Abstract
Objectives:
Cardioplegia is essential for adequate myocardial protection. There continues to remain ambiguity regarding the ideal cardioplegia for adequate myocardial protection in congenital heart surgery. This study compares clinical outcomes using St Thomas II solution and Del Nido cardioplegia in neonates undergoing cardiac surgery.
Methods:
All neonates (<30 days) from 2011 to 2017 who underwent surgery requiring cardioplegic arrest were analyzed retrospectively. We divided the cohort into two groups depending on cardioplegia received, as group A (Blood cardioplegia with St Thomas II solution, n = 56) and group B (Del Nido cardioplegia, n = 48). Various demographic, intraoperative, early postoperative, and discharge variables were analyzed.
Results:
Two groups were similar in age, gender, pre-operative diagnosis, and risk category. Cardiopulmonary bypass (CPB) time (P = 0.002), aortic cross-clamp (ACC) time (P = 0.018), and the number of doses of cardioplegia (P < 0.001) were significantly lower with Del Nido group. Though vasoactive inotropic score (VIS) (P = 0.036) was high during the first 24 h in the immediate postoperative period in group A, there was no difference in early mortality among both groups (P = 0.749). Both groups did not show significant differences related to various postoperative and discharge variables.
Conclusion:
When compared to St. Thomas solution, the use of Del Nido cardioplegia solution in neonates is associated with a significant decrease in CPB and ACC times and VIS in the first 24 h after surgery. The choice of cardioplegia (St Thomas/Del Nido) in neonates does not affect early mortality and early postoperative clinical outcomes.
Keywords: Clinical outcomes, Del Nido cardioplegia, neonatal congenital heart surgery, St Thomas blood cardioplegia, vasoactive ionotropic score
INTRODUCTION
The majority of congenital heart surgeries involve diastolic cardiac arrest as one of the strategies to maintain adequate myocardial protection.[1] Hence, cardioplegia forms the prime basis to perform the surgical repair. The development of cardioplegia was a significant milestone in the history of cardiac surgery. Since the development of potassium citrate solution by Melrose et al.,[2] numerous cardioplegia solutions have been developed and used in various centers worldwide.
Among various types of cardioplegia solutions, blood cardioplegia with St Thomas II and Del Nido cardioplegia are the most commonly used in neonatal cardiac operations. Blood cardioplegia with St Thomas II solution is an extracellular cardioplegia with high potassium concentration, which acts predominantly by depolarizing the cell membrane. This method of arrest involves perfusing repetitive dosing of cardioplegia once every 20--30 min. Repetitive dosing may interrupt the operation and may result in inadequate myocardial protection if the timing of cardioplegia is not appropriately followed.
The neonatal heart prefers single dose cardioplegia in various experimental studies.[3,4] Pedro Del Nido et al. at the University of Pittsburgh[5] developed a single dose cardioplegia, which provides adequate myocardial protection for more extended periods as compared to conventional cardioplegia techniques. Del Nido cardioplegia, a crystalloid based extracellular cardioplegia solution, predominantly exerts its effect by depolarizing the cell membrane. Though the potassium concentration is lower compared to St. Thomas solution, its action is prolonged because of magnesium and lignocaine as additives. Lignocaine is a sodium channel blocker that increases the myocyte refractory period. Further, it also prevents the negative effect of hyperkalemic depolarized arrest through its repolarizing effect. Both these additives have also been proven to improve myocardial recovery.
The needs of an immature myocardium and its response to ischemia remain contradictory. Immature myocardium of neonates is known to be more tolerant to ischemia[6,7] and their needs to achieve cardioplegic arrest differs from the adult heart in some studies.[8] This tolerance of immature myocardium to ischemia is enhanced in the presence of hypothermia.[9] Whereas, few studies have demonstrated it to be less tolerant to ischemia.[10] Furthermore, the dosing interval of cardioplegia in immature myocardium also remains contradictory, with few studies favoring single-dose and few in favor of multidose strategies.[11] Hence the choice of cardioplegia, the dosing strategies may result in variable clinical outcomes in neonates.
Since there remains ambiguity regarding the optimum cardioplegic solution for adequate myocardial protection in neonates, we compared our experience of using either blood cardioplegia with St Thomas II solution or Del Nido cardioplegia in neonates undergoing cardiac surgery.
MATERIALS AND METHODS
The study protocol was reviewed and approved by the technical advisory committee (SCT/IEC/1326/DECEMBER-2018) and the Institutional Ethics Committee of our institution. The study is retrospective by design, and data is collected from the institutional database and the medical records department. All neonates (<30 days) who underwent congenital heart surgery from 2011 to 2017, requiring cardiopulmonary bypass (CPB) and cardioplegic arrest, were included. Those who underwent surgery without CPB or where a cardioplegic arrest was not required were excluded from the study.
The study population consisted of two groups based on the type of cardioplegia solution administered. Group A – patients who received blood cardioplegia with St. Thomas II solution and Group B – patients who received Del Nido cardioplegia solution [Table 1]. There was no age bias, as the study population includes only neonates.
Table 1.
Composition of cardioplegia solutions
| Group A | Group B | ||
|---|---|---|---|
| Blood Cardioplegia in St. Thomas solution | Del Nido cardioplegia | ||
| Blood: Crystalloid 4: 1 | Blood: Crystalloid 1: 4 | ||
| Ringer’s lactate | 320 ml | Plasmalyte-A | 500 ml |
| Mannitol | 20 ml | KCl (2 meq/ml) | 6.5 ml |
| Bicarbonate | 20 ml | NaHCO3 | 6.5 ml |
| St. Thomas solution 40 ml | Lidocaine 2% | 3.25 ml | |
| KCl 16 mmol | Mannitol | 8.15 ml | |
| MgCl2 16 mmol | MgSO4 50% | 2 ml | |
| Procaine 1 mmol | |||
KCl – Potassium chloride, MgCl2 – Magnesium chloride, MgSO4 – Magnesium Sulfate, NaHCO3 – Sodium bicarbonate
Surgical procedure and cardioplegia protocol
All operations were performed by three senior surgeons, with expertise in neonatal cardiac surgery to avoid operator bias. The same perfusion team has handled all the above neonatal surgeries. There was no change in anesthesia and perfusion protocol during the study period. All operations were performed by primary median sternotomy, systemic hypothermia, cardioplegic myocardial protection with topical ice-cold saline cooling. There were no significant changes in inotrope and postoperative management strategies among both groups in the intraoperative and postoperative periods.
At our institute, St Thomas II cardioplegia was used until 2014. Since outcomes following Del Nido cardioplegia were promising, we changed our approach to myocardial protection to Del Nido cardioplegia since 2014. All three surgeons changed the approach at the same time.
We prepared the St Thomas II cardioplegia as four parts of blood taken from the arterial outlet with one part of the Ringer's lactate solution to which St. Thomas type 2 solution is added. Two strengths of the solution---high strength for initiation dose and low strength for maintenance dose were prepared. Cardioplegia is delivered through a cardioplegia delivery system at 6--8°C at a dose of 20 ml/kg for initiation and 10 ml/kg for maintenance once every 30 min.
We prepared Del Nido cardioplegia with one part of blood and four parts of the crystalloid (Plasmalyte A) solution [Table 1], which was delivered at 6--8°C. Cardioplegia is delivered at a dose of 20--30 ml/kg for induction and 10 ml/kg for maintenance of cardiac arrest once every 90--120 min. depending on the duration of the procedure or return of cardiac activity. In both the groups, cardioplegia was delivered through aortic root by antegrade approach.
Common congenital anomalies included the Dextro-Transposition of Great Arteries (D-TGA) and obstructed total anomalous pulmonary venous connection (TAPVC). The intraoperative and postoperative variables like Cardiopulmonary bypass (CPB) time, Aortic cross-clamp (ACC) time, the number of cardioplegia doses, post-CPB and postoperative (within first 24 h) arrhythmias, postoperative blood transfusion, vasoactive inotropic score (VIS) within first 48 h were collected from intensive care unit charts.
Postoperative outcomes assessed were low cardiac output syndrome (LCOS) in the first 24 h, the number of ventilatory hours, ICU stay, the total duration of hospital stay, and renal dysfunction. Left ventricular function was assessed by echocardiography before discharge. We defined early mortality as death within 30 days of operation. LCOS was defined as the presence of oliguria of less than 1 ml/kg/h or anuria with a rising trend of lactate level and an increase in inotropic score in the first 24 h. Though cardiac index, inflammatory markers, and markers of myocardial injury like troponin levels are better indicators of low cardiac output in the immediate postoperative period, these variables were not studied as we routinely do not measure these at our institution.
Statistical analysis
All collected data paired among both groups and analyzed using SPSS software (SPSS Inc, Chicago, Illinois, USA) and data are represented as mean with standard deviation or median with an interquartile range as appropriate. Further variables were assessed using the Student's t-test, Fischer's exact t-test, and Mann--Whitney test for significance. A P value of less than 0.05 is considered as statistically significant.
RESULTS
A total of 104 neonates underwent congenital heart operations under CPB and cardioplegic arrest from 2011 to 2017. 56 neonates received blood cardioplegia with St Thomas II solution (Group A), and 48 neonates received Del Nido cardioplegia (Group B). Three neonates with TAPVC had heterotaxy syndrome. Demographic characteristics between Groups A and B are depicted in Table 2. Cardiopulmonary bypass time (Group A 219 ± 51 min vs. Group B 184 ± 60 min, P = 0.002) and ACC (Group A 122 ± 33 min vs. Group B 106 ± 36 min, P = 0.018) times were significantly lower in group B as compared to group A as shown in Figure 1.
Table 2.
Demographic characteristics of both groups
| Variable | Group A (n=56, 53.8%) | Group B (n=48, 46.2%) | Total (n=104) | P * |
|---|---|---|---|---|
| Age (in days), mean (SD) | 17.8 (7.6) | 17 (9) | 17.4 (8.4) | 0.627 |
| Male, n (%) | 46 (82.1) | 39 (81.3) | 85 (81.7) | 1.00 |
| BSA (m2), mean (SD) | 0.19 (0.02) | 0.19 (0.02) | 0.19 (0.02) | 0.29 |
| Pre-operative Diagnosis, n (%) | ||||
| D-TGA | 38 (67.9) | 32 (66.7) | 70 (67.3) | - |
| Obstructed TAPVC | 15 (26.8) | 15 (31.3) | 30 (28.8) | - |
| Heterotaxy with obstructed TAPVC | 3 (5.4) | - | 3 (2.9) | - |
| HLHS | - | 1 (2.1) | 1 (1) | - |
| RACHS category, n (%) | ||||
| RACHS 3 | 31 (55.4) | 29 (60.4) | 60 (57.7) | - |
| RACHS 4 | 25 (44.6) | 18 (37.5) | 43 (41.3) | - |
| RACHS 5 | 0 | 1 (2.1) | 1 (1) | - |
Group A – Blood Cardioplegia with St. Thomas solution, Group B – Del Nido cardioplegia. BSA – Body Surface area, D-TGA: D – Transposition of Great Arteries, HLHS – Hypoplastic Left heart Syndrome, RACHS – Risk Adjusted Congenital Heart Surgery, SD – Standard deviation, TAPVC – Total Anomalous Pulmonary Venous Connection. *Student’s t-test
Figure 1.
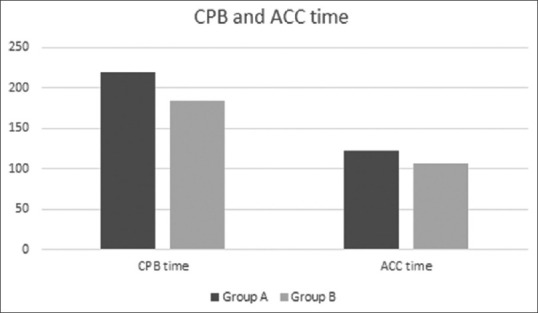
Comparison of CPB and ACC time (min) among both groups. ACC – Aortic cross clamp, CPB – Cardio pulmonary bypass
A variable which has shown its applicability in predicting mortality and morbidity in postoperative setting is the vasoactive inotropic score (VIS). On comparison of VIS for the first 24 h and the subsequent 24 h, VIS during the first 24 h (St. Thomas group A - 10, IQR 7.5--10; Del Nido group - B 7.5, IQR 5--10) was found to be significantly lower in neonates operated with Del Nido cardioplegia. Whereas there appears to be a clinically significant reduction of inotropic support in group A in the next 24 h. However, this reduction was not observed in group B. Hence, VIS in both the groups was similar in the subsequent 24 h and did not maintain this significant difference.
Though blood transfusion requirement was higher during the first 24 h in group B, there exists no significant difference among both groups. Similarly, no significant difference was observed in the incidence of LCOS and postoperative renal dysfunction (serum creatinine levels) among both groups. There was no significant difference between the two groups for the number of ventilatory hours, ICU stay, and hospital stay [Table 3]. The incidence of postoperative arrhythmias is less in group B, but we did not observe a statistically significant difference.
Table 3.
Comparison analysis of various intra-operative and post-operative variables among two groups
| Variable | Group A (n=56, 53.8%) | Group B (n=48, 46.2%) | Total (n=104) | P * |
|---|---|---|---|---|
| Cardio pulmonary bypass (CPB) time (min), mean (SD) | 219.23 (51.3) | 184.7 (60) | 203.29 (57.87) | 0.002 |
| Aortic cross clamp (ACC) time (min), mean (SD) | 122.29 (33.3) | 106.02 (35.9) | 114.78 (35.31) | 0.018 |
| Number of doses of cardioplegia, median (IQR) | 3 (3-4) | 1 (1-2) | 2 (2-3) | <0.001 |
| Priming Volume (ml), mean (SD) | 306.95 (55.78) | 319.52 (49.18) | 312.75 (52.96) | 0.229 |
| Post-op blood transfusion during first 24 hrs. (ml), median (IQR) | 30 (2.5-40) | 30 (14.75-58.75) | 30 (10-50) | 0.185 |
| Vasoactive inotropic score (VIS) first 24 hrs., median (IQR) | 10 (7.5-15) | 7.5 (5-12.5) | 9 (6.62-13.37) | 0.036 |
| Vasoactive inotropic score (VIS) next 24 hrs., median (IQR) | 9 (5-10) | 7.5 (5-10) | 7.5 (5-10) | 0.592 |
| Mechanical ventilation (hrs.), Median (IQR) | 91 (66.25-117) | 89 (68-129) | 90 (67.25-119.75) | 0.876 |
| Mean ICU stay (days), Median (IQR) | 9.5 (8-13.75) | 10.5 (7-14.75) | 10 (7-14) | 0.749 |
| Hospital stay (days), Median (IQR) | 14 (12-21) | 14 (10-18.75) | 14 (11-20) | 0.310 |
| Post-op Arrhythmias, n (%) | 9 (16.1) | 8 (16.7) | 17 (16.3) | 1 |
| Defibrillation, n (%) | 3 (5.4) | 0 | 3 (2.9) | 0.247 |
| Low cardiac output syndrome, n (%) | 23 (41.1) | 17 (35.4) | 40 (38.5) | 0.686 |
| Post-op creatinine (mg/dl), mean (SD) | 0.76 (0.4) | 0.85 (0.31) | 0.8 (0.36) | 0.176 |
| Post-op platelet count (L/c.mm), mean (SD) | 1.15 (0.59) | 1.33 (0.67) | 1.23 (0.63) | 0.134 |
| 30-day mortality, n (%) | 6 (10.7) | 4 (8.3) | 10 (9.6) | 0.749 |
Group A – Blood cardioplegia with St. Thomas solution, Group B – Del Nido cardioplegia solution. ICU – Intensive care Unit, IQR – Inter Quartile Range, SD – Standard Deviation. *Student’s t-test, Fischer’s exact t-test, Mann-Whitney test
Early mortality in this study was 10.4% (10), of which six are from group A and four from group B. Even though number of deaths in group B are less than group A, there was no statistically significant difference. The survival trend among both groups is shown in Figure 2. Subgroup analysis based on the diagnosis of D-TGA and obstructed TAPVC did not reveal any significant difference in mortality between the two groups. A detailed description of mortality is included in Supplementary Table 1.
Figure 2.
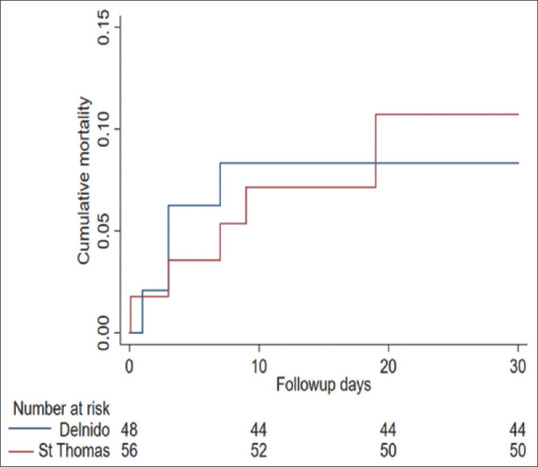
Kaplan-Meier curve showing survival trend in both groups
Of six deaths in group A, three patients had heterotaxy syndrome with obstructed TAPVC and duct dependent pulmonary circulation. They presented with severe acidosis, desaturation, and maintained on prostaglandin infusion. One patient developed sepsis with occult gastrointestinal bleed following arterial switch procedure. Of the four mortality in group B, one patient had HLHS and underwent stage 1 Norwood with Sano shunt. This patient required Extracorporeal membrane oxygenation (ECMO) support in the postoperative period, and we could not wean off ECMO. Another neonate who had D-TGA with an interrupted aortic arch continued to remain in severe low cardiac output in the postoperative period, and we could not institute ECMO.
Comment
Over the decades, various strategies have developed for safe and effective myocardial protection.[5] Cardioplegia forms the cornerstone of myocardial preservation. Pediatric immature myocardium is structurally, physiologically, and metabolically different from the mature adult heart, and many researchers have focused attention on developing the best myocardial protection for pediatric congenital heart surgeries.[1,12,13] Even though significant advances in cardioplegia strategies resulted in improved outcomes, new methods continue to emerge,[14] indicating a continuous quest to identify an ideal cardioplegic solution to achieve the best results.
Multiple cardioplegic solutions were developed to protect immature myocardium without causing significant metabolic changes by focusing on specific intracellular processes.[15,16,17,18] Elaborative animal and experimental studies have targeted various biochemical and molecular mechanisms during the ischemic period of surgery, contributing to progress in this field. Despite this progress, there is still no consensus on the best cardioplegic strategy.[4,19,20]
Expert pediatric cardiac surgical centers worldwide continue to use various solutions depending on their experience and outcome over the years. Introduction of Del Nido cardioplegia developed by Pedro Del Nido et al. in the early 1990s has led to a change in the practice of cardioplegic arrest in many centers.[5] Though Del Nido solution has the potential benefit of arresting heart for a prolonged period with reduced arrhythmogenic potential because of its lignocaine and magnesium as content, the use of Del Nido solution for cardioplegia is still not universal.[21]
Studies have shown St. Thomas II cardioplegia as being both effective and ineffective for neonatal hearts.[4,10,13] Initial experimental animal studies were inconclusive, as neonatal hearts preferred single dose cardioplegia in some studies, while no difference was observed with multidose cardioplegia in other studies.[3,6] Better myocardial protection can be achieved postoperatively by reducing energy consumption during ischemia, which can be attained by a decrease in myocardial excitability and intracellular calcium ion concentration.[21]
There are few retrospective studies and one randomized controlled trial comparing the Del Nido solution's effectiveness to others in the pediatric population, and none specifically studied among neonatal hearts.[22,23] Retrospective studies on the infants have shown similar clinical outcomes in both groups without proven superiority over other.[11,23] This study compares the use of St Thomas II solution to Del Nido solution exclusively among neonates undergoing complex procedures, as they represent the most immature form of the myocardium, eliminating differential maturity of different hearts as age advances.
Charette et al.[11] showed Del Nido solution to be safer for prolonged periods, with no significant impact on postoperative outcomes. A recent study by Lenoir et al.[24] compared Del Nido versus blood cardioplegia in adult aortic surgery and found interesting results on prolonged periods of cardioplegic arrest. In their study, patients with myocardial ischemia for more than 180 min. were found to have higher median creatine kinase MB isotype in Del Nido group. Hence, they concluded that Del Nido cardioplegia solution might be associated with increased myocardial injury, especially during prolonged ischemia times, however, this did not translate into clinical outcomes in their study.
Pourmoghadam et al.[23] compared Del Nido solution with non-Del Nido solution in infantile and neonatal hearts with similar clinical outcomes. The use of the Del Nido solution was, however, associated with the advantage of less interruption of operation and lower volume of cardioplegia. There was no significant superiority in terms of postoperative clinical outcomes or early morbidity and mortality in either cardioplegia strategies.
Though CPB and ACC times are significantly lower in neonates who received Del Nido cardioplegia as they receive less frequent dosing (Prolonged action), these cannot be attributed entirely to lesser interruption of operation to administer cardioplegia alone but can also be related to probable “learning curve effect.”
We observed similar results in other studies comparing these cardioplegia strategies. Even the dosing volume was lower in the Del Nido group. In this study, we repeated the Del Nido solution at intervals of 90 min. This duration was similar to Charette et al.[11] and higher than Pourmoghadam et al.[23] (60 min.). We did not observe the return of activity during this period or increased incidence of ventricular arrhythmias during recovery. We presume that myocardial protection was maintained during these prolonged intervals, though there was no quantitative evidence to support the same as we do not routinely perform troponin levels during operation. Transesophageal echocardiography is not routinely performed for neonates, whereas epicardial echo performed did not reveal significant ventricular dysfunction or other gross abnormalities.
The incidence of LCOS was more in group A (St Thomas II) than in group B (Del Nido), although not statistically significant. The randomized trial by Talwar et al.[22] has shown better preservation of myofibrillar architecture and preservation of cardiac indices with the Del Nido solution. Their study population involved older children who underwent elective surgeries for ventricular septal defects and Tetralogy of Fallot. Pediatric myocardium in their group is more mature than neonatal myocardium, and surgeries are less complex requiring lesser CPB times.
Our study demonstrated that the mean vasoactive inotropic scores (VIS) in neonates of group A during the first 24 h were higher as compared to group B, and it is statistically significant. These findings are consistent with the results of Pourmoghadam et al.[23] Whereas vasoactive scores assessed for the subsequent 24 h did not reveal any difference. The higher VIS score in group A neonates probably suggests a higher degree of myocardial depression, which gradually resolved over the next 48 h of operation. The incidence of postoperative arrhythmias is higher in group A and comparable to group B. This protective effect might be because of lignocaine (increases the myocyte refractory period) in group B neonates.[25] Although the myocardial function is depressed more in group A than group B in the immediate postoperative period, the incidence of early mortality is similar in both groups.[26,27]
Even though early postoperative clinical parameters (first 24 h) were unfavorable in group A, late postoperative clinical outcomes were similar in both groups without significant differences. Mean ventilatory days did not differ significantly among both groups. Besides, the length of ICU stay and total hospital stay were similar in both groups. These findings are consistent with the final clinical outcomes of Pourmoghadam et al,[23] whereas in contrast with findings of trial by Talwar et al.[22] These contrast findings could have been due to different sets of cohorts, including older children and complexity of operating condition (RACHS 2 & 3), whereas similar findings could have been possible because of a similar patient population. Though there was no significant difference among both groups in late outcomes regarding cardioplegia strategy, it is difficult to conclude the effect of cardioplegia solution on outcomes.
CONCLUSIONS
Del Nido cardioplegia is associated with favorable early (first 24 h) clinical outcomes. Use of Del Nido cardioplegia is associated with significantly decreased vasoactive inotropic score and decreased incidence of low cardiac output only during the first 24 h after surgery. After the first 24 h, postoperative clinical outcomes are similar to both Del Nido and ST Thomas cardioplegia solution.
Limitations
This is a single-center retrospective study. The small sample size precludes sophisticated statistical analyses and may lack the power to detect significant differences in outcome. The study is retrospective, objective analysis of myocardial function by enzyme assays was not possible in this study. However, if biomarkers for myocardial ischemia or injury have been measured, it would have provided weightage to our observations. As study groups were from two different time periods, changes over time can have a “learning curve effect” and act as a confounder for the variables measured.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We sincerely thank Mr. Manas Chacko for help in statistical analysis. We express our gratitude to Mrs. Beegum Thaslim, chief of clinical perfusion for providing expert advice in drafting the article.
REFERENCES
- 1.Talwar S, Keshri V, Choudhary S, Airan B. Myocardial protection in neonates and infants: What have we learnt? Where do we go? J Heart Circ. 2015:1. [Google Scholar]
- 2.Melrose DG, Dreyer B, Bentall HH, Baker JB. Elective cardiac arrest. Lancet. 1955;269:21–2. doi: 10.1016/s0140-6736(55)93381-x. [DOI] [PubMed] [Google Scholar]
- 3.Sawa Y, Matsuda H, Shimazaki Y, Kadoba K, Onishi S, Nakada T, et al. Comparison of single dose versus multiple dose crystalloid cardioplegia in neonate.Experimental study with neonatal rabbits from birth to 2 days of age. J Thorac Cardiovasc Surg. 1989;97:229–34. [PubMed] [Google Scholar]
- 4.Kohman LJ, Veit LJ. Single-dose versus multidose cardioplegia in neonatal hearts. J Thorax Cardiovasc Surg. 1994;107:1512–8. [PubMed] [Google Scholar]
- 5.Matte GS, del Nido PJ. History and use of del Nido cardioplegia solution at Boston Children's Hospital. J Extra Corpor Technol. 2012;44:98–103. [PMC free article] [PubMed] [Google Scholar]
- 6.Bove EL, Stammers AH. Recovery of left ventricular function after hypothermic global ischemia.Age-related differences in the isolated working rabbit heart. J Thorac Cardiovasc Surg. 1986;91:115–22. [PubMed] [Google Scholar]
- 7.Mayer JE. Myocardial preservation in the immature heart. In: Piper HM, Preusse CJ, editors. Ischemia-Reperfus Card Surg. Dordrecht: Springer Netherlands; 1993. pp. 279–91. [Google Scholar]
- 8.Hiramatsu T, Zund G, Schermerhorn ML, Shinóka T, Miura T, Mayer JE., Jr Age differences in effects of hypothermic ischemia on endothelial and ventricular function. Ann Thorac Surg. 1995;60:S501–4. doi: 10.1016/0003-4975(95)00814-4. [DOI] [PubMed] [Google Scholar]
- 9.Doenst T, Schlensak C, Beyersdorf F. Cardioplegia in pediatric cardiac surgery: Do we believe in magic? Ann Thorac Surg. 2003;75:1668–77. doi: 10.1016/s0003-4975(02)04829-4. [DOI] [PubMed] [Google Scholar]
- 10.Wittnich C, Peniston C, Ianuzzo D, Abel JG, Salerno TA. Relative vulnerability of neonatal and adult hearts to ischemic injury. Circulation. 1987;76:V156–60. [PubMed] [Google Scholar]
- 11.Charette K, Gerrah R, Quaegebeur J, Chen J, Riley D, Mongero L, et al. Single dose myocardial protection technique utilizing del Nido cardioplegia solution during congenital heart surgery procedures. Perfusion. 2012;27:98–103. doi: 10.1177/0267659111424788. [DOI] [PubMed] [Google Scholar]
- 12.Allen BS. Pediatric myocardial protection: Where do we stand? J Thorac Cardiovasc Surg. 2004;128:11–3. doi: 10.1016/j.jtcvs.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Magovern JA, Pae WE, Jr, Miller CA, Waldhausen JA. The immature and the mature myocardium.Responses to multidose crystalloid cardioplegia. J Thorac Cardiovasc Surg. 1988;95:618–24. [PubMed] [Google Scholar]
- 14.Kotani Y, Tweddell J, Gruber P, Pizarro C, Austin EH, 3rd, Woods RK, et al. Current cardioplegia practice in pediatric cardiac surgery: A North American multiinstitutional survey. Ann Thorac Surg. 2013;96:923–9. doi: 10.1016/j.athoracsur.2013.05.052. [DOI] [PubMed] [Google Scholar]
- 15.Ginther RM, Gorney R, Forbess JM. Use of del Nido cardioplegia solution and a low-prime recirculating cardioplegia circuit in pediatrics. J Extra Corpor Technol. 2013;45:46–50. [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien JD, Howlett SE, Burton HJ, O’Blenes SB, Litz DS, Friesen CL. Pediatric cardioplegia strategy results in enhanced calcium metabolism and lower serum troponin T. Ann Thorac Surg. 2009;87:1517–23. doi: 10.1016/j.athoracsur.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 17.Handy JR, Jr, Dorman BH, Cavallo MJ, Hinton RB, Roy RC, Crawford FA, et al. Direct effects of oxygenated crystalloid or blood cardioplegia on isolated myocyte contractile function. J Thorac Cardiovasc Surg. 1996;112:1064–72. doi: 10.1016/S0022-5223(96)70108-3. [DOI] [PubMed] [Google Scholar]
- 18.Amark K, Berggren H, Björk K, Ekroth A, Ekroth R, Nilsson K, et al. Blood cardioplegia provides superior protection in infant cardiac surgery. Ann Thorac Surg. 2005;80:989–94. doi: 10.1016/j.athoracsur.2005.03.095. [DOI] [PubMed] [Google Scholar]
- 19.Caputo M, Modi P, Imura H, Pawade A, Parry AJ, Suleiman MS, et al. Cold blood versus cold crystalloid cardioplegia for repair of ventricular septal defects in pediatric heart surgery: A randomized controlled trial. Ann Thorac Surg. 2002;74:530–4. doi: 10.1016/s0003-4975(02)03695-0. discussion 535. [DOI] [PubMed] [Google Scholar]
- 20.Modi P, Suleiman M-S, Reeves B, Pawade A, Parry AJ, Angelini GD, et al. Myocardial metabolic changes during pediatric cardiac surgery: A randomized study of 3 cardioplegic techniques. J Thorac Cardiovasc Surg. 2004;128:67–75. doi: 10.1016/j.jtcvs.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 21.Dobson GP, Jones MW. Adenosine and lidocaine: A new concept in nondepolarizing surgical myocardial arrest, protection, and preservation. J Thorac Cardiovasc Surg. 2004;127:794–805. doi: 10.1016/s0022-5223(03)01192-9. [DOI] [PubMed] [Google Scholar]
- 22.Talwar S, Bhoje A, Sreenivas V, Makhija N, Aarav S, Choudhary SK, et al. Comparison of del Nido and St Thomas cardioplegia solutions in pediatric patients: A prospective randomized clinical trial. Semin Thorac Cardiovasc Surg. 2017;29:366–74. doi: 10.1053/j.semtcvs.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Pourmoghadam KK, Ruzmetov M, O’Brien MC, Piggott KD, Plancher G, Narasimhulu SS, et al. Comparing del Nido and conventional cardioplegia in infants and neonates in congenital heart surgery. Ann Thorac Surg. 2017;103:1550–6. doi: 10.1016/j.athoracsur.2016.10.070. [DOI] [PubMed] [Google Scholar]
- 24.Lenoir M, Bouhout I, Jelassi A, Cartier R, Poirier N, El-Hamamsy I, et al. Del Nido cardioplegia versus blood cardioplegia in adult aortic root surgery. J Thorac Cardiovasc Surg. 2020 doi: 10.1016/j.jtcvs.2020.01.022. S0022-5223(20)30235-X. doi: 10.1016/j.jtcvs. 2020.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Buel ST, Striker CW, O’Brien JE. del Nido versus St.Thomas cardioplegia solutions: A single-center retrospective analysis of post cross-clamp defibrillation rates. J Extra Corpor Technol. 2016;48:67–70. [PMC free article] [PubMed] [Google Scholar]
- 26.Kansy A, Tobota Z, Maruszewski P, Maruszewski B. Analysis of 14,843 neonatal congenital heart surgical procedures in the European Association for Cardiothoracic Surgery Congenital Database. Ann Thorac Surg. 2010;89:1255–9. doi: 10.1016/j.athoracsur.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs JP, He X, Mayer JE, Jr, Austin EH, 3rd, Quintessenza JA, Karl TR, et al. Mortality trends in pediatric and congenital heart surgery: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2016;102:1345–52. doi: 10.1016/j.athoracsur.2016.01.071. [DOI] [PubMed] [Google Scholar]


