap (mEq/L) | 22 | aHUS/TMA gene panel | Negative |
| Lactate dehydrogenase (IU/L) | 798 |
He was readmitted 1 day later with acute abdominal pain, with imaging evidence of small bowel obstruction. Exploratory laparotomy with small bowel resection of a mid-jejunal perforation and primary anastomosis was done. The pathology of the bowel revealed transmural necrosis (presumably ischemic) with perforation and acute serositis (Figure 1). On postoperative day 5, the patient developed acute bilateral vision loss. Magnetic resonance imaging revealed foci of recent ischemia involving the deep right frontal centrum semiovale, left capsular region, and posterior left temporal lobe. Ophthalmology exam revealed a severe diffusely ischemic retina consistent with TMA or intravascular coagulopathy. Due to the acute vision loss, he was started on high-dose methylprednisolone and transferred to our hospital for further evaluation and management.
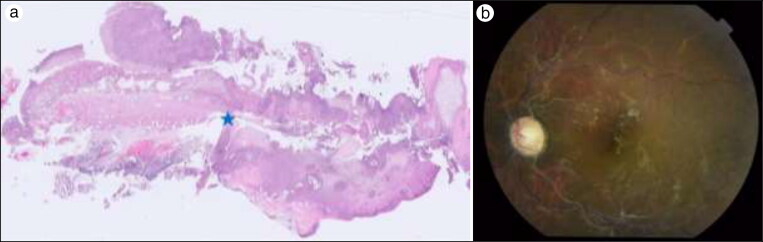
Figure 1.
(a) Small bowel segment with transmural necrosis of the wall (right half of the image), perforation with discontinuity of the muscular layer (indicated by blue star), and acute serositis (light microscopy, hematoxylin-eosin stain, 100× magnification). (b) Fundus photograph, left eye, showing optic nerve head pallor, diffusely sclerotic retinal arteries (silver wiring), venous tortuosity, collateral vessel formation along the superior and inferior vascular arcades and optic nerve head, and scattered hard exudates within the macula. Similar findings were apparent in the other eye.
In light of his catastrophic presentation involving multiple organs suspicious for TMA, a kidney biopsy was done and revealed ischemic glomerular injury (Figure 2). We believed our patient had a clinical presentation consistent with COVID-19–associated systemic TMA. He was initiated on eculizumab 900 mg weekly for four doses while continuing a tapering course of corticosteroids. At last check, the patient reported increasing urine output, though still requiring dialysis. Unfortunately, his vision remains poor despite intravitreal bevacizumab, with no light perception in his right eye and hand motion vision in his left.
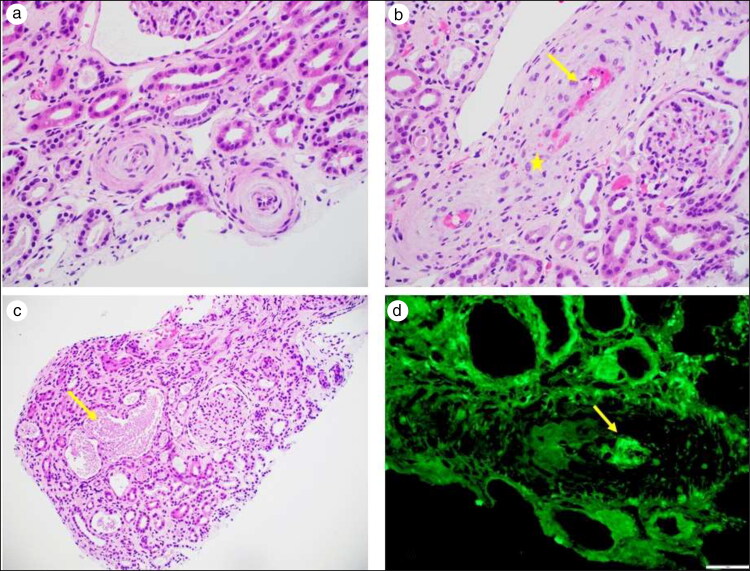
Figure 2.
Renal biopsy. (a) Arterioles with concentric intimal fibrosis and mucoid changes with swollen endothelial cells resulting in near luminal occlusion (light microscopy, hematoxylin-eosin stain, 400× magnification). (b) Interlobar artery with fibrin deposition (arrow) and fragmented red blood cells (star) in the vascular wall (light microscopy, hematoxylin-eosin stain, 400× magnification). (c) Tubular necrosis with sloughing of the tubular epithelial cells (arrow) (light microscopy, hematoxylin-eosin stain, 200× magnification). (d) Immunofluorescence microscopy with fibrinogen highlighting the presence of intravascular fibrin thrombus (immunofluorescence microscopy, fibrinogen, 400× magnification).
DISCUSSION
COVID-19 infection elicits pathologic syndromes far afield from the respiratory tissues that the virus primarily targets. Despite being the least common of the renal manifestations of COVID-19, TMA has the worst prognosis, with nearly all patients requiring dialytic support.3 The earliest mention of TMA was noted in June 2020, which involved a 69-year-old woman who decompensated rapidly and was found to have TMA by day 12. A kidney biopsy revealed evidence of intravascular thrombi.4
Endothelial damage is key to TMA, which has also been noticed with SARS-CoV-2. Interestingly, SARS-CoV-2 has been shown to bind to mannose binding lectin–associated serine protease-2 of the lectin pathway to generate lung injury.5 A prospective cohort observational study identified several pathologic variants of complement factor H, C5b-9, ADAMTS-13 in patients with COVID-19 infections who were found to have atypical hemolytic uremic syndrome.2 Our patient had essentially normal complement factor levels. In an autopsy series from the United Kingdom, thrombotic features were noted in at least one of the major organ systems in all their autopsy cases.6
Although our patient had markedly elevated blood pressure at presentation, malignant hypertension–associated TMA is unlikely, as he did not have any neurologic or cardiac manifestations or papilledema at presentation but instead exhibited ischemic retinal injury and bowel perforation in addition to pulmonary and renal manifestations as part of a systemic TMA.7 In a meta-analysis of 22 studies, 31 patients were identified, of whom one-third had small bowel ischemia and two-thirds required laparotomy and bowel resection.8 Retinal vasculature may be involved due to hypercoagulability, as part of a systemic TMA and a vasculitis-like process due to endothelial cell invasion by virus.9
Eculizumab, a C5b convertase inhibitor, has been shown to be the definitive treatment for atypical hemolytic uremic syndrome. It has been used off label in four critically ill patients from Italy with severe COVID-19 infections, where it rapidly improved the overall outcome.10 A case report from Turkey reported the first successful treatment of an adult COVID-19 patient with TMA treated with eculizumab.11 A high degree of clinical suspicion of microangiopathic disease coupled with early detection and aggressive therapeutic measures with plasmapheresis or complement pathway inhibitors is paramount to a successful outcome.
References
- 1.Da Silva RL. Viral-associated thrombotic microangiopathies. Hematol Oncol Stem Cell Ther. 2011;4(2):51–59. doi: 10.5144/1658-3876.2011.51. [DOI] [PubMed] [Google Scholar]
- 2.Magro C, Mulvey JJ, Berlin D, et al. . Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Ng JH, Bijol V, Jhaveri KD, Wanchoo R.. Pathology of COVID-19-associated acute kidney injury. Clin Kidney J. 2021;14(Suppl 1):i30–i39. doi: 10.1093/ckj/sfab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jhaveri KD, Meir LR, Chang BF, et al. . Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98(2):509–512. doi: 10.1016/j.kint.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavriilaki E, Brodsky RA.. Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily. Br J Haematol. 2020;189(6):e227–e230. doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]
- 6.Hanley B, Naresh KN, Roufosse C, et al. . Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thind G, Kailasam K.. Malignant hypertension as a rare cause of thrombotic microangiopathy. BMJ Case Rep. 2017;1–3. doi: 10.1136/bcr-2017-220457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keshavarz P, Rafiee F, Kavandi H, et al. Ischemic gastrointestinal complications of COVID-19: a systematic review on imaging presentation. Clin Imaging. 2021;73:86–95. doi: 10.1016/j.clinimag.2020.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen S, Kannan NB, Kumar J, et al. . Retinal manifestations in patients with SARS-CoV-2 infection and pathogenetic implications: a systematic review. Int Ophthalmol. 2021;41:1–14. doi: 10.1007/s10792-021-01996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diurno F, Numis FG, Porta G, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 11.Safak S, Aksoy E, Dirim AB, et al. . Successful treatment of a COVID-19 patient with thrombotic microangiopathy. Clin Kidney J. 2021;14(4):1287–1288. doi: 10.1093/ckj/sfab02. [DOI] [PMC free article] [PubMed] [Google Scholar]